Abstract
Objectives:
Retinaldehyde inhibits adipogenesis, increases metabolic rate, reduces weight gain, and improves tolerance to a glucose load. We assessed the effects of citral – an inhibitor of retinaldehyde dehydrogenase (the primary enzyme metabolizing retinaldehyde), on body weight, glucose tolerance, fasting plasma glucose and insulin levels, metabolic rate, adipocyte size, and morphology in a diet-induced model of obesity.
Materials and Methods:
Out of the 5 groups of 6-week-old male Sprague–Dawley rats, 4 were maintained on an energy-intense, palatable, diet for a period of – 42 days, while 1 served as the control. After obesity had been induced, 3 groups were treated with daily doses of citral (10, 15, and 20 mg/kg body weight) for a period of 28 days. They were then subjected to metabolic experiments. Body weight, fasting plasma glucose, glucose tolerance to an intraperitoneal glucose load, metabolic rate, and adipocyte size were assessed.
Results:
Citral-treated groups showed a dose-dependent reduction in body weight gain. They significantly had lower fasting glucose levels, improved glucose tolerance, lower fasting plasma glucose, higher metabolic rate, and smaller adipocytes after drug administration.
Conclusion:
The findings suggest that citral increased energy dissipation (and also reduced lipid accumulation) consequently preventing and ameliorating diet-induced obesity. In addition it improved insulin sensitivity and glucose tolerance. In the current scenario of increasing prevalence of obesity and diabetes, citral may prove as novel agent in its management.
Keywords: Citral, obesity, retinaldehyde, type 2 diabetes mellitus
Introduction
Obesity usually results from an energy imbalance due to excessive energy intake and/or insufficient energy expenditure. Although not an immediate lethal disease by itself, it is a significant risk factor associated with a range of serious noncommunicable diseases and conditions, such as coronary heart disease, liver and kidney disease, sleep apnea, depression, hypertension, osteoarthritis, and type 2 diabetes mellitus (T2DM).[1,2] In fact, body weight has been recognized as the single most important predictor of the development of T2DM.[3] As high as 80% of the cases of T2DM could be attributed to the combined effect of inactivity and high body weight.[3] The incidence of obesity is increasing at an alarming rate throughout the world.[4] Sedentary lifestyles, environmental factors, and change in dietary habits are the prime contributors to this sudden surge.[5] Adipose tissue plays a major role in regulating whole body insulin resistance. The increase in the prevalence of obesity has been accompanied by a parallel increase in the prevalence of T2DM. The prevalence of diabetes for all age-groups worldwide was estimated to be 2.8% in 2000 and is projected to be 4.4% in 2030 (most of which will be T2DM). The total number of people with diabetes is projected to rise from 171 million in 2000 to 366 million in 2030, with India, China, and USA being the top 3 countries estimated to have the highest numbers of people with diabetes.[6]
Amelioration of obesity improves insulin resistance in a large number of patients. The treatment of obesity can include both lifestyle interventions and/or pharmacological therapy. Lifestyle modification is considered the safest method of inducing a persistent weight loss. However, compliance with conventional weight-management programs is low, leading to physicians increasingly becoming reliant on drug therapy.[7] There is therefore a constant demand for safe and effective pharmacological options.
The role of retinoids in adipose tissue is variable. Retinol-binding protein 4 (RBP4) is one adipokine implicated in the development of obesity-linked T2DM.[8] Retinaldehyde suppresses differentiation of fibroblasts into adipocytes and slows down maturation.[9] Retinoic acid in turn promotes maturation, consequently increasing glucose intolerance and insulin resistance.[9] Enzymes of this pathway are therefore potential targets in the management of both obesity and the consequent insulin resistance.
