Abstract
Passiflora foetida L. (Passifloraceae), a widely growing perennial climber, has been used in traditional medicine for treating many ailments. The objective of the present study was to evaluate the effects of ethanolic extract of P. foetida (EEPF) whole plant on gastric ulcer. The antiulcer effects of EEPF at 100 and 200 mg/kg doses were evaluated on ethanol and aspirin-induced gastric ulcer models. The antioxidant parameters and histological changes in gastric tissue of ulcer rats were also determined in both the models. P. foetida treatment significantly (P < 0.01) reduced the ulcer index and significantly (P < 0.01) increased the gastric pH of both ethanol and aspirin-induced ulcer rats. P. foetida showed significant (P < 0.01) reduction in lipid peroxidation and increase in reduced glutathione levels. The observations confirm that EEPF whole plant has antiulcer and antioxidant activities.
Keywords: Antioxidant, antiulcer activity, Passiflora foetida
Introduction
Gastric ulcer is a break in the tissue lining of the stomach. Most ulcers can be cured without complications; however, in some cases peptic ulcers can develop, such as in penetration, perforation, bleeding (hemorrhage), and obstruction.[1,2] Ethanol and aspirin-induced gastric ulcer models have been widely used for the evaluation of gastroprotective activity. Acute treatment with ethanol increases oxidative stress, DNA damage, xanthine oxidase activity and malondialdehyde levels, and decreases the total glutathione content in gastric mucosal cells.[3] Aspirin-induced ulcer is mediated through tissue damaging free radicals which are produced from the conversion of hydroperoxyl to hydroxy fatty acids, which leads to cell destruction.[4] It has been found that oxygen-derived free radicals are implicated in the mechanism of acute and chronic ulceration in the gastric mucosa and scavenging these free radicals can play an appreciable role in healing the ulcer.[5] Before the introduction of potent antiulcerogenic agents, i.e., H2-receptor antagonist, proton pump inhibitors, etc., plant remedies were widely employed for the treatment of various symptoms of peptic ulcer. There has been renewed interest in identifying new antiulcer drugs from natural sources. Passiflora foetida L. (Passifloraceae), commonly known as passion fruit, is an exotic fast growing perennial climber occurring in USA and extended to India. Traditionally, the plant has been used for its properties like antiproliferative, sedative, anti-anxiety, antibacterial, leishmanicidal, antispasmodic, emetic, dressing for wounds and antiulcer.[6–8] In this preliminary study, an attempt has been made to evaluate the effects of P. foetida whole plant on experimentally induced gastric ulcers.
Materials and Methods
Extraction and Qualitative Chemical Evaluation
The plants were collected from Azhagar kovil, and were identified and authenticated by Dr. D. Stephen, Taxonomist, American College, Madurai. About 300 g of the plant powder was extracted with 1.5 l of ethanol at room temperature for 7 days and the mixture was stirred every 24 h.[8] The ethanolic extract of P. foetida (EEPF) was evaporated (at 40°C) and the sticky blackish green substance (3.73%) was subjected to qualitative chemical tests to evaluate the various phytoconstituents.[9]
Animals
Wister rats of either sex (150–200 g) were properly housed in separate cages at controlled room temperature (24 ± 2°C; relative humidity 60–70%) in a 12-h light dark cycle. They were fed with standard pellet diet and water ad libitum. The IAEC of Ultra College of Pharmacy permitted this pharmacological study (approval no. UCP/IAEC/ 2008/030).
Antiulcer Activity
Ulcer induced by absolute ethanol
The rats were divided into four groups of six each. Group I (toxicant control) received absolute ethanol (1 ml/animal); Group II was treated with ranitidine (100 mg/kg); Groups III and IV were treated with EEPF 100 and 200 mg/kg, respectively.[10] The rats were fasted for 24 h and they received 1 ml of absolute ethanol orally. Standard and test drugs were administered orally 30 min before the ethanol dose. The animals were sacrificed after 1 h of ulcerogen administration, and their stomachs were excised and the gastric contents were aspirated. The contents were subjected to centrifugation at 1000 rpm for 10 min and then analyzed for pH (digital pH meter). The stomachs were washed with normal saline and kept in 10% formalin for the determination of ulcer index and histological studies.[11,12]
Ulcer index is determined as follows:
Ulcer index = 10/x, where “x” is total mucosal area/total ulcerated area.
