Abstract
Multipotent neural stem/progenitor cells (NS/NPCs) that are capable of generating neurons and glia offer enormous potential for treating neurological diseases. Adult NS/NPCs that reside in the mature mammalian brain can be isolated and expanded in vitro, and could be a potential source for autologous transplantation to replace cells lost to brain injury or disease. When these cells are transplanted into the normal brain, they can survive and become region-specific cells. However, it has not been reported whether these cells can survive for an extended period and become functional cells in an injured heterotypic environment. In this study, we tested survival, maturation fate, and electrophysiological properties of adult NS/NPCs after transplantation into the injured rat brain. NS/NPCs were isolated from the subventricular zone of adult Fisher 344 rats and cultured as a monolayer. Recipient adult Fisher 344 rats were first subjected to a moderate fluid percussive injury. Two days later, cultured NS/NPCs were injected into the injured brain in an area between the white matter tracts and peri-cortical region directly underneath the injury impact. The animals were sacrificed 2 or 4 weeks after transplantation for immunohistochemical staining or patch-clamp recording. We found that transplanted cells survived well at 2 and 4 weeks. Many cells migrated out of the injection site into surrounding areas expressing astrocyte or oligodendrocyte markers. Whole cell patch-clamp recording at 4 weeks showed that transplanted cells possessed typical mature glial cell properties. These data demonstrate that adult NS/NPCs can survive in an injured heterotypic environment for an extended period and become functional cells.
Key words: neural stem/progenitor cells, subventricular zone, transplantation, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is the leading cause of death and disability of persons under the age of 45 years in the U.S. The recovery from such injuries is severely hindered due to the limited ability of the adult brain to replace damaged neurons lost to injury. Recent experimental successes in cell replacement in models of Parkinson's disease and other neurodegenerative diseases have inspired TBI researchers to investigate this approach for treating the injured brain (Studer et al., 1998).
The therapeutic potential of cell transplantation is based on the speculation that transplanted cells can differentiate into region-specific cells and integrate into the host tissue to replace lost cells in the injured brain; alternatively, transplanted cells could provide neurotransmitters or trophic factors in local CNS tissue to facilitate survival or regeneration of host cells (Bjorklund et al., 2000; Gates et al., 2000). Recent findings show that the mature mammalian CNS harbors multipotent stem cells that can differentiate into a variety of specialized cells throughout life (Gage et al., 1998; Lois and Alvarez-Buylla, 1993). These findings highlight the potential therapeutic value of adult-derived neural stem and progenitor cells (NS/NPCs) in treating neurological disorders and the injured brain (Gage et al., 1995; McKay, 1997).
NS/NPCs in the adult mammalian CNS are primarily confined to the subventricular zone (SVZ) surrounding the lateral ventricle and the dentate gyrus (DG) of the hippocampus (Altman and Das, 1965; Lois and Alvarez-Buylla, 1993). These adult-derived stem cells express low levels of the major histocompatibility complex antigens (Klassen et al., 2003) and display high survival rates following transplantation into the adult striatum of hemiparkinsonian rats (Dziewczapolski et al., 2003). Such properties make these cells potential candidates for neuronal cell replacement therapy. The survival and differentiation of these adult NS/NPCs following transplantation has been known to depend on both the intrinsic properties of the grafted cells and the regional environmental cues in the host. In normal conditions, cultured adult NS/NPCs can differentiate into region-specific neurons when transplanted into neurogenic regions such as the SVZ or hippocampus (Gage et al., 1995; Suhonen et al., 1996). However, when transplanted into non-neurogenic sites, these cells differentiate primarily into glia or remain undifferentiated (Dziewczapolski et al., 2003; Gage et al., 1995; Herrera et al., 1999; Richardson et al., 2005a). This suggests that the in vivo fate of transplanted cells is regulated by the intrinsic properties of grafted cells and the local environmental cues in the host.
To date, few studies have attempted to examine the behavior of adult NS/NPCs in the injured mature CNS, and it is not clear to what extent changes in the CNS microenvironment following injury affect the fate of transplanted adult NS/NSCs, and whether these cells can become anatomically and functionally integrated into an injured heterotypic environment. In this study, we tested the long-term survival and maturation fate of adult NS/NPCs after transplantation into the injured peri-lesion cortex in animals following TBI. We also assessed the electrophysiological properties of the transplanted cells using whole-cell patch-clamp techniques.
Methods
Animals
Two- to three-month-old male Fisher 344 rats weighing approximately 160–250 g were used in this study. The Fisher 344 rat is an inbred strain that allows for immunocompatible allografting without using immunosuppressants. The animals were housed in an animal facility, with a 12-h light/dark cycle, and water and food provided ad libitum. All procedures were approved by the VCU Institutional Animal Care and Use Committee.
Cell isolation and culturing
Cultures were derived from 2-month-old rats. The rat brains were dissected from deeply anesthetized rats that were sacrificed by cervical dislocation. For each culture set, multiple brains were pooled together and placed in sterile PBS containing 1% penicillin/streptomycin (P/S) solution on ice and transferred to a sterile tissue culture hood. NS/NPCs were isolated and cultured following a protocol described previously (Richardson et al., 2005b). Briefly, the SVZs were dissected, minced, and enzymatically treated with papain. After passing through ascending gauged needles, cell suspensions were suspended and washed with sterile phosphate-buffered saline (PBS). The NS/NPCs were then enriched with modified Percoll gradient protocol (Palmer et al., 1995) and subsequently plated to poly-L-ornithine- and laminin-coated flasks. The cells were cultured as a monolayer in DMEM-F12 medium containing 20 ng/mL bFGF and 1% N2 supplement. Fresh medium was changed every 3 days. Once confluent, the cells were replated. The second-passage cells were used for transplantation.
To examine the cell phenotype at the time of transplantation, a parallel set of cells were plated in a coated 24-well plate for each batch of culture. On the day of transplantation the cells that were plated on the 24-well plates were fixed and processed for immunofluorescence staining with cell type-specific markers, including glial fibrillary acidic protein (GFAP, an astrocyte marker), RIP (a mature oligodendrocyte marker), NG2 (an immature oligodendrocyte marker), nestin (a neural stem and progenitor cell marker), NeuN (a mature neuronal marker), and Tuj1 (an immature neuronal marker), following our published protocol (Richardson et al., 2005a).
Cell labeling
In order to study survival and differentiation of transplanted cells, cultured cells were labeled with BrdU. BrdU (10 μM) was added into the culture medium for 72 h before cells were harvested and used for transplantation. For electrophysiological study, cultured cells were transduced with the lentiviral vector pLL3.7 encoding internal reporter enhanced green fluorescent protein (EGFP), which allows easy identification of the transduced cells. This lentiviral vector has been widely used to efficiently and stably transduce NS/NPCs and generate transgenic animals. Packaging, purification, and titer determination of the lentivirus were performed as described previously (Hu et al., 2006). For cell transduction, 24 h after NS/NPCs were re-plated and attached to the culture flask, 100 μL of pLL3.7 lentivirus (1×108 TU/mL) was added to the culture medium. One day later, the virus-containing medium was removed and the cells were grown in culture conditions as described above until confluent. Fluorescent activated cell sorting showed that approximately 90% of NS/NPCs were labeled with EGFP.
