Abstract
GD25 cells lacking β1 integrins or expressing β1A with mutations of conserved cytoplasmic tyrosines (Y783, Y795) to phenylalanine have poor directed migration to platelet-derived growth factor or lysophosphatidic acid when compared with GD25 cells expressing wild-type β1A. We studied the effects of v-src on these cells. Transformation with v-src caused tyrosine and serine phosphorylation of wild-type β1A but not of Y783/795F doubly mutated β1A. v-src-transformed cells had rounded and/or fusiform morphology and poor assembly of fibronectin matrix. Adhesion to fibronectin or laminin and coupling of focal contacts to actin-containing cytoskeleton were preserved in transformed Y783/795F cells but lost on transformation when β1A was wild type. Transformed Y783/795F cells also retained ability, albeit limited, to migrate across filters, whereas transformed cells with wild-type β1A were unable to transverse filters. Studies of single tyrosine mutants showed that the more important tyrosine for retaining ability to adhere, assemble focal contacts, and migrate is Y783. These results suggest that overactive phosphorylation of cytoplasmic residues of β1A, particularly Y783, accounts in part for the phenotype of v-src-transformed cells.
Rous sarcoma virus (RSV) is the first known tumor virus, recognized for its ability to cause sarcomas in chickens (1). The transforming protein of RSV, v-src, is a cytoplasmic tyrosine kinase with constitutive activity (2, 3). v-src-transformed fibroblasts exhibit several properties related to cell adhesion, including morphological changes, increased growth rate, loss of anchorage dependence of growth, loss of focal contacts, and decreased synthesis and deposition of extracellular matrix (3–7). v-src causes extensive protein tyrosine phosphorylation of cellular proteins (3, 8), including tyrosines in the NPXY motifs of the cytoplasmic domain of the β1A integrin subunit (9–11). The role of phosphorylation of β1 integrins in transformation by v-src is not known. We have studied the effects of v-src on mouse GD25 cells lacking β1A (12) or expressing the β1A splice variant of β1 (13) or β1A with Tyr-to-Phe mutations in the NPXY motifs [Y783F, Y795F, and Y783/795F cells (14)]. The results suggest that overactive phosphorylation of cytoplasmic residues of β1A, particularly Y783, contributes to the transformed phenotype of v-src-transformed cells.
Materials and Methods
Cells.
Wild-type β1A (13) or β1As with mutations of the cytoplasmic domain (14) were expressed in the β1-null GD25 fibroblastic cell line as described. It should be noted that, after differentiation from β1-null stem cells, the GD25 clone had been immortalized with simian virus 40 large T antigen (12). v-src was expressed in cells by cotransfecting with pZeoSV2 (Invitrogen) and pMv-src (15), in which expression of v-src is driven by the long terminal repeats of Moloney mouse leukemia virus. The cotransfection was accomplished with Lipofectamine (GIBCO/BRL). Selection was performed with 300 μg/ml Zeocin (Invitrogen). After 2–3 weeks, surviving clones were isolated and expanded. Clones were monitored for morphology by phase microscopy and for protein tyrosine phosphorylation and expression of Src by Western blotting. Two separate clones of each v-src-transformed cell type were analyzed. The experiments and photomicrographs shown were chosen as representative of differences constantly observed among the different cells. 3T3 cells transformed with v-src were prepared as described (15).
Assays.
Cell adhesion assays, flow cytometry, and fluorescence microscopic studies were performed as previously described (14). For assembly of exogenous fibronectin, cells were incubated with FITC-labeled fibronectin in the presence of 1-oleoyl-lysophosphatidic acid (LPA) (Avanti Polar Lipids) for 1 h at 37°C, beginning 4 h after culturing on defined substrate in medium with 0.2% fatty acid free albumin. For phosphotyrosine localization with the PY20 monoclonal antibody (Transduction Laboratories, Lexington, KY), 1 mM sodium orthovanadate was present throughout the fixation, permeabilization, and immunofluorescence staining steps. Western blotting was performed as previously described (16). Monoclonal antibodies against Src (clone 327) and phosphotyrosine (clone 4G10) were from Oncogene Science and Upstate Biotechnology (Lake Placid, NY), respectively. Antibody MB1.2 to mouse β1 was generously provided by B. Chan (University of Western Ontario, London, ON, Canada) or purchased from Chemicon. Rabbit polyclonal antibodies against cytoplasmic domain of human β1A were generously provided by E. Ruoslahti (Burnham Institute, La Jolla, CA).