Ulcer induced by aspirin
The rats were divided into four groups of six each like the absolute ethanol-induced ulcer models, but the control and drug-treated animals received aspirin (100 mg/kg p.o.) instead of ethanol. The rats were fasted for 24 h with free access to water, the standard and test drugs were administered orally 30 min before the aspirin dose.[13] The animals were sacrificed after 5 h of ulcerogen administration and their stomachs were excised, then the procedure followed was similar to that for absolute ethanol-induced ulcer model.
Histopathological Evaluation
After the standard processing, the ulcerated gastric tissues were examined under the microscope for histopathological changes such as inflammation, infiltration, and erosion.[5]
Antioxidant Activity
The lipid peroxides (LPOs) of stomach mucosa were determined indirectly by thiobarbituric acid reactive substances (TBARS) formation. Reduced glutathione concentration was read off a standard curve and expressed as μg GSH/g of wet tissue. Alkaline phosphatase activity was assayed using the method of Kind and King.[14–16]
Statistical Analysis
The treated groups were compared with the toxicant group; results were expressed as a mean ± SD of six animals in each group. The results were analyzed statistically using one-way analysis of variance (ANOVA) followed by Dunnett's test. P < 0.05 when compared with toxicant group was considered as significant.
Results
The phytochemical screening of the EEPF showed the presence of alkaloids, phenols, carbohydrates, proteins, phytosterols, phenolic compounds, tannins, and flavonoids. In ethanol-induced ulcer model, EEPF significantly (P < 0.01) reduced the ulcer index at 100 and 200 mg/kg doses to 64.30 and 71.66%, respectively. In aspirin-induced ulcer model, the EEPF treatment at 100 and 200 mg/kg significantly (P < 0.01) inhibited the ulcer index to 67.83 and 79.45%, respectively, when compared with toxicant control rats. The EEPF also significantly (P < 0.01) increased gastric pH of ulcer rats [Table 1]. In both the ulcer models, EEPF (100 and 200 mg/kg) significantly (P < 0.01) reduced the lipid peroxidation and alkaline phosphatase levels when compared to toxicant group. The results also show that EEPF significantly (P < 0.01) increased the reduced glutathione levels [Table 2]. Histopathological evaluation of alcohol and aspirin-induced ulcer models showed perforated ulcer, deep ulceration of granular epithelium and loss of the histological structure, almost reducing the submucosa. EEPF at 100 mg/kg showed partial healing of ulcer with few inflammatory cells and at 200 mg/kg showed the healed ulcer, normal mucosa, and no inflammatory cells [Figures 1 and 2].
Table 1.
Effect of Passiflora foetida L. on pH and ulcer index of absolute ethanol and aspirin-induced ulcer models
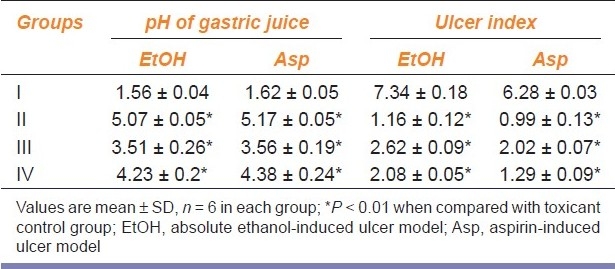
Table 2.
Antioxidant effect of Passiflora foetida L. on absolute ethanol and aspirin-induced ulcer models
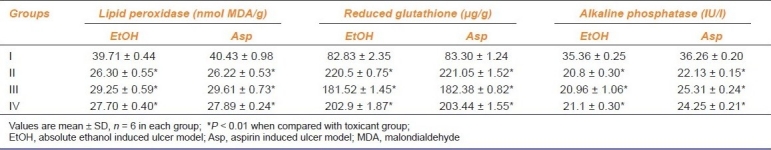
Figure 1.
