Abstract
Background:
It has been established that sublingual (SL) route of misoprostol has a great potential to be developed for medical abortion, but there is dearth of evidence to reveal satisfaction rate and safety profile among patients of oral and SL routes. Thus, this study was conducted to provide an insight into the acceptability and safety profile of the same.
Materials and Methods:
A randomized controlled trial was carried out by giving 200 mg mifepristone orally, followed by administration of 600 μg misoprostol orally to 50 women and sublingually to 50 women. The primary endpoints of study were measurements of acceptability and safety profile parameters (average blood loss, nausea, vomiting, diarrhea, hot flushes, fever) of both the groups. The secondary endpoints of the study were number of doses required for complete abortion, success rate and the induction to evacuation interval in both the groups.
Results:
SL route of administration was more acceptable than the oral route (P = 0.009). Average blood loss was higher in the oral group than in the SL group (P = 0.001). Amongst the side effects, 34% in the SL group and 52% in the oral group had nausea (P = 0.264), 22% in the SL group and 44% in the oral group had vomiting (P = 0.031), 48% in the SL group and 86% in the oral group had diarrhea (P < 0.05), hot flushes were presented by 24% in the SL group and 50% in the oral group (P < 0.05), fever was presented by 20% in the SL group and 44% in the oral group (P < 0.05), and the number of cases aborted with only one dose was higher (86%) in the SL group as compared to 63% in the oral group (P = 0.004). The evacuation (success) rates were 92% in the SL group and 84% in the oral group (P = 0.218) and the mean ± SD induction to evacuation intervals in the SL and oral groups were 5.6 ± 4.54 hours and 9.44 ± 5.61 hours, respectively (P = 0.0002).
Conclusion:
The SL route had fewer undesirable effects, was more satisfactory, required less number of doses and was more acceptable to the patient compared to the oral route.
Keywords: Adverse effect, early fetal demise, oral route, misoprostol, sublingual route
Introduction
Recently, medical management has been explored as an alternative to surgical management of miscarriage after early fetal demise and placed it over surgical management as the latter has more side effects. Moreover, the future fertility of the women may be jeopardized.[1] Chung et al. reported an overall complication rate of 6.6% with the surgical method.[2] It has been established by various trials that vaginal route of misoprostol is superior to oral route for medical termination of pregnancy, as it is absorbed by vaginal mucosa, the hepatic metabolism is bypassed which results in better efficacy and lesser side effects.[3–6] Sublingual (SL) route of administration of misoprostol may have similar pharmacokinetic characteristics and avoids the painful, uncomfortable vaginal administration. It is more convenient to take and offers more privacy during the abortion process and is preferred by women.[4] Moreover, the vaginal route of administration may not be acceptable to many women due to religious and social reasons.
A pharmacokinetic and a pilot study by Tang et al. has suggested that taken sublingually, misoprostol have better bioavailability and efficacy.[7,8] Some more studies reported uterine tonus to be better with the SL route.[9,10] On the other hand, some of them also suggested that SL misoprostol is associated with a higher incidence of adverse effects, especially diarrhea, fever and chills.[1,8] Recently, Wagaarchchi et al.[11] and Tang et al.[12] have studied the use of SL route of administration of misoprostol in early fetal demise and concluded it to have better efficacy, acceptability and lesser side effects.
After several clinical trials of SL misoprostol, it has been established as a method of medical abortion,[7–10] but there is a dearth of evidences to show the safety profile and acceptability of this route in previous studies. The present study was undertaken to evaluate and compare the acceptability and safety profile of oral and SL misoprostol for uterine evacuation following early fetal demise.
Materials and Methods
Study Design
The study was an open-label, randomized, parallel-group controlled trial. The study protocol was approved by ethics committee of the Delhi University Faculty of Medical Sciences. Women who were eligible for the study were thoroughly counseled and informed written consents were taken.
Patients
Patients were recruited for the study based on the following.
Inclusion criteria
Pregnant women with first trimester pregnancy.
Patient with known last menstrual period.
Confirmed missed miscarriage or anembryonic pregnancy by ultrasonography.
Good general health, hemoglobin level > 9 g/dl.
Confirmed intrauterine gestational sac.
Women willing to use the barrier method of contraception until the first menses after termination of pregnancy.
Exclusion criteria
Age: <16 years and >35 years, Hb < 9 g/dl.
Heavy smokers using >10 cigarettes a day.
Presence of cardiovascular disease, adrenal insufficiency, long-term use of glucocorticoids, bleeding disorders.
History of asthma, epilepsy, glaucoma, any allergy toward mifepristone or misoprostol or any prostaglandins.
