Abstract
Objectives
We described the results from the Swiss National Vaccination Coverage Survey (SNVCS) 2005–2007, a survey designed to monitor immunization coverage of children and adolescents residing in Switzerland in each canton within a three-year period.
Methods
The SNVCS is a cross-sectional survey using a two-stage sampling design targeting children aged 2, 8, and 16 years. Families of selected children were contacted by mail and telephone. Coverage was determined via vaccination cards or vaccination summary forms.
Results
A total of 25 out of 26 cantons participated in the survey, with 8,286 respondents for children aged 24–35 months, 10,314 respondents for children aged 8 years, and 9,301 respondents for teenagers aged 16 years. Compared with data from 1999–2003, coverage estimates for toddlers remained unchanged for diphtheria, tetanus, pertussis, poliomyelitis, and Haemophilus influenzae type b vaccines at three doses, but increased five percentage points to 86%–87% for measles-mumps-rubella at one dose and was 71% at two doses. Coverage for measles, mumps, and rubella were 89%–90% at one dose and 75% at two doses for 8-year-olds, and 94% and 76% for the two dosages, respectively, for 16-year-olds. Linguistic region and nationality were highly correlated with being vaccinated against measles for the two younger age groups.
Conclusion
Despite the increase in vaccine coverage, measles vaccination is still low, and the World Health Organization goal to eliminate measles by 2010 was not achieved in Switzerland. More efforts are needed by the cantons and the central government to increase vaccination coverage.
Switzerland is situated in the center of Western Europe, surrounded by Germany, Austria, France, and Italy. Although its population size is modest at 7.6 million inhabitants, this small federalist country has three major language regions and is divided into 26 cantons, which consist of municipalities, the number varying from three to almost 400 municipalities per canton. There are 19 cantons in the German-speaking region, six cantons in the French-speaking region (in two of these cantons, two-thirds are French-speaking and one-third are German-speaking), and one canton in the Italian-speaking region.
The Swiss Immunization Schedule recommends two doses of measles-mumps-rubella (MMR) vaccine for children before 2 years of age, with catch-up shots available during the school year for children lacking the two doses.1 Despite this effort, three large measles outbreaks plagued Switzerland in 1997, 2003, and 2006–2009. Two hundred thirty-five cases of measles were reported in 1997 within a sentinella notification surveillance system (an estimated 6,400 cases extrapolated for Switzerland), of which 84% of the patients were unvaccinated for measles.2 In 2003, 614 cases were registered in which 79% of the patients were younger than 16 years of age and 88% were not vaccinated against measles.3 Most recently, between November 2006 and September 2009, 4,415 cases of measles were confirmed with three distinct outbreak waves, where 93% were not vaccinated and 5% were not fully vaccinated.4 Measles outbreaks in Austria, Germany, and the United States in 2008 and Israel in 2003 have been linked to measles strains originating in Switzerland.5–8 In 2008, during the European Soccer Championship League in which Switzerland was one of the two host countries, a warning was issued for those planning to attend the championship to be vaccinated against measles.9
Because available data on vaccination coverage were very limited and concerned with potential epidemic outbreaks, the Swiss Federal Office of Public Health (SFOPH) sponsored a national effort between 1999 and 2003 to determine the vaccination coverage in each of the 26 cantons. The Institute of Social and Preventive Medicine (ISPM) at the University of Zurich was responsible for the development and implementation of this endeavor. The survey had two major goals: (1) to determine vaccination coverage and factors affecting vaccination in three different age groups: toddlers aged 24–35 months, children at school entry (i.e., kindergarten, first grade, second grade, or third grade), and children at school departure (seventh, eighth, or ninth grade); and (2) to establish a feasible survey methodology that could be implemented in every canton, using the existing infrastructure where possible.10 In short, two different methods were applied to sample toddlers and schoolchildren based on available sampling frames and preexisting infrastructures. Hence, data collection for the toddlers and schoolchildren varied, as families of toddlers were directly contacted for participation, while data for schoolchildren were submitted via school doctors, nurses, or teachers.
