Abstract
Bedside monitoring of cerebral metabolism in traumatic brain injury (TBI) with microdialysis is gaining wider clinical acceptance. The objective of this study was to examine the relationship between the fundamental physiological neuromonitoring modalities intracranial pressure (ICP), cerebral perfusion pressure (CPP), brain tissue oxygen (PbtO2), and cerebrovascular pressure reactivity index (PRx), and cerebral chemistry assessed with microdialysis, with particular focus on the lactate/pyruvate (LP) ratio as a marker of energy metabolism. Prospectively collected observational neuromonitoring data from 97 patients with TBI, requiring neurointensive care management and invasive cerebral monitoring, were analyzed. A linear mixed model analysis was used to account for individual patient differences. Perilesional tissue chemistry exhibited a significant independent relationship with ICP, PbtO2 and CPP thresholds, with increasing LP ratio in response to decrease in PbtO2 and CPP, and increase in ICP. The relationship between CPP and chemistry depended upon the state of PRx. Within the studied physiological range, tissue chemistry only changed in response to increasing ICP or drop in PbtO2<1.33 kPa (10 mmHg). In agreement with previous studies, significantly higher levels of cerebral lactate (p<0.001), glycerol (p=0.013), LP ratio (p<0.001) and lactate/glucose (LG) ratio (p=0.003) were found in perilesional tissue, compared to “normal” brain tissue (Mann-Whitney test). These differences remained significant following adjustment for the influences of other important physiological parameters (ICP, CPP, PbtO2, PbtCO2, PRx, and brain temperature; mixed linear model), suggesting that they may reflect inherent tissue properties related to the initial injury. Despite inherent biochemical differences between less-injured brain and “perilesional” cerebral tissue, both tissue types exhibited relationships between established physiological variables and biochemistry. Decreases in perfusion and oxygenation were associated with deteriorating neurochemistry and these effects were more pronounced in perilesional tissue and when cerebrovascular reactivity was impaired.
Key words: autoregulation, brain injury, CPP, ICP, microdialysis
Introduction
Severe traumatic brain injury (TBI) remains a significant clinical problem and despite improvements in clinical management is still associated with a high rate of unfavorable outcome. Monitoring of intracranial pressure (ICP) and cerebral perfusion pressure (CPP) is now regarded as the standard of care and is used to guide therapy. ICP reflects changes in the volume of the individual intracranial contents induced by trauma, such as edema or increased cerebral blood volume (CBV), and analysis of the ICP waveform can provide additional information as to the state of craniospinal compensation or compliance and cerebrovascular reactivity (autoregulation) by providing secondary indices. CPP and cerebrovascular reactivity jointly serve as a surrogate estimate of cerebral blood flow (CBF), which is also affected by other factors. Despite some remaining controversies surrounding the validity of ICP/CPP-guided management, especially in terms of improving outcome (Cremer, 2008; Cremer et al., 2005; Guidelines for the management of severe traumatic brain injury, 3rd edition, 2007) it is now accepted by many as the current “gold standard” of neuromonitoring. Despite the obvious advantage of ICP monitoring as a simple continuous bedside method it is not very sensitive in detecting regional and focal cerebral ischemia, which may play a crucial role in determining neurological outcome (Chesnut et al., 1993; Coles et al., 2004; Graham et al., 1989). In recent years other invasive neuromonitoring techniques aimed at assessing local tissue oxygenation and energy metabolism have been introduced and are gaining wider clinical acceptance. Brain tissue oximetry measures the partial pressure of oxygen (PbtO2) in the small area of brain surrounding the sensor, which is a product of delivery, diffusion, and consumption of the oxygen by cerebral tissue. Microdialysis allows in vivo assessment of several key energy metabolites, also in the direct vicinity of the implanted catheter. Despite the unique additional information provided by these methods, there is still substantial controversy on how to interpret and utilize the findings and even on how these parameters relate to more established monitoring modalities.
The aim of this study was to evaluate in a large prospective observational data set how focal parameters of energy metabolism measured by cerebral microdialysis relate to cerebral perfusion, ICP, and cerebral oxygenation, and whether the state of cerebrovascular pressure reactivity has any effect on these relationships. In particular, we aimed to assess the effects of decreasing CPP and PbtO2 on energy metabolism and to examine whether increasing ICP in the presence of clinically adequate levels of CPP may lead to metabolic derangement.
Methods
Observational monitoring data from 97 consecutive patients admitted with TBI between years 2001 and 2006, requiring neurointensive care management and ICP monitoring with addition of cerebral microdialysis, were prospectively collected. Data collection and analysis was approved by Local Ethical Committee and by the hospital Research and Development Department as part of our ongoing head injury program, and for all patients consent to the use of data was obtained from the next of kin or other legal representative. All patients were managed according to a standard CPP/ICP-driven protocol (Menon, 1999), with the aim of maintaining ICP <20 mm Hg and CPP >70 mm Hg (the latter target was only recently changed to >60 mm Hg following the evidence on the complications associated with higher CPP targets and subsequent guidelines) (Guidelines for the management of severe traumatic brain injury, 3rd edition, 2007; Robertson et al., 1999). Treatment options were introduced in a stepwise fashion, when the above targets were not met, and included head elevation, sedation and analgesia, muscular relaxants, colloid and crystalloid infusion therapy ventriculostomy, osmotic agents, moderate hyperventilation (PaCO2≥4.0 kPa), mild-to-moderate hypothermia (35–33°C), CPP augmentation with inotropes, barbiturates, and decompressive craniectomy.
Monitoring
Mean arterial pressure (MAP) was measured invasively via an arterial line with the transducer zeroed at the level of the heart. ICP, tissue oxygen sensors, and microdialysis catheters were inserted via triple lumen access device (Technicam, Newton Abbot, U.K.), placed routinely in the frontal area of the right hemisphere, unless clinical circumstances dictated another position. In all patients the position of the catheters was checked on the next available post-insertion CT scan and classified by the proximity to traumatic cerebral lesions as being positioned in macroscopically “normal” or “perilesional” (next to but not within traumatic parenchymal lesions) brain tissue. The definition of “normal” brain (less-injured brain) implied macroscopically (radiologically) normal brain tissue surrounding the microdialysis catheter. In most cases it corresponded to the diffuse injury pattern according to the CT Marshall grade classification, although in some cases it also applied to patients with evacuated mass lesions, where the catheter was positioned in macroscopically “normal” brain. Such definition of “normal” brain was based on previous microdialysis publications and consensus reports (Bellander et al., 2004; Engstrom et al., 2005; Vespa et al., 2007). Although traumatic penumbra cannot be reliably defined on simple CT scan and there are no universally accepted guidelines concerning optimal pericontusional catheter placement, we classified all catheters placed within 0.5–1.5 cm of the edge of radiographic abnormality (hemorrhagic or ischemic contusion) as “pericontusional”. ICP was measured with a parenchymal ICP microsensor (Codman, Raynham, MA) in all patients and CPP was calculated as the difference between MAP and ICP. In addition, 59 patients had a Neurotrend™ sensor (Codman, Raynham, MA), for continuous measurement of cerebral extracellular PO2 (PbtO2), pH (pHbt), PCO2 (PbtCO2), and temperature (tempb). All data were digitalized and captured by a bedside computer, with a sampling rate of 30 Hz, using software developed in house (ICM+, University of Cambridge, U.K.). The software, apart from recording ICP, CPP, MAP, and parameters measured by the Neurotrend sensor, allows online calculation of several ICP waveform-derived indices. The index used in this study for characterization of cerebrovascular pressure reactivity – the PRx index – represents the moving correlation coefficient between 30 time-averaged (over consecutive 8 sec periods) recordings of ICP and MAP (Czosnyka and Pickard, 2004; Czosnyka et al., 1997). Preserved cerebral vascular reactivity is characterized by negative values of the PRx (negative correlation between ICP and MAP) as increases in blood pressure lead to compensatory vasoconstriction aimed at preservation of the adequate level of CBF. On the contrary, positive PRx values imply impairment in physiological vascular response with increases in MAP leading to “passive” dilatation of arterioles and increase in CBV manifesting as concomitant rise in ICP (positive correlation between ICP and MAP).