Uterine anomaly, multifetal gestation, extrauterine pregnancy.
Presence of intrauterine device.
Any evidence of septic abortion/any infection.
Transvaginal scan showing thin endometrial echo suggesting complete miscarriage.
The study included 100 women up to 12 weeks pregnancy with confirmed fetal demise (anembryonic pregnancy and missed abortion) and randomized by computer model.
The women were randomized to two groups of 50 each. After administering 200 mg mifepristone on day 1 to all 100 cases, group 1 was given 600 μg misoprostol SL on day 3 and group 2 was given 600 μg misoprostol orally on day 3. The women in group 1 were instructed to put misoprostol under the tongue themselves and were not allowed to have food or drink for 20 minutes and not to swallow the tablet.
The women in group 2 were instructed to swallow three 200 μg tablets with water. Following misoprostol administration, pulse, blood pressure, temperature, and systemic symptoms were monitored hourly. The women were observed for a further 6 hours and a repeat hemoglobin assay was done on day 7. Follow-up ultrasonography was done whenever required.
Study Procedure
Whenever women expelled products of conception or bled vaginally, they underwent a vaginal examination to assess the degree of expulsion, which was determined to be complete on ultrasound. The evacuation was considered complete when the women no longer had active vaginal bleeding, and had a closed cervical os and an empty uterine cavity on ultrasound. The evacuation was considered incomplete when vaginal bleeding continued, the cervical os remained open, and products of conception were visible on ultrasound.
The women who did not expel products of conception within 12 hours of the first dose of misoprostol were given up to three supplemental 400-μg doses at 3-hour intervals, sublingually or orally depending on their group. Those who received the maximum misoprostol regimen and did not expel products of conception within 4 hours of taking the last 400-μg dose by either route underwent surgical evacuation under intravenous sedation and paracervical block.
After complete uterine evacuation, whether medical or surgical, the women were kept under observation for 6 hours and then discharged. They returned 7 days later to assess their hemoglobin level and any difference compared to the baseline value was noted. They all had a routine checkup 2 weeks after discharge.
Study Endpoints
The primary endpoints of study were measurements of acceptability and safety. The safety profile parameters were average blood loss, nausea, vomiting, diarrhea, hot flushes and fever in both the groups. Regimen acceptability was defined as whether they would consider the same method of induction again, if needed.[13]
The secondary endpoints of the study were number of doses required for complete abortion and the induction to evacuation interval in both the groups, defined as the time between the first dose of misoprostol and the time when the products of conception were expelled.
Sample Size Calculation
Sample size was calculated manually based on the study by Saxena et al.[14] For calculation of power and sample size, induction to evacuation time was used which gave a sample size of 100. Power of study was 80% with a margin of error of 5% and effect size was 7.85.
Statistical Analysis
For comparing the groups for complete evacuation, nausea, vomiting, diarrhea, hot flushes, fever, and acceptability, chi-square test was applied. For calculating the number of doses required for complete abortion, Fisher's exact test was applied. Success variation of one dose between groups was calculated using Gaussian test. For finding the induction to evacuation interval, Student's “t” test was applied. For calculating the blood loss score, Mann Whitney U test was applied. P value <0.05 was considered significant.
Results
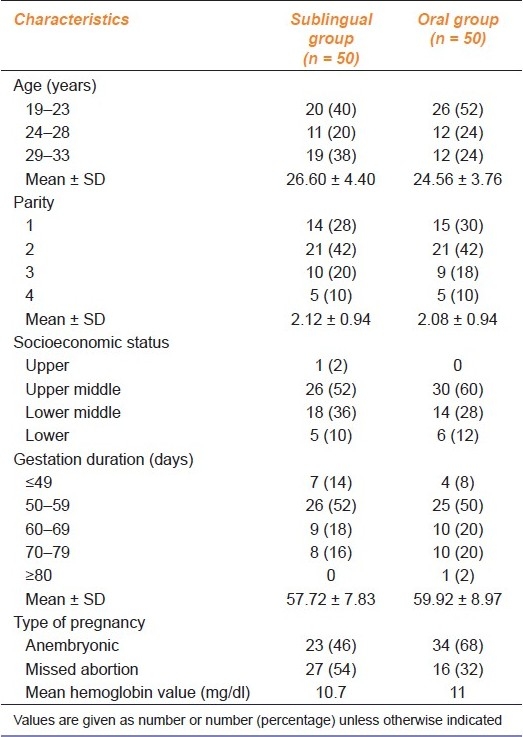
As mentioned in Table 1, baseline characteristics of both the groups in terms of demographic profile, socioeconomic status, age, parity, period of gestation, type of pregnancy and hemoglobin value were comparable.
