Abstract
Objectives
We assessed whether extra-immunization can serve as a clinical indicator for fragmentation of care.
Methods
Using public-use files of the 1999–2003 National Immunization Survey, we classified children 19–35 months of age by their vaccination providers for the degree of fragmentation of care as ordered from lowest with one vaccine provider, to increasing fragmentation with multiple providers in one facility type, to multiple providers in more than one facility type. Extra-immunization was defined conservatively based on the year-specific recommendations of the Advisory Committee on Immunization Practices (ACIP) for immunizations due before 18 months of age. Of note, 1999–2003 transitioned from oral to inactivated poliovirus vaccines.
Results
The rate for extra-immunization was 9.4% (95% confidence interval [CI] 9.2, 9.7). Of single vaccines, the rate for polio vaccine was highest (5.7%, 95% CI 5.5, 6.0). Extra-immunization was lowest for the 69% of children with only one vaccination provider (6.4%, 95% CI 6.1, 6.7), was higher in children who had more than one vaccination provider with one vaccination facility type (13.9%, 95% CI 13.2, 14.6), and highest with more than one facility type (24.1%, 95% CI 22.5, 25.6). Logistic regression (including race/ethnicity, language, provider type, survey year, and a parent-held immunization record) confirmed that multiple providers (adjusted odds ratio [AOR] = 2.30), multiple facility types (AOR=4.67), Spanish language (AOR=1.29), and race/ethnicity (black AOR=1.16, Hispanic AOR=1.31) were each associated with extra-immunization. Excluding poliovirus vaccine from the analysis, AORs for multiple providers and multiple facility types increased to 3.64 and 8.95, respectively.
Conclusions
Extra-immunization is associated with receiving immunizations from multiple providers and multiple facility types.
The American Academy of Pediatrics defines the medical home as one that provides care to infants, children, and adolescents that is accessible, continuous, comprehensive, family-centered, coordinated, compassionate, and culturally effective. Explicitly noted in the American Academy of Pediatrics-defined services that a medical home should provide are continuity and needed immunizations.1,2 However, fragmentation of care can result in the failure to deliver care and overutilization of resources.3,4 The medical home with continuity of care is associated with the delivery of needed care, including immunizations.4–8 Medical homes offer up a solution to that fragmentation.8–10 As compared with other avenues of care, medical homes are equipped to achieve complete and timely immunization.5,8,11
Early work in the development of regional immunization registries indicated that approximately 5% of children have received at least one unnecessary immunization by 2 years of age.12 This finding has been termed “extra-immunization”13–16 or “overvaccination.”17 While the use of some combination vaccines can result in acceptable extra-immunization, missing information regarding previous vaccine status can lead to additional extra-immunization as a result of appropriate efforts of medical providers to assure the child's up-to-date immunization status. Extra-immunization may better reflect medical care fragmentation than underimmunization. The latter can also reflect parental choice and lack of access to medical care in addition to fragmentation of medical care. Thus, extra-immunization may serve as a clinical indicator18 and may, along with other clinical indicators, serve a purpose in testing claims that for a given population of patients, they are truly residing in medical homes and, for a given medical home, it is truly eliminating fragmentation of care. Using validated data collected over five years, we sought to determine if fragmentation of care was inversely associated with extra-immunization.
METHODS
We analyzed the public-use files of the National Immunization Survey (NIS) from 1999 to 2003. Problems with duplicate entries and unclear reporting rules with combination vaccines were addressed beginning with the 1999 NIS dataset.16 The National Center for Immunization and Respiratory Diseases of the Centers for Disease Control and Prevention sponsors these surveys, and the National Center for Health Statistics conducts them. The methods of these annual surveys have been published elsewhere.19 In brief, these are validated, stratified, random-digit-dialed telephone surveys of households with children 19–35 months of age. Information is collected through computer-assisted telephone interview techniques. Immunization information is collected directly from the identified immunization providers for the surveyed children. Adjustments to design variables are made by the Centers for Disease Control and Prevention for biases resulting from nonresponse and non-telephone households. The 1999–2003 surveys included 111,730 children, representing a cohort of 5,756,583 children (the average population of children 19–35 months of age during the five-year period in the U.S.).
Our main outcome variable was extra-immunization defined as present or absent. We used the provider-based record data available from NIS to assess the frequency of extra-immunization. We defined extra-immunization to allow the largest number of vaccines and based our definition on each year's published immunization schedule, including the minimum interval schedule between doses for each vaccine (i.e., the “catch-up schedule”).20–24 We defined extra-immunization with diphtheria and tetanus toxoids and pertussis vaccine, whole or acellular (DTxP) and Haemophilus influenzae type b vaccine as >4 doses each. Doses counted as DTxP included diphtheria and tetanus toxoids, diphtheria-tetanus-acellular pertussis (DTaP), and diphtheria-tetanus-pertussis (DTP). We defined extra-immunization with polio vaccine as >3 doses of either inactivated polio vaccine (IPV) or oral polio vaccine in any combination. We defined extra-immunization with hepatitis B (Hep B) vaccine as >3 doses from 1999 to 2001 and four doses from 2002 to 2003, when the first Hep B vaccine was given in the initial week of life. (Beginning in 2002, the Advisory Committee on Immunization Practices [ACIP] provided permissive language allowing four doses of Hep B vaccine to support newborn immunization and the subsequent use of combination Hep B vaccines.)
