Abstract
Objectives
We described the uptake and coverage rates of meningococcal conjugate vaccine (MCV4); tetanus-diphtheria-acellular pertussis vaccine (Tdap); and quadrivalent human papillomavirus vaccine (HPV4) in North Dakota using the North Dakota Immunization Information System (NDIIS).
Methods
We analyzed all available MCV4, Tdap, and HPV4 doses given after vaccine licensure and through December 31, 2009, obtained from the NDIIS to identify trends and patterns in vaccine administration. We analyzed all data by administration date, age group, and health-care provider type. We also calculated missed opportunities to complete all recommended vaccines among vaccinated adolescents.
Results
For adolescents aged 13–17 years, 69.2% had ≥1 dose of Tdap and 62.8% had ≥1 dose of MCV4. Of females aged 13–17 years, 42.8% initiated the HPV4 vaccination series and 24.9% received ≥3 HPV4 doses. Only 48.7% of males aged 13–17 years received both Tdap and MCV4 at the same visit, and only 11.5% of females aged 13–17 years received Tdap, MCV4, and HPV4 doses at the first visit.
Conclusions
The NDIIS is useful in tracking adolescent vaccine uptake. The immunization rates for all three routinely recommended adolescent vaccines are rising in North Dakota, although at different paces. Providers should be educated about the importance of not missing opportunities to vaccinate, and school-based vaccination clinics should be used to reach adolescents who are less likely to have preventive care visits.
Prior to 2005, tetanus-diphtheria vaccine (Td) was the only routinely recommended vaccination for adolescents.1 Large-scale immunization campaigns were not routinely in place for adolescents except in unique circumstances, such as disease outbreaks and catch-up vaccination campaigns.2 Starting in 2005, for the first time, new vaccines were licensed and recommended specifically for adolescents. Providing access to the newly recommended adolescent vaccines required new approaches that differed from those for infants and children.
Between 2005 and 2006, the Advisory Committee on Immunization Practices (ACIP) recommended routine meningococcal conjugate vaccine (MCV4) and tetanus-diphtheria-acellular pertussis vaccine (Tdap) for all children aged 11 and 12 years, as well as quadrivalent human papillomavirus vaccine (HPV4) for females aged 11 and 12 years.3–5 Catch-up vaccination was recommended for adolescents who did not receive these vaccines at 11 and 12 years of age. In late 2009, the U.S. Food and Drug Administration (FDA) licensed a bivalent human papillomavirus vaccine (HPV2) and expanded the indication for HPV4 to include males, with a permissive recommendation by the ACIP.6 HPV4 in males and HPV2 were not analyzed in this study.
National immunization rates are tracked using a variety of survey tools, including the National Immunization Survey (NIS), the National Health Interview Survey, and the Behavioral Risk Factor Surveillance System. Starting in 2006, the NIS conducted national surveys to determine adolescent vaccine rates and is the only national survey to estimate adolescent vaccination coverage.7 The NIS data are useful in determining overall trends of adolescent vaccine uptake, but there are limitations to the data. It takes a year to collect the target sample size per area and, as a result, NIS data are reported for the previous year, leaving a gap between changes in the vaccination schedule and evaluation by NIS. The NIS-Teen is powered to achieve an expected 95% confidence interval (CI) per state of about 66% for 13- to 17-year-olds and 69% for females only. The NIS is not designed to provide precise estimates for smaller geographic areas or for population subgroups within states.8
Without detailed, real-time data, an immunization program's ability to implement new strategic initiatives to increase immunization coverage is limited. Monitoring the uptake of new vaccines is critical to understanding where future resources and efforts need to be directed. Ideally, immunization information systems, such as the North Dakota Immunization Information System (NDIIS), provide supplementary data that the NIS is not designed to obtain by providing local-level data and a timely surveillance system that allows immunization programs to analyze the impact of decisions, such as school immunization requirements.
We analyzed information from the NDIIS to determine the number of doses of each vaccine administered since licensure; the vaccination rates for Tdap, MCV4, and HPV4; trends for the three vaccines, including rates by age group; whether adolescents are missing opportunities to receive vaccines; the percentage of adolescents up-to-date (UTD) on all three vaccines; and doses administered by provider type.