Citral, a mixture of cis and trans isomers (geranial and neral) of 3,7-dimethyl-2,6-octadienal, is a naturally occurring aliphatic aldehyde of the terpene series. It is the main component (approximately 80%) of lemongrass oil, and is found in all citrus fruits and used extensively in the food, cosmetic, and detergent industries. Lemongrass (Cymbopogon citrates) is the prime commercial source of this oil. It is an evergreen plant that grows widely in Asia and is used in oriental households as a herb. Known to possess antiseptic, antimicrobial, anti-inflammatory, carminative, diuretic, and central nervous system–stimulating effects, citral is effective against prostate gland tumor in various strains of rats.[10,11] It is nontoxic and does not possess carcinogenic potential in mice and rats.[12]
Citral competitively inhibits E1, E2, and E3 isozymes of retinaldehyde dehydrogenase (Raldh), thereby increasing retinaldehyde concentrations in the adipose tissue.[13]
The current epidemic of obesity across the world has been attributed to changing lifestyles and food consumption patterns. Increased intake of highly palatable, energy-intense diets, the so-called supermarket foods, has compounded the problem.[14]
Our experiments were therefore on a diet-induced model similar to one described by Rolls et al.[15] Diet-induced models are widely criticized because they do not attain the extreme obesity seen in leptin-deficient genetic models. However, obesity due to genetic causes is rare in humans too. In addition, the total role of leptins on energy metabolism is still unclear, which may have had bearing on our experiments. We reasoned a diet-induced model would parallel the human situation and would be a better reflection of the real world human situation and investigated the effects of citral on various metabolic parameters of rats maintained on an energy-intense diet.
Materials and Methods
Animal Studies
All procedures employed had prior approval and were in strict conformity with the guidelines of the National Center for Laboratory Animal Sciences.
Citral was delivered to the rats using pure ethanol (Sigma-Aldrich, Bangalore, India) as the vehicle. A total of 50 male Sprague-Dawley rats, 4 weeks of age were matched for body weight and randomly allocated to the experimental group (n=30, 78–82 g), which received citral and the modified diet, the drug control group (n=10, 75–84 g), which received the vehicle and modified diet, and the diet control group (n=10, 72–84 g), which received none. The experimental group was further divided into 3 subgroups A, B, and C, which received 10, 15, and 20 mg/kg body weight citral, respectively. The rats were housed in small plastic cages (approx. 40 cm × 15 cm) under conditions of controlled lighting (12:12 h light:dark cycle) and temperature, 24°C-25°C and humidity 45%–65%.
In the beginning, the experimental and drug control groups were subjected to dietary manipulation. To develop obesity they were given free access to an energy-intense, palatable, “fattening” diet, and water ad libitum similar to the one designed by Rolls et al.[15] The diet consisted of three commonly consumed energy-intense foods (flavored potato chips (Pepsico Inc.), chocolate chip cookies (Parle Ltd.), and butter flavored cookies (Britannia Ltd.). The diet and its approximate composition and energy content are given in Table 1.
Table 1.
Approximate composition of the and calorific value of the diet given to the rats

The food intake was measured by weighing the leftovers on a daily basis for each cage. To ensure absence of vitamin deficiency, a vitamin supplement from Glaxo Smith Kline was added to the bottle of water.
The diet control group was fed the standard laboratory diet made in-house from milk, grams, nuts, and pulses (homogenized and made into small pellets), which were placed on the cage. The approximate calorific value of this composition was established at 8.0 kJ/g. After a period of 6 weeks, the rats on modified diet were found to be significantly heavier compared with the diet control group.
From the seventh week onward, the experimental group was divided into 3 subgroups (A, B, and C) who were administered citral, once daily in the morning, as a solution of citral (wt/mL = 0.8928) and absolute alcohol (Sigma–Aldrich) in the ratio of 1:10 by oral gavage. The doses of the 3 groups were 10, 15, and 20 mg/kg body weight for groups A, B, and C, respectively. After 4 weeks of citral administration, rats were subjected to metabolic experiments.
