Abstract
Fetal alcohol spectrum disorder (FASD) is the most common preventable cause of mental retardation in the USA. Ethanol impairs neuronal survival and function by two major mechanisms: 1) it inhibits insulin signaling required for viability, metabolism, synapse formation, and acetylcholine production; and 2) it functions as a neurotoxicant, causing oxidative stress, DNA damage and mitochondrial dysfunction. Ethanol inhibition of insulin signaling is mediated at the insulin receptor (IR) level and caused by both impaired receptor binding and increased activation of phosphatases that reverse IR tyrosine kinase activity. As a result, insulin activation of PI3K-Akt, which mediates neuronal survival, motility, energy metabolism, and plasticity, is impaired. The neurotoxicant effects of ethanol promote DNA damage, which could contribute to mitochondrial dysfunction and oxidative stress. Therefore, chronic in utero ethanol exposure produces a dual state of CNS insulin resistance and oxidative stress, which we postulate plays a major role in ethanol neurobehavioral teratogenesis. We propose that many of the prominent adverse effects of chronic prenatal exposure to ethanol on CNS development and function may be prevented or reduced by treatment with peroxisome-proliferated activated receptor (PPAR) agonists which enhance insulin sensitivity by increasing expression and function of insulin-responsive genes, and reducing cellular oxidative stress.
Keywords: Insulin signaling, mitochondria, oxidative stress, energy metabolism, insulin sensitizer, insulin resistance, central nervous system
The Public Health Problem
Gestational exposure to alcohol is the leading preventable cause of mental retardation in North America. As many as 7 per 1000 women binge drink during pregnancy, and even higher percentages consume alcohol at various times during pregnancy. Binge drinking has not declined among women of child-bearing age in the USA.1 The syndrome caused by maternal consumption of alcohol during pregnancy is termed, “Fetal Alcohol Spectrum Disorders” (FASD).2 FASD is not one entity, but rather a collection of heterogeneous disorders that range broadly in terms of severity and outcomes.3 Fetal alcohol syndrome (FAS) is the most severe form of FASD, and associated with intrauterine growth restriction, central nervous system (CNS) malformations, mental retardation, and craniofacial and skeletal defects, whereas less severe effects of prenatal alcohol exposure have been classified as alcohol-related birth defects and alcohol-related neurodevelopmental disorders.4 The economic burden of FASD/FAS is high5, and despite public health efforts, the incidence rates have not declined in the past decade.6 Epidemiologic data indicate that in the US, FAS rates range from 0.2-1.5 per 1,000 live births, whereas alcohol-related birth defects and alcohol-related neuro-developmental disorders occur in approximately 0.9% of live births.7
The tendency to abuse alcohol during pregnancy could be consequential to heavy chronic or binge alcohol abuse during adolescence. In this regard, attendant sustained structural and functional abnormalities in the brain8-11, including deficits in performing executive, visual-spatial12-14, and working memory tasks15, might contribute to poor judgment concerning alcohol and drug misuse during pregnancy. Increased tendency to abuse alcohol during child-bearing years, including its inadvertent misuse during pregnancy, could account for the high prevalence rates of attention deficit hyperactivity disorder (ADHD) in the United States.16-18 Children with ADHD and deficits in visual-spatial, fine motor, and cerebellar learning are more challenged8,19-21, and if their problems go unrecognized or ignored, the risk for engaging in aberrant, socially unacceptable behaviors, including drug and alcohol dependence and abuse as adolescents or young adults, also increases.22-26 Experimentally, it has been demonstrated that alcohol misuse during adolescence does increase the propensity to consume alcohol as adults.27 In essence, alcohol abuse in adolescents and young adults establishes a vicious cycle whereby impaired judgment and cognition increase the risk of causing pre-natal alcohol exposure. Long-term consequences of prenatal alcohol exposure range from behavioral abnormalities to learning disabilities and ADHD, to mental retardation.19,20,28-31 Presumably, structural and functional CNS abnormalities mediate the increased tendency of adolescents and young people to participate in high-risk behaviors, including alcohol abuse during pregnancy.
Prenatal Ethanol Exposure Impairs CNS Growth and Development
Heavy gestational exposure to alcohol can be teratogenic, resulting in gross abnormalities in the developing CNS2,17,32-35 including microencephaly, white matter hypomyelination, hydrocephalus, cerebellar hypoplasia, neuronal migration disorders, and neuroglial heterotopias.5,32,36-43 Moderate levels of prenatal alcohol exposure tend to be less harmful, although they still lead to structural and functional abnormalities, including altered gene expression in the brain40,44-46 and impairments in cognitive, behavioral, and motor functions.47 Ethanol mediates its neurotoxic effects on proliferating and immature neuronal cells by causing permanent structural and functional abnormalities that promote cell death41,48-50 and impair neurotransmission and plasticity.27,51-55 Correspondingly, prenatal ethanol exposure causes sustained cognitive-motor deficits in children, adolescents, and young adults.20,28 29
Although the mechanisms are not entirely understood, based on the known targets of alcohol neurotoxicity and structure-function relationships, long-term cognitive deficits are likely due to impaired function of the hippocampus and anterior cingulate region of the frontal lobe. Sustained motor deficits could be caused by structural and functional impairments in the cerebellum. Behavioral abnormalities are probably caused by loss of neurons within hypothalamic-limbic brain structures. Our research has focused on the role of impaired insulin and insulin-like growth factor (IGF) signaling as mediators of neuro-developmental abnormalities caused by chronic gestational exposure to alcohol.40,42,50,56 Others have examined the contributions of impaired IGF actions in relation to sustained deficits in neuro-cognitive function caused by prenatal alcohol exposure.57-62 Herein, we review the importance of insulin and IGF signaling in relation to CNS neuronal function, and the mechanisms and potential consequences of ethanol-impaired insulin/IGF signaling in brain. While emerging data suggest alcohol-mediated epigenetic modifications in gene expression may serve as underlying mechanisms of the brain structural abnormalities and associated aberrant behaviors27,63-67, it is noteworthy that at least some of these effects are linked to perturbations in IGF, in particular IGF-2 expression and function in the CNS.67,68
Insulin Regulates Growth, Viability and Function in CNS Neurons by Signaling through Insulin Receptor Substrate Molecules
In the CNS, neuronal survival, energy metabolism, and plasticity are critical for maintaining cognitive and motor functions, and regulated through the actions of insulin and IGF types 1 and 2. Insulin, IGF-1 and IGF-2, and their corresponding receptors are abundantly expressed in various cell types throughout the brain, including neurons.69-71 The highest brain levels of insulin and IGF polypeptide and receptor expression are distributed in the hypothalamus, temporal lobe, and cerebellum, which notably represent major targets of ethanol neurotoxicity. Insulin promotes neurite outgrowth, protein synthesis, neuronal cytoskeletal protein expression, and nascent synapse formation.72 The stimulatory effects of insulin are mediated through complex intracellular signaling pathways, beginning with ligand binding and activation of the intrinsic receptor tyrosine kinase (RTK).69,72-78 Insulin RTK phosphorylates specific cytosolic molecules, including one of its major substrates, insulin receptor substrate, type 1 (IRS-1). Tyrosyl phosphorylated IRS-1 (PY-IRS-1) transmits intracellular signals that mediate growth, metabolic function, and viability by interacting with downstream src-homology 2 (SH2)-containing molecules through specific motifs located in the C-terminal region of IRS-1. Phosphorylation of tyrosine residue 897 within the YVNI motif of IRS-1 (897YVNI) enables binding to the growth-factor receptor-bound protein 2 (Grb2) adapter molecule, whereas phosphorylation of tyrosine 1180 (1180YIDL motif) enables IRS-1 interaction with Syp protein tyrosine phosphatase, and phosphorylation of tyrosine residues 613 and 942 (613YMPM and 942YMKM motifs) leads to IRS-1 binding to the p85 subunit of phosphatidylinositol-3 kinase (PI3K). Grb2 binds to PY-IRS-1 via its SH2 domain, and to a prolinerich region of son-of-sevenless (SOS) through its SH3 domain. Sos complexed with PY-IRS-1 and Grb2, interacts with Ras-GDP, catalyzes a GDP/GTP exchange on Ras, which promotes sequential activation of p21ras, mitogen-activated protein kinase kinase (MAPKK), and MAPK. Erk MAPK activation directly contributes to growth factor-stimulated mitogenesis, neuritic sprouting, and gene expression. Tyrosyl phosphorylated Syp acts as an adapter protein between the Grb2-SOS complex and the epidermal growth factor (EGF) or platelet-derived growth factor (PDGF) receptor, whereas catalytically inactive (non-phosphorylated) Syp inhibits MAPK activity. Therefore, the catalytic substrates of Syp may help regulate mitogenic signals and cell cycle progression. The binding of PY-IRS-1 to p85 stimulates glucose uptake and inhibits apoptosis by signaling through Akt/Protein kinase B. In addition, insulin signaling through PI3K phosphorylates and thereby inactivates glycogen synthase kinase 3β (GSK-3β). GSK-3β has roles in energy metabolism, cell survival, and phosphorylation of neuronal cytoskeletal proteins. A very similar signaling cascade exists for IGF-1.