Metabolic Labeling and Immunoprecipitation.
Cells were metabolically labeled with [32P]orthophosphate (1.5 mCi/ml medium) (Amersham) for 5 h in the presence of 100 μM sodium orthovanadate in phosphate-free medium. Immunoprecipitation of β1 from labeled cells was performed as described (9) with slight modifications. Briefly, cells were incubated on ice in lysis buffer containing 100 mM sodium chloride, 2 mM EDTA, 1 mM sodium orthovanadate, 0.5 mM sodium molybdate, protease inhibitor mixture (Boehringer Mannheim), 10 mM Tris⋅HCl (pH 7.6), and 0.5% (wt/vol) 3-[(3-cholamidopropyl)dimethylammonio]-1-peopanesulfonate. After centrifugation, the supernatants were precleared with agarose beads coupled with antibodies to rat IgG and IgM (American Qualex, La Mirada, CA) and subsequently incubated with rat MB1.2 antibody against mouse β1. Immune complexes were collected with the anti-rat Ig beads. For deglycosylation with peptide/N-glycosidase F (PNGaseF) (New England Biolabs), the samples were treated with the manufacturer's denaturing buffer at 100°C for 10 min, and one-tenth volume each of 10% Nonidet P-40 and 10× reaction buffer were added. Samples were incubated for 1 h at 37°C after addition of 1,000 units PNGaseF. Proteins were analyzed by SDS/PAGE. The gel was fixed and dried or bands were transferred onto poly(vinylidene difluoride) (PVDF) membranes (Amersham Pharmacia). Gel or membrane was subjected to autoradiography to detect phosphorylated proteins. Phosphoamino acid analysis of acid-hydrolyzed phosphorylated proteins excised from the PVDF membranes was performed as described previously (17, 18).
Cell Migration.
Cell migration assays were performed in modified Boyden chambers containing Nucleopore polycarbonate membranes (5- or 8-μm pore size; Costar) (14, 16). The filters were soaked overnight in 10 μg/ml fibronectin rinsed with PBS containing 0.2% (wt/vol) fatty acid free albumin, air dried, and then placed in the chamber. Platelet-derived growth factor or LPA in medium containing 0.2% fatty-acid free albumin was added to the lower compartment of the chambers. Cells suspended in DMEM containing 0.2% fatty-acid free albumin were introduced into the upper compartment. The chambers were then incubated for 6 or 16 h at 37°C. The filters were fixed and stained, and the cells that had migrated to the lower surface were counted at ×400. In each experiment, two areas from each of two wells were counted. Values are the mean ± SD of cells per 0.16-mm2 field.
Results
General Characterization of v-src-Transformed Cells.
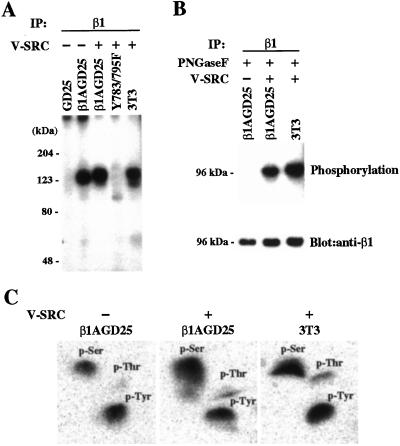
All v-src-transfectants had comparably increased expression of Src when compared with nontransfected parental cells and increased phosphorylation of many cellular proteins as assessed by immunoblotting of cell extracts with anti-Src and antiphosphotyrosine, respectively (not shown). All transfectants proliferated more rapidly than the parental cells, lacked contact inhibition, and grew past confluence as aggregates of cells that were loosely associated with the culture dish or floated free in the medium (not shown). Flow cytometry demonstrated that expression of cell-surface β1A did not change significantly after v-src transformation (not shown). Multiple attempts to demonstrate reactivity of wild-type β1 with antiphosphotyrosine in extracts of v-src-transformed β1A-GD25 failed, by using either immunoprecipitation with anti-β1 followed by immunoblotting with antiphosphotyrosine or vice versa. However, immunoprecipitation with anti-β1 after metabolic labeling with [32P]orthophosphate yielded phosphorylated proteins in β1A-GD25 and v-src-transformed β1A-GD25 and 3T3 cells but not in β1A-deficient GD25 cells or v-src-transformed Y783/795F cells (Fig. 1A). The phosphorylated protein in nontransformed β1A-GD25 cells was 123 kDa, 5 kDa smaller than the phosphorylated