Standard CMA 71 or 70 microdialysis catheters (10 mm membrane) were used in all patients and were perfused with central nervous system (CNS) perfusion fluid at the standard rate of 0.3 μL/min, with a sampling interval of 1 h. A methodological study comparing recovery using CMA 70 and 71 catheters has been performed earlier by a senior author of the manuscript and demonstrated comparable recovery rates, when using either of the catheters (Hutchinson et al., 2005). Analysis for glucose, lactate, pyruvate, and glutamate or glycerol was performed using bedside CMA 600 or ISCUS analyzers (all microdialysis equipment and perfusate was provided by CMA Microdialysis, Solna, Sweden). The system time of bedside computers and microdialysis analyzers were synchronised prior to the start of monitoring.
Data processing and statistical analysis
All data were examined for known artefacts associated with temporary disconnection of monitoring, flushing of the arterial line, and refilling of perfusion fluid, which were manually removed. Microdialysis values outside of the analytical range of CMA-600 or ISCUS analyzers (CMA, Solna, Sweden) were also deemed erroneous and excluded from the analysis. Data from the first 2 h following insertion of the microdialysis catheter and Neurotrend sensor were omitted, to minimize the influence of artefacts related to the insertion injury. This period was extended to 12 h for glutamate, based on the universal prolonged normalization of this metabolite following initial injury of catheter insertion. Timing of microdialysis measurements was adjusted to account for 17 min transit time from the catheter tip to the collecting vial. Microdialysis and continuously recorded physiological parameters were averaged over 60 min periods preceding microdialysis sampling time points, and merged into a single data set, resulting in 10,863 data points each representing 1 h of monitoring.
Statistical analysis was performed using SPSS 15.0 software (SPSS inc., Chicago, IL). Individual patients' trends were plotted and examined for time-related relationships between parameters. This exploratory stage was supplemented by calculating correlations between parameters, both within patient and for the pooled data set. To average the data and minimize imbalance caused by a potential strong influence of values from individual patients, predictor variables of interest were split into bins and dependent variables were averaged by patient/bin/day of monitoring, resulting in a comparable numbers of averaged values per each patient/bin. The following thresholds, considered clinically and physiologically relevant, were used to create data bins: CPP (mm Hg) <60, 60.1–70, 70.1–80, 80.1–90, >90 mm Hg; ICP (mm Hg) <15, 15.1–20, 20.1–25, 25.1–30, >30 mm Hg); PbtO2 (kPa) <1.33 (≈10 mm Hg), 1.34–1.99 (≈10.01–15 mm Hg), 2–2.66 (≈15.01 – 20 mm Hg), >2.6 mm Hg; PRx <− 0.2 (good cerebrovascular reactivity), >0.2 (impaired cerebrovascular reactivity). PRx data from the range of >−0.2 – <0.2 was considered inconclusive with respect to the state of cerebrovascular reactivity. Analysis was primarily focused on the lactate to pyruvate (LP) ratio as the most commonly used microdialysis parameter and independent of variations in recovery. However, significant differences in other markers are also reported. Non-parametric (Mann-Whitney) or parametric methods (ANOVA and independent samples T-test) were used, depending upon data distribution and variance. In addition, as an alternative approach to further minimize individual patient bias and to account for important covariates, linear mixed model analysis was applied to data. In these models, patients represented random effects and data bins, whereas catheter location and covariates represented fixed effects. When adjustment for covariate effect was performed, the mean values of covariates were used, which in all cases represented normal physiological value. A p-value of ≤0.05 was considered significant for all procedures and tests.
Results
Of the 97 patients studied 71 (73%) were male and 26 (27%) were female. The median (range) age of patients was 37 (16, 73) years. Based on the initial post-resuscitation pre-intubation Glasgow Coma Scale (GCS) score, assessed either by pre-hospital or hospital teams depending upon the place of intubation, 75 (77%) patients sustained severe (GCS 3–8), 16 (17%) patients sustained moderate (GCS 9–12) and 6 (6%) patients sustained mild brain injury. All patients with initially mild or moderate injury subsequently deteriorated and required intubation and intensive care. According to the initial admission CT scan, 58 (60%) patients had diffuse brain injury and 39 (40%) patients had a mass lesion, which in 23 (24%) of patients was later evacuated. At 6 months post-injury 26 (27%) patients were dead, 24 (25%) had unfavorable (Glasgow outcome scale [GOS] scores 2–3) and 40 (41%) had favorable outcome (GOS scores 4–5). Seven (7%) patients were lost to follow-up. Analysis of microdialysis catheter location on post-insertion CT scan found that 69 (71%) catheters were positioned in macroscopically “normal” (diffuse injury) brain tissue and 28 (29%) catheters were in close proximity to a traumatic parenchymal lesion (catheter tip within 0.5–1.5 cm from the edge of radiographic abnormality; a “perilesional” location). No catheters were placed directly into contusions. Mean (±SD) duration of microdialysis monitoring was 7 (±4) days.
Examination of individual monitoring trends using time of injury as the starting point revealed a high variation in the temporal relationship between different parameters, both between patients and within the same patient (e.g., between different days of monitoring for the same patient). Both non-parametric (Spearman's rank) and parametric (Pearson) correlations between microdialysis and other neuromonitoring parameters varied from non-significant to highly significant, depending upon patient/time epoch chosen. Even for periods of significant correlation, no firm temporal relationship was present, i.e., changes in chemistry followed or were preceded by changes in ICP, CPP, or PRx with no sustained order or pattern. However, the data revealed consistent differences between microdialysis parameters depending upon microdialysis catheter position. Further analysis, including averaging parameters per patient and applying mixed model with adjustment for potential confounders (ICP, CPP, PRx, PbtO2, PbtCO2, and brain temperature) confirmed this observation, by finding a significant difference between catheter locations for lactate, LP ratio, LG ratio, and glycerol (Table 1). Differences between “normal” and “perilesional” locations for glucose and pyruvate, which were significant with patient averaged values, lost their significance in the mixed model, following adjustment for other parameters. Because of these differences between catheter locations, all further analysis included site location as an independent factor.
Table 1.