Ethanol Inhibits Neuronal Responses to Growth Factors
Ethanol-induced developmental arrest in the CNS may be due to impaired responses to various growth factors.56,58,61,79,80 For example, ethanol inhibits bFGF-, PDGF-AA-, PDGF-BB-, NGF, and IGF-1-stimulated proliferation and cell cycle progression in neural cells.61,81-83 Ethanol inhibition of neuronal proliferation can be mediated by reduced levels of growth factor receptor expression, growth factor stimulated receptor autophosphorylation, abolishment of the association between growth factor receptor and Ras GTPase-activating protein (Ras-GAP), and inhibition of Erk MAPK activation.82 Major consequences of ethanol-impaired neuronal responses to growth factor stimulation include reduced viability (increased cell death), motility, adhesion, mitochondrial function, and acetylcholine homeostasis. 40,54,83-88 Importantly, many of these adverse effects of ethanol are mediated by reduced phosphorylation and consequently increased activation of GSK-3β.42,50, 89-93
Ethanol Inhibits Insulin and IGF1 Signaling Through IRS-1 in Immature Neuronal Cells
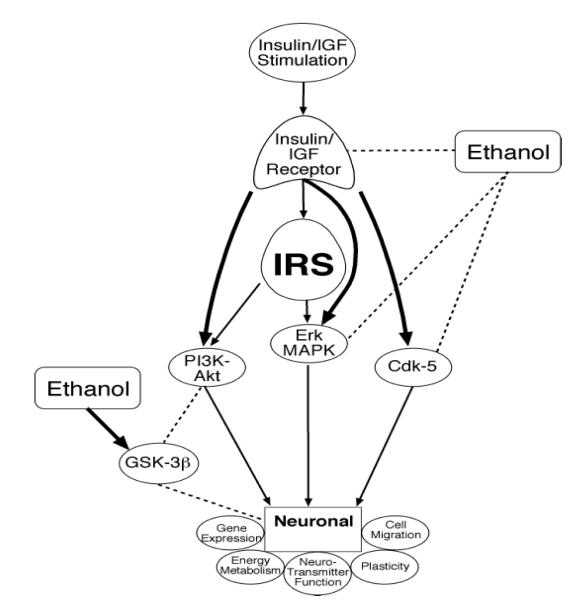
Since insulin and IGFs have significant roles in regulating many functions in the immature brain, understanding how ethanol inhibits the corresponding signaling pathways will likely provide important mechanistic clues regarding the pathogenesis of FASD and its associated CNS developmental abnormalities. Previous studies demonstrated that ethanol inhibits phosphorylation and activation of insulin and IGF RTKs, as well as down-stream signaling through IRS-1.94-98 In addition, ethanol inhibits G-protein expression 99, cyclic AMP-dependent signaling100, IRS-1-associated PI3 kinase94,98,101,102, and second messenger cascades such as calcium phospholipid-dependent protein kinases (PKC).103 PI3 kinase is important for signaling cell survival through Akt/protein kinase B (PKB).104 Therefore, inhibition of insulin signaling through PI3 kinase could account for the increased apoptosis observed in ethanol-exposed CNS cells. Apart from it’s inhibitory effects on PI3K-Akt signaling, ethanol promotes neuronal apoptosis by increasing intracellular Ca++ release105, activating pro-death signaling through Bax, Bad, GSK-3β, and caspases, or by inhibiting survival signaling through Bcl-2.46,50,93,98,101,106,107 Since the signaling networks corresponding to a number of trophic factor receptors expressed in brain converge downstream to modulate MAPK, PI3K-Akt, Bax, Bad, Bcl-2, GSK-3β, and caspases, ethanol’s adverse effects on neuronal growth, survival, energy metabolism, and plasticity could be mediated by inhibition of one or more different receptors, including insulin and IGF-1. Studies utilizing immature brains (mainly cerebella) and cultured neuronal cells demonstrated that ethanol inhibits insulin and IGF signaling at multiple points within the cascade, beginning at the receptor level and extending downstream through pathways that regulate growth, survival, energy metabolism, neuronal migration, and plasticity (Figure 1).88,101,108-111 Ethanol’s inhibitory effects on insulin/IGF stimulated neuronal survival through blockage of downstream signaling through PI3 kinase-Akt50,88,108,112 promote both apoptosis88,106,108 and mitochondrial dysfunction.50,84,85,108,112 At proximal points, ethanol inhibition of insulin and IGF stimulated survival signaling in brain is mediated at the receptor level by two distinct mechanisms: 1) reduced receptor binding and attendant activation of receptor tyrosine kinases (RTK) and corresponding downstream signaling through PI3 kinase-Akt40; and 2) increased activation of phosphatases that negatively regulate RTK (PTP-1b) and PI3 kinase (PTEN) and increase GSK-3β activity.56,98,102 Therefore, chronic ethanol exposure causes insulin/IGF resistance in the developing brain.
FIG. 1.
Schematic of ethanol’s adverse effects on insulin/IGF signaling in the brain. Ethanol inhibits phosphorylation and activation of insulin/IGF-1 receptor tyrosine kinases, as well as downstream mediators of neuronal growth, survival, plasticity, energy metabolism, migration, and neurotransmitter function. In addition, ethanol stimulates GSK-3β activity through inhibition of PI3K and activation of PTEN phosphatase.
Ethanol Impairs Mitochondrial (Mt) Function
Ethanol toxicity perturbs the structural and functional integrity of mitochondria in brain.50,112-118 Experimental chronic ethanol feeding results in oxidative modification of MtDNA, manifested by increased 8-hydroxydeoxyguanosine (8-OHdG) incorporation, reduced MtDNA content, and increased MtDNA single-strand breaks.84,112,119-122 Ethanol-induced MtDNA damage and impaired Mt function increase cellular sensitivity to toxins, and promote Mt permeability transition resulting in necrosis or apoptosis.84,112,121,122 These adverse effects of ethanol are likely mediated by increased oxygen free radical production, lipid peroxidation, and inhibition of Mt glutathione.116,123-126 Ethanol metabolism by the microsomal monoxygenase system, involving the alcohol-inducible cytochrome P450 2E1 could contribute to oxidative cellular injury through hydroxylethyl radical formation.127,128 Therefore, with regard to the CNS, we hypothesize that ethanol-induced MtDNA damage and impaired Mt function cause defects in energy metabolism and oxidative phosphorylation, leading to reduced neuronal viability, as well as compromised activities required for synaptic plasticity and cognitive/motor functions. In this regard, experimental results demonstrated that chronic ethanol exposure during development results in significantly reduced expression of mitochondria-encoded cytochrome c oxidase and ATP synthase, and increased expression of NADPH oxidase.50,84,112 These effects lead to reduced ATP production, and increased indices of oxidative stress, including DNA damage and lipid peroxidation in the developing brain.84,115,118 In addition to impairing mitochondrial function, chronic ethanol exposure increases cellular stress by promoting free radical generation, endoplasmic reticulum stress, and pro-apoptosis signaling, and inhibiting survival pathways.50,54,92,112,115-117,125,129-132
The extent to which these mechanisms mediate the teratogenic effects of prenatal alcohol exposure has been demonstrated through the use of anti-oxidants to minimize the adverse effects of ethanol on brain development and function.116,117,120,126,130,133
Downstream Consequences of Ethanol-Impaired Insulin/IGF Signaling in the CNS
Neuronal integrity and function in the CNS are highly influenced by insulin and IGF signaling. For example, CNS neuronal survival, energy metabolism, and plasticity, which are critical for maintaining cognitive and motor functions, are regulated through the actions of insulin and IGF types 1 and 2.72 In this regard, insulin promotes neurite outgrowth, protein synthesis, neuronal cytoskeletal protein expression, and nascent synapse formation.134,135 Insulin and IGF-1 regulate the expression and phosphorylation of tau 136,137, an important neuronal cytoskeletal protein, while ethanol-impaired signaling through insulin or IGF-1 increases GSK-3β phosphorylation of tau, which is pathologic and contributes to neurodegeneration.138,139 Insulin and IGF-1 signaling also regulates choline acetyltransferase (ChAT)40,140,141, which is required for acetylcholine biosynthesis.
Acetylcholine is a major neurotransmitter that mediates cognitive-motor functions in the brain and is deficient in brains of chronic ethanol-exposed animal models.40,129,142-145 Although ethanol has demonstrated inhibitory effects on many neurotransmitter systems that modulate neuronal activity and plasticity51-53,129,146-150, herein we emphasize the adverse effects of ethanol on cholinergic systems because of their widespread distribution in the brain and their regulation by insulin/IGF signaling.40,72,151
In aggregate, the data suggest that an important mechanism by which ethanol impairs neuronal survival, growth, neurotransmitter function and plasticity is to inhibit the actions of insulin and IGFs in the developing brain. On a cellular basis, compromise of insulin/IGF signal transduction networks leads to increased neuronal apoptosis, mitochondrial dysfunction with deficits in energy metabolism, oxidative stress, activation of pro-death and pro-stress signaling, and deficits in cholinergic function, all of which are features of FASD.40,56,84 Therefore, it is likely that ethanol inhibition of insulin and IGF signaling in the brain significantly contributes to the cognitive and motor impairments associated with FASD. Together, these observations led us to the hypothesis that insulin sensitizer agents such as peroxisome-proliferator activated receptor agonists that both enhance expression and function of downstream targets of insulin/IGF signaling and reduce cellular stress may have therapeutic application in the context of FASD.