Table 1.
Characteristics of the patients
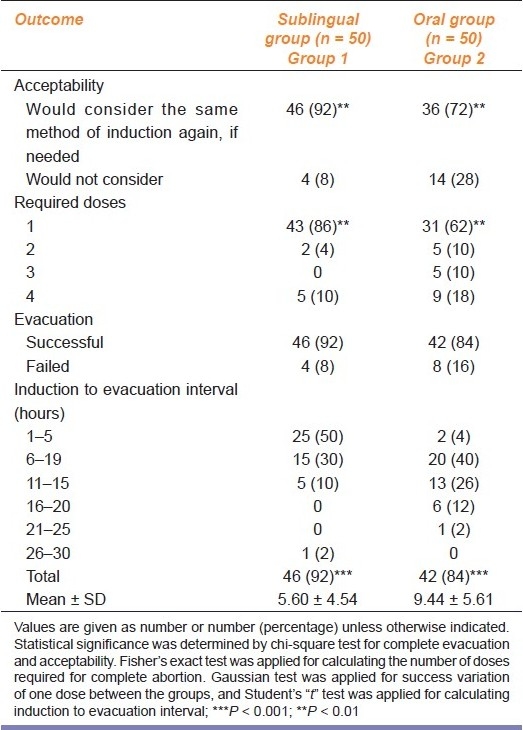
SL route of administration was more acceptable [Table 2] to the cases than the oral route (χ2 = 6.78; P = 0.009; df = 1).
Table 2.
Outcome of the study on oral and SL misoprostol for uterine evaluation following early fetal demise
Average blood loss [Table 3] was higher in oral group than in SL group. Results were statistically highly significant (P = 0.001).
Table 3.
Side effects observed in patients receiving oral and SL misoprostol
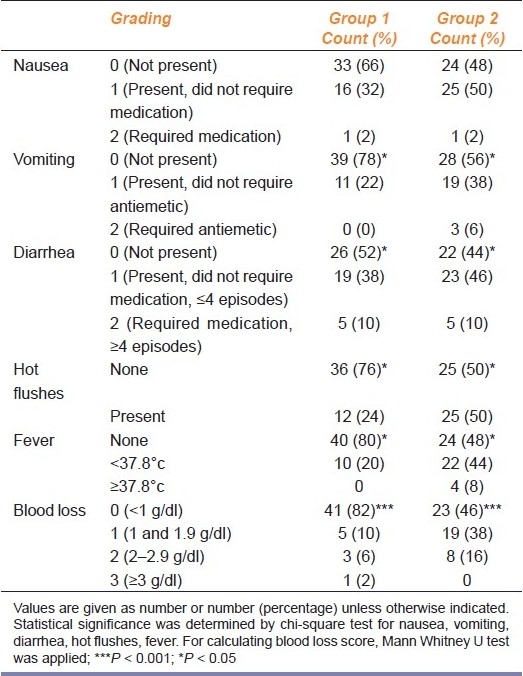
SL group cases had fewer side effects [Table 3] than the cases of oral group. Amongst the side effects, 17 (34%) cases in SL group and 26 (52%) cases in oral group had nausea (P = 0.264), 11 (22%) cases in SL group and 22 (44%) cases in oral group had vomiting (P = 0.031), 24 (48%) cases in SL group and 28 (86%) cases in oral group had diarrhea (P < 0.05), hot flushes was presented by 12 (24%) cases in SL group and 25 (50%) cases in oral group (P < 0.05), and 10 (20%) cases in SL group and 22 (44%) cases in oral group had fever (P < 0.05).
Maximum number of cases aborted with only one dose of oral/SL misoprostol; however, cases requiring higher doses of misoprostol were more in group 2 (oral group). Difference of required doses in groups 1 and 2 was statistically significant (P = 0.004).
Complete abortion rate in SL group was 46 (92%), while it was 42 (84%) in the oral group. The abortion rate was higher in SL group. However, difference was not statistically significant (P = 0.218).
In group 1, 46 (92%) cases were satisfied with the regimen while 4 (8%) cases were dissatisfied. Failure of regimen was the only cause for rejection of regimen in group 1; in group 2, 36 (72%) cases were satisfied while 14 (28%) cases were dissatisfied. Eight (16%) cases rejected the regimen due to the failure of the method while 6 (12%) cases rejected the regimen due to non-acceptance of side effect.
Therefore, SL route of administration was more acceptable to the cases than the oral route. Difference in level of satisfaction in both the groups was statistically significant (P = 0.009).