METHODS
The NDIIS was established in 1988 using a modem connection and has been Web-based since 2001. The NDIIS's primary purpose is to provide complete and accurate vaccination histories to health-care providers, schools, and disease investigators. The NDIIS stores information on all segments of the population, including children, adolescents, and adults. The NDIIS captures data about immunizations given in North Dakota and consolidates information about immunizations received from multiple providers.
The NDIIS has high provider participation, with more than 90% of immunization providers enrolled and entering immunization information. Fifty-five percent of vaccine encounters are entered within one day and almost 95% are entered within 30 days. Immunization providers are required by state law to enter immunization data for children aged 18 years and younger into the NDIIS.9 NDIIS vaccination coverage rates for 2008 and 2009 were within the NIS 95% CIs. Because of high provider participation and timely data, the NDIIS data provide in-depth, real-time answers about the uptake of adolescent vaccines that help programmatic decision-making when used in conjunction with the NIS.
The North Dakota Department of Health receives funding from the Centers for Disease Control and Prevention as an Immunization Information System Sentinel Site, which helps support research activities using NDIIS data.10
We restricted analyses to patients receiving at least one dose of Tdap, MCV4, or HPV4. For HPV4, an initiated vaccine series was defined as having ≥1 HPV4 dose, partial vaccination was defined as having between ≥1 HPV4 dose and <3 HPV4 doses, and full vaccination was defined as having ≥3 HPV4 doses. At least 24 weeks between the first and third doses of HPV4 vaccine are needed to complete the series.5
All records containing a date of vaccination with MCV4, Tdap, or HPV4 were queried from the NDIIS as of December 31, 2009. Missed or delayed opportunities were defined as having a Tdap, MCV4, or HPV4 dose administered and not receiving the other recommended adolescent vaccine(s) on the same day. We calculated adolescents receiving no vaccines by determining the total number of 13- to 17-year-olds who received Tdap, HPV4, or MCV4 and subtracting that from the U.S. Census number. Information obtained in the query included birth date, county, gender, vaccine name, provider name, and date of administration. Illogical data, including doses given before licensure, incomplete information, and illogical values according to the NDIIS, were removed from the analysis. We used the 2008 U.S. Census estimate for North Dakota to estimate the number of people in each age group.11 There were 41,678 adolescents aged 13–17 years in North Dakota according to the U.S. Census.
Adolescents were analyzed as of Quarter 4 2009. Adolescents aged 11–18 years were born between January 1, 1991, and December 31, 1998. Adolescents aged 13–17 years were born between January 1, 1992, and December 31, 1996.
RESULTS
Doses administered by year
In North Dakota, 37.4% (n=22,165) of the HPV4 doses were administered in 2008 and 28.5% (n=16,906) were administered in 2009. Of the total Tdap doses given, 27.5% (n=31,701) were administered in 2008 and 32.3% (n=37,250) were administered in 2009. Of the total MCV4 doses given, 27.8% (n=16,023) were administered in 2008 and 22.2% (n=12,800) were administered in 2009 (data not shown).
Vaccine coverage by age and vaccine type
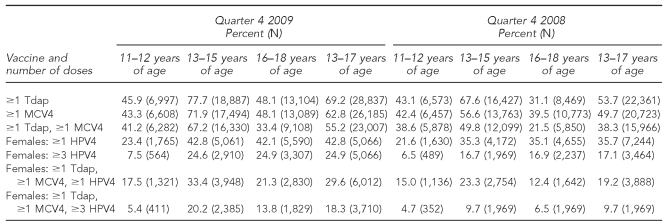
As shown in Table 1, excluding the 11- to 12-year age group, which remained similar, adolescents aged 13–17 years were more likely to have received Tdap, HPV4, and MCV4 in Quarter 4 2009 than in Quarter 4 2008. Adolescents between the ages of 11 and 12 years were likely to be in the process of being vaccinated and, as a result, less likely than older adolescents to have received the recommended vaccines. In both 2008 and 2009, adolescent females were more likely to receive ≥1 dose of Tdap and ≥1 dose of MCV4 than ≥1 dose of HPV4.
Table 1.