Peroxisome-proliferator Activated Receptors (PPAR)
PPAR-α, δ, and γ, are nuclear hormone receptors that bind to DNA and regulate gene transcription in a broad range of cells and tissues.152-155 PPARs are regulated by ligand binding and mediate their effects by heterodimerizing with the retinoid × receptor.152-155 PPARs have important roles in regulating adipocyte growth and differentiation, insulin responsiveness, and cardiovascular function.152-155 For example, the enhanced insulin sensitivity imparted by PPAR-γ agonists led to their current use in the treatment of type 2 diabetes mellitus.156 In addition, PPAR agonists can modulate vascular function, resulting in vasorelaxation and lowered blood pressure through increased release of nitric oxide.157,158 Correspondingly, dominant-negative mutations in the PPAR-γ gene result in early onset hypertension and insulin resistance in humans.159 In addition to their actions on endocrine signaling, PPARs help protect cells from the adverse effects of lipid peroxidation.154,155,160 Lipid peroxidation is a recognized consequence of oxidative stress in the brain, and increased levels of lipid peroxidation products have been detected in brains with neurodegeneration161-164, as well as in disease states that cause mitochondrial dysfunction and oxidative stress.84,165
Potential Therapeutic Role for PPAR Agonists in FASD
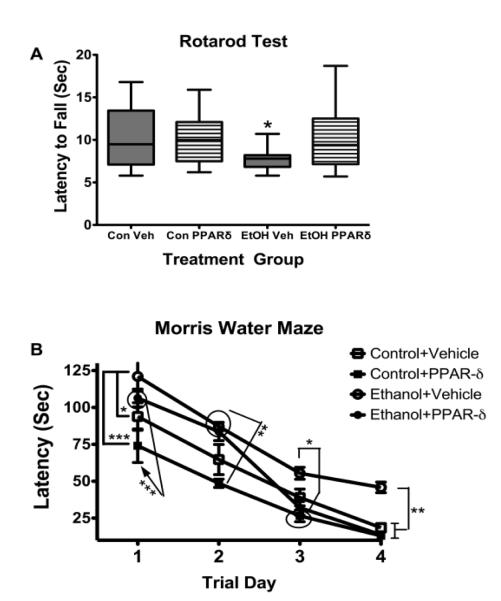
To begin examining potential therapeutic effects of PPAR agonists in relation to ethanol-induced neuro-cognitive deficits, we generated an in vivo model in which Long Evans rat pups were treated with ethanol (3 mg/g) or vehicle by intraperitoneal injection.166,167 (50 μl) on postnatal days (P) 4, P6, and P8. Rats in both groups were also treated with vehicle or a PPAR-delta (δ agonist (L-165,041; 2 μg/Kg) by i.p. injection on P5, P7, and P9. We previously showed that L-165,041 treatment could prevent insulin resistance-mediated neurodegeneration141 and alcohol-induced liver disease168 in vivo. On P16 the rats were evaluated by rotarod tests of latency to fall using an incremental fixed speed protocol (10 trials, 1-5 rpm)169, and data from Trials 7-10 (speed= 5 rpm) were grouped and analyzed by two-way ANOVA with the Bonferroni post-hoc test. From P27 to P30, rats were evaluated by Morris Water Maze testing as previously described.141 In brief, rats were subjected to 3 daily trials in which the latencies (seconds) required to locate and land on the platform were recorded. On Day 1, the platform was visible, but on Days 2-4, the platform was submerged. On Days 3 and 4, the water entry quadrants were randomized for each trial. Morris water maze data were grouped and analyzed by repeated measures mixed models ANOVA (diet × treatment × trial day) using area-under-the-curve (AUC) calculations corresponding to performance over the 3 trials each day 141. For both studies, 8 male rats were included in each sub-group.
Rotarod performance differed significantly among the groups as demonstrated by two-way ANOVA (F=4.98, P=0.028 for interaction of group by treatment; F=4.754, P=0.031 for treatment effect; F=3.04, P=0.084 for group effect). The Bonferroni post-hoc test demonstrated that vehicle-treated ethanol exposed rats had a significantly shorter mean latency to fall compared with all other groups (P<0.05; Figure 2A). In essence, L-165,041 treatment of ethanol-exposed rats increased the mean latency to fall, resulting in an overall performance that was similar to controls treated with either vehicle or the PPAR-δ agonist. With regard to the Morris water maze tests, all groups exhibited gradual improvements in latency required to locate the platform. The repeated measures mixed model ANOVA test demonstrated highly significant inter-group performance differences due to treatment effects (F=20.83, P<0.0001) and trial day/time (F=95.72, P<0.0001). Furthermore, Bonferroni post-hoc tests revealed significant differences in mean latency for locating the platform between the Control+Vehicle and Ethanol+Vehicle groups on Trial Days 1 (P<0.05) and 4 (P<0.01), between the Control+PPAR-δ and Ethanol+Vehicle groups on Trial Days 1 (P<0.001), 2 (P<0.001), 3 (P<0.05), and 4 (P<0.01), and between the Control+PPAR-δ and Ethanol+PPAR-δ groups on Trial Days 1 (P<0.001), 2 (P<0.01), and 4 (P<0.01). In essence, ethanol treatment significantly impaired performance on the Morris water maze tests relative to controls that were treated with either vehicle or the PPAR-δ agonist. Importantly, PPAR-δ agonist treatment improved Morris water maze performance in ethanol exposed rats such that, on Trial Days 3 and 4 when the platform was hidden and the entry points were randomized, their mean latencies needed to locate the platform were similar to controls, and significantly shorter than in the ethanol+vehicle group (Figure 2B).
FIG. 2A & 2B.
Therapeutic benefit of PPAR-delta (δ treatment in an ethanol exposure model. Long Evans rat pups were treated with ethanol (3 mg/g) or vehicle (Veh) by i.p. injection (50 μl) on postnatal days (P) 4, 6, and 8. Rats were also treated with vehicle or L-165,041 (2 μg/Kg), a PPAR-δ agonist by i.p. injection on P5, P7, and P9. Eight male rats were included in each sub-group. (A) On P16, the rats were evaluated by rotarod testing of latency to fall using an incremental (1-5 rpm) fixed speed protocol. Rotarod data from trials 7-10 (speed= 5 rpm) were grouped and analyzed by two-way ANOVA (F=4.98, P=0.028 for interaction of group by treatment; F=4.754, P=0.031 for treatment effect; F=3.04, P=0.084 for group effect). The Bonferroni post-hoc test revealed that the mean latency to fall was significantly shorter for the Ethanol (EtOH) +Veh group compared with all other groups (P<0.05). (B) From P27 to P30, rats were evaluated by Morris Water Maze testing with 3 trials per day. On Day 1, the platform was visible, but on Days 2-4, the platform was submerged. On Days 3 and 4, the water entry quadrant was randomized. The latency for locating and landing on the platform was recorded. Data were analyzed using area-under-the-curve (AUC) calculations corresponding to performance over the 3 trials each day. The graph depicts the mean ± S.E.M. of AUC latencies for each group on each day of testing. Inter-group comparisons were made using repeated measured mixed model ANOVA (F=20.83, P<0.0001 for treatment; and F=95.72, P<0.0001 for Trial Day). Post-hoc Bonferroni tests demonstrated significant differences between Control+Vehicle and Ethanol+Vehicle on Trial Days 1 (P<0.05) and 4 (P<0.01), between Control+ PPAR-δ and Ethanol+Vehicle on Trial Days 1 (P<0.001), 2 (P<0.001), 3 (P<0.05), and 4 (P<0.01), and Control+PPAR-δx and Ethanol+PPAR-δ on Trial Days 1 (P<0.001), 2 (P<0.01), and 4 (P<0.01). (*P<0.05; **P<0.01; ***P<0.001)
These findings suggest that early treatment with a PPAR-δ agonist can reduce the severity or prevent ethanol-mediated cognitive-motor deficits. Moreover, these results support our main hypothesis.