The induction to evacuation interval in the SL group was 5.60 ± 4.54 hours and in the oral group was 9.44 ± 5.61 hours. Difference in mean induction abortion interval in both the groups was statistically very highly significant (P = 0.0002).
Discussion
With good antenatal care and more awareness and the advent of routine early pregnancy scanning, diagnosis of early fetal demise (anembryonic pregnancy or missed abortion) has increased. Although investigators have used various prostaglandin analogues to initiate uterine expulsion, misoprostol (a prostaglandin E1 analogue) is currently favored because of its safety, low cost, availability, stability at room temperature and oral route of delivery. Addition of mifepristone to misoprostol makes the myometrium more sensitive to the stimulatory effects of prostaglandin, which aids in uterine evacuation.[15,16] Use of medical method with mifepristone and misoprostol has been evaluated in several studies, but there is still a dilemma in the available literature regarding side effects and acceptability with these routes of misoprostol administration. The present study was conducted with the aim to evaluate the efficacy and side effects of oral and SL misoprostol in the management of early fetal demise.
Primarily, Tang et al. explored a new route of administration of misoprostol, i.e. SL route. Recently, it has been established by several studies that SL route of administration of misoprostol demonstrated a great potential to be developed into a method of medical abortion.[17]
This study gives an insight into the safety profile of these routes, which was always an area of focus for clinicians before its clinical application.
In the present study, difference in the hemoglobin level was found to be statistically significant in the oral group. Number of cases having higher degree of blood loss was more in the oral group. In both the groups, there was no significant change in the hemoglobin level, while only one case had severe blood loss with a hemoglobin difference of 3.1 g/dl and required blood transfusion and surgical evacuation. Possible explanation of excessive blood loss was type 2 pregnancy (missed abortion), longer period of gestation (72 days) and requirement of higher doses (1800 μg).
Although pain is inherent with the process of abortion and has been found to be most common complaint by the patients in various studies, tolerability was variable from patient to patient. Cramping pain in the abdomen was the most common side effect of mifepristone-misoprostol regimen but could be managed with oral analgesics. Lower abdominal pain was tolerable to most of the cases in both the groups. However, cases requiring analgesics were more in the oral group. None of the cases in either group required parental analgesics.
Lesser occurrence of nausea and vomiting in the present study appears to be due to the fact that maximum cases (86%) in this group aborted with a single dose (600 μg) of misoprostol, while most of the previous studies used higher doses (600-1800 μg).[8,12,13] When the frequency of GI side effects was compared in both the groups, it was found to be higher in the oral group, as the dose requirement of misoprostol for complete abortion was more in the oral group. In the present study, none of the cases in group 1 had fever more than 1000°F, while 8% (four cases) in group 2 had fever >1000°F, which appears to be due to due to the requirement of higher doses to abort (four doses in three cases and three doses in one case). Higher occurrence of fever in the other mentioned studies[8,12] appears to be due to the use of higher doses of misoprostol.
In the present study, 92% cases were satisfied and 8% cases were dissatisfied with the regimen in the SL group. Complete abortion and failure rate of the regimen was 92 and 8%, respectively. Thus, acceptability of the regimen was solely dependent on the success of the treatment as side effects were tolerable to all cases. However, in group 2, 72% cases (36 out of 50) were satisfied while 28% cases (14 out of 50) were dissatisfied. Among these 14 cases, failure of treatment was the reason for dissatisfaction of 8 cases; remaining 6 cases were dissatisfied with the regimen due to intolerable side effects, especially bleeding and GI side effects. Therefore, efficacy of the regimen is the determining factor for the acceptability of the method in most cases in both the groups. Rate of acceptability in the present study is the same as reported in earlier studies.[8,12]
Therefore, it can be concluded that SL route of administration of misoprostol is better in terms of efficacy, tolerability and acceptability than the oral route.
Many studies have been performed earlier on this, but are few in number to come to any conclusion. The present study with more clear selection criteria and study method tries to lay the platform to carry out further studies.
Small sample size and open label were the limitations of the study. Further double-blind studies are needed with a large sample population to elucidate the safety and optimal dosage of misoprostol.