Patient Averaged Median Values and Estimated Marginal Means from Mixed Model (after Adjustment for Covariates) of Microdialysis Parameters by Catheter Location with Intergroup Comparison
| |
Patient averaged values (median[IQR]) |
Estimated marginal means (standard error) |
||||
|---|---|---|---|---|---|---|
| Monitoring parameter | Normal brain | Perilesional brain | Mann-Whitney test p-value | Normal brain | Perilesional brain | Mixed model p-value |
| Glucose (mmol/L) n=97 | 1.1 (1.6) | 0.6 (1.1) | 0.08* | 1.6 (0.2) | 1.1 (0.3) | 0.23 |
| Lactate (mmol/L) n=97 | 2.8 (1.3) | 4.2 (2.4) | <0.001* | 3.0 (0.2) | 4.0 (0.3) | 0.03* |
| Pyruvate (μmol/L) n=97 | 110.1 (59.6) | 123.6 (63.6) | 0.07* | 117.4 (7.4) | 128.9 (9.9) | 0.36 |
| Glutamate (μmol/L) n=75 | 3.2 (3.0) | 2.5 (9.3) | 0.14 | 4.9 (2.3) | 10.5 (3.2) | 0.16 |
| Glycerol (μmol/L) n=72 | 52.9 (33.4) | 148.3 (157.0) | 0.013* | 74.3 (18.6) | 135.5 (24.6) | 0.05* |
| LP ratio n=97 | 25.0 (6.1) | 31.3 (13.2) | <0.001* | 26.1 (1.2) | 31.9 (1.6) | 0.05* |
| LG ratio n=97 | 3.9 (8.0) | 8.7 (17.5) | 0.003* | 5.2 (2.2) | 14.4 (3.0) | 0.02* |
| Ph n=56 | 7.2 (0.1) | 7.2 (.2) | 0.555 | 7.2 (0.01) | 7.2 (0.01) | 0.9 |
| PbtO2 (kPa) n=59 | 2.7 (1.3) | 2.8 (1.7) | 0.335 | 2.8 (0.2) | 2.4 (0.3) | 0.15 |
Adjustment for ICP, CPP, PRx, PbtO2 (with the exception of model when PbtO2 is dependent variable), pCO2, and brain temperature
n, number of patients; IQR, interquartile range.
p<0.05.
CPP, PRX, and microdialysis
Plotting of patient averaged data by CPP bins revealed a modest increase in LP ratio, as CPP reduced (Fig. 1). This trend was more prominent in “perilesional” tissue, where the difference was significant (ANOVA, p=0.019). There were not enough data to make reliable conclusions about the <60 mm Hg CPP bin because of relative lack of data points (averaged CPP <60 accounted for only 1.8% of all monitoring time). No significant difference in other parameters was detected with this method. However, when mixed model analysis was applied, a significant increase in lactate (p<0.001) and glycerol (p<0.001) as well as decrease in glucose (p=0.01), pyruvate (p<0.001), and pHbt (p<0.001) were predicted.
FIG. 1.
Relationship between CPP (mm Hg, binned) and LP ratio. (A) Observed LP ratio values (mean, 95% CI); black squares depict data from “perilesional” and white squares depict data from “normal” brain tissue. (B) Predicted values (mean±SE) generated by mixed linear model after controlling for possible confounders; black circles represent “perilesional” tissue values and white circles represent “normal tissue”.
Good autoregulation was observed in 373 (44%) and impaired autoregulation in 487 (56%) of valid cases (patient days). Patients' autoregulation status changed from day to day and therefore individual patients could contribute to both groups.
When two autoregulation groups (deranged and “intact” PRx) were compared, with microdialysis parameters averaged by patient/day, significantly higher levels of lactate and glycerol in the deranged autoregulation cohort were only seen in “perilesional” tissue (lactate 3.88±1.4 mmol/L vs. 4.34±1.9 mmol/L, p=0.03 and glycerol 132.6±132.3 μmol/L vs. 186.8±191.8 μmol/L for good and deranged cerebrovascular reactivity, respectively) with no difference between groups in “normal” brain tissue.
When each CPP bin was split by the state of autoregulation, in perilesional tissue the values of LP ratio were consistently higher in all bins (Fig. 2A), with this difference reaching significance at lower CPP values (60–70 mm Hg, p=0.022, Mann-Whitney test), and only in the higher PRx (disturbed vascular reactivity) group did an overall difference between CPP bins remain significant (ANOVA, p=0.003). Mixed model analysis, adjusted for patients and covariates, predicted higher values of LP ratio, both in “normal” and “perilesional” tissue for each CPP bin when PRx was deranged (Fig. 2B). The observed and predicted LP ratio values are presented in Table 2. Higher lactate and lower pyruvate values were also predicted for both catheter locations in deranged compared to preserved cerebrovascular reactivity groups. Predicted glutamate levels in less-injured tissue were higher in low CPP bins when derangement in vascular reactivity was present (p<0.001) (Fig. 3).
FIG. 2.
Relationship between LP ratio and CPP (mm Hg, binned) depending upon the state of cerebrovascular pressure reactivity and microdialysis catheter location. Black squares and circles represent preserved (PRx<0.2) and white circles represent deranged cerebrovascular pressure reactivity (PRx>0.2). (A) Observed values; (B) Values predicted by mixed linear model after adjusting for within-patient variability and covariates.
Table 2.
Observed and Predicted (Mixed Linear Model): LP Ratio Values, Stratified, and Compared within and between CPP Bins Depending upon State of Cerebrovascular Pressure Reactivity
| |
|
Normal brain, CPP bins (mm Hg) |
|
Perilesional brain,CPP (mm Hg) |
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 60–70 n=322 | 70–80 n=421 | 80–90 n=367 | >90 n=207 | p-value (ANOVA) | 60–70 n=135 | 70–80 n=188 | 80–90 n=176 | >90 n=123 | p-value (ANOVA) | ||
| Observed LP ratio by autoregulation category Mean (±SD) | Overall | 27.4 (±11.0) | 27.0 (±9.9) | 27.0 (±9.6) | 26.4 (±8.3) | 0.798 | 34.8 (±10.8) | 33.3 (±10.4) | 32.0 (±9.3) | 30.8 (±7.5) | 0.019* |
| Good autoregulation | 26.2 (±10.8) | 26.3 (±9.7) | 25.2 (±7.5) | 26.7 (±10.7) | 0.591 | 31.4 (±9.5) | 31.9 (±10.5) | 28.8 (±8.1) | 27.3 (±3.4) | 0.251 | |
| Impaired autoregulation | 26.7 (±11.3) | 26.7 (±9.6) | 26.4 (±9.8) | 26.5 (±8.7) | 0.992 | 37.3 (±12.7) | 34.3 (±11.3) | 31.6 (±9.1) | 30.9 (±7.5) | 0.003* | |
| Within bin comparison (Mann-Whitney p-value) | 0.66 | 0.56 | 0.62 | 0.75 | 0.022* | 0.174 | 0.07* | 0.1 | |||
| Predicted LP ratio by autoregulation category (EMM) Mean (±SE) | Overall | 25.1 (±0.9) | 26.6 (±1.1) | 25.1 (±2) | 25.4 (±0.8) | <0.001* | 34.7 (±1.3) | 33.3 (±1.3) | 30.8 (±1.3) | 29.9 (±1.3) | <0.001* |
| Good autoregulation | 23.8 (±1.0) | 24.1 (±0.9) | 23.4 (±1.0) | 24.7 (±1.2) | <0.001* | 25.9 (±1.7) | 27.2 (±1.5) | 26.1 (±1.6) | 25.8 (±2.0) | <0.001* | |
| Impaired autoregulation | 27.2 (±0.9) | 26.1 (±0.9) | 25.8 (±1.0) | 26.1 (±0.9) | 35.7 (±1.5) | 33.7 (±1.4) | 30.6 (±1.4) | 29.9 (±1.4) | |||
n=number of observations in each bin.
p <0.05.