We defined extra-immunization with a measles-containing vaccine as >2 doses occurring on or after 12 months of age (discounting any dose before 12 months of age, the catch-up schedule and ACIP measles-mumps-rubella vaccine recommendation allows two doses separated by 28 days occurring after 12 months of age).25 We defined extra--immunization with varicella zoster vaccine as >1 dose. Other than the initial doses of Hep B and measles-containing vaccines, as explained previously, we did not test for initial dose or minimum interval violations. Because of the large amount of extra-immunization specific to polio vaccine that may have resulted for reasons peculiar to the polio vaccine and concurrent changes in recommendations for that vaccine during the period examined, we constructed another outcome variable that did not include polio vaccine extra-immunization.
Medical home served as the main exposure variable. As a proxy for medical home, we used the NIS-collected number and type of immunization providers. Immunization provider is not necessarily an individual clinician but, rather, one reporter of immunizations (e.g., an office or clinic). The NIS classified immunization providers by both number and facility type. We constructed a composite variable to capture both multiple providers and multiple facility types (e.g., public, private, hospital, military, mixed, other, unknown, and missing). If the record listed only one immunization provider and the provider facility type was missing (about 11% were missing), then we assumed that child had only “one provider, one facility type.” If the record indicated more than one provider but only one facility type or facility type missing, then we coded that child as having “multiple providers, same facility type.” If the record explicitly listed the child as having multiple facility types, then we coded this as “multiple facility types” regardless of how many providers were indicated.
For race/ethnicity, we used a composite race variable defined by the NIS as Hispanic, non-Hispanic white, non-Hispanic black, and non-Hispanic other. We categorized those respondents indicating multiple races/ethnicities in the “all others” category if they did not indicate Hispanic ethnicity. We also constructed a summary variable that dichotomized race/ethnicity into non-Hispanic white vs. nonwhite.
NIS data provided three potential socioeconomic status variables: poverty status, household income, and maternal education. Because maternal education had full reporting and the other variables had substantial missing values, we chose maternal education as the measure of socioeconomic status for our analyses.
The NIS data contained a variable reporting whether the child's vaccine providers reported vaccinations to a state or community immunization registry. This variable was coded in NIS data as “all providers,” “some but not all providers,” “no providers,” and “unknown.”
We modeled the effect of the presence of a medical home on extra-immunization using logistic regression. We selected the variables for further analyses based on magnitude of the effect of that variable on extra-immunization in the bivariate analyses. The final model included medical home (provider number and facility type), race/ethnicity, survey language, survey year, maternal education, and parent-held immunization records. We used adjusted odds ratios (AORs) to report the results of analyses controlling for variables in the full model. The final multivariable model did not include vaccine registry use because of the large number of unknown values over the years, averaging 21.6% and ranging from 38.6% in 1999 to 16.5% in 2001.
Each year's NIS dataset included weights appropriate for inferences to the population of children 19–35 months of age in that year in the U.S. To analyze our five-year dataset while avoiding overweighting of observations, we divided the weight for each observation by five. This had the effect of making the weighted dataset the average of the target population of children during the time period studied.
We performed our data extraction and recoding using SAS® version 9.126 and conducted analyses appropriate for this multistage, complex survey using Stata® version 8.0.27 Stata permitted the inclusion of the survey design variables into the analysis and, thus, addressed the complex sampling appropriately to achieve the best approximate variances for population estimates. All rates reported, unless otherwise stated, were weighted to reflect population-based estimates.
