Abstract
Three laboratories participated with their laboratory-specific microbiologic growth assays (MA) in the NHANES 2007–2008 to assess whether the distributions of serum (n = 2645) and RBC folate (n = 2613) for the same one-third sample of participants were comparable among laboratories. Laboratory (L) 2 produced the highest and L1 the lowest serum and RBC folate geometric means (nmol/L) in the NHANES sample (serum: L1, 39.5; L2, 59.2; L3, 47.7; and RBC: L1, 1120; L2, 1380; L3, 1380). Each laboratory produced different reference intervals for the central 95% of the population. Pearson correlation coefficients were highest between L3 and L1 (serum, r = 0.95; RBC, r = 0.92) and lowest between L2 and L1 (serum, r = 0.81; RBC, r = 0.65). Notable procedural differences among the laboratories were the Lactobacillus rhamnosus microorganism (L1 and L3: chloramphenicol resistant, L2: wild type) and the calibrator [L1: [6S]5-methyltetrahydrofolate (5-methylTHF), L2: [6R,S] 5-formyltetrahydrofolate ([6R,S] 5-formylTHF), L3: folic acid (FA)]. Compared with 5-methylTHF as calibrator, the folate results were 22–32% higher with FA as calibrator and 8% higher with 5-formylTHF as calibrator, regardless of the matrix (n = 30 serum, n = 28 RBC). The use of different calibrators explained most of the differences in results between L3 and L1 but not between L2 and L1. The use of the wild-type L. rhamnosus by L2 appeared to be the main reason for the differences in results between L2 and the other 2 laboratories. These findings indicate how assay variations influence MA folate results and how those variations can affect population data. To ensure data comparability, better assay harmonization is needed.
Introduction
Serum and RBC folate concentrations are important nutritional status markers. The NHANES has measured these concentrations for over 30 y. The introduction of mandatory folic acid (FA)10 fortification in 1998 contributed to appreciable increases in blood folate concentrations in the U.S. population (1). This meant continued monitoring of folate status through NHANES. Since 1991, that monitoring had been done with the Bio-Rad QuantaPhase II radioassay (Bio-Rad Laboratories), but in 2007, the manufacturer discontinued its production. For NHANES 2007–2008, CDC selected the traditional microbiologic growth assay (MA) using Lactobacillus rhamnosus (formerly known as L. casei) to measure serum and RBC folate levels.
Important improvements were made to the MA in the 1960s, such as the development of a chloramphenicol-resistant strain of the organism, eliminating the need for sterilization or aseptic addition and enabling the use of disposable labware; the ability to cryopreserve the inoculum, providing standardized growth curves for hundreds of assays; and the introduction of automated microtiter plate technology, miniaturizing the assay and providing dramatically improved efficiency in absorbance readings (2). These improvements made it possible to use the MA in a high-throughput routine setting. The MA is sensitive and therefore requires only small sample volumes; it is known to measure all folate vitamers equally (3, 4), whereas clinical protein binding assays may have different recoveries for different folate vitamers (4–7). It is also a comparatively inexpensive assay. Some countries use the MA in their national nutrition surveys for population monitoring and it is of interest to know whether population results among laboratories can be compared. There is currently no guidance on using a specific MA protocol or calibrator and countries implement the MA for their surveys expecting comparable results when the same type of methodology is used.
It is widely known that clinical folate assays using mainly a competitive protein binding format lack good agreement, particularly for RBC folate (2, 8, 9). Little information is available on the comparability of MAs across laboratories. Small sample sets have shown that mean results produced by different laboratories were not always in agreement (8, 9). The causes for such disagreement, however, have remained uninvestigated. It is not known how method differences affect the distributions of folate concentrations in the population or the classification of the population regarding folate status. This complicates any attempt to compare data from different laboratories.
We set out to address the above questions by conducting a comprehensive method comparison study among 3 expert laboratories using their laboratory-specific MA to analyze serum and whole blood samples from the same one-third sample of NHANES 2007–2008 participants. Our study allows for the first time, to our knowledge, to generate percentile distributions and reference intervals from 3 different MA to investigate potential differences among laboratories. To identify potential sources of differences among these 3 laboratories, calibrators and quality control (QC) samples were exchanged and available reference materials were analyzed.
Participants and Methods
NHANES 2007#x20132008 sample.
Serum and EDTA whole blood samples were collected in the NHANES mobile examination center during 2007–2008 and a one-third sample of participants 1 y and older was used for this comparison study. An aliquot of EDTA whole blood was either diluted (1/11) with 1% ascorbic acid solution [for laboratory (L)1 and 3] or frozen directly (for L2, which added ascorbic acid solution later). Each laboratory received aliquots of frozen serum (n = 2645) and whole blood/hemolysate (n = 2613) via dry ice shipments weekly. All respondents gave their informed consent and the NHANES protocol was reviewed and approved by the National Center for Health Statistics Institutional Review Board.
Sample exchange study.
