Abstract
Background
Early B cell factor (EBF) family members are transcription factors known to have important roles in several aspects of vertebrate neurogenesis, including commitment, migration and differentiation. Knowledge of how EBF family members contribute to neurogenesis is limited by a lack of detailed understanding of genes that are transcriptionally regulated by these factors.
Results
We performed a microarray screen in Xenopus animal caps to search for targets of EBF transcriptional activity, and identified candidate targets with multiple roles, including transcription factors of several classes. We determined that, among the most upregulated candidate genes with expected neuronal functions, most require EBF activity for some or all of their expression, and most have overlapping expression with ebf genes. We also found that the candidate target genes that had the most strongly overlapping expression patterns with ebf genes were predicted to be direct transcriptional targets of EBF transcriptional activity.
Conclusions
The identification of candidate targets that are transcription factor genes, including nscl-1, emx1 and aml1, improves our understanding of how EBF proteins participate in the hierarchy of transcription control during neuronal development, and suggests novel mechanisms by which EBF activity promotes migration and differentiation. Other candidate targets, including pcdh8 and kcnk5, expand our knowledge of the types of terminal differentiated neuronal functions that EBF proteins regulate.
Background
Throughout animal development, many processes must occur coordinately, including patterning, commitment, differentiation and migration of progenitor cells. In the nervous system in particular, these processes are exceedingly complex and depend on the coordinated expression of many sets of genes. A detailed understanding of gene regulation, including knowledge of the hierarchy of transcriptional activity and the types of genes that different transcription factors target, is therefore a critical foundation for understanding nervous system development. One group of transcription factors expressed strongly in the developing nervous system is the early B cell factor (EBF; also called Collier/Olf/Ebf (COE), and Olf/Ebf (O/E)) family of zinc finger helix-loop-helix proteins.
The EBF family includes EBF1, 2, 3 and O/E-4 in mammals [1-6], with EBF2 and EBF3 being the known family members in Xenopus [7,8], and ZCOE2 a family member in zebrafish [9]. Invertebrate members of this family include Collier in Drosophila, and UNC-3 in Caenorhabditis elegans [10,11]. EBF family proteins contain a DNA binding domain (a zinc finger coordination motif), which can also participate in dimerization and transactivation, an atypical helix-loop-helix domain, which is critical for formation of homo- and heterodimers, and a carboxy-terminal domain, which is important for transactivation [2,4,12].
EBF proteins influence multiple processes during development of multiple lineages, including neurons (reviewed in [13,14]). One of their functions is a role in stabilizing neuronal cell commitment. For example, Dubois et al. [7] showed that EBF2 can affect neuronal progenitor cell commitment in early Xenopus embryos by reinforcing the expression of the proneural basic helix-loop-helix (bHLH) transcription factor NGNR-1, and by maintaining the expression of delta1. In addition, in developing chick spinal cord, electroporated mouse Ebf1 drives expression of Ngn1 and Ngn2 [15].
EBF proteins also have critical roles in neuronal cell differentiation. For example, overexpression of ebf2 and ebf3 leads to ectopic expression of neuronal-specific markers like n-tubulin and nf-m in Xenopus embryos [7,8], suggesting that EBF2 and EBF3 may drive specific aspects of the neuronal differentiation program. Consistent with this, in Ebf1 null mouse striatum, early neuronal cells show abnormal expression of several genes, indicating disruption of the process of differentiation [16]. EBF proteins have also been shown to regulate aspects of cell differentiation in both Drosophila and C. elegans ventral nerve cord [17,18], as well as in early chick spinal cord, where electroporated mouse Ebf1 promotes expression of numerous neuronal markers [15].
EBF proteins have been shown to regulate neurite formation and axon guidance, including thalamocortical fibers in the mouse lateral ganglionic eminence [16,19], olfactory axons projecting to the mouse olfactory bulb [20], as well as motor neurons in C. elegans [11]. EBF proteins also are critical for neuronal cell migration. For example, EBF factors regulate the migration of gonadotropin releasing hormone (GnRH-1)-synthesizing neurons from the olfactory epithelium to the hypothalamus [21], the migration of Purkinje neurons from the anterior cortical transitory zone to beneath the external granular layer in cerebellar cortex [22], and the migration of facial branchiomotor neurons in the hindbrain [23]. Furthermore, when Ebf1 is misexpressed in chick spinal cord, neuroepithelial progenitors migrate toward the mantle layer faster than normal, and the expression levels of NF and R-cadherin are upregulated [15].
Ebf genes are strongly expressed in differentiating central and peripheral neurons throughout development [1,7,8,24], and clearly govern diverse aspects of neuronal development. However, it is not fully understood how these functions are executed since there has not previously been a systematic analysis of EBF transcriptional targets involved in neuronal development. The goals of this study were as follows. First, we performed a microarray analysis to identify candidate targets of EBF3 activity in the developing Xenopus nervous system. Second, we analyzed the expression of the candidate targets, to compare their expression with the ebf genes and to gain an understanding of where in the embryo they may function. Third, we performed gain- and loss-of-function studies of EBF2 and EBF3 in Xenopus embryos to analyze in vivo the dependence of the discovered candidate targets on EBF activity, and to confirm the microarray results in vivo. Finally, we assessed which candidate target genes are likely to be direct targets of EBF3, and which are indirect targets, to better understand the hierarchy of transcriptional control by EBF proteins. Many genes previously demonstrated to be required for neuronal development are strongly upregulated by EBF, but were not previously known to be targets of EBF transcriptional activity. These targets include transcription factors, cell structural proteins, an ion channel protein, and a gene involved in transforming growth factor (TGF)-beta signaling. The variety of targets found expands our knowledge of the kinds of processes EBF proteins regulate, and reinforces the idea that EBF proteins can influence many aspects of neuronal development because they direct expression of several different functional classes of genes. The discovered candidate targets open a new window to understanding the broader scope of EBF functions.
Materials and methods
Generation of hormone-inducible constructs
To generate hormone-inducible constructs, a hormone-binding domain of the human glucocorticoid receptor (hGR) was fused to the coding region of Xenopus ebf3 (or ebf2), with a myc tag epitope between hGR and the coding region (Additional file 1A) [25,26]. The ebf3 coding region was obtained by excision from pBS-Xebf3 [8] with the restriction enzyme DraI, which produces a DNA fragment with blunt ends. The hormone-binding domain was obtained from the pCS2+hGR-MT vector (hGR with myc tag) by cutting with XbaI and treatment with Klenow (Promega, Madison, WI, USA) to generate blunt ends followed by dephosphorylation of the 3' ends with CIP (New England Biolabs, Ipswich, MA, USA). The excised ebf3 was then subcloned into the pCS2+hGR-MT vector. To generate hGR-XEBF2 construct, pBS-Xebf2 [8] was used as a template and the following primers containing XhoI and XbaI sites were used to amplify ebf2: 5'-GGCCCTCGAGATGGATCCAATCCA-3' and 5'-GGCCTCTAGATTCACATGGGCACC-3' (residues in italics are XhoI and XbaI sites, respectively; bold sequences are the ebf2 sequence). The amplified products were digested with XhoI and XbaI and subcloned into the pCS2+hGR-MT vector. Following sequence verification, the in vivo expression of these fusion proteins was verified by injecting capped mRNAs into Xenopus embryos at the one-cell stage and then performing western blotting with lysates of gastrula stage embryos with 9E10 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), which recognizes the myc epitope (data not shown).
Microinjection of RNA and morpholinos
The following constructs were used as DNA templates to make capped mRNA: pCS2+Noggin [27], pCS2+hGR [28], pCS2+hGR-MT-Xebf2, pCS2+hGR-MT-Xebf3, pCS2+MT-DN-Xebf, and pCS2+nβgal [29]. Capped mRNA was generated in vitro using the Message mMachine kit (Ambion, Austin, TX, USA). Antisense morpholino oligonucleotides (MO) were designed by Gene Tools (Philomath, OR, USA), and directed against a region at or near the translational start site of ebf2 (5'-GCGCTTTGTCTCTCAAGGCAGTTCC-3') and ebf3 (5'-GTATATTTTCCTGAATCCCAAACAT-3').
For microarray experiments, 1 ng of hGR-XEBF3 mRNA and 0.2 ng Noggin mRNA were co-injected into Xenopus embryos at the one-cell stage. Alternatively, 0.4 ng hGR mRNA and 0.2 ng Noggin mRNA were co-injected in control embryos. At stage 9, animal caps were dissected from the embryo, using either a Gastromaster or a hypodermic needle tip. Animal caps were treated with 30 μM dexamethasone (DEX) in 1× Marc's modified Ringer solution (MMR) for 4.5 hours before harvesting of total RNA (Additional file 1B).
For all other microinjections, a volume of 4 nl or 5 nl containing capped mRNA or MOs was injected into one blastomere of two-cell stage embryos in the following amounts: hGR (0.5 ng), hGR-XEBF2 (0.5 ng for overexpression, 0.1 ng for the MO rescue), hGR-XEBF3 (0.5 ng), DN-XEBF (2 ng), MyoD-hGR (0.5 ng), nβgal (30 pg), EBF2 MO (15 ng), EBF3 MO (15 ng) and standard control MO (30 ng). In the MO experiments, both EBF2 MO and EBF3 MO were co-injected. For all injections nβgal capped mRNA was co-injected as a tracer. Embryos were grown until neurula or tail bud stages [30]. hGR-XEBF2, hGR-XEBF3 and MyoD-hGR injected embryos were treated with 30 μM DEX from the gastrula stage (stage 11/11.5) to the neurula stage (stage 14/15). Embryos were then fixed with 4% paraformaldehyde in phosphate-buffered saline for 30 minutes. After washing embryos three times with phosphate-buffered saline, X-gal staining was performed as described [31], followed by post-fixation in 4% paraformaldehyde for 1 hour at room temperature or overnight at 4°C.
Microarray analysis
Total RNA was isolated from animal caps with the RNeasy mini kit (Qiagen, Germantown, MD, USA). This RNA was used to perform two-color microarray analysis on the Xenopus Agilent microarray by the University of Utah Microarray core facility. Fluorescently labeled cRNA, containing either cyanine 3-CTP or cyanine 5-CTP, was generated using the Agilent Two-Color Quick Amp Labeling kit (catalog number 5190-0444). Next, microarray hybridizations were performed using Agilent surehyb hybridization chambers. Slides were then scanned in an Agilent Technologies G2505B microarray scanner at 5 μm resolution. Finally, TIF files were generated from the scanned microarray image, and loaded into Agilent Feature Extraction Software version 9.5.1. LOWESS-normalized data from the Feature Extraction software was filtered to remove control features and features flagged as 'nonuniform'. The LOWESS-normalized intensity values were loaded into Genesifter software (Geospiza, Seattle, WA, USA) for analysis. A t-test was applied to data from four biological replicate experiments. P-values from the t-test were adjusted for multiple testing using the Benjamini and Hochberg method. Genes were selected that showed at least two-fold differential expression and had adjusted P-values < 0.05.
In situ hybridization
The following constructs were used to generate antisense RNA probes: pBS-Xebf2 [8], pBS-Xebf3 [8], pBS-Sox2 [32], PCDH8 (IMAGE ID 6955713, ATCC), Peripherin (IMAGE ID 4959167, ATCC), GREB1 (IMAGE ID 5569934, ATCC), pBS-XNF-M [8], KCNK5 (IMAGE ID 6863628, ATCC), NSCL-1 (IMAGE ID 5514274, ATCC), pBS-XNeuroD [33], AML1 (IMAGE ID 4963637, ATCC), Activin beta B (IMAGE ID 5440215, ATCC), EMX1 (IMAGE ID 6957219, ATCC). Antisense RNA probe was generated in vitro using SP6, T7 or T3 RNA polymerase (Ambion) and labeled with digoxigenin-11-UTP (Roche, Indianapolis, IN, USA). Whole mount in situ hybridization was performed on the fixed and X-gal stained embryos as described [34,35].