RESULTS
Sample characteristics
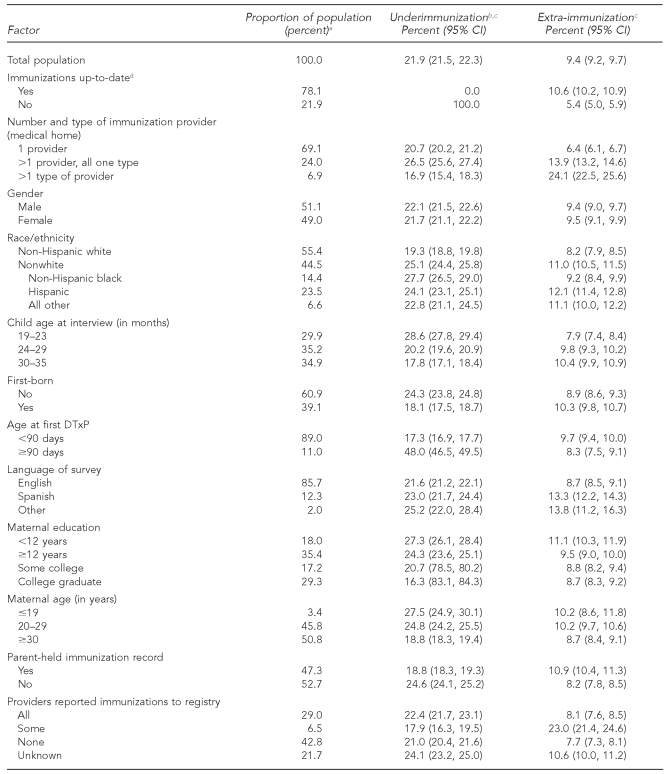
Table 1 shows that the weighted overall rate of extra-immunization was 9.4% (95% confidence interval [CI] 9.2, 9.7) compared with underimmunization (21.9%, 95% CI 21.5, 22.3). Of the sample, 49.0% of respondents were female, 14.4% were black, 23.5% were Hispanic, and 6.6% were from another or multiple racial/ethnic minority groups. Most children (69.1%) had a single immunization provider, with 24.0% having >1 provider with the same facility type and 6.9% having >1 provider from multiple facility types.
Table 1.
Characteristics of children aged 19–35 months and associations with underimmunization and extra-immunization: 1999–2003 National Immunization Survey
aProportions are based on weighted observations. Percentages may not add up to 100 due to rounding.
bUndervaccinated is defined as not having at least four DTxP, three polio, one measles-containing, three hepatitis B, and three Haemophilus influenzae type b vaccines by the time of the survey.
cFor both undervaccinated and extra-immunized, this is a yes/no characterization. Note that one can be both undervaccinated and have extra-immunization because of the combination of vaccines.
dUp-to-date is the receipt of at least four DTxP, three polio, one measles-containing, three hepatitis B, and three Haemophilus influenzae type b vaccines by the time of the survey.
CI = confidence interval
DTxP = diphtheria and tetanus toxoids and pertussis vaccine, whole or acellular
Bivariate associations
The association of extra-immunization with the number of providers and facility type was large and consistent. As shown in Table 1, the rate of extra-immunization with one provider was only 6.4% (95% CI 6.1, 6.7) whereas children who had more than one provider with all providers in the same facility type had an extra-immunization rate of 13.9% (95% CI 13.2, 14.6). Children who had been vaccinated in multiple facility types had the highest risk of extra-immunization (24.1%, 95% CI 22.5, 25.6). The extra-immunization rate for nonwhite children was 11.0% (95% CI 10.5, 11.5) as compared with non-Hispanic white children (8.2%, 95% CI 7.9, 8.5). Hispanic children were most likely to be overvaccinated (12.1%, 95% CI 11.4, 12.8).
Assuming that the language in which the parent completed the survey was that parent's preferred language, the rate of extra-immunization with English-speaking people (85.7% of the population) was 8.7% (95% CI 8.5, 9.1), while the extra-immunization rate with Spanish-speaking people (12.3% of the population) was 13.3% (95% CI 12.2, 14.3) (Table 1).
Maternal education and maternal age did have a modest association with extra-immunization, with less education and younger maternal age each associated with higher extra-immunization rates. Other factors traditionally associated with underimmunization did not show substantive associations with extra-immunization. Variables included the child's age, the timing of the first DTxP, and birth order. Not being up-to-date was associated with a lower rate of extra-immunization (5.4%, 95% CI 5.0, 5.9). Having parent-held immunization records was associated both with lower underimmunization rates (18.8%) and increased extra-immunization rates (10.9%) (Table 1).
We examined those children whose providers reported contributing data to an immunization registry and found that when all providers of immunizations reported to the registry, extra-immunization decreased to 8.1% (95% CI 7.6, 8.5); however, if only some providers reported to an immunization registry, the extra-immunization rate climbed to 23.0% (95% CI 21.4, 24.6). The lowest extra-immunization rate (7.7%, 95% CI 7.3, 8.1) occurred in children who had no provider reporting to a registry. The rates for all providers reporting and no providers reporting did not differ significantly. However, for a substantial number of children, it was unknown whether immunizations were reported to a registry (Table 1).
