Abstract
Objective:
The aqueous extract of Celosia argentea var. cristata L. leaves at 100, 200, and 400 mg/kg body weight (b.w.) was investigated against cadmium (Cd)-induced oxidative stress in Wistar rats. The in vitro antioxidant of the extract was evaluated using ammonium thiocyanate, reducing power, and membrane stabilizing models.
Materials and Methods:
For the in vivo study, 30 male rats (Rattus norvegicus) weighing 138.02 ± 7.02 g were completely randomized into 6 groups (A–F) of 5 animals each. Animals in groups A and B received 0.5 ml of distilled water and the same volume containing 8 mg/kg b.w. of Cd, respectively, for 7 days orally. Animals in groups C, D, E, and F were treated like those in group B except that they received 100 mg/kg b.w. of ascorbic acid, and 100, 200, and 400 mg/kg b.w. of the extract, respectively, in addition to Cd.
Results:
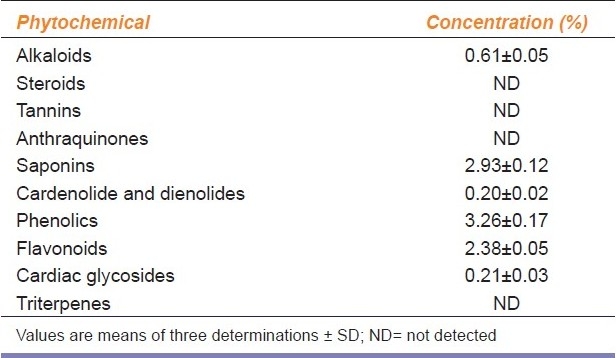
Phytochemical screening revealed the presence of alkaloids (0.61%), saponins (2.93%), cardiac glycosides (0.21%), cardenolides (0.20%), phenolics (3.26%), and flavonoids (2.38%). A total of 10 mg/ml of the extract inhibited linoleic acid oxidation by 67.57%. The highest reducing power was 100 mg/ml as against 10 mg/ml for ascorbic acid. In addition, 2 mg/ml of the extract produced a membrane stabilizing activity of 63.49% as against 77.46% for indomethacin. Compared with the distilled water control group, the administration of Cd alone significantly (P < 0.05) decreased the alkaline phosphatase activity of the rat liver and brain. This decrease was accompanied by a corresponding increase in the serum enzyme. The simultaneous administration of the extract and Cd produced an enzyme activity that compared favorably (P > 0.05) with the animals that received Cd and ascorbic acid. In addition, the reduction in the superoxide dismutase and catalase activity of the liver and brain of the animals, serum uric acid, albumin and bilirubin, and also the increase in the serum malondialdehyde content in animals treated with Cd alone was attenuated by the extract; the values compared well (P > 0.05) with those simultaneously administered with Cd and ascorbic acid.
Conclusion:
Overall, the results indicated that the aqueous extract of C. argentea leaves attenuated Cd-induced oxidative stress in the animals, with the best result at 400 mg/kg b.w. The antioxidant activity of the extract may be attributed to the phenolic and flavonoid components of the extract. The induction of antioxidant enzymes and scavenging of free radicals may account for the mechanism of action of the extract as an antioxidant.
Keywords: Amaranthaceae, antioxidant, cadmium, Celosia argentea, free radicals, oxidative stress
Introduction
Cadmium (Cd), a xenobiotic metal, is ubiquitously present in the environment. It can enter human and animal bodies via different industrial products, environmental pollution, air, contaminated foods, and water. Food and cigarette have also been shown to be the biggest sources of Cd in the general population.[1] Cadmium is capable of inducing irreparable damage to vital organs of the animals such as the liver and kidney during acute and chronic exposures.[2] It is also widely accepted that Cd causes tissue damage by membrane lipid peroxidation because of its ability to generate free radicals and inhibit or reduce the activity of antioxidant enzymes like glutathione peroxidase, glutathione reductase, catalase, and superoxide dismutase (SOD).[2] Cadmium and oxidative stress seem to be related, as the production of oxygen free radicals responsible for its toxicity has been found to be stimulated in Cd -treated rats.[2]
In recent times, there has been an upsurge of interest in the therapeutic potentials of medicinal plants as antioxidants in reducing tissue injuries caused by free radicals.[3] Various forms of antioxidant products such as butylated hydroxytoluene, butylated hydroxyanisole, tertiary butylated hydroxyquinone, and garlic acid esters have been formulated to offer protection against oxidative stress, but they are often too costly. In addition, the synthetic antioxidants have been implicated in promoting negative health effects such as radio-sensitization, increased toxicity of other chemicals, mutagenic activity, and tumor yield from chemical carcinogens.[4] Hence, strong restrictions placed on their application have necessitated the need to search for naturally occurring antioxidants. Consequently, many plants have been investigated in the search for novel antioxidants. Yet the need to find more information concerning the antioxidant potentials of plant species continues.
