Abstract
Trace eyeblink conditioning (with a trace interval ≥500 msec) depends on the integrity of the hippocampus and requires that participants develop awareness of the stimulus contingencies (i.e., awareness that the conditioned stimulus [CS] predicts the unconditioned stimulus [US]). Previous investigations of the relationship between trace eyeblink conditioning and awareness of the stimulus contingencies have manipulated awareness or have assessed awareness at fixed intervals during and after the conditioning session. In this study, we tracked the development of knowledge about the stimulus contingencies trial by trial by asking participants to try to predict either the onset of the US or the onset of their eyeblinks during differential trace eyeblink conditioning. Asking participants to predict their eyeblinks inhibited both the acquisition of awareness and eyeblink conditioning. In contrast, asking participants to predict the onset of the US promoted awareness and facilitated conditioning. Acquisition of knowledge about the stimulus contingencies and acquisition of differential trace eyeblink conditioning developed approximately in parallel (i.e., concurrently).
Memory is composed of several different abilities that depend on different brain systems (Schacter and Tulving 1994; Squire and Zola 1997; Gabrieli 1998). Declarative memory depends on the hippocampus and anatomically related structures in the medial temporal lobe and diencephalon and supports the capacity for conscious recollection of facts and events. Nondeclarative memory supports a collection of nonconscious learning abilities that are independent of the medial temporal lobe and are expressed through performance as, for example, skill and habit learning and simple forms of conditioning.
Delay classical conditioning of the eyeblink response is a quintessential example of nondeclarative memory and is perhaps the most thoroughly studied example of associative learning in vertebrates (Lavond et al. 1993; Woodruff-Pak and Steinmetz 2000). In single-cue, delay eyeblink classical conditioning, the conditioned stimulus (CS) is presented and remains on until the unconditioned stimulus (US; a mild puff of air to the eye) is presented. The two stimuli overlap and coterminate. After repeated CS–US pairings, reflexive eyeblink CRs are emitted immediately prior to the onset of the US. In differential delay conditioning, two conditioned stimuli are used. One CS (the CS+) is consistently paired with the US. The other CS (the CS−) is always presented alone. The magnitude of conditioning is calculated as the percentage of CS+ trials in which a CR is emitted minus the percentage of CS− trials in which a CR is emitted.
Single-cue delay eyeblink conditioning is intact in amnesic patients (Daum et al. 1989; Daum and Ackermann 1994; Gabrieli et al. 1995) and in experimental animals with bilateral hippocampal lesions (Schmaltz and Theios 1972). Differential delay eyeblink conditioning is also preserved in amnesic patients (Clark and Squire 1998). In addition, in humans, knowledge that the CS predicts the US is not necessary to acquire delay eyeblink conditioning, using either the single-cue (Papka et al. 1997) or the differential procedure (Clark and Squire 1998, 1999).
Trace eyeblink conditioning differs from delay conditioning in that a silent (or trace) interval separates the termination of the CS (or CS+ in the case of the differential procedure) from the onset of the US. Unlike delay eyeblink conditioning, trace eyeblink conditioning depends on the integrity of the hippocampus in both humans and experimental animals (with a trace interval ≥500 ms; Moyer et al. 1990; Kim et al. 1995; McGlinchey-Berroth et al. 1997; Clark and Squire 1998). In humans, successful trace eyeblink conditioning requires that participants develop awareness of the stimulus contingencies, that is, awareness that the CS predicts the US (Clark and Squire 1998, 1999). For example, in one study, only individuals who demonstrated knowledge of the stimulus contingencies on a postsession questionnaire exhibited differential trace conditioning (Clark and Squire 1998). The importance of awareness for trace conditioning has also been demonstrated for single-cue trace conditioning (Manns et al. 2000). Further, in the case of single-cue trace eyeblink conditioning, the degree of awareness after 10 conditioning trials predicted the overall success of conditioning across the entire session (Manns et al. 2000).