HYPOTHESIS
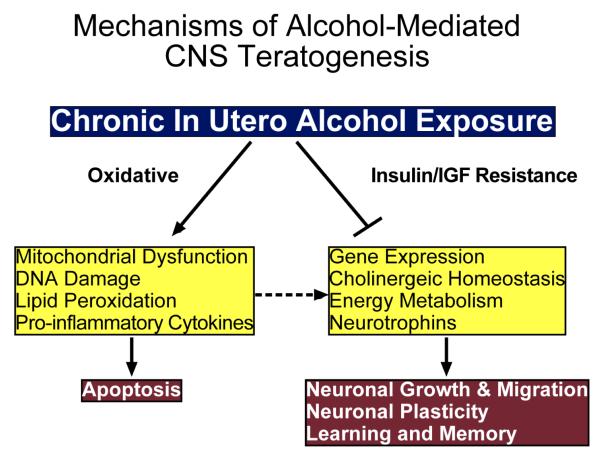
The experimental evidence to date suggests a dual mechanism underlies ethanol-mediated CNS neuronal death during development, namely: 1) impaired survival signaling through brain insulin (IRβ) and probably also IGF-1 receptors, as evidenced by reduced levels of tyrosine phosphorylated (PY) IRβ, IRβ tyrosine kinase (TK) activity, PY-insulin receptor substrate-1 (IRS-1), and PI3 kinase-Akt; and 2) increased oxidative stress that is principally mediated by the combined effects of mitochondrial dysfunction (Figure 3), increased generation of reactive oxygen species (ROS), lipid peroxidation, and DNA damage. Our working hypothesis is that chronic gestational exposure to ethanol produces long-lasting and progressive abnormalities in CNS structure and function due to persistent impairments in insulin signaling caused by CNS insulin resistance. We propose that by circumventing upstream problems in the insulin signaling cascade, i.e. bypassing receptor functions, activating downstream pathways, and altering gene expression, we will be able to therapeutically rescue CNS neuronal cells from ethanol-mediated injury/degeneration.
FIG. 3.
Dual mechanisms hypothesis of alcohol-mediated CNS teratogenesis. Chronic gestational exposure to ethanol causes oxidative stress and insulin/IGF resistance in the brain. The oxidative stress is caused by mitochondrial dysfunction, which promotes DNA damage, lipid peroxidation, pro-inflammatory cytokine activation, and apoptosis. Insulin/IGF resistance in brain impairs gene expression required for neuronal genesis, myelin maintenance, cell migration, neurotransmitter function, and energy metabolism. Oxidative stress and pro-inflammatory cytokine activation exacerbate the adverse effects of insulin/IGF resistance. Together, these abnormalities lead to deficits in neuronal plasticity, learning and memory.
Funding Acknowledgement
Supported by AA-02666, AA-02169, AA-11431, AA-12908, and AA-16126 from the National Institutes of Health
REFERENCES
- 1.Alcohol use among women of childbearing age--United States, 1991-1999. MMWR Morb Mortal Wkly Rep. 2002;51:273–6. [PubMed] [Google Scholar]
- 2.Riley EP, McGee CL. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med (Maywood) 2005;230:357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- 3.Astley SJ. Fetal alcohol syndrome prevention in Washington State: evidence of success. Paediatr Perinat Epidemiol. 2004;18:344–51. doi: 10.1111/j.1365-3016.2004.00582.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoyme HE, May PA, Kalberg WO, et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 institute of medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet. 2004;127:42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- 6.Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230:389–93. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- 7.Stromland K, Pinazo-Duran MD. Ophthalmic involvement in the fetal alcohol syndrome: clinical and animal model studies. Alcohol Alcohol. 2002;37:2–8. doi: 10.1093/alcalc/37.1.2. [DOI] [PubMed] [Google Scholar]
- 8.Monti PM, Miranda R, Jr., Nixon K, et al. Adolescence: booze, brains, and behavior. Alcohol Clin Exp Res. 2005;29:207–20. doi: 10.1097/01.alc.0000153551.11000.f3. [DOI] [PubMed] [Google Scholar]
- 9.Brown SA, Tapert SF. Adolescence and the trajectory of alcohol use: basic to clinical studies. Ann N Y Acad Sci. 2004;1021:234–44. doi: 10.1196/annals.1308.028. [DOI] [PubMed] [Google Scholar]
- 10.Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–52. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapert SF, Schweinsburg AD, Barlett VC, et al. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–86. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- 12.Tapert SF, Pulido C, Paulus MP, Schuckit MA, Burke C. Level of response to alcohol and brain response during visual working memory. J Stud Alcohol. 2004;65:692–700. doi: 10.15288/jsa.2004.65.692. [DOI] [PubMed] [Google Scholar]
- 13.Townshend JM, Duka T. Binge drinking, cognitive performance and mood in a population of young social drinkers. Alcohol Clin Exp Res. 2005;29:317–25. doi: 10.1097/01.alc.0000156453.05028.f5. [DOI] [PubMed] [Google Scholar]
- 14.Tapert SF, Brown GG, Kindermann SS, Cheung EH, Frank LR, Brown SA. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol Clin Exp Res. 2001;25:236–45. [PubMed] [Google Scholar]
- 15.Sher L. Functional magnetic resonance imaging in studies of neurocognitive effects of alcohol use on adolescents and young adults. Int J Adolesc Med Health. 2006;18:3–7. doi: 10.1515/ijamh.2006.18.1.3. [DOI] [PubMed] [Google Scholar]
- 16.Burd L, Klug MG, Martsolf JT, Kerbeshian J. Fetal alcohol syndrome: neuropsychiatric phenomics. Neurotoxicol Teratol. 2003;25:697–705. doi: 10.1016/j.ntt.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 17.O’Malley KD, Nanson J. Clinical implications of a link between fetal alcohol spectrum disorder and attention-deficit hyperactivity disorder. Can J Psychiatry. 2002;47:349–54. doi: 10.1177/070674370204700405. [DOI] [PubMed] [Google Scholar]
- 18.Coles CD. Fetal alcohol exposure and attention: moving beyond ADHD. Alcohol Res Health. 2001;25:199–203. [PMC free article] [PubMed] [Google Scholar]
- 19.Coffin JM, Baroody S, Schneider K, O’Neill J. Impaired cerebellar learning in children with prenatal alcohol exposure: a comparative study of eyeblink conditioning in children with ADHD and dyslexia. Cortex. 2005;41:389–98. doi: 10.1016/s0010-9452(08)70275-2. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan-Estrin M, Jacobson SW, Jacobson JL. Neurobehavioral effects of prenatal alcohol exposure at 26 months. Neurotoxicol Teratol. 1999;21:503–11. doi: 10.1016/s0892-0362(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 21.Ma X, Coles CD, Lynch ME, et al. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res. 2005;29:1214–22. doi: 10.1097/01.alc.0000171934.22755.6d. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JL, Leff M. Children of substance abusers: overview of research findings. Pediatrics. 1999;103:1085–99. [PubMed] [Google Scholar]
- 23.Baumbach J. Some implications of prenatal alcohol exposure for the treatment of adolescents with sexual offending behaviors. Sex Abuse. 2002;14:313–27. doi: 10.1177/107906320201400403. [DOI] [PubMed] [Google Scholar]
- 24.Young NK. Effects of alcohol and other drugs on children. J Psychoactive Drugs. 1997;29:23–42. doi: 10.1080/02791072.1997.10400168. [DOI] [PubMed] [Google Scholar]
- 25.Barr HM, Bookstein FL, O’Malley KD, Connor PD, Huggins JE, Streissguth AP. Binge drinking during pregnancy as a predictor of psychiatric disorders on the Structured Clinical Interview for DSM-IV in young adult offspring. Am J Psychiatry. 2006;163:1061–5. doi: 10.1176/ajp.2006.163.6.1061. [DOI] [PubMed] [Google Scholar]
- 26.Olson HC, Streissguth AP, Sampson PD, Barr HM, Bookstein FL, Thiede K. Association of prenatal alcohol exposure with behavioral and learning problems in early adolescence. J Am Acad Child Adolesc Psychiatry. 1997;36:1187–94. doi: 10.1097/00004583-199709000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–31. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- 28.O’Hare ED, Kan E, Yoshii J, et al. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport. 2005;16:1285–90. doi: 10.1097/01.wnr.0000176515.11723.a2. [DOI] [PubMed] [Google Scholar]
- 29.Aronson M, Hagberg B. Neuropsychological disorders in children exposed to alcohol during pregnancy: a follow-up study of 24 children to alcoholic mothers in Goteborg, Sweden. Alcohol Clin Exp Res. 1998;22:321–4. doi: 10.1111/j.1530-0277.1998.tb03655.x. [DOI] [PubMed] [Google Scholar]
- 30.Goodlett CR, Lundahl KR. Temporal determinants of neonatal alcohol-induced cerebellar damage and motor performance deficits. Pharmacol Biochem Behav. 1996;55:531–40. doi: 10.1016/s0091-3057(96)00248-1. [DOI] [PubMed] [Google Scholar]