Reagents
All reagents were obtained from Sigma–Aldrich, Bangalore, India and Merck , Mumbai , India unless otherwise indicated. Citral was obtained as a 95% pure mixture from HT Corporation, Kolkata. The purity was verified by gas chromatography.
Body Weight and Girth
Body weight (measured daily) was used as an indicator of growth.[16] The girth at the level of the kidneys was measured as an indicator of abdominal fat.[15]
Fasting Glucose, Insulin, and Glucose Tolerance Tests
Serum glucose were measured once at the end of the sixth week and then at the end of the experiment when insulin levels were also measured. The rats were fasted overnight and the glucose levels were measured 12 h later. Serum insulin was measured by using rat insulin EIA kit (Cayman Chem., Ann Arbour, Michigan, USA).
Immediately after, glucose tolerance was tested with an intraperitoneal glucose load of 25% (w/v) dextrose injection (0.004 mL/g body weight) delivered with an insulin syringe and measuring glucose levels 0, 30, 60, 90, and 150 min after the load. Glucose levels were measured by the “tail method” using a glucometer (Accucheck, Roche Diagnostics, Mumbai, India).
Metabolic Rate
Metabolic rate was computed in metabolic cages after measuring O2 and CO2 consumption using the Weir equation.[17]
Adipocyte Size, Morphology, Triglyceride, and Serum Cholesterol Assay
Perinephric fat was isolated after sacrificing the rats at the end of all the experiments. They were hematoxylin–eosin stained by standard procedures. Adipocyte size was quantified using imageJ software. Triglyceride content in adipose tissue was measured by lysing cells using sonification in an enzymatic buffer and then using a colorimetric assay. Serum cholesterol and triglycerides were measured using the cholesterol oxidase–phenyl aminophenazone (CHOD–PAP) and the glyceraldehyde-3-phosphate-phenyl aminophenazone (GPO–PAP) colorimetric assays, respectively.
Statistical Analysis
The data were analyzed using Graphpad statistical software (Graphpad Inc., La Jolla, California, USA) and Sigmaplot 11 software. It was assumed that the populations had Gaussian distributions using the method of Kolmogorov and Smirnov. In all cases a two-tailed P value < 0.01 was considered significant. In case of bodyweight P < 0.05 was considered significant. Student's unpaired t test and one-way ANOVA was used for statistical analysis as relevant.
Results
Body Weight and Abdominal Girth
The weekly mean body weight for all the 5 groups (from the seventh week) during the experimental period is shown in Figure 1. At the end of the sixth week, the diet modified groups had a significantly higher mean body weight (175.5 ± 7.4 g) than the diet control group (mean body weight = 166.4 ± 5.2) (P < 0.05), whereas there was no difference between experimental and drug control groups.
Figure 1.

Effect of citral on body weight. Mean body weight of the rats during the experiment. Final body weights of the experimental groups, Group A (248.300 ± 6.3 g), Group B (241.600 ± 4.600 g), and Group C (236.000 ± 8.069 g) were significantly less than the drug control (269.2 ± 17.4 g) group. All data are mean ± SD. P < 0.001 (one-way ANOVA, Holm–Sidak method)
During citral administration there was a decrease in the rate of weight gain of the experimental group. At the end of drug administration the 3 experimental groups showed a significantly less mean body weight (Group A= 248.300 ± 6.3 g, Group B = 241.600 ± 4.600 g, Group C = 236.000 ± 8.069 g) than the drug control group (269.2 ± 17.4 g) (one-way ANOVA, Holm–Sidak method, P < 0.001). The amount of weight loss increased with the dose of drug administered as shown in Figure 1.
At the end of the sixth week, there was no significant difference in abdominal girth between experimental and drug control groups. Abdominal girth was significantly less in the experimental groups (Group A = 12.9 ± 0.8 cm, Group B = 12.3 ± 0.7 cm, Group C = 11.4 ± 0.6 cm) than the drug control group (13.3 ± 0.4 cm) (one-way ANOVA, Holm–Sidak method, P < 0.05) at the end of experiment. This difference also increased with the dose of the drug administered [Figure 2].