An important question concerns the temporal relationship between trace conditioning and the development of awareness. It is not currently known whether awareness of the stimulus contingencies precedes, follows, or parallels the acquisition of trace conditioning. To address this question, we tracked the development of knowledge about the stimulus contingencies trial by trial by asking participants to try to predict the onset of the US. Specifically, participants watched a silent movie during differential trace eyeblink conditioning and pressed a button whenever during the session they believed that the US was about to occur. Participants should be able to perform this task to the extent that they are aware that the CS predicts the US. We also asked a second group of participants to press a button during the conditioning session whenever they believed that they were about to blink. Previous work indicates that participants tend not to become aware of the link between the CS and their eyeblinks (Clark and Squire 1998). Moreover, attention-demanding secondary tasks can interfere with trace eyeblink conditioning (Clark and Squire 1999). Accordingly, it seemed possible that asking participants to predict their eyeblinks might slow the development of awareness about the CS–US relationship and might permit better resolution of the temporal relationship between the acquisition of awareness and the acquisition of trace conditioning.
RESULTS
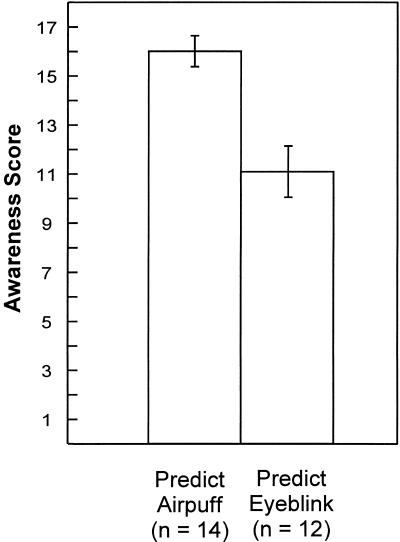
The Predict Airpuff and Predict Eyeblink groups performed similarly on the 10 true or false questions that asked about the content of the movie (mean number of items correct ± SEM = 9.5 ± 0.2 and 9.3 ± 0.2, for the Predict Airpuff and Predict Eyeblink groups, respectively). On the critical 17 true or false questions that asked about the relationships between the CS+, the CS−, and the US, participants in the Predict Airpuff group answered more questions correctly than participants in the Predict Eyeblink group (Fig. 1; mean number of items correct ± SEM = 15.6 ± 0.6 and 11.1 ± 1.0, for the Predict Airpuff and Predict Eyeblink groups, respectively; t[24] = 3.84, P < 0.01). Thus, the Predict Airpuff group learned more about the stimulus contingencies than did the Predict Eyeblink Group.
Figure 1.
Number of correct responses to 17 true or false questions given at the end of the conditioning session. The questions asked about the relationship between the CS+, the CS−, and the US. During the session, participants in the Predict Airpuff group pressed a button whenever they believed the US was about to occur. Participants in the Predict Eyeblink group pressed a button whenever they believed they were about to blink. Brackets show SEM.
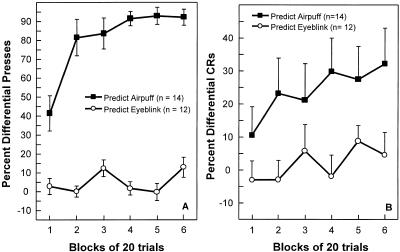
The button-press responses reveal how knowledge of the stimulus contingencies developed during the course of the conditioning session. Figure 2A shows for both groups the differential percentage button presses (percentage of CS+ trials on which the button was pressed minus the percentage of CS− trials on which a button was pressed) across the six blocks of trials. A trial was considered to begin at the onset of the CS+ or CS− and to end 1250 msec later, at US onset. Participants in the Predict Airpuff group quickly learned to predict the onset of the US by pressing the button primarily on CS+ trials. In contrast, participants in the Predict Eyeblink group did not reliably press the button more on CS+ trials than on CS− trials (main effect of group, F[1, 24] = 130.03, P < 0.001). Indeed, for the Predict Eyeblink group, the mean differential percentage of button presses across the six blocks was only marginally above zero (mean ± SEM = 4.75% ± 2.4%; t[11] = 2.00, P = 0.07). Despite these marked differences in percentage differential button presses, the two groups exhibited similar latencies between the onset of the CS and the button press (for the trials in which the button was pressed) both on CS+ trials (mean latency across six blocks ± SEM = 600 ± 43 msec and 662 ± 30 msec for the Predict Eyeblink and Predict Airpuff groups, respectively; t[24] = 1.24, P > 0.1) and on CS− trials (552 ± 45 msec and 553 ± 45 msec; t[22] = 0.02, P > 0∼.1).