protein with an apparent mass of 128 kDa in v-src-transformed β1A-GD25 and 3T3 cells. The phosphorylated protein in nontransformed β1A-GD25 cells was also smaller than the band in the same lane immunoblotted with anti-β1, whereas the phosphorylated protein in v-src-transformed Y7831795F and 3T3 cells comigrated with immunoblotted β1 (not shown). To test further whether the phosphorylated band in nontransformed β1A-GD25 cells was β1A, we compared the immunoprecipitated phosphorylated proteins after removal of carbohydrate with PNGaseF (19). Phosphorylated proteins in v-src-transformed β1A-GD25 cells and 3T3 cells were degraded to apparent masses of 96 kDa after deglycosylation and were identical to deglycosylated β1 detected by immunoblotting (Fig. 1B). In contrast, a phosphorylated protein of 96 kDa was not found in the PNGaseF-treated immunoprecipitate of β1A-GD25 cells, although the 96-kDa band was detected with anti-β1. Thus, we conclude that the phosphorylated 123-kDa protein in β1A-GD25 cells is not β1A but a protein that associates with β1A in nontransformed cells. The identity of this protein is at present not known. Phosphoamino acid analysis revealed that phosphorylated β1A in v-src-transformed β1A-GD25 cells and 3T3 cells contained an approximately equivalent amount of phosphotyrosine and phosphoserine and a lesser amount of phosphothreonine (Fig. 1C). The 123-kDa phosphorylated protein that coimmunoprecipitated with β1A from nontransformed β1A-GD25 cells also was phosphorylated on tyrosine and serine (Fig. 1C).
Figure 1.
Phosphorylation of β1 integrin. (A) Autoradiogram of SDS/PAGE gel of [32P]orthophosphate-labeled proteins immunoprecipitated with monoclonal antibody MB1.2 to β1 from lysates of metabolically labeled nontransformed GD25 cells lacking β1A, nontransformed β1A-GD25 cells, v-src-transformed β1A-GD25 cells, v-src-transformed Y783/795F cells, and v-src-transformed 3T3 cells. Samples were reduced. The positions of the molecular mass markers are indicated (Left). (B) (Upper) Autoradiogram of poly(vinylidene difluoride) membrane of immunoprecipitates from lysates of nontransformed β1A-GD25 cells and v-src-transformed β1A-GD25 cells and 3T3 cells after PNGaseF treatment. (Lower) The same membrane immunoblotted with rabbit antibody against cytoplasmic domain of β1A. The positions of the molecular mass markers are indicated (Left). (C) Phosphoamino acid analysis of the bands of 123–128 kDa immunoprecipitated from nontransformed β1A-GD25 and v-src-transformed β1A-GD25 and 3T3 cells shown in A. Shown are phosphorimages of two-dimensional TLC plates. Positions of unlabeled phosphotyrosine, phosphoserine, and phosphothreonine, detected with ninhydrin, are indicated.
Cell Adhesion.
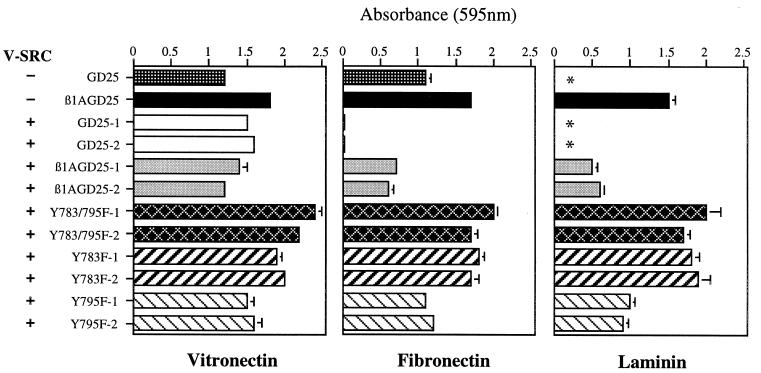
Nontransformed GD25 and β1A-GD25 cells (Fig. 2) and Y783F, Y795F, and Y783/795F cells (not shown; see ref. 14) all adhered to vitronectin or fibronectin after 1 h, probably through the αVβ3 integrin for GD25 cells and a combination of αVβ3 and α5β1A for the other cells (13, 14). GD25 cells, which lack α6β1 (13, 14), did not adhere to laminin-1, whereas cells expressing wild-type or mutant β1A did (Fig. 2; not shown). After transformation with v-src, GD25, β1A-GD25, Y783F, Y795F, and Y783/795F cells all adhered to vitronectin (Fig. 2). Transformation with v-src caused GD25 and β1A-GD25 cells to lose the ability to adhere to fibronectin, nearly completely in the case of GD25 cells (Fig. 2). β1A-GD25 cells also adhered less well to laminin-1 after transformation (Fig. 2). Adhesion of v-src-transformed Y795F cells to fibronectin or laminin-1 was also less compared with nontransformed Y795F cells (Fig. 2 and data not shown). v-src-transformed Y783/795F and Y783F cells, in contrast, adhered as well to fibronectin or laminin-1 as nontransformed counterparts (Fig. 2 and data not shown).