Percentage of adolescents 13–17 years of age vaccinated with Tdap, MCV4, and HPV4 vaccines, by age group: Quarter 4 of 2008 and 2009, North Dakota
Tdap = tetanus-diphtheria-acellular pertussis
MCV4 = meningococcal conjugate
HPV4 = quadrivalent human papillomavirus
Comparison with the NIS
For adolescents aged 13–17 years in Quarters 2 and 3 in 2009, the NDIIS results for all vaccines were within the 95% CIs of the NIS, although toward the lower end of the NIS results. According to the 2009 NIS, 71.6% (95% CI 65.4, 77.1) of adolescents had received ≥1 dose of Tdap compared with 65.4% in the NDIIS. Coverage with ≥1 dose of MCV4 was estimated at 66.0% (95% CI 59.5, 72.0) in the NIS compared with 59.8% in the NDIIS. For ≥1 dose of HPV4, the vaccination estimate was 45.1% (95% CI 36.0, 54.6) in the NIS compared with 40.9% in the NDIIS. For ≥3 doses of HPV4, the NIS estimate was 31.7% (95% CI 23.6, 41.1) compared with 24.0% in the NDIIS (data not shown).
Missed opportunities during vaccination visits
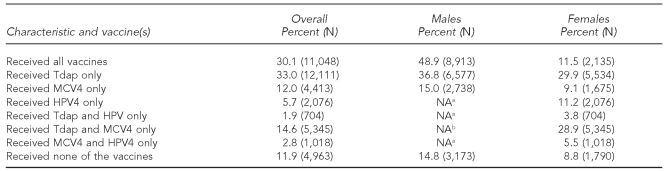
Among males aged 13–17 years, 51.1% either missed or delayed an opportunity to receive both Tdap and MCV4 at the same time (Table 2). Of those with a missed opportunity, 70.6% missed an opportunity to receive MCV4 and 29.4% missed an opportunity to receive Tdap.
Table 2.
Percentage of adolescents 13–17 years of age who missed opportunities to be vaccinated, by gender: Quarter 4 2009, North Dakota
aAt the time of the study, HPV4 was not approved for males.
bFor males “Received all vaccines” and “Received Tdap and MCV4 only” are the same.
Tdap = tetanus-diphtheria-acellular pertussis
MCV4 = meningococcal conjugate
HPV4 = quadrivalent human papillomavirus
NA = not applicable
Of females aged 13–17 years, 11.5% received Tdap, MCV4, and HPV4 on the first visit; 28.9% missed only HPV4; 29.9% missed both HPV4 and MCV4; and 9.1% missed HPV4 and Tdap. Only 11.2% missed MCV4 and Tdap and received HPV4. Adolescent females aged 13–17 years who received all three vaccines on the first visit were more likely (64.6% considered UTD) to complete the recommended series than those who did not receive all three vaccines on the first visit (19.3% UTD) (data not shown).
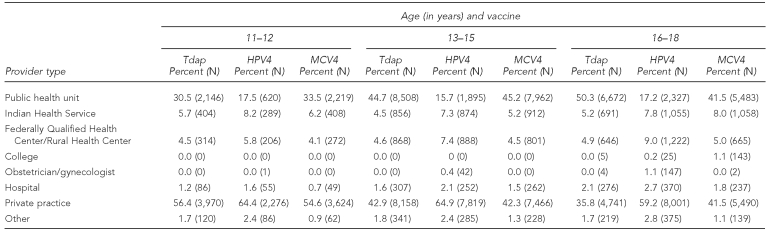
Provider type
As shown in Table 3, adolescents aged 11–18 years were more likely to receive Tdap and MCV4 than HPV4 at public health units (PHUs). The type of provider administering vaccine did not differ significantly between MCV4 and Tdap, except for adolescents aged 16–18 years. For adolescents 16–18 years of age, 50.3% of Tdap doses and 41.5% of MCV4 doses were administered at PHUs. HPV4 doses were more likely to be administered at private providers than at PHUs, with 59%–65% of HPV4 doses administered at private practices for all age groups. Indian Health Service facilities accounted for fewer than 10% of doses administered for each type of vaccine in each age group.
Table 3.