Figure 2.
(A) Percentage differential button presses across six blocks of 20 trials (the percentage of CS+ trials on which participants pressed the button minus the percentage of CS− trials on which participants pressed the button). During the conditioning session, participants in the Predict Airpuff group pressed a button whenever they believed the US was about to occur. Participants in the Predict Eyeblink group pressed the button whenever they believed they were about to blink. (B) Percentage differential CRs across the six blocks of 20 trials (the percentage of CS+ trials on which participants emitted an eyeblink minus the percentage of CS− trials on which an eyeblink was emitted). Brackets show SEM.
Figure 2B shows for both groups the differential percent CRs (the percentage of CS+ trials on which a CR was emitted minus the percentage of CS− trials on which a CR was emitted) across the six blocks of 120 trials. The Predict Airpuff group demonstrated robust conditioning (mean percentage CRs across six blocks ± SEM = 24.0 ± 7.6%; t[13] = 3.16, P < 0.01). Conditioning performance improved from 11% differential CRs during the first block of 20 trials to 32% differential CRs during the sixth block of 20 trials. In contrast, participants in the Predict Eyeblink group demonstrated little acquisition of differential responding, and the mean percentage of CRs across the conditioning session was not significantly above zero (mean ± SEM = 1.8 ± 3.3%; t[11] = 0.54, P > 0.2). In addition, across the conditioning session, the Predict Eyeblink group emitted fewer differential CRs than the Predict Airpuff group (F[1,24] = 6.31, P < 0.05).
We also determined the level of conditioned responding separately for CS+ and CS− trials. This analysis indicated that the difference between the two groups in differential conditioning resulted from the fact that the Predict Airpuff group emitted more conditioned responses on CS+ trials than did the Predict Eyeblink group (mean percentage CRs across all six blocks ± SEM = 66.3% ± 4.4% and 39.4% ± 4.3% for the Predict Eyeblink and Predict Airpuff groups, respectively; t[24] = 4.35, P < 0.001). On CS− trials, the percentage CRs emitted by the two groups were similar (block mean ± SEM = 37.4% ± 5.6% and 42.4% ± 5.5%, for the Predict Eyeblink and Predict Airpuff groups, respectively; t[24] = 0.62, P > 0.1). These data indicate that the Predict Eyeblink group's lack of discriminative responding was due to poor responding on CS+ trials and not to continued elevated responding on CS− trials.
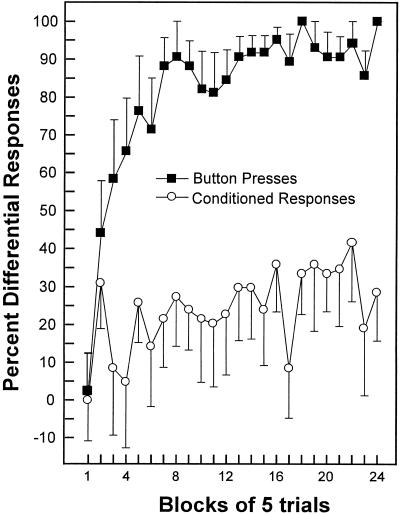
For the Predict Airpuff group, the button presses provided a trial by trial measure of each participant's knowledge (or awareness) of the stimulus contingencies. Figure 3 compares the development of knowledge about stimulus contingencies to the development of conditioning performance itself. The acquisition of awareness (as measured by differential button presses on CS+ and CS− trials) and the acquisition of conditioning (as measured by differential eye-blinks) developed approximately in parallel (i.e., concurrently). It is also possible that awareness preceded conditioning. However, there is no indication in the data that awareness developed after conditioning.
Figure 3.
Percentage differential button presses and percentage differential CRs across 24 blocks of five trials for the Predict Airpuff group (n = 14). During the conditioning session, participants pressed a button to try to predict the onset of the US. Brackets show SEM.