Figure 2.
Attachment of v-src-transformed GD25 cells expressing no β1A, wild-type β1A, or mutant β1A on vitronectin, fibronectin, or laminin-1. Bars represent the mean of attachment activity quantified by spectrophotometric analysis at 595 nm after staining of adherent cells with bromophenol blue. Error bars represent ± SD of quadruplicate experiments. Absorbance because of nonspecific cell adhesion as measured on albumin-coated wells was less than 0.1 and has been subtracted. Two different clones in each v-src-transformed mutant (clones 1 and 2) were analyzed. GD25, β1-deficient cells; β1GD25, GD25 cells expressing wild-type B1A; other cells are designated by mutation(s). *, β1A-deficient GD25 cells had no detectable adherence to laminin-1.
Assembly of Fibronectin Matrix.
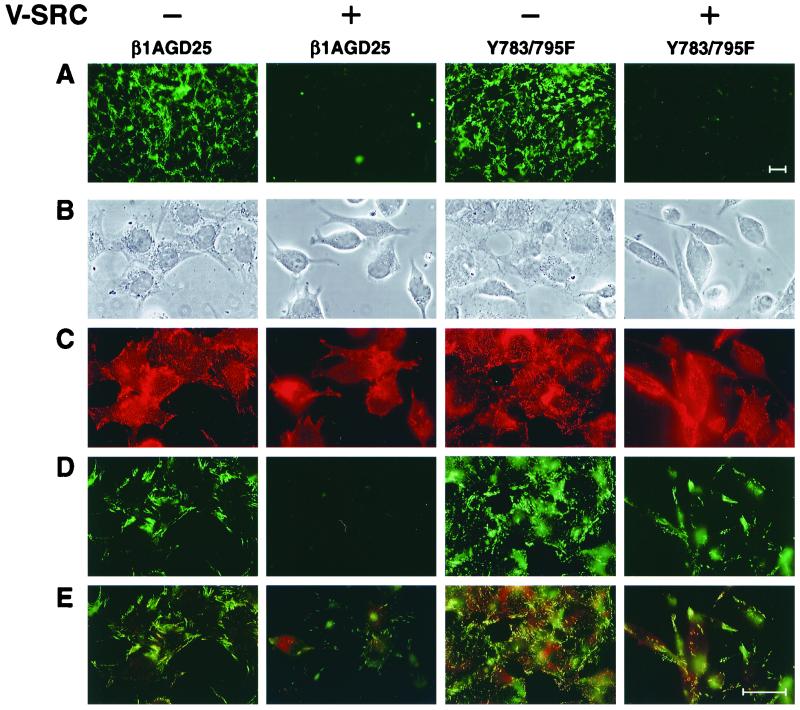
v-src transformation is known to cause a marked loss of fibronectin matrix by a variety of cell types (20). After 3 days in 10% FBS, no deposited fibrils of fibronectin could be detected by immunofluorescence staining of v-src-transformed β1A-GD25 or Y783/795F cells, whereas nontransformed cells had widespread deposition of fibronectin in a fibrillar network (Fig. 3A).
Figure 3.
