Abstract
Objective:
To study the effects of N-acetylcysteine (NAC) and atorvastatin on endothelial dysfunction in patients with systemic lupus erythematosus (SLE).
Materials and Methods:
Thirty-two SLE patients and age, sex-matched 10 healthy control subjects were studied. The patients were between 17 and 65 years of age and positive for diagnostic tests, such as antinuclear antibodies (ANA). Photoplethysmogram (PPG) detects the changes in the amount of light absorbed by hemoglobin, which reflects changes in the blood volume. Pulse wave analysis was performed at rest, 30 s, 90 s after shear stress, and 10 min after 300 μm of salbutamol inhalation.
Results:
Stiffness index (SI) of patients before the treatment was 8.46±2.78 cm/s and of controls was 6.07±1.4 cm/s (P = 0.002) and that of reflection index (RI) was 73±13 for patients and 65±7 for controls (P = 0.001). The percentage change in RI after salbutamol inhalation for controls and patients were -16±6 and -7±4 (P = 0.001), respectively, indicating the presence of endothelial dysfunction. The percentage decrease in RI after salbutamol inhalation was from -2.36±0.76 to ?7.92±1.46 in patients treated with N-acetylcysteine (NAC, P = 0.007). The percentage decrease in RI after salbutamol inhalation was from ?6.361.21 to -9.92±1.21 in patients treated with atorvastatin (P = 0.05). This indicated the improvement in endothelial function. There was decrease in C-reactive protein (CRP) from 1.03±0.72 mg/dL to 0.52±0.22 mg/dL and that of malondialdehyde (MDA) from 11.20±4.07 nmol/mL to 8.81±2.79 nmol/mL with N-acetylcysteine treatment (P < 0.05). The CRP was decreased from 1.11±0.92 mg/dL to 0.440.16 mg/dL (P = 0.05) and that of MDA was decreased from 9.37±3.29 nmol/mL to 8.51±3.27 nmol/mL after treatment with atorvastatin. It showed improvement in oxidative stress with these treatments.
Conclusion:
The presence of arterial stiffness indicated endothelial dysfunction. There was reduction in RI and SI with treatment of N-acetylcysteine and atorvastatin suggesting improvement in endothelial dysfunction. There was decrease in CRP (a marker of inflammation) and MDA after treatment with N-acetylcysteine suggesting improvement in endothelial dysfunction. There was reduction in CRP after treatment with atorvastatin, suggesting improvement in endothelial function. Improvement in endothelial dysfunction is associated with decreased incidence of cardiovascular and cerebrovascular accidents.
Keywords: C-reactive protein, reflection index, salbutamol, stiffness index
Introduction
The vascular endothelium lines the entire cardiovascular system and forms the biological interface between the flowing blood and various tissues. Endothelial dysfunction[1] is characterized by a shift of the actions of the endothelium toward reduced vasodilatation, a proinflammatory state, and prothrombic properties, which finally pertains to the abnormalities in the regulation of the lumen of vessels. Endothelial dysfunction has been a finding associated with conditions, such as atherosclerosis,[2] diabetes,[3] hypertension,[4] hypercholesterolemia, high homocysteine, angina,[5] congestive heart failure, oxidative stress,[6] aging,[7] and cigarette smoking.[8] The precise mechanism inducing endothelial dysfunction is not clear, but several investigators found correlations between endothelial dysfunction and oxidative stress.[6] In the context of vascular diseases, endothelial dysfunction has been defined as the blunting of vasodilator response and is particularly due to, reduced NO activity.[9] Recent studies have shown that women with systemic lupus erythematosus (SLE) have a high prevalence of coronary artery disease[10] with the incidence of myocardial infarction up to 52-fold higher compared with age-matched women without SLE.[11] Arterial vascular disease resulting from atherosclerosis contributes to significant cardiovascular and cerebrovascular morbidity and mortality in SLE patients.[11] The preventive strategies directed toward increased availability of endogenous NO and/or decreased inactivation of NO by oxidative stress[12] proved to be useful in improving endothelial function.