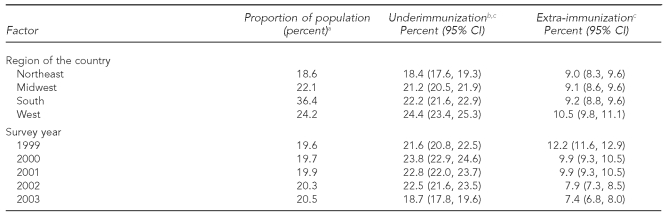
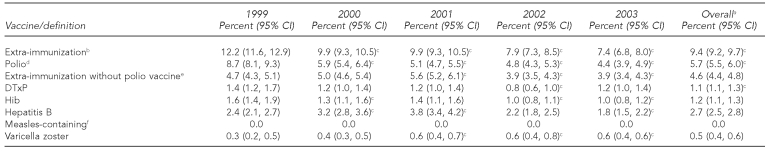
We found a significant trend of decreasing extra-immunization during the calendar years (Table 2). The extra-immunization rate decreased from 12.2% (95% CI 11.6, 12.9) in 1999 to 7.4% (95% CI 6.8, 8.0) in 2003. This decrease was largely explained by the decreasing rate of extra-immunization with polio vaccine, which varied from 8.7% in 1999 to 4.4% in 2003. During the same time period, extra-immunization not including polio vaccine decreased modestly, though significantly, from 4.7% in 1999 to 3.9% in 2003, with the maximum extra-immunization moving from 1999 (with polio vaccine) to 2001 (without polio vaccine).
Table 2.
Extra-immunization of children aged 19–35 months by vaccine and definition of extra-immunization: National Immunization Survey, 1999–2003
aPolio extra-immunization is defined as >3 doses of polio vaccine.
bThe sums of the individual vaccines do not add up to the “any vaccine” sum, reflecting that individuals may be overvaccinated with more than one vaccine.
cIndicates different when compared with 1999
dExtra-immunization is defined as DTxP (>4 doses), hepatitis B vaccine (>3 doses and, for 2002–2003, >4 doses if the first dose was in the first week of life), Hib (>4 doses), measles-containing vaccine (>2 doses after 12 months of life), polio vaccine (>3 doses), and varicella zoster vaccine (>1 dose).
eExtra-immunization without polio vaccine is defined the same as extra-immunization except that polio extra-immunization is not included.
fOver the five years, only seven of 111,730 children received more than two measles-mumps-rubella vaccinations, ranging from three in 1999 to zero in 2002. This resulted in estimated proportions and 95% CIs that were rounded to zero.
CI = confidence interval
DTxP = diphtheria and tetanus toxoids and pertussis vaccine, whole or acellular
Hib = Haemophilus influenzae type b vaccine
Stratified analyses
In a stratified analysis of provider and facility type by race/ethnicity, provider number and facility type classification had a large effect on extra-immunization by racial/ethnic minority group status. With just one provider, the rates of extra-immunization dropped to <9% for any of the racial/ethnic minority group classifications. All racial/ethnic minority groups had increased rates of extra-immunization with more than one provider, including those with more than one provider of one facility type and even more so with more than one type of facility (Table 3). Hispanic children with providers at more than one type of facility had the highest rates of extra-immunization at 29.7% (95% CI 26.1, 33.3), with non-Hispanic white children in the same category having an extra-immunization rate of 21.3% (95% CI 19.4, 23.1). Similarly, the use of parent-held immunization records was associated with increased extra-immunization in Hispanic children (13.7%) (data not shown).
Table 3.
Extra-vaccination of children aged 19–35 months according to race/ethnicity, by language and provider/facility type: National Immunization Survey, 1999–2003
aIncludes Hispanic, black, and all other races/ethnicities (including multiple races/ethnicities)
bIndicates different from non-Hispanic white race/ethnicity at p<0.05
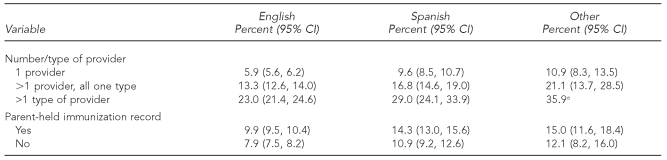
Examining extra-immunization by language as well as provider number and facility type (Table 4), we found that Spanish language combined with more than one provider but of one type and more than one facility type resulted in higher rates of extra-immunization of 16.8% (95% CI 14.6, 19.0) and 29.0% (95% CI 24.1, 33.9), respectively. The use of a means of communication between providers, the parent-held immunization record, is associated with increased extra-immunization. The use of Spanish as the preferred language for the survey was associated with an increase in extra-immunization with parent-held immunization records (14.3%, 95% CI 13.0, 15.6) compared with English language (9.9%, 95% CI 9.5, 10.4).
Table 4.
Extra-immunization of children aged 19–35 months by number and type of provider, parent-held immunization record, and language: National Immunization Survey, 1999–2003
aAs the number of observations for this cell were <100, the statistical guidance for the National Immunization Survey recommends that no statistical inferences be made.