Deposition of fibronectin in 3-day culture and colocalization of exogenous fibronectin and β1A in short-term assay in nontransformed and v-src-transformed β1A-GD25 or Y783/795F cells. (A) Immunofluorescence of fibronectin matrix after 3 days of culture in serum-containing medium. (B–E) Assembly of FITC-fibronectin during a 1-h period beginning 4 h after seeding of cells on surfaces coated with fibronectin. After fixation, staining for β1 integrin was performed by using antibody MB1.2 and lissamine rhodamine-labeled anti-rat IgG. The same field was photographed for cellular morphology by phase (B), β1A (C), FITC-fibronectin (D), and double fluorescence of β1A (red) and FITC-fibronectin (green) (E). (Bar = 35 μm.)
We sought to relate loss of a fibronectin matrix to shape of cells, localization of β1A, and binding of FITC-fibronectin in a short-term assay (Fig. 3 B–E). v-src-transformed cells adherent to fibronectin were refractile and usually bipolar, in contrast to their untransformed counterparts, which were flatter, less refractile, and often polygonal in shape (Fig. 3B). Compared with the numerous punctate focal contacts staining for β1 in nontransformed β1A-GD25 and Y783/795F cells, there was less frequent punctate staining of β1 in v-src-transformed Y783/795F cells, usually in association with long polar extensions, and no punctate staining of β1A in transformed β1A-GD25 cells (Fig. 3C; also see Fig. 4). Similar results were obtained with cells cultured on laminin-1 or vitronectin (not shown). Nontransformed β1A-GD25 and Y783/795F cells grown on coverslips coated with fibronectin (Fig. 3E) or laminin-1 (not shown) assembled numerous short fluorescent fibrils of FITC-labeled plasma fibronectin over a 1-hour period, often colocalizing with β1A (14). v-src-transformed β1A-GD25 cells did not assemble fibronectin when cultured on fibronectin (Fig. 3D) or laminin-1 (not shown). Short thin fibrils of FITC-fibronectin were assembled at cell edges of v-src-transformed Y783/795F cells on fibronectin-coated (Fig. 3D) or laminin-1-coated (not shown) substrates, although assembly was decreased when compared with nontransfected parental cells. In the transient assay, v-src-transformed Y795F cells, like v-src-transformed β1A-GD25 cells, did not assemble fibronectin, whereas transformed Y783F cells assembled short, thin fibrils of fibronectin like transformed Y783/795F cells (not shown). β1A in punctate focal contacts of transformed Y783/795F cells was associated, in part, with fibronectin fibrils (Fig. 3E). We previously found that expression of wild-type or tyrosine-mutated β1A overcomes the suppression of fibronectin matrix assembly when GD25 cells are cultured on vitronectin (21). However, none of the v-src-transformed cells, including Y783/795F cells, assembled FITC-fibronectin when cultured on vitronectin (not shown).
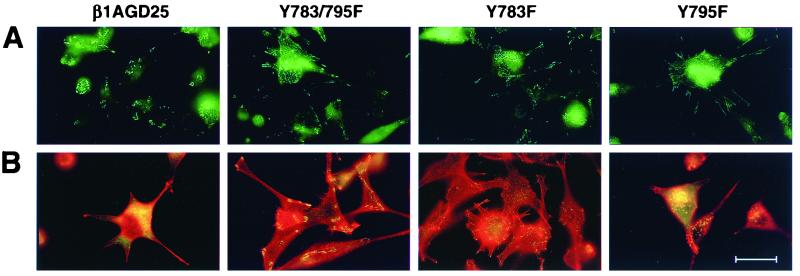
Figure 4.
Visualization of focal contacts and associated structures in v-src-transformed β1A-GD25 or mutant β1A cells on fibronectin-coated substrata. (A) Single immunofluorescent detection of β1A. (B) Double immunofluorescent detection of vinculin (green) and rhodamine-phalloidin (red). β1A-GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s). Microscopic analysis of multiple cells in multiple fields indicated the β1- and vinculin-containing focal contacts were present in >40% of transformed Y783F and Y783/795F cells but in <5% of β1A cells and <20% of Y795F cells. (Bar = 30 μm.)
Focal Contacts and Actin Cytoskeleton.