FIG. 3.
Predicted response of (A) extracellular glycerol (μmol/L) and (B) glutamate (μmol/L) to changes in CPP, depending upon the cerebrovascular pressure reactivity (white circles indicate deranged and black circles preserved pressure reactivity [PRx]).
ICP and microdialysis
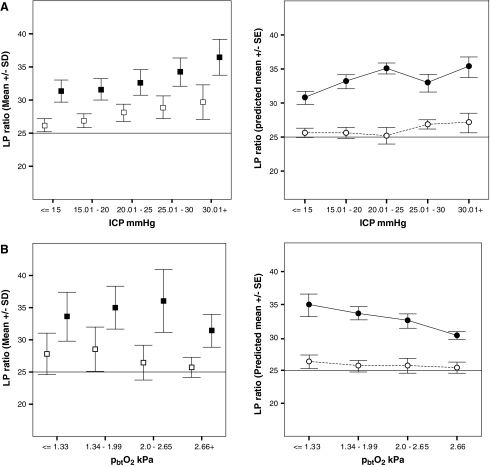
In the “normal” cerebral tissue, higher ICP bins were associated with increasing mean values of LP ratio and decreasing levels of extracellular pyruvate. A similar and more pronounced trend was seen in “perilesional” tissue, where in addition, a significant incremental increase in lactate was observed. There were no consistent or significant differences in other variables, including glycerol, glutamate, PbtO2, and pHbt. However, the differences for lactate, pyruvate, and LP ratio remained significant, even when individual patient influences and important covariates (including CPP, PRx, PbtO2, PbtCO2, and brain temperature) were accounted for by the mixed linear model for both catheter locations (Fig. 4A).
FIG. 4.
(A) Observed (left panel) and predicted (right panel) values by mixed linear model (after adjustment for confounders and within-patient variability) of LP ratio by ICP (mmHg) bins, stratified by catheter location (black, “perilesional”; white, “normal” tissue); n=97 patients. (B) Observed (left) and predicted (right) values of LP ratio by PbtO2 (kPa) bins stratified by tissue type (black, “perilesional”; white, “normal” tissue); n=59 patients.
PbtO2 and microdialysis
No significant differences for any microdialysis parameters were seen between PbtO2 cohorts, when patient/day mean values were analyzed. When influences of other covariates were controlled in the model, a modest but statistically significant increase in the LP ratio was predicted by the model for lower PbtO2 bins in both tissue types (Fig. 4B).
Discussion
The objective of this study was to examine the relationship between several neuromonitoring modalities, including the well established and those introduced into clinical practice more recently, as well as an association between catheter or sensor location and tissue biochemistry following TBI. The results of the study suggest that there is substantial difference in several baseline biochemical parameters between macroscopically “normal” or less-injured brain and “perilesional” brain tissue. This difference cannot be attributed to an effect of other important confounders, such as CPP, ICP, PbtO2, PRx, PbtCO2, and brain temperature. Some of these parameters do, however, exhibit an individual relationship with microdialysis markers, more so in the perilesional cerebral areas.
Location of the microdialysis catheter
Cerebral microdialysis has been used to monitor extracellular tissue chemistry in patients with TBI for over decade, and the most commonly used markers are glucose, lactate, pyruvate, glycerol, and glutamate as well as derived ratios: LP and LG ratios. The LP ratio is believed to reflect the state of cerebral oxidative metabolism and increases when the metabolic phase following glycolysis—aerobic metabolism of pyruvate in tricarboxylic acid (TCA) cycle in mitochondria—cannot take place because of either lack of oxygen or other reasons causing mitochondrial respiratory chain failure. Being a ratio of two metabolites, rather than absolute concentration, it is not dependent upon factors affecting microdialysis recovery, and it is often considered a marker of tissue ischemia. The LP ratio has been clearly shown to increase in situations in which cerebral ischemia is present (Bauer et al., 2004; Kett-White et al., 2002) and it is found to correlate with other parameters used to detect brain ischemia, e.g., cerebral oxygen extraction fraction (OEF) using O2 positron emission tomography (PET) (Hutchinson et al., 2002) and brain tissue oxygenation using direct PbtO2 monitoring (Hlatky et al., 2004a). On the other hand, it has been reported that the proportion of time when the LP ratio is raised above “ischemic” values by far exceeds the true incidence of cerebral ischemia detected by other methods (Vespa et al., 2005) and therefore, such LP ratio elevations may play an independent role and reflect tissue injury or non-ischemic metabolic crisis. Differences in baseline chemistry between relatively normal brain tissue and perilesional brain have been reported before (Engstrom et al., 2005) and whereas some previous studies suggest that abnormal chemistry in perilesional tissue may reflect evolving injury (Hillered et al., 1990; Hlatky et al., 2004b) and that such tissue may be more sensitive to physiological insults compared to “normal” brain (Nordstrom et al., 2003), others imply that pericontusional tissue may exhibit highly abnormal baseline biochemical values with little or no dependence upon other parameters (Vespa et al., 2007), and that therefore these metabolic disturbances possibly reflect the severity of initial injury.
Our data, revealing sustained differences in the levels of lactate, LP ratio, LG ratio, and glycerol between two catheter locations, which remained even after controlling for possible influences from confounding parameters, supports the presence of baseline differences between “normal” and “perilesional” tissue types. Higher glycerol levels in perilesional tissue, as a marker of cellular membrane breakdown, may reflect the degree of structural injury, manifesting in cellular death or apoptosis, although contribution from extracerebral sources of glycerol cannot be excluded, especially taking into account the higher likelihood of blood–brain barrier disruption in more injured tissue, even though no glycerol-containing medications (Berger et al., 2005) were used in these patients. Higher lactate, LP, and LG ratios are more likely to indicate persistent metabolic derangement, e.g., acquired and sustained mitochondrial dysfunction (Verweij et al., 2000). The latter suggestion is further supported by the lack of significant differences in glucose, pyruvate, and PbtO2 levels between two sides, pointing more toward intrinsic mitochondrial failure, rather than toward substrate delivery failure or tissue hypoxia. Despite these baseline differences, both tissue types exhibited biochemical variability in respect to thresholds in other important physiological parameters as discussed subsequently.