Real-time quantitative PCR
For real-time quantitative PCR (RT-QPCR) experiments, 1 ng hGR-XEBF3 mRNA and 0.2 ng Noggin mRNA were co-injected into Xenopus cells at the one-cell stage and animal caps were isolated at stage 9. The animal caps were divided into four groups. The control group received no treatment (-C-D). The second group was treated with 30 μM DEX alone for 3 hours (-C+D), and the third group was treated with 5 μg/ml cycloheximide (CHX) alone for 3.5 hours (+C-D). Finally, the fourth group was treated with 5 μg/ml CHX for 30 minutes and then 30 μM DEX was added for 3 hours (+C+D). Total RNA was purified from animal caps with Trizol (Invitrogen) and then genomic DNAs were removed with the RNeasy mini kit (Qiagen).
To make cDNA from the isolated total RNA from animal caps, the SuperScript III reverse transcriptase (Invitrogen) was used according to the manufacturer's instructions, and then QPCR was performed using Power SYBR Green PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA) on a 7900HT Real Time PCR System (Applied Biosystems). Alternatively, the Superscript III Platinum two step RT-QPCR kit and SYBR Green (Invitrogen) were used to make cDNA and to generate the PCR solution, and QPCR was performed on the same 7900HT Real Time PCR system. MacVector Software was used to design the gene-specific primers (Additional file 2). The relative gene expression level was determined by normalizing the threshold cycle (Ct) of each gene to the Ct of histone H4. One Ct difference indicates a two-fold difference in the initial cDNA template amount. Finally, expression levels were normalized by setting the expression level in the condition of -C+D to 100.
Results
Identification of candidate targets of EBF3 in animal caps
To identify transcriptional targets of EBF3, we performed a microarray screen comparing the transcripts expressed in Xenopus animal cap ectoderm with and without active Xenopus EBF3 protein. To control EBF3 activity, we generated a hormone-inducible fusion protein (hGR-XEBF3) that can be regulated by the hormone DEX (Additional file 1A) [26]. We verified that the fusion protein hGR-XEBF3 replicates EBF3 activity in vivo by demonstrating that hGR-XEBF3 activated by DEX treatment can induce ectopic expression of n-tubulin (data not shown) and neurofilament-m (nf-m) (Figure 1H), similar to unmodified EBF3 [8]. For all microarrays, we injected mRNA encoding hGR-XEBF3 into one-cell stage embryos, collected animal caps at the blastula stage, and treated with DEX for 4.5 hours (to activate hGR-XEBF3 and induce target gene expression), followed by isolation of total RNA (Additional file 1B). Control animal caps without active hGR-XEBF3 were generated in the same way, but without the addition of DEX.
Figure 1.
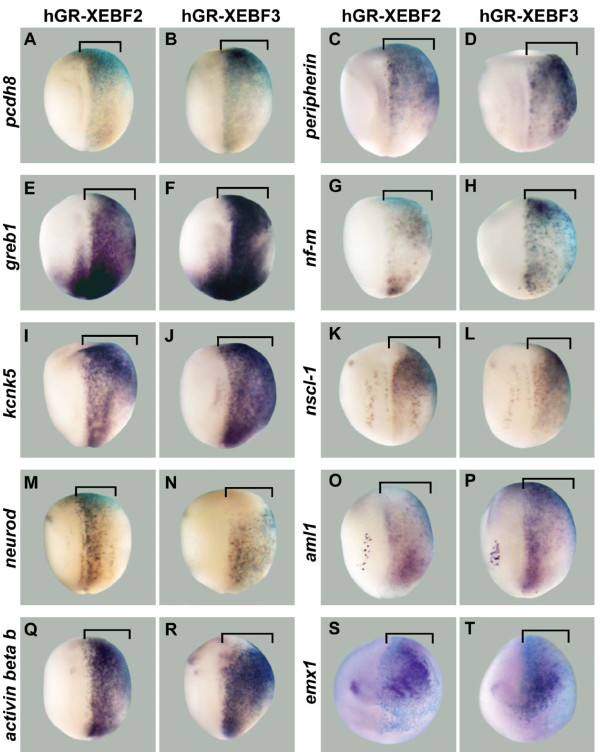
Candidate target genes upregulated by overexpression of EBF2 or EBF3. hGR-XEBF2 or hGR-XEBF3 mRNA was injected into one cell of two-cell stage embryos, followed by DEX treatment from the late gastrula stage (stage 11/11.5) to the neurula stage (stage 14/15). β-Galactosidase (β-gal) mRNA was co-injected as a marker of the injected side. In all panels the right side is the injected side, showing the light blue color of X-gal staining. (A-T) The expression levels of pcdh8 (A,B), peripherin (C,D), greb1 (E,F), nf-m (G,H), kcnk5 (I,J), nscl-1 (K,L), neurod (M,N), aml1 (O,P), activin beta b (Q,R), and emx1 (S,T) are strongly upregulated by EBF2 and EBF3 (brackets). Panels (A-R) show dorsal views, while (S,T) show anterior views.
EBF proteins are involved in the development of B cells, adipocytes, and muscle cells, as well as of neurons (reviewed in [13,14,36]), and the activities of EBF are tissue specific [37]. Since we were interested primarily in neuronal-specific targets of EBF activity, we co-injected embryos with Noggin mRNA to neuralize the animal caps [27]. Agilent Xenopus microarrays were used to compare target gene expression levels in DEX-treated animal caps to those in control DEX-untreated animal caps in four independent experiments. To exclude genes that had their expression levels affected by the hormone DEX alone, we performed a separate, control microarray analysis using animal caps treated with DEX expressing control hGR versus untreated animal caps expressing hGR-XEBF3 (Additional file 1B). After removal of the small number of genes that were affected by DEX itself, we found 602 genes that were upregulated more than two-fold, and 504 genes that were downregulated more than two-fold by EBF3 activity (Additional file 3; Gene Expression Omnibus (GEO) accession [GEO:GSE25734]). We found 242 genes upregulated more than five-fold, and 115 genes downregulated more than five-fold.
In cases of incomplete annotation for the Xenopus microarray, we used NCBI UniGene or BLAST to identify homologs in other species and determine likely gene identity. The list of candidate targets includes genes with known functions in neurons, as well as genes with established roles in other tissue types, including muscle. Since EBF proteins have not previously been implicated in vertebrate muscle development, an analysis of EBF3 targets with functions in muscle tissue are described in a separate study (YS Green and ML Vetter, submitted). An indication of the integrity of our screen was the strong upregulation of nf-m, a known EBF3 target gene [8] (Table 1). As a whole, the results of this array provide an expansive data set for future study of EBF3 activity in Xenopus.
Table 1.
Candidate targets of EBF activity
| Gene name | Function | FC1 (microarray) |
FC2 (RT-QPCR) |
GenBank |
|---|---|---|---|---|
| pcdh8 | Transmembrane protein | 66 | 93a | BC074360 |
| nr2f2 | Nuclear receptor TF | 47 | ND | BC078057 |
| wnt3a | Wnt signaling ligand | 45 | ND | L07538 |
| peripherin | Type III intermediate filament | 37 | 32a | BC056020 |
| greb1 | Estrogen-regulated gene | 32 | 21b | BC043838 |
| hoxd10 | Homeodomain TF | 31 | ND | BC061944 |
| nf-m | Type IV intermediate filament | 28 | 29b | BC078128 |
| kcnk5 | K+ ion channel | 27 | 6b | BC084931 |
| nscl-1 | bHLH TF | 26 | 7b | BC084434 |
| neurod | bHLH TF | 26 | 164b | BC072996 |
| en-2 | Homeodomain TF | 24 | ND | X62974 |
| aml1 | Runt-related TF | 22 | 24a | BC057739 |
| activin beta b | TGF-beta superfamily member | 21 | 154b | S61773 |
| emx1 | Homeodomain TF | 16 | 15b | BC077629 |
The genes chosen for analysis are shown with their known functional roles. FC1 refers to the average fold change in expression by microarray analysis after DEX treatment for 4.5 hours compared to control, in four replicate experiments. FC2 refers to the fold change in expression after DEX treatment for 3 hours compared to control, as detected by RT-QPCR using independent samples (see Figure 6 and Additional file 6 for details). aPerformed three replicate experiments; bperformed once. FC, fold change; ND, not determined; TF, transcription factor.
Classes of candidate target genes with predicted neuronal function
The list of targets with predicted neuronal function was very promising, so for further analysis, we selected from the microarray results 14 genes that were more than 10-fold upregulated and that we predicted to be involved in neuronal development, based on expression patterns and functions known from the published literature, as well as on our own observations with whole mount in situ hybridization (WM-ISH; discussed below). Although the number of downregulated genes in our array results is comparable to the number upregulated, we focused on upregulated genes for the remaining analysis to identify potential mediators of EBF function in the nervous system. The selected EBF3 candidate target genes were classified based on their known or predicted functions, and these are summarized in Table 1. To confirm the microarray results for these genes, reverse transcriptase PCR (RT-PCR) was performed using independent animal caps from those used in the microarray experiments. For each tested gene, these RT-PCR results showed significant upregulation of gene expression by activated hGR-XEBF3, matching the results found with the microarray analysis (data not shown). As described in detail later, for a subset of the genes we performed RT-QPCR using independent samples, and also found upregulation of gene expression (Table 1). To determine whether neuralization of the animal caps was required for EBF regulation of candidate target genes, we performed an additional microarray experiment using the same conditions, but without Noggin mRNA co-injection (Additional file 4; GEO accession [GEO:GSE27084]). We found that all 14 genes were still upregulated by hGR-XEBF3 in the presence of DEX, suggesting that they can be activated by EBF activity in both neuralized and non-neuralized ectoderm.
After classification, we found that a large number of the selected candidate EBF targets are transcription factors - for example, NR2F2 (nuclear receptor subfamily 2, group F, member 2, also called COUP-TFII) [38], HOXD10 (homeobox D10, also called HOX4D) [39], NSCL-1 (neuronal stem cell leukemia, also called XHEN1 and NHLH1) [40], NeuroD [33], EN-2 (engrailed 2) [41], AML1 (acute myeloid leukemia, also called RUNX1) [42,43], and EMX1 [44]. The fact that we find many transcription factors strongly upregulated by EBF proteins suggests that there are multiple levels of transcriptional control that involve the activity of EBF proteins. For the most part, the remainder of the strongly upregulated candidate targets that we chose are involved in cell structure and neuronal function, including PCDH8 (protocadherin 8) [45], WNT3a [46], Peripherin (also called XIF3) [47] and NF-M (Neurofilament-M) [48], KCNK5 (potassium channel subfamily K member 5, also called TASK2) [49], and Activin beta B (also called INHBB) [50], reinforcing the idea that EBF proteins are involved in neuronal differentiation during development, as well as performing various functions in mature neurons.