Lateral fluid percussion injury
A group of male Fisher 344 rats approximately 3 months old was subjected to a moderate lateral fluid percussion injury (FPI) or sham injury following our previously described protocol (Sun et al., 2007). Briefly, adult rats were anesthetized in an acrylic glass chamber with 3% isoflurane, intubated, and ventilated with 2% isoflurane in a gas mixture (30% oxygen and 70% N2), and secured in a stereotaxic frame. After a midline incision and exposure of the skull, a 4.9-mm craniotomy was made on the left parietal bone half way between the lambda and bregma sutures. A Luer-lock syringe hub was affixed to the craniotomy site with cyanoacrylate glue and further cemented with dental acrylic to the skull, at which point the anesthesia was switched off. Once the animal regained consciousness showing toe and tail reflexes, the Luer-lock fitting filled with saline was connected to a pre-calibrated fluid percussion device and a 2.1±0.02 atm fluid pulse was administered. Sham animals went through the same surgical procedure without receiving the fluid pulse. After injury, the Luer-lock fitting was removed, the animal was returned to the surgical table, the incision was sutured, and righting time was assessed. Once recovered from anesthetic under a thermal blanket, the animals were returned to a clean cage.
Cell transplantation
At 2 days following brain injury, animals received cell injection. Specifically, cultured NS/NPCs at the second passage that were labeled with BrdU or lentiviral EGFP were harvested, dissociated into single cells with trypsin, and followed by concentration with PBS to a final concentration of 4×104 cells/μL and stored on ice. The animals were anesthetized and ventilated with 2% isoflurane, and secured in a stereotaxic frame. The skull was cleaned and the FPI craniotomy site exposed. Cells were then injected into the injury ipsilateral to the peri-injury cortical region at the following coordinates: AP −3/−4.0 mm, ML −3.5 mm, DV −3 mm. A 10-μL Hamilton syringe with a 26-G beveled needle was mounted on an automated syringe driver to deliver 2 μL of cells for each injection site at a rate of 0.5 μL/min. To prevent backflow along the needle track, the needle was left in situ for 5 min prior to and after cell injection. Control animals received PBS injection only.
Tissue processing and immunohistochemistry
To identify the survival and differentiation of the transplanted cells, the rats were perfused at 2 or 4 weeks following cell injection (n=5 in the injured group at each time point, n=4 in the sham group at each time point). The animals were euthanized with sodium pentobarbital and transcardially perfused with PBS, followed by 4% paraformaldehyde in PBS, and the brains were dissected and post-fixed in 4% paraformaldehyde for 48 h at 4°C. The brains were cut coronally at 50 μm with a vibratome through the tissue encompassing the injection site. Sequential sections were collected in 24-well plates filled with PBS plus 0.01% sodium azide and stored at 4°C.
To identify transplanted cells, the sections were processed for BrdU immunohistochemistry following our previously published protocol (Sun et al., 2007). Briefly, the sections were washed with PBS, and denatured with 50% formamide for 60 min at 65°C followed by a rinse in 2×saline-sodium citrate buffer (SSC), and then incubated with 2 N HCl for 30 min at 37°C. After denaturing, the sections were washed with PBS and endogenous peroxidase was blocked using 3% H2O2. After overnight serum blocking with 5% normal horse serum, the sections were incubated with mouse anti-BrdU antibody (1:200; Dako North America, Inc., Carpenteria, CA) in PBST (PBS with 0.4% Triton-X) plus 5% normal horse serum at 4°C for 48 h. After rinsing with PBST, the sections were incubated with HRP-conjugated anti-mouse IgG (1:200; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C and visualized with diaminobenzidine (DAB). Sections were mounted on glass slides, lightly counterstained with cresyl violet, and cover-slipped.
To determine the differentiation fate of the transplanted cells, parallel sections were processed for immunofluorescence double labeling using antibodies against BrdU and markers for immature neurons (Tuj1), mature neurons (NeuN), neural stem cells and astrocytes (nestin and GFAP), immature astrocytes (vimentin), macrophages, and activated microglial cells (ED1), as well as oligodendrocytes (Olig2, which recognizes both mature and immature oligodendrocytes). Two sections per brain were stained for each cell type-specific marker. The staining procedure was similar to the BrdU staining described above. The primary antibodies used were mouse anti-NeuN (1:500; Chemicon International, Temecula, CA), mouse anti-nestin (1:500; Millipore, Billerica, MA), rabbit anti-Tuj1 (1:1000; Covance Inc., Princeton, NJ), mouse anti-ED1 (1:500; Chemicon), mouse anti-vimentin (1:500; Dako North America) combined with sheep anti-BrdU (1:200; Biodesign International, Saco, ME), and rabbit anti-GFAP (1:1000; Dako North America), rabbit anti-Olig2 (1:2000; kindly provided by Drs. John Alberta and Chuck Stiles, Dana-Farber Cancer Institute, Boston, MA), with mouse anti-BrdU (1:200; Dako North America). The secondary antibodies used were Alexa Fluor 488 anti-sheep IgG, Alex Fluor 488 or 568 anti-mouse IgG, or Alex Fluor 568 anti-rabbit IgG (1:200; Molecular Probes, Eugene, OR). After DNA denaturing, endogenous peroxidase and serum blocking, the sections were incubated with primary antibodies for 72 h at 4°C with constant agitation. After washing, the sections were then incubated with the secondary antibodies overnight at 4°C. Finally, the sections were mounted on glass slides and cover-slipped with Vectashield (Vector Laboratories, Burlingame, CA).
Stereological quantification of BrdU-labeled transplanted cells
To quantify the number of surviving transplanted cells, BrdU-stained sections were examined with an Olympus Image System CAST program. The optical fractionator method was used to estimate the total number of BrdU-positive cells following our previously described method (Sun et al., 2009). This design-based stereological method to estimate cell numbers has been widely used by our lab. To do this, the region of interest was outlined using a 4×objective. A 60×oil-immersion objective was used for cell counting. Within the region of interest, an optical dissector counting frame was used to count BrdU-positive cells at predetermined regular x, y intervals. The area (a) of the counting frame was known relative to the stage-stepping intervals over the section, the sampling fraction (asf)=a (frame)/a (x,y step). The dissector height (h) was known relative to the section thickness (t). With these parameters, the number of total cell counts (n) was estimated as n=ΣQ −·t/h·1/asf·1/ssf, where ssf was the section-sampling fraction (0.25 in this study), and ΣQ − was the number of cells counted. For each rat brain, an average of six sequential sections that contained BrdU-positive cells spaced 300 μm apart were examined by a blinded observer.