CPP, cerebrovascular pressure reactivity (PRx), and microdialysis
The importance of an adequate cerebral perfusion following traumatic brain injury is well recognized. However the search for optimum levels of CPP is continuing. Several targets have been proposed and revised and as the body of evidence on harmful effects of too low, but equally too high CPP grows, and a number of observations that adequate CPP levels may vary considerably between individual patients increases, other monitoring modalities are more often referred to in an attempt to individualize therapy. Observed patients' monitoring trends in this study confirm a highly individual relationship between CPP and microdialysis parameters, both between patients and within different time periods in the same patient. Although by far the most common pattern was the one with near simultaneous changes in CPP and chemistry, with LP ratio rising during or just after reduction in CPP, no change or minimal change in chemistry with substantial CPP drop or biochemical changes without any changes in cerebral perfusion pressure were also observed. Averaging of the data and comparing the values for different thresholds allows a more general estimate of the relationship among variables, although at the expense of losing sensitivity to short-term changes, which may be important, but obscured by the averaging process. Nevertheless, this approach has been applied before to relationships between CPP and microdialysis parameters, producing somewhat different results. Nordstrom and associates (2003), in 50 patients treated according to the Lund Concept following evacuation of the mass lesion, found that only CPP levels <50 mm Hg and only in the worse position were associated with significant increases in lactate and LP ratio. Stahl and associates (2001), reported gradual normalization of tissue chemistry in 48 patients treated with controlled reduction in CPP according to Lund Concept, despite CPP being <60 mm Hg 30% of time, even though better biochemistry toward the end of monitoring period seemed to be associated with higher mean CPP values. Vespa and associates (2007), in a smaller data set (n=8 pericontusional catheters) found, applying a mixed-effects model, that perilesional tissue exhibited a higher LP ratio with no significant difference between CPP thresholds above or below 60 mm Hg. Together, these reports may suggest that changes in tissue biochemistry only take place at very low CPP levels, possibly <50 mm Hg, although data from other studies (Poon et al., 2002), suggest that much higher thresholds may be appropriate for more severely injured patients requiring maximum therapy. In our data set, there was an insufficient number of monitoring hours with CPP falling <60 mm Hg, although because CPP data was averaged hourly, a considerable amount of hourly CPP values equal to or slightly above 60 mm Hg did contain periods of lower CPP. Within chosen CPP bins, we observed a significant increase in LP ratio with decreasing CPP, as well as adverse changes in other biochemical parameters when the mixed-effects model was applied. Adequacy of CBF to metabolic demand of the tissue depends not only on CPP, but also on the ability of cerebral vasculature to maintain and regulate CBF across the range of perfusion pressures and metabolic needs, known as cerebral autoregulation. When autoregulation is impaired, even levels of CPP above clinically accepted thresholds may not be sufficient for tissue demands. On the other hand, deranged metabolism may lead to secondary impairment in autoregulation and inadequacy of substrate delivery. Observations on the relationship between cerebral autoregulation and metabolism have been reported previously (Poon et al., 2005; Reinert et al., 2007) and Chan and associates (2005) even suggested using the relationship between CPP and cerebral glutamate as a measure of autoregulatory capacity. We have used the PRx index, which has been validated against more established indices (Czosnyka et al., 1998), relates to patients' outcome (Czosnyka et al., 2002, 2005), and has been used to estimate optimum ranges of CPP (Steiner et al., 2002). Overall comparison of metabolic parameters between preserved and deranged autoregulation, only revealing significant differences in lactate and glycerol in perilesional sites, did not suggest a substantial effect of autoregulation on energy metabolism. However, this is likely to occur because at higher levels of CPP, even when autoregulation is deranged, CBF may be adequate for the energy demands of the tissue. With decreasing CPP, the state of autoregulation starts to have more a noticeable effect on cerebral metabolism. We found that when autoregulation was preserved, the difference between CPP bins lost significance, whereas when autoregulation was deranged the LP ratio increase with CPP reduction was even more pronounced. Interestingly, this relationship was only seen in perilesional tissue, but not in “normal” brain, where there were no significant changes in chemistry across studied CPP ranges for either state of autoregulation. This may support the belief that less-injured tissue is more resistant to CPP challenges, and that lower CPP levels may need to be sustained before metabolic changes are evident in “normal” cerebral tissue. In addition, the mixed model predicted consistently higher values of LP ratio in each CPP bin when cerebrovascular pressure reactivity was deranged compared to when it was intact. However, mixed-effects model analysis also suggested that deterioration in energy metabolism associated with lower CPP values was seen only in perilesional tissue and only when autoregulation is impaired. In such cases with deranged vascular reactivity, higher values of CPP (e.g., >90 mm Hg as observed in our data) may be necessary to achieve adequate tissue perfusion, and therefore improvements in metabolism (LP ratio), although the reported relationship is purely observational and would require prospective interventional studies to prove causality prior to recommending universal clinical application.
ICP and microdialysis
Increasing ICP is arguably the most important manifestation of pathophysiological crisis following acute TBI, culminating in brain edema, and is strongly linked to mortality after TBI. It is largely responsible for the reduction in CPP following TBI and is a common target of therapeutic interventions. Increases in ICP may be caused by derangements in cerebral metabolism, but can in turn lead to further exacerbation in substrate delivery and energy production, contributing to a vicious cycle of brain edema. The nature of the relationship between ICP and metabolism poses several important questions. First, do changes in tissue biochemistry precede intracranial pressure rises and as such can they be detected with monitoring metabolism by microdialysis? Second, can elevation in ICP affect the recovery of metabolites by microdialysis and to what extent? And finally, does elevation of ICP matter if clinically adequate levels of CPP are maintained? Reports from several authors suggest that metabolic derangements detectable with microdialysis can indeed precede substantial rises in ICP (Belli et al., 2008; Boret et al., 2006). This relationship was also seen in some of our patients, however, far more common observations included (near) simultaneous changes in ICP and LP ratio or other metabolic parameters, or episodes where ICP elevations were not associated with any change in biochemistry, before or after an event. Rising interstitial pressure associated with brain edema may affect recovery of metabolites, by either increasing flow of fluid and substances into the catheter or preventing their movement into tissue. Further methodological studies are required to evaluate the presence and degree of this effect, however, one solution aimed at more accurate results in the presence of intracranial hypertension may be to use ratios, rather than single metabolites' levels. A debate on the relative importance of ICP versus CPP is ongoing between proponents of ICP- and CPP-oriented treatment approaches. Reports on good outcome in patients in whom the ICP was persistently high but satisfactory levels of CPP were maintained (Young et al., 2003) as well as observations from other pathologies in which very high ICP may be associated with minimal clinical symptoms and metabolic changes (Agren-Wilsson et al., 2005), question the importance of ICP in the presence of adequate CPP. However, maintenance of adequate CPP in the presence of uncontrolled intracranial pressure is difficult and associated with risks and complications (Robertson et al., 1999) and in most cases both ICP- and CPP-directed strategies can be used. Our ICP bin averaged data suggested that both “perilesional” and “normal” tissue exhibit progressively higher values of LP ratio with increase in ICP. When the influence of other important covariates and individual patients' effects are controlled for by the mixed-effects model, the trend remains, with significantly higher predicted LP ratio values associated with ICP cohorts >20 mm Hg, compared to relatively normal ICP <15 mm Hg, with the most noticeable difference for ICP >30 mm Hg, for both tissue types. One possible underlying mechanism for such differences, especially when influence from CPP and oxygenation is excluded, is microcirculatory impairment associated with ICP increases or tissue edema.
In summary, our data suggest that overall higher ICP levels are independently associated with significantly worse energy metabolism, although at the individual patient level temporal relationship can be highly individual. Therefore, the impact of ICP on patients' conditions may need to be assessed in each case and evaluation of cerebral metabolism may provide some useful information both in terms of warning of impeding ICP rise and assessing an impact of ICP level on the patient's condition, thereby assisting with therapeutic decisions.