Celosia argentea var. cristata L. (Amaranthaceae), known as red “soko” (Yoruba, Western Nigeria), is a herbaceous, vegetable plant grown in Nigeria. It has red pigments on the leaves which differentiate it from the green variety, green “soko.” The stem is about 60 cm tall, typically simple and erect. The leaves which may be about 8 cm long and 4 cm broad are alternate, glabrous, and petiolate. The chaffy, terminal inflorescence is about 12 cm long in a mature plant. The flowers which may be variously colored are typically red in some instances.[5]
Although Odukoya et al.[6] had evaluated the in vitro antioxidant activity of selected Nigerian green leafy vegetables including C. argentea var. cristata, the antioxidant models (linoleic acid system using ferric thiocyanate, total phenolic content expressed as tannic acid equivalent, TAE, determination of the ascorbic acid content, and DPPH radical scavenging activity) employed are different from the ones used in this study. Furthermore, green “soko” was used instead of the red “soko” in the present study. In addition, the study did not evaluate the in vivo antioxidant activity of the green “soko.” Therefore, in this study, the potential of the aqueous extract of C. argentea var. cristata L. leaves (CAVCL) as a natural antioxidant supplement was investigated. This is with the view to providing a cheap, readily available, and rich source of phytochemicals (with antioxidant properties) essential for good health. The in vitro study was carried out using several antioxidant models such as the linoleic acid system entailing ammonium thiocyanate, reducing power and membrane stabilizing activity, as well as the in vivo activity against Cd ion-induced oxidative stress.
Materials and Methods
Chemicals
Para-Nitrophenylphosphate, ascorbic acid, cadmium chloride, and adrenalin were products of Sigma Chemical Co. (St. Louis, MO, USA), BDH Chemicals Ltd. (Poole, UK), Merck Chemical Co. (Germany), and Laborate Chemical Co. (India), respectively. Assay kits for uric acid, albumin, and bilirubin were products of Fortress® Diagnostics Ltd. (Antrim, UK). All other chemicals used were of analytical grade.
Plant Material
The plant material was purchased from an open market (Obada) in Oro, Kwara State, Nigeria. It was authenticated at the herbarium unit of the Forest Research Institute of Nigeria (FRIN) Ibadan, Nigeria. A voucher specimen (F.H.I. 108372) was deposited at the herbarium of the Institute.
Animals
A total of thirty, male albino rats (Rattus norvegicus) weighing 138.02 ± 7.02 g were obtained from the Animal Holding Unit of the Department of Biochemistry, University of Ilorin, Nigeria. They were acclimatized for 7 days to standard housing condition (temperature 28–30°C, relative humidity 40–45%, and a 12t/12 h light-dark cycle). Tap water and pellets (Savannah Feeds, Ilorin, Nigeria) were provided ad libitum. Approval by the ethics committee was obtained. The animals were handled humanely in accordance with the guidelines of the Ethical Committee on the Care and Use of Laboratory Animals of the Department of Biochemistry, University of Ilorin, Nigeria, as well as that of European Convention for the Protection of Vertebrate Animals and Other Scientific Purposes (ETS no. 123).[7]
Preparation of the Extract
The plant leaves were oven dried at 40°C to a constant weight. The dried leaves were then pulverized with an electric blender. A portion of the powder (100 g) was extracted in 1000 ml of distilled water at room temperature for 48 h. The extract was then filtered using Whatman's no. 1 filter paper (Maidstone, UK). The filtrate was thereafter concentrated on a water bath to give a yield of 15.74 g.
Phytochemical Screening of the Extract
The aqueous extract of CAVCL was screened for alkaloids, steroids, anthraquinones, cardenolides, phenolics flavonoids, tannins, triterpenes, glycosides, and saponins.[8] Furthermore, quantitative determination was carried out as described for alkaloids, saponins, cardenolides, glycosides, total phenolics, and total flavonoids.[9]
In vitro Antioxidant Activity
Ammonium thiocyanate assay
The in vitro antioxidant activity of the extract was determined using the ammonium thiocyanate assay described by Lee et al.[10] Briefly, 500 μl of the extract, 200 μl of appropriately diluted linoleic acid (25 mg/ml in 99% ethanol) and 400 μl of 50 mM phosphate buffer (pH 7.4) were mixed together and incubated at 40°C for 15 min. An aliquot (100 μl) of the solution was mixed with another solution containing 3 ml of 70% ethanol, 100 μl of ammonium thiocyanate (300 mg/ml distilled water), and 100 μl of ferrous chloride (2.45 mg/ml in 3.5% hydrochloric acid). The resultant solution was shaken and incubated at room temperature for 3 min after which the absorbance was read at 500 nm. The linoleic acid emulsion without the extract served as the control while ascorbic acid was used as a reference antioxidant. The inhibition of the linoleic acid emulsion was calculated from the following expression:

where Abs = Absorbance.
Assay of the reducing power of the extract
The assay of the reducing power of the extract was carried out according to the method described by Yildrim et al.[11] Briefly, 0.5 ml of the extract was mixed with 2.5 ml of phosphate buffer (0.2 M, pH 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was incubated at 50°C for 30 min after which 2.5 ml of 10% trichloroacetic acid was added. The resultant solution was centrifuged at 503 g for 10 min. The supernatant(s) were collected and mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% ferric chloride after which the absorbance was read at 700 nm. An ascorbic acid solution was used as the reference antioxidant. An increase in the absorbance of the mixture indicates an enhanced reducing power.