Additionally, school vaccination programs differ for each canton, ranging from diverse vaccination policies in the schools to age at which vaccination cards were checked. So, despite our efforts to adapt the existing infrastructure, data collection for the schoolchildren was complex and the comparability of the results among the cantons was inadequate.
Based on this experience, the methodology was reevaluated and improved for the next period of data collection in the Swiss National Vaccination Coverage Survey (SNVCS). The major changes in the SNVCS included (1) targeting children aged 2, 8, and 16 years; (2) employing the same data collection methodology used in 1999–2003 for the toddlers for all three age groups to increase comparability among the cantons; and (3) collecting data in all 26 cantons within a three-year cycle. We evaluated vaccination coverage for the SNVCS survey period 2005–2007.
METHODS
The cantons were allowed to designate their year of participation and define the level of their responsibility in the SNVCS 2005–2007. Nine cantons conducted the survey in 2005, eight in 2006, and six in 2007. Two cantons conducted the survey within two years during the survey period. One canton declined to participate while another was unable to implement the survey for toddlers due to lack of time. Participation year for a canton was designated by the year that data collection was conducted. Permission from the Office of Data Protection was obtained in all 26 cantons.
Sampling
Depending on the available sampling frame, either cluster or simple random sampling was used for all age groups. In 12 cantons where cluster sampling was applied, a list of the number of children born in each municipality in each canton served as the sampling frame. Using a self-weighted sampling design, the municipalities were first selected, then the children, with the number of children depending on the targeted population size. Because a central registry exists in four cantons, either the central office of registry in each canton randomly selected the children or ISPM selected the children via simple random sampling. Due to the low number of municipalities, all municipalities in seven cantons were requested to submit information on all residents in the targeted age groups. Once this information was compiled, simple random sampling was applied to select the children. Because of financial constraints and organizational simplification, the school health nurses continued to collect data for the schoolchildren in three cantons in the first, second, third, eighth, and ninth grades. While applying the same data collection method, three other cantons conducted the survey independently and shared their databases at a later time point.
Data collection
The information requested from the municipalities or the central office of registry included names of the child and parents; date of birth, gender, address, and nationality of the child; and occupation and telephone numbers for the parents, when possible. All families of selected children were then invited to participate by mail, which included an introductory letter and a prepaid return envelope. Families were asked to send a copy of or the original vaccination card. Four to five weeks later, a reminder was sent to all nonrespondents, followed by a final telephone attempt, which included five to six calls at different hours during the week. If necessary, primary care physicians were contacted to confirm questionable data from the vaccination cards, or to obtain records that were stored in the office, after acquiring permission from the participating families. Vaccination information that was not obtained from a vaccination card or through the physician's office was not included in the database. Time of response and reasons for nonparticipation were recorded when possible (data not shown).
In three cantons where collaboration with school health nurses was sought, the survey was coordinated with the routine health check-up, with data collected after the recommended vaccination was administered in the schools. After summarizing the vaccination information on a form or copying the vaccination cards, one canton transmitted the data electronically while the other two cantons submitted hard copies of the data. Data were submitted for all children in the second or third grades (those aged 7–9 years) and eighth or ninth grades (those aged 14–16 years) in two cantons. In the remaining canton, data were collected from 74 classes in the first grade (those aged 6–7 years) and 24 classes in the eighth grade.
Data extracted from the vaccination cards included dates of administration of all doses of diphtheria (Di), tetanus (Te), pertussis (Per), polio (Pol), Haemophilus influenzae type b (Hib), MMR, hepatitis B, hepatitis A, varicella, pneunomococcal, meningococcal conjugate group C, and tick-borne encephalitis vaccines. For two of the three cantons in which data were collected via school health nurses, vaccinations received were summarized by the number of doses for a particular antigen for each child. Only coverage for the first nine aforementioned antigens are examined in this article.