To identify potential sources of differences among these laboratories, in 2008 we conducted a sample exchange study. Each laboratory provided calibrators and QC samples to the other 2 laboratories and each laboratory analyzed available reference materials. The calibrators from L1 included several folate stock solutions: [6S]5-methyltetrahydrofolate (5-methylTHF), 100 mg/L (218 μmol/L), Merck Eprova (Merck & Cie), used as a calibrator in the MA; [6S]5-formyltetrahydrofolate ([6S]5-formylTHF), 100 mg/L (211 μmol/L), Merck Eprova; FA (pteroylglutamic acid), 100 mg/L (227 μmol/L), Merck Eprova; and FA, 500 μg/L (1133 nmol/L), Sigma (Sigma Chemicals), 2 different lot numbers. The calibrator from L2 was [6R,S]5-formylTHF (racemic), 23 μmol/L (Sigma). The calibrator from L3 was FA, 100 μg/L (227 nmol/L) (Sigma). QC samples included 3 levels each of serum and whole blood hemolysate pools from L1, 2 levels of serum pools from L2, and 4 levels each of serum and whole blood hemolysate pools from L3. The reference materials were from the National Institute of Standards and Technology (NIST) and the National Institute for Biological Standards and Controls (NIBSC). They included 3 levels of the NIST SRM 1955 serum material (10), 1 level of the NIBSC 03/178 serum material (11), and 1 level of the NIBSC 95/528 whole blood material (12). Additionally, 20 randomly selected NHANES serum and whole blood samples were also analyzed by each laboratory as part of the sample exchange study.
Laboratory MA protocols.
We allowed the expert laboratories to use their laboratory-specific supplies, calibrators, and MA protocols, because this would be the case if different countries conducted their national nutrition surveys. Table 1 summarizes the procedural differences among the 3 laboratories. L1 and 3 performed a 96-well plate assay using chloramphenicol-resistant cryo-preserved L. rhamnosus (ATCC 27773 or NCIB 10463) (3, 13). L1 used 5-methylTHF as a calibrator (concentration verified spectrophotometrically at 290 nm). L3 used FA (concentration verified spectrophotometrically at 283 nm). Both laboratories prepared an 11-point calibration curve covering the range of 0–1.0 nmol/L with 8 replicates/point in each assay. L1 used polynomial regression (3rd degree), whereas L3 used 4-parameter logistic curve fitting. Both laboratories used a robotic work station to dilute and dispense samples and reagents into the 96-well plate. L1 prepared 4 replicates/sample at 2 dilutions using 0.5% sodium ascorbate (1/100 and 1/200 for serum and 1/140 and 1/280 for whole blood hemolysate). L3 prepared 4 replicates per sample at 2 dilutions using 0.5% sodium ascorbate (1/160 and 1/320 for both serum and whole blood hemolysate samples). But L3 analyzed each blood sample twice, so that 8 estimates contributed to the final reported value. L3 also incubated the thawed whole blood hemolysate for 30 min at room temperature before performing the dilutions. L1 omitted this step; it was shown previously not to influence the results (14). L1 and 3 incubated sealed 96-well plates for 42–45 h at 37°C and measured turbidity at 590 nm using a microplate reader. L2 performed a 96-well plate assay using wild-type, cryo-protected L. rhamnosus (ATCC 7469) and 5-formylTHF as a calibrator (concentration verified spectrophotometrically at 282 nm) (15). Because the microorganism is not antibiotic-resistant, sterile labware was used and reagents were filtered through a 0.22-μm microfilter to prevent bacterial contamination. A 7-point calibration curve (logit-log fit) was prepared for every 5 unknown samples covering the range of 0.04–0.27 nmol/L, with 2 replicates/point (n = 14). Sample dilutions and dispensing were performed manually using 8-channel pipettes. Samples were tested at 7 dilutions from 1/40 to 1/2560 with 2 replicates/dilution. Results from 2 or more consecutive dilutions with acceptable agreement (<20% CV) were used in the calculation of the final reported value. L2 prepared hemolysates from thawed whole blood by 1/10 dilution with 0.1 mol/L potassium phosphate buffer (pH 6.3) containing 1% ascorbic acid and incubated the hemolysates for 60 min at 37°C. A shorter incubation time of 16–20 h at 37°C was sufficient for the growth of the wild-type L. rhamnosus. Turbidity was measured at 600 nm.
TABLE 1.
Procedural differences between the folate microbiological assays performed in the 3 laboratories
| L1 | L2 | L3 | |
| L. rhamnosus | Chloramphenicol resistant (ATCC 27773) | Wild-type (ATCC 7469) | Chloramphenicol resistant (ATCC 27773) |
| Folate calibrator | 5-MethylTHF from Eprova | 5-FormylTHF (racemic) from Sigma | FA from Sigma |
| WB1 hemolysate | WB diluted 1/11 with 1% ascorbic acid within 1 h of phlebotomy (hemolysate pH, 3.8) | WB frozen within 1 h of phlebotomy; at the time of analysis diluted 1/10 with 0.1 mol/L K3PO4 (pH 6.3) with 1% ascorbic acid (hemolysate pH, 4.5) and incubated for 60 min/37°C | WB diluted 1/11 with 1% ascorbic acid within 1 h of phlebotomy (hemolysate pH, 3.8); at the time of analysis incubated for 30 min/RT1 |
| Dilutions/sample (diluent), n | 2 (0.5% Na ascorbate) | 7 (0.1 mol/L K3PO4, pH 6.3 with 0.1% ascorbic acid) | 2 (0.5% Na ascorbate) |
| Replicates/dilution, n | 2 | 2 | 4 |
| Incubation time at 37°C | 43–45 h | 16–20 h | 42 h |
| Curve fitting | Polynomial regression (3rd order) | Logit-log fitting | 4 Parameter logistic |
| Internal QC | 3 Serum/3 WB QC pools, 2 serum/2 WB blind QC pools | 2 Serum QC pools (containing 1% ascorbic acid), no WB QC | 4 Serum/4 WB QC pools |
| Use of international reference materials | NIST SRM 1955, NIBSC 03/178, NIBSC 95/528; minimum twice annually | NIST SRM 1955; twice annually | NIST SRM 1955; 6 times annually |
| Serum folate assay between-run imprecision (concentration range, n runs) | 6.9–8.6% (6.7–49 nmol/L, 59–78 runs) | 4.0–9.3% (23–82 nmol/L, 116 runs) | 5.2–6.8% (7.4–81 nmol/L, 64–73 runs) |
| RBC folate assay between-run imprecision (concentration range, n runs) | 6.7–13% (407–1570 nmol/L, 36–105 runs) | Data not available | 6.2–7.7% (397–1110 nmol/L, 64 runs) |
RT, room temperature, WB, whole blood.