EBF2 and EBF3 are sufficient for the expression of candidate neuronal targets in vivo
We previously showed that the protein sequences of Xenopus EBF2 and EBF3, as well as their functions in neuronal development in Xenopus, are very similar [8]. For these reasons, we included both EBF2 and EBF3 in the experiments that follow. In order to both confirm the microarray data and to determine if EBF2 and EBF3 are sufficient for the expression of the candidate target genes in vivo, we examined the expression levels of candidates after overexpression of hGR-XEBF2 and hGR-XEBF3. Overexpression was achieved by injection of mRNA for hGR-XEBF2 or hGR-XEBF3 into one cell of two-cell stage embryos, followed by treatment of the embryos with DEX from the gastrula stage (stage 11/11.5) to the neurula stage (stage 14/15). The expression level of candidate target genes was then examined by WM-ISH. We found that 10 of the 14 candidate target genes were upregulated by overexpression of EBF2 and EBF3 (Figure 1). These were pcdh8 (20/23 embryos by EBF2, 27/28 embryos by EBF3), peripherin (15/15, 35/35), greb1 (genes regulated by estrogen in breast cancer; 12/12, 10/10), nf-m (15/15, 35/38), kcnk5 (11/11, 39/40), nscl-1 (9/9, 33/40), neurod (20/21, 39/39), aml1 (12/12, 11/11), activin beta b (11/11, 37/40), and emx1 (11/13, 28/32) (Figure 1). However, four genes were not consistently upregulated by EBF3. The expression of en-2 (10/19) and hoxd10 (30/44) was downregulated, while the expression of nr2f2 was upregulated (19/73) in some embryos but downregulated (30/73) in others, and the expression of wnt3a (24/24) was not changed by EBF3 (data not shown). We therefore believe that these four genes are unlikely to be in vivo targets of EBF activity at this stage of early nervous system development, and we have excluded them from the experiments that follow. The fact that the expression levels of 10 genes among 14 candidates are upregulated by overexpression of EBF2 and EBF3 in the intact embryo supports the microarray data, and further shows that EBF2 and EBF3 activity are sufficient to drive expression of these candidate genes in vivo.
EBF2 and EBF3 are required for the expression of candidate targets in vivo
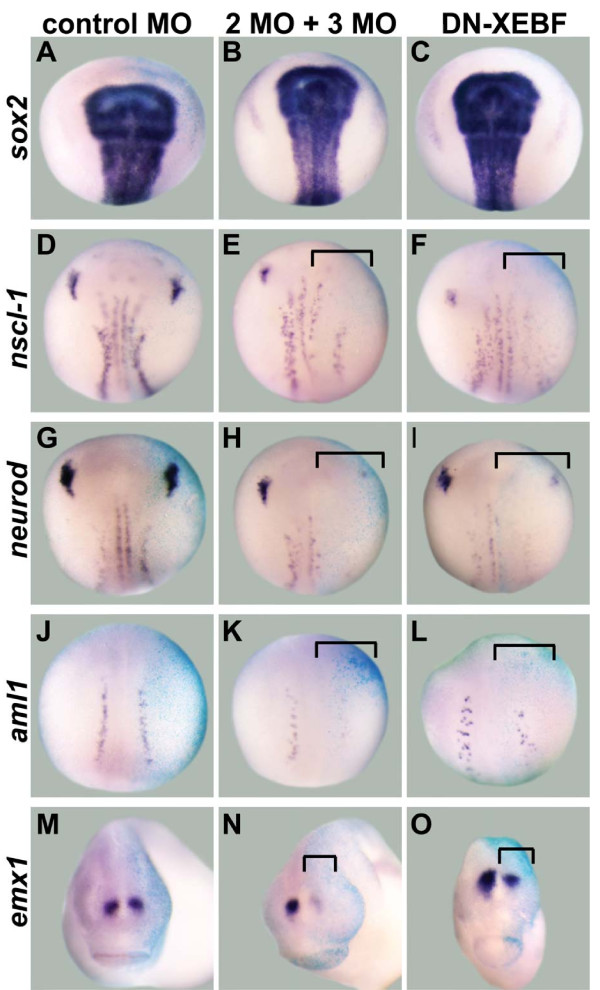
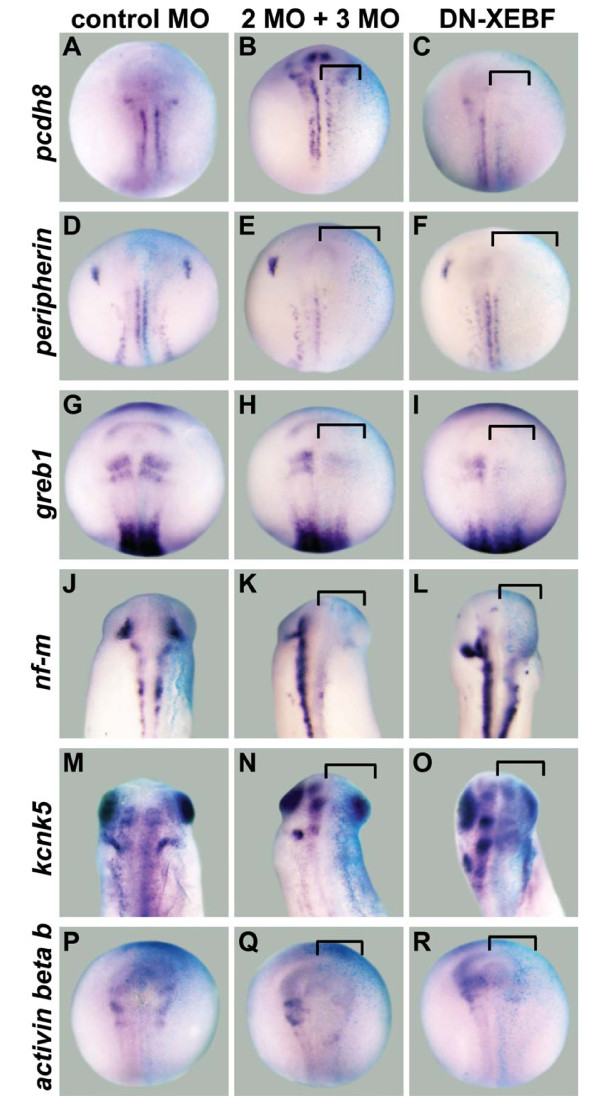
To determine if the expression of our identified candidate target genes is dependent on EBF2 and EBF3 in vivo, we examined the expression levels of candidate targets after knockdown of EBF2 and EBF3 expression using translation blocking antisense MOs. EBF2 MO and EBF3 MO were co-injected into one cell of two-cell stage embryos and the expression levels of endogenous candidate target genes were examined at the neurula stage (stage 15/16) or tailbud stage (stages 25 to 28), when expression of candidate target genes is apparent (Figures 2 and 3). We first confirmed that expression of the neural plate marker sox2 was not changed (10/10 embryos; Figure 2B), indicating that knockdown of EBF2 and EBF3 did not affect early global neuronal development in the early embryos. After co-injection of EBF2 MO and EBF3 MO, the expression of nscl-1 (10/11), neurod (13/15), aml1 (7/12), emx1 (12/15), pcdh8 (12/13), peripherin (11/11), greb1 (11/12), nf-m (14/16), kcnk5 (10/15) and activin beta b (5/10) were downregulated (Figures 2 and 3). Control MO did not change the expression levels of these genes (Figures 2 and 3). The expression of peripherin was partially rescued by co-injecting EBF2 MO and EBF3 MO together with mRNA for hGR-XEBF2, which does not have overlapping sequence with the MOs, and then treating with DEX from the gastrula stage (stage 11.5) to the neurula stage (stage 15/16) (Additional file 5). This control demonstrates that the EBF2 MO and EBF3 MO specifically block EBF activity.
Figure 2.
Downregulation of transcription factor candidate target genes after knockdown of EBF2 and EBF3. One cell of two-cell stage embryos was injected with either control MO, both EBF2 MO and EBF3 MO (2MO + 3MO), or dominant negative Xenopus EBF3 (DN-XEBF) mRNA. β-Galactosidase (β-gal) mRNA was co-injected as a marker of the injected side. In all panels the right side is the injected side, showing the light blue color of X-gal staining. (A-C) The expression of sox2 was not changed in all three conditions. (D-O) The expression of nscl-1 (E,F), neurod (H,I), aml1 (K,L), and emx1 (N,O) is downregulated by EBF2 MO and EBF3 MO, and by DN-XEBF (brackets), while control MO does not change their expression levels (D,G,J,M). Panels (A-L) show dorsal views of neurula stage embryos (stage 15/16), and (M-O) are anterior views of tail bud stage embryos (stages 25 to 28).
Figure 3.
Downregulation of non-transcription factor candidate target genes after knockdown of EBF2 and EBF3. One cell of two-cell stage embryos was injected with either control MO, both EBF2 MO and EBF3 MO (2MO + 3MO), or dominant negative Xenopus EBF3 (DN-XEBF) mRNA. β-Galactosidase (β-gal) mRNA was co-injected as a marker of the injected side. In all panels the right side is the injected side, showing the light blue color of X-gal staining. (A-R) The expression of pcdh8 (B,C), peripherin (E,F), greb1 (H,I), nf-m (K,L), kcnk5 (N,O), and activin beta b (Q,R) is downregulated by EBF2 MO and EBF3 MO, and by DN-XEBF (brackets), while control MO does not change their expression levels (A,D,G,J,M,P). Panels (A-I) and (P-R) are neurula stage embryos (stage 15/16), and (J-O) are tail bud stage embryos (stages 25 to 28). All panels show dorsal views.
To confirm the MO results, we generated a dominant negative Xenopus EBF3 construct (DN-XEBF). This DN-XEBF (amino acids 349 to 598) lacks the DNA binding domain in the amino-terminal region, but it has an intact dimerization domain [4,7,12]. Since EBF1, 2, and 3 can form homodimers or heterodimers in vitro [2,4,12], this DN-XEBF is predicted to block the function of both EBF2 and EBF3 by forming non-functional dimers. Similar to our MO data, injection of mRNA encoding DN-XEBF led to downregulation of the expression of nscl-1 (5/13 embryos), neurod (12/18), aml1 (6/14), emx1 (7/15), pcdh8 (8/18), peripherin (8/13), greb1 (8/14), nf-m (10/19), kcnk5 (7/19) and activin beta b (8/17) (Figures 2 and 3) while sox2 expression was not changed by DN-XEBF at the neurula stage (13/14) (Figure 2C). However, the level of downregulation of candidate target genes was weaker than that obtained by MO injection, perhaps because some endogenous EBF protein is able to form normal dimers even in the presence of DN-XEBF. In addition, a majority of embryos became bent toward the injected side at the tailbud stage because this side was smaller than the uninjected side (data not shown), suggesting changes in the development of other tissues. Taken together, these function-blocking experiments with MOs and DN-XEBF suggest that EBF2 and EBF3 are required for the expression of our neuronal candidate targets in vivo.
Comparison of the expression patterns of EBF2, EBF3 and their candidate targets in the Xenopus nervous system
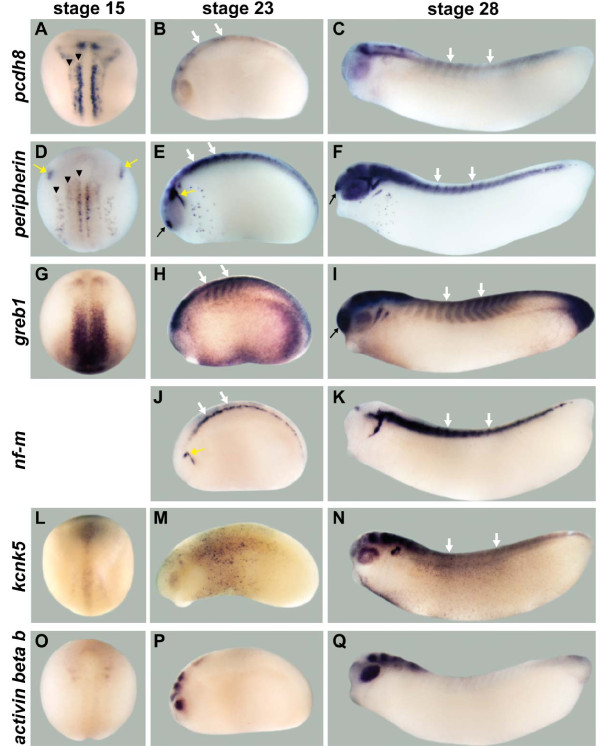
To determine if the functional relationships we identified above might be indicative of in vivo genetic relationships between ebf genes and candidate targets, and to determine if the candidate targets have expression patterns consistent with a role in neuronal development, we compared the expression domains of ebf2 and ebf3 with those of candidate target genes by WM-ISH at four different stages in early Xenopus embryos: stage 12.5 (data not shown), 15, 23 and 28 (Figures 4 and 5). We chose these stages because the expression of ebf2 is visible from stage 12.5, the expression of ebf3 is clearly visible at stage 15, and their expression continues beyond stage 28 [7,8]. ebf2 and ebf3 are expressed in very similar neuronal tissues (Figure 4A-F). At stage 15, both are expressed in the three stripes (medial, intermediate, and lateral) of primary neurons in the neural plate and trigeminal placodes. At stage 23, both are expressed in the trigeminal placodes, the olfactory placodes, spinal cord, and neural crest derivatives, including branchial arches. By stage 28, their expression expands to encompass much of the developing brain.