Quantification for double-labeled cells
To quantify the percentage of BrdU-labeled transplanted cells that had differentiated into the various cell types, the sections were examined by confocal microscopy. Because cells in the center of the injection were packed together, only cells outside the center were examined to assess the co-labeling of BrdU with cell type-specific markers. A minimum of 100 BrdU-positive cells per brain were examined for each marker. Each BrdU-positive cell was manually examined in its full “z” dimension, and only those cells for which the BrdU-positive nucleus was unambiguously associated with a given cell type-specific marker was considered double-labeled. The percentage of double-labeled cells was calculated as the number of BrdU+/GFAP+, BrdU+/NeuN+, BrdU+/ED1+, or BrdU+/Oligo2+ cells against the total number of BrdU+ cells.
Electrophysiological studies
To assess the electrophysiological properties of the transplanted cells, injured animals received injection of lentiviral EGFP-transduced NS/NPCs at 2 days post-injury. At 4 weeks following cell injection, rat brain slices were prepared as described previously (McQuiston, 2007). Briefly, the rats were deeply anesthetized with ketamine (200 mg/kg) and xylazine (20 mg/kg), transcardially perfused with ice-cold buffer (consisting of in mM: sucrose 230, KCl 2.5, CaCl 1, MgCl2 4, NaHPO4 1, NaHCO3 25, and glucose 25) and sacrificed by decapitation. The brains were then removed and 350-μm thick coronal slices encompassing the cell injection site were cut with a vibratome 3000 (Ted Pella Inc., Redding CA). The sections were incubated in a holding chamber at 35°C containing artificial cerebrospinal fluid (CSF) consisting of in mM: NaCl 125, KCl 3.0, CaCl 1.2, MgCl2 1.2, NaHPO4 1.2, NaHCO3 25, and glucose 25, bubbled with 95% O2/5% CO2. After 30 min the slices were permitted to return to room temperature. Grafted EGFP-labeled NS/NPCs were identified through the combination of differential interference contrast (DIC) videomicroscopy and fluorescence microscopy. More specifically, the slices were submerged and perfused in a glass-bottom recording chamber with oxygenated artificial CSF (32–34°C), which was mounted on a fixed stage under an Olympus BX51WI microscope equipped with DIC optics. Cells in the slice were visualized using transmitted near-infrared light (>775 nm) with a 20×(0.95 NA) water-immersion objective. The EGFP-expressing cells were identified by epifluorescence (150 W xenon lamp; Osram, Munich, Germany; EGFP-Endow EGFP bandpass emission filter set [excitation filter 470/40 bandpass, dichromatic beam splitter 495 long-pass, emission filter 525/50 bandpass]; Chroma Technology, Rockingham, VT). Once EGFP-positive cells were identified, patch pipettes (containing in mM: KMeSO4 135, NaCl 8, MgATP 2, NaGTP 0.3, HEPES 10, and BAPTAK4 0.1 [pH 7.25]) were guided to the cell body under the 20×objective. Membrane potential responses of the cell were recorded with an A-M Systems Model 2400 patch-clamp amplifier and further amplified and filtered with a Brownlee 440 amplifier. To examine the membrane electrical properties of transplanted cells, we measured the resting membrane potential, input resistance, and membrane potential responses to current injection. Electrophysiological data were digitized by a PCI-6221 A/D board (National Instruments, Austin, TX), and stored and analyzed on a personal computer using WCP software (Dr. J. Dempster, University of Strathclyde, Glasgow, Scotland).
Morris Water Maze (MWM)
To test whether cell transplantation affected the cognitive recovery of the recipients, the animals were tested in the MWM at 20–24 days post-injury (18–22 days following transplantation). MWM testing was performed following our previously published protocol (Sun et al., 2007). In the MWM test, goal latency was used as the primary dependent variable. Path length and swim speed were also analyzed. Prior to MWM testing, a visual platform test was performed to confirm that the visual system of the animal was not impaired. Briefly, the animals were placed in a large circular tank containing opaque water and allowed to swim freely to find the hidden goal platform (1 cm below the water's surface) in order to escape from the water. Each animal was tested four times each day. For each trial, the animal was randomized to one of four starting positions (N, E, S, and W). MWM performance was recorded using a computerized video tracking system, and latency to find the platform, total distance swum to reach the goal platform, and swim speed were calculated for each trial. Upon finding the platform, the animals were left there for 30 sec before being removed from the maze and placed in a warming cage to dry. Animals that did not find the platform after 120 sec were placed on the platform for 30 sec and then removed from the maze. In this study, 15 injured rats (9 with cells and 6 with vehicle) and 13 sham rats (7 with cells and 6 with vehicle) were included.
Statistical analysis
The data generated were analyzed using SPSS software. For MWM data analysis, the data were analyzed using a split-plot analysis of variance (ANOVA) [treatment×day], comparing the effect of group on goal latency. A Fisher LSD test was performed to allow for pair-wise group contrasts. Swim speed was also analyzed using a one-way ANOVA. For cell quantification a one-way ANOVA with post-hoc Fisher LSD test or the Student's t-test with an applied Bonferroni correction for multiple groups was utilized, with p values <0.05 considered statistically significant. Data are presented as mean±standard error of the mean (SEM) in all figures.
Results
Cell phenotypes of transplanted cells
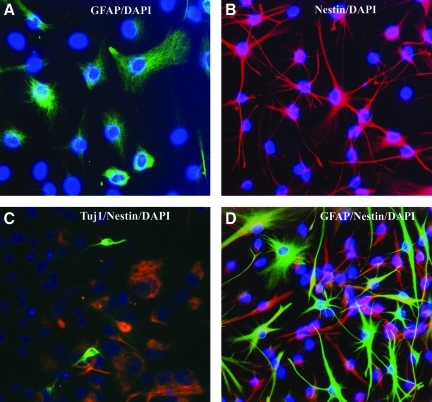
NS/NPCs used for transplantation were isolated from the SVZs of the adult rats. Cells were cultured as a monolayer, expanded in vitro, and used after two passages. To examine the phenotype of cells used for transplantation, at the second passage some cells were plated on a 24-well plate and were processed for immunocytochemistry on the same day when the same passage cells were used for transplantation. Immunofluorescence staining showed that many cells were nestin- or GFAP-positive, and very few cells expressed Tuj1 (Fig. 1). No other markers were stained. To quantify the percentage of cells expressing each marker, a minimum of 100 4,6-diamino-2-phenylindole (DAPI)-positive cells were examined in three random fields under a 20×objective. Of all cultures, about 70% of the cells were labeled with nestin, 65% of the cells were labeled with GFAP, 62% co-expressed both nestin and GFAP, and fewer than 3% of the cells were labeled with Tuj1.
FIG. 1.
Expanded subventricular zone (SVZ) cells in vitro. After 14 days of in vitro expansion, cultured cells were immunostained with cell nuclear dye 4,6-diamino-2-phenylindole (DAPI, blue) and astrocyte marker glial fibrillary acidic protein (GFAP, A and D, green), progenitor cell marker nestin (B, C, and D, red), and immature neuronal marker Tuj1 (C, green).