Figure 2.

Effect of citral on abdominal girth: Mean abdominal girth of Group A (12.9 ± 0.8 cm), Group B (12.3 ± 0.7 cm), and Group C (11.4 ± 0.6 cm), drug control (13.3 ± 0.4 cm), and diet control groups (13.3 ± 0.4 cm) at the end of experiment. Abdominal girth of the experimental groups is significantly less than the drug control group P < 0.05 (oneway ANOVA, Holm–Sidak method)
Fasting Plasma Glucose, Insulin, and Glucose Tolerance Tests
Four weeks after drug administration, the experimental groups had a significantly lower plasma glucose after overnight fasting (Group A = 82.1 ± 2.6 mg/dL, Group B = 81.9 ± 2.6 mg/dL, Group C = 81.4 ± 2.3 mg/dL) than the drug control groups (85 ± 2.3 mg/dL) (P = 0.01) [Figure 3]. This significant difference did not exist before drug administration. ANOVA also showed a significant dose dependent difference between the drug-treated groups (P = 0.01).
Figure 3.
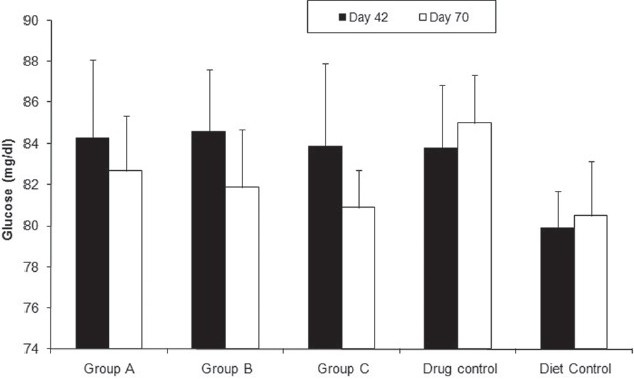
Effect of citral on fasting plasma glucose level. Fasting plasma glucose of Group A (82.1 ± 2.6 mg/dL), Group B (81.9 ± 2.6 mg/dL), Group C (81.4 ± 2.3 mg/dL), drug control (85 ± 2.3 mg/dL) and diet control groups (80.5 ± 2.4 mg/dL)at the end of the sixth week (filled columns) and the end of experiment (unfilled columns). Fasting plasma glucose of the experimental groups is significantly less than the drug control group. P = 0.01 (one-way ANOVA, Holm–Sidak method). All data are mean ± SD
At the end of experiment we performed an intraperitoneal glucose load test. The drug-treated rats showed better tolerance, reaching lower peaks and returning to the baseline values faster [Figure 4].
Figure 4.
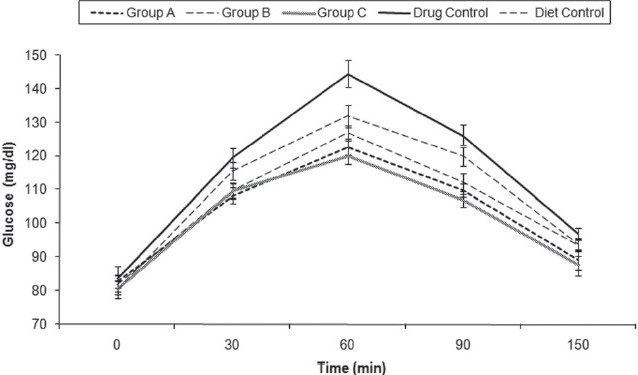
Effect of citral on glucose tolerance. Glucose tolerance test. Mean peak glucose levels of Group A (127 ± 4 mg/dL), Group B (123 ± 3mg/dL), Group C (120 ± 3 mg/dL), drug control (145 ± 4 mg/dL), and diet control groups (132 ± 4 mg/dL) at the end of experiment. Mean glucose levels are shown. All data are mean ± SD. Mean peak glucose levels of Group A, Group B, and Group C are significantly lower than the drug control (one-way ANOVA, Holm–Sidak method, P < 0.05)
Insulin assays showed that the plasma insulin levels were significantly lower in the drug control group [Figure 5].