Figure 4.
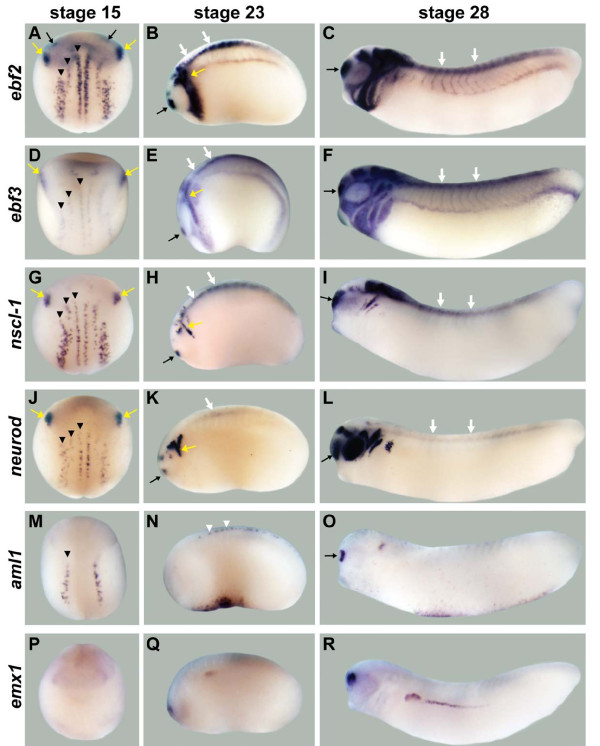
Neuronal expression for ebf genes and transcription factor candidate target genes. (A-F) ebf2 (A-C) and ebf3 (D-F) are expressed in multiple regions of the developing nervous system, including the trigeminal placodes (yellow arrows), olfactory placodes (black arrows), some domains in the brain, the spinal cord (white arrows), and neural crest derivatives like the branchial arches. (G-L) nscl-1 (G-I) and neurod (J-L) are expressed in the trigeminal placodes and three stripes of primary neurons in the neural plate (black arrowheads) at stage 15, and are strongly expressed in the trigeminal placodes, olfactory placodes, and spinal cord at stage 23. At stage 28, nscl-1 is expressed in the olfactory placodes, some domains in the midbrain/hindbrain, spinal cord and cranial ganglia IX and X. At stage 28, neurod is expressed in the olfactory placodes, retina, otic placodes, cranial ganglia, spinal cord and some domains in the brain. (M-O) aml1 is expressed in the lateral primary neuron stripe at stage 15, sensory neurons of the spinal cord (white arrowheads) at stage 23, and the olfactory placodes and otic placodes at stage 28. (P-R) emx1 is weakly expressed in the prospective forebrain region at stage 15. At stages 23 and 28, this gene is expressed in the dorsal forebrain region. Stage 15 embryos show dorsal views except (P) (anterior view). Stage 23 and 28 embryos show lateral views.
Figure 5.
Neuronal expression for non-transcription factor candidate target genes. (A-C) pcdh8 is expressed in medial and intermediate stripes (arrowheads) of primary neurons, in one posterior stripe between the two stripes of primary neurons, and in the anterior domain of the neural plate at stage 15, and in the spinal cord (white arrows) and some domains in the brain at stages 23 and 28. (D-F) peripherin is expressed in the trigeminal placodes (yellow arrows) and three stripes of primary neurons (arrowheads) at stage 15, and in the trigeminal placodes, olfactory placodes (black arrow), spinal cord, retina and many domains in the brain at stages 23 and 28. (G-I) greb1 is expressed as a band in the prospective midbrain/hindbrain region at stage 15, and in the midbrain/hindbrain region and spinal cord at stage 23, and in the olfactory placodes, spinal cord, and many domains in the brain at stage 28. (J,K) nf-m is not expressed at stage 15, but at stages 23 and 28 it is expressed in the trigeminal placodes and spinal cord. (L-N) kcnk5 is weakly expressed from anterior to posterior along the dorsal midline, with stronger expression in the anterior end of the neural fold at stage 15. It is expressed in retina, otic placode, and several domains in the brain at stages 23 and 28, and in spinal cord at stage 28. (O-Q) activin beta b is expressed in two bands in the prospective midbrain/hindbrain region and diffusely throughout the anterior neural plate at stage 15, and it is expressed in the retina and some brain domains at stages 23 and 28. Stage 15 embryos show dorsal views, and stages 23 and 28 embryos show lateral views.
First, we compared the expression patterns of ebf genes and the candidate targets that are known transcription factors since a number of candidate target genes fell into this category (Figure 4). The expression patterns of both bHLH transcription factors, nscl-1 (Figure 4G-I) [40] and neurod (Figure 4J-L) [33], overlap strongly with those of ebf2 and ebf3 in the three stripes of primary neurons, trigeminal placodes, olfactory placodes and spinal cord. In Xenopus embryos, the neuronal expression of aml1 (Figure 4M-O) [42,43] is limited to sensory neurons, including the lateral stripe at stage 15, sensory neurons in the spinal cord at stage 23, and olfactory placode at stage 28, and this expression pattern overlaps with the expression of ebf2 and ebf3. The expression of emx1 (Figure 4P-R) [44] in the dorsal forebrain region at stages 23 and 28 overlaps with the expression of ebf2 and ebf3.
Second, we compared the expression patterns of ebf genes and the candidate targets that do not have transcriptional activity, and that were predicted to play a role in cell structure and neuronal function (Figure 5). The expression of pcdh8 (Figure 5A-C) does partially overlap with that of ebf2 and ebf3 in two stripes of primary neurons, spinal cord, midbrain, and hindbrain. The neuronal intermediate filament genes peripherin (Figure 5D-F) [51] and nf-m (Figure 5J,K) [52] show strongly overlapping expression patterns with ebf2 and ebf3 in the three stripes of primary neurons, trigeminal placodes, olfactory placodes and spinal cord from the earliest stages in which they are expressed. The expression patterns of greb1 (Figure 5G-I), kcnk5 (Figure 5L-N) and activin beta b (Figure 5O-Q) [50] do not overlap with those of ebf2 and ebf3 at stage 15, but partially overlap in some domains in the brain or the spinal cord at stages 23 or 28.
In summary, the candidate EBF target genes show complex patterns of expression during nervous system development with different degrees of overlap with the EBF factors. For example, some were highly overlapping, nscl-1, neurod, aml1, peripherin, and nf-m, while others showed more limited overlap, such as emx1, pcdh8, greb1, kcnk5, and activin beta b. The genes pcdh8, greb1, and kcnk5 have not previously been described during Xenopus neuronal development.
Identification of direct candidate targets for EBF3 in animal caps by RT-QPCR
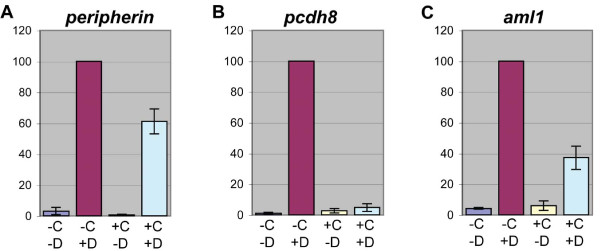
To better understand the transcriptional interaction between EBF3 and its candidate target genes, we sought to identify which genes are direct transcriptional candidate targets and which are indirect transcriptional candidate targets. We used an approach similar to the microarray analysis, with DEX treatment of animal caps to drive activation of hGR-XEBF3, but we added CHX to block protein synthesis, so that only direct EBF3 candidate targets should be transcribed. Animal caps were collected at stage 9 after injection of hGR-XEBF3, and divided into four groups: untreated control (-C-D), DEX alone (-C+D), CHX alone (+C-D), and both CHX and DEX (+C+D). All animal caps were collected after a 3.5-hour incubation. CHX treatment lasted the entire 3.5 hours, while DEX treatment started after a 30-minute delay to allow time for CHX to take effect. The expression level of each candidate target was examined by RT-QPCR (Table 1 and Figure 6; Additional file 6). We normalized the expression level of candidate target genes with that of histone h4, and for Figure 6 and Additional file 6 we set the normalized expression level in the condition of -C+D to 100% (see Materials and methods).
Figure 6.
The identification of direct and indirect candidate targets of EBF3 by RT-QPCR. hGR-XEBF3 mRNA and Noggin mRNA were injected into one-cell stage embryos, and animal caps were collected at the blastula stage (stage 9). The animal caps were divided into four groups, based on CHX and DEX treatment: -C-D, -C+D, +C-D, and +C+D. After a 3.5-hour incubation with CHX and/or a 3-hour incubation with DEX, total RNA was isolated from each animal cap group. RT-QPCR was conducted with the isolated total RNA. The expression level was normalized with the expression level of histone h4 and then normalized to the expression level of -C+D, for each gene, at 100 arbitrary units. (A-C) The expression levels of all candidate target genes in controls (-C-D and +C-D) are very low compared to the DEX-treated condition of -C+D. (A) The expression level of peripherin in +C+D (61%) is only partially reduced compared to -C+D, indicating that the majority of its expression is controlled by EBF3 directly. (B) The expression level of pcdh8 in +C+D (5%) is much lower than in -C+D and is similar to the levels of the control conditions, indicating that it is an indirect candidate target. (C) The expression level of aml1 in +C+D (37%) is lower than the expression level in -C+D but higher than levels of the control conditions, indicating that its expression is partially controlled by EBF3 directly. Error bars represent standard error of the mean. The results for the remaining candidate target genes are shown in Additional file 6. N = 3 replicates, 20 to 30 animal caps per condition.
After treatment with both CHX and DEX, candidate targets that had expression levels of less than 10% of the level in animal caps treated with DEX alone (or candidates that had expression levels similar to untreated controls, even if that was greater than 10% of the DEX alone condition) were considered to be indirect candidate targets. These indirect targets are pcdh8, kcnk5, activin beta b, neurod and greb1 (Figure 6B; Additional file 6D-G). Candidate target genes with expression levels of greater than 50% of the levels in animal caps treated with DEX alone are candidate targets for which the majority of their expression is directly controlled by EBF3. These include peripherin, emx1 and nf-m (Figure 6A; Additional file 6AB). Finally, there are genes with expression levels between 10 and 50% of the level in animal caps treated with DEX alone. These genes, including aml1 and nscl-1, likely have some expression that is under direct regulation by EBF3 (Figure 6C; Additional file 6C), suggesting that their expression is controlled not only by EBF3 directly but also through other targets of EBF3.
Discussion
To better understand the range of activities that are driven by EBF transcription factors during neural development, we used a systematic approach to identify candidate target genes of EBF activity in Xenopus. In this study, we emphasize candidate targets that were highly upregulated by EBF activity by microarray analysis and with potential functions in neuronal development. Our microarray screen for targets of EBF transcriptional activity revealed many genes that were previously not known to be targets of EBF activity. Ten of the genes showed upregulation after EBF overexpression, and downregulation after EBF knockdown in neurula or tailbud stage embryos, demonstrating their in vivo dependence on EBF activity. In addition, all ten of these genes partially overlap in their expression with ebf genes, consistent with regulation by EBF proteins during neural development. Since some of these genes are direct targets with partial dependency on EBF activity, others are indirect candidate targets, and most have only partially overlapping expression with ebf genes, it is clear that EBF proteins are part of a more complex transcriptional regulatory network involved in driving their expression. The candidate target genes we identified by microarray analysis, but did not characterize further, also have the potential to reveal involvement of EBF proteins in additional activities, and this list of genes should aid future research into EBF functions.