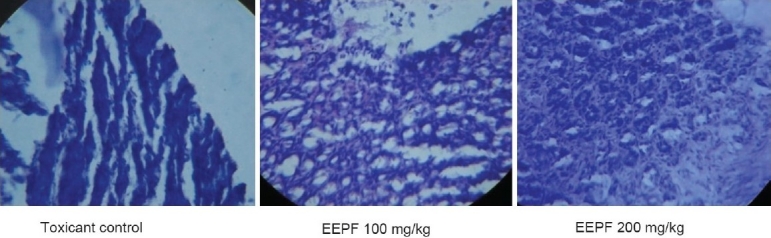
Histopathology of stomach of absolute ethanol-induced ulcer models
Figure 2.
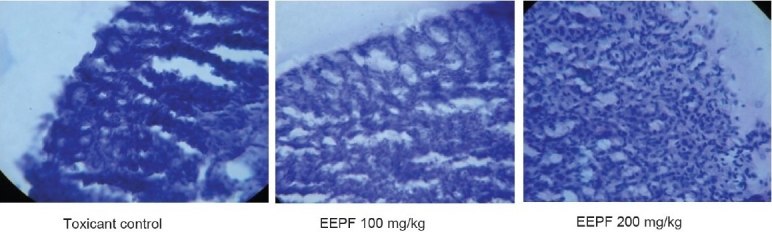
Histopathology of the stomach of aspirin-induced ulcer models
Discussion
The genesis of ethanol-induced gastric lesions is multifactorial with the depletion of gastric wall mucous content as one of the involved factors. It is also associated with significant production of free radicals, leading to an increased oxidative stress and damage to the cell and cell membrane.[17] Aspirin causes a dose-dependent reduction in mucosal prostaglandin E2 and prostaglandin I2 biosynthesis accompanied by an increase in the mean of gastric ulceration. It is therefore reasonable to assume that the observed gastric mucosal lesion induced by aspirin is due to deficiency of mucosal prostaglandin.[18] The present study reveals that both the doses of EEPF significantly (P < 0.01) reduced the ulcer index and increased the gastric pH of ethanol and aspirin-induced ulcer models. Lipid peroxidation is a free radical mediated process, which has been implicated in a variety of disease states. It involves the formation and propagation of free radicals, the uptake of oxygen and rearrangement of double bond in unsaturated lipids, which eventually result in destruction of membrane lipids. The release of alkaline phosphatase enzyme has been suggested to play a role in tissue necrosis associated with various models of gastrointestinal ulceration such as absolute alcohol and aspirin-induced ulcer. Increased activity of this enzyme may be found in damaged tissues.[16] EEPF at both the dose levels significantly (P < 0.01) reduced the LPOs and alkaline phosphatase levels of ulcer animals. Antioxidant enzymes such as glutathione are present in oxygen handling cells, which are the first line of cellular defense against oxidative injury. They decrease the gastric mucosal damaging effect of absolute alcohol and aspirin. There was a significant (P < 0.01) increase of glutathione activity in EEPF pretreated rats. Hence, the antioxidant activity of EEPF may be one of the important defensive factors involved in its antiulcer effect. The present study reveals that P. foetida exhibits both antiulcer and antioxidant properties.