Percentage of doses administered for Tdap, MCV4, and HPV4 vaccines, by age group and provider type: Quarter 4 2009, North Dakota
Tdap = tetanus-diphtheria-acellular pertussis
HPV4 = quadrivalent human papillomavirus
MCV4 = meningococcal conjugate
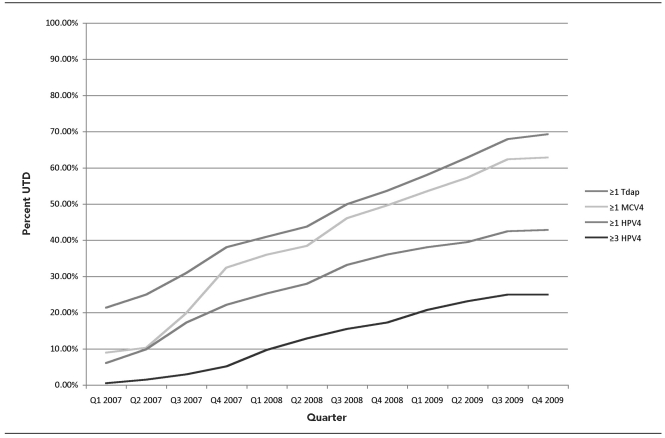
Immunization trends in North Dakota
The immunization rates for all three routinely recommended adolescent vaccines have been rising in North Dakota, although at different paces depending on the vaccine (Figure). Adolescents were more likely to receive Tdap and MCV4 than HPV4. HPV4 vaccine has been administered to the ACIP-recommended groups, but coverage levels remain low. Many of the vaccine recipients have not received the full series. More than one-third of HPV4 recipients who could have completed the series if they had remained on schedule were only partially vaccinated. There has been a steady rise in HPV4 doses administered, but a substantial increase has yet to be observed.
Figure.
Percentage of adolescents 13–17 years of age up-to-date for Tdap, MCV4, and HPV4 vaccines in North Dakota through Quarter 4 2009
Tdap = tetanus-diphtheria-acellular pertussis
MCV4 = meningococcal conjugate
HPV4 = quadrivalent human papillomavirus
UTD = up-to-date
Providers have been missing opportunities to vaccinate adolescents. Only half of males who received either Tdap or MCV4 received both vaccines at the same visit, and only 11% of females received Tdap, MCV4, and HPV4 at the first visit. Adolescents who did not receive all recommended vaccines on the first visit were less likely to receive all recommended vaccines. At PHUs, adolescents were more likely to receive Tdap and MCV4 than HPV4. The majority of HPV4 vaccine has been administered within the private sector.
DISCUSSION
The NDIIS data are consistent with the NIS, which has shown HPV4 coverage levels to be lower than Tdap and MCV4 coverage levels.12 The NDIIS results show the importance of missed opportunities in adolescent vaccinations and the greater reliance on the private sector for HPV4 than for Tdap and MCV4.
There are multiple reasons for HPV4 to have greater reliance on the private sector and to have lower coverage. For one, it is the most commonly refused vaccine routinely administered in the United States, and most states do not mandate HPV4 for school entry.13, North Dakota should implement education programs for providers and parents about the safety of HPV4 and the importance of all adolescent vaccines.15, Assessment, Feedback, Incentives, and Exchange (AFIX), a program designed to provide intervention and education to immunization providers, has been shown to help improve childhood immunization rates, and providing AFIX to adolescent providers could be a cost-effective way to educate providers and increase adolescent vaccination rates.17 Unlike Tdap and MCV4, which have been required since the 2008–2009 school year, North Dakota does not require HPV4 at middle school entry.18 School entry requirements have been shown to be effective at increasing coverage of required vaccines shortly after their adoption and could be an effective way to increase the number of females vaccinated with HPV4, as they have with the hepatitis B vaccine.19, However, an HPV4 vaccine requirement is a politically charged issue that makes an HPV4 school entry requirement less feasible than Tdap and MCV4 school mandates.21 With the ACIP making a permissive recommendation of HPV4 for males, these issues will become even more complicated in the future.
Another barrier to being vaccinated against HPV4 is cost, which is rising due to the number and expense of vaccines. HPV4 is currently the most expensive vaccine on the market.22, The Guide to Community -Preventive Services recommends reducing out-of-pocket expenses for vaccines by providing insurance coverage, reducing copayments, or paying for vaccines and/or administration fees.24 North Dakota has provided free vaccines to children aged 18 years and younger who are Medicaid-eligible, uninsured, American Indian/Alaska Native, or underinsured through the Vaccines for Children (VFC) program, as these vaccines were recommended by ACIP.25 However, the VFC program does not cover insured children with high deductibles or the cost of an office visit with a physician. These barriers need to be addressed to help increase vaccination coverage.