Drugs Used
One group received N-acetylcysteine (Mucomix; Samarth, Indore, India) 600 mg 3 times a day for 2 weeks. The other group received atorvastatin (Altovas; Servocare Life SC, Chandigarh, India) 10 mg a day for 2 weeks. The patients were also on regular medicines that are used to treat SLE, such as hydroxychloroquine, prednisolone, azathioprine, and methotrexate.
The aim is to study the endothelial function in patients with SLE and healthy controls and also to study the efficacy of N-acetylcysteine and atorvastatin, on endothelial dysfunction in SLE patients.
Materials and Methods
The present study was conducted in the Department of Clinical Pharmacology and Therapeutics, Nizam's Institute of Medical Sciences (NIMS), Hyderabad, from October 2004 to July 2005 . All the patients with confirmed diagnosis of SLE attending the outpatient unit of the Department of Rheumatology were requested to participate in the study. The normal healthy controls were the attendants of these patients. The study protocol was approved by the Ethics Committee of the institute and all the trial participants gave their informed consent before enrollment.
Inclusion Criteria
SLE patients diagnosed by a consultant rheumatologist, were selected as per the criterion of the presence of 4 or more American College of Rheumatology criteria for classification.[13]
Exclusion Criteria
Patients who had severe hypertension, diabetes, hypercholesteremia, or a history of stroke were excluded from the study.
All SLE patients underwent diagnostic tests for SLE status, namely ANA (anti-nuclear antibodies), anti-DNA, anticardiolipin antibodies, and other laboratory tests, before the study. All patients underwent detailed clinical examination and systemic examination and were selected based on the SLE disease activity index (SLEDAI).[14]
The endothelial function was assessed by digital photoplethysmography (PPG) analyzer (ENDOWIN, M/S Genesis Medical System, Hyderabad).
Patients were asked to withhold their medication for at least 24 h before test. The patients were also asked not to drink caffeine-containing drinks and abstain from smoking 24 h before test. PPG was recorded by an observer always in the morning between 8 AM and 10 AM. The subject was examined in supine position after a 10-min rest on bed; the 3 recordings of basal pulse rate and blood pressure were recorded in supine position at 2-min intervals. PPG was recorded by placing the probe on the right index finger. The plethysmographic sensor measures digital waveforms.[15] Sufficient waveforms were stored for 15 s with automatic gain. The reflection index (RI, no units) and stiffness index (SI) were calculated automatically from the shape of the pulse waveforms, using software. The RI and SI were measured for controls and patients before starting the experiment. These values were treated as baseline values.
A blood pressure cuff was placed over the middle of the forearm. The cuff was inflated to a pressure of 250 mmHg for 4.5 min. The endothelial function was assessed twice using PPG analyzer at 30 s, 90 s after deflating the cuff.
Then the subject was asked to inhale 400 μg of salbutamol from a spacer after 15 min of shear stress test. The endothelial function was reassessed after 15 min. The percentage fall in RI at the end of each test was used to assess the endothelial-dependent vasodilator[16] function. During the first visit, endothelial function[17] was assessed and 6 mL of blood was collected. The hemodynamic parameters, pulse rate, systolic blood pressure, mean arterial pressure, diastolic blood pressure, and pulse pressure were recorded before and after the tests.
The patients were divided into 2 groups; one group of 23 patients received 600 mg of N-acetylcysteine 3 times a day for 2 weeks, while other group of 9 patients received atorvastatin, 10 mg/day for 2 weeks. Both groups of patients were asked to continue regular medications they were receiving for treatment of SLE.
They were asked to come for the second visit after 2 weeks. At the second visit, the same procedure was repeated and compliance was assessed and adverse events were recorded. The compliance was assessed by pill count method. The patients who had not taken more than 80% of the total study medication were considered noncompliant.
At each visit, blood samples were collected in clean dry test tubes. The test tubes were then centrifuged at 2000 rpm for 10 min; serum was separated and collected in aliquots labeled with patient code and date of collection and was stored at 20°C in a refrigerator for the analysis of serum malondialdehyde (MDA)[18] and C-reactive protein (CRP).[19]
Measurement of MDA[18] provides a sensitive index of the lipid peroxidation and is an indirect marker of oxidative stress. CRP was measured by latex agglutination method[19] in each visit.