Although participants in the Predict Eyeblink group showed little evidence of differential conditioning (Fig. 2B) and did not press the button more on CS+ trials than on CS−trials (Fig. 2A), they did demonstrate some ability to predict their eyeblinks. That is, on CS+ and CS− trials they blinked more frequently when they pressed the button than when they did not (mean percentage blinks ± SEM = 69.8% ± 4.3% and 40.7% ± 5.7%, respectively; t[13] = 3.64, P < 0.01). Eyeblinks that occurred during the intertrial interval were not recorded. Thus, the Predict Eyeblink group did demonstrate some success at the task they were assigned to perform. Note, however, that the ability of these participants to predict eyeblinks was similar on CS+ and CS− trials. Accordingly, the ability to predict eyeblinks is not evidence for awareness of the stimulus contingencies. Rather, participants may sometimes be able to detect the sensation of an impending eyeblink, regardless of the trial type. In addition, they may sometimes tend to emit an eyeblink immediately after predicting one.
DISCUSSION
Participants in the Predict Airpuff group acquired accurate knowledge of the relationships between the CS+, CS−, and US, as measured both by the 17 critical true or false questions about the stimulus contingencies and by their ability to predict the US by pressing a button from trial to trial. Indeed, the Predict Airpuff group pressed the button reliably and accurately after about the first 20 conditioning trials (Fig. 2A). Further, the Predict Airpuff group exhibited reliable trace eyeblink conditioning. In contrast, participants in the Predict Eyeblink group developed little awareness of the stimulus contingencies and did not reliably press the button more on CS+ trials than on CS− trials. In addition, the Predict Eyeblink group showed little or no evidence of differential trace eyeblink conditioning.
For the Predict Airpuff group, the acquisition of conditioning appeared to parallel the acquisition of knowledge about the stimulus contingencies. That is, the acquisition of awareness and the acquisition of conditioning developed approximately in parallel (i.e., concurrently). For the first five trials, the percentage differential button presses and the percentage differential CRs were both nearly at zero (Fig. 3). During trials 11–15, the differential button presses reached 58%, and the differential CRs reached 9%. From trial 16 to the end of the conditioning session (trial 120), the rate of increase in percentage differential CRs was similar to the increase in percentage differential button presses (slope ± SEM = 1.25 ± 0.37 and 1.67 ± 0.58 for the button presses and CRs, respectively).
There appear to be two ways to understand the performance curves for button presses and CRs. First, the two measures might have reached different asymptotes. During the final 60 trials, participants in the Predict Airpuff group exhibited >90% differential button pressing. In contrast, the maximum level of differential eyeblink conditioning was about 35%. If conditioning performance was asymptotic, then the performance curves for button pressing and differential eyeblinks progressed to asymptote at approximately the same rate. Alternatively, a more likely possibility is that differential conditioning would have continued to improve with further training until the level of differential eyeblink performance approached the level of differential button pushes. Indeed, differential eyeblink conditioning can reach a level of >85% in rabbits given many days of training (Berger and Orr 1983). In either case, there appears to be a close connection between awareness and trace conditioning.
It seemed possible that participants in the Predict Airpuff group were advantaged by the instructions, which explicitly directed their attention to the relationship between the CS+, the CS−, and the airpuff US. Accordingly, we compared the performance of participants in the Predict Airpuff group to the performance of individuals who watched a silent movie during trace conditioning but without any special instructions (Trace 1000 group; Clark and Squire 1998). The Predict Airpuff group exhibited greater differential conditioning across the 120-trial session, emitting 17.9% more differential CRs overall (mean percentage differential CRs for blocks 1–6 = 10.5, 23.1, 21.1, 29.7, 27.4, and 32.1 and −1.7, −3.1, 2.8, 8.3, 14.4, and 14.7 for the Predict Airpuff and Trace 1000 groups, respectively; F[1,26] = 5.01, P < 0.05). Thus, the differential conditioning displayed by the Predict Airpuff group was higher than it would have been had participants not been explicitly directed to predict the occurrence of the airpuff. Perhaps directing the attention of participants in the Predict Airpuff group to the airpuff US increased awareness of the conditioning stimuli and led to greater differential trace conditioning. Indeed, participants in the Predict Airpuff group scored significantly higher on the 17 true or false questions about the stimulus contingencies than did the participants in the earlier study who received no special instructions and did not press a button during the conditioning session (mean number correct ± SEM = 15.6 ± 0.6 and 13.3 ± 0.8, for the Predict Airpuff and Trace 1000 groups, respectively, t[26] = 2.37, P < 0.05).