Brain tissue oxygen and microdialysis
Brain tissue oximetry provides unique information on focal cerebral oxygen content. However the interpretation of the PbtO2 levels in isolation is difficult. High PbtO2 may imply both adequate oxygen delivery and decreased metabolism caused by either reduced demand or barriers to oxygen delivery (Menon et al., 2004) and low levels can reflect increased consumption or inadequate delivery. Previous studies have linked low levels of PbtO2 with abnormal microdialysis values (Hlatky et al., 2004a; Robertson et al., 1998; Valadka et al., 1998). However, no consistent improvement in cerebral chemistry, and in particular in LP ratio as measure of oxidative phosporylation, has been demonstrated by improving cerebral oxygenation either by increasing CPP (Johnston et al., 2005) or fraction of inspired oxygen (FiO2) (Reinert et al., 2003; Tolias et al., 2004) even despite other signs of physiological improvement (Nortje et al., 2007). Whereas this may be because of persistent mitochondrial dysfunction not yet amenable to therapeutic interventions, it may also reflect both delivery problems and treatment thresholds. We observed generally higher levels of LP ratio in each brain tissue oxygen bin in “pericontusional” tissue, compared to “normal” brain, implying baseline differences in energy metabolism between these tissue types. Mixed model analysis, however, predicted steady increase in LP ratio with declining PbtO2 (Fig. 4) for “perilesional” location, and significantly higher LP ratio even in “normal” brain at PbtO2 levels <1.33 kPa (10 mm Hg). This may suggest that “perilesional” tissue may be at risk at higher levels of decreasing PbtO2, but equally may demonstrate better response to treatment improving tissue oxygenation at these levels, which may be still adequate for “normal” cerebral tissue. On the other hand, “perilesional” tissue may exhibit intrinsic metabolic “failure”, which may explain persistently high values of LP ratio in the presence of adequate CBF and PbtO2, which do not respond to therapeutic manoeuvers.
Limitations
The results of the study are limited by its observational nature, making accurate cause–effect interpretations impossible. Duration of monitoring could have had an effect on monitoring parameters, with a general trend for improvement with time. However, whereas most patients tend to improve with time, some can have late or ongoing physiological derangements. The requirement for prolonged ICP and microdialysis monitoring could indicate the latter and therefore we felt that omitting these data was inappropriate. Evaluation of catheter position in the brain, although performed at the time of monitoring for clinical purposes, was not blinded. Moreover, the exact location and size of “traumatic penumbra” or “pericontusional” regions of tissue are very difficult to define, unlike the more clearly delineated penumbra of ischemic stroke, and therefore possibility of bias based on judging catheter positions cannot be completely ruled out, although we attempted to minimize error by adhering to a standardized “distance from the edge of contusion” value. Averaging of the data, as has been mentioned previously, may lead to obscuring important, but short-lived changes, as well as concealing longitudinal trends and temporal changes. Furthermore, data from periods of abnormal physiology were limited, as such episodes triggered corrective therapeutic interventions and therefore the majority of observed deviations from normal physiology represented failure of treatment to achieve targets, because of severity of TBI and associated physiological derangements, rather than periods of non-treatment. In view of a large amount of monitoring data and duration of the data collection, it was not possible to provide reliable conclusions on treatment effects based on the available data set, although it would be important to address in a separate, prospective study.
The choice of physiological thresholds was arbitrary, although aimed at clinically accepted levels, and choosing other “cutoffs” may have produced different results. Other physiological conditions (Hopwood et al., 2005; Vespa et al., 2002) and monitoring parameters (Eide et al., 2007) are likely to have effects or exhibit relationships with tissue chemistry that are not accounted for by this study.
Conclusions
This study shows that derangements in physiological parameters are associated with biochemical perturbations. Intracranial hypertension and cerebral hypoperfusion adversely affect cerebral metabolism, an effect that is enhanced in perilesional tissue and in the presence of impaired cerebrovascular reactivity.
However, within individual patients, deranged chemistry occurs even when physiological targets are being met, especially in perilesional tissue. Whereas the baseline differences can reflect the injury type and severity, variability in response to physiological challenges can provide an important feedback to steer the treatment and individualize targets of conventional monitoring modalitites. This supports the use of microdialysis as a monitor for these patients, especially as a means of individualizing and “fine-tuning” of the therapy. Further prospective studies are required to establish interventions that may lead to improved biochemical end-points.
Acknowledgments
We thank Dr. Peter Smielewski for help with digital data capture. Ivan Timofeev received a Codman Inc. grant, The Evelyn Trust grant, the Medical Research Council RESCUEicp trial grant, and a BP-TNK Kapitza Scholarship. Peter Hutchinson received an Academy of Medical Sciences/Health Foundation, Senior Surgical Scientist Fellowship. Dr. Keri Carpenter received support from the Medical Research Council Acute Brain Injury Programme Grant and the National Institute for Health Research Biomedical Research Centre, Cambridge. Study support (Medical Research Council Grant Nos. G9439390 ID 65883 and G0600986 ID79068).
Author Disclosure Statement
ICM+ is software for brain monitoring (www.neurosurg.cam.ac.uk/icmplus), licensed by University of Cambridge, Cambridge Enterprise Ltd. Dr. M. Czosnyka has financial interest in a part of the licensing fee.