Statistical analysis.
For each laboratory, we calculated descriptive statistics (mean ± SE, geometric mean ± SE, selected percentiles) for the one-third NHANES sample. We calculated the prevalence of low or high folate values for each laboratory using various cutoff levels. We determined the Pearson correlation coefficients and assessed agreement among the laboratories by Deming regression and Bland-Altman difference plot analysis by using Microsoft Excel, with a clinical statistical analysis plug-in (Analyze-it; Analyze-it-Software). All statistical comparisons were evaluated at a significance level of α = 0.05.
Results
NHANES 2007#x20132008 sample.
Both serum and RBC folate geometric means (nmol/L) were highest in L2 and lowest in L1 (Table 2). The same was true for selected percentiles for serum folate. For RBC folate, L2 produced the lowest values of the 3 laboratories at lower percentiles and the highest values at and above the median. The central 95% reference intervals (2.5th and 97.5th percentiles, nmol/L) for serum folate were different for each laboratory: 12.6–107 (L1), 18.0–178 (L2), and 14.5–138 (L3). The same was true for RBC folate: 506–2600 (L1), 460–3660 (L2), and 617–3370 (L3).
TABLE 2.
Descriptive statistics for serum and RBC folate results for the 3 laboratories, NHANES 2007–2008
| Serum folate, nmol/L, n = 2645 | RBC folate, nmol/L, n = 2613 | |||||
| L1 | L2 | L3 | L1 | L2 | L3 | |
| Mean ± SE | 45.8 ± 0.54 | 70.0 ± 0.85 | 55.7 ± 0.62 | 1210 ± 10.5 | 1570 ± 16.2 | 1500 ± 12.7 |
| Geomean ± SE | 39.5 ± 0.42 | 59.2 ± 0.68 | 47.7 ± 0.53 | 1120 ± 8.73 | 1380 ± 14.0 | 1380 ± 10.9 |
| Selected percentiles | ||||||
| 1 | 10.8 | 14.0 | 12.0 | 430 | 382 | 533 |
| 2.5 | 12.6 | 18.0 | 14.5 | 506 | 460 | 617 |
| 10 | 19.4 | 27.0 | 21.7 | 682 | 686 | 825 |
| 25 | 27.8 | 41.0 | 33.0 | 873 | 1020 | 1080 |
| 50 | 40.6 | 61.0 | 49.9 | 1120 | 1420 | 1370 |
| 75 | 56.9 | 88.0 | 71.2 | 1430 | 1940 | 1760 |
| 90 | 76.0 | 122 | 94.8 | 1800 | 2580 | 2270 |
| 97.5 | 107 | 178 | 138 | 2600 | 3660 | 3370 |
| 99 | 149 | 238 | 159 | 3210 | 4380 | 3880 |
Few NHANES participants had serum and RBC folate levels below the traditional cutoff value that could indicate deficiency (17): 2, 1 and 2 of a total of 2645 participants had serum folate values < 7 nmol/L when analyzed by L1, 2, and 3, respectively; 2, 12, and 1 of a total of 2613 participants had RBC folate values < 318 nmol/L, respectively.
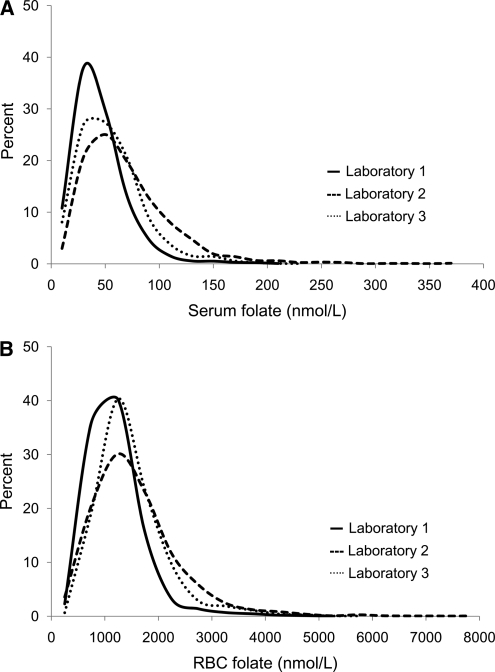
When we used folate concentrations at selected percentile values obtained by L1 as cutoff values, proportions for low- or high-folate concentrations were different across laboratories (Table 3). For example, at the 2.5th percentile for L1, the proportions for low serum folate levels were one-half and one-fifth for L2 and 3, respectively; the proportions for low RBC folate values were one-third and one-and-a half for L2 and 3. This difference also appears in the frequency distribution curves obtained by the 3 laboratories for serum and RBC folate (Fig. 1). The distribution curves for L2 were widest and most positively skewed.
TABLE 3.