The performance of the Predict Eyeblink group was strikingly different from the performance of the Predict Airpuff group. A simple change in instructions (predict eyeblinks instead of predict airpuffs) reduced awareness of the stimulus contingencies and prevented conditioning. This finding is reminiscent of the observation that a secondary distraction task (counting odd digits) prevented trace eyeblink conditioning (Trace-Distraction group; Clark and Squire, 1999). Indeed, the overall level of differential eyeblink conditioning displayed by the Predict Eyeblink group across all 120 trials was similar to that observed in the presence of a secondary distraction task (mean percentage CRs SEM = 1.75% ± 3.3% and −0.90% ± 2.8% for the Predict Eyeblink group and the Distraction group, respectively; t[19] = 0.58, P > 0.2). The participants in the Predict Eyeblink group pressed the button whenever they believed they were about to blink, which required them to monitor for impending eyeblinks not only during the short interval following the CS but during the entire conditioning session. In addition, their attention was focused on their internal state rather than on external events (i.e., airpuffs). Their poor score (11.1) on the 17 critical questions about the stimulus contingencies suggests that this sustained internal monitoring left few resources available for attending to the external stimulus contingencies. In any case, this group gained little knowledge of the stimulus contingencies and also exhibited little evidence of differential conditioning. Thus, these results further support the important role of awareness for trace eyeblink conditioning.
Trace eyeblink conditioning has been demonstrated previously to be related to awareness of the stimulus contingencies. These previous investigations have either manipulated participant awareness (Clark and Squire 1999), assessed awareness at the end of the conditioning session (Clark and Squire 1998), or assessed awareness at fixed intervals during and after the conditioning session (Manns et al. 2000). In this study, we assessed awareness trial by trial, making it possible to inspect the relationship between awareness and conditioning on every trial of the conditioning session. The results confirm a close connection between awareness and trace eyeblink conditioning and suggest that the development of awareness and conditioning developed concurrently.
One view of the relationship between the acquisition of awareness and the acquisition of trace conditioning is that both reflect interaction between the hippocampus and other brain structures that is optimal for the acquisition of declarative memory (Manns et al. 2000). Note, however, that the neural systems supporting the development of awareness and trace conditioning are not identical. The development of knowledge about the stimulus contingencies depends on the activation of reciprocal connections between the hippocampus and neocortex. Successful trace conditioning depends additionally on the participation of the cerebellum (Woodruff-Pak et al. 1985). If awareness were directly contributing to the eyeblink response, participants should have awareness of their eyeblink responses to the CS+. Yet individuals who become aware of the stimulus contingencies and who exhibit successful trace conditioning are usually unable to report their responses to the CS+ (i.e., they are unaware that they are blinking to the CS+; Papka et al. 1997; Clark and Squire 1998). Moreover, in our study the level of differential eyeblink conditioning was well below the level of differential button pushes (Fig. 3). These observations suggest that the conditioned eyeblink responses are formed and generated, at least in part, independently of the development of awareness of the stimulus contingencies. Indeed, studies of experimental animals suggest that the eyeblink conditioned response is formed in and driven by the cerebellum (Thompson and Krupa 1994; Woodruff-Pak et al. 1985). In the case of trace eyeblink conditioning, the cerebellum may, in addition, require critical input from other structures, including the hippocampus and neocortex, to acquire the conditioned response. If so, the development of awareness would serve as a good indicator that the hippocampus and neocortex are being effectively engaged by the task and working with the cerebellum to accomplish successful trace conditioning.