Multivariate analyses
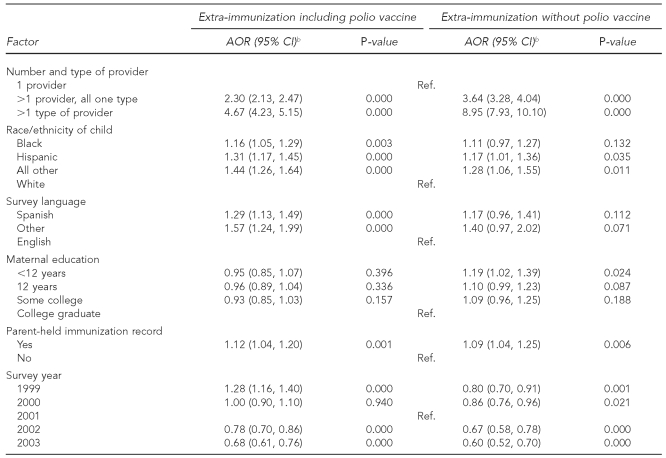
Logistic regression resulted in the AORs displayed in Table 5. These results indicated a modest increase in risk for extra-immunization was associated with being from a racial/ethnic minority group (AOR range: 1.16–1.44), having a preferred survey language of Spanish (AOR=1.29), and having a parent-held immunization record (AOR=1.12). The strongest risk factors by far were the presence of more than one provider even in the same facility type (AOR=2.30, 95% CI 2.13, 2.47) and the presence of more than one provider in more than one facility type (AOR=4.67, 95% CI 4.23, 5.15), compared with having only one provider.
Table 5.
Logistic regression model of odds of children aged 19–35 months being extra-immunized: National Immunization Survey, 1999–2003a
aIncludes all children in the sample from 1999 to 2003. Because of missing values, the total number for this analysis was 111,664 unweighted and 1,754,222 weighted.
bThe AORs presented are those controlling for the effect of all other variables in the table.
AOR = adjusted odds ratio
CI = confidence interval
Ref. = referent group
Because of the large effect of polio vaccine on extra-immunization rates and the decreasing rate of polio vaccine extra-immunization with time, we ran the logistic model again with the outcome of extra-immunization other than polio vaccine. In this model, the effect of race/ethnicity and language was attenuated, with black race/ethnicity and Spanish language no longer significant; however, the effect of multiple providers and multiple facility types was dramatically increased. In the full model, the presence of more than one provider in the same facility type (AOR=3.64, 95% CI 3.28, 4.04) and the presence of more than one provider in more than one facility type (AOR=8.95, 95% CI 7.93, 10.10) resulted in a 50% increase and almost doubling of the odds of extra-immunization, respectively.
We conducted analyses examining the data for significant interactions by survey year. For the outcome of extra-immunization, no significant interaction was found between race/ethnicity and survey year. There was an interaction between the survey year 1999 and >1 provider in the same facility (AOR=0.76, 95% CI 0.63, 0.85). Again, however, with the outcome of extra-immunization without polio vaccine, there was a significant interaction in 1999 between >1 provider in multiple facilities (AOR=1.57, 95% CI 1.10, 2.24). No other interaction was found by year and provider (data not shown).
DISCUSSION
We used number of immunization providers and provider types as an indicator of a medical home (one immunization provider) and increasing fragmentation of care as the number of providers increased and when the child moved between facility types. More than 30% of children had more than one provider or more than one facility type providing immunizations. Multiple vaccine providers and multiple facility types represent an absence of a key aspect of the medical home: continuity. Patients with this lack of continuity accounted for much of extra-immunization. While we found that the extra-immunization rate for U.S. children aged 19–35 months was 9.4% and that children from racial/ethnic minority groups were more likely to be overvaccinated (11.0%, 95% CI 10.5, 11.5), if all children in the U.S. had a single provider (i.e., a medical home), the extra-immunization rate would decrease to 6.4% for all U.S. children (7.6% for nonwhite children and 8.5% for Hispanic children). This rate is a decrease in extra-immunization of at least 30% overall and a decrease of 50% from the next category—those with multiple providers all of one type. These analyses do not imply that each patient should have a single individual providing their care. In the NIS dataset, “one provider” refers to one place providing immunizations (e.g., an office or a clinic).
Extra-immunization may result from a number of causes including a lack of documentation or problems with the communication of previous immunizations, mismanagement of a lapse in the sequence of immunizations, or simply a misunderstanding of the routine childhood vaccination schedule itself. It is worth noting in this analysis that the parent-held immunization records are associated with being both less likely to be underimmunized and more likely to be overimmunized, probably as a result of records that are not current.28 It is surprising to see such an increase in extra-immunization when moving from public to private provision of vaccines (i.e., multiple types of providers). We hypothesize that moving between facilities represents a large barrier to communication. Supporting the hypothesis that communication is a major issue in extra-immunization is the finding that having only some immunization providers reporting to a registry is worse than having all or none reporting to a registry. When no providers and all providers report to a registry, information is known. When only some providers report to a registry, it is easier to have incomplete information without realizing it.