Statistical Analysis
The statistical analysis was carried out with Sigma graph pad software, USA Version-4. All the data were presented as mean ± standard deviation. Within group analysis was carried out by using paired t test and between group analyses by unpaired t test. For statistical significance, the probability value of less than 0.05 was considered as significant.
Results
In the present study group, of the total 42 subjects, 32 were patients with SLE and 10 were healthy controls. There were 1 male and 9 females in the control group. In the patient group, 2 were males and 30 were females. The age range of patients was 29 ± 8 years and in the control group, 24 ± 6 years. Disease characteristics (according to SLEDAI) and baseline hemodynamic parameters of all the participants are shown in Tables 1 and 2a, respectively.
Table 1.
Disease characteristics of SLE patients before starting the treatment
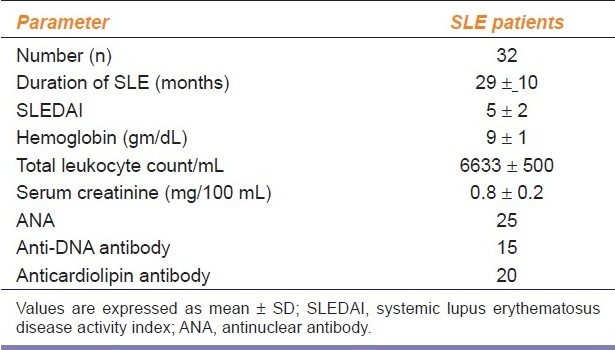
Table 2a.
Hemodynamic parameters in healthy controls and SLE patients
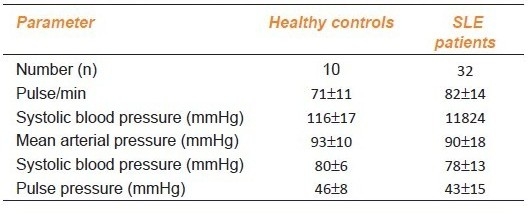
Baseline Hemodynamic Parameters and Endothelial Function
There was no statistically significant difference in any of the above hemodynamic parameters between the patients and the healthy control subjects [Table 2a]. The SI, a marker of large arterial stiffness was found to be significantly high in patients, than in healthy control subjects [Table 2b]. The RI, which is a marker of tone of peripheral artery was also found to be significantly raised in patients as compared to healthy controls [Table 2b].
Table 2b.
Endothelial function parameters in healthy controls and SLE patients
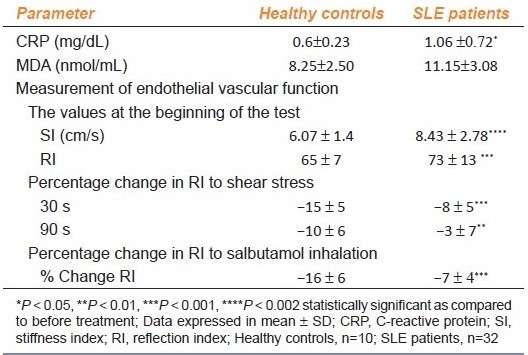
The two tests used to evaluate endothelial-dependent function, such as shear stress and salbutamol challenge, revealed the presence of a significant endothelial dysfunction in patients as compared to that in the controls. The observed percentage fall in the mean RI at 30 and 90 s after shear stress in the patients was significantly less as compared to the healthy control group [Table 2b]. The percentage fall in RI after salbutamol inhalation was also significantly higher in the healthy controls than in the patients, suggesting the presence of endothelial dysfunction [Table 2b].