Figure 5.
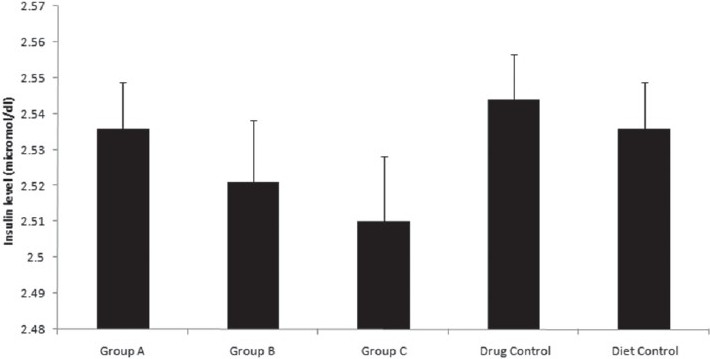
Effect of citral on serum insulin levels. Serum insulin levels of Group A (2.536 ± 0.012 micromol/dL), Group B (2.521 ± 0.018 micromol/dL), Group C (2.51 ± 0.019 micromol/dL), drug control (2.544 ± 0.012 micromol/dL), and diet control groups (2.537 ± 0.012 micromol/ dL) at the end of experiment. Insulin levels are significantly lower for the experimental groups. One-way ANOVA, Holm–Sidak method, P < 0.001. All data are mean ± SD
Body Metabolism
We questioned if the decrease in fat accumulation were a result of decreased energy intake or increased energy expenditure. Metabolic rate, body temperature, and respiratory quotient were significantly higher in the drug-treated group suggesting greater energy dissipation [Figure 6].
Figure 6.
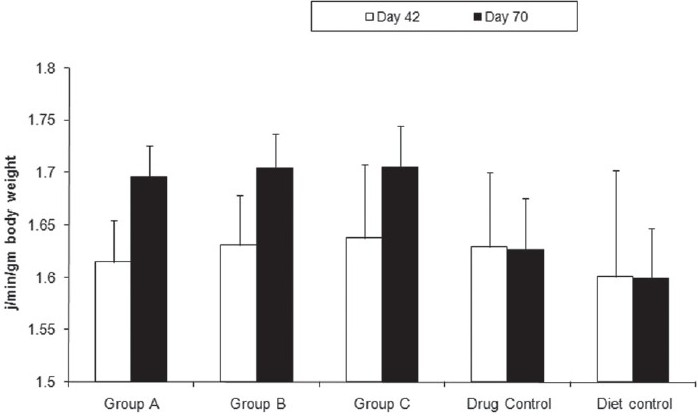
Effect of citral on metabolic rate. Metabolic rate (calculated by Weir equation) of Group A (1.69 ± 0.024 J/min/g body weight), Group B (1.71 ± 0.025 J/min/g body weight), Group C (1.71 ± 0.029 J/min/g body weight), drug control (1.63 ± 0.05 J/min/g body weight), and diet control groups (1.60 ± 0.05 J/min/g -body weight) at the end of experiment. Metabolic rate is significantly higher for the experimental groups. Oneway ANOVA, Holm–Sidak method, P < 0.001 All data are mean ± SD
Adipocyte Morphology and abdominal Fat Deposition, Triglyceride Content in Serum and Cells
The effects of citral on the adipocyte morphology were studied by Hematoxylin-Eosin (H & E) staining of a sample of periniephric fat after the rats had been sacrificed. Histopathologic examination showed that the adipocytes were smaller in the drug-treated group [Figure 7]. However, no adverse effect on perinephric fat, such as edema or inflammation, was found. Triglyceride assay did not show any significant difference in lipid accumulation within the cells. Serum cholesterol and triglycerides too did not show any significant difference between the experimental and drug control groups. Mean serum cholesterol of the experimental Group A (3.31 ± 0.09 mmol/L), Group B (3.25 ± 0.09 mmol/L), and Group C (3.27 ± 0.09) was not significantly different from that of the drug control group (3.35 ± 0.10) (one-way ANOVA, Holm–Sidak method, P > 0.05) We did not find any significant difference in the triglyceride levels also (Group A (0.89 ± 0.08 mmol/L), Group B (0.92 ± 0.08 mmol/L), Group C (0.94 ± 0.07 mmol/L), drug control (0.92 ± 0.06 mmol/L), diet control (0.86 ± 0.07 mmol/L)).