Survival of transplanted adult rat NS/NPCs
The feasibility of adult-derived NS/NPCs as a cell source for transplantation was first tested in sham animals. Survival of cells in the sham brain was assessed at 2 and 4 weeks following transplantation. Subsequently, in the pilot study, we tested the optimal post-injury time point for cell transplantation and the most favorable location for cell survival in an injured adult brain. Rat NS/NPCs were transplanted into the injured CA3 region or peri-lesional cortical white matter at 2, 7, or 14 days post injury, and sacrificed 1 or 2 weeks later. Transplanted cells showed better survival when transplanted at 2 days post-injury at the peri-lesion cortical-white matter interface (data not shown). Subsequently, this time point and location were used for the current study.
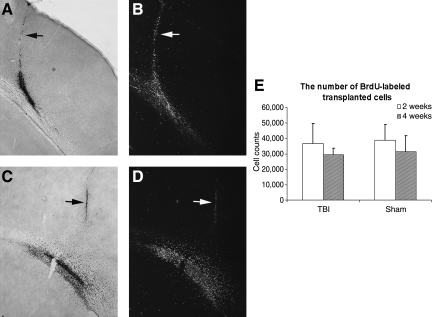
To assess the survival of adult rat NS/NPCs, recipients were sacrificed at 2 or 4 weeks following cell injection. Sequential coronal sections encompassing the injection site from both injured and sham groups were processed for BrdU immunostaining to identify transplanted cells that were labeled with BrdU in vitro before transplantation. At 2 weeks following transplantation, BrdU-positive grafted cells were mostly located at the cortex-white matter interface (Fig. 2A and B). At 4 weeks following transplantation, BrdU-labeled cells were more scattered compared to what was found at 2 weeks. Many BrdU-labeled cells migrated out of the injection site into the surrounding areas (Fig. 2C and D). At both 2 and 4 weeks, there were few cells remaining along the injection needle tract.
FIG. 2.
Survival of transplanted cells. Cultured adult rat multipotent neural stem/progenitor cells (NS/NPCs) were labeled with 5-bromo-2-deoxyuridine (BrdU) in vitro 3 days before being used for transplantation and subsequent identification with BrdU immunostaining. (A and B) Coronal section from an animal sacrificed 2 weeks following transplantation. Grafted cells were mostly located at the cortex-white matter interface. (C and D) Coronal section taken from an animal sacrificed at 4 weeks following transplantation. Compared to 2 weeks, at 4 weeks many BrdU-labeled cells had migrated out of the injection site into the surrounding areas. The arrow indicates the injection needle tract. (A and C) Bright-field view. (B and D) Phase-contrast view. (E) Stereological assessment of the number of surviving cells at 2 and 4 weeks following transplantation. The number of cells presented at both time points was similar in sham and injured brains. The total number of BrdU-labeled cells at 4 weeks post-transplantation was lower compared to at 2 weeks in both the sham and injured groups; however, no statistical significance was found (TBI, traumatic brain injury).
To estimate the total number of transplanted BrdU-labeled cells that were present at 2 or 4 weeks after injection, six 50-μm-thick coronal sections through the injection site spaced 300 μm apart were quantified using an unbiased stereological optical fractionator method as previously described (Sun et al., 2009, 2010). A total of 8×104 cells (4×104×2 μL) were injected initially. At 2 and 4 weeks following transplantation, we counted 3.7×104±1.3×104 cells and 2.9×104±0.4×104 cells in the injured groups, respectively, whereas 3.9×104±1.1×104 and 3.1×104±1×104 in the sham groups were counted, respectively (Fig. 2E). The number of remaining transplanted cells at 4 weeks was lower than the number of cells at 2 weeks in both the sham and injured groups; however, the difference was not statistically significant.
Fate of transplanted NS/NPCs
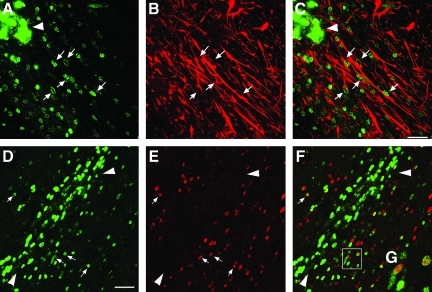
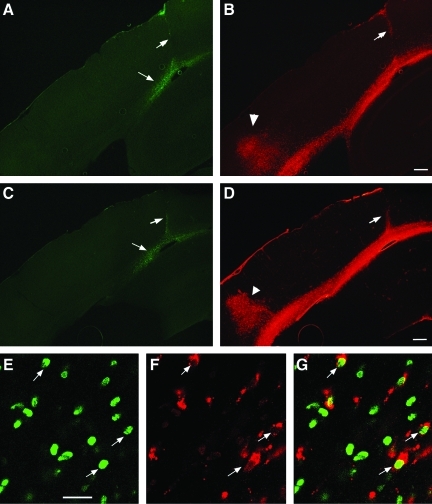
To assess the differentiation fate of the transplanted cells, sequential coronal sections taken 4 weeks post-transplantation were processed for double-immunofluorescence staining for BrdU and either the neuronal markers (Tuj1 or NeuN), the neural astrocytic markers (nestin or GFAP), the macrophage/activated migroglia marker ED1, or the oligodendrocyte marker Olig2. The sections were examined using confocal microscopy. We found that BrdU-labeled transplanted cells at the injection center were mostly non-differentiated cells with weak nestin positivity in some of the cells. No co-labeling with any other above-mentioned markers was found (data not shown). Away from the injection center, many BrdU-labeled transplanted cells were scattered along the white matter tract, and predominantly differentiated into GFAP-labeled astrocytes showing typical astrocyte morphology (Fig. 3A–C). In this area, many BrdU-labeled cells were co-labeled with Olig2 (Fig. 3D–F), a marker for both mature and immature oligodendrocytes (Ligon et al., 2004). Strong ED1-positive staining was found along the injection needle tract, white-matter tract, and the cortical lesion center in the injured groups, indicating that injection and TBI induced an extensive inflammatory response (Fig. 4B). Some BrdU-labeled nuclei were co-labeled with ED-1, indicating that either transplanted cells were becoming inflammatory cells, or were being taken up by inflammatory cells. TBI and cell implantation also initiated strong glial responses at those regions as revealed by vimentin staining (Fig. 4D). Injury-induced host tissue reaction in the white-matter tract overlapped, particularly with the cell graft site. In order to better examine the fate of transplanted cells, we quantified the percentage of double-labeled BrdU-positive cells that migrated away from the injection center with cell type-specific markers. We found that the percentages of double-labeled BrdU/GFAP, BrdU/Olig2, and BrdU/ED1 cells were approximately 44%, 13%, and 21%, respectively, in the injured brain. In the peri-lesion cortical region, few BrdU-positive cells were found ambiguously co-labeled with the mature neuronal marker NeuN (data not shown). No BrdU-Tuj1 co-labeling was found. Similar maturational phenotypes of the transplanted cells were found in sham animals.
FIG. 3.