Statistical analysis
The collected data were first weighted to account for sampling design; adjusted for nonresponse; and poststratified with respect to nationality, gender, and urbanicity where possible. Urbanicity was defined following the guidelines used by the Swiss Federal Statistical Office. Participation was considered only when concrete vaccination information was obtained either from vaccination cards, school health nurses, or primary care physicians. Undocumented vaccinations based on parental recall were not accepted. Imputations for missing information were only conservatively conducted for nationality and gender variables when the municipalities did not supply this information, but names of the children were known.
In the three cantons where data had been collected by the school health nurses, information on nationality was missing in one canton, and on gender in two cantons. We performed Chi-square analysis, with p<0.05 considered significant. Using the survey option in Stata® version 10.1,11 we calculated coverage estimates and performed univariate logistic regression with measles-containing vaccine coverage in regard to nationality, linguistic region, time of participation, and data collection year.
RESULTS
Participation
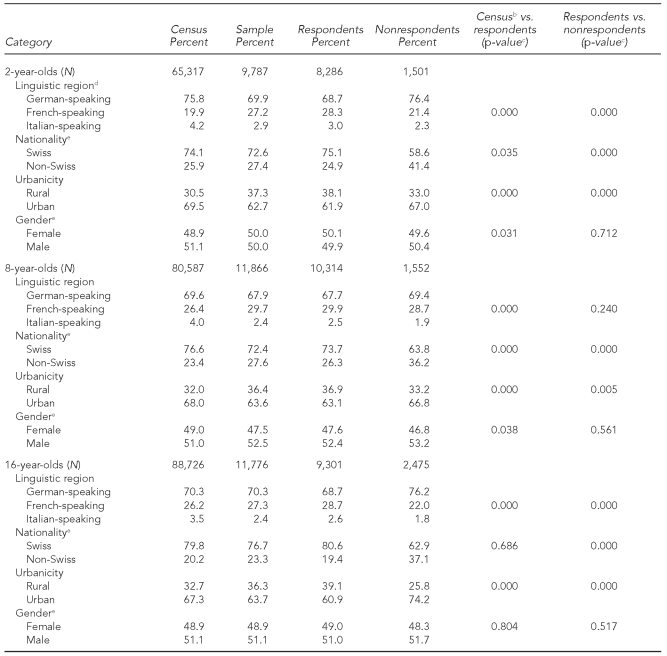
Table 1 shows demographic characteristics for the sample size, including respondents and nonrespondents in the survey, unweighted with the population data for 2005–2007. There were significant differences (p<0.05) between the participants and the census data and participants and nonrespondents in almost all four categories. After poststratification, these differences were corrected, such that the proportions for the respondents corresponded to those from the population data.
Table 1.
A comparison of demographic characteristics (unweighted) between participants in the Swiss National Vaccination Coverage Survey and the general Swiss population for three age groups: 2005–2007a
aDoes not include two cantons for toddlers and one canton for schoolchildren
bCensus data stemmed from the Swiss Federal Statistical Office, municipalities, or statistics office in the cantons, between 2005 and 2007.
cChi-square analysis was used to test for the difference between respondents and census data, and respondents and nonrespondents.
dThe German-speaking region includes 18 cantons, the French-speaking region includes six cantons, and the Italian-speaking region includes one canton.
eSome data are missing.
Not including the three cantons in which data were collected via school health nurses, participation level was 84.7% (8,286/9,787) for 2-year-olds, 84.1% (7,312/8,691) for 8-year-olds, and 80.1% (7,088/8,847) for 16-year-olds. For the three cantons using school health nurses, the response was 94.6% (3,002/3,175) for schoolchildren in the younger age group and 75.6% (2,213/2,929) for schoolchildren in the older age group (data not shown).