Several previous studies, including EBF gain- and loss-of-function studies in Drosophila, C. elegans, ebf mutant mice, chicks, Xenopus, and in cell lines, have revealed a number of neuronal genes that are regulated by EBF transcriptional activity [2,7,8,15-17,19,53-56]. With the exception of nf-m, the list of candidate EBF target genes that we selected for analysis does not include these previously described EBF-regulated genes. However, among the additional genes from the microarray that are upregulated by EBF activity we found lhx9 and lmo4, which are homologous to apterous, a known target of Collier in Drosophila [17]. It is likely that we did not recognize the presence of other known EBF target genes in our array results due to the incomplete annotation of the Xenopus microarray. Another explanation could be that expression of these targets is driven by EBF in the more differentiated environments of each independent experiment, but not in the relatively naïve environment of Xenopus animal caps. Future improvements in Xenopus annotation should make it possible to help distinguish between these two explanations.
We find in our Xenopus EBF screens comparable numbers of strongly downregulated genes and strongly upregulated genes. This parallels the important roles shown for EBF proteins functioning as transcriptional repressors to help determine cell fate in cell types such as B cells in mice [37,57,58] and ASI chemosensory neurons in C. elegans [55]. As part of a future study, it will be interesting to see the extent to which our candidate downregulated genes are related to cell fate.
EBF proteins are involved in the development of multiple lineages, including B cells and adipocytes. Interestingly, in our array analysis we found genes with known roles in non-neuronal tissue types like myod, which regulates muscle development, or lmo2 and hex, which are involved in leukocyte development. These genes were upregulated even after animal caps were neuralized with Noggin. Thus, in the relatively naïve environment of animal cap ectoderm it may be permissive for EBF target genes from multiple lineages to be expressed. EBF proteins have been proposed to function as pioneer factors for B cell genes in hematopoietic lineages [37]. Therefore, EBF factors may alter chromatin to permit genes involved in lineage specification and differentiation to be expressed. There have been screens performed to identify targets of EBF proteins in non-neuronal cell types such as B cells and adipocytes [37,59,60]. A detailed comparison of our array results with these studies is difficult since limited annotation of the Xenopus arrays precludes systematic cross-species comparisons.
EBF regulation of multiple transcription factor genes suggests involvement in extensive transcriptional networks for neuronal development
Our finding that several transcription factors are among the strongest candidate targets of EBF activity expands the potential means by which EBF activity could exert its many effects on neuronal development, and also suggests some interesting new potential functions. The bHLH transcription factor NSCL-1 can drive expression of the proneural bHLH transcription factor NGNR-1, which is important for neuronal cell commitment in Xenopus embryos [40,61]. In chick and mouse, NSCL-1 can promote neuronal cell differentiation, and migration of cellular populations, including GnRH-1 neurons [62-64]. Interestingly, Ebf2 knockout mice show a migration defect of GnRH-1 neurons [21]. We find that the expression of nscl-1 is partially under the direct control of EBF activity, and that the expression patterns of nscl-1 and ebf genes strongly overlap. This suggests that EBF activity may act through NSCL-1 to regulate neuronal cell commitment, differentiation or migration.
The well-known functions of the proneural bHLH transcription factor NeuroD in multiple species show that it is involved primarily in differentiation, but also acts to regulate cell fate, cell migration and cell survival [33,65-68]. This study and previous studies show that neurod expression is very similar to that of ebf genes [7,8,33]. Previous studies showed that neurod is both downstream of EBF2 and upstream of ebf2 and ebf3 in Xenopus embryos [7,8,28,65]. Our present data suggest that neurod is also an indirect candidate target of EBF3. Together, these results support and expand the concept of multiple transcriptional interactions between EBF proteins and NeuroD [7,8,28,65].
AML1, a runt related transcription factor, is known to be expressed in neurons, including cortical progenitors, olfactory receptor progenitors and neurons in the dorsal root ganglia and to be involved in differentiation and cell type specification of several types of sensory and motor neurons, including neurons in the dorsal root ganglia [42,69-71]. Interestingly, AML1 is known to cooperate with EBF proteins in B cell development [72]. We find that aml1 is partially under the direct control of EBF activity, and that the expression patterns of aml1 and ebf genes overlap strongly in the nervous system. Thus, AML1 and EBF proteins may also act cooperatively in promoting neuronal differentiation.
In multiple species, the homeobox transcription factor Emx1 is strongly expressed in the developing forebrain, and the EMX1 protein is present in the axons of the olfactory neurons [44,73,74]. Compared to Emx2 knockout mice, Emx1 knockout mice show only minor defects in brain development [75-77]. However, Emx1 and Emx2 double mutant mice show more severe defects than Emx2 knockout mice, including defects of neuronal differentiation and thalamocortical pathfinding [78], similar to those found in Ebf1 knockout mice [16]. Since we find emx1 to be a strong, direct candidate target of EBF proteins, and emx1 and ebf genes are both strongly expressed in the forebrain, EBF proteins may control cell differentiation and axon growth in part by driving expression of emx1.
EBF proteins drive expression of candidate targets involved in multiple aspects of neuronal differentiation
The candidate targets that are not transcription factors illuminate some of the ways that EBF activity could help regulate late steps of neuronal differentiation and neuronal function. Peripherin and NF-M are important components of neuronal intermediate filaments, which help to form the cytoskeleton in the cell body and neurites of neurons [79-82]. We find that the majority of expression of peripherin and nf-m is controlled directly by EBF3, and that their expression strongly overlaps with that of ebf genes. These discoveries correlate with previous evidence showing axonal pathfinding defects of thalamocortical and olfactory receptor neurons in Ebf null mice, pathfinding defects of motor neurons in C. elegans UNC-3 mutants, and problems with dendritic arborization in Drosophila Collier mutants [11,16,19,20,53,54,83]. These correlations both support a role for EBF proteins in axon growth or stability and provide a potential additional route for exploration of how EBF proteins can affect this important process.
PCDH8 is a transmembrane calcium-dependant adhesion molecule. The rat homolog Arcadlin affects the number of dendritic spines in cultured hippocampal neurons [84] and is required for activity-induced long-term potentiation [85]. We find that EBF proteins positively regulate the expression of a gene that is likely the Xenopus pcdh8 homolog (based on sequence similarity and similar range of gene expression with mouse Pcdh8 in midbrain, hindbrain and spinal cord [86]). We show that pcdh8 is an indirect candidate target of EBF activity, and that pcdh8 and ebf expression patterns overlap in the brain and spinal cord, suggesting that EBF proteins may be involved in synaptic plasticity by controlling the expression of pcdh8, which would be a new function for EBF proteins in the nervous system.
KCNK5 is a K+ channel that is sensitive to extracellular pH, and in rat kidney cells it functions to stabilize bicarbonate transport and control cell volume [49,87,88]. It appears to be involved in maintaining the membrane potential of chemoreceptor cells in mouse brainstem [89]. We find that Xenopus kcnk5 is indirectly upregulated by EBF activity. In addition, we find overlap between ebf and kcnk5 gene expression in the midbrain and hindbrain at the tailbud stage. Regulation of this gene represents a previously unknown function for EBF transcriptional activity.
Activin beta B forms homodimers, or heterodimers with Activin beta A. Activins are ligands of the TGF-beta superfamily, which are involved in differentiation in tissues from many systems, including the reproductive system [90-93]. Activin beta B is expressed in the developing brain and retina ([50,94,95] and our data), but its function in neuronal development is not yet clear. Our study shows that the activin beta b gene is likely an indirect candidate target of EBF proteins, and that its expression precedes that of ebf genes in midbrain, hindbrain and retina. These results suggest that EBF activity may maintain the expression of activin beta b instead of initiating its expression.
The protein GREB1 is thought to be involved in the estrogen-induced growth of breast cancer cells [96,97], but its function is not known, and its expression pattern in animal development has not been previously described. We find that greb1 is expressed in several tissues, including neurons and muscle cells, during Xenopus development. Overlapping expression with ebf genes is limited to the spinal cord and a few brain regions at tailbud stages, and we find that the expression of greb1 is controlled by EBF proteins indirectly. Our findings demonstrate a potential relationship between these genes and a possible role for GREB1 in neuronal development.
Candidate targets that overlap most with ebf genes tend to be direct targets
Interestingly, the candidate targets having direct dependency on EBF3 for their expression, based on our CHX experiments (Figure 6; Additional file 6), tended to have the most overlap with ebf genes in their WM-ISH expression patterns (Figures 4 and 5). These included the genes that code for axonal structural proteins (peripherin and nf-m) and two that code for transcription factors (aml1 and nscl-1). In contrast, the genes that appear to be indirect candidate targets tended to be those that have the least overlap with ebf genes in their expression patterns. These included pcdh8, kcnk5, activin beta b, and greb1. An exception to this rule was neurod, which has extensive overlap with ebf genes, but is an indirect target. This could reflect the fact that neurod is also upstream of ebf genes [7,8,28,65]. These results strengthen the conclusion that in vivo expression of the candidate target genes determined to have direct dependency on EBF3 transcriptional activity is likely to be heavily dependent on EBF activity.
EBF proteins function in cell commitment and differentiation in the development of multiple lineages
EBF proteins have been implicated in the specification, commitment, and differentiation of specific cell types by regulating the expression of both genes in transcriptional regulatory networks as well as genes involved in the functional activities of a cell type, consistent with what we found in our analysis. For example, EBF1 is known to participate in B cell specification, commitment, and differentiation by inducing the expression of transcription factors like E2A (Tcf3) and Pax5, and non-transcription factors like CD79a (mb-1) and VpreB [37,60,98,99]. EBF proteins similarly participate in adipocyte differentiation, by inducing the expression of both types of genes [59,100,101].
In B cells EBF1 acts cooperatively with other transcription factors, including AML1 (RUNX1), E2A, Pax5, and Foxo1, by driving expression of overlapping sets of target genes ([102] and reviewed in [36,103-105]). Interestingly, these transcription factors are themselves targets of EBF1 in B cells. Similar mechanisms are found during neuronal subtype development in the Drosophila ventral nerve cord [17]. The fact that AML1 is also one of our candidate targets, and that most of our transcription factor candidate targets have extensive and early co-expression with ebf2 and ebf3, suggests that EBF proteins may act cooperatively with their transcription factor targets for the expression of some genes in Xenopus neuronal development. Cooperative regulation of gene expression could be part of positive feedback mechanisms that solidify cell commitment choices and differentiated states.
EBF2 and EBF3 appear to share most candidate targets during early Xenopus development
In the developing mouse nervous system, there is evidence for areas of overlap in the functions of different members of the EBF family. For example, Ebf2 and Ebf3 knockout mice have similar phenotypes for olfactory axon growth [20], and Ebf1 null mice do not show severe defects in the brain regions expressing multiple EBF family members concomitantly [1,2,5,16,98]. However, there is also evidence for distinct functions of different members, including the fact that Ebf1 is the only member expressed in the embryonic striatum and Ebf1 null mice show defective neuronal cell differentiation in that region [1,16]. In addition, Ebf2 null mice show defective migration of GnRH-1 neurons even though Ebf1 is also expressed in those neurons [21,106]. In Xenopus, the previously known functions of EBF2 and EBF3 are very similar during early development [7,8]. They are both important for neuronal differentiation, including control of the expression of the neuronal specific markers N-tubulin, N-CAM, and NF-M. Supporting the similarity of the roles of these two genes, we find that the ten candidate targets of EBF3 that were upregulated by hGR-XEBF3 in Xenopus animal caps and in vivo could also be upregulated by hGR-XEBF2 in vivo. Although there is interesting evidence for some differences in expression patterns and functions of EBF2 and EBF3 [7,8], our results support the idea that EBF2 and EBF3 have largely redundant functions at the transcriptional level.