Glial differentiation of transplanted rat multipotent neural stem/progenitor cells (NS/NPCs) in the injured brain at 4 weeks following transplantation. (A–C) Astrocytes: confocal micrograph showing double-labeling of 5-bromo-2-deoxyuridine (BrdU; green) and glial fibrillary acidic protein (GFAP, red). Many BrdU-labeled transplanted cells migrated away from the injection center (arrowhead) along the white-matter tract and differentiated into spindle-shaped GFAP-labeled astrocytes (arrows; scale bar=50 μm). (D–G) Oligodendrocytes: confocal micrograph showing double-labeling of transplanted cells with BrdU (green) and Olig2 (red). Arrows indicate that many BrdU-positive transplanted cells away from the injection center (arrowhead) were co-labeled with Olig2 (E, red). Merged image shows co-localization of BrdU and Olig2 (F; scale bar=50 μm). (G) Highlighted image of the boxed area in F showing co-labeling of BrdU and Olig2.
FIG. 4.
Traumatic brain injury (TBI)- and transplantation-induced focal inflammatory and glial reaction at 4 weeks following cell implantation. (A–D) Inflammatory cell reaction as labeled by ED1 staining (B, red) at the cortical injury site (arrowhead), and along the white-matter tract, as well as the cell injection needle tract (arrow). Vimentin-stained glial reaction at the injured cortex, white-matter tract, and the needle tract (D, red). Note 5-bromo-2-deoxyuridine (BrdU)-labeled transplanted cells (A and C, green, arrows) at the site of the peri-cortical-white matter inferface (scale bar=300 μm). (E–G) Confocal images of BrdU-labeled transplants (green) co-labeled with ED1 (red). G represents the merged BrdU and ED1 staining (scale bar=40 μm).
Electrophysiological properties of transplanted NS/NPCs
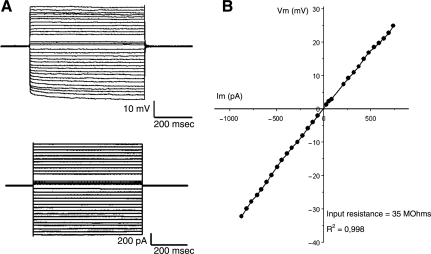
To assess whether transplanted cells can become mature and functional cells in the injured microenvironment, a group of injured animals received NS/NPCs labeled with lentiviral-EGFP at 2 days post-injury. Four weeks following transplantation, hippocampal slices were prepared for electrophysiological investigations. Whole-cell patch-clamp recordings were obtained from EGFP-expressing cells that were fluorescently visualized in the hippocampal slices as small, flat, and often spindle-shaped somata that displayed low contrast under DIC optics. Injection of rectangular current pulses into the soma of some EGFP-positive cells produced membrane potential changes that varied linearly with current amplitudes (Fig. 5). Some EGFP-positive cells produced membrane responses with current-voltage relationships that rectified either inwardly or outwardly. The resting membrane potential of the recorded EGFP-positive cells was −75±4 mV, and they had input resistances varying from 35 to 160 MΩ (mean 108±22 MΩ; n=5). Importantly, none of the recorded EGFP-positive cells exhibited any active membrane potential responses such as action potentials, low threshold spikes, or plateau potentials in response to depolarizing current injections. Taken together, the EGFP-positive cells displayed electrophysiological responses consistent with glia and not neurons (Sontheimer, 1994).
FIG. 5.
Electrophysiological properties of transplanted multipotent neural stem/progenitor cells (NS/NPCs) at 4 weeks following transplantation. (A) The membrane potential response (top) to current injection (bottom). (B) The amplitude of the membrane potential responses is plotted against the current injected. The input resistance was low and the current-voltage relationship was linear, consistent with mature glial cell but not neuronal electrophysiological properties.
Cognitive function
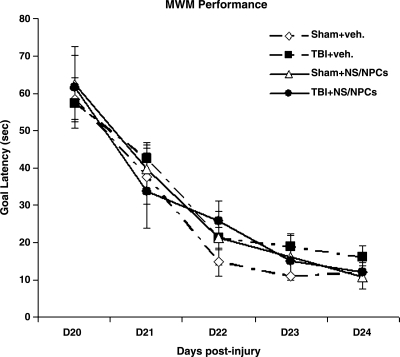
In TBI studies, Sprague-Dawley rats are the most commonly used animal strain. Moderate lateral fluid percussive injury (LFPI) in this strain induces significant cognitive deficits that can be measured with the MWM test. We previously found that compared to Sprague-Dawley rats, Fisher 344 rats had a higher mortality rate and more acute post-TBI seizure attacks, but fewer cognitive deficits following a moderate LFPI (Reid et al., 2010). In the current study, cognitive function was tested at 20–24 days following LFPI (18–22 days post-transplantation). Latency to find the goal platform was analyzed for each day of MWM training using a one-way ANOVA. No significant difference in the mean latency to reach the platform was found between injured and sham animals, nor did animals receive cells or vehicle injections (Fig. 6). This suggests that the injury level of severity used in the current study had no effect on cognitive performance in Fisher 344 rats, similarly to what we reported previously (Reid et al., 2010).
FIG. 6.
Cognitive function. The graph compares the Morris water maze (MWM) performance of sham and injured animals receiving injection of vehicle or adult rat multipotent neural stem/progenitor cells (NS/NPCs). Compared to sham animals, injured animals did not show any significant difference in cognitive deficits as assessed by goal latency through days 20–24 following injury. The animals that received cells did not show any difference compared to the vehicle-treated groups (TBI, traumatic brain injury; veh., vehicle).
Discussion
The results of the current study demonstrated that NS/NPCs isolated from the adult brain were capable of surviving for an extended period in an acutely injured environment. After being transplanted into the peri-injury cortical region, these cells migrated along the white-matter tract becoming region-specific astrocytes or oligodendrocytes, as shown by immunohistochemistry as well as electrophysiologically. This suggests that adult-derived NS/NPCs can become region-specific functional cells in the injured adult brain.
Using transplantation to replace lost cells in the injured brain and to restore function is a challenging but attractive strategy. To date, published studies in this area have primarily utilized cells derived from embryonic or fetal tissue (Gao et al., 2006; Sinson et al., 1996; Wallenquist et al., 2009). However, due to a host of biological, technical, and ethical issues, many problems have been encountered in the use of these cells, including cell availability, the risk of tumor formation, and immunological rejection (Molcanyi et al., 2007; Riess et al., 2007). To overcome such problems, in recent years, adult-derived cells that could serve as a donor source for autologous transplantation for TBI therapy have been explored. Thus far, the majority of studies investigating adult-derived cells have focused on the potential of bone marrow mesenchymal cells (Li and Chopp, 2009; Qu et al., 2009). However, the reported beneficial effect of bone marrow cells in the injured brain largely relies on the effect of modulating the injured host brain rather than direct cell replacement, as limited cell survival and differentiation have been observed in the brain (Mahmood et al., 2001b, 2004; Li and Chopp, 2009). Here we show that NS/NPCs derived from the adult brain can survive for an extended period and become region-specific functional cells following transplantation into the injured adult brain.