As described in ref. 14, nontransformed β1A-GD25, Y783/795F, Y783F, and Y795F cells all have a well-developed F-actin-containing cables that terminate in β1A- and vinculin-containing focal contacts. In v-src-transformed GD25 cells expressing wild-type or Y795F mutant β1A, β1A was present in circular podosomes, as reported previously for a variety of src-transformed cells (4, 11, 22, 23) (Fig. 4A). Actin-containing stress fibers and vinculin-containing focal contacts were largely absent from v-src transformed β1A-GD25 and Y795F cells cultured on fibronectin (Fig. 4B) or laminin-1 (not shown). In v-src-transformed Y783/795F or Y783F cells adherent on fibronectin (Fig. 4) or laminin-1 (not shown), some β1A- and vinculin-containing focal contacts were retained, especially at the ends of the long cellular extensions; fine F-actin networks were also present although much less developed than in nontransformed parental cells. Phosphotyrosine-containing proteins also could be demonstrated in focal contacts of v-src-transformed Y783/795F or Y783F cells (not shown). Phosphotyrosine-containing proteins in v-src-transformed β1A-GD25 cells localized to podosomes (not shown). On a vitronectin-coated substratum, anti-vinculin localized to podosomes in all v-src-transformed cells, including Y783/795F and Y783F cells, and no focal β1 could be detected (not shown).
Cell Migration.
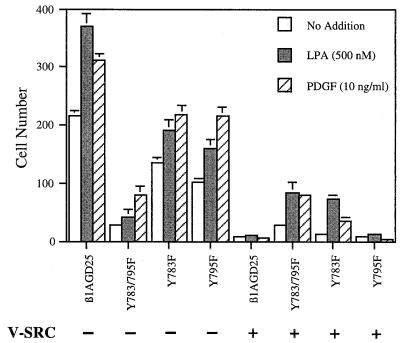
v-src transformation causes increased ruffling of the plasma membrane in vitro and increased metastatic capacity in vivo (5, 6) but also results in loss of directed cell migration in a concentration gradient (chemotaxis) (24). Chemotaxis requires the fine control of cellular association with and release from the extracellular matrix; integrins play a central role in such associative and dissociative events (25–27). Chemotaxis was assayed as number of cells passing through filters with 5-μm pores coated with fibronectin (Fig. 5). As reported (14, 16), nontransformed β1A-GD25 cells migrated more efficiently across filters in response to platelet-derived growth factor or LPA than Y783/795F cells, whereas Y783F and Y795F cells had intermediate migration (Fig. 5). v-src-transformation caused loss of the migratory ability of β1A-GD25 and Y795F cells (Fig. 5). In contrast, v-src-transformed Y783/795F retained fully the limited ability to migrate, and migration of transformed Y783F, although diminished, was not lost completely (Fig. 5). Similar differences between v-src-transformed β1A-GD25 or Y795F cells, and v-src-transformed Y783/795F or Y783F cells were found when filters with 8-μm diameter pores (rather than 5-μm diameter pores) were tested (not shown).
Figure 5.
Cell migration through fibronectin-coated 5-μm filters in response to LPA or platelet-derived growth factor (PDGF). LPA (500 nM) or PDGF (10 ng/ml) was in the lower chamber. Each represents the mean of cell number per 0.16-mm2 field. Error bars indicate ± SD of quadruplicate determinations. β1A-GD25, GD25 cells expressing wild-type β1A; other cells are designated by mutation(s).
Discussion
Src-type kinases are known to participate in integrin-mediated signaling (28), suggesting that tyrosine phosphorylation of β1A may be important for function and dysregulated on transformation with v-src. Our findings demonstrate that the Y783F mutant of β1A, which changes the phenol side chain in the first cytoplasmic NPXY motif to a benzyl side chain that cannot be phosphorylated, allows v-src-transformed GD25 cells to preserve some phenotypic features of nontransformed cells. GD25 cells expressing β1A with the Y783F mutation without or with the Y795F mutation on transformation with v-src retained β1-dependent cell adhesion and a semblance of focal contact formation and actin cytoskeletal organization that were otherwise lost in cells expressing wild-type β1A or β1A with the Y795F mutation. Transformed cells with the Y783F mutation also retained the ability to bind fibronectin in short-term assays, although this fibronectin could not be retained in a fibrillar network. In the chemotaxis assay, transformed cells with Y783F or Y783/795F mutant β1A migrated, whereas transformed cells with wild-type or Y795F mutant β1A did not.
v-src phosphorylates Y783 in vitro in the context of both a synthetic β1-peptide containing Y783 and intact β1 (10). These findings indicate that tyrosines of β1A cytoplasmic domain are potentially direct substrates for v-src in v-src-transformed cells. Phosphorylated β1 integrins interact less well with fibronectin via integrin extracellular domains or with talin via intracellular domains (10). Tyrosine phosphorylation of β1 also increases affinity for the SH2 domain of PI-3-kinase (11), which is a substrate of v-src and may regulate actin filament rearrangement on activation (29). Thus, presence or absence of overactive phosphorylation of Y783 may explain findings of loss of adhesivity, formation of focal contacts and F-actin bundles, and directed migration in v-src-transformed cells expressing wild-type β1A and preservation of such functions in v-src-transformed cells expressing β1A with the Y783F mutation.