While evidence exists that extra-immunization with tetanus toxoid,29 pneumococcal polysaccharide,30 and meningococcal polysaccharide31,32 can result in an increase in local or systemic reactions to vaccination, extra-immunization with other vaccines is unlikely to harm the recipient. In fact, with some combination vaccines, extra-immunization is permitted. However, other extra-immunizations incur costs to the parent, child, provider, and payers; provide a source of confusion and frustration for providers, parents, and patients; and are an unnecessary source for claims regarding adverse events and harms. It should be noted that underimmunization is more prevalent than extra-immunization, and underimmunization is a public health problem while extra-immunization is primarily an administrative failure.
Feikema et al. found rates of extra-immunization approaching 21%,13 but at the time the NIS database was of questionable quality. Since that time, the NIS data quality has improved (e.g., redundant vaccine entries have been corrected).14 Using the improved database, Strine et al. found extra-immunization rates of 10%–14%.16 We studied the same two years as Strine et al. did, as well as three additional years since the study; our findings were consistent with those found by Strine et al. Our findings for the two overlapping years would have been identical to Strine et al., but we treated the measles-containing vaccine differently, allowing two doses after one year of life and discounting all doses given in the first year of life, consistent with current ACIP recommendations. While Feikema et al. found an association with racial/ethnic disparity, the year's NIS database used (1997) had flaws that overestimated extra-immunization. We examined subsequent years and sought to understand the underlying mechanisms of the racial/ethnic disparity in extra-immunization. Our analyses found that while racial/ethnic minority groups were more likely to be overvaccinated, much of the effect was mediated through multiple providers and multiple types of providers. Thus, our study better supports extra-immunization as a clinical indicator for fragmentation of care.
Both Mell et al. and Davis evaluated the trends in polio vaccine dosing for children aged 19–35 months who were born between 1994 and 1997 in several large health maintenance organizations.14,15 They found that extra-immunization with polio vaccine began to decline before the introduction of IPV, leveled off during the introduction of IPV, and continued to fall afterward, suggesting that the change in recommendations elevated awareness of the current schedule and that the need to inject the current form of vaccine may be somewhat protective against extra-immunization. Our analyses confirmed these findings at the national level and showed that the extra-immunization with polio vaccine has continued to decline, while extra-immunization with vaccines other than polio has been more stable. The extra-immunization with polio vaccine appears to differ from extra-immunization with other vaccines, and analyses without polio extra-immunization included showed an even greater effect of fragmentation of care.
Extra-immunization is neither well-recognized nor regularly examined, but it appears to occur more frequently among vulnerable and at-risk populations, including the underprivileged racial/ethnic minority groups, non-English speakers, and those dependent on fragmented sources of care. While extra-immunization itself is not a significant medical problem, it does serve as a clinical indicator for fragmentation of care and lack of continuity of care, and can serve as a test of the integrity of a medical home. The validity of this measure is supported by the consistency of extra-immunization's association with multiple providers and with other indicators of problematic communication. Extra-immunization is not associated or only weakly associated with predictors of underimmunization that are patient-related, such as late DTP, maternal education, and birth order. This finding supports that extra-immunization is measuring aspects of care that are different from underimmunization. Additional research is needed to examine the relationship between extra-immunizations and other measures of quality, including continuity of care.
Limitations
Our study had several limitations. The NIS is a cross-sectional survey; as such, the associations found were not necessarily causal. Certain factors associated with extra-immunization were not captured by the survey, including the type of record keeping used in the practice. Furthermore, the survey data indicating extra-immunization may lead us to conclude incorrectly that extra-immunization occurred when, in fact, a legitimate reason existed for an additional dose of vaccine. Potential examples include repeat dosing of a vaccine in the event of a lot recall or a delayed recognition of incorrect storage,33 or in the correction of an inappropriate split-dosing with DTxP.34 Another reason for additional doses could be recognition of minimum interval violations, though the strong association of over-immunization with multiple providers makes that explanation unlikely.35 Alternatively, we may have missed doses given that were never recorded in the surveyed providers' records. This has been shown to be more likely as the record is fragmented across providers.36 Thus, the rates of extra-immunization may very well have been underestimated.
The use of a single provider of vaccines as a surrogate for the presence of a medical home could be flawed if that provider does not provide comprehensive care. Certainly, children could have multiple medical homes over time related to changes in insurance or residence. Furthermore, just as the survey data may miss certain vaccinations, the survey data may also have vaccine doses entered in duplicate, with errors in the administration dates resulting in the appearance of extra-immunization when it did not occur. Finally, the NIS only includes households that have landlines, yet the proportion of households with mobile or cell phones only is growing, particularly among individuals with young children.