One of the major issues confounding transplantation is the survival of transplanted cells. The fate of transplanted cells is affected by the nature of the cells and the local host environment. Following TBI, the injured tissue undergoes a transient disruption of the blood–brain barrier accompanied by an influx of polymorphonuclear leukocytes at 24 h post-injury, followed by the subsequent entry of macrophages by 24–48 h (Lenzlinger et al., 2001; Soares et al., 1995). This early response is associated with the release of inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and transforming growth factor-β (TGF-β). Additionally, a number of trophic factors are elevated during the early stages post-TBI. For example, nerve growth factor (NGF) is increased at 3 days after TBI (Boockvar et al., 2005), and we previously found increased basic fibroblast growth factor (bFGF) and endothelial growth factor (EGF) expression levels at 2 days post-injury (unpublished data). After the regression of this acute phase of inflammation in the injured brain, astrocyte proliferation peaks at approximately 3 days post-TBI, followed by new microvasculature and glia limitans formation in the later stages post-injury (Hill-Felberg et al., 1999). Therefore, the injury response has an early and a late phase, each of which is characterized by its own distinct microenvironment. However, what is unclear is which of these environments is more conducive for cell transplant survival. Within a different post-injury environment, published studies using cell sources ranging from embryonic murine (Boockvar et al., 2005; Hoane et al., 2004; Philips et al., 2001; Riess et al., 2002; Wallenquist et al., 2009), or fetal stem cell lines (Hagan et al., 2003; Shear et al., 2004; Tate et al., 2002, 2009; Wennersten et al., 2004), to bone marrow cells (Mahmood et al., 2001a, 2001b, 2003; 2006; Qu et al., 2009), have reported varying degrees of cell survival after transplantation into the injured brain, both at early and later post-injury time points. Many studies have indicated that post-injury timing and location of implantation are important factors determining the fate of transplanted NS/NPCs. In our preliminary studies, we tested implanting NS/NPCs into different locations, including the peri-lesion area between the cortical-white matter interface, injured cortex, and CA3 regions, and found that the peri-lesion white matter-cortical interface was most suitable for cell survival. We also tested different post-injury time points for transplantation, and found that grafting at 2 days post-injury had better cell survival compared to transplantation at 7 or 14 days post-injury (data not included). The results are similar to some from previously reported studies using other cell sources (Boockvar et al., 2005; Riess et al., 2002). Using the stereological cell quantification method, we determined the remaining number of transplanted cells at 2 and 4 weeks following transplantation, and found around 46% of total injected cells and 36% in the injured brain, respectively, and 48% and 39% in the sham brain, respectively. Statistical analysis revealed that the cell survival rate at 4 weeks was not significantly reduced compared to that seen at 2 weeks, in both the sham and injured groups. Previously published studies have reported varying degrees of survival rates of cells derived from adult rat brains, ranging from 60% in the intact or dopamine-denervated striatum at 5 weeks following transplantation (Dziewczaploski et al., 2003), to 35–41% when transplanted into the intact dentate gyrus at 8–12 weeks (Gage et al., 1995). Our data are comparable to the results of these studies.
Adult-brain-derived NS/NPCs as a cell source for transplantation have been tested in the normal brain and in a Parkinson's model, as well as in a stroke model (Dziewczapolski et al., 2003; Gage et al., 1995; Herrera et al., 1999; Richardson et al., 2005a, 2005b). Cultured adult rat NS/NPCs, when transplanted into neurogenic regions such as the SVZ or dentate gyrus of the hippocampus, can differentiate into region-specific neurons (Gage et al., 1995; Suhonen et al., 1996), but when transplanted into non-neurogenic sites, these cells differentiate primarily into glia or remain undifferentiated (Dziewczapolski et al., 2003; Gage et al., 1995; Herrera et al., 1999; Richardson et al., 2005a). These data support the notion that the in vivo fate of transplanted cells is regulated by the intrinsic properties of grafted cells and the local environmental cues in the host. In the current study, we utilized NS/NPCs derived from the SVZ region of adult rats, and used cells at low passages, ensuring minimal fate commitment at the time of transplantation. Immunostaining of parallel cultured cells showed that the majority of cells used for in vivo study were nestin- and GFAP-positive neural stem cells, which indicated that the cells transplanted were able to differentiate into both neurons and glia. In the current study, we grafted cells into the peri-lesion white matter-cortical interface and observed that transplanted cells migrated away from the injection site, mostly along white-matter tracts of the corpus callosum. The majority of the cells expressed astrocyte marker GFAP, with a smaller proportion expressing oligodendrocyte marker Olig2, and very few cells located in the cortical region expressing neuronal marker NeuN. When the electrophysiological properties of the transplanted cells were examined with single-cell patch-clamp recordings, the cells displayed glial properties, suggesting that the transplanted cells that migrated out of the injection site were becoming mature functional glial cells in the white-matter tract.
In transplantation studies, graft rejection is one of the major concerns for long-term cell survival. To reduce graft rejection, immunosuppressants are routinely used, especially when donor and recipient cells are coming from different species. In the current study, due to the inbred nature of Fisher 344 rats, immunosuppressants were not used. However, at 4 weeks following transplantation, extensive inflammatory responses and glial reactions induced by TBI and transplantation were observed at the injury site, as well as along the needle tract as demonstrated by ED1 and vimentin staining. Among the remaining transplanted cells at 4 weeks after implantation, approximately 21% of BrdU-labeled cells were co-labeled with macrophage/activated microglial marker ED1. Morphologically, it is difficult to determine whether BrdU/ED1 double-labeled cells were indeed differentiated transplanted cells, or were ED1-positive cells that engulfed BrdU-labeled transplants. In any case, the presence of BrdU/ED1 double-labeling indicated a graft-evoked host inflammatory response at the site of cell transplantation produced by the injection procedure and TBI, as demonstrated by strong ED1-positivity along the injection needle tract and trauma lesion center. This suggests that although autologous transplants enjoy a longer survival in the injured brain without using immunosuppressants, anti-inflammation modulation may still be needed for better long-term graft survival.
Previous studies have shown that transplantation of NS/NPCs derived from fetal or bone marrow can improve functional recovery following experimental brain injury (Gao et al., 2006; Mahmood et al., 2001b; Riess et al., 2002; Sinson et al., 1996). In the current study, we examined cognitive function in experimental subjects. However, we did not find any changes in cell-treated animals compared to controls. In the current study, we used Fisher 344 rats as both donors and recipients to avoid the use of immunosuppressants. As we have shown previously, Fisher 344 rats are extremely susceptible to fluid percussive injury, and exhibit a higher mortality rate compared to Sprague-Dawley rats, and this strain of rats also shows fewer cognitive deficits compared to Sprague-Dawley rats (Reid et al., 2010). Similarly, in the current study no significant cognitive deficits were observed in the injured animals compared to sham animals; however, this does not completely rule out a possible beneficial effect of transplanted adult NS/NPCs on functional recovery of injured animals. Future studies will explore other more sensitive functional assessment paradigms.
The results of the current study demonstrated that NS/NPCs derived from the adult brain were capable of surviving for an extended period in an acutely-injured environment. After being transplanted into the peri-injury white matter-cortical interface, cells preferentially migrated along the white-matter tract and became region-specific astrocytes or oligodendrocytes, as shown by marker expression as well as electrophysiologically. This suggests that adult-derived NS/NPCs can become region-specific functional cells in an injured environment.