Immunization coverage
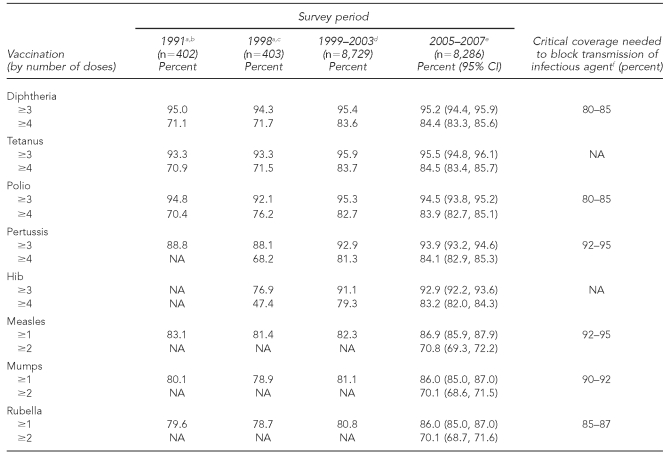
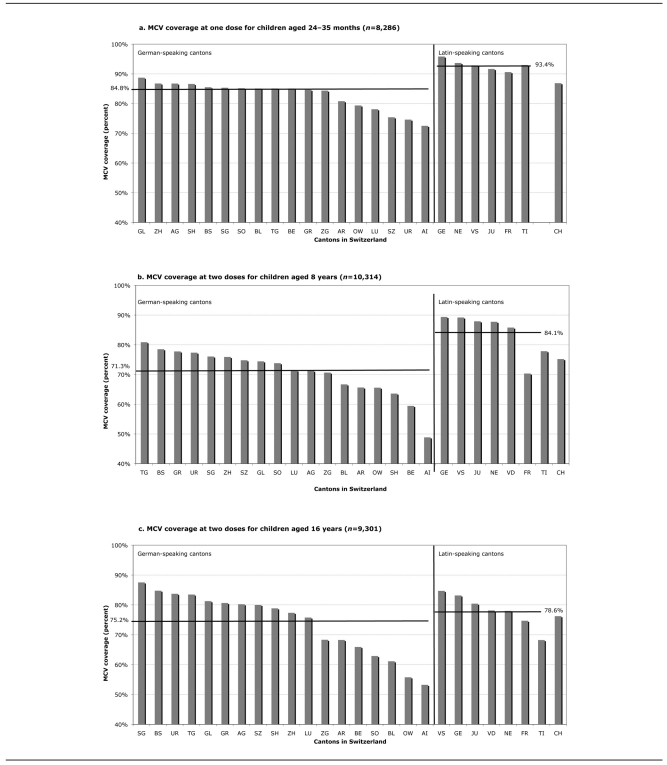
As shown in Table 2, coverage for Di, Te, Per, and Pol vaccines for toddlers did not change between 1999–2003 and 2005–2007, remaining around 84% at four doses; there was an increase for Hib at four doses, from 79.3% in 1999–2003 to 83.2% in 2005–2007. For measles, mumps, and rubella, coverage rose from 82.3% to 86.9%, from 81.1% to 86.0%, and from 80.8% to 86.0%, respectively, between the two study periods. The range for measles-containing vaccine coverage was 72.5%–95.8% at one dose, with five cantons having coverage ≤80% and all six cantons in the French- and Italian-speaking regions having coverage ≥90% at one dose (Figure).
Table 2.
Vaccination coverage of children aged 24–35 months in Switzerland, 2005–2007
aUsing a nationally representative survey conducted with samples proportionally reflecting the different linguistic regions; data collection included direct contact with the parents.
bSource: Minder CH, Steffen R. [Immunization for toddlers: a representative survey of vaccination coverage in Switzerland 1991]. Bull BAG/OFSP 1992;32:504-7. German.
cSource: Swiss Federal Office of Public Health. [Immunization for toddlers: a representative survey of vaccination coverage in Switzerland 1998]. Bull BAG/OFSP 1999;20:356-61. German.
dSurvey conducted in 26 cantons; data collection included direct contact with the parents. Source: Lang P, Piller U, Steffen R. Vaccination coverage of children in Switzerland, 1999–2003. Zurich: University of Zurich Institute of Social and Preventive Medicine; 2005.
eSurvey conducted in 24 cantons; data collection included direct contact with the parents.
fSource: Anderson RM, May RM. Immunisation and herd immunity. Lancet 1990;335:641-5.