CONCLUSIONS
A medical home is recommended for all children and having a medical home is associated with improved health outcomes, including decreased underimmunization. Extra-immunization is associated with fragmentation of care and the lack of a medical home. Extra-immunization could prove a more valuable and accessible measure of the medical home than underimmunization, as it is more closely linked to care provision, less closely linked to parental actions, and more easily measured. Extra-immunization can serve as a clinical indicator of medical care fragmentation and, thus, support quality improvement efforts in constructing and sustaining medical homes.
Footnotes
Paul Darden has acted as a consultant to Pfizer in 2010 and Sanofi Pasteur in 2009; he has no ongoing relationship with either company.
REFERENCES
- 1.American Academy of Pediatrics Medical Home Initiatives for Children with Special Needs Project Advisory Committee. Policy statement: organizational principles to guide and define the child health care system and/or improve the health of all children. Pediatrics. 2004;113(5 Suppl):1545–7. [PubMed] [Google Scholar]
- 2.American Academy of Pediatrics Medical Home Initiatives for Children with Special Needs Project Advisory Committee. The medical home. Pediatrics. 2002;110(1 Pt 1):184–6. [Google Scholar]
- 3.Cabana MD, Jee SH. Does continuity of care improve patient outcomes? J Fam Pract. 2004;53:974–80. [PubMed] [Google Scholar]
- 4.Heagarty MC, Robertson LS, Kosa J, Alpert JJ. Some comparative costs in comprehensive versus fragmented pediatric care. Pediatrics. 1970;46:596–603. [PubMed] [Google Scholar]
- 5.Allred NJ, Wooten KG, Kong Y. The association of health insurance and continuous primary care in the medical home on vaccination coverage for 19- to 35-month-old children. Pediatrics. 2007;119(Suppl 1):S4–11. doi: 10.1542/peds.2006-2089C. [DOI] [PubMed] [Google Scholar]
- 6.Christakis DA. Does continuity of care matter? Yes: consistent contact with a physician improves outcomes. West J Med. 2001;175:4. doi: 10.1136/ewjm.175.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christakis DA, Mell L, Wright JA, Davis R, Connell FA. The association between greater continuity of care and timely measles-mumps-rubella vaccination. Am J Public Health. 2000;90:962–5. doi: 10.2105/ajph.90.6.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith PJ, Santoli JM, Chu SY, Ochoa DQ, Rodewald LE. The association between having a medical home and vaccination coverage among children eligible for the Vaccines for Children program. Pediatrics. 2005;116:130–9. doi: 10.1542/peds.2004-1058. [DOI] [PubMed] [Google Scholar]
- 9.Cooley WC, McAllister JW, Sherrieb K, Kuhlthau K. Improved outcomes associated with medical home implementation in pediatric primary care. Pediatrics. 2009;124:358–64. doi: 10.1542/peds.2008-2600. [DOI] [PubMed] [Google Scholar]
- 10.Starfield B. The Medical Home Index applies primarily to children with special health care needs. Ambul Pediatr. 2004;4:192. doi: 10.1367/1539-4409(2004)4<192b:LTTE>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Pediatrics Ad Hoc Task Force on Definition of the Medical Home. The medical home. Pediatrics. 1992;90:774. [PubMed] [Google Scholar]
- 12.Yawn BP, Yawn RA, Geier GR, Xia Z, Jacobsen SJ. The impact of requiring patient authorization for use of data in medical records research. J Fam Pract. 1998;47:361–5. [PubMed] [Google Scholar]
- 13.Feikema SM, Klevens RM, Washington ML, Barker L. Extraimmunization among US children. JAMA. 2000;283:1311–7. doi: 10.1001/jama.283.10.1311. [DOI] [PubMed] [Google Scholar]
- 14.Davis RL. Vaccine extraimmunization—too much of a good thing? JAMA. 2000;283:1339–40. doi: 10.1001/jama.283.10.1339. [DOI] [PubMed] [Google Scholar]
- 15.Mell LK, Davis RL, Mullooly JP, Black SB, Shinefield HR, Zangwill KM, et al. Polio extraimmunization in children younger than 2 years after changes in immunization recommendations. Pediatrics. 2003;111:296–301. doi: 10.1542/peds.111.2.296. [DOI] [PubMed] [Google Scholar]
- 16.Strine TW, Barker LE, Jain RB, Washington ML, Chu SY, Mokdad AH. Extraimmunization in children through 2000. JAMA. 2002;287:588–9. doi: 10.1001/jama.287.5.588-a. [DOI] [PubMed] [Google Scholar]