Acknowledgments
Sponsored by the A.D. William Fund (Sun), National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) grant NS062369 (Sun), NIH/NINDS grant NS055086 (Sun), and the Virginia Commonwealth Neurotrauma Initiative Trust Fund (RFP07-302, Sun). Microscopy work was performed at the VCU–Department of Anatomy and Neurobiology Microscopy Facility, supported in part by funding from NIH/NINDS center core grant 5P30NS047463.
Author Disclosure Statement
No competing financial interest is involved.
References
- Altman J. Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Bjorklund A. Kirik D. Rosenblad C. Georgievska B. Lundberg C. Mandel R.J. Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- Boockvar J.A. Schouten J. Royo N. Millard M. Spangler Z. Castelbuono D. Snyder E. O'Rourke D. McIntosh T. Experimental traumatic brain injury modulates the survival, migration, and terminal phenotype of transplanted epidermal growth factor receptor-activated neural stem cells. Neurosurgery. 2005;56:163–171. doi: 10.1227/01.neu.0000145866.25433.ff. [DOI] [PubMed] [Google Scholar]
- Dziewczapolski G. Lie D.C. Ray J. Gage F.H. Shults C.W. Survival and differentiation of adult rat-derived neural progenitor cells transplanted to the striatum of hemiparkinsonian rats. Exp. Neurol. 2003;183:653–664. doi: 10.1016/s0014-4886(03)00212-7. [DOI] [PubMed] [Google Scholar]
- Gage F.H. Coates P.W. Palmer T.D. Kuhn H.G. Fisher L.J. Suhonen J.O. Peterson D.A. Suhr S.T. Ray J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl. Acad. Sci. USA. 1995;92:11879–11883. doi: 10.1073/pnas.92.25.11879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage F.H. Kempermann G. Palmer T.D. Peterson D.A. Ray J. Multipotent progenitor cells in the adult dentate gyrus. J. Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gao J. Prough D.S. McAdoo D.J. Grady J.J. Parsley M.O. Ma L. Tarensenko Y.I. Wu P. Transplantation of primed human fetal neural stem cells improves cognitive function in rats after traumatic brain injury. Exp. Neurol. 2006;201:281–292. doi: 10.1016/j.expneurol.2006.04.039. [DOI] [PubMed] [Google Scholar]
- Gates M.A. Fricker-Gates R.A. Macklis J.D. Reconstruction of cortical circuitry. Prog. Brain Res. 2000;127:115–156. doi: 10.1016/s0079-6123(00)27008-8. [DOI] [PubMed] [Google Scholar]
- Hagan M. Wennersten A. Meijer X. Holmin S. Wahlberg L. Mathiesen T. Neuroprotection by human neural progenitor cells after experimental contusion in rats. Neurosci. Lett. 2003;351:149–152. doi: 10.1016/j.neulet.2003.07.021. [DOI] [PubMed] [Google Scholar]
- Herrera D.G. Garcia-Verdugo J.M. Varez-Buylla A. Adult-derived neural precursors transplanted into multiple regions in the adult brain. Ann. Neurol. 1999;46:867–877. doi: 10.1002/1531-8249(199912)46:6<867::aid-ana9>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hill-Felberg S.J. McIntosh T.K. Oliver D.L. Raghupathi R. Barbarese E. Concurrent loss and proliferation of astrocytes following lateral fluid percussion brain injury in the adult rat. J. Neurosci. Res. 1999;57:271–279. doi: 10.1002/(SICI)1097-4547(19990715)57:2<271::AID-JNR13>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Becerra G.D. Shank J.E. Tatko L. Pak E.S. Smith M. Murashov A.K. Transplantation of neuronal and glial precursors dramatically improves sensorimotor function but not cognitive function in the traumatically injured brain. J. Neurotrauma. 2004;21:163–174. doi: 10.1089/089771504322778622. [DOI] [PubMed] [Google Scholar]
- Hu W. Huang J. Mahavadi S. Li F. Murthy K.S. Lentiviral siRNA silencing of sphingosine-1-phosphate receptors S1P1 and S1P2 in smooth muscle. Biochem. Biophys. Res. Commun. 2006;343:1038–1044. doi: 10.1016/j.bbrc.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Klassen H. Imfeld K.L. Ray J. Young M.J. Gage F.H. Berman M.A. The immunological properties of adult hippocampal progenitor cells. Vision Res. 2003;43:947–956. doi: 10.1016/s0042-6989(03)00094-4. [DOI] [PubMed] [Google Scholar]
- Lenzlinger P.M. Morganti-Kossmann M.C. Laurer H.L. McIntosh T.K. The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 2001;24:169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- Ligon K.L. Alberta J.A. Kho A.T. Weiss J. Kwaan M.R. Nutt C.L. Louis D.N. Stiles C.D. Rowitch D.H. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J. Neuropathol. Exp. Neurol. 2004;63:499–509. doi: 10.1093/jnen/63.5.499. [DOI] [PubMed] [Google Scholar]
- Li Y. Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci. Lett. 2009;456:120–123. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois C. Alvarez-Buylla A. Proliferating subventricular zone cells in the adult mammalian forebrain can differentiate into neurons and glia. Proc. Natl. Acad. Sci. USA. 1993;90:2074–2077. doi: 10.1073/pnas.90.5.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Chopp M. Intravenous administration of marrow stromal cells (MSCs) increases the expression of growth factors in rat brain after traumatic brain injury. J. Neurotrauma. 2004;21:33–39. doi: 10.1089/089771504772695922. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Lu M. Chopp M. Treatment of traumatic brain injury in adult rats with intravenous administration of human bone marrow stromal cells. Neurosurgery. 2003;53:697–702. doi: 10.1227/01.neu.0000079333.61863.aa. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Qu C. Goussev A. Chopp M. Long-term recovery after bone marrow stromal cell treatment of traumatic brain injury in rats. J. Neurosurg. 2006;104:272–277. doi: 10.3171/jns.2006.104.2.272. [DOI] [PubMed] [Google Scholar]
- Mahmood A. Lu D. Wang L. Li Y. Lu M. Chopp M. Treatment of traumatic brain injury in female rats with intravenous administration of bone marrow stromal cells. Neurosurgery. 2001a;49:1196–1203. [PubMed] [Google Scholar]
- Mahmood A. Lu D. Yi L. Chen J.L. Chopp M. Intracranial bone marrow transplantation after traumatic brain injury improving functional outcome in adult rats. J. Neurosurg. 2001b;94:589–595. doi: 10.3171/jns.2001.94.4.0589. [DOI] [PubMed] [Google Scholar]
- McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. doi: 10.1126/science.276.5309.66. [DOI] [PubMed] [Google Scholar]