References
- Agren-Wilsson A. Eklund A. Koskinen L.O. Bergenheim A.T. Malm J. Brain energy metabolism and intracranial pressure in idiopathic adult hydrocephalus syndrome. J. Neurol. Neurosurg. Psychiatr. 2005;76:1088–1093. doi: 10.1136/jnnp.2004.042838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R. Gabl M. Obwegeser A. Galiano K. Barbach J. Mohsenipour I. Neurochemical monitoring using intracerebral microdialysis during cardiac resuscitation. Intensive Care Med. 2004;30:159–161. doi: 10.1007/s00134-003-2015-5. [DOI] [PubMed] [Google Scholar]
- Bellander B.M. Cantais E. Enblad P. Hutchinson P. Nordstrom C.H. Robertson C. Sahuquillo J. Smith M. Stocchetti N. Ungerstedt U. Unterberg A. Olsen N.V. Consensus meeting on microdialysis in neurointensive care. Intensive Care Med. 2004;30:2166–2169. doi: 10.1007/s00134-004-2461-8. [DOI] [PubMed] [Google Scholar]
- Belli A. Sen J. Petzold A. Russo S. Kitchen N. Smith M. Metabolic failure precedes intracranial pressure rises in traumatic brain injury: a microdialysis study. Acta Neurochir. (Wien). 2008;150:461–469. doi: 10.1007/s00701-008-1580-3. ; discussion 470. [DOI] [PubMed] [Google Scholar]
- Berger C. Sakowitz O.W. Kiening K.L. Schwab S. Neurochemical monitoring of glycerol therapy in patients with ischemic brain edema. Stroke. 2005;36:e4–6. doi: 10.1161/01.STR.0000151328.70519.e9. [DOI] [PubMed] [Google Scholar]
- Boret H. Fesselet J. Meaudre E. Gaillard P.E. Cantais E. Cerebral microdialysis and P(ti)O2 for neuro-monitoring before decompressive craniectomy. Acta Anaesthesiol Scand. 2006;50:252–254. doi: 10.1111/j.1399-6576.2005.00862.x. [DOI] [PubMed] [Google Scholar]
- Chan T.V. Ng S.C. Lam J.M. Poon W.S. Gin T. Monitoring of autoregulation using intracerebral microdialysis in patients with severe head injury. Acta Neurochir. 2005;95(Suppl):113–116. doi: 10.1007/3-211-32318-x_24. [DOI] [PubMed] [Google Scholar]
- Chesnut R.M. Marshall L.F. Klauber M.R. Blunt B.A. Baldwin N. Eisenberg H.M. Jane J.A. Marmarou A. Foulkes M.A. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216–222. doi: 10.1097/00005373-199302000-00006. [DOI] [PubMed] [Google Scholar]
- Coles J.P. Fryer T.D. Smielewski P. Chatfield D.A. Steiner L.A. Johnston A.J. Downey S.P. Williams G.B. Aigbirhio F. Hutchinson P.J. Rice K. Carpenter T.A. Clark J.C. Pickard J.D. Menon D.K. Incidence and mechanisms of cerebral ischemia in early clinical head injury. J. Cereb. Blood Flow Metab. 2004;24:202–211. doi: 10.1097/01.WCB.0000103022.98348.24. [DOI] [PubMed] [Google Scholar]
- Cremer O.L. Does ICP monitoring make a difference in neurocritical care? Eur. J. Anaesthesiol. 2008;42(Suppl):87–93. doi: 10.1017/S0265021507003237. [DOI] [PubMed] [Google Scholar]
- Cremer O.L. van Dijk G.W. van Wensen E. Brekelmans G.J. Moons K.G. Leenen L.P. Kalkman C.J. Effect of intracranial pressure monitoring and targeted intensive care on functional outcome after severe head injury. Crit. Care Med. 2005;33:2207–2213. doi: 10.1097/01.ccm.0000181300.99078.b5. [DOI] [PubMed] [Google Scholar]
- Czosnyka M. Pickard J.D. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatr. 2004;75:813–821. doi: 10.1136/jnnp.2003.033126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnyka M. Balestreri M. Steiner L. Smielewski P. Hutchinson P.J. Matta B. Pickard J.D. Age, intracranial pressure, autoregulation, and outcome after brain trauma. J Neurosurg. 2005;102:450–454. doi: 10.3171/jns.2005.102.3.0450. [DOI] [PubMed] [Google Scholar]
- Czosnyka M. Smielewski P. Kirkpatrick P. Laing R.J. Menon D. Pickard J.D. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–17. doi: 10.1097/00006123-199707000-00005. discussion 17–19. [DOI] [PubMed] [Google Scholar]
- Czosnyka M. Smielewski P. Kirkpatrick P. Piechnik S. Laing R. Pickard J.D. Continuous monitoring of cerebrovascular pressure-reactivity in head injury. Acta Neurochir. 1998;71(Suppl):74–77. doi: 10.1007/978-3-7091-6475-4_23. [DOI] [PubMed] [Google Scholar]
- Czosnyka M. Smielewski P. Piechnik S. Pickard J.D. Clinical significance of cerebral autoregulation. Acta Neurochir. 2002;81(Suppl):117–119. doi: 10.1007/978-3-7091-6738-0_30. [DOI] [PubMed] [Google Scholar]
- Eide P.K. Bentsen G. Stanisic M. Stubhaug A. Association between intracranial pulse pressure levels and brain energy metabolism in a patient with an aneurysmal subarachnoid haemorrhage. Acta Anaesthesiol. Scand. 2007;51:1273–1276. doi: 10.1111/j.1399-6576.2007.01433.x. [DOI] [PubMed] [Google Scholar]
- Engstrom M. Polito A. Reinstrup P. Romner B. Ryding E. Ungerstedt U. Nordstrom C.H. Intracerebral microdialysis in severe brain trauma: the importance of catheter location. J Neurosurg. 2005;102:460–469. doi: 10.3171/jns.2005.102.3.0460. [DOI] [PubMed] [Google Scholar]
- Graham D.I. Ford I. Adams J.H. Doyle D. Teasdale G.M. Lawrence A.E. McLellan D.R. Ischaemic brain damage is still common in fatal non–missile head injury. J. Neurol. Neurosurg. Psychiatr. 1989;52:346–350. doi: 10.1136/jnnp.52.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidelines for the management of severe traumatic brain injury. J Neurotrauma. (3rd) 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- Hillered L. Persson L. Ponten U. Ungerstedt U. Neurometabolic monitoring of the ischaemic human brain using microdialysis. Acta Neurochir. (Wien). 1990;102:91–97. doi: 10.1007/BF01405420. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Valadka A.B. Goodman J.C. Contant C.F. Robertson C,S. Patterns of energy substrates during ischemia measured in the brain by microdialysis. J. Neurotrauma. 2004a;21:894–906. doi: 10.1089/0897715041526195. [DOI] [PubMed] [Google Scholar]
- Hlatky R. Valadka A.B. Goodman J.C. Robertson C.S. Evolution of brain tissue injury after evacuation of acute traumatic subdural hematomas. Neurosurgery. 2004b;55:1318–1323. doi: 10.1227/01.neu.0000143029.42638.2c. discussion 1324. [DOI] [PubMed] [Google Scholar]
- Hopwood S.E. Parkin M.C. Bezzina E.L. Boutelle M.G. Strong A.J. Transient changes in cortical glucose and lactate levels associated with peri-infarct depolarisations, studied with rapid-sampling microdialysis. J. Cereb. Blood Flow Metab. 2005;25:391–401. doi: 10.1038/sj.jcbfm.9600050. [DOI] [PubMed] [Google Scholar]
- Hutchinson P.J. Gupta A.K. Fryer T.F. Al-Rawi P.G. Chatfield D.A. Coles J.P. O'Connell M.T. Kett–White R. Minhas P.S. Aigbirhio F.I. Clark J.C. Kirkpatrick P.J. Menon D.K. Pickard J.D. Correlation between cerebral blood flow, substrate delivery, and metabolism in head injury: a combined microdialysis and triple oxygen positron emission tomography study. J. Cereb. Blood Flow Metab. 2002;22:735–745. doi: 10.1097/00004647-200206000-00012. [DOI] [PubMed] [Google Scholar]