CI = confidence interval
NA = not available
Hib = Haemophilus influenzae type b
Figure.
MCV coverage of children in Switzerland, 2005–2007, by cantons and regionsa
aHorizontal lines show the coverage mean for each region. Cantons are identified by their abbreviations.
MCV = measles-containing vaccine
TI = the only Italian-speaking canton
CH = Switzerland
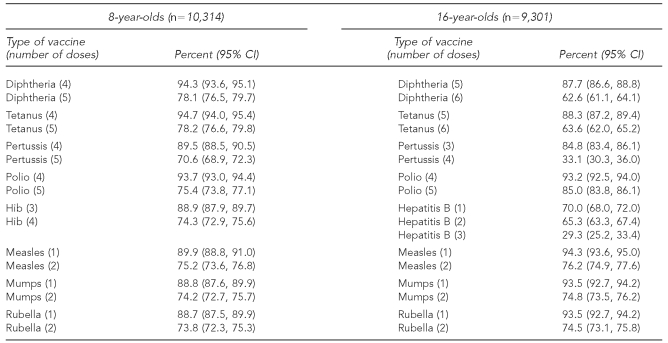
Coverage estimates for schoolchildren are depicted in Table 3. In the 25 participating cantons, the range of coverage for 8-year-olds was 75.4%–78.2% at five doses of Di, Te, and Pol vaccines. Coverage for fourth and fifth doses of Per vaccine were 89.5% and 70.6%, respectively. As shown in the Figure, no canton had measles-containing vaccine coverage ≥90% at two doses, with the minimum at 48.8% and the maximum at 87.7%. The highest estimates were found primarily in the French- and Italian-speaking regions.
Table 3.
Vaccination coverage of schoolchildren aged 8 and 16 years: Switzerland, 2005–2007a
aData were collected in 25 of 26 cantons. Data were collected by the school health nurses in three cantons and by contacting the families directly in 22 cantons.
CI = confidence interval
Hib = Haemophilus influenzae type b
For 16-year-olds, coverage was 62.6% for Di vaccine and 63.6% for Te vaccine at six doses; 33.1% for Per vaccine at four doses; and 76.2%, 74.8%, and 74.5% for measles, mumps, and rubella vaccines, respectively, at two doses (Table 3). While all 25 cantons had coverage of ≥90% at one dose of measles vaccine, no canton surpassed this figure at two doses, with coverage ranging from 53.2% to 87.5%. Measles-containing vaccine coverage levels were similar among the different linguistic regions, although lower coverage estimates were found in the German-speaking region (Figure).
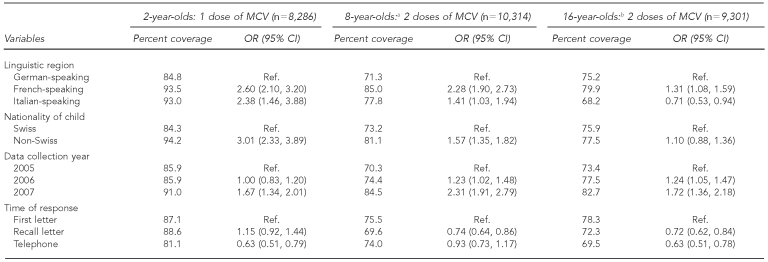
The univariate logistic regression in Table 4 shows that the child's nationality and linguistic region were correlated with measles coverage for the two younger age groups. The proportion of children up-to-date for measles vaccination was greater in the French- and Italian-speaking regions than in the German-speaking region; Swiss children had a lower chance of being vaccinated for measles than children of foreign nationality. For teenagers, there was no correlation between nationality and measles-containing vaccine coverage at two doses. In contrast, 79.9% (odds ratio [OR] = 1.31, 95% confidence interval [CI] 1.08, 1.59) of teenagers living in the French-speaking region had received two doses of measles-containing vaccine, compared with 75.2% of teens in the German-speaking region; the reverse was true for the Italian-speaking region, where only 68.2% (OR=0.71, 95% CI 0.53, 0.94) of 16-year-olds were up-to-date.