- 31.Thomas JD, Burchette TL, Dominguez HD, Riley EP. Neonatal alcohol exposure produces more severe motor coordination deficits in high alcohol sensitive rats compared to low alcohol sensitive rats. Alcohol. 2000;20:93–9. doi: 10.1016/s0741-8329(99)00080-4. [DOI] [PubMed] [Google Scholar]
- 32.Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol Res Health. 2001;25:185–91. [PMC free article] [PubMed] [Google Scholar]
- 33.Abel EL. Prenatal effects of alcohol. Drug Alcohol Depend. 1984;14:1–10. doi: 10.1016/0376-8716(84)90012-7. [DOI] [PubMed] [Google Scholar]
- 34.O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W. Prenatal alcohol exposure and attention, learning and intellectual ability at 14 years: A prospective longitudinal study. Early Hum Dev. 2007;83:115–23. doi: 10.1016/j.earlhumdev.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 35.O’Callaghan FV, O’Callaghan M, Najman JM, Williams GM, Bor W. Maternal alcohol consumption during pregnancy and physical outcomes up to 5 years of age: a longitudinal study. Early Hum Dev. 2003;71:137–48. doi: 10.1016/s0378-3782(03)00003-3. [DOI] [PubMed] [Google Scholar]
- 36.Tarelo-Acuna L, Olvera-Cortes E, Gonzalez-Burgos I. Prenatal and postnatal exposure to ethanol induces changes in the shape of the dendritic spines from hippocampal CA1 pyramidal neurons of the rat. Neurosci Lett. 2000;286:13–6. doi: 10.1016/s0304-3940(00)01075-2. [DOI] [PubMed] [Google Scholar]
- 37.Ozer E, Sarioglu S, Gure A. Effects of prenatal ethanol exposure on neuronal migration, neuronogenesis and brain myelination in the mice brain. Clin Neuropathol. 2000;19:21–5. [PubMed] [Google Scholar]
- 38.Volk B. Cerebellar histogenesis and synaptic maturation following pre- and postnatal alcohol administration. An electron-microscopic investigation of the rat cerebellar cortex. Acta Neuropathol. 1984;63:57–65. doi: 10.1007/BF00688471. [DOI] [PubMed] [Google Scholar]
- 39.Edwards HG, Dow-Edwards DL. Craniofacial alterations in adult rats prenatally exposed to ethanol. Teratology. 1991;44:373–8. doi: 10.1002/tera.1420440403. [DOI] [PubMed] [Google Scholar]
- 40.Soscia SJ, Tong M, Xu XJ, et al. Chronic gestational exposure to ethanol causes insulin and IGF resistance and impairs acetylcholine homeostasis in the brain. Cell Mol Life Sci. 2006;63:2039–56. doi: 10.1007/s00018-006-6208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarren SK, Alvord EJ, Sumi SM, Streissguth AP, Smith DW. Brain malformations related to prenatal exposure to ethanol. J Pediatr. 1978;92:64–7. doi: 10.1016/s0022-3476(78)80072-9. [DOI] [PubMed] [Google Scholar]
- 42.de la Monte SM, Tong M, Carlson RI, et al. Ethanol inhibition of aspartyl-asparaginyl-beta-hydroxylase in fetal alcohol spectrum disorder: potential link to the impairments in central nervous system neuronal migration. Alcohol. 2009;43:225–40. doi: 10.1016/j.alcohol.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumada T, Jiang Y, Cameron DB, Komuro H. How does alcohol impair neuronal migration? J Neurosci Res. 2007;85:465–70. doi: 10.1002/jnr.21149. [DOI] [PubMed] [Google Scholar]
- 44.Bonthius DJ, Pantazis NJ, Karacay B, et al. Alcohol exposure during the brain growth spurt promotes hippocampal seizures, rapid kindling, and spreading depression. Alcohol Clin Exp Res. 2001;25:734–45. [PubMed] [Google Scholar]
- 45.Ge Y, Belcher SM, Light KE. Alterations of cerebellar mRNA specific for BDNF, p75NTR, and TrkB receptor isoforms occur within hours of ethanol administration to 4-day-old rat pups. Brain Res Dev Brain Res. 2004;151:99–109. doi: 10.1016/j.devbrainres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Ge Y, Belcher SM, Pierce DR, Light KE. Altered expression of Bcl2, Bad and Bax mRNA occurs in the rat cerebellum within hours after ethanol exposure on postnatal day 4 but not on postnatal day 9. Brain Res Mol Brain Res. 2004;129:124–34. doi: 10.1016/j.molbrainres.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 47.Kelly SJ, Pierce DR, West JR. Microencephaly and hyperactivity in adult rats can be induced by neonatal exposure to high blood alcohol concentrations. Exp Neurol. 1987;96:580–93. doi: 10.1016/0014-4886(87)90220-2. [DOI] [PubMed] [Google Scholar]
- 48.Clarren SK, Smith DW. The fetal alcohol syndrome. N Engl J Med. 1978;298:1063–7. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- 49.de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch Neurol. 1988;45:990–2. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- 50.de la Monte SM, Wands JR. Chronic gestational exposure to ethanol impairs insulin-stimulated survival and mitochondrial function in cerebellar neurons. CMLS, Cell Mol Life Sci. 2002;59:882–93. doi: 10.1007/s00018-002-8475-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:304–12. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 52.Kelm MK, Criswell HE, Breese GR. Calcium release from presynaptic internal stores is required for ethanol to increase spontaneous gamma-aminobutyric acid release onto cerebellum Purkinje neurons. J Pharmacol Exp Ther. 2007;323:356–64. doi: 10.1124/jpet.107.126144. [DOI] [PubMed] [Google Scholar]
- 53.Olney JW, Farber NB, Wozniak DF, Jevtovic-Todorovic V, Ikonomidou C. Environmental agents that have the potential to trigger massive apoptotic neurodegeneration in the developing brain. Environ Health Perspect. 2000;108(Suppl 3):383–8. doi: 10.1289/ehp.00108s3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med (Maywood) 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- 55.Miller MW, Mooney SM. Chronic exposure to ethanol alters neurotrophin content in the basal forebrain-cortex system in the mature rat: effects on autocrine-paracrine mechanisms. J Neurobiol. 2004;60:490–8. doi: 10.1002/neu.20059. [DOI] [PubMed] [Google Scholar]
- 56.de la Monte SM, Xu XJ, Wands JR. Ethanol inhibits insulin expression and actions in the developing brain. Cell Mol Life Sci. 2005;62:1131–45. doi: 10.1007/s00018-005-4571-z. [DOI] [PubMed] [Google Scholar]
- 57.Breese CR, D’Costa A, Sonntag WE. Effect of in utero ethanol exposure on the postnatal ontogeny of insulin-like growth factor-1, and type-1 and type-2 insulin-like growth factor receptors in the rat brain. Neuroscience. 1994;63:579–89. doi: 10.1016/0306-4522(94)90551-7. [DOI] [PubMed] [Google Scholar]
- 58.Resnicoff M, Rubini M, Baserga R, Rubin R. Ethanol inhibits insulin-like growth factor-1-mediated signalling and proliferation of C6 rat glioblastoma cells. Lab Invest. 1994;71:657–62. [PubMed] [Google Scholar]
- 59.Dees WL, Srivastava V, Hiney JK. Actions and interactions of alcohol and insulin-like growth factor-1 on female pubertal development. Alcohol Clin Exp Res. 2009;33:1847–56. doi: 10.1111/j.1530-0277.2009.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGough NN, Thomas JD, Dominguez HD, Riley EP. Insulin-like growth factor-I mitigates motor coordination deficits associated with neonatal alcohol exposure in rats. Neurotoxicol Teratol. 2009;31:40–8. doi: 10.1016/j.ntt.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo J, Miller MW. Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev. 1998;27:157–67. doi: 10.1016/s0165-0173(98)00009-5. [DOI] [PubMed] [Google Scholar]
- 62.Tateno M, Ukai W, Ozawa H, et al. Ethanol inhibition of neural stem cell differentiation is reduced by neurotrophic factors. Alcohol Clin Exp Res. 2004;28:134S–8S. doi: 10.1097/01.alc.0000133538.40841.36. [DOI] [PubMed] [Google Scholar]
- 63.Biermann T, Reulbach U, Lenz B, et al. N-methyl-D-aspartate 2b receptor subtype (NR2B) promoter methylation in patients during alcohol withdrawal. J Neural Transm. 2009;116:615–22. doi: 10.1007/s00702-009-0212-2. [DOI] [PubMed] [Google Scholar]
- 64.Ishii T, Hashimoto E, Ukai W, et al. Epigenetic regulation in alcohol-related brain damage. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2008;43:705–13. [PubMed] [Google Scholar]
- 65.Ouko LA, Shantikumar K, Knezovich J, Haycock P, Schnugh DJ, Ramsay M. Effect of alcohol consumption on CpG methylation in the differentially methylated regions of H19 and IG-DMR in male gametes: implications for fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1615–27. doi: 10.1111/j.1530-0277.2009.00993.x. [DOI] [PubMed] [Google Scholar]