Proportions of low or high folate concentrations for the 3 laboratories, NHANES 2007–2008
| L1 | L2 | L3 |
| Serum folate results, n = 2645 | ||
| <12.61 nmol/L, % (n) | ||
| 2.4 (63) | 1.2 (33) | 0.4 (11) |
| <27.82 nmol/L, % (n) | ||
| 24.8 (657) | 18.1 (479) | 10.6 (281) |
| <40.63 nmol/L, % (n) | ||
| 50 (1318) | 63.9 (1691) | 75.2 (1990) |
| >56.94 nmol/L, % (n) | ||
| 24.9 (658) | 41.4 (1096) | 54.2 (1434) |
| >1075 nmol/L, % (n) | ||
| 2.5 (66) | 6.1 (162) | 14.8 (391) |
| RBC folate results, n = 2613 | ||
| <5061 nmol/L, % (n) | ||
| 2.5 (65) | 0.7 (18) | 3.8 (99) |
| <8732 nmol/L, % (n) | ||
| 25.0 (653) | 11.8 (308) | 17.6 (461) |
| <11203 nmol/L, % (n) | ||
| 50 (1295) | 71.7 (1873) | 69.8 (1823) |
| >14304 nmol/L, % (n) | ||
| 24.7 (645) | 45.1 (1179) | 49.4 (1290) |
| >26005 nmol/L, % (n) | ||
| 2.5 (65) | 5.9 (153) | 9.9 (258) |
2.5th percentile.
25th percentile.
50th percentile.
75th percentile.
97.5th percentile. Based on data from L1.
FIGURE 1.
Frequency distribution curves for serum (A) and RBC folate (B) results from NHANES 2007–2008.
Pearson correlation coefficients were highest between L3 and 1 and lower between L2 and 1 and between L2 and 3 (Table 4). Deming regression analysis showed proportional bias between L3 and 1 and proportional and constant bias between L2 and 1 and between L2 and 3 (Table 4; Supplemental Figs. 1 and 2). Bland-Altman analysis showed a similar relative bias for serum and RBC folate between L3 and 1 (~20%) but different relative biases between L2 and 1 or L2 and 3 for serum and RBC folate.
TABLE 4.
Comparison of serum and RBC folate results between the 3 laboratories, NHANES 2007–2008
| Comparison | Serum folate, n = 2645 | RBC folate, n = 2613 |
| Pearson correlation coefficient (r), P-value | ||
| L2 vs.1 | 0.80, <0.0001 | 0.65, <0.0001 |
| L3 vs.1 | 0.95, <0.0001 | 0.92, <0.0001 |
| L2 vs.3 | 0.82, <0.0001 | 0.69, <0.0001 |
| Deming regression slope (95% CI) | ||
| L2 vs.1 | 1.74 (1.54–1.94) | 1.90 (1.75–2.05) |
| L3 vs.1 | 1.16 (1.08–1.23) | 1.25 (1.21–1.29) |
| L2 vs.3 | 1.46 (1.36–1.56) | 1.39 (1.30–1.49) |
| Deming regression intercept (95% CI) | ||
| L2 vs.1 | −9.43 (−17.9 to −0.95) | −735 (−901–569) |
| L3 vs.1 | 2.76 (−0.39–5.90) | −16.0 (−59.0–26.9) |
| L2 vs.3 | −11.2 (−16.4 to −6.04) | −512 (−643 to −381) |
| Bland-Altman relative bias (95% limits of agreement) | ||
| L2 vs.1 | 39% (−15–94%) | 21% (−57–99%) |
| L3 vs.1 | 19% (−10–48%) | 21% (−12–53%) |
| L2 vs.3 | 21% (−35–77%) | 1% (−73–75%) |
Sample exchange study.
Similar to the NHANES 2007–2008 participants, L2 produced the highest and L1 the lowest serum folate results with the QC samples and the NIST SRM 1995 and NIBSC 03/178 reference materials. However, considerable sample-to-sample variability appeared in the ratio of serum results from the 2 laboratories (1.19–2.95) (Table 5). L2 produced both lower and higher results compared with L1 for RBC folate (0.84–1.54). L3 produced variably higher serum folate (1.04–1.80) and consistently higher RBC folate (1.10–1.34) results compared with L1.
TABLE 5.
Comparison of serum and RBC folate results from the sample exchange study between the laboratories
| Folate concentration, nmol/L | Ratio | |||||
| L1 | L2 | L3 | L2:L1 | L3:L1 | L2:L3 | |
| Serum QC pools | ||||||
| L1 – Low QC | 8.7 | 11 | 10.9 | 1.26 | 1.25 | 1.01 |
| L1 – Medium QC | 25.2 | 34 | 31.8 | 1.35 | 1.26 | 1.07 |
| L1 – High QC | 49.1 | 82 | 64.5 | 1.67 | 1.31 | 1.27 |
| L2 – Low QC | 16 | No data | 18.4 | n/a | 1.15 | n/a |
| L2 – High QC | 45.9 | No data | 60.1 | n/a | 1.31 | n/a |
| L3 – Low QC | 5.8 | 9 | 7.1 | 1.55 | 1.23 | 1.26 |
| L3 – Medium QC | 12.0 | 22 | 15.8 | 1.83 | 1.32 | 1.39 |
| L3 – High QC | 21.1 | 36 | 24.6 | 1.71 | 1.17 | 1.46 |
| L3 – Ultrahigh QC | 45.8 | 135 | 82.6 | 2.95 | 1.80 | 1.63 |
| Whole blood QC pools | ||||||
| L1 – Low QC | 199 | 167 | 219 | 0.84 | 1.10 | 0.76 |
| L1 – Medium QC | 244 | 312 | 325 | 1.28 | 1.33 | 0.96 |
| L1 – High QC | 369 | 567 | 482 | 1.54 | 1.31 | 1.18 |
| L3 – Low QC | 162 | 214 | 187 | 1.32 | 1.27 | 1.04 |
| L3 – Medium QC | 307 | 389 | 354 | 1.27 | 1.27 | 1.00 |
| L3 – High QC | 307 | 486 | 330 | 1.58 | 1.18 | 1.34 |
| L3 – Ultrahigh QC | 478 | 559 | 527 | 1.17 | 1.21 | 0.96 |
| Reference materials | ||||||
| NIBSC 03/178 (serum)1 | 10.8 | 14 | 12.3 | 1.30 | 1.14 | 1.14 |
| NIBSC 95/528 (whole blood)2 | 20.9 | 30.7 | 28.0 | 1.47 | 1.34 | 1.10 |
| NIST SRM 1955 (serum)3 | ||||||
| Level 1 | 5.11 ± 0.814 | 6.08 ± 0.545 | 5.30 ± 0.574 | 1.19 | 1.04 | 1.15 |
| Level 2 | 11.3 ± 1.334 | 14.3 ± 0.525 | 11.7 ± 1.694 | 1.27 | 1.04 | 1.22 |
| Level 3 | 39.0 ± 4.144 | 47.4 ± 4.215 | 41.1 ± 3.944 | 1.22 | 1.05 | 1.15 |
Assigned content is 12.1 nmol/L and was obtained by LC-MS/MS.