Effects of N-acetylcysteine and Atorvastatin Treatment in SLE Patients
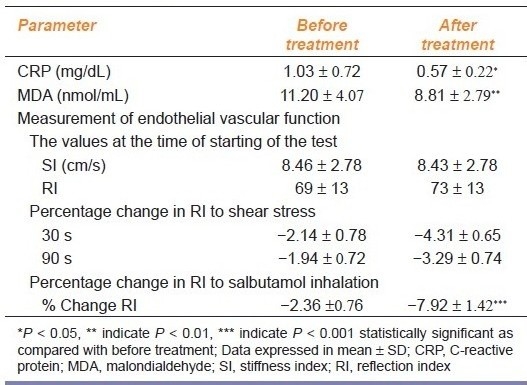
Administration of N-acetylcysteine did not significantly alter any other hemodynamic parameter [Table 3a]; however, N-acetylcysteine treatment significantly lowered both CRP and serum MDA Table 3b]. There was no significant change in basal SI or RI after N-acetylcysteine treatment in SLE patients. Also there was no significant change in the percentage fall in RI at 30 and 90 s after shear stress after N-acetylcysteine treatment [Table 3b]. However, there was a significant decrease in the percentage of RI after salbutamol inhalation in patients treated with N-acetylcysteine, suggesting improvement in the endothelial dysfunction particularly of the resistance vessel [Table 3b].
Table 3a.
Hemodynamic parameters before and after N-acetylcysteine in SLE patients
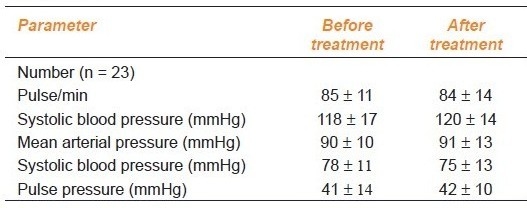
Table 3b.
Endothelial function parameters before and after N-acetylcysteine in SLE patients (n=32)
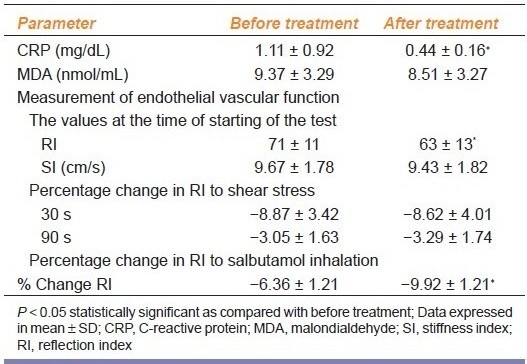
Atorvastatin did not alter hemodynamic parameter in patients [Table 4a]. Atorvastatin treatment produced significant reduction in CRP without affecting the value of MDA in SLE patients [Table 4b]. Also it significantly reduced RI without affecting SI [Table 4b]. The percentage fall in RI at 30 and 90 s after shear stress was also not altered significantly, however, the percentage change in RI after salbutamol inhalation was significantly decreased [Table 4b], suggesting improvement in the endothelial dysfunction after the treatment with atorvastatin.
Table 4a.
Hemodynamic parameters before and after atorvastatin in SLE patients
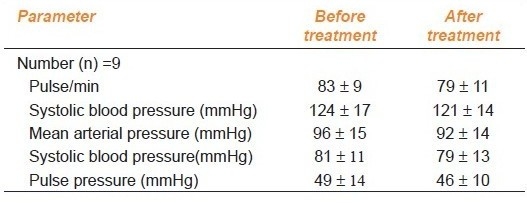
Table 4b.
Endothelial function parameters before and after atorvastatin in SLE patients
All the patients participated in the study showed good compliance with both the treatments and none of them developed any side effects.
Discussion
Epidemiological and clinical studies have shown that, microvascular diseases, in SLE, are responsible for cardiovascular mortality and morbidity. In the present study we assessed SI (a marker of arterial stiffness) and RI (a marker of small artery tone), to study the endothelial dysfunction. Endothelial dysfunction is one of the early markers of atherosclerosis that is observed in SLE.[20] We also observed increased arterial stiffness in patient groups with increased CRP as compared to that in normal subjects.