Assay of the membrane stabilizing activity of the extract
The method described by Sadique et al.[12] was employed for the assay of the membrane stabilizing activity of the extract. The assay mixture consisted of 2 ml of hyposaline (0.25% (w/v), 1 ml of 0.15 M sodium phosphate buffer (pH = 7.4), 1.0 ml each of 2 mg/ml of indomethacin solution (reference drug) or 2 mg/ml of the extract, and 2% (v/v) erythrocyte suspension in 0.5 ml of isosaline, to give a total assay volume of 4.5 ml. The control was prepared as described earlier except that the drug (or extract) was omitted whereas the drug control and the extract control (4.5 ml) lacked erythrocyte suspension. The volume of the reaction mixture designated as the drug control, extract control, and the control were made up with 0.5, 0.5, and 1.0 ml of isosaline, respectively, to give a total volume of 4.5 ml each. The experiment was replicated five times. The reaction mixtures were incubated at 56°C for 30 min. The tubes were thereafter cooled in running tap water and then centrifuged at 895 g for 15 min. The supernatants were collected followed by the reading of the absorbance of the released hemoglobin at 560 nm. The percentage membrane stabilizing activity was calculated from the following expression:
100 - [100 × (Drug test value - Drug control value)/(Control value)].
The drug test values were then replaced by the extract test values to obtain the percentage membrane stability for the extract.
Animal Grouping
The animals were randomized into six treatment groups (A–F) of five rats each. The groupings were as follows: group A (control) received 0.5 ml of distilled water, group B received 8 mg/kg body weight (b.w.) of Cd,[2] group C, 8 mg/kg b.w. of Cd and 100 mg/kg b.w. of ascorbic acid. Animals in groups D–F were simultaneously administered with 8 mg/kg b.w. of Cd each and 100, 200, and 400 mg/kg b.w. of the aqueous extract of CAVCL, respectively. Ascorbic acid and the extract were administered orally using a metal oropharyngeal cannula. Water and pellets were provided ad libitum throughout the experimental period. All the animals were sacrificed 24 h after 7 days of treatment.
Preparation of Serum and Tissue Homogenates
The procedure described by Yakubu et al.[13] was used. Briefly, under ether anesthesia, rats were made to bleed into centrifuge tubes, through a cut made on their slightly displaced jugular veins. The veins were displaced to prevent blood contamination with interstitial fluid. The blood samples were left to clot for 10 min at room temperature. Clear serum was then collected with the aid of a Pasteur pipette following centrifugation at 503 g for 10 min (Uniscope Laboratory Centrifuge, model SM800B; Surgifriend Medicals, Essex, UK). The sera were used immediately for the biochemical analyses. The animals were quickly dissected and the liver and brain removed. Weighed organs were homogenized in an ice-cold 0.25 M sucrose solution (1:5 w/v) and centrifuged at 1,398 g for 15 min. The supernatants obtained were stored frozen overnight and used for biochemical analyses.
Determination of Biochemical Parameters
Total protein, total and conjugated bilirubin, albumin, uric acid, SOD, catalase, lipid peroxidation, and alkaline phosphatase (ALP) were determined in accordance with the principles described by Tietz.[14]
Statistical Analysis
Results were expressed as mean ± SD of five replicates.Data obtained were subjected to one-way Analysis of Variance (ANOVA) and complemented with Student's t-test. The statement of statistical significance was based on P < 0.05 (Statistical Calculator StatPac® v3.0; StatPac Inc. Bloomington, MN, USA).
Results
The aqueous extract of CAVCL showed positive results for alkaloids, saponins, phenolics, flavonoids, glycosides, cardenolides, and dienolides. Quantitative analysis revealed that phenolics were the highest followed by saponins and flavonoids while alkaloids, glycosides, cardenolides, and dienolides were weakly present [Table 1].
Table 1.
Phytochemical constituents of aqueous extract of Celosia argentea leaves
In the ammonium thiocyanate model, the extract produced a higher inhibition of linoleic acid oxidation (67.57%) compared to the reference antioxidant, ascorbic acid (64.62%) [Figure 1].
Figure 1.
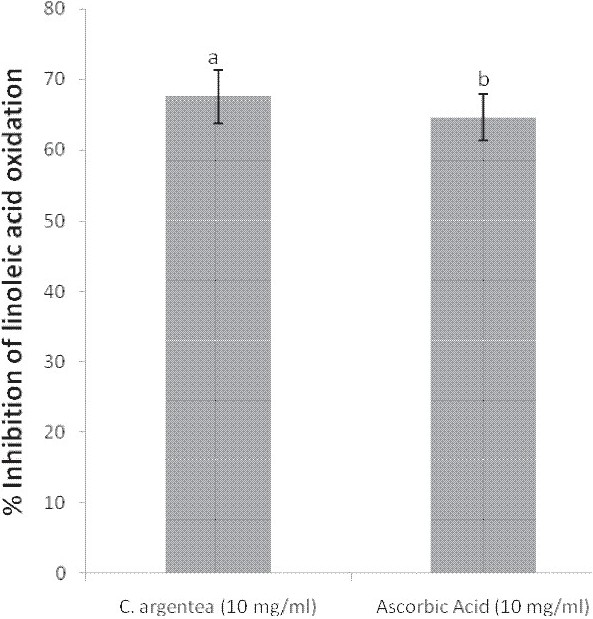
Percentage inhibition of linoleic acid oxidation by the aqueous extract of Celosia argentea leaves and ascorbic acid
The extract and the reference antioxidant (ascorbic acid) produced a contrasting reducing power activity. For example, although the reducing power of ascorbic acid decreased with an increase in concentration, that of the extract increased with concentration. In addition, the most effective reducing power for ascorbic acid was 10 mg/ml as against 100 mg/ml for CAVCL [Figure 2].