Figure 7.

Effect of citral on adipose tissue. Representative stained sections of perinephric adipose tissue from drug control group (right) and experimental Group A (10 mg/kg body weight; left). The drug-treated group shows smaller adipocytes than the drug control group. (1000× magnification, oil immersion lens)
Discussion
The current combined epidemics of diabetes and obesity have focused attention on potential molecules that can provide an effective cure with minimal side effects. Currently available antiobesity medications attack the body fat dilemma in 3 different ways. They stimulate metabolism, suppress appetite, or impede digestion of fat. A variety of compounds have been isolated from plant sources, which have the potential of being antiobesity and/or antidiabetic agents.[18]
Citral is one such plant extract that is cheap, widely available, and with no known harmful effects.[12] We demonstrated that citral administration significantly lowered body weight gain and abdominal fat mass in rats on a high-energy diet in a dose-dependent manner.
Initially, in our attempt to create a diet-induced obesity model, we fed 2 groups with an energy-intense diet. The rats on the energy-intense diet took a slightly shorter time to achieve a significant difference than the periods reported in other studies.[19] One explanation could be that this diet contained monosodium gluconate, which leads to obesity in rats.[20]
The addition of citral did not produce any difference in total food intake and the percentage of each category of food remained similar. Thus, citral did not alter the food preference proving that it had no effects on appetite and approximate energy intake was same. Total energy input being same the decrease in weight gain must be a result of either decreased fat absorption or greater energy dissipation. A colorimetric assay did not show any significant difference in triglyceride content of drug-treated and control groups. Serum cholesterol and triglyceride levels too did not have a significant difference. Metabolic rate, temperature, and respiratory quotient were raised suggesting increased metabolism. Although not conclusive, these data suggest increased metabolic rate has the dominant role in suppressing weight gain.
Citral significantly decreased serum insulin levels. The decrease in insulin levels was accompanied by improved glucose tolerance and lower fasting plasma glucose levels, suggesting an insulin-sensitizing action of citral. Hyperinsulinemia results from an elevated caloric intake. It has been postulated that this hyperinsulinemic state can cause the development of insulin resistance in adipocytes. At the same time, insulin resistance exacerbates the abnormalities in hepatic fat metabolism.[21] Citral may thus reduce the possibility of progression from impaired glucose tolerance to frank diabetes by correcting the initial insulin resistance. The exact site of insulin sensitization is yet to be determined. It is conclusively proved that citral accumulates retinaldehyde.[8] Retinaldehyde activates the peroxisome proliferator–activated response (PPAR) receptors leading to an increase in serum adiponectin levels, which sensitizes both the liver and the muscle.[8] It is not yet known if citral acts on any other tissue other than adipose tissue directly or indirectly to modulate whole-body insulin resistance.
It is suggested that insulin resistance develops as a result of excessive adipose tissue and the adipokines it secretes.[21] There is therefore the possibility that the improved sensitivity to insulin could be a result of the decreased fat. However, insulin resistance due to increased adiposity hardly develops in a diet-induced model, such as ours. It is most likely that the increased insulin sensitivity is a separate action mediated through other mechanisms.