Table 4.
Univariate logistic regression of diverse factors and being up-to-date with MCV at one dose for children aged 24–35 months and at two doses for children aged 8 and 16 years: Switzerland, 2005–2007
aFor the 8-year-olds, information on nationality was missing in one canton (n=9,486) and time of response was missing in three cantons (n=7,312).
bFor the 16-year-olds, information on nationality was missing in one canton (n=8,396) and time of response was missing in three cantons (n=7,088).
MCV = measles-containing vaccine
OR = odds ratio
CI = confidence interval
Ref. = reference group
Data collection year also appeared to be correlated with being up-to-date for measles vaccination. Children who were recruited in 2007 seemed to have a higher measles-containing vaccine coverage than those in 2005. Time of response in the survey was also correlated with measles-containing vaccine coverage, with children whose parents participated after receiving the first letter having higher coverage than those recruited afterward.
DISCUSSION
The Swiss Immunization Schedule recommends two doses of MMR and four doses of Di, Te, Per, Pol, and Hib vaccine for children before 2 years of age, with catch-up shots available during the school years.1 Comparison of the national immunization coverage among toddlers for measles for the two most recent survey periods revealed that coverage increased from 82% in 1999-–2003 to 87% at one dose and 71% at two doses in 2005–2007.10 The recurring measles outbreaks, together with more intense efforts by the SFOPH and the cantons, probably contributed to this rise. Despite the increase, measles-containing vaccine coverage is far from 95% for two doses, the level necessary to attain herd immunity to eliminate this disease.12 Our results show that for measles vaccination at one dose, only six cantons had coverage ≥90% for toddlers, and no canton had measles-containing vaccine coverage of ≥90% at two doses for schoolchildren.
An epidemiologic assessment of measles between 2006 and 2007 by Muscat et al. confirmed that Switzerland is among the top five countries responsible for 85% of the recorded measles cases in Europe, contributing 27% of the total cases in 2007 with the highest incidence rate of 78.8 and 58.2 per 100,000 inhabitants in children aged 5–9 and 10–14 years, respectively.13 Between November 2006 and September 2009, there were 4,415 reported cases of measles in Switzerland, resulting in an incidence rate of 58 per 100,000 inhabitants, with one canton supplying 24% of the total cases. In January 2009, a 12-year-old girl from France, who was hospitalized in Geneva, died from encephalitis due to measles infection.14 To reach the goal of measles elimination in the European region by 2010, efforts to achieve this goal were intensified. These efforts included aggressive vaccination information campaigns and media exposure, participation in the European vaccination week, strengthened measles surveillance, commitment from the Chief Medical -Officers to achieve coverage ≥95%, and political support from Parliament with consideration for introducing compulsory measles immunization, as required in the U.S. at school entry.15–17
Based on the World Health Organization (WHO) guidelines to control outbreaks of vaccine-preventable childhood diseases, the SFOPH defined operational goals for coverage levels for the Swiss National Immunization Program as follows: coverage ≥95% nationally and ≥90% in each canton at three doses of Di, Te, Per, and Pol vaccines for toddlers, at four doses for children aged 5–7 years, and at five doses for those aged 16 years.18 For the most part, the reported coverage estimates at the national and canton level for Di, Te, and Pol for 2- and 8-year-olds have reached this goal, but those for the 16-year-olds are still too low. Of particular concern is the low coverage rate for Per for schoolchildren, despite the change from -whole-cell to acellular vaccine and the recommendation of a fourth and fifth dose in 1996 in the Swiss Immunization Schedule.