The previous studies also supported our results.[21,22] It is also found that systemic vasculitis has been reversed by the administration of immunosuppressive therapy.[20] It is also known that age,[7] height,[7] smoking,[8] alcoholism, diabetes,[23] systolic blood pressure,[5] diastolic blood pressure,[5] mean arterial pressure,[5] pulse pressure,[5] and markers of autoimmune disease, such as ANA,[24] CRP,[19] and MDA[18] had good correlation with raised arterial stiffness. This is significantly associated with an improvement of pulse pressure and endothelial function.
Endothelial derived nitric oxide contributes to the resting tone[24] of the arteries. Recent studies have demonstrated that inhibition of basal nitric oxide increases RI and SI, reflecting abnormalities of arterial wave reflections and arterial stiffness.[25] Further in endothelial dysfunction, there was premature inactivation of circulating inhibitors[26] of nitric oxide. Endothelial dysfunction has been reported in both resistance and conduit arteries in subjects with SLE.[27] In the present study, endothelial-dependent vasodilator response to salbutamol in patients with SLE was blunted as compared to that in controls, suggesting a possible endothelial dysfunction.
One of the possible causes for the endothelial dysfunction in our study group may be due to increased oxidative stress. Oxidative stress can promote the production of free radicals. These free radicals attack cell membrane bound lipids and result in the formation of lipid peroxidation products.[28] Increased lipid peroxidation in anticardiolipin-positive SLE patients[29] suggest that the presence of these antibodies can be associated with increased cardiovascular morbidity. In addition, the super oxide radical formed from as a result of oxidative stress, reacts with NO to yield hydroxyl and peroxyl radicals, which results in premature inactivation of NO, thereby decreasing the bioavailability of NO, also aggravation of endothelial dysfunction.[30]
In our study, we observed elevated levels of serum CRP (an indirect marker of oxidative stress) in SLE patients as compared to that in the controls. We also observed blunting of vasodilator response to salbutamol in patients with elevated CRP levels. Therefore, it is likely that endothelial dysfunction in our study group could be due to increased oxidative stress.
In particular, there was an improvement in the peripheral artery vasodilator endothelial function, as shown by the more percentage falls in the RI after treatment with NAC and statin to provocative administration of salbutamol. However, there was no significant percentage fall in RI or SI (a marker of large arterial stiffness) to shear stress test. This is further substantiated by our finding of a significant reduction in plasma MDA and CRP level in patients receiving (an indirect marker of oxidative stress) N-acetylcysteine and atorvastatin.
Ascorbic acid represents one of the most prominent antioxidants; exerting beneficial effects by inhibiting lipid peroxidation and by reducing endothelial dysfunction.[31] In one randomized controlled trial,[32] treatment with N-acetylcysteine significantly reduced cardiovascular events by 40% in the treated group compared with the healthy control group.
Conclusion
Our study involves the evaluation of arterial stiffness and estimation of RI and SI using well-established noninvasive device. We demonstrated increase in arterial stiffness in patients with SLE, which was associated with the presence of endothelial dysfunction. Salbutamol inhalation test, which demonstrates the endothelial dysfunction mainly of small and resistance vessels, was impaired in patients with SLE.
Administration of N-acetylcysteine, which is known to increase synthesis of glutathione, had antioxidant property in patients with SLE. This was associated with reduction in CRP and serum MDA levels, a marker of oxidative stress. Thus N-acetylcysteine administration improved endothelial dysfunction in patients with SLE.