Assigned content is 28.3 nmol/ampoule and is a consensus value obtained from laboratories using microbiological assays or radioassays.
Assigned content is 5.28 nmol/L (level 1), 14 nmol/L (level 2), and 44 nmol/L (level 3) and represents orientation values obtained by the CDC MA.
Mean ± SD ( = 40).
Mean ± SD ( = 5).
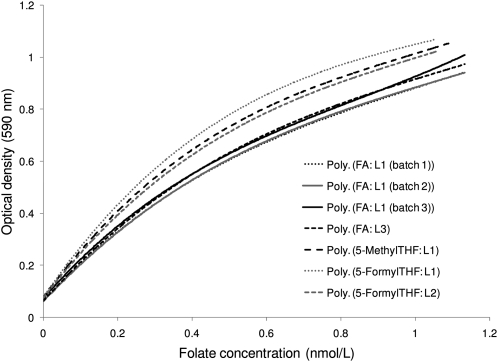
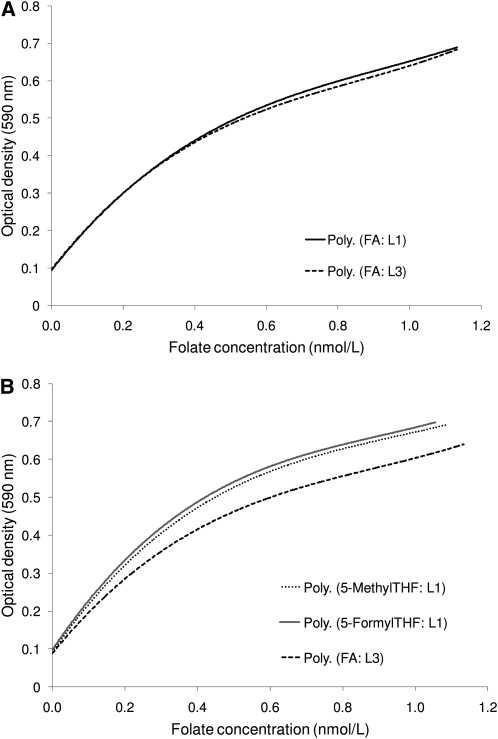
To compare the response of the chloramphenicol-resistant L. rhamnosus for different folate compounds, L1 generated multiple calibration curves in the same assay using 7 folate calibrators from each laboratory (Fig. 2). All 4 FA calibrators (3 from L1 and 1 from L3) produced overlapping response curves. The 2 reduced folate calibrators, 5-methylTHF from L1 and 5-formylTHF from L1 and 2, produced comparable response curves, but the response was higher than for FA, particularly at higher folate concentrations. L3 had similar findings when it generated multiple calibration curves in the same assay: overlapping response curves for 2 FA calibrators (Fig. 3A) and higher response curves for 5-methylTHF and 5-formylTHF compared with FA (Fig. 3B).
FIGURE 2.
L. rhamnosus polynomial (3rd order) calibration curves generated by L1 using 7 folate calibrators from the 3 laboratories.
FIGURE 3.
L. rhamnosus polynomial (3rd order) calibration curves generated by L3 using 2 different FA calibrators (A) or using 2 reduced folate calibrators (5-methylTHF and 5-formylTHF) and FA (B).
In the same assay in which L1 generated 7 calibration curves, it measured 20 serum and whole blood samples each from NHANES, QC samples from each laboratory, and all available reference materials for a total of 30 serum samples and 28 whole blood samples. Folate results calculated with each of the 4 FA calibrators were consistently 22–33% higher than results calculated with the 5-methylTHF calibrator for serum and RBC folate (Table 6). Results calculated with each of the 2 5-formylTHF calibrators were within 10% of results calculated with the 5-methylTHF calibrator (8% lower to 8% higher).
TABLE 6.