Conclusions
We have found multiple EBF candidate targets using a systematic approach in Xenopus embryos. The expression patterns of direct candidate targets of EBF3 have strong overlap with ebf gene expression, while candidate targets having largely indirect dependency on EBF3 are expressed in less overlapping patterns, suggesting more complex modes of regulation. The novel candidate target genes suggest new potential routes for EBF transcription factors to carry out their previously known functions of neuronal cell commitment, differentiation, neurite formation and migration, and also suggest some new potential functions of EBF activity.
Abbreviations
bHLH: basic helix-loop-helix; CHX: cycloheximide; COE: Collier/Olf/Ebf; DEX: dexamethasone; EBF: early B cell factor; GEO: Gene Expression Omnibus; GnRH: gonadotropin releasing hormone; hGR: human glucocorticoid receptor; MO: morpholino; nf-m: neurofilament-m; O/E: Olf/Ebf; RT-PCR: reverse transcriptase PCR; RT-QPCR: real-time quantitative PCR; TGF: transforming growth factor; WM-ISH: whole mount in situ hybridization.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
YSG participated in the design of these experiments, performed the experiments, analyzed most of the data, and wrote the manuscript. MLV guided the design of the experiments and aided the writing and data analysis. Both authors approved the final manuscript.
Supplementary Material
Hormone inducible constructs and scheme of microarray screens. (A) Schematic diagram of the hormone inducible constructs used in the microarrays. (B) Schematic representation of the microarray screen comparing transcripts in DEX-treated animal caps (with activated hGR-XEBF3) and untreated animal caps (without activated hGR-XEBF3), as well as the control array to analyze the effects of DEX treatment. The scheme shown represents the arrays performed in the presence of Noggin mRNA.
Primer sequences used for RT-QPCR.
Microarray results table showing genes up- or downregulated more than two-fold by EBF3 activity in the presence of Noggin.
Microarray results table showing genes up- or downregulated more than two-fold by EBF3 activity in the absence of Noggin.
Expression of peripherin in embryos treated with EBF2 MO and EBF3 MO can be rescued by co-injection of hGR-XEBF2. One cell of two-cell stage embryos was injected with EBF2 MO, EBF3 MO and mRNA encoding hGR-XEBF2, followed by DEX treatment (or no treatment in controls) from the late gastrula stage (stage 11.5) to the neurula stage (stage 15/16). β-Galactosidase (β-gal) mRNA was co-injected as a marker of the injected side. In both panels the right side is the injected side (brackets). In control embryos (without DEX treatment) peripherin expression was downregulated either strongly (3/7, shown in (A)) or weakly (4/7, not shown) compared to the uninjected side due to the MO effect. In the majority of DEX-treated embryos peripherin expression was either rescued (16/34, shown in (B)), or only weakly downregulated (17/34, not shown). Both panels show dorsal views.
Additional identification of direct and indirect candidate targets of EBF3 by RT-QPCR. Expression levels for the remaining candidate target genes tested by RT-QPCR after CHX and DEX treatment (those not shown in Figure 6). (A,B) The expression levels of emx1 (88%) and nf-m (56%) in +C+D are slightly lower than in -C+D, indicating that the majority of their expression is controlled by EBF3 directly. (C) The expression level of nscl-1 (46%) in +C+D is lower than in -C+D but higher than that in the two controls, indicating that its expression is under partial direct control of EBF3. (D-G) The expression levels of kcnk5 (20%), activin beta b (10%), neurod (8%) and greb1 (1%) in +C+D are similar to control levels (D) or much lower (less than 10%) than in -C+D (E-G). N = 20 to 30 animal caps per condition.
Contributor Information
Yangsook S Green, Email: yangsook@neuro.utah.edu.
Monica L Vetter, Email: monica.vetter@neuro.utah.edu.
Acknowledgements
We thank Brett Milash for assistance with the microarray data analysis. We thank Kathryn Moore, Jianmin Zhang, and Eric Green for critical comments on the manuscript. We thank Richard Harland for the pCS2+Noggin construct, Ombretta Pozzoli for the pCS2+hGR-MT-Xebf3 construct, and David Hutcheson for the pCS2+hGR-MT construct. This work was funded by NIH grant EY012274 to MLV.
References
- Garel S, Marin F, Mattei MG, Vesque C, Vincent A, Charnay P. Family of Ebf/Olf-1-related genes potentially involved in neuronal differentiation and regional specification in the central nervous system. Dev Dyn. 1997;210:191–205. doi: 10.1002/(SICI)1097-0177(199711)210:3<191::AID-AJA1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Wang SS, Tsai RY, Reed RR. The characterization of the Olf-1/EBF-like HLH transcription factor family: implications in olfactory gene regulation and neuronal development. J Neurosci. 1997;17:4149–4158. doi: 10.1523/JNEUROSCI.17-11-04149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudrycki K, Stein-Izsak C, Behn C, Grillo M, Akeson R, Margolis FL. Olf-1-binding site: characterization of an olfactory neuron-specific promoter motif. Mol Cell Biol. 1993;13:3002–3014. doi: 10.1128/mcb.13.5.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J, Belanger C, Travis A, Turck CW, Grosschedl R. Cloning and functional characterization of early B-cell factor, a regulator of lymphocyte-specific gene expression. Genes Dev. 1993;7:760–773. doi: 10.1101/gad.7.5.760. [DOI] [PubMed] [Google Scholar]
- Malgaretti N, Pozzoli O, Bosetti A, Corradi A, Ciarmatori S, Panigada M, Bianchi ME, Martinez S, Consalez GG. Mmot1, a new helix-loop-helix transcription factor gene displaying a sharp expression boundary in the embryonic mouse brain. J Biol Chem. 1997;272:17632–17639. doi: 10.1074/jbc.272.28.17632. [DOI] [PubMed] [Google Scholar]
- Wang SS, Betz AG, Reed RR. Cloning of a novel Olf-1/EBF-like gene, O/E-4, by degenerate oligo-based direct selection. Mol Cell Neurosci. 2002;20:404–414. doi: 10.1006/mcne.2002.1138. [DOI] [PubMed] [Google Scholar]
- Dubois L, Bally-Cuif L, Crozatier M, Moreau J, Paquereau L, Vincent A. XCoe2, a transcription factor of the Col/Olf-1/EBF family involved in the specification of primary neurons in Xenopus. Curr Biol. 1998;8:199–209. doi: 10.1016/S0960-9822(98)70084-3. [DOI] [PubMed] [Google Scholar]
- Pozzoli O, Bosetti A, Croci L, Consalez GG, Vetter ML. Xebf3 is a regulator of neuronal differentiation during primary neurogenesis in Xenopus. Dev Biol. 2001;233:495–512. doi: 10.1006/dbio.2001.0230. [DOI] [PubMed] [Google Scholar]
- Bally-Cuif L, Dubois L, Vincent A. Molecular cloning of Zcoe2, the zebrafish homolog of Xenopus Xcoe2 and mouse EBF-2, and its expression during primary neurogenesis. Mech Dev. 1998;77:85–90. doi: 10.1016/S0925-4773(98)00144-0. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Valle D, Dubois L, Ibnsouda S, Vincent A. Collier, a novel regulator of Drosophila head development, is expressed in a single mitotic domain. Curr Biol. 1996;6:707–718. doi: 10.1016/S0960-9822(09)00452-7. [DOI] [PubMed] [Google Scholar]
- Prasad BC, Ye B, Zackhary R, Schrader K, Seydoux G, Reed RR. unc-3, a gene required for axonal guidance in Caenorhabditis elegans, encodes a member of the O/E family of transcription factors. Development. 1998;125:1561–1568. doi: 10.1242/dev.125.8.1561. [DOI] [PubMed] [Google Scholar]
- Hagman J, Gutch MJ, Lin H, Grosschedl R. EBF contains a novel zinc coordination motif and multiple dimerization and transcriptional activation domains. EMBO J. 1995;14:2907–2916. doi: 10.1002/j.1460-2075.1995.tb07290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois L, Vincent A. The COE - Collier/Olf1/EBF - transcription factors: structural conservation and diversity of developmental functions. Mech Dev. 2001;108:3–12. doi: 10.1016/S0925-4773(01)00486-5. [DOI] [PubMed] [Google Scholar]
- Liberg D, Sigvardsson M, Akerblad P. The EBF/Olf/Collier family of transcription factors: regulators of differentiation in cells originating from all three embryonal germ layers. Mol Cell Biol. 2002;22:8389–8397. doi: 10.1128/MCB.22.24.8389-8397.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Dominguez M, Poquet C, Garel S, Charnay P. Ebf gene function is required for coupling neuronal differentiation and cell cycle exit. Development. 2003;130:6013–6025. doi: 10.1242/dev.00840. [DOI] [PubMed] [Google Scholar]
- Garel S, Marin F, Grosschedl R, Charnay P. Ebf1 controls early cell differentiation in the embryonic striatum. Development. 1999;126:5285–5294. doi: 10.1242/dev.126.23.5285. [DOI] [PubMed] [Google Scholar]
- Baumgardt M, Miguel-Aliaga I, Karlsson D, Ekman H, Thor S. Specification of neuronal identities by feedforward combinatorial coding. PLoS Biol. 2007;5:e37. doi: 10.1371/journal.pbio.0050037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad B, Karakuzu O, Reed RR, Cameron S. unc-3-dependent repression of specific motor neuron fates in Caenorhabditis elegans. Dev Biol. 2008;323:207–215. doi: 10.1016/j.ydbio.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garel S, Yun K, Grosschedl R, Rubenstein JL. The early topography of thalamocortical projections is shifted in Ebf1 and Dlx1/2 mutant mice. Development. 2002;129:5621–5634. doi: 10.1242/dev.00166. [DOI] [PubMed] [Google Scholar]
- Wang SS, Lewcock JW, Feinstein P, Mombaerts P, Reed RR. Genetic disruptions of O/E2 and O/E3 genes reveal involvement in olfactory receptor neuron projection. Development. 2004;131:1377–1388. doi: 10.1242/dev.01009. [DOI] [PubMed] [Google Scholar]
- Corradi A, Croci L, Broccoli V, Zecchini S, Previtali S, Wurst W, Amadio S, Maggi R, Quattrini A, Consalez GG. Hypogonadotropic hypogonadism and peripheral neuropathy in Ebf2-null mice. Development. 2003;130:401–410. doi: 10.1242/dev.00215. [DOI] [PubMed] [Google Scholar]
- Croci L, Chung SH, Masserdotti G, Gianola S, Bizzoca A, Gennarini G, Corradi A, Rossi F, Hawkes R, Consalez GG. A key role for the HLH transcription factor EBF2COE2, O/E-3 in Purkinje neuron migration and cerebellar cortical topography. Development. 2006;133:2719–2729. doi: 10.1242/dev.02437. [DOI] [PubMed] [Google Scholar]
- Garel S, Garcia-Dominguez M, Charnay P. Control of the migratory pathway of facial branchiomotor neurones. Development. 2000;127:5297–5307. doi: 10.1242/dev.127.24.5297. [DOI] [PubMed] [Google Scholar]
- Davis JA, Reed RR. Role of Olf-1 and Pax-6 transcription factors in neurodevelopment. J Neurosci. 1996;16:5082–5094. doi: 10.1523/JNEUROSCI.16-16-05082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DA, Vetter ML. The bHLH factors Xath5 and XNeuroD can upregulate the expression of XBrn3d, a POU-homeodomain transcription factor. Dev Biol. 2001;232:327–338. doi: 10.1006/dbio.2001.0178. [DOI] [PubMed] [Google Scholar]