Indeed, retinaldehyde, which is hypothesized to increase following citral administration, interacts with peroxisome proliferator gamma receptors, which improve insulin resistance in adipose tissue but not muscle.[9] Thiazolidinediones (a new class of antidiabetic drugs) too increase insulin sensitivity by engaging the same receptor.
Retinoids play a stellar role in adipocyte differentiation and metabolism. The ratio of retinaldehyde and retinoic acid seems important in regulating the rate of differentiation of adipose tissue. Retinoic acid speeds up the process, while retinaldehyde retards it. The balance between retinaldehyde and retinoic acid is determined by factors, such as the concentration of vitamin A in the body, the expression and activity of enzymes that metabolize retinaldehyde and retinoic acid, other retinol-modifying enzymes (such as esterases and hydrolases), as well as retinol-binding proteins and the redox status in cells.[22–24] Retinaldehyde regulates adipocyte metabolism by regulating RXR-α and PPAR-γ in turn suppressing adipogenic gene expression, adipocyte maturation, increasing fat metabolism and increasing secretion of adiponectin.[9] Adiponectin is a multifunctional protein that exerts pleiotropic insulin-sensitizing effects. It lowers hepatic glucose production and increases glucose uptake and fatty acid oxidation in skeletal muscle.[25,26]
We did not prove that retinaldehyde accumulated in the adipose tissue of the treated rats. Retinaldehyde dehydrogenase-deficient mice (Raldh–/–) which possess excessive concentration of retinaldehyde in their adipose tissues show a similar decrease in body weight gain in response to a high-fat diet as our citral-administered rats.[9] These rats possess better glucose tolerance, lower fasting plasma glucose levels, and increased metabolic rate compared with the wild type. In our experiment, the citral-treated group had similar manifestations. Taking into account this similarity, the block in retinaldehyde metabolism appears the most plausible mechanism. Citral being a close structural congener of retinoids may possess some affinity for the retinoid receptor. However, it is least likely to possess intrinsic activity and would for all purposes act as an antagonist.
Our study proves that citral has the potential to be used as an antiadipogenic agent. The lack of toxicity and other effects, such as its antimicrobial properties, gives it an advantage. The perils of interfering with retinoid metabolism must, however, be considered. Retinoids, especially retinoic acid are essential for cellular differentiation and function. The consequences of long-term administration of citral must be adequately probed and its effects on body systems other than adipose tissue must be observed. The suitability of the oral route must also be examined as citral's antimicrobial properties may have an undesirable effect on the gut flora.
In conclusion, the study demonstrates that the administration of citral leads to suppressed accumulation of abdominal fat, weight gain to a high-energy diet, hyperinsulinemia, and also improved glucose tolerance. Metabolic studies further suggest these are a result of increased energy production, although decreased lipid accumulation could also have a role. Considering that the consumption of high-fat and energy-intense diets have been implicated as reasons behind the current epidemic of diabetes and obesity, our findings suggest the possibility that citral could ameliorate these lifestyle diseases.