Figure 2.
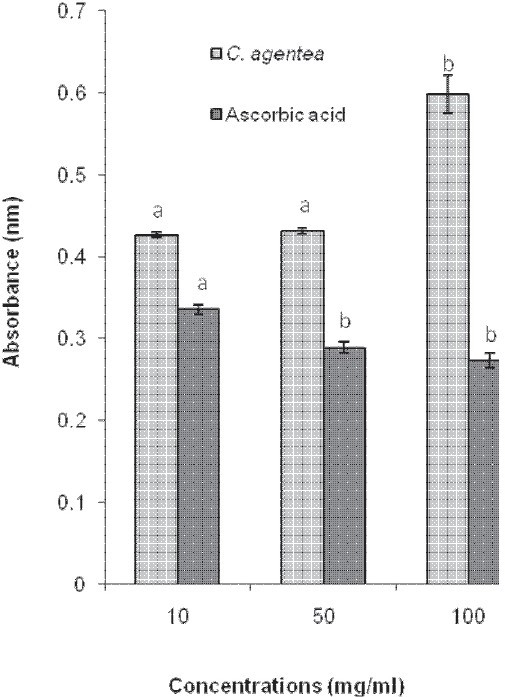
Reducing power activity of the aqueous extract of Celosia argentea leaves and ascorbic acid
The percentage membrane stabilizing activity of the extract was lower than that of the reference anti-inflamatory drug, indomethacin. Furthermore, the extract displayed activity of 63.49% which was lower than that of indomethacin (77.46%) in this study [Figure 3].
Figure 3.
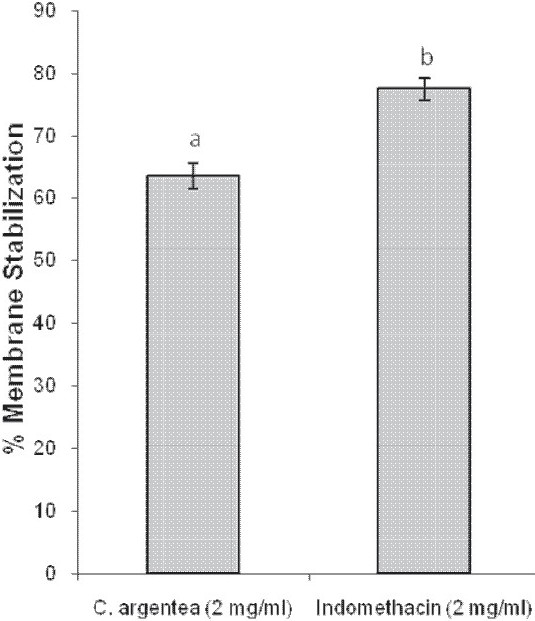
Membrane stabilizing activity of the aqueous extract of Celosia argentea leaves and indomethacin
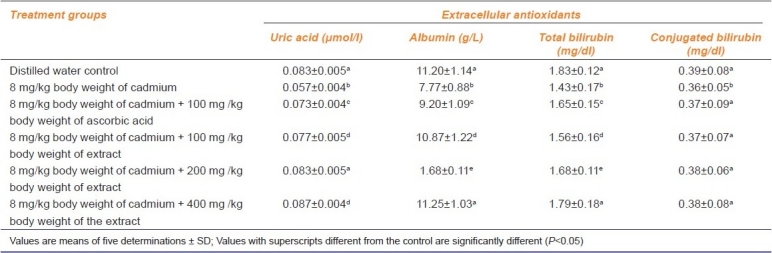
The levels of uric acid which were significantly decreased (P < 0.05) in the Cd-treated animals extended to the animals treated with Cd and ascorbic acid as well as 100 mg/kg b. w. of the extract. In contrast, although the animals treated with Cd and 200 mg/kg b. w. of the extract produced uric acid levels that compared well (P > 0.05) with the distilled water-treated controls, the 400 mg/kg b.w. of the extract produced significantly higher levels of uric acid [Table 2]. Similarly, albumin and total bilirubin levels were reduced in all the treatment groups except in the animals simultaneously administered with Cd and 400 mg/kg b.w. of the extract where the values compared well (P > 0.05) with the distilled water-administered control group. Again, the conjugated bilirubin level decreased only in the Cd-treated animals whereas the levels in the other treatment groups compared favorably (P > 0.05) with the distilled water control animals.
Table 2.