- Kolm PJ, Sive HL. Efficient hormone-inducible protein function in Xenopus laevis. Dev Biol. 1995;171:267–272. doi: 10.1006/dbio.1995.1279. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, Harland RM. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Logan MA, Steele MR, Van Raay TJ, Vetter ML. Identification of shared transcriptional targets for the proneural bHLH factors Xath5 and XNeuroD. Dev Biol. 2005;285:570–583. doi: 10.1016/j.ydbio.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Chitnis A, Henrique D, Lewis J, Ish-Horowicz D, Kintner C. Primary neurogenesis in Xenopus embryos regulated by a homologue of the Drosophila neurogenic gene Delta. Nature. 1995;375:761–766. doi: 10.1038/375761a0. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. Normal Table of Xenopus laevis (Daudin): a Systematical and Chronological Survey of the Development from the Fertilized Egg Till the End of Metamorphosis. New York: Garland Publishing; 1994. [Google Scholar]
- Turner DL, Weintraub H. Expression of achaete-scute homolog 3 in Xenopus embryos converts ectodermal cells to a neural fate. Genes Dev. 1994;8:1434–1447. doi: 10.1101/gad.8.12.1434. [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Matsui M, Nakanishi S, Sasai Y. Xenopus Zic-related-1 and Sox-2, two factors induced by chordin, have distinct activities in the initiation of neural induction. Development. 1998;125:579–587. doi: 10.1242/dev.125.4.579. [DOI] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Kanekar S, Perron M, Dorsky R, Harris WA, Jan LY, Jan YN, Vetter ML. Xath5 participates in a network of bHLH genes in the developing Xenopus retina. Neuron. 1997;19:981–994. doi: 10.1016/S0896-6273(00)80391-8. [DOI] [PubMed] [Google Scholar]
- Harland RM. In situ hybridization: an improved whole-mount method for Xenopus embryos. Methods Cell Biol. 1991;36:685–695. doi: 10.1016/s0091-679x(08)60307-6. [DOI] [PubMed] [Google Scholar]
- Lukin K, Fields S, Hartley J, Hagman J. Early B cell factor: regulator of B lineage specification and commitment. Semin Immunol. 2008;20:221–227. doi: 10.1016/j.smim.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber T, Mandel EM, Pott S, Gyory I, Firner S, Liu ET, Grosschedl R. Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription- independent poising of chromatin. Immunity. 2010;32:714–725. doi: 10.1016/j.immuni.2010.04.013. [DOI] [PubMed] [Google Scholar]
- van der Wees J, Matharu PJ, de Roos K, Destree OH, Godsave SF, Durston AJ, Sweeney GE. Developmental expression and differential regulation by retinoic acid of Xenopus COUP-TF-A and COUP-TF-B. Mech Dev. 1996;54:173–184. doi: 10.1016/0925-4773(95)00471-8. [DOI] [PubMed] [Google Scholar]
- Dolle P, Izpisua-Belmonte JC, Falkenstein H, Renucci A, Duboule D. Coordinate expression of the murine Hox-5 complex homoeobox-containing genes during limb pattern formation. Nature. 1989;342:767–772. doi: 10.1038/342767a0. [DOI] [PubMed] [Google Scholar]
- Bao J, Talmage DA, Role LW, Gautier J. Regulation of neurogenesis by interactions between HEN1 and neuronal LMO proteins. Development. 2000;127:425–435. doi: 10.1242/dev.127.2.425. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, de la Torre JR, Holt C, Harland RM. Cephalic expression and molecular characterization of Xenopus En-2. Development. 1991;111:715–724. doi: 10.1242/dev.111.3.715. [DOI] [PubMed] [Google Scholar]
- Park BY, Saint-Jeannet JP. Expression analysis of Runx3 and other Runx family members during Xenopus development. Gene Expr Patterns. 2010;10:159–166. doi: 10.1016/j.gep.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey WD Jr, Pepling ME, Horb ME, Thomsen GH, Gergen JP. A Xenopus homologue of aml-1 reveals unexpected patterning mechanisms leading to the formation of embryonic blood. Development. 1998;125:1371–1380. doi: 10.1242/dev.125.8.1371. [DOI] [PubMed] [Google Scholar]
- Pannese M, Lupo G, Kablar B, Boncinelli E, Barsacchi G, Vignali R. The Xenopus Emx genes identify presumptive dorsal telencephalon and are induced by head organizer signals. Mech Dev. 1998;73:73–83. doi: 10.1016/S0925-4773(98)00034-3. [DOI] [PubMed] [Google Scholar]
- Strehl S, Glatt K, Liu QM, Glatt H, Lalande M. Characterization of two novel protocadherins (PCDH8 and PCDH9) localized on human chromosome 13 and mouse chromosome 14. Genomics. 1998;53:81–89. doi: 10.1006/geno.1998.5467. [DOI] [PubMed] [Google Scholar]
- Wolda SL, Moody CJ, Moon RT. Overlapping expression of Xwnt-3A and Xwnt-1 in neural tissue of Xenopus laevis embryos. Dev Biol. 1993;155:46–57. doi: 10.1006/dbio.1993.1005. [DOI] [PubMed] [Google Scholar]
- Sharpe CR. Developmental expression of a neurofilament-M and two vimentin-like genes in Xenopus laevis. Development. 1988;103:269–277. doi: 10.1242/dev.103.2.269. [DOI] [PubMed] [Google Scholar]
- Sharpe CR, Pluck A, Gurdon JB. XIF3, a Xenopus peripherin gene, requires an inductive signal for enhanced expression in anterior neural tissue. Development. 1989;107:701–714. doi: 10.1242/dev.107.4.701. [DOI] [PubMed] [Google Scholar]
- Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem. 1998;273:30863–30869. doi: 10.1074/jbc.273.47.30863. [DOI] [PubMed] [Google Scholar]
- Dohrmann CE, Hemmati-Brivanlou A, Thomsen GH, Fields A, Woolf TM, Melton DA. Expression of activin mRNA during early development in Xenopus laevis. Dev Biol. 1993;157:474–483. doi: 10.1006/dbio.1993.1150. [DOI] [PubMed] [Google Scholar]
- Gervasi C, Stewart CB, Szaro BG. Xenopus laevis peripherin (XIF3) is expressed in radial glia and proliferating neural epithelial cells as well as in neurons. J Comp Neurol. 2000;423:512–531. doi: 10.1002/1096-9861(20000731)423:3<512::AID-CNE13>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Gervasi C, Szaro BG. Sequence and expression patterns of two forms of the middle molecular weight neurofilament protein (NF-M) of Xenopus laevis. Brain Res Mol Brain Res. 1997;48:229–242. doi: 10.1016/s0169-328x(97)00096-x. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Vincent A. Control of multidendritic neuron differentiation in Drosophila: the role of Collier. Dev Biol. 2008;315:232–242. doi: 10.1016/j.ydbio.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Jinushi-Nakao S, Arvind R, Amikura R, Kinameri E, Liu AW, Moore AW. Knot/Collier and cut control different aspects of dendrite cytoskeleton and synergize to define final arbor shape. Neuron. 2007;56:963–978. doi: 10.1016/j.neuron.2007.10.031. [DOI] [PubMed] [Google Scholar]
- Kim K, Colosimo ME, Yeung H, Sengupta P. The UNC-3 Olf/EBF protein represses alternate neuronal programs to specify chemosensory neuron identity. Dev Biol. 2005;286:136–148. doi: 10.1016/j.ydbio.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Persson P, Manetopoulos C, Lagergren A, Nygren J, Gisler R, Axelson H, Sigvardsson M. Olf/EBF proteins are expressed in neuroblastoma cells: potential regulators of the Chromogranin A and SCG10 promoters. Int J Cancer. 2004;110:22–30. doi: 10.1002/ijc.20092. [DOI] [PubMed] [Google Scholar]
- Pongubala JM, Northrup DL, Lancki DW, Medina KL, Treiber T, Bertolino E, Thomas M, Grosschedl R, Allman D, Singh H. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nat Immunol. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- Thal MA, Carvalho TL, He T, Kim HG, Gao H, Hagman J, Klug CA. Ebf1-mediated down-regulation of Id2 and Id3 is essential for specification of the B cell lineage. Proc Natl Acad Sci USA. 2009;106:552–557. doi: 10.1073/pnas.0802550106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerblad P, Mansson R, Lagergren A, Westerlund S, Basta B, Lind U, Thelin A, Gisler R, Liberg D, Nelander S, Bamberg K, Sigvardsson M. Gene expression analysis suggests that EBF-1 and PPARgamma2 induce adipogenesis of NIH-3T3 cells with similar efficiency and kinetics. Physiol Genomics. 2005;23:206–216. doi: 10.1152/physiolgenomics.00015.2005. [DOI] [PubMed] [Google Scholar]
- Mansson R, Tsapogas P, Akerlund M, Lagergren A, Gisler R, Sigvardsson M. Pearson correlation analysis of microarray data allows for the identification of genetic targets for early B-cell factor. J Biol Chem. 2004;279:17905–17913. doi: 10.1074/jbc.M400589200. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/S0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Kruger M, Ruschke K, Braun T. NSCL-1 and NSCL-2 synergistically determine the fate of GnRH-1 neurons and control necdin gene expression. EMBO J. 2004;23:4353–4364. doi: 10.1038/sj.emboj.7600431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid T, Kruger M, Braun T. NSCL-1 and -2 control the formation of precerebellar nuclei by orchestrating the migration of neuronal precursor cells. J Neurochem. 2007;102:2061–2072. doi: 10.1111/j.1471-4159.2007.04694.x. [DOI] [PubMed] [Google Scholar]
- Xie W, Yan RT, Ma W, Wang SZ. Enhanced retinal ganglion cell differentiation by ath5 and NSCL1 coexpression. Invest Ophthalmol Vis Sci. 2004;45:2922–2928. doi: 10.1167/iovs.04-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Lim JW, Yellajoshyula D, Chang LW, Kroll KL. Neurogenin and NeuroD direct transcriptional targets and their regulatory enhancers. EMBO J. 2007;26:5093–5108. doi: 10.1038/sj.emboj.7601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Pleasure SJ, Collins AE, Noebels JL, Naya FJ, Tsai MJ, Lowenstein DH. Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc Natl Acad Sci USA. 2000;97:865–870. doi: 10.1073/pnas.97.2.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. NeuroD-null mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development. 2001;128:417–426. doi: 10.1242/dev.128.3.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge W, He F, Kim KJ, Blanchi B, Coskun V, Nguyen L, Wu X, Zhao J, Heng JI, Martinowich K, Tao J, Wu H, Castro D, Sobeih MM, Corfas G, Gleeson JG, Greenberg ME, Guillemot F, Sun YE. Coupling of cell migration with neurogenesis by proneural bHLH factors. Proc Natl Acad Sci USA. 2006;103:1319–1324. doi: 10.1073/pnas.0510419103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Sigrist M, de Nooij JC, Taniuchi I, Jessell TM, Arber S. A role for Runx transcription factor signaling in dorsal root ganglion sensory neuron diversification. Neuron. 2006;49:379–393. doi: 10.1016/j.neuron.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Theriault FM, Nuthall HN, Dong Z, Lo R, Barnabe-Heider F, Miller FD, Stifani S. Role for Runx1 in the proliferation and neuronal differentiation of selected progenitor cells in the mammalian nervous system. J Neurosci. 2005;25:2050–2061. doi: 10.1523/JNEUROSCI.5108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault FM, Roy P, Stifani S. AML1/Runx1 is important for the development of hindbrain cholinergic branchiovisceral motor neurons and selected cranial sensory neurons. Proc Natl Acad Sci USA. 2004;101:10343–10348. doi: 10.1073/pnas.0400768101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier H, Ostraat R, Gao H, Fields S, Shinton SA, Medina KL, Ikawa T, Murre C, Singh H, Hardy RR, Hagman J. Early B cell factor cooperates with Runx1 and mediates epigenetic changes associated with mb-1 transcription. Nat Immunol. 2004;5:1069–1077. doi: 10.1038/ni1119. [DOI] [PubMed] [Google Scholar]