North Dakota data show that providers are missing opportunities to give adolescents all three recommended vaccines. Providers and parents should be educated about the importance of vaccination with all recommended vaccines during a visit. In a national survey, physicians identified adolescents having few preventive care visits as a barrier to immunization.26, This barrier has become apparent as the vast majority of adolescents require two or three additional visits to receive all recommended vaccines on time.28 Increasing HPV4 vaccination coverage will require additional visits, and as the NDIIS data show, missing an opportunity to receive HPV4 vaccination when other vaccinations are given will increase the likelihood of undervaccination.
According to NDIIS data, more than one-third of females who initiated the HPV4 series and are eligible to complete the series have not received all three doses. To reduce the number of adolescents not completing the recommended vaccine series, immunization providers should review their Immunization Information System (IIS) record at each clinic visit, and the IIS and providers should incorporate reminder/recall into their practices.29, A review of the literature on reminder/recall found it to be an effective way to increase immunizations for both children and adults in 80% of all reviewed studies.31
Eliminating missed opportunities and adding reminder/recall will improve rates, but new strategies need to be used to increase the number of adolescents initiating the vaccine series. Even if every adolescent who had received at least one dose of MCV4, Tdap, or HPV4 in North Dakota had been fully UTD, more than 10% of adolescents had not received any doses of these vaccines (Table 2). Strategies such as school immunization clinics and increased enforcement of school immunization requirements could help immunization providers reach a greater percentage of the population.32 Studies show that well-organized school-based vaccination clinics can help achieve high immunization rates.33 Collaboration between public and private immunization providers to plan and implement school vaccination clinics, along with subsidized vaccines, would help reach adolescents who are less likely to be vaccinated.
Limitations
There were several limitations to this study. First, North Dakota has a small population and the study findings are not necessarily representative of other states. Second, the NDIIS contains more adolescents than census data suggest are in North Dakota. This discrepancy is due to duplicate records and because people who move to North Dakota and receive an immunization are added to the NDIIS, but the NDIIS does not currently have a way to track people who move out of state. Therefore, the NDIIS overestimates population size and, as a result, underestimates immunization rates when NDIIS population estimates are used as a denominator.
Third, we used U.S. Census numbers for the denominator to prevent downward bias in the coverage estimates. The analysis for missed opportunities did not take into account whether an adolescent had a health-care visit in which no vaccines were given or the adolescent received vaccines (e.g., influenza) and, as a result, missed all three vaccines. Older adolescents may have received Td and would not currently be recommended for Tdap, resulting in lower Tdap coverage. It is possible that some MCV4 doses were mistakenly recorded as meningococcal polysaccharide vaccine (MPSV4) and Tdap as Td, especially shortly after licensure.
Lastly, although more than 95% of immunization providers are using the NDIIS and are required by law to enter immunization information into the NDIIS for children aged 18 years and younger, it is possible that, early on, providers entered adolescent immunizations less frequently than childhood immunizations. An increase in reporting over time would lead to an exaggerated apparent increase in the vaccination rates trend.
CONCLUSIONS
Despite these limitations, the NDIIS is useful for tracking vaccine uptake/coverage, and NDIIS adolescent vaccination coverage rates for 2008 and 2009 were within the NIS 95% CIs. The NDIIS has high provider participation and more than 90% of immunization data are entered within one month. These strengths allow the NDIIS to be used to estimate vaccination coverage trends. Further evaluation should be conducted to determine whether adolescents are missing opportunities to be vaccinated with MCV4, Tdap, or HPV4 when receiving other vaccines (e.g., influenza and catch-up varicella), or if adolescents are having clinic visits but are not being vaccinated.