Comparison of regression parameters for serum and RBC folate results produced by L1 by using different folate calibrators
| Different folate calibrators (y) vs. 5-methylTHF calibrator from L1 (x) | ||||||
| FA, Sigma lot 1 (L1)1 | FA, Sigma lot 2 (L1)2 | FA, Eprova (L1)2 | FA, Sigma (L3)3 | 5-formylTHF, Eprova (L1)4 | 5-formylTHF, Sigma (L2)5 | |
| Serum samples (n = 30) | ||||||
| Deming slope (95% CI) | 1.24 (1.19, 1.29) | 1.31 (1.26, 1.35) | 1.33 (1.28, 1.37) | 1.22 (1.18, 1.26) | 0.92 (0.92, 0.93) | 1.08 (1.07, 1.09) |
| Deming intercept (95% CI) | −0.33 (−1.36, 0.70) | 0.21 (−0.68, 1.11) | 0.17 (−0.73, 1.07) | 0.77 (−0.09, 1.62) | 0.42 (0.21, 0.63) | 0.16 (0.00, 0.32) |
| Pearson correlation coefficient, P-value | 1.00, <0.0001 | 1.00, <0.0001 | 1.00, <0.0001 | 1.00, <0.0001 | 1.00, <0.0001 | 1.00, <0.0001 |
| Whole blood samples (n = 28) | ||||||
| Deming slope (95% CI) | 1.25 (1.22, 1.28) | 1.31 (1.28, 1.33) | 1.33 (1.30, 1.36) | 1.23 (1.22, 1.23) | 0.93 (0.91, 0.95) | 1.08 (1.08, 1.09) |
| Deming intercept (95% CI) | −12.2 (−22.8, −1.53) | −0.91 (−10.2, 8.36) | −2.31 (−12.7, 8.11) | 8.40 (4.56, 12.3) | 5.50 (−1.73, 12.7) | 1.88 (0.81, 2.94) |
| Pearson correlation coefficient, P-value | 1.00, <0.0001 | 1.00, <0.0001 | 1.00, <0.0001 | 1.00, <0.0001 | 1.00, <0.0001 | 1.00, <0.0001 |
FA, 100 mg/L (227 mol/L), Merck Eprova.
FA, 500 g/L (1133 nmol/L), Sigma (Sigma Chemicals), 2 different lot numbers.
FA, 100 g/L (227 nmol/L), Sigma.
5-formylTHF (racemic), 23 mol/L, Sigma.
5-formylTHF, 100 mg/L (211 mol/L), Merck Eprova.
L1 evaluated whether the different procedures used to prepare whole blood hemolysates could explain the differences in results between the laboratories. Using freshly collected EDTA whole blood from 3 blood donors, RBC folate levels (mean, nmol/L) did not differ regardless of whether the hemolysate was prepared according to the procedure used by L1 and 3 (1/11 dilution with 1% ascorbic acid; hemolysate pH of 3.8) and incubated for up to 2 h at 37°C (0 h: 344, 1 h: 344, 2 h: 323, respectively), or whether it was prepared according to the L2 procedure (1/10 dilution with 0.1 mol/L potassium phosphate buffer containing 1% ascorbic acid; hemolysate pH of 4.5) and incubated for up to 2 h at 37°C (0 h: 352, 1 h: 349, 2 h: 370, respectively).
Discussion
Monitoring of biochemical indicators over time as part of population surveys requires stable laboratory methods. To allow comparability of population survey data among countries, assays used in population monitoring must achieve comparable results. Earlier studies on small sample sets indicated, however, that MA performed in different laboratories produced inconsistent results (8, 9). This is the first investigation to our knowledge that used a large sample set to assess the magnitude of differences in distributions of serum and RBC folate results among laboratories using MA. This study also attempted for the first time to our knowledge to identify potential sources for the differences between laboratories.
Using the NHANES 2007–2008 one-third sample, we observed distinct differences in serum and RBC folate levels, means and reference intervals, among the 3 laboratories. L3 produced serum and RBC folate results well correlated (r > 0.9) with results from L1 but ~20% higher across the entire concentration range (Table 4); 95% of serum and RBC folate samples analyzed by L3 produced results between 10% lower to 50% higher than results produced by L1 (limits of agreement). The correlation of folate results between L2 and the other 2 laboratories was lower (r ~0.8 for serum and ~0.7 for RBC). Although L2 generally produced the highest results of the 3 laboratories, this was not the case at lower RBC folate concentrations. This different response by matrix was also seen in the different relative bias for serum and RBC folate between L2 and 1 (39 and 21%, respectively) or L2 and 3 (21 and 1%, respectively). The limits of agreement on relative bias estimates for comparisons that included L2 were much wider than for the comparison of L1 and 3, reflecting the wider scatter of points.
The increased folate status of the U.S. population since the introduction of FA fortification resulted in only a few cases with low serum or RBC folate levels indicative of deficiency, making a comparison of prevalence deficiency estimates across the 3 MA difficult. When we used folate concentrations at selected percentiles from L1 as cutoff values, the 3 laboratories arrived at different proportions of low or high folate levels (Table 3). Without doubt, differences in assays can lead to differences in folate status interpretation.
The 2 most distinguishing procedural differences among the 3 laboratory MA were the L. rhamnosus microorganism and the folate calibrator. Smaller differences between the 3 assays, not expected to be as influential on folate results, were the number of dilutions/sample, the number of replicates/dilution, and the curve-fitting algorithm. Common features of all 3 assays were the similar assay imprecision, the use of multi-level matrix-based QC samples for daily internal QC, and the periodic use of reference materials.
Older reports found an equivalent response of L. rhamnosus to different folate vitamers (3, 4). More recently, small differences have been observed in the response of the chloramphenicol-resistant L. rhamnosus to different folate vitamers (17), with the microorganism responding slightly better to reduced folates compared with FA. The sample exchange study confirmed and extended the recent observation. It showed that different stock solutions of FA (whether prepared in one or different laboratories) produced overlapping calibration curves (Fig. 2). In addition, different stock solutions of 5-methylTHF and 5-formylTHF (prepared in different laboratories) produced comparable calibration curves. But those curves were slightly higher compared with the FA calibration curves, regardless of whether the assay was conducted in L1 or L3.