MATERIALS AND METHODS
Participants
The participants were employees or volunteers at the San Diego Veterans Affairs Medical Center or were respondents to an advertisement placed in the local newspaper. The 26 participants were assigned to one of two groups. One group (Predict Airpuff) pressed a button during the conditioning session each time they believed that an airpuff was about to occur. These 14 individuals (three men and 11 women) averaged (mean ± SEM) 67.1 ± 2.4 yr of age (range: 49–79), had 16.1 ± 0.4 yr of education, and obtained scores of 23.4 ± 0.7 and 61.6 ± 1.1 on the information and vocabulary subscales of the WAIS-R, respectively. The other group (Predict Eyeblink) pressed a button during the conditioning session each time they believed that they were about to blink their eyes. These 12 individuals (six men and six women) averaged 67.8 ± 3.2 yr of age (range: 47–79), had 15.1 ± 1.0 yr of education, and obtained scores of 20.5 ± 1.9 and 51.5 ± 4.0 on the information and vocabulary subscales of the WAIS-R, respectively. The two groups were similar on all these measures except vocabulary (P < 0.05).
Apparatus and Procedure
All participants were told that they were taking part in a study of how distraction affects learning and memory and that they would be distracted by tones, static noise (white noise), and airpuffs. After giving informed consent, participants were seated in a darkened room, 0.7 m from a television monitor, and were told that they would be watching a silent movie (“The Gold Rush”), which they should try to remember. During the presentation of the silent movie, 120 differential trace conditioning trials were administered with an intertrial interval of 10–15 sec. The conditioning session consisted of six blocks of 20 trials. For 10 trials in each block, one conditioned stimulus (the CS+) was consistently paired with the airpuff. For the other 10 trials in each block, the other conditioned stimulus (the CS−) was presented alone. No more than two CS+ or CS− trials occurred in succession. Further, each block of five trials contained either two or three CS+ and CS−trials.
The CS+ was always an 85-dB, 1-KHz tone, and the CS− was an 85-dB burst of white noise. Both stimuli were delivered through headphones. The unconditioned stimulus (US) was a 3-psi airpuff delivered to the left eye through a nozzle attached to modified sunglasses that were worn by the participants. The sunglasses also held an infrared reflective sensor for recording eyeblinks. On CS+ trials, the tone was presented for 250 msec and was followed by a 1000-msec trace interval. At the end of the trace interval, the US was presented for 100 msec. On CS− trials, the static noise was presented alone for 250 msec.
Only eyeblinks that occurred between 750 ms after the onset of the CS and before the onset of the US were scored as CRs. This latency criterion was used to filter out nonassociative responding and voluntary responding (purposeful or volitional blinking; for a similar approach to the scoring of human eyeblink conditioning data, see Daum et al. 1993; Finkbiner and Woodruff-Pak 1991). To score CRs, the mean eyeblink amplitude in response to the first 10 US presentations was first calculated for each participant. For a response to a CS+ or CS− to be scored as a CR for that participant, the maximum eyeblink amplitude had to be 20% of this mean. This criterion was used instead of some lower threshold because our pilot work indicated that with the infrared eyeblink measurement method an eyeball movement, like that involved in scanning the television monitor, could register as high as 16% of the unconditioned response amplitude in the absence of an actual eyeblink. Button pushes that occurred during the 1250 msec between the onset of the CS+ and the onset of the US were considered to have occurred on CS+ trials. Button pushes that occurred within 1250 msec after the onset of the CS− were considered to have occurred on CS− trials.
Participants in both groups were asked to hold a small momentary switch button. Button pushes were relayed to a computer, where they were synchronized and displayed with the eyeblink waveform. Participants in the Predict Airpuff group were instructed to press the button whenever they believed that the airpuff was about to occur. Participants in the Predict Eyeblink group were instructed to press the button whenever they believed that they were about to blink.
Following the conditioning session, participants were given a series of true or false questions. The first 10 questions asked about events in the movie. The critical questions were 17 items concerning the temporal relationships between the CS+, the CS−, and the US. For example, participants were asked (true or false): “I believe the airpuff came immediately before the tone”; “I believe the tone usually came before the static noise”; “I believe the tone and airpuff were always closely related in time.” All 17 questions appear in Appendix A of Clark and Squire (1999).
Acknowledgments
This work was supported by the Medical Research Service of the Department of Veterans Affairs and NIMH grant MH24600. We thank Shauna Stark for her assistance.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL lsquire@ucsd.edu; FAX (858) 552-7457.
Article and publication are at www.learnmem.org/cgi/doi/10.1101/lm.33400.