Effect of administration of cadmium and aqueous extract of Celosia argentea leaves on some extracellular antioxidants of rats
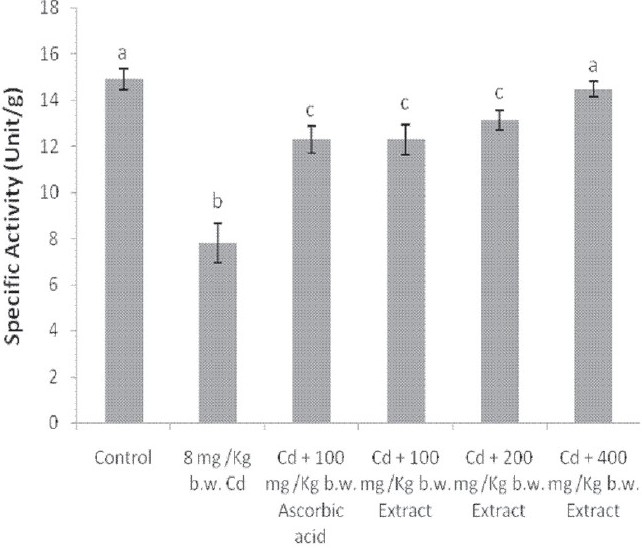
The administration of Cd alone significantly reduced the activities of catalase and SOD in the liver and brain of the animals [Figures 4–7]. Except for the catalase activity of the liver where the simultaneous administration of Cd and ascorbic acid compared well with the animals treated with Cd alone [Figure 4], the enzyme activity increased in the brain of the animals [Figure 5]. Similar increases were produced in the SOD activity of the liver and brain of the animals [Figures 6 and 7]. In addition, a simultaneous administration of Cd and the graded doses of the extract significantly increased the activities of catalase and SOD in the liver and brain of the animals [Figures 4–7]. Furthermore, the activity of SOD in the rat brain of animals administered with Cd and 400 mg/kg b.w. of the extract compared favorably with the distilled water-treated controls [Figure 7].
Figure 4.
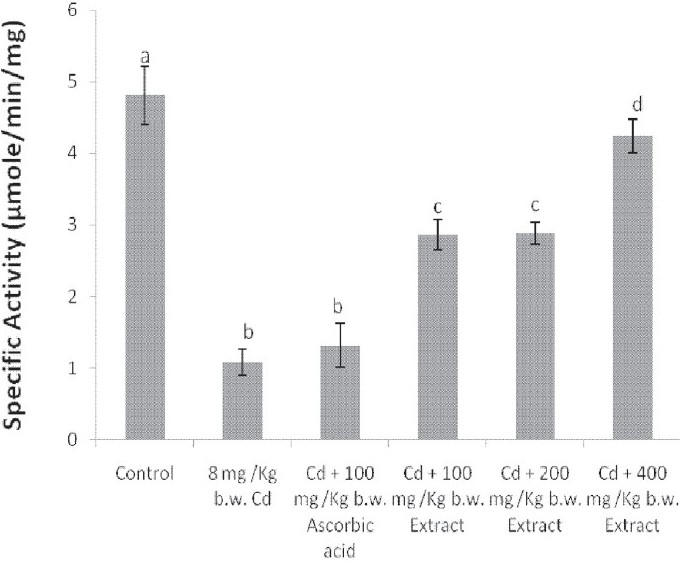
Effect of the simultaneous administration of cadmium and the aqueous extract of Celosia argentea leaves on the catalase activity of rat liver
Figure 7.
Effect of the simultaneous administration of cadmium and the aqueous extract of Celosia argentealeaves on the superoxide dismutase activity of rat brain
Figure 5.
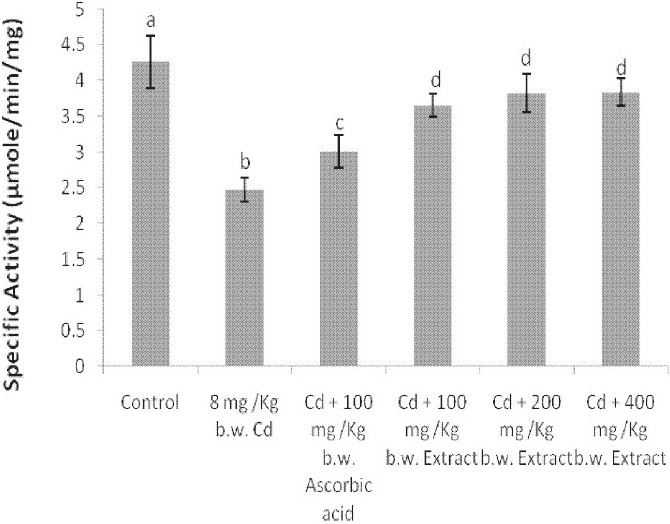
Effect of the simultaneous administration of cadmium and the aqueous extract of Celosia argentea leaves on the catalase activity of rat brain
Figure 6.