Higher calibration curves will result in lower calculated folate concentrations. The proportional difference (~20%) in serum and RBC folate between L1 (using 5-methylTHF as a calibrator) and 3 (using FA as a calibrator) may therefore be due mainly to the differences in the calibrators (Table 6). Use of different calibrators in L1 (5-methylTHF) and 2 (5-formylTHF), however, did not appear to explain the differences in serum and RBC folate results (Table 6). The different procedure to prepare whole blood hemolysates for L2 also did not seem to explain the differences in folate level compared with L1 and 3. The use of the wild-type L. rhamnosus by L2 appeared to be the main reason for differences in results between L2 and the other 2 laboratories.
The chloramphenicol-resistant L. rhamnosus yields a higher response to reduced folate forms than to FA. Also, the wild-type microorganism seems to respond differently to serum and RBC folate than does the chloramphenicol-resistant microorganism, particularly as folate concentrations increase. There have been at least 2 known changes that could contribute to these effects: the properties of the microorganism could have been slightly changed in the process of rendering it antibiotic resistant and subculturing the wild-type microorganism in L2 over the last 40 y could have changed its properties. Tamura et al. (18) have shown that the form of folate used during the development of methotrexate resistance affects the response of the assay organism to folates. A similar phenomenon could have occurred with the chloramphenicol-resistant microorganism, making it respond slightly better to reduced folates. Further research will be required to find the reasons for the responses of microorganisms to folate species. The current data cannot be used to conclude whether one organism should be regarded as superior to the other. Forty years ago, when the chloramphenicol-resistant strain was evaluated as a tool for assessing folate status, blood folate concentrations generated with the new organism were generally comparable to those obtained by the standard method using the wild-type microorganism; however, there was heterogeneity in the data, probably as a result of the variability of the assay (19–22).
The measurement of available reference materials as part of the sample exchange study (Table 5) was of limited value, because the assigned values for each material were derived from different assays and there is currently no serum or whole blood reference material available that has certified concentrations for total folate assigned by a higher-order liquid chromatography-tandem MS (LC-MS/MS) reference measurement procedure. However, efforts to generate such a serum material by NIST are underway. This is the first study to our knowledge to investigate the magnitude of differences across the entire distribution of serum and RBC folate results among laboratories using MA and to conduct systematic investigations to identify potential sources for the differences. Using FA as a calibrator produced ~25% higher serum and RBC folate results than using 5-methylTHF as a calibrator. The majority of folate in blood is in the form of 5-methylTHF. Unmetabolized FA appears in serum after a larger bolus dose of FA from foods or supplements and its concentration in fasting individuals is usually small compared with the total folate (23). Thus, using 5-methylTHF as a calibrator is expected to produce more accurate results than the FA calibrator and is recommended. However, the laboratory has to pay attention to the purity of this compound, expeditiously handle the preparation of stock solutions, and use appropriate antioxidants and store stock solutions in a −70°C freezer to ensure their stability (2, 17).
The question whether the wild-type or the chloramphenicol-resistant microorganism produces more accurate results cannot be satisfactorily settled until serum- and whole blood-based reference materials with certified values for total folate by LC-MS/MS are available. In the interim, NHANES continues to monitor folate status employing an MA that uses the chloramphenicol-resistant microorganism and 5-methylTHF as a calibrator and an LC-MS/MS assay for additional information on folate species. Other countries that wish to compare their folate population data to that of the US can harmonize their MA by using the same microorganism and calibrator.
Supplementary Material
Acknowledgments
We thank the following laboratory members: Donna LaVoie, Bridgette Haynes, Neelima Paladugula, and Daniel Rabinowitz (CDC’s National Center for Environmental Health), Regina Dempsey (Trinity College), and Kelley E. Johnston (University of Alabama). C.M.P., D.A.L., E.A.Y., M.F.P. (recently deceased), and C.L.J. designed the overall research project; C.M.P., M.Z., A.M.M., T.T., and D.A.L. conducted most of the research; C.M.P., M.Z., and D.A.L. analyzed the majority of the data; C.M.P. wrote the initial draft, which was modified after feedback from all coauthors; and C.M.P. has primary responsibility for content. All authors read and approved the final manuscript.
Footnotes
Supported by the Office of Dietary Supplements, NIH. The findings and conclusions in this report are those of the authors and do not necessarily represent the official views or positions of the CDC/Agency for Toxic Substances and Disease Registry, the NIH, or the Department of Health and Human Services.
Supplemental Figures 1 and 2 are available from the “Online Supporting Material” link in the online posting of the article and from the same link in the online table of contents at jn.nutrition.org.
Abbreviations used: FA, folic acid; 5-formylTHF, [6S]5-formyltetrahydrofolate; L, laboratory; LC-MS/MS, light chromatography tandem MS; MA, microbiologic growth assay; 5-methylTHF, [6S]5-methyltetrahydrofolate; NIBSC, National Institute for Biological Standards and Controls; NIST, National Institute of Standards and Technology; QC, quality control.