Conclusion
To conclude, the ethanolic extract of P. foetida Linn. has been found to have antiulcerogenic effect, which could be related to its antioxidant potential. More work is required for a clear understanding of the mechanism of action with chemically identified active principle.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Kochman ML, Elta GH. Gastric ulcers - when is enough, enough. Gastroenterology. 1993;105:1583. [PubMed] [Google Scholar]
- 2.Chan FK, Leung WK. Peptic ulcer disease. Lancet. 2002;360:933–41. doi: 10.1016/s0140-6736(02)11030-0. [DOI] [PubMed] [Google Scholar]
- 3.Cola Miranda M, Barbastefano V, Hiruma-Lima CA, Calvo TR, Vilegas W, Brito AR. Antiulcerogenic activity of indigofera truxillensis kunth. Biota Neotrop. 2006;6:3. [Google Scholar]
- 4.Sannomiya M, Fonseca VB, Dasilva MA, Rocha LR, Dossantos LC, Hiruma-Lima CA, et al. Flavonoids and antiulcerogenic activity from Brysonima crassa leaves extracts. J Ethnopharmacol. 2005;97:1–6. doi: 10.1016/j.jep.2004.09.053. [DOI] [PubMed] [Google Scholar]
- 5.Umamaheshwari M, Asokkumar K, Rathidevi R, Sivashanmugam AT, Subhadra DV, Ravi TK. Antiulcer and in vitro antioxidant activities of Jasminum grandiflorum L. J Ethnopharmacol. 2007;110:464–70. doi: 10.1016/j.jep.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal M, editor. Austin, Texas: American Botanical Council, America; 1997. Klein, trans.German Commission Therapeutic Monographs on Medicinal Herbs for Human Use. [Google Scholar]
- 7.Nadkarni KM. Indian Materia Medica. 1996;1:924. [Google Scholar]
- 8.Mohanasundari C, Natarajan D, Srinivasan K, Umamaheshwari S, Ramachandran A. Antibacterial properties of Passiflora foetida a common exotic medicinal plant. Afr J Biotechnol. 2007;6:2650–3. [Google Scholar]
- 9.Khandelwal KR. 11th ed. Pune: Nirali Prakashan; 2004. Practical Pharmacognosy; pp. 149–56. [Google Scholar]
- 10.Anandan R, Jayakar B, Jeganathan S, Manavalan R, Senthilkumar R. Effect of ethanol extract of fruits of Passiflora foetida Linn.on CCl4 induced hepatic injury in rats. J Pharm Res. 2009;2:413–5. [Google Scholar]
- 11.Morimoto M, Shimohara K, Oshima S, Sukamoto T. Effects of the new anti ulcer agent KB-5492 on experimental gastric mucosal lesions and gastric mucosal defensive factors, as compared to those of teprenone and cimitidine. Jpn J Pharmacol. 1991;57:495–505. doi: 10.1254/jjp.57.495. [DOI] [PubMed] [Google Scholar]
- 12.Sing R, Madan J, Rao HS. Anti ulcer activity of black pepper against absolute ethanol induced gastric mucosal damage in mice. Pharmacog Mag. 2008;4:232–5. [Google Scholar]
- 13.Muniappan M, Sundarraj T. Anti-inflammatory and antiulcer activites of Bambusa arundinacea. J Ethnopharmacol. 2003;88:161–7. doi: 10.1016/s0378-8741(03)00183-1. [DOI] [PubMed] [Google Scholar]
- 14.Khalil H, Ismail H, Taye A, Kamel M. Gastroprotective effect of Lippa nodiflora L. extracts in ethanol induced gastric lesions. Pharmacog Mag. 2007;3:973–1296. [Google Scholar]
- 15.Sedlak J, Lindsay RH. Estimation of total protein- bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:1192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 16.Kind PR, King EJ. Determination of serum alkaline phosphatase. Clin Path. 1954;7:322. doi: 10.1136/jcp.7.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Narayan S, Devi RS, Jainu M, Sabitha KE, Shyamala DC. Protective effect of a polyherbal drug ambrex in ethanol induced gastric mucosal lesions in experimental rats. Indian J Pharmacol. 2003;36:34–7. [Google Scholar]
- 18.Ologundudu A, Lawal AO. The antiulcerogenic activity of aqueous extract of Carica papaya fruits on aspirin induced ulcer in rats. Int J Toxicol. 2008;5:2. [Google Scholar]