Atorvastatin, a HMG-CoA reductase inhibitor, has antioxidant property associated with reduction in CRP, a marker of inflammation and thus causes improvement in endothelial function in patients with SLE. Thus our study has shown that N-acetylcysteine and atorvastatin reduce oxidative stress and improve endothelial function in patients with SLE.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Ingami T, Naruse M, Hoover R. Endothelium as an endocrine organ. Ann Rev Physiol. 1995;57:171–89. doi: 10.1146/annurev.ph.57.030195.001131. [DOI] [PubMed] [Google Scholar]
- 2.Anderson TJ, Gerhard MD, Meridith IT, Charbonneau F, Delegrange D, Creager MA, et al. Systemic nature of endothelial dysfunction in atherosclerosis. Am J Cardiol. 1995;75:71–4. doi: 10.1016/0002-9149(95)80017-m. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone MT, Creager SJ, Scales KM, Cusco JA, Creager MA. Impaired endothelium-dependant vasodilatation in patients with Insulin-dependent diabetes mellitus. Circulation. 1993;88:2510–6. doi: 10.1161/01.cir.88.6.2510. [DOI] [PubMed] [Google Scholar]
- 4.Panja JA, Quyyumi AA, Brush JE, Epstein SE. Abnormal endothelium-dependant vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–7. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan A, Mullins PA, Taylor G, Petch MC, Schofield PM. Both endothelium-dependant and endothelium-independent function is impaired in patients with angina pectoris and normal coronary angiograms. Eur Heart J. 1997;18:60–8. doi: 10.1093/oxfordjournals.eurheartj.a015119. [DOI] [PubMed] [Google Scholar]
- 6.Henning B, Chow CK. Lipid peroxidation and endothelial cell injury: Implications in atherosclerosis. Free Radic Biol Med. 1998;4:106–9. doi: 10.1016/0891-5849(88)90070-6. [DOI] [PubMed] [Google Scholar]
- 7.Gerhard M, Roddy MA, Creager SJ, Creager MA. Aging progressively impairs endothelial dependant vasodilatation in forearm resistance vessels of humans. Hypertension. 1996;27:849–53. doi: 10.1161/01.hyp.27.4.849. [DOI] [PubMed] [Google Scholar]
- 8.Cholon S, Moreno HJ, Benowitz NI, Hoffman BB, Blaschke TF. Nicotine impairs endothelial dependent vasodilatation in forearm resistance vessels of humans. Clin Pharmacol Ther. 2000;67:391–7. doi: 10.1067/mcp.2000.105153. [DOI] [PubMed] [Google Scholar]
- 9.Peterson TE, Poppa V, Ueba H, Wu A, Yan C, Berk CB. Opposing effects of reactive oxygen species and cholesterol on endothelial nitric oxide synthase and endothelial cell caveole. Circ Res. 1999;85:29–37. doi: 10.1161/01.res.85.1.29. [DOI] [PubMed] [Google Scholar]
- 10.Bruce IN, Gladman DD, Urowitz MB. Detection and modification of risk factors for coronary artery disease in patients with SLE. A quality improvement study. Clin Exp Rheumatol. 1998;16:435–40. [PubMed] [Google Scholar]
- 11.Bonetti PO, Lerman LO, Lerman A. Endothelial dysfunction: A marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol. 2003;23:168–75. doi: 10.1161/01.atv.0000051384.43104.fc. [DOI] [PubMed] [Google Scholar]
- 12.Ceriello A, Quatraro A, Caretta F, Varano R, Guigliano D. Evidence of possible role of oxygen free radicals in the abnormal functional arterial vasomotion in insulin-dependent diabetics. Diabetes Metab. 1990;16:318–22. [PubMed] [Google Scholar]
- 13.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of Systemic Lupus Erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 14.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of SLEDAI. A disease activity index for lupus patients. The committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35:630–40. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 15.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, et al. Nitric Oxide is responsible for flow-mediated dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91:1314–9. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 16.Dawes M, Chowienczyk PJ, Ritter JM. Effects of inhibition of the L-argnine/nitric oxide pathway on vasodilatation caused by beta-agonists in human forearm. Circulation. 1997;95:2293–7. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- 17.El-Magadmi M, Bodill H, Ahmad Y, Durrington PN, Mackness M, Walker M, et al. Systemic Lupus Erythematosus: An independent risk factor for endothelial dysfunction in women. Circulation. 2004;110:399–404. doi: 10.1161/01.CIR.0000136807.78534.50. [DOI] [PubMed] [Google Scholar]