In the absence of v-src, cells expressing β1A with the Y783F mutation have more focal contacts and a tendency to assemble more fibronectin than cells expressing wild-type β1A (14). These results and the finding that β1A-integrins with the dual Y783/795F mutation are defective in activation of focal adhesion kinase (30) indicate that phosphorylation of Y783 is functionally important in untransformed cells. Similar double Tyr-to-Phe mutations in the mouse gene for β3 result in attenuated outside-in signaling by αΙΙbβ3 and platelet function (31).
β1 in v-src-transformed and vanadate-treated β1A-GD25 or 3T3 cells was phosphorylated on serine as well as on tyrosine. A similar phosphoamino acid pattern was reported previously for integrins immunoprecipitated from src-transformed chick embryonic fibroblasts with the CSAT monoclonal antibody (9). S785, two residues downstream from Y783, has been demonstrated to be phosphorylated in F9 stem cells (32). Sequences homologous to the Y783KS785 in β1A are YKE in β3 and FKS in β2. The tyrosine homologous to Y783 of β1A can be demonstrated to be phosphorylated in β3 with antiphosphotyrosine (31, 33). The serine in β2 homologous to S785 of β1A has also been shown to be phosphorylated (34). Thus, we hypothesize that there is diphosphorylation of Y783 and S785 in β1A, and such diphosphorylation accounts for the enigma that we could not detect tyrosine phosphorylation of β1 of v-src-transformed cells with antiphosphotyrosine.
It has been suggested that phosphorylation–dephosphorylation cycles for Y783 (14) and S785 (32) are required for movement of β1A-integrins in and out of focal contacts. Our results raise the possibility that phosphorylations of the two residues are coupled. The parsimonious explanation for the observation that phosphorylations of both tyrosine and serine are up-regulated in v-src-transformed cells is a Src-activated dual specificity kinase. Coordinately regulated phosphorylation to form diphosphorylated β1A would distinguish β1A from β3, in which the serine is glutamic acid, a phosphoserine mimic, and β2, in which the tyrosine is phenylalanine and cannot be phosphorylated.
Acknowledgments
We gratefully acknowledge Julie Nowlen for technical assistance with cell culture, Mats Johansson and Maximilian Schneller for advice, Olga Göransson for technical advice in phosphoamino acid analysis, Bosco Chan and Erkki Ruoslahti for gifts of antibodies, and Marcy Salmon for preparation of the manuscript. This work was supported by National Institutes of Health Grants HL21644 and HL56396, fellowship funds from the Cell Science Research Foundation (T.S.), and a grant from the Swedish National Research Foundation.