- 66.Haycock PC. Fetal alcohol spectrum disorders: the epigenetic perspective. Biol Reprod. 2009;81:607–17. doi: 10.1095/biolreprod.108.074690. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Balaraman Y, Wang G, Nephew KP, Zhou FC. Alcohol exposure alters DNA methylation profiles in mouse embryos at early neurulation. Epigenetics. 2009;4:500–11. doi: 10.4161/epi.4.7.9925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Haycock PC, Ramsay M. Exposure of mouse embryos to ethanol during preimplantation development: effect on DNA methylation in the h19 imprinting control region. Biol Reprod. 2009;81:618–27. doi: 10.1095/biolreprod.108.074682. [DOI] [PubMed] [Google Scholar]
- 69.Gammeltoft S, Fehlmann M, Van OE. Insulin receptors in the mammalian central nervous system: binding characteristics and subunit structure. Biochimie. 1985;67:1147–53. doi: 10.1016/s0300-9084(85)80113-9. [DOI] [PubMed] [Google Scholar]
- 70.Goodyer CG, De SL, Lai WH, Guyda HJ, Posner BI. Characterization of insulin-like growth factor receptors in rat anterior pituitary, hypothalamus, and brain. Endocrinology. 1984;114:1187–95. doi: 10.1210/endo-114-4-1187. [DOI] [PubMed] [Google Scholar]
- 71.Hill JM, Lesniak MA, Pert CB, Roth J. Autoradiographic localization of insulin receptors in rat brain: prominence in olfactory and limbic areas. Neuroscience. 1986;17:1127–38. doi: 10.1016/0306-4522(86)90082-5. [DOI] [PubMed] [Google Scholar]
- 72.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. J Alzheimers Dis. 2005;7:45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 73.Myers MG, White MF. The new elements of insulin signaling. Insulin receptor substrate-1 and proteins with SH2 domains. Diabetes. 1993;42:643–50. doi: 10.2337/diab.42.5.643. [DOI] [PubMed] [Google Scholar]
- 74.Kido Y, Nakae J, Accili D. Clinical review 125: The insulin receptor and its cellular targets. J Clin Endocrinol Metab. 2001;86:972–9. doi: 10.1210/jcem.86.3.7306. [DOI] [PubMed] [Google Scholar]
- 75.Del Valle L, Wang JY, Lassak A, et al. Insulin-like growth factor I receptor signaling system in JC virus T antigen-induced primitive neuroectodermal tumors--medulloblastomas. J Neurovirol. 2002;8(Suppl 2):138–47. doi: 10.1080/13550280290101111. [DOI] [PubMed] [Google Scholar]
- 76.Folli F, Saad MJ, Kahn CR. Insulin receptor/IRS-1/PI 3-kinase signaling system in corticosteroid-induced insulin resistance. Acta Diabetol. 1996;33:185–92. doi: 10.1007/BF02048541. [DOI] [PubMed] [Google Scholar]
- 77.Nakae J, Kido Y, Accili D. Distinct and overlapping functions of insulin and IGF-I receptors. Endocr Rev. 2001;22:818–35. doi: 10.1210/edrv.22.6.0452. [DOI] [PubMed] [Google Scholar]
- 78.Giovannone B, Scaldaferri ML, Federici M, et al. Insulin receptor substrate (IRS) transduction system: distinct and overlapping signaling potential. Diabetes Metab Res Rev. 2000;16:434–41. doi: 10.1002/1520-7560(2000)9999:9999<::aid-dmrr159>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 79.Xu YY, Bhavani K, Wands JR, de la Monte SM. Ethanol inhibits insulin receptor substrate-1 tyrosine phosphorylation and insulin-stimulated neuronal thread protein gene expression. Biochem J. 1995;310:125–32. doi: 10.1042/bj3100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mauceri HJ, Lee WH, Conway S. Effect of ethanol on insulin-like growth factor-II release from fetal organs. Alcohol Clin Exp Res. 1994;18:35–41. doi: 10.1111/j.1530-0277.1994.tb00877.x. [DOI] [PubMed] [Google Scholar]
- 81.Luo J, Miller MW. Differential sensitivity of human neuroblastoma cell lines to ethanol: correlations with their proliferative responses to mitogenic growth factors and expression of growth factor receptors. Alcohol Clin Exp Res. 1997;21:1186–94. [PubMed] [Google Scholar]
- 82.Luo J, Miller MW. Platelet-derived growth factor-mediated signal transduction underlying astrocyte proliferation: site of ethanol action. J Neurosci. 1999;19:10014–25. doi: 10.1523/JNEUROSCI.19-22-10014.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luo J, West JR, Cook RT, Pantazis NJ. Ethanol induces cell death and cell cycle delay in cultures of pheochromocytoma PC12 cells. Alcohol Clin Exp Res. 1999;23:644–56. [PubMed] [Google Scholar]
- 84.Chu J, Tong M, de la Monte SM. Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol (Berl) 2007;113:659–73. doi: 10.1007/s00401-007-0199-4. [DOI] [PubMed] [Google Scholar]
- 85.de la Monte SM, Wands JR. Mitochondrial DNA damage and impaired mitochondrial function contribute to apoptosis of insulin-stimulated ethanol-exposed neuronal cells. Alcohol Clin Exp Res. 2001;25:898–906. [PubMed] [Google Scholar]
- 86.Miller MW. A longitudinal study of the effects of prenatal ethanol exposure on neuronal acquisition and death in the principal sensory nucleus of the trigeminal nerve: interaction with changes induced by transection of the infraorbital nerve. J Neurocytol. 1999;28:999–1015. doi: 10.1023/a:1007088021115. [DOI] [PubMed] [Google Scholar]
- 87.West JR, Goodlett CR, Bonthius DJ, Hamre KM, Marcussen BL. Cell population depletion associated with fetal alcohol brain damage: mechanisms of BAC-dependent cell loss. Alcohol Clin Exp Res. 1990;14:813–8. doi: 10.1111/j.1530-0277.1990.tb01820.x. [DOI] [PubMed] [Google Scholar]
- 88.Zhang FX, Rubin R, Rooney TA. Ethanol induces apoptosis in cerebellar granule neurons by inhibiting insulin-like growth factor 1 signaling. J Neurochem. 1998;71:196–204. doi: 10.1046/j.1471-4159.1998.71010196.x. [DOI] [PubMed] [Google Scholar]
- 89.Acquaah-Mensah GK, Kehrer JP, Leslie SW. In utero ethanol suppresses cerebellar activator protein-1 and nuclear factor-kappa B transcriptional activation in a rat fetal alcohol syndrome model. J Pharmacol Exp Ther. 2002;301:277–83. doi: 10.1124/jpet.301.1.277. [DOI] [PubMed] [Google Scholar]
- 90.Carter JJ, Tong M, Silbermann E, et al. Ethanol impaired neuronal migration is associated with reduced aspartyl-asparaginyl-beta-hydroxylase expression. Acta Neuropathol. 2008 doi: 10.1007/s00401-008-0377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chakraborty G, Saito M, Mao RF, Wang R, Vadasz C, Saito M. Lithium blocks ethanol-induced modulation of protein kinases in the developing brain. Biochem Biophys Res Commun. 2008;367:597–602. doi: 10.1016/j.bbrc.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo J. GSK3beta in ethanol neurotoxicity. Mol Neurobiol. 2009;40:108–21. doi: 10.1007/s12035-009-8075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Takadera T, Ohyashiki T. Glycogen synthase kinase-3 inhibitors prevent caspase-dependent apoptosis induced by ethanol in cultured rat cortical neurons. Eur J Pharmacol. 2004;499:239–45. doi: 10.1016/j.ejphar.2004.07.115. [DOI] [PubMed] [Google Scholar]
- 94.Sasaki Y, Wands JR. Ethanol impairs insulin receptor substrate-1 mediated signal transduction during rat liver regeneration. Biochem Biophys Res Commun. 1994;199:403–9. doi: 10.1006/bbrc.1994.1243. [DOI] [PubMed] [Google Scholar]
- 95.de la Monte SM, Ganju N, Tanaka S, et al. Differential effects of ethanol on insulin-signaling through the insulin receptor substrate-1. Alcohol Clin Exp Res. 1999;23:770–7. doi: 10.1097/00000374-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 96.Banerjee K, Mohr L, Wands JR, de la Monte SM. Ethanol inhibition of insulin signaling in hepatocellular carcinoma cells. Alcohol Clin Exp Res. 1998;22:2093–101. [PubMed] [Google Scholar]
- 97.Mohr L, Tanaka S, Wands JR. Ethanol inhibits hepatocyte proliferation in insulin receptor substrate 1 transgenic mice. Gastroenterology. 1998;115:1558–65. doi: 10.1016/s0016-5085(98)70036-8. [DOI] [PubMed] [Google Scholar]
- 98.Xu J, Yeon JE, Chang H, et al. Ethanol impairs insulin-stimulated neuronal survival in the developing brain: role of PTEN phosphatase. J Biol Chem. 2003;278:26929–37. doi: 10.1074/jbc.M300401200. [DOI] [PubMed] [Google Scholar]
- 99.Diehl AM, Yang SQ, Cote P, Wand GS. Chronic ethanol consumption disturbs G-protein expression and inhibits cyclic AMP-dependent signaling in regenerating rat liver. Hepatology. 1992;16:1212–9. [PubMed] [Google Scholar]