- 18.Placer ZA, Crushman LL, Johnson BC. Estimation of products of lipid peroxidation (malondialdehyde) in biochemical systems. J Biol Chem. 1966;16:359. doi: 10.1016/0003-2697(66)90167-9. [DOI] [PubMed] [Google Scholar]
- 19.Koh KK, Schenke WH, Walclawiw MA, Csako G, Cannon RO., III Statin attenuates increase in C-reactive protein during estrogen replacement therapy in postmenopausal women. Circulation. 2002;105:1531–3. doi: 10.1161/01.cir.0000013837.81710.da. [DOI] [PubMed] [Google Scholar]
- 20.Heibel RH, O′Toole JD, Curtiss EI, Medsger TA, Jr, Reddy SP, Shaver JA. Coronary arteritis in Systemic Lupus Erythematosus. Chest. 1976;69:700–3. doi: 10.1378/chest.69.5.700. [DOI] [PubMed] [Google Scholar]
- 21.Scholze A, Rinder C, Beige J, Riezler R, Zidek W, Tepel M. Acetylcysteine Reduces Plasma Homocysteine concentration and improves pulse pressure and endothelial function in patients with end-stage renal failure. Circulation. 2004;109:369–74. doi: 10.1161/01.CIR.0000109492.65802.AD. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Perera O, Pérez-Sala D, Navarro-Antolín J, Sánchez-Pascuala R, Hernández G, Díaz C, et al. Effect of the 3-hydroxy-3methylglutaryl- Coa reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–9. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowienczyk PJ, Kelly RP, MacCallum H, Millasseau SC, Andersson TL, Gosling RG, et al. Photoplethysmographic assessment of pulse wave reflection: Blunted response to endothelial dependent beta-2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol. 1999;34:2007–14. doi: 10.1016/s0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- 24.Chan TM, Yu PM, Tsang KL, Cheng IK. Endothelial cell binding by human polyclonal anti-DNA antibodies: Relationship to disease activity and endothelial functional alterations. Clin Exp Immunol. 1995;100:506–13. doi: 10.1111/j.1365-2249.1995.tb03730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venugopal SK, Devaraj S, Yuhanna I, Shaul P, Jialal I. Demonstration that C-reactive protein decreases eNOS expression and bioactivity in human aortic endothelial cells. Circulation. 2002;106:1439–41. doi: 10.1161/01.cir.0000033116.22237.f9. [DOI] [PubMed] [Google Scholar]
- 26.Heinloth A, Heermeier K, Raff U, Wanner C, Galle J. Stimulation of NADPH oxidase by oxidized low-density lipoprotein induces proliferation of human vascular endothelial cells. J Am Soc Nephrol. 2000;11:1819–25. doi: 10.1681/ASN.V11101819. [DOI] [PubMed] [Google Scholar]
- 27.Guzik TJ, Mussa S, Gastaldi D, Sadowski J, Ratnatunga C, Pillai R, et al. Mechanisms of increased vascular superoxide production in humans: role of NAD(P)H oxidase and endothelial nitric oxide synthase. Circulation. 2002;105:1656–62. doi: 10.1161/01.cir.0000012748.58444.08. [DOI] [PubMed] [Google Scholar]
- 28.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 29.Munro R, Morrison E, McDonald AG, Hunter JA, Madhok R, Capell HA. Effect of disease modifying agents on the lipid profiles of patients with rheumatoid arthritis. Ann Rheum Dis. 1997;56:374–7. doi: 10.1136/ard.56.6.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Signorelli SS, Neri S, Di Pino L, Costa MP, Pennisi G, Digrandi D, et al. Oxidative stress and endothelial damage in patients with asymptomatic carotid atherosclerosis. Clin Exp Med. 2001;1:9–12. doi: 10.1007/s10238-001-8002-7. [DOI] [PubMed] [Google Scholar]
- 31.Halliwell B, Zaho K, Whiteman M. Nitric oxide and peroxyl nitrite. The ugly, the uglier and not the so good: A personal review of recent controversies. Free Radic Res. 1999;31:651–69. doi: 10.1080/10715769900301221. [DOI] [PubMed] [Google Scholar]
- 32.Tepel M, van der Giet M, Statz M, Jankowski J, Zidek W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: A randomized, controlled trial. Circulation. 2003;107:992–5. doi: 10.1161/01.cir.0000050628.11305.30. [DOI] [PubMed] [Google Scholar]