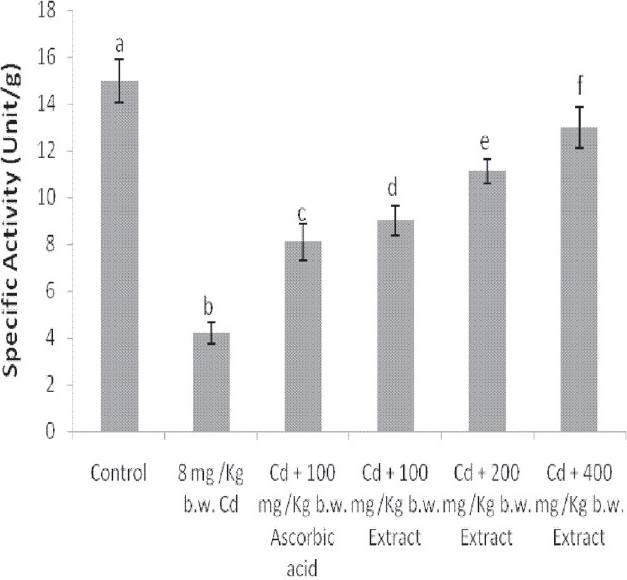
Effect of the simultaneous administration of cadmium and the aqueous extract of Celosia argentealeaves on the superoxide dismutase activity of rat liver
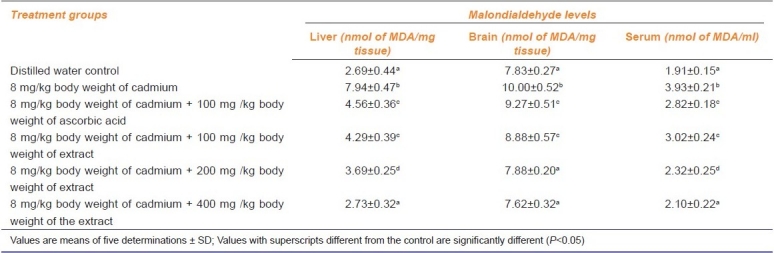
Compared with the distilled water control animals, administration of Cd alone significantly increased the levels of MDA in the liver, brain and serum of the animals by 2.95, 1.28 and 2.05 folds respectively. These increases were reduced by ascorbic acid and the graded doses of the extract with the 400 mg/kg b. w. of the extract comparing favourably with the distilled water control animals [Table 3].
Table 3.
Effect of administration of cadmium and aqueous extract of Celosia argentea leaves on the malondialdehyde levels of rat liver, brain and serum
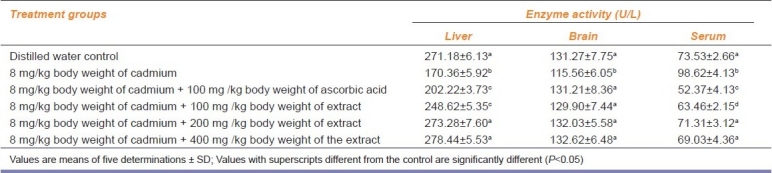
The administration of Cd alone significantly reduced the ALP activity in the liver of the animals. The trend obtained for the simultaneous administration of Cd and ascorbic acid as well as Cd and 100 mg/kg b.w. of the extract was similar to the administration of Cd alone. In contrast, however, the 200 and 400 mg/kg b.w. of the extract reversed this trend with the enzyme activity comparing well with that of distilled water controls [Table 4]. Again, the ALP activity of the rat brain was reduced by Cd alone. The enzyme activity was not affected by other treatment groups [Table 4]. Furthermore, although Cd alone increased the serum ALP activity of the animals, all the other treatment groups decreased the serum enzyme activity except the simultaneous administration of Cd and 400 mg/kg b.w. of the extract. The Cd and 400 mg/kg b.w. of the extract produced an enzyme activity that compared favorably with the distilled water control animals [Table 4].
Table 4.
Effect of administration of cadmium and aqueous extract of Celosia argentea leaves on the alkaline phosphatase activity of rat liver, brain and serum
Discussion
The screening studies for antioxidant properties of medicinal and food plants have been performed increasingly for the last few decades with the hope of finding an effective remedy for several present-day diseases and means to delay aging symptoms.[3] The disorders related to the excessive oxidation of cellular substrates (oxidative stress) include type II diabetes, neurodegenerative diseases, and some types of cancer.[15] Furthermore, there is also a huge demand for natural antioxidants in food and related industries, for replacing the synthetic preservatives.[16]
Secondary metabolites from medicinal plants function as small molecular weight antioxidants through direct antiradical, chain-breaking of the free radical propagation and interaction with transition metals. Other mechanisms include the inhibition of ROS-generating enzymes such as xanthine oxidase, inducing nitric oxide synthase, and improving the endogenous cellular antioxidant mechanisms such as the up-regulation of the activity of SOD.[17] Furthermore, phenolic compounds function as high-level antioxidants because they possess the ability to adsorb and neutralize free radicals as well as quench reactive oxygen species. Flavonoids, as one of the most diverse and widespread groups of natural compounds, are also probably the most natural phenolics capable of exhibiting in vitro and in vivo antioxidant activities. In addition, plant flavonoids which show an antioxidant activity in vitro also function as antioxidants in vivo. Again, a strong relationship between the total phenolic content and antioxidant activity in fruits, vegetables, grain products, and plant subjects of ethnopharmacological treatments has also been reported.[16] Therefore, the in vitro antioxidative effect of the aqueous extract of C. argentea leaves may be due to the phenolic and flavonoid components.
The higher percentage inhibition of linoleic acid oxidation by the extract compared with the reference drug, ascorbic acid, suggests a marked and higher antioxidant activity. Furthermore, the percentage inhibition of linoleic acid oxidation (67.57%) obtained for the red “soko” variety of C. argentea var. cristata in this study is similar to that of green “soko” (68.41%) reported by Odukoya et al.[6]
At physiological pH (7.4), ferrous ions (Fe2+), in the presence of oxygen and phosphate ions (PO42-), exist only transiently before being auto-oxidized to ferric ion (Fe3+). During this process, an electron is transferred from iron to oxygen to form a superoxide radical anion and hydroperoxyl radical (HO2·) by Fenton reaction. The concentration-dependent, high reducing power of the aqueous extract of C. argentea leaves suggests that the extract possessed the ability to be effective, under physiological conditions, in reducing the transition state of iron and consequently, the rate at which superoxide and hydroperoxyl radicals are generated from the metal. A strong relationship between the total phenolic content and reducing activity in fruits and vegetables has been reported.[11] Therefore, the reducing power of the extract may be attributed to its phenolic content.