- 100.Gordon AS, Collier K, Diamond I. Ethanol regulation of adenosine receptor-stimulated cAMP levels in a clonal neural cell line: an in vitro model of cellular tolerance to ethanol. Proc Natl Acad Sci U S A. 1986;83:2105–8. doi: 10.1073/pnas.83.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de la Monte SM, Ganju N, Banerjee K, Brown NV, Luong T, Wands JR. Partial rescue of ethanol-induced neuronal apoptosis by growth factor activation of phosphoinositol-3-kinase. Alcohol Clin Exp Res. 2000;24:716–26. [PubMed] [Google Scholar]
- 102.Yeon JE, Califano S, Xu J, Wands JR, De La Monte SM. Potential role of PTEN phosphatase in ethanol-impaired survival signaling in the liver. Hepatology. 2003;38:703–14. doi: 10.1053/jhep.2003.50368. [DOI] [PubMed] [Google Scholar]
- 103.Domenicotti C, Paola D, Lamedica A, et al. Effects of ethanol metabolism on PKC activity in isolated rat hepatocytes. Chem Biol Interact. 1996;100:155–63. doi: 10.1016/0009-2797(96)03696-4. [DOI] [PubMed] [Google Scholar]
- 104.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–5. doi: 10.1126/science.275.5300.661. [see comments] [DOI] [PubMed] [Google Scholar]
- 105.Bhave SV, Hoffman PL. Ethanol promotes apoptosis in cerebellar granule cells by inhibiting the trophic effect of NMDA. J Neurochem. 1997;68:578–86. doi: 10.1046/j.1471-4159.1997.68020578.x. [DOI] [PubMed] [Google Scholar]
- 106.Ikonomidou C, Bittigau P, Ishimaru MJ, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–60. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 107.Olney JW, Ishimaru MJ, Bittigau P, Ikonomidou C. Ethanol-induced apoptotic neurodegeneration in the developing brain. Apoptosis. 2000;5:515–21. doi: 10.1023/a:1009685428847. [DOI] [PubMed] [Google Scholar]
- 108.de la Monte SM, Neely TR, Cannon J, Wands JR. Ethanol impairs insulin-stimulated mitochondrial function in cerebellar granule neurons. Cell Mol Life Sci. 2001;58:1950–60. doi: 10.1007/PL00000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hallak H, Seiler AE, Green JS, Henderson A, Ross BN, Rubin R. Inhibition of insulin-like growth factor-I signaling by ethanol in neuronal cells. Alcohol Clin Exp Res. 2001;25:1058–64. [PubMed] [Google Scholar]
- 110.Camp MC, Mayfield RD, McCracken M, McCracken L, Alcantara AA. Neuroadaptations of Cdk5 in cholinergic interneurons of the nucleus accumbens and prefrontal cortex of inbred alcohol-preferring rats following voluntary alcohol drinking. Alcohol Clin Exp Res. 2006;30:1322–35. doi: 10.1111/j.1530-0277.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 111.Rajgopal Y, Vemuri MC. Ethanol induced changes in cyclin-dependent kinase-5 activity and its activators, P35, P67 (Munc-18) in rat brain. Neurosci Lett. 2001;308:173–6. doi: 10.1016/s0304-3940(01)02011-0. [DOI] [PubMed] [Google Scholar]
- 112.Ramachandran V, Perez A, Chen J, Senthil D, Schenker S, Henderson GI. In utero ethanol exposure causes mitochondrial dysfunction, which can result in apoptotic cell death in fetal brain: a potential role for 4- hydroxynonenal. Alcohol Clin Exp Res. 2001;25:862–71. [PubMed] [Google Scholar]
- 113.Bannigan J, Cottell D. Ethanol teratogenicity in mice: an electron microscopic study. Teratology. 1984;30:281–90. doi: 10.1002/tera.1420300216. [DOI] [PubMed] [Google Scholar]
- 114.Farrar RP, Seibert C, Gnau K, Leslie SW. Development of tolerance in brain mitochondria for calcium uptake following chronic ethanol ingestion. Brain Res. 1989;500:374–8. doi: 10.1016/0006-8993(89)90334-x. [DOI] [PubMed] [Google Scholar]
- 115.Goodlett CR, Horn KH. Mechanisms of alcohol-induced damage to the developing nervous system. Alcohol Res Health. 2001;25:175–84. [PMC free article] [PubMed] [Google Scholar]
- 116.Henderson GI, Chen JJ, Schenker S. Ethanol, oxidative stress, reactive aldehydes, and the fetus. Front Biosci. 1999;4:D541–50. doi: 10.2741/henderson. [DOI] [PubMed] [Google Scholar]
- 117.Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther. 2001;298:737–43. [PubMed] [Google Scholar]
- 118.Xu YJ, Liu P, Li Y. Effect of alcohol on brain mitochondria development of mouse embryos. Wei Sheng Yan Jiu. 2005;34:61–3. [PubMed] [Google Scholar]
- 119.Cahill A, Wang X, Hoek JB. Increased oxidative damage to mitochondrial DNA following chronic ethanol consumption. Biochem Biophys Res Commun. 1997;235:286–90. doi: 10.1006/bbrc.1997.6774. [DOI] [PubMed] [Google Scholar]
- 120.Lee RD, An SM, Kim SS, et al. Neurotoxic effects of alcohol and acetaldehyde during embryonic development. J Toxicol Environ Health A. 2005;68:2147–62. doi: 10.1080/15287390500177255. [DOI] [PubMed] [Google Scholar]
- 121.Pastorino JG. Potentiation by chronic, ethanol treatment of the mitochondrial permeability transition. Biochem Biophys Res Commun. 1999;265:405–9. doi: 10.1006/bbrc.1999.1696. [DOI] [PubMed] [Google Scholar]
- 122.Wieland P, Lauterburg BH. Oxidation of mitochondrial proteins and DNA following administration of ethanol. Biochem Biophys Res Commun. 1995;213:815–9. doi: 10.1006/bbrc.1995.2202. [DOI] [PubMed] [Google Scholar]
- 123.Almansa I, Fernandez A, Garcia-Ruiz C, et al. Brain mitochondrial alterations after chronic alcohol consumption. J Physiol Biochem. 2009;65:305–12. doi: 10.1007/BF03180583. [DOI] [PubMed] [Google Scholar]
- 124.Cherian PP, Schenker S, Henderson GI. Ethanol-mediated DNA damage and PARP-1 apoptotic responses in cultured fetal cortical neurons. Alcohol Clin Exp Res. 2008;32:1884–92. doi: 10.1111/j.1530-0277.2008.00769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Green CR, Watts LT, Kobus SM, Henderson GI, Reynolds JN, Brien JF. Effects of chronic prenatal ethanol exposure on mitochondrial glutathione and 8-iso-prostaglandin F2alpha concentrations in the hippocampus of the perinatal guinea pig. Reprod Fertil Dev. 2006;18:517–24. doi: 10.1071/rd05128. [DOI] [PubMed] [Google Scholar]
- 126.Siler-Marsiglio KI, Pan Q, Paiva M, Madorsky I, Khurana NC, Heaton MB. Mitochondrially targeted vitamin E and vitamin E mitigate ethanol-mediated effects on cerebellar granule cell antioxidant defense systems. Brain Res. 2005;1052:202–11. doi: 10.1016/j.brainres.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 127.Albano E, French SW, Ingelman-Sundberg M. Hydroxyethyl radicals in ethanol hepatotoxicity. Front Biosci. 1999;4:D533–40. doi: 10.2741/albano. [DOI] [PubMed] [Google Scholar]
- 128.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–76. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 129.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 130.Heaton MB, Mitchell JJ, Paiva M. Amelioration of ethanol-induced neurotoxicity in the neonatal rat central nervous system by antioxidant therapy. Alcohol Clin Exp Res. 2000;24:512–8. [PubMed] [Google Scholar]
- 131.Kim JH, Kim JE, Kim HJ, et al. Ethanol decreases the expression of mitochondrial cytochrome c oxidase mRNA in the rat. Neurosci Lett. 2001;305:107–10. doi: 10.1016/s0304-3940(01)01820-1. [DOI] [PubMed] [Google Scholar]
- 132.Wallin C, Puka-Sundvall M, Hagberg H, Weber SG, Sandberg M. Alterations in glutathione and amino acid concentrations after hypoxia-ischemia in the immature rat brain. Brain Res Dev Brain Res. 2000;125:51–60. doi: 10.1016/s0165-3806(00)00112-7. [DOI] [PubMed] [Google Scholar]
- 133.Heaton MB, Moore DB, Paiva M, Madorsky I, Mayer J, Shaw G. The role of neurotrophic factors, apoptosis-related proteins, and endogenous antioxidants in the differential temporal vulnerability of neonatal cerebellum to ethanol. Alcohol Clin Exp Res. 2003;27:657–69. doi: 10.1097/01.ALC.0000060527.55252.71. [DOI] [PubMed] [Google Scholar]
- 134.Mill JF, Chao MV, Ishii DN. Insulin, insulin-like growth factor II, and nerve growth factor effects on tubulin mRNA levels and neurite formation. Proc Natl Acad Sci U S A. 1985;82:7126–30. doi: 10.1073/pnas.82.20.7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Puro DG, Agardh E. Insulin-mediated regulation of neuronal maturation. Science. 1984;225:1170–2. doi: 10.1126/science.6089343. [DOI] [PubMed] [Google Scholar]