Acknowledgments
We acknowledge the help of Dr. S. Ray and his staff at IICB for their help in conducting the experiments.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Pi-Sunyer X. The medical risks of obesity. Postgrad Med. 2009;121:21–33. doi: 10.3810/pgm.2009.11.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Isomma B. A major health hazard: The metabolic syndrome. Life Sci. 2003;73:2395–2411. doi: 10.1016/s0024-3205(03)00646-5. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–7. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 4.Obesity: Preventing and managing the global epidemic.Report of a WHO consultation on obesity. Geneva: World Health Organisation; 2000. World Health Organisation. [PubMed] [Google Scholar]
- 5.Wilding JP. Causes of obesity. Pract Diab Int. 2001;18:288–91. [Google Scholar]
- 6.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 7.Hardeman W, Griffin S, Johnston M, Kinmonth AL, Wareham NJ. Interventions to prevent weight gain: A systematic review of psychological models and behaviour change methods. Int J Obes Relat Metab Disord. 2000;24:131–43. doi: 10.1038/sj.ijo.0801100. [DOI] [PubMed] [Google Scholar]
- 8.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–62. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 9.Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, et al. Retinaldehyde represses adipogenesis and diet-induced obesity. Nat Med. 2007;13:695–702. doi: 10.1038/nm1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carbajal D, Casaco A, Arruzazabala L, Gonzalez R, Tolon Z. Pharmacological study of Cymbopogon citrates leaves. J Ethnopharmacol. 1989;25:103–7. doi: 10.1016/0378-8741(89)90049-4. [DOI] [PubMed] [Google Scholar]
- 11.Scolnik MD, Servadio C, Abramovici A. Comparative study of experimentally induced benign and atypical hyperplasia in the ventral prostrate of different rat strains. J Androl. 1994;15:287–97. [PubMed] [Google Scholar]
- 12.Vinitketkumnuen U, Puatanchokchai R, Kongtawelert P. Antimutagenicity of lemongrass (Cymbopogon citratus stapf) to various known mutagens in salmonella mutation assay. Mutat Res. 1994;341:71–5. doi: 10.1016/0165-1218(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 13.Kikonyogo A, Abriola DP, Dryjanski M, Pietruszko R. Mechanism of inhibition of aldehyde dehydrogenase by citral, a retinoid antagonist. Eur J Biochem. 1999;262:704–12. doi: 10.1046/j.1432-1327.1999.00415.x. [DOI] [PubMed] [Google Scholar]
- 14.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–4. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 15.Rolls BJ, Rowe EA, Turner RC. Persistent obesity in rats following a period of consumption of a mixed, high-energy diet. J Physiol. 1980;298:415–27. doi: 10.1113/jphysiol.1980.sp013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes PC, Tanner JM. A longitudinal study of the growth of the black hooded rat: Methods of measurement and rates of growth for skull, limbs, pelvis, nose-rump and tail lengths. J Anat. 1970;106:349–70. [PMC free article] [PubMed] [Google Scholar]
- 17.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol (Lond) 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasani-Ranjbar S, Nayebi N, Larijani B, Abdollahi M. A systematic review of the efficacy and safety of herbal medicines used in the treatment of obesity. World J Gastroenterol. 2009;15:3073–85. doi: 10.3748/wjg.15.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitts GC, Bull LS. Exercise, dietary obesity, and growth in the rat. Am J Physiol. 1977;232:R38–44. doi: 10.1152/ajpregu.1977.232.1.R38. [DOI] [PubMed] [Google Scholar]
- 20.Bunyan J, Murrell EA, Shah PP. The induction of obesity in rodents by means of monosodium glutamate. Br J Nutr. 1976;35:25–39. doi: 10.1079/bjn19760005. [DOI] [PubMed] [Google Scholar]
- 21.Zammit VA. Insulin stimulation of hepatic triacylglycerol secretion in the insulin-replete state: Implications for the etiology of peripheral insulin resistance. Ann N Y Acad Sci. 2002;967:52–65. doi: 10.1111/j.1749-6632.2002.tb04263.x. [DOI] [PubMed] [Google Scholar]
- 22.Napoli JL. Retinoic acid: Its biosynthesis and metabolism. Prog Nucleic Acid Res Mol Biol. 1999;63:139–88. doi: 10.1016/s0079-6603(08)60722-9. [DOI] [PubMed] [Google Scholar]
- 23.Duester G, Mic FA, Molotkov A. Cytosolic retinoid dehydrogenases govern ubiquitous metabolism of retinol to retinaldehyde followed by tissue-specific metabolism to retinoic acid. Chem Biol Interact. 2003;143-144:201–10. doi: 10.1016/s0009-2797(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 24.Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–19. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–53. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 26.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]