- Briata P, Di Blas E, Gulisano M, Mallamaci A, Iannone R, Boncinelli E, Corte G. EMX1 homeoprotein is expressed in cell nuclei of the developing cerebral cortex and in the axons of the olfactory sensory neurons. Mech Dev. 1996;57:169–180. doi: 10.1016/0925-4773(96)00544-8. [DOI] [PubMed] [Google Scholar]
- Simeone A, Gulisano M, Acampora D, Stornaiuolo A, Rambaldi M, Boncinelli E. Two vertebrate homeobox genes related to the Drosophila empty spiracles gene are expressed in the embryonic cerebral cortex. EMBO J. 1992;11:2541–2550. doi: 10.1002/j.1460-2075.1992.tb05319.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M, Mansouri A, Simeone A, Boncinelli E, Gruss P. Dentate gyrus formation requires Emx2. Development. 1996;122:3893–3898. doi: 10.1242/dev.122.12.3893. [DOI] [PubMed] [Google Scholar]
- Qiu M, Anderson S, Chen S, Meneses JJ, Hevner R, Kuwana E, Pedersen RA, Rubenstein JL. Mutation of the Emx-1 homeobox gene disrupts the corpus callosum. Dev Biol. 1996;178:174–178. doi: 10.1006/dbio.1996.0207. [DOI] [PubMed] [Google Scholar]
- Yoshida M, Suda Y, Matsuo I, Miyamoto N, Takeda N, Kuratani S, Aizawa S. Emx1 and Emx2 functions in development of dorsal telencephalon. Development. 1997;124:101–111. doi: 10.1242/dev.124.1.101. [DOI] [PubMed] [Google Scholar]
- Bishop KM, Garel S, Nakagawa Y, Rubenstein JL, O'Leary DD. Emx1 and Emx2 cooperate to regulate cortical size, lamination, neuronal differentiation, development of cortical efferents, and thalamocortical pathfinding. J Comp Neurol. 2003;457:345–360. doi: 10.1002/cne.10550. [DOI] [PubMed] [Google Scholar]
- Lin W, Szaro BG. Neurofilaments help maintain normal morphologies and support elongation of neurites in Xenopus laevis cultured embryonic spinal cord neurons. J Neurosci. 1995;15:8331–8344. doi: 10.1523/JNEUROSCI.15-12-08331.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand BT, Mendez MG, Pugh J, Delsert C, Goldman RD. A role for intermediate filaments in determining and maintaining the shape of nerve cells. Mol Biol Cell. 2003;14:5069–5081. doi: 10.1091/mbc.E03-06-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia ML, Lobsiger CS, Shah SB, Deerinck TJ, Crum J, Young D, Ward CM, Crawford TO, Gotow T, Uchiyama Y, Ellisman MH, Calcutt NA, Cleveland DW. NF-M is an essential target for the myelin-directed "outside-in" signaling cascade that mediates radial axonal growth. J Cell Biol. 2003;163:1011–1020. doi: 10.1083/jcb.200308159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A, Gervasi C, Szaro BG. Neurofilament content is correlated with branch length in developing collateral branches of Xenopus spinal cord neurons. Neurosci Lett. 2006;403:283–287. doi: 10.1016/j.neulet.2006.04.055. [DOI] [PubMed] [Google Scholar]
- Hattori Y, Sugimura K, Uemura T. Selective expression of Knot/Collier, a transcriptional regulator of the EBF/Olf-1 family, endows the Drosophila sensory system with neuronal class-specific elaborated dendritic patterns. Genes Cells. 2007;12:1011–1022. doi: 10.1111/j.1365-2443.2007.01107.x. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Tanaka H, Sugiura H, Okamura K, Sakaguchi T, Tran U, Takemiya T, Mizoguchi A, Yagita Y, Sakurai T, De Robertis EM, Yamagata K. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-cadherin endocytosis via TAO2beta and p38 MAP kinases. Neuron. 2007;56:456–471. doi: 10.1016/j.neuron.2007.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Andreasson KI, Sugiura H, Maru E, Dominique M, Irie Y, Miki N, Hayashi Y, Yoshioka M, Kaneko K, Kato H, Worley PF. Arcadlin is a neural activity-regulated cadherin involved in long term potentiation. J Biol Chem. 1999;274:19473–19479. doi: 10.1074/jbc.274.27.19473. [DOI] [PubMed] [Google Scholar]
- Makarenkova H, Sugiura H, Yamagata K, Owens G. Alternatively spliced variants of protocadherin 8 exhibit distinct patterns of expression during mouse development. Biochim Biophys Acta. 2005;1681:150–156. doi: 10.1016/j.bbaexp.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Warth R, Barriere H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R, Guy N, Bendahhou S, Lesage F, Poujeol P, Barhanin J. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA. 2004;101:8215–8220. doi: 10.1073/pnas.0400081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriere H, Belfodil R, Rubera I, Tauc M, Lesage F, Poujeol C, Guy N, Barhanin J, Poujeol P. Role of TASK2 potassium channels regarding volume regulation in primary cultures of mouse proximal tubules. J Gen Physiol. 2003;122:177–190. doi: 10.1085/jgp.200308820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci USA. 2010;107:2325–2330. doi: 10.1073/pnas.0910059107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itman C, Mendis S, Barakat B, Loveland KL. All in the family: TGF-beta family action in testis development. Reproduction. 2006;132:233–246. doi: 10.1530/rep.1.01075. [DOI] [PubMed] [Google Scholar]
- Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction. 2006;132:191–206. doi: 10.1530/rep.1.01074. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Kumar TR, Vassalli A, Bickenbach JR, Roop DR, Jaenisch R, Bradley A. Functional analysis of activins during mammalian development. Nature. 1995;374:354–356. doi: 10.1038/374354a0. [DOI] [PubMed] [Google Scholar]
- Smith JC, Price BM, Van Nimmen K, Huylebroeck D. Identification of a potent Xenopus mesoderm-inducing factor as a homologue of activin A. Nature. 1990;345:729–731. doi: 10.1038/345729a0. [DOI] [PubMed] [Google Scholar]
- Roberts VJ, Barth SL, Meunier H, Vale W. Hybridization histochemical and immunohistochemical localization of inhibin/activin subunits and messenger ribonucleic acids in the rat brain. J Comp Neurol. 1996;364:473–493. doi: 10.1002/(SICI)1096-9861(19960115)364:3<473::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams TL, Scheurer D, Adler R. Activin family members in the developing chick retina: expression patterns, protein distribution, and in vitro effects. Dev Biol. 1999;210:107–123. doi: 10.1006/dbio.1999.9268. [DOI] [PubMed] [Google Scholar]
- Ghosh MG, Thompson DA, Weigel RJ. PDZK1 and GREB1 are estrogen-regulated genes expressed in hormone-responsive breast cancer. Cancer Res. 2000;60:6367–6375. [PubMed] [Google Scholar]
- Rae JM, Johnson MD, Scheys JO, Cordero KE, Larios JM, Lippman ME. GREB 1 is a critical regulator of hormone dependent breast cancer growth. Breast Cancer Res Treat. 2005;92:141–149. doi: 10.1007/s10549-005-1483-4. [DOI] [PubMed] [Google Scholar]
- Lin H, Grosschedl R. Failure of B-cell differentiation in mice lacking the transcription factor EBF. Nature. 1995;376:263–267. doi: 10.1038/376263a0. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Cotta CV, Stephan RP, deGuzman CG, Klug CA. Enforced expression of EBF in hematopoietic stem cells restricts lymphopoiesis to the B cell lineage. EMBO J. 2003;22:4759–4769. doi: 10.1093/emboj/cdg464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerblad P, Lind U, Liberg D, Bamberg K, Sigvardsson M. Early B-cell factor (O/E-1) is a promoter of adipogenesis and involved in control of genes important for terminal adipocyte differentiation. Mol Cell Biol. 2002;22:8015–8025. doi: 10.1128/MCB.22.22.8015-8025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED. Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol Cell Biol. 2007;27:743–757. doi: 10.1128/MCB.01557-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YC, Jhunjhunwala S, Benner C, Heinz S, Welinder E, Mansson R, Sigvardsson M, Hagman J, Espinoza CA, Dutkowski J, Ideker T, Glass CK, Murre C. A global network of transcription factors, involving E2A, EBF1 and Foxo1, that orchestrates B cell fate. Nat Immunol. 2010;11:635–643. doi: 10.1038/ni.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagman J, Lukin K. Early B-cell factor 'pioneers' the way for B-cell development. Trends Immunol. 2005;26:455–461. doi: 10.1016/j.it.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Mandel EM, Grosschedl R. Transcription control of early B cell differentiation. Curr Opin Immunol. 2010;22:161–167. doi: 10.1016/j.coi.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Singh H, Medina KL, Pongubala JM. Contingent gene regulatory networks and B cell fate specification. Proc Natl Acad Sci USA. 2005;102:4949–4953. doi: 10.1073/pnas.0500480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray S. Development of luteinizing hormone releasing hormone neurones. J Neuroendocrinol. 2001;13:3–11. doi: 10.1046/j.1365-2826.2001.00609.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Hormone inducible constructs and scheme of microarray screens. (A) Schematic diagram of the hormone inducible constructs used in the microarrays. (B) Schematic representation of the microarray screen comparing transcripts in DEX-treated animal caps (with activated hGR-XEBF3) and untreated animal caps (without activated hGR-XEBF3), as well as the control array to analyze the effects of DEX treatment. The scheme shown represents the arrays performed in the presence of Noggin mRNA.
Primer sequences used for RT-QPCR.
Microarray results table showing genes up- or downregulated more than two-fold by EBF3 activity in the presence of Noggin.
Microarray results table showing genes up- or downregulated more than two-fold by EBF3 activity in the absence of Noggin.
Expression of peripherin in embryos treated with EBF2 MO and EBF3 MO can be rescued by co-injection of hGR-XEBF2. One cell of two-cell stage embryos was injected with EBF2 MO, EBF3 MO and mRNA encoding hGR-XEBF2, followed by DEX treatment (or no treatment in controls) from the late gastrula stage (stage 11.5) to the neurula stage (stage 15/16). β-Galactosidase (β-gal) mRNA was co-injected as a marker of the injected side. In both panels the right side is the injected side (brackets). In control embryos (without DEX treatment) peripherin expression was downregulated either strongly (3/7, shown in (A)) or weakly (4/7, not shown) compared to the uninjected side due to the MO effect. In the majority of DEX-treated embryos peripherin expression was either rescued (16/34, shown in (B)), or only weakly downregulated (17/34, not shown). Both panels show dorsal views.
Additional identification of direct and indirect candidate targets of EBF3 by RT-QPCR. Expression levels for the remaining candidate target genes tested by RT-QPCR after CHX and DEX treatment (those not shown in Figure 6). (A,B) The expression levels of emx1 (88%) and nf-m (56%) in +C+D are slightly lower than in -C+D, indicating that the majority of their expression is controlled by EBF3 directly. (C) The expression level of nscl-1 (46%) in +C+D is lower than in -C+D but higher than that in the two controls, indicating that its expression is under partial direct control of EBF3. (D-G) The expression levels of kcnk5 (20%), activin beta b (10%), neurod (8%) and greb1 (1%) in +C+D are similar to control levels (D) or much lower (less than 10%) than in -C+D (E-G). N = 20 to 30 animal caps per condition.