Acknowledgments
My deep sense of gratitude to Zeashan Haider Zaidi, Lecturer, Biostatistics, Department of Community Medicine, ELMC and H, for his support and guidance in statistical work.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Graziosi GM, Mol BW, Ankun WM, Bruinse HW. Management of early pregnancy loss. Int J Gynecol Obstet. 2004;86:337–46. doi: 10.1016/j.ijgo.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Chung T, Leung P, Cheung LP, Haines C, Chang AM. A medical approach to management of spontaneous abortion using misoprostol. Acta Obstet Gynecol Scand. 1997;76:248–51. [PubMed] [Google Scholar]
- 3.El-Refacy H, Rajasekar D, Abdalla M, Calder L, Templeton A. Induction of abortion with mifepristone (RU 486) and oral or vaginal misoprostol. N Eng J Med. 1995;332:983–7. doi: 10.1056/NEJM199504133321502. [DOI] [PubMed] [Google Scholar]
- 4.Ho PC, Ngai SW, Liu KL, Wong GC, Lee SW. Vaginal misopristol compared with oral misopristol in termination of second trimester pregnancy. Obstet Gynecol. 1997;90:735–8. doi: 10.1016/S0029-7844(97)00419-5. [DOI] [PubMed] [Google Scholar]
- 5.Carbonell JL, Varela L, Velazeo A, Fernandez C. The use of misopristol for termination of early pregnancy. Contraception. 1997;55:165–8. doi: 10.1016/s0010-7824(97)00020-6. [DOI] [PubMed] [Google Scholar]
- 6.Carbonell JL, Varela L, Tanda R, Cabezas E, Sanctez C. Early abortion with 800 Ìg of misopristol by vaginal route. Contraception. 1999;59:219–25. doi: 10.1016/s0010-7824(99)00032-3. [DOI] [PubMed] [Google Scholar]
- 7.Tang OS, Soohweer H, Seyberth HW, Lee SW, Ho PG. Pharmacokinetics of different routes of administration of misopristol. Hum Reprod. 2002;17:332–6. doi: 10.1093/humrep/17.2.332. [DOI] [PubMed] [Google Scholar]
- 8.Tang OS, Miao BY, Lee SW, Chung Ho. Pilot study on the use of repeated doses of sublingual misoprostol in termination of pregnancy up to 12 weeks gestation: Efficacy and acceptability. Hum Reprod. 2002;17:654–8. doi: 10.1093/humrep/17.3.654. [DOI] [PubMed] [Google Scholar]
- 9.Aronsson A, Bygdeman M, Gemzell-Danielsson K. Effects of misoprostol on uterine contractility following different routes of administration. Hum Reprod. 2004;19:81–4. doi: 10.1093/humrep/deh005. [DOI] [PubMed] [Google Scholar]
- 10.Meckstroth KR, Whitaker AK, Bertisch S, Goldberg AB, Danney PD. Misoprostol administered by epithelial routes: Drug absorption and uterine response. Obstet Gynecol. 2006;108:582–90. doi: 10.1097/01.AOG.0000230398.32794.9d. [DOI] [PubMed] [Google Scholar]
- 11.Waggarachchi PT, Ashok PW, Smith N, Templeton A. Medical management of early fetal demise using sublingual Misoprostol. Br J Obstet Gynaecol. 2002;109:462–5. doi: 10.1111/j.1471-0528.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 12.Tang OS, Lau WN, Ng EH, Lee SW, Ho PC. A prospective randomized study to compare the use of repeated doses of vaginal with sublingual misoprostol in the management of first trimester silent miscarriages. Hum Reprod. 2003;18:176–81. doi: 10.1093/humrep/deg013. [DOI] [PubMed] [Google Scholar]
- 13.Shetty A, Mackie L, Danielian P, Rice P, Templeton A. Sublingual compared with oral misoprostol in term labour induction: A randomised controlled trial. Br J Obstet Gynaecol. 2002;109:645–50. doi: 10.1111/j.1471-0528.2002.01459.x. [DOI] [PubMed] [Google Scholar]
- 14.Saxena P, Salhan S, Sarda N. Comparison between the sublingual and oral route of misoprostol for pre-abortion cervical priming in first trimester abortions. Hum Reprod. 2004;19:77–80. doi: 10.1093/humrep/deh001. [DOI] [PubMed] [Google Scholar]
- 15.Tang OS, Gemzell-Danielsson K, Ho PC. Misoprostol: Pharmacokinetic profiles, effects on the uterus and side-effects. Int J Gynecol Obstet. 2007;99:160–7. doi: 10.1016/j.ijgo.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 16.Ashok PW, Penney GC, Flett GM, Templeton A. An effective regimen for early medical abortion: A report of 2000 consecutive cases. Hum Reprod. 1998;13:2962–5. doi: 10.1093/humrep/13.10.2962. [DOI] [PubMed] [Google Scholar]
- 17.Schaff EA, Fieldings SL, Eisinger SH, Stadalius LS, Fuller L. Low does Mifepristone followed by vaginal misoprostol at 48 hours for abortion upto 63 days. Contraceptions. 2000;61:41–6. doi: 10.1016/s0010-7824(99)00119-5. [DOI] [PubMed] [Google Scholar]