Raising Per catch-up vaccination in this age group should be a priority, as adolescents and especially adults have long been recognized as a source of Bordetella pertussis transmission in young, unprotected infants, in addition to the problems of waning immunity over time and vaccine efficacy lower than the desired coverage needed for herd immunity.19–22 As with measles, because of the suboptimal coverage level, circulation of the pathogen is unavoidable and can have tragic consequences, as in the case of the 2-year-old in one canton who died from a Per infection in 2009, the first mortality case in 10 years.23
Although not presented in this article, there was a general increase in coverage at the canton level, with only two cantons exhibiting a possible decrease for all eight targeted antigens for toddlers; one canton for 8-year-olds for all except Per and Hib vaccines; and one canton for teenagers for Di, Te, and Pol vaccines. The decrease could have been due to use of complementary and alternative medicine (e.g., homeopathy), a change in the school vaccination program, and/or a change in the survey methodology. Studies, including the last survey in 1999–2003, have shown the negative influence of complementary and alternative medicine on vaccination coverage and the potential impact of schools on vaccination programs.10,24–28 Race/ethnicity and nationality are also highly correlated with measles coverage, which has also been observed in many other studies.29–33
Unlike these studies, our results showed that children in the two younger age groups whose nationality was not Swiss were better vaccinated against measles than Swiss children. Research by Mixer et al. confirmed that awareness of the controversy surrounding MMR vaccination was higher among white mothers whose toddlers had lower MMR coverage, as compared with mothers of other racial/ethnic minority groups whose children had higher MMR coverage.34 There was no difference for 16-year-olds, as we see that logistic problems and access to health service may be barriers to being fully immunized. Furthermore, many of these children of foreign background do not have their vaccination records from their native country, although some have said that they were fully vaccinated before coming to Switzerland. Without any written records, we had to assume that these vaccinations were not administered. This problem is similar to record scattering, as documentations are incomplete due to multiple health-care providers, resulting in a negative impact on vaccination coverage.35,36 Record scattering increases the risk of losing immunization records and hinders the evaluation and improvements of vaccination coverage.
The highest immunization coverage estimates were found most often in the Latin-speaking region. With the most recent measles epidemic, evidence shows that measles incidence is inversely proportional to vaccination coverage, with the lowest incidence in the Latin-speaking region.4 Furthermore, vaccination for Di is mandatory in three Latin-speaking cantons (one canton only until 2008), which could have also influenced the higher immunization coverage among these regions. The difference in immunization rates for compulsory and facultative vaccinations has been established in other studies.37–39
While the French-speaking cantons appeared to have higher coverage than the German-speaking ones for the two younger age groups, this impact was not observed for adolescents. Rather, immunization coverage for teenagers appears to be influenced more by school vaccination programs, which are confounded by the fact that all French-speaking cantons have school vaccination programs, unlike in the German- and Italian-speaking regions where it varies by canton; this association was also observed in the first survey.10 Furthermore, alternative immunization plans adopted by complementary and alternative medicine practitioners recommend MMR vaccination for children to be delayed until 10–14 years of age, and only if protection has not yet been acquired through natural infection.40,41 In-depth multivariate analyses are currently being executed to examine the impact of some of these important factors.
Limitations
The coverage estimates obtained were based on the number of doses administered, which could be an overestimation, as has been shown in studies analyzing coverage by validity of vaccination doses and/or age-appropriate vaccination.42–46 Overestimation and vaccinations not timely administered will leave some children unprotected. As mentioned previously, coverage estimates could also be an underestimation, as vaccinations not documented in the submitted immunization cards were not taken into consideration. Recall bias was eliminated, as only data documented in the vaccination cards or provided by the primary care physicians or school health nurses were included in the analysis.
Although participation was quite high at 80%–85%, more effort is still needed to increase response level, as our study and other published literature have shown it to be associated with coverage.47,48 Our survey used a mix-mode data collection method (via mailings and telephone), which has been observed to produce less biased results with limited cost.49,50 However, due to greater volume of solicitation for participation in surveys and for commercial purposes, resistance to these contacts has risen, with more families blocking publication of their numbers in telephone books and at the municipality level.
In addition to increasing participation at the children's level, participation from cantons and municipalities must also be consistently maintained. Between 2005 and 2007, only one canton declined to participate; currently, data are being collected for the SNVCS for 2008–2010, with participation uncertain for three cantons. Although participation by municipalities was high at 97.3% between 2005 and 2007, reservations to release requested information have increased, with the minimum in one canton in the current cycle at an unacceptable 80%. Furthermore, coverage estimates ascertained from a three-year study cycle may be less sensitive to detecting changes in national coverage levels. More support at the political level for the SNVCS is required.