- 136.de la Monte SM, Chen GJ, Rivera E, Wands JR. Neuronal thread protein regulation and interaction with microtubule-associated proteins in SH-Sy5y neuronal cells. Cell Mol Life Sci. 2003;60:2679–91. doi: 10.1007/s00018-003-3305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–53. doi: 10.1074/jbc.272.31.19547. [DOI] [PubMed] [Google Scholar]
- 138.Kosik KS, Shimura H. Phosphorylated tau and the neurodegenerative foldopathies. Biochim Biophys Acta. 2005;1739:298–310. doi: 10.1016/j.bbadis.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 139.Tsujio I, Tanaka T, Kudo T, et al. Inactivation of glycogen synthase kinase-3 by protein kinase C delta: implications for regulation of tau phosphorylation. FEBS Lett. 2000;469:111–7. doi: 10.1016/s0014-5793(00)01234-5. [DOI] [PubMed] [Google Scholar]
- 140.Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer’s disease. J Alzheimers Dis. 2006;9:13–33. doi: 10.3233/jad-2006-9102. [DOI] [PubMed] [Google Scholar]
- 141.de la Monte SM, Tong M, Lester-Coll N, Plater M, Jr., Wands JR. Therapeutic rescue of neurodegeneration in experimental type 3 diabetes: Relevance to Alzheimer’s disease. J Alzheimers Dis. 2006;10:89–109. doi: 10.3233/jad-2006-10113. [DOI] [PubMed] [Google Scholar]
- 142.Arendt T. Impairment in memory function and neurodegenerative changes in the cholinergic basal forebrain system induced by chronic intake of ethanol. J Neural Transm Suppl. 1994;44:173–87. doi: 10.1007/978-3-7091-9350-1_13. [DOI] [PubMed] [Google Scholar]
- 143.Cohen AC, Tong M, Wands JR, de la Monte SM. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol Clin Exp Res. 2007;31:1558–73. doi: 10.1111/j.1530-0277.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 144.Dymecki J, Medynska E, Walski M. The influence of ethanol intoxication on cholinergic axon terminals in the rat brain. (Morphometric evaluation) Exp Pathol. 1982;22:73–83. doi: 10.1016/s0232-1513(82)80028-5. [DOI] [PubMed] [Google Scholar]
- 145.West JR, Dewey SL, Cassell MD. Prenatal ethanol exposure alters the post-lesion reorganization (sprouting) of acetylcholinesterase staining in the dentate gyrus of adult rats. Brain Res. 1984;314:83–95. doi: 10.1016/0165-3806(84)90178-0. [DOI] [PubMed] [Google Scholar]
- 146.Diamond I, Gordon AS. Cellular and molecular neuroscience of alcoholism. Physiol Rev. 1997;77:1–20. doi: 10.1152/physrev.1997.77.1.1. [DOI] [PubMed] [Google Scholar]
- 147.Eravci M, Schulz O, Grospietsch T, et al. Gene expression of receptors and enzymes involved in GABAergic and glutamatergic neurotransmission in the CNS of rats behaviourally dependent on ethanol. Br J Pharmacol. 2000;131:423–32. doi: 10.1038/sj.bjp.0703596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hamid A, Wani NA, Kaur J. New perspectives on folate transport in relation to alcoholism-induced folate malabsorption--association with epigenome stability and cancer development. FEBS J. 2009;276:2175–91. doi: 10.1111/j.1742-4658.2009.06959.x. [DOI] [PubMed] [Google Scholar]
- 149.Lancaster FE. Alcohol, nitric oxide, and neurotoxicity: is there a connection?--a review. Alcohol Clin Exp Res. 1992;16:539–41. doi: 10.1111/j.1530-0277.1992.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 150.Savage DD, Montano CY, Otero MA, Paxton LL. Prenatal ethanol exposure decreases hippocampal NMDA-sensitive [3H]-glutamate binding site density in 45-day-old rats. Alcohol. 1991;8:193–201. doi: 10.1016/0741-8329(91)90806-8. [DOI] [PubMed] [Google Scholar]
- 151.Breese CR, D’Costa A, Booze RM, Sonntag WE. Distribution of insulin-like growth factor 1 (IGF-1) and 2 (IGF-2) receptors in the hippocampal formation of rats and mice. Adv Exp Med Biol. 1991;293:449–58. doi: 10.1007/978-1-4684-5949-4_40. [DOI] [PubMed] [Google Scholar]
- 152.Kliewer SA, Lehmann JM, Milburn MV, Willson TM. The PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signaling pathways. Recent Prog Horm Res. 1999;54:345–67. discussion 67-8. [PubMed] [Google Scholar]
- 153.Schoonjans K, Staels B, Auwerx J. The peroxisome proliferator activated receptors (PPARS) and their effects on lipid metabolism and adipocyte differentiation. Biochim Biophys Acta. 1996;1302:93–109. doi: 10.1016/0005-2760(96)00066-5. [DOI] [PubMed] [Google Scholar]
- 154.Gilde AJ, Van Bilsen M. Peroxisome proliferator-activated receptors (PPARS): regulators of gene expression in heart and skeletal muscle. Acta Physiol Scand. 2003;178:425–34. doi: 10.1046/j.1365-201X.2003.01161.x. [DOI] [PubMed] [Google Scholar]
- 155.Lee CH, Olson P, Evans RM. Minireview: lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology. 2003;144:2201–7. doi: 10.1210/en.2003-0288. [DOI] [PubMed] [Google Scholar]
- 156.Savkur RS, Miller AR. Investigational PPAR-gamma agonists for the treatment of Type 2 diabetes. Expert Opin Investig Drugs. 2006;15:763–78. doi: 10.1517/13543784.15.7.763. [DOI] [PubMed] [Google Scholar]
- 157.Tao L, Liu HR, Gao E, et al. Antioxidative, antinitrative, and vasculoprotective effects of a peroxisome proliferator-activated receptor-gamma agonist in hypercholesterolemia. Circulation. 2003;108:2805–11. doi: 10.1161/01.CIR.0000097003.49585.5E. [DOI] [PubMed] [Google Scholar]
- 158.Zhao X, Quigley JE, Yuan J, Wang MH, Zhou Y, Imig JD. PPAR-alpha activator fenofibrate increases renal CYP-derived eicosanoid synthesis and improves endothelial dilator function in obese Zucker rats. Am J Physiol Heart Circ Physiol. 2006;290:H2187–95. doi: 10.1152/ajpheart.00937.2005. [DOI] [PubMed] [Google Scholar]
- 159.Barroso I, Gurnell M, Crowley VE, et al. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–3. doi: 10.1038/47254. [DOI] [PubMed] [Google Scholar]
- 160.Bishop-Bailey D, Warner TD. PPARgamma ligands induce prostaglandin production in vascular smooth muscle cells: indomethacin acts as a peroxisome proliferator-activated receptor-gamma antagonist. Faseb J. 2003;17:1925–7. doi: 10.1096/fj.02-1075fje. [DOI] [PubMed] [Google Scholar]
- 161.Markesbery WR, Carney JM. Oxidative alterations in Alzheimer’s disease. Brain Pathol. 1999;9:133–46. doi: 10.1111/j.1750-3639.1999.tb00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Montine KS, Kim PJ, Olson SJ, Markesbery WR, Montine TJ. 4-hydroxy-2-nonenal pyrrole adducts in human neurodegenerative disease. J Neuropathol Exp Neurol. 1997;56:866–71. doi: 10.1097/00005072-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 163.Sayre LM, Zelasko DA, Harris PL, Perry G, Salomon RG, Smith MA. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J Neurochem. 1997;68:2092–7. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 164.de la Monte SM, Wands JR. Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J Alzheimers Dis. 2006;9:167–81. doi: 10.3233/jad-2006-9209. [DOI] [PubMed] [Google Scholar]
- 165.Ghafourifar P, Klein SD, Schucht O, et al. Ceramide induces cytochrome c release from isolated mitochondria. Importance of mitochondrial redox state. J Biol Chem. 1999;274:6080–4. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- 166.de Licona HK, Karacay B, Mahoney J, McDonald E, Luang T, Bonthius DJ. A single exposure to alcohol during brain development induces microencephaly and neuronal losses in genetically susceptible mice, but not in wild type mice. Neurotoxicology. 2009;30:459–70. doi: 10.1016/j.neuro.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 167.Li Z, Zharikova A, Vaughan CH, et al. Intermittent high-dose ethanol exposures increase motivation for operant ethanol self-administration: possible neurochemical mechanism. Brain Res. 1310:142–53. doi: 10.1016/j.brainres.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pang M, de la Monte SM, Longato L, et al. PPARdelta agonist attenuates alcohol-induced hepatic insulin resistance and improves liver injury and repair. J Hepatol. 2009;50:1192–201. doi: 10.1016/j.jhep.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Monville C, Torres EM, Dunnett SB. Comparison of incremental and accelerating protocols of the rotarod test for the assessment of motor deficits in the 6-OHDA model. J Neurosci Methods. 2006;158:219–23. doi: 10.1016/j.jneumeth.2006.06.001. [DOI] [PubMed] [Google Scholar]