Literature Cited
- 1.Pfeiffer CM, Johnson CL, Jain RB, Yetley EA, Picciano MF, Rader JI, Fisher KD, Mulinare J, Osterloh JD. Trends in blood folate and vitamin B12 concentrations in the United States, 1988–2004. Am J Clin Nutr. 2007;86:718–27 [DOI] [PubMed] [Google Scholar]
- 2.Pfeiffer CM, Fazili Z, Zhang M. Folate analytical methodology. : Bailey LB, editor Folate in health and disease. 2nd ed. Boca Raton: CRC Press, Taylor and Francis Group; 2010. p. 517–74 [Google Scholar]
- 3.O’Broin S, Kelleher B. Microbiological assay on microtitre plates of folate in serum and red cells. J Clin Pathol. 1992;45:344–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shane B, Tamura T, Stokstad ELR. Folate assay: a comparison of radioassay and microbiological methods. Clin Chim Acta. 1980;100:13–9 [DOI] [PubMed] [Google Scholar]
- 5.Blackmore S, Pfeiffer C, Hamilton MS, Lee A. Recoveries of folate species from serum pools sent to participants of the UK NEQAS Haematinics scheme in February and March 2004. Clin Chim Acta. 2005;355:S459 [Google Scholar]
- 6.Fazili Z, Pfeiffer CM, Zhang M. Comparison of serum folate species analyzed by LC-MS/MS with total folate measured by microbiologic assay and BioRad radioassay. Clin Chem. 2007;53:781–4 [DOI] [PubMed] [Google Scholar]
- 7.Fazili Z, Pfeiffer CM, Zhang M, Jain RB, Koontz D. Influence of 5,10-methylene-tetrahydrofolate reductase polymorphism on whole blood folate concentrations measured by LC-MS/MS, microbiologic assay and BioRad radioassay. Clin Chem. 2008;54:197–201 [DOI] [PubMed] [Google Scholar]
- 8.Gunter EW, Bowman BA, Caudill SP, Twite DB, Adams MJ, Sampson EJ. Results of an international round robin for serum and whole-blood folate. Clin Chem. 1996;42:1689–94 [PubMed] [Google Scholar]
- 9.Pfeiffer CM, Gunter EW, Caudill SP. Comparison of serum and whole blood folate measurements in 12 laboratories: an international study. 53rd Annual Meeting of the American Association of Clinical Chemists, San Diego, CA, July 2001. Clin Chem. 2001;47 S6:A62 [Google Scholar]
- 10.Satterfield MB, Sniegoski LT, Sharpless KE, Welch MJ, Hornikova A, Zhang N-F, Pfeiffer CM, Fazili Z, Zhang M, et al. Development of a new standard reference material: SRM 1955 (homocysteine and folate in human serum). Anal Bioanal Chem. 2006;385:612–22 [DOI] [PubMed] [Google Scholar]
- 11.Thorpe SJ, Heath A, Blackmore S, Lee A, Hamilton M, O'Broin S, Nelson BC, Pfeiffer CM. An international standard for serum vitamin B12 and serum folate: international collaborative study to evaluate a batch of lyophilized serum for B12 and folate content. Clin Chem Lab Med. 2007;45:380–6 [DOI] [PubMed] [Google Scholar]
- 12.Thorpe SJ, Sands D, Heath AB, Hamilton M, Blackmore S, Barrowcliffe T. An international standard for whole blood folate: evaluation of a lyophilised haemolysate in an international collaborative study. Clin Chem Lab Med. 2004;42:533–9 [DOI] [PubMed] [Google Scholar]
- 13.Molloy AM, Scott JM. Microbiological assay for serum, plasma, and red cell folate using cryopreserved, microtiter plate method. Methods Enzymol. 1997;281:43–53 [DOI] [PubMed] [Google Scholar]
- 14.Fazili Z, Pfeiffer CM, Zhang M, Jain RB. Erythrocyte folate extraction and quantitative determination by LC/MS/MS: comparison of results with microbiologic assay. Clin Chem. 2005;51:2318–25 [DOI] [PubMed] [Google Scholar]
- 15.Tamura T. Microbiological assay of folates. Folic acid metabolism in health and disease. Picciano MF, Stokstad ELR, Gregory JF, III, New York: Wiley-Liss; 1990. p. 121–37 [Google Scholar]
- 16.Life Sciences Research Office. Assessment of folate nutritional status of the U.S. population based on data collected in the second National Health and Nutrition Examination Survey, 1976–1980. Prepared for the Center for Food Safety and Nutrition. FDA. Rockville (MD): Federation of American Societies for Experimental Biology; 1984 [Google Scholar]
- 17.Pfeiffer CM, Fazili Z, McCoy LF, Gunter EW. Determination of folate vitamers in human serum by stable-isotope dilution tandem mass spectrometry and comparison to radioassay and microbiologic assay. Clin Chem. 2004;50:423–32 [DOI] [PubMed] [Google Scholar]
- 18.Tamura T, Baggott JE, Johnston KE, Li Q-J, Antony AC. The form of folate affects the mechanisms of methotrexate resistance in Enterococcus faecium. Microbiology. 1997;143:2639–46 [DOI] [PubMed] [Google Scholar]
- 19.Davis RE, Nicol DJ, Kelly A. An automated method for the measurement of folate activity. J Clin Pathol. 1970;23:47–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millbank L, Davis RE, Rawlins M, Waters AH. Automation of the assay of folate in serum and whole blood. J Clin Pathol. 1970;23:54–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chanarin I, Kyle R, Stacey J. Experience with microbiological assay for folate using a chloramphenicol-resistant L. casei strain. J Clin Pathol. 1972;25:1050–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Broin JD, Scott JM, Temperly IJ. A comparison of serum folate estimations using two different methods. J Clin Pathol. 1973;26:80–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalmbach RD, Choumenkovitch SF, Troen AM, D'Agostino R, Jacques PF, Selhub J. Circulating folic acid in plasma: relation to folic acid fortification. Am J Clin Nutr. 2008;88:763–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.