Abbreviations
- LPA
1-oleoyl-lysophosphatidic acid
- PNGaseF
peptide/N-glycosidase F
References
- 1.Rous P. J Exp Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jove R, Hanafusa H. Annu Rev Cell Biol. 1987;3:31–56. doi: 10.1146/annurev.cb.03.110187.000335. [DOI] [PubMed] [Google Scholar]
- 3.Parsons J T, Weber M J. Curr Top Microbiol Immunol. 1989;147:79–127. doi: 10.1007/978-3-642-74697-0_3. [DOI] [PubMed] [Google Scholar]
- 4.Burridge K, Fath K, Kelly T, Nuckolls G, Turner C E. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- 5.Chambers A F, Tuck A B. Anticancer Res. 1988;8:861–872. [PubMed] [Google Scholar]
- 6.Golden A, Brugge J S. In: The Oncogene Handbook. Reddy E P, Skalka A M, Curran T, editors. Amsterdam: Elsevier; 1988. pp. 149–173. [Google Scholar]
- 7.Weisberg E, Sattler M, Ewaniuk D S, Salgia R. Crit Rev Oncog. 1997;8:343–358. doi: 10.1615/critrevoncog.v8.i4.40. [DOI] [PubMed] [Google Scholar]
- 8.Brown M T, Cooper J A. Biochim Biophys Acta. 1996;1287:121–149. doi: 10.1016/0304-419x(96)00003-0. [DOI] [PubMed] [Google Scholar]
- 9.Hirst R, Horwitz A, Buck C, Rohrschneider L. Proc Natl Acad Sci USA. 1986;83:6470–6474. doi: 10.1073/pnas.83.17.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapley P, Horwitz A, Buck C, Duggan K, Rohrschneider L. Oncogene. 1989;4:325–333. [PubMed] [Google Scholar]
- 11.Johansson M W, Larsson E, Lüning B, Pasquale E B, Ruoslahti E. J Cell Biol. 1994;126:1299–1309. doi: 10.1083/jcb.126.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fässler R, Pfaff M, Murphy J, Noegel A A, Johansson S, Timpl R, Albrecht R. J Cell Biol. 1995;128:979–988. doi: 10.1083/jcb.128.5.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fässler R. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakai T, Zhang Q, Fässler R, Mosher D F. J Cell Biol. 1998;141:527–538. doi: 10.1083/jcb.141.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson P J, Coussens P M, Danko A V, Shalloway D. Mol Cell Biol. 1985;5:1073–1083. doi: 10.1128/mcb.5.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai T, Peyruchaud O, Fässler R, Mosher D F. J Biol Chem. 1998;273:19378–19382. doi: 10.1074/jbc.273.31.19378. [DOI] [PubMed] [Google Scholar]
- 17.Kamps M K. Methods Enzymol. 1991;201:21–27. doi: 10.1016/0076-6879(91)01005-m. [DOI] [PubMed] [Google Scholar]
- 18.Boyle W J, van der Geer P, Hunter T. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- 19.Schneller M, Vuori K, Ruoslahti E. EMBO J. 1997;16:5600–5607. doi: 10.1093/emboj/16.18.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaheri A, Mosher D F. Biochim Biophys Acta. 1978;516:1–25. doi: 10.1016/0304-419x(78)90002-1. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Q, Sakai T, Nowlen J, Hayashi I, Fässler R, Mosher D F. J Biol Chem. 1999;274:368–375. doi: 10.1074/jbc.274.1.368. [DOI] [PubMed] [Google Scholar]
- 22.Tarone G, Cirilo D, Giancotti F G, Comoglio P M, Marchisio P C. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 23.Gavazzi I, Nermut M V, Marchisio P C. J Cell Sci. 1989;94:85–99. doi: 10.1242/jcs.94.1.85. [DOI] [PubMed] [Google Scholar]
- 24.Kundra V, Soker S, Zetter B R. Oncogene. 1994;9:1429–1435. [PubMed] [Google Scholar]
- 25.Huttenlocher A, Sandborg R R, Horwitz A F. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 26.Lauffenburger D A, Horwitz A F. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 27.Palecek S P, Loftus J C, Ginsberg M H, Lauffenburger D A, Horwitz A F. Nature (London) 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- 28.Yamada K M, Miyamoto S. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- 29.Cantley L C, Auger K R, Carpenter C, Duckworth B, Graziani A, Kapeller R, Soltoff S. Cell. 1991;64:281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- 30.Wennerberg K, Armulik A, Sakai T, Karlsson M, Fässler R, Schaefer E M, Mosher D F, Johansson S. Mol Cell Biol. 2000;20:5758–5765. doi: 10.1128/mcb.20.15.5758-5765.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Law D A, DeGuzman F R, Heiser P, Ministri-Madrid K, Killeen N, Phillips D R. Nature (London) 1999;401:808–811. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 32.Mulrooney J, Foley K, Vineberg S, Barreuther M, Grabel L. Exp Cell Res. 2000;258:332–341. doi: 10.1006/excr.2000.4964. [DOI] [PubMed] [Google Scholar]
- 33.Blystone S D, Lindberg F P, Williams M P, McHugh K P, Brown E J. J Biol Chem. 1996;271:31458–31462. doi: 10.1074/jbc.271.49.31458. [DOI] [PubMed] [Google Scholar]
- 34.Valmu L, Hilden T J, van Willigen G, Gahmberg C G. Biochem J. 1999;33:119–125. [PMC free article] [PubMed] [Google Scholar]