The effect of anti-inflammatory drugs including herbal preparations on the stabilization of the erythrocyte membrane exposed to heat and hypotonic solution has been studied extensively. The erythrocyte membrane resembles the lysosomal membrane, and therefore, the effect of drugs on the stabilization of erythrocyte could be extrapolated to the stabilization of the lysosomal membrane.[18] Therefore, the high membrane stabilizing activity of the extract in this study suggests that C. argentea has the potential to protect the erythrocyte membrane from free radical damage. Flavonoids, tannins, and saponins have been reported to exert profound in vitro and in vivo stabilizing effect on the lysosomes of experimental animals.[12] Tannins and saponins stabilize the erythrocyte membrane by binding cations and other biomolecules.[19] Therefore, the membrane stabilizing activity of the extract may be due to the presence of flavonoids and or saponins since tannins were not detected in the extract. The findings on the aqueous extract of C. argentea leaves in this study are similar to those reported by Sadique et al.[12] and Olugbenga et al.[20]
Certain heavy metals including cadmium have been reported to generate reactive oxygen species.[21] The extracellular fluid of the body contains certain preventive, low molecular weight antioxidants such as transferin and albumin that bind extracellular iron and copper (metals involved in free radical generation) in a nonreactive state. Similarly, the presence of bilirubin and uric acid also scavenge free radicals in the extracellular body fluid. These antioxidants ensure limited survival of reactive oxygen species such as superoxide and H2O2 in the extracellular fluid by binding the metals and thus diminishing their ability to accelerate lipid peroxidation.[22] Loss of these antioxidants observed in the cadmium-treated animals only indicated biological oxidative stress.[21] The attenuation of the loss of these molecules by the extract suggests an antioxidant activity which may be attributed to the phenolics including flavonoids. The findings in this study are similar to those of Karuna et al.[15] following the administration of the aqueous extract of Phyllanthus amarus to rats.
The enzymic antioxidant systems such as catalase, superoxide dismutase, glutathione reductase, glutathione peroxidase, and glucose-6-phosphate dehydrogenase play a coordinated role in the prevention of oxidative damage by reactive oxygen species. SOD is one of the chief cellular defence enzymes that dismutate superoxide radicals to water and oxygen. Catalases on the other hand are heme-containing proteins that protect the cells from toxic effects of reactive oxygen species by converting hydrogen peroxide to water and molecular oxygen. SOD is also another metalloenzyme requiring zinc for structural stability and copper for enzymatic activity. The cadmium-induced inhibition of metalloenzymes has been reported to result from the displacement of these metals from the active site of these enzymes.[23] Therefore, the reduction in the SOD activity in the tissues of animals treated with cadmium alone may be due to the displacement of these metals from the enzymes. Again, the reduction in the activities of SOD and catalase following the administration of cadmium alone may also suggest oxidative stress. Such reduction in the activity of catalase by cadmium alone in this study is similar to the findings of Beytut and Aksakal.[3] Furthermore, the attenuation of the activities of SOD and catalase by the aqueous extract of C. argentea leaves buttresses the antioxidant potential of the plant. It is possible that the extract might have acted as a scavenger of free radical products of cadmium metabolism or might have played a role in enhancing the synthesis of the antioxidant enzymes which in turn protect the cells from reactive oxygen species. This may also complement the antioxidant defence mechanism of the animals. The increase in the activities of antioxidant enzymes by the aqueous extract of C. argentea leaves is similar to that reported by Karunna et al.[15] following the administration of the aqueous extract of P. armarus to rats.
ALP, a membrane-bound enzyme, is normally used to assess the integrity of the cells following the administration of chemical compounds.[24] It also plays an important role in maintaining cell membrane permeability. A significant reduction in the ALP activity of the liver and brain of rats in this study, following the administration of cadmium alone, could have resulted from the labilization of the plasma membrane by the oxidative products of cadmium metabolism. This was further supported by a corresponding increase in the serum enzyme of the animals treated with cadmium alone. The altered membrane could be the consequence of the peroxidation of unsaturated fatty acids. This is more likely since the level of malondialdehyde, a widely used indicator of lipid peroxidation, was also increased. Cadmium has been reported to be toxic to vital organs such as the liver, kidney, testes, and brain via the induction of lipid peroxidation.[25] In contrast, the significant reversal in the activity of ALP in the liver, brain, and serum of the animals as well as the levels of malondialdehyde by the extract in this study suggests a possible preventive role from oxidative damage by cadmium.