REFERENCES
- Berger TW, Orr WB. Hippocampectomy selectively disrupts discrimination reversal conditioning of the rabbit nictitating membrane response. Behav Brain Res. 1983;8:49–68. doi: 10.1016/0166-4328(83)90171-7. [DOI] [PubMed] [Google Scholar]
- Clark RE, Squire LR. Classical conditioning and brain systems: A key role for awareness. Science. 1998;280:77–81. doi: 10.1126/science.280.5360.77. [DOI] [PubMed] [Google Scholar]
- ————— Human eyeblink classical conditioning: Effects of manipulating awareness of the stimulus contingencies. Psychol Sci. 1999;10:14–18. [Google Scholar]
- Daum I, Ackerman H. Frontal-type memory impairment associated with thalamic damage. Int J Neurosci. 1994;75:187–198. doi: 10.3109/00207459408986030. [DOI] [PubMed] [Google Scholar]
- Daum I, Channon S, Canavan AG. Classical conditioning in patients with severe memory problems. J Neurol Neurosurg Psychiatry. 1989;52:47–51. doi: 10.1136/jnnp.52.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daum I, Schugens MM, Ackermann H, Lutzenberger W, Dichgans J, Birbaumer N. Classical conditioning after cerebellar lesions in humans. Behav Neurosci. 1993;107:748–756. doi: 10.1037//0735-7044.107.5.748. [DOI] [PubMed] [Google Scholar]
- Finkbiner, R.G. and Woodruff-Pak, D.S. Classical eyeblink conditioning in adulthood: Effects of age and interstimulus interval on acquisition in the trace paradigm. Psychol. Aging 6: 109–117. [DOI] [PubMed]
- Gabrieli JDE. Cognitive neuroscience of human memory. Annu Rev Psychol. 1998;49:87–115. doi: 10.1146/annurev.psych.49.1.87. [DOI] [PubMed] [Google Scholar]
- Gabrieli JD, McGlinchey-Berroth R, Carrillo MC, Gluck MA, Cermak LS, Disterhoft JF. Intact delay-eyeblink classical conditioning in amnesia. Behav Neurosci. 1995;109:819–827. doi: 10.1037//0735-7044.109.5.819. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Lavond DG, Kim JJ, Thompson RF. Mammalian brain substrates of aversive classical conditioning. Annu Rev Psychol. 1993;44:317–342. doi: 10.1146/annurev.ps.44.020193.001533. [DOI] [PubMed] [Google Scholar]
- Manns JR, Clark RE, Squire LR. Awareness predicts the magnitude of single-cue trace eyeblink conditioning. Hippocampus. 2000;10:181–186. doi: 10.1002/(SICI)1098-1063(2000)10:2<181::AID-HIPO7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- McGlinchey-Berroth R, Carrillo MC, Gabrieli JDE, Brawn CM, Disterhoft JF. Impaired trace eyeblink conditioning in bilateral, medial-temporal lobe amnesia. Behav Neurosci. 1997;100:243–252. doi: 10.1037//0735-7044.111.5.873. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eyeblink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- Papka M, Ivry RB, Woodruff-Pak DS. Eyeblink classical conditioning and awareness revisited. Psychol Sci. 1997;8:404–408. [Google Scholar]
- Schacter DL, Tulving E, editors. Memory Systems 1994. Cambridge, MA: MIT Press; 1994. [Google Scholar]
- Schmaltz LW, Theios J. Acquisition and extinction of a classically conditioned response in hippocampectomized rabbits (Oryctolagus cuniculus) J Comp Physiol Psychol. 1972;79:328–333. doi: 10.1037/h0032531. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Amnesia, memory and brain systems. Phil Trans R Soc Lond B. 1997;352:1663–1673. doi: 10.1098/rstb.1997.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RF, Krupa DJ. Organization of memory traces in the mammalian brain. Annu Rev Neurosci. 1994;17:519–549. doi: 10.1146/annurev.ne.17.030194.002511. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Lavond DG, Thompson RF. Trace conditioning: Abolished by cerebellar nuclear lesions but not lateral cerebellar cortex aspirations. Brain Res. 1985;348:249–260. doi: 10.1016/0006-8993(85)90443-3. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Steinmetz JE, editors. Eyeblink classical conditioning: Human. Boston, MA: Kluwer Academic; 2000. [Google Scholar]