Adolescent vaccination rates are increasing in North Dakota, but a large percentage of the adolescent population remains unvaccinated or undervaccinated, especially for HPV4. Immunization providers need to continue educating adolescents and their parents about the importance of these vaccines, along with the safety profile of the vaccines and the risk of disease. Health-care providers should use every opportunity to check vaccination status in and enter all immunization data into their IIS, as provider participation is critical in making IISs successful. Vaccine uptake should continue to be monitored to determine areas of strength and weakness. Along with interventions already underway, immunization strategies such as reminder/recall, school entry requirements, and school-based clinics could be used to reach a higher percentage of the population.
This study showed that IISs can be used to determine adolescent vaccination coverage. Future IIS studies also should examine racial/ethnic disparities and the effect of VFC eligibility on immunization coverage. This study did not review pockets of underimmunized groups within North Dakota or the effect of exemptions on immunization rates. Future studies should consider these issues.
References
- 1.Recommended childhood and adolescent immunization schedule— United States January–June 2004. MMWR Morb Mortal Wkly Rep. 2004;53(01):Q1–4. [PubMed] [Google Scholar]
- 2.Hall S, Galil K, Watson B, Seward J. The use of school-based vaccination clinics to control varicella outbreaks in two schools. Pediatrics. 2000;105:e17. doi: 10.1542/peds.105.1.e17. [DOI] [PubMed] [Google Scholar]
- 3.Bilukha OO, Rosenstein N. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2005;54(RR-7):1–21. [PubMed] [Google Scholar]
- 4.Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2006;55(RR-3):1–34. [PubMed] [Google Scholar]
- 5.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER. Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 6.Recommended immunization schedules for persons aged 0 through 18 years—United States, 2010. MMWR Morb Mortal Wkly Rep. 2010;58(51&52):1–4. [PubMed] [Google Scholar]
- 7.Shefer A, Santoli J, Singleton JA. Measuring vaccination coverage—where are we now and where are we going? J Public Health Manag Pract. 2007;13:541–3. doi: 10.1097/01.PHH.0000296127.47143.26. [DOI] [PubMed] [Google Scholar]
- 8.Smith PJ, Battaglia MP, Huggins VJ, Hoaglin DC, Roden AS, Khare M, et al. Overview of the sampling design and statistical methods used in the National Immunization Survey. Am J Prev Med. 2001;20(4 Suppl):17–24. doi: 10.1016/s0749-3797(01)00285-9. [DOI] [PubMed] [Google Scholar]
- 9.Immunization data. N.D.C.C. §23-01.05.3 (2010) [cited 2011 Mar 17] Available from: URL: http://www.legis.nd.gov/cencode/t23c01.pdf.
- 10.Centers for Disease Control and Prevention (US). Q&A about sentinel sites: what are IIS sentinel sites? [cited 2010 Apr 15] Available from: URL: http://www.cdc.gov/vaccines/programs/iis/activities/sentinel-sites.htm#what.
- 11.Census Bureau (US). Population estimates: state single year of age and sex population estimates: April 1, 2009, to July 1, 2009 [cited 2010 Jan 7] Available from: URL: http://www.census.gov/popest/states/asrh/stasrh.html.
- 12.National state and local area vaccination coverage among adolescents aged 13–17 years— United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(36):997–1001. [PubMed] [Google Scholar]
- 13.Freed GL, Clark SJ, Butchart AT, Singer DC, Davis MM. Parental vaccine safety concerns in 2009. Pediatrics. 2010;125:654–9. doi: 10.1542/peds.2009-1962. [DOI] [PubMed] [Google Scholar]
- 14.National Vaccine Advisory Committee. Mandates for adolescent immunizations: recommendations from the National Vaccine Advisory Committee. Am J Prev Med. 2008;35:145–51. doi: 10.1016/j.amepre.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009;302:750–7. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 16.Forhan SE, Gottlieb SL, Sternberg MR, Xu F, Datta SD, McQuillan GM, et al. Prevalence of sexually transmitted infections among female adolescents aged 14 to 19 in the United States. Pediatrics. 2009;124:1505–12. doi: 10.1542/peds.2009-0674. [DOI] [PubMed] [Google Scholar]
- 17.LeBaron CW, Mercer JT, Massoudi MS, Dini E, Stevenson J, Fischer WM, et al. Changes in clinic vaccination coverage after institution of measurement and feedback in 4 states and 2 cities. Arch Pediatr Adolesc Med. 1999;153:879–86. doi: 10.1001/archpedi.153.8.879. [DOI] [PubMed] [Google Scholar]
- 18.Inoculation required before admission to school. N.D.C.C. §23-07.17.1 [cited 2011 Mar 17] Available from: URL: http://www.legis.nd.gov/cencode/t23c07.pdf.