- 17.Rabo E. [Risk of overvaccination against tetanus—who is deciding: the National Board of Health and Welfare or the manufacturer?] Lakartidningen. 1989;86:615–6. [PubMed] [Google Scholar]
- 18.Mainz J. Defining and classifying clinical indicators for quality improvement. Int J Qual Health Care. 2003;15:523–30. doi: 10.1093/intqhc/mzg081. [DOI] [PubMed] [Google Scholar]
- 19.Zell ER, Ezzati-Rice TM, Battaglia MP, Wright RA. National Immunization Survey: the methodology of a vaccination surveillance system. Public Health Rep. 2000;115:65–77. doi: 10.1093/phr/115.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Recommended childhood immunization schedule—United States, 1999. MMWR Morb Mortal Wkly Rep. 1999;48(1):12–6. [PubMed] [Google Scholar]
- 21.Recommended childhood immunization schedule—United States, 2000. MMWR Morb Mortal Wkly Rep. 2000;49(2):35–8. 47. [PubMed] [Google Scholar]
- 22.Recommended childhood immunization schedule—United States, 2001. MMWR Morb Mortal Wkly Rep. 2001;50(1):7–10. 19. [PubMed] [Google Scholar]
- 23.Recommended childhood immunization schedule—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(2):31–3. [PubMed] [Google Scholar]
- 24.Recommended childhood and adolescent immunization schedule. MMWR Morb Mortal Wkly Rep. 2003;52(4):Q1–4. United States, 2003 [published erratum appears in MMWR Morb Mortal Wkly Rep 2003;52(9):191] [PubMed] [Google Scholar]
- 25.Watson JC, Hadler SC, Dykewicz CA, Reef S, Phillips L. Measles, mumps, and rubella—vaccine use and strategies for elimination of measles, rubella, and congenital rubella syndrome and control of mumps: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1998;47(RR-8):1–57. [PubMed] [Google Scholar]
- 26.SAS Institute, Inc. SAS®: Version 9.1 for Windows. Cary (NC): SAS Institute, Inc; 2008. [Google Scholar]
- 27.StataCorp. Stata®: Version 8.0. College Station (TX): StataCorp; 2004. [Google Scholar]
- 28.McElligott JT, Darden PM. Are patient-held vaccination records associated with improved vaccination coverage rates? Pediatrics. 2010;125:e467–72. doi: 10.1542/peds.2009-0835. [DOI] [PubMed] [Google Scholar]
- 29.Jones AE, Melville-Smith M, Watkins J, Seagroatt V, Rice L, Sheffield F. Adverse reactions in adolescents to reinforcing doses of plain and adsorbed tetanus vaccines. Community Med. 1985;7:99–106. doi: 10.1093/oxfordjournals.pubmed.a043782. [DOI] [PubMed] [Google Scholar]
- 30.Borgono JM, McLean AA, Vella PP, Woodhour AF, Canepa I, Davidson WL, et al. Vaccination and revaccination with polyvalent pneumococcal polysaccharide vaccines in adults and infants. Proc Soc Exp Biol Med. 1978;157:148–54. doi: 10.3181/00379727-157-40010. [DOI] [PubMed] [Google Scholar]
- 31.Borrow R, Joseph H, Andrews N, Longworth E, Martin S, Peake N, et al. Reduced antibody response to revaccination with meningococcal serogroup A polysaccharide vaccine in adults. Vaccine. 2000;19:1129–32. doi: 10.1016/s0264-410x(00)00317-0. [DOI] [PubMed] [Google Scholar]
- 32.MacLennan J, Obaro S, Deeks J, Williams D, Pais L, Carlone G, et al. Immune response to revaccination with meningococcal A and C polysaccharides in Gambian children following repeated immunisation during early childhood. Vaccine. 1999;17:3086–93. doi: 10.1016/s0264-410x(99)00139-5. [DOI] [PubMed] [Google Scholar]
- 33.Advisory Committee on Immunization Practices update: report of PedvaxHIB lots with questionable immunogenicity. MMWR Morb Mortal Wkly Rep. 1992;41(46):878–9. [PubMed] [Google Scholar]
- 34.Kroger AT, Atkinson WL, Marcuse EK, Pickering LK. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) [published erratum appears in MMWR Morb Mortal Wkly Rep 2006;55(48):1303] MMWR Recomm Rep. 2006;55(RR-15):1–48. [PubMed] [Google Scholar]
- 35.Luman ET, Shaw KM, Stokley SK. Compliance with vaccination recommendations for U.S. children [published erratum appears in Am J Prev Med 2008;35:319] Am J Prev Med. 2008;34:463–70. doi: 10.1016/j.amepre.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 36.Smith PJ, Stevenson J. Racial/ethnic disparities in vaccination coverage by 19 months of age: an evaluation of the impact of missing data resulting from record scattering. Stat Med. 2008;27:4107–18. doi: 10.1002/sim.3223. [DOI] [PubMed] [Google Scholar]