- McQuiston A.R. Effects of mu-opioid receptor modulation on GABAB receptor synaptic function in hippocampal CA1. J. Neurophysiol. 2007;97:2301–2311. doi: 10.1152/jn.01179.2006. [DOI] [PubMed] [Google Scholar]
- Molcanyi M. Riess P. Bentz K. Maegele M. Hescheler J. Schafke B. Trapp T. Neugebauer E. Klug N. Schafer U. Trauma-associated inflammatory response impairs embryonic stem cell survival and integration after implantation into injured rat brain. J. Neurotrauma. 2007;24:625–637. doi: 10.1089/neu.2006.0180. [DOI] [PubMed] [Google Scholar]
- Palmer T.D. Ray J. Gage F.H. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol. Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- Philips M.F. Mattiasson G. Wieloch T. Bjorklund A. Johansson B.B. Tomasevic G. Martinez-Serrano A. Lenzlinger P.M. Sinson G. Grady M.S. McIntosh T.K. Neuroprotective and behavioral efficacy of nerve growth factor-transfected hippocampal progenitor cell transplants after experimental traumatic brain injury. J. Neurosurg. 2001;94:765–774. doi: 10.3171/jns.2001.94.5.0765. [DOI] [PubMed] [Google Scholar]
- Qu C. Xiong Y. Mahmood A. Kaplan D.L. Goussev A. Ning R. Chopp M. Treatment of traumatic brain injury in mice with bone marrow stromal cell-impregnated collagen scaffolds. J. Neurosurg. 2009;111:658–665. doi: 10.3171/2009.4.JNS081681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W.M. Rolfe A. Register D. Levasseur J.E. Churn S.B. Sun D. Strain-related differences after experimental traumatic brain injury in rats. J. Neurotrauma. 2010;27:1243–1253. doi: 10.1089/neu.2010.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson R.M. Broaddus W.C. Holloway K.L. Fillmore H.L. Grafts of adult subependymal zone neuronal progenitor cells rescue hemiparkinsonian behavioral decline. Brain Res. 2005a;1032:11–22. doi: 10.1016/j.brainres.2004.10.043. [DOI] [PubMed] [Google Scholar]
- Richardson R.M. Broaddus W.C. Holloway K.L. Sun D. Bullock M.R. Fillmore H.L. Heterotypic neuronal differentiation of adult subependymal zone neuronal progenitor cells transplanted to the adult hippocampus. Mol. Cell Neurosci. 2005b;28:674–682. doi: 10.1016/j.mcn.2004.11.013. [DOI] [PubMed] [Google Scholar]
- Riess P. Molcanyi M. Bentz K. Maegele M. Simanski C. Carlitscheck C. Schneider A. Hescheler J. Bouillon B. Schafer U. Neugebauer E. Embryonic stem cell transplantation after experimental traumatic brain injury dramatically improves neurological outcome, but may cause tumors. J. Neurotrauma. 2007;24:216–225. doi: 10.1089/neu.2006.0141. [DOI] [PubMed] [Google Scholar]
- Riess P. Zhang C. Saatman K.E. Laurer H.L. Longhi L.G. Raghupathi R. Lenzlinger P.M. Lifshitz J. Boockvar J. Neugebauer E. Snyder E.Y. McIntosh T.K. Transplanted neural stem cells survive, differentiate, and improve neurological motor function after experimental traumatic brain injury. Neurosurgery. 2002;51:1043–1052. doi: 10.1097/00006123-200210000-00035. [DOI] [PubMed] [Google Scholar]
- Shear D.A. Tate M.C. Archer D.R. Hoffman S.W. Hulce V.D. Laplaca M.C. Stein D.G. Neural progenitor cell transplants promote long-term functional recovery after traumatic brain injury. Brain Res. 2004;1026:11–22. doi: 10.1016/j.brainres.2004.07.087. [DOI] [PubMed] [Google Scholar]
- Sinson G. Voddi M. McIntosh T.K. Combined fetal neural transplantation and nerve growth factor infusion: effects on neurological outcome following fluid-percussion brain injury in the rat. J. Neurosurg. 1996;84:655–662. doi: 10.3171/jns.1996.84.4.0655. [DOI] [PubMed] [Google Scholar]
- Soares H.D. Hicks R.R. Smith D. McIntosh T.K. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J. Neurosci. 1995;15:8223–8233. doi: 10.1523/JNEUROSCI.15-12-08223.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H. Voltage-dependent ion channels in glial cells. Glia. 1994;11:156–172. doi: 10.1002/glia.440110210. [DOI] [PubMed] [Google Scholar]
- Studer L. Tabar V. McKay R.D. Transplantation of expanded mesencephalic precursors leads to recovery in parkinsonian rats. Nat. Neurosci. 1998;1:290–295. doi: 10.1038/1105. [DOI] [PubMed] [Google Scholar]
- Suhonen J.O. Peterson D.A. Ray J. Gage F.H. Differentiation of adult hippocampus-derived progenitors into olfactory neurons in vivo. Nature. 1996;383:624–627. doi: 10.1038/383624a0. [DOI] [PubMed] [Google Scholar]
- Sun D. Bullock M.R. Altememi N. Zhou Z. Hagood S. Rolfe A. McGinn M.J. Hamm R. Colello R.J. The effect of epidermal growth factor in the injured brain after trauma in rats. J. Neurotrauma. 2010;27:923–938. doi: 10.1089/neu.2009.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. Bullock M.R. McGinn M.J. Zhou Z. Altememi N. Hagood S. Hamm R. Colello R.J. Basic fibroblast growth factor-enhanced neurogenesis contributes to cognitive recovery in rats following traumatic brain injury. Exp. Neurol. 2009;216:56–65. doi: 10.1016/j.expneurol.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun D. McGinn M.J. Zhou Z. Harvey H.B. Bullock M.R. Colello R.J. Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 2007;204:264–272. doi: 10.1016/j.expneurol.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Tate C.C. Shear D.A. Tate M.C. Archer D.R. Stein D.G. LaPlaca M.C. Laminin and fibronectin scaffolds enhance neural stem cell transplantation into the injured brain. J. Tissue Eng. Regen. Med. 2009;3:208–217. doi: 10.1002/term.154. [DOI] [PubMed] [Google Scholar]
- Tate M.C. Shear D.A. Hoffman S.W. Stein D.G. Archer D.R. LaPlaca M.C. Fibronectin promotes survival and migration of primary neural stem cells transplanted into the traumatically injured mouse brain. Cell Transplant. 2002;11:283–295. [PubMed] [Google Scholar]
- Wallenquist U. Brannvall K. Clausen F. Lewen A. Hillered L. Forsberg-Nilsson K. Grafted neural progenitors migrate and form neurons after experimental traumatic brain injury. Restor. Neurol. Neurosci. 2009;27:323–334. doi: 10.3233/RNN-2009-0481. [DOI] [PubMed] [Google Scholar]
- Wennersten A. Meier X. Holmin S. Wahlberg L. Mathiesen T. Proliferation, migration, and differentiation of human neural stem/progenitor cells after transplantation into a rat model of traumatic brain injury. J. Neurosurg. 2004;100:88–96. doi: 10.3171/jns.2004.100.1.0088. [DOI] [PubMed] [Google Scholar]