- 19.Averhoff F, Linton L, Peddecord KM, Edwards C, Wang W, Fishbein D. A middle school immunization law rapidly and substantially increases immunization coverage among adolescents. Am J Public Health. 2004;94:978–84. doi: 10.2105/ajph.94.6.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson TR, Fishbein DB, Ellis PA, Edlavitch SA. The impact of a school entry law on adolescent immunization rates. J Adolesc Health. 2005;37:511–6. doi: 10.1016/j.jadohealth.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther. 2007;82:760–3. doi: 10.1038/sj.clpt.6100397. [DOI] [PubMed] [Google Scholar]
- 22.Freed GL, Cowan AE, Gregory S, Clark SJ. Variation in provider vaccine purchase prices and payer reimbursement. Pediatrics. 2008;122:1325–31. doi: 10.1542/peds.2008-2038. [DOI] [PubMed] [Google Scholar]
- 23.Hammer LD, Curry ES, Harlor AD, Laughlin JJ, Leeds AJ, Lessin HR, et al. Policy statement increasing immunization coverage. Pediatrics. 2010;125:1295–304. doi: 10.1542/peds.2010-0743. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (US). Universally recommended vaccinations: reducing client out-of-pocket costs for vaccinations [cited 2010 Nov 2] Available from: URL: http://www.thecommunityguide.org/vaccines/universally/clientoutofpocketcosts.html.
- 25.Centers for Disease Control and Prevention (US). VFC operations guide [cited 2010 Nov 4] Available from: URL: http://www.cdc.gov/vaccines/programs/vfc/operations-guide.htm.
- 26.Rand CM, Shone LP, Albertin C, Auinger P, Klien J, Szilagyi PG. National health care visit patterns of adolescents: implications for delivery of new adolescent vaccines. Arch Pediatr Adolesc Med. 2007;161:252–9. doi: 10.1001/archpedi.161.3.252. [DOI] [PubMed] [Google Scholar]
- 27.Oster NV, McPhillips-Tangum CA, Averhoff F, Howell K. Barriers to adolescent immunization: a survey of family physicians and pediatricians. J Am Board Fam Pract. 2005;18:13–9. doi: 10.3122/jabfm.18.1.13. [DOI] [PubMed] [Google Scholar]
- 28.Rand CM, Szilagyi PG, Albertin C, Auinger P. Additional health care visits needed among adolescents for human papillomavirus vaccine delivery within medical homes: a national study. Pediatrics. 2007;120:461–6. doi: 10.1542/peds.2007-0012. [DOI] [PubMed] [Google Scholar]
- 29.Minkovitz CS, Belote AD, Higman SM, Serwint JR, Weiner JP. Effectiveness of a practice-based intervention to increase vaccination rates and reduce missed opportunities. Arch Pediatr Adolesc Med. 2001;155:382–6. doi: 10.1001/archpedi.155.3.382. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention (US). Universally recommended vaccinations: client reminder & recall systems [cited 2010 Nov 2] Available from: URL: http://www.thecommunityguide.org/vaccines/universally/clientreminder.html.
- 31.Szilagyi PG, Bordley C, Vann JC, Chelminski A, Kraus RM, Margolis PA, et al. Effect of patient reminder/recall interventions on immunization rates. JAMA. 2000;284:1820–7. doi: 10.1001/jama.284.14.1820. [DOI] [PubMed] [Google Scholar]
- 32.Lee GM, Lorick SA, Pfoh E, Kleinman K, Fishbein D. Adolescent immunizations: missed opportunities for prevention. Pediatrics. 2008;122:711–7. doi: 10.1542/peds.2007-2857. [DOI] [PubMed] [Google Scholar]
- 33.Lindley MC, Boyer-Chu L, Fishbein DB, Kolasa M, Middleman AB, Wilson T, et al. The role of schools in strengthening delivery of new adolescent vaccinations. Pediatrics. 2008;121(Suppl 1):S46–54. doi: 10.1542/peds.2007-1115F. [DOI] [PubMed] [Google Scholar]