The present study revealed that aqueous extract of C. argentea var. cristata leaves, the red “soko,” attenuated the depleted biochemical parameters investigated. This suggests an antioxidant activity for the plant extract. The in vitro and in vivo antioxidant activity of the plant could be due, at least, in part to the phenolics in the extract. Therefore, C. argentea var. cristata leaves are a potential source of cheap, natural antioxidants and a possible replacement for currently available, toxic synthetic antioxidants. The highest dose of the extract, i.e., 400 mg/kg b.w. was the most effective among the doses investigated. The antioxidant activity may be the mechanism of the potential of the drug against free radical-mediated diseases. Work is in progress on the isolation and characterization of the antioxidant bioactive principle(s) in C. argentea var. cristata leaves.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Ololade IA, Ologundudu A. Concentration and bioavailability of cadmium by some plants. Afr J Biotechnol. 2007;6:1916–21. [Google Scholar]
- 2.Jamba L, Nehru B, Bansal MP. Redox modulation of selenium binding proteins by cadmium exposures in mice. Mol Cell Biochem. 1997;177:169–75. doi: 10.1023/a:1006869623864. [DOI] [PubMed] [Google Scholar]
- 3.Jimoh FO, Babalola SA, Yakubu MT. Assessment of antioxidant potentials of Cnidoscolous chayamansa. Pharm Biol. 2009;47:903–9. [Google Scholar]
- 4.Kahl R. Synthetic antioxidants: Biochemical actions and interference with radiation, toxic compounds, chemical mutagens and chemical carcinogens. Toxicol. 1984;33:185–228. doi: 10.1016/0300-483x(84)90038-6. [DOI] [PubMed] [Google Scholar]
- 5.Iwu MM. Florida, USA: CRC Press, LLC; 1993. Handbook of African Medicinal plants; p. 435. [Google Scholar]
- 6.Odukoya OA, Inya-Agha SI, Segun FI, Sofidiya MO, Ilori OO. Antioxidant activity of selected Nigerian green leafy vegetable. Am J Food Technol. 2007;2:169–75. [Google Scholar]
- 7.European Convention for the protection of vertebrate animals used for experimental and other scientific purposes. European Treaty Series, Strasbourg, ETS-123. 2005 [Google Scholar]
- 8.Harbone JB. London: Chapman and Hall Ltd; 1973. Phytochemical methods; pp. 148–89. [Google Scholar]
- 9.El-Olemy MM, Al-Muhtadi FJ, Afifi AA. A Laboratory Manual. Riyadh, Saudi Arabia: King Saud University Press; 1994. Experimental Phytochemistry; pp. 3–137. [Google Scholar]
- 10.Lee JC, Kim HR, Jim KJ. Antioxidant property of an extract of the stem of Opuntia ficus-indica Var.saboten. J Agric Food Chem. 2002;50:6490–6. doi: 10.1021/jf020388c. [DOI] [PubMed] [Google Scholar]
- 11.Yildrim A, Mavi A, Kara AA. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J Agric Food Chem. 2001;9:4083–9. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- 12.Sadique J, Al-Raqobah WA, Bugharith ME, El-Gindy AR. The bioactivity of certain medicinal plants on the stabilization of RBC membrane system. Fitoterapia. 1989;60:525–32. [Google Scholar]
- 13.Yakubu MT, Akanji MA, Oladiji AT. Aphrodisiac potentials of aqueous extract of Fadogia agrestis (Schweinf.Ex Heirn) stem in male albino rats. Asian J Androl. 2005;7:399–404. doi: 10.1111/j.1745-7262.2005.00052.x. [DOI] [PubMed] [Google Scholar]
- 14.Tietz NW. 3rd ed. Philadelphia, USA: W. B. Saunders Company; 1995. Clinical guide to laboratory tests. [Google Scholar]
- 15.Karuna R, Reddy SS, Baskar R, Saralakumari K. Antioxidant potential of aqueous extract of Phyllanthus armarus in rat. Indian J Pharmacol. 2009;14:64–7. doi: 10.4103/0253-7613.51342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dorman HJ, Kosar M, Karlos K, Holm Y, Hittuner R. Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties and cultivars. J Agric Food Chem. 2003;51:4563–9. doi: 10.1021/jf034108k. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: How should you do it and what do the results mean? Br J Pharmacol. 2004;142:231–55. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyedapo OO, Akinpelu BA, Orefuwa SO. Anti-inflammatory effect of Theobroma cacao, root extract. Trop Med Plants. 2004;5:161–6. [Google Scholar]
- 19.Oyedapo OO. Biological activity of Phyllanthus armarus extract on Pragrow-Dawley rats. Nig J Biochem Mol Biol. 2001;26:202–26. [Google Scholar]
- 20.Olugbenga M, Fafunso MA, Makinde JM. Membrane stabilizing activity: A possible mechanism of action of the anti-inflammatory property of Gongronema latifolium leaves. Int J Biomed Health Sci. 2005;1:1–4. [Google Scholar]
- 21.Iszard MB, Liu J, Klassen CD. Effect of several metallothionein on inducers of oxidative stress defense mechanisms in rats. Toxicol. 1995;101:25–33. doi: 10.1016/0300-483x(95)03118-y. [DOI] [PubMed] [Google Scholar]
- 22.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–28. [PubMed] [Google Scholar]
- 23.Environmental Health Criteria. Vol. 134. Cadmiu, Geneva: World Health Organization; 1992. World Health Organization; pp. 111–2. [Google Scholar]
- 24.Wright PJ, Plummer DT. The use of urinary enzyme measurement to detect renal damages caused by nephrotoxic compounds. Biochem Pharmacol. 1974;12:65–8. doi: 10.1016/0006-2952(74)90314-1. [DOI] [PubMed] [Google Scholar]
- 25.Robarts K, Wosfold P. Cadmium: Toxicity and analysis, a review. Analyst. 1991;116:549–68. doi: 10.1039/an9911600549. [DOI] [PubMed] [Google Scholar]