- Hutchinson P.J. O'Connell M.T. Nortje J. Smith P. Al-Rawi P.G. Gupta A.K. Menon D.K. Pickard J.D. Cerebral microdialysis methodology––evaluation of 20 kDa and 100 kDa catheters. Physiol. Meas. 2005;26:423–428. doi: 10.1088/0967-3334/26/4/008. [DOI] [PubMed] [Google Scholar]
- Johnston A.J. Steiner L.A. Coles J.P. Chatfield D.A. Fryer T.D. Smielewski P. Hutchinson P.J. O'Connell M.T. Al-Rawi P.G. Aigbirihio F.I. Clark J.C. Pickard J.D. Gupta A.K. Menon D.K. Effect of cerebral perfusion pressure augmentation on regional oxygenation and metabolism after head injury. Crit. Care Med. 2005;33:189–195. doi: 10.1097/01.ccm.0000149837.09225.bd. discussion 255–187. [DOI] [PubMed] [Google Scholar]
- Kett–White R. Hutchinson P.J. Al-Rawi P.G. Gupta A.K. O'Connell M.T. Pickard J.D. Kirkpatrick P.J. Extracellular lactate/pyruvate and glutamate changes in patients during per-operative episodes of cerebral ischaemia. Acta. Neurochir. 2002;81(Suppl):363–365. doi: 10.1007/978-3-7091-6738-0_92. [DOI] [PubMed] [Google Scholar]
- Menon D.K. Cerebral protection in severe brain injury: physiological determinants of outcome and their optimisation. Br. Med. Bull. 1999;55:226–258. doi: 10.1258/0007142991902231. [DOI] [PubMed] [Google Scholar]
- Menon D.K. Coles J.P. Gupta A.K. Fryer T.D. Smielewski P. Chatfield D.A. Aigbirhio F. Skepper J.N. Minhas P.S. Hutchinson P.J. Carpenter T.A. Clark J.C. Pickard J.D. Diffusion limited oxygen delivery following head injury. Crit. Care Med. 2004;32:1384–1390. doi: 10.1097/01.ccm.0000127777.16609.08. [DOI] [PubMed] [Google Scholar]
- Nordstrom C.H. Reinstrup P. Xu W. Gardenfors A. Ungerstedt U. Assessment of the lower limit for cerebral perfusion pressure in severe head injuries by bedside monitoring of regional energy metabolism. Anesthesiology. 2003;98:809–814. doi: 10.1097/00000542-200304000-00004. [DOI] [PubMed] [Google Scholar]
- Nortje J. Coles J.P. Timofeev I. Fryer T.D. Aigbirhio F.I. Smielewski P. Outtrim J.G. Chatfield D.A. Pickard J.D. Hutchinson P.J. Gupta A.K. Menon D.K. Effect of hyperoxia on regional oxygenation and metabolism after severe traumatic brain injury: preliminary findings. Crit Care Med. 2008;36:273–281. doi: 10.1097/01.CCM.0000292014.60835.15. [DOI] [PubMed] [Google Scholar]
- Poon W.S. Ng S.C. Chan M.T. Lam J.M. Lam W.W. Cerebral blood flow (CBF)-directed management of ventilated head-injured patients. Acta Neurochir. 2005;95(Suppl):9–11. doi: 10.1007/3-211-32318-x_2. [DOI] [PubMed] [Google Scholar]
- Poon W.S. Ng S.C. Chan M.T. Leung C.H. Lam J.M. Neurochemical changes in ventilated head-injured patients with cerebral perfusion pressure treatment failure. Acta Neurochir. 2002;81(Suppl):335–338. doi: 10.1007/978-3-7091-6738-0_85. [DOI] [PubMed] [Google Scholar]
- Reinert M. Andres R.H. Fuhrer M. Muller A. Schaller B. Widmer H. Online correlation of spontaneous arterial and intracranial pressure fluctuations in patients with diffuse severe head injury. Neurol. Res. 2007;29:455–462. doi: 10.1179/016164107X164175. [DOI] [PubMed] [Google Scholar]
- Reinert M. Barth A. Rothen H.U. Schaller B. Takala J. Seiler R.W. Effects of cerebral perfusion pressure and increased fraction of inspired oxygen on brain tissue oxygen, lactate and glucose in patients with severe head injury. Acta Neurochir. (Wien) 2003;145:341–349. doi: 10.1007/s00701-003-0027-0. [DOI] [PubMed] [Google Scholar]
- Robertson C.S. Gopinath S.P. Uzura M. Valadka A.B. Goodman J.C. Metabolic changes in the brain during transient ischemia measured with microdialysis. Neurol. Res. 20. 1998;1(Suppl):S91–94. doi: 10.1080/01616412.1998.11740618. [DOI] [PubMed] [Google Scholar]
- Robertson C.S. Valadka A.B. Hannay H.J. Contant C.F. Gopinath S.P. Cormio M. Uzura M. Grossman R.G. Prevention of secondary ischemic insults after severe head injury. Crit. Care Med. 1999;27:2086–2095. doi: 10.1097/00003246-199910000-00002. [DOI] [PubMed] [Google Scholar]
- Stahl N. Ungerstedt U. Nordstrom C.H. Brain energy metabolism during controlled reduction of cerebral perfusion pressure in severe head injuries. Intensive Care Med. 2001;27:1215–1223. doi: 10.1007/s001340101004. [DOI] [PubMed] [Google Scholar]
- Steiner L.A. Czosnyka M. Piechnik S.K. Smielewski P. Chatfield D. Menon D.K. Pickard J.D. Continuous monitoring of cerebrovascular pressure reactivity allows determination of optimal cerebral perfusion pressure in patients with traumatic brain injury. Crit. Care Med. 2002;30:733–738. doi: 10.1097/00003246-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Tolias C.M. Reinert M. Seiler R. Gilman C. Scharf A. Bullock M.R. Normobaric hyperoxia–induced improvement in cerebral metabolism and reduction in intracranial pressure in patients with severe head injury: a prospective historical cohort-matched study. J. Neurosurg. 2004;101:435–444. doi: 10.3171/jns.2004.101.3.0435. [DOI] [PubMed] [Google Scholar]
- Valadka A.B. Goodman J.C. Gopinath S.P. Uzura M. Robertson CS. Comparison of brain tissue oxygen tension to microdialysis-based measures of cerebral ischemia in fatally head-injured humans. J. Neurotrauma. 1998;15:509–519. doi: 10.1089/neu.1998.15.509. [DOI] [PubMed] [Google Scholar]
- Verweij B.H. Muizelaar J.P. Vinas F.C. Peterson P.L. Xiong Y. Lee C.P. Impaired cerebral mitochondrial function after traumatic brain injury in humans. J. Neurosurg. 2000;93:815–820. doi: 10.3171/jns.2000.93.5.0815. [DOI] [PubMed] [Google Scholar]
- Vespa P. Bergsneider M. Hattori N. Wu H.M. Huang S.C. Martin N.A. Glenn T.C. McArthur D.L. Hovda D.A. Metabolic crisis without brain ischemia is common after traumatic brain injury: a combined microdialysis and positron emission tomography study. J. Cereb. Blood Flow Metab. 2005;25:763–774. doi: 10.1038/sj.jcbfm.9600073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vespa P. Martin N.A. Nenov V. Glenn T. Bergsneider M. Kelly D. Becker D.P. Hovda D.A. Delayed increase in extracellular glycerol with post-traumatic electrographic epileptic activity: support for the theory that seizures induce secondary injury. Acta Neurochir. 2002;81(Suppl):355–357. doi: 10.1007/978-3-7091-6738-0_90. [DOI] [PubMed] [Google Scholar]
- Vespa P.M. Phelan K.O. McArthur D. Miller C. Eliseo M. Hirt D. Glenn T. Hovda D.A. Pericontusional brain tissue exhibits persistent elevation of lactate/pyruvate ratio independent of cerebral perfusion pressure. Crit. Care Med. 2007;35:1153–1160. doi: 10.1097/01.CCM.0000259466.66310.4F. [DOI] [PubMed] [Google Scholar]
- Young J.S. Blow O. Turrentine F. Claridge J.A. Schulman A. Is there an upper limit of intracranial pressure in patients with severe head injury if cerebral perfusion pressure is maintained? Neurosurg. Focus. 2003;15:E2. doi: 10.3171/foc.2003.15.6.2. [DOI] [PubMed] [Google Scholar]