Finally, the participants in our survey were significantly different from the census data and the nonrespondents. Although statistical adjustments were made, including poststratification, they may not have been enough to account for nonresponse bias and the different sampling methodology. However, from our study, only 0.3% of nonrespondents who gave a reason for not participating fundamentally opposed vaccination.
CONCLUSIONS
Between 2005 and 2007, vaccination coverage for toddlers remained relatively stable for Di, Te, Per, Pol, and Hib but increased for MMR. However, coverage still does not convey herd immunity, and the WHO goal to eliminate measles by 2010 was not achieved in Switzerland. Due to recurring measles outbreaks and numerous exportations of measles to other countries, it is vital that more efforts are taken to increase vaccination coverage for measles to ≥95%.
Acknowledgments
The authors thank the Chief Medical Officers and their staffs in all 25 Swiss cantons for their collaboration, along with the families and doctors who participated in the survey. The authors also thank Hanspeter Jauss from the Institute of Social and Preventive Medicine for technical assistance. Funding was provided by the Cantons and the Swiss Federal Office of Public Health.
Footnotes
Robert Steffen has accepted honoraria and travel grants from various producers of vaccines, but none relating to pediatric vaccines listed in this article.
REFERENCES
- 1.Swiss Federal Office of Public Health.; Advisory Board on Immunization (EKIF). [Swiss Immunization Schedule 2010 guidelines and recommendations] Bern (Switzerland): SFOPH; 2010. German. [Google Scholar]
- 2.Paget WJ, Zimmermann H, Vorkauf H. A national measles epidemic in Switzerland in 1997: consequences for the elimination of measles by the year 2007. Euro Surveill. 2000;5:17–20. doi: 10.2807/esm.05.02.00025-en. [DOI] [PubMed] [Google Scholar]
- 3.Richard JL, Boubaker K, Doutaz M, Schubiger G. [Mandatory notifications of measles cases in Switzerland: strong increase in the number of cases in spring 2003] Schweizerische Aerztezeitung. 2003;84:1439–44. German. [Google Scholar]
- 4.Richard JL, Masserey Spicher V. Large measles epidemic in Switzerland from 2006 to 2009: consequences for the elimination of measles in Europe. Euro Surveill. 2009;14:19443. [PubMed] [Google Scholar]
- 5.Schmid D, Holzmann H, Schwarz K, Kasper S, Kuo HW, Aberle SW, et al. Measles outbreak linked to a minority group in Austria, 2008. Epidemiol Infect. 2010;138:415–25. doi: 10.1017/S0950268809990604. [DOI] [PubMed] [Google Scholar]
- 6.Pfaff G, Mezger B, Santibanez S, Hoffmann U, Maassen S, Wagner U, et al. Measles in south-west Germany imported from Switzerland—a preliminary outbreak description [published erratum appears in Euro Surveill 2008;13:8068] Euro Surveill. 2008;13:8044. [PubMed] [Google Scholar]
- 7.Outbreak of measles— San Diego California: January–February 2008. MMWR Morb Mortal Wkly Rep. 2008;57(8):203–6. [PubMed] [Google Scholar]
- 8.Stein-Zamir C, Zentner G, Abramson N, Shoob H, Aboudy Y, Shulman L, et al. Measles outbreaks affecting children in Jewish ultra-orthodox communities in Jerusalem. Epidemiol Infect. 2008;136:207–14. doi: 10.1017/S095026880700845X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richard J, Masserey-Spicher V, Santibanez S, Mankertz A. Measles outbreak in Switzerland—an update relevant for the European Football Championship (EURO 2008) Euro Surveill. 2008;13:8043. [PubMed] [Google Scholar]
- 10.Lang P, Piller U, Steffen R. Vaccination coverage of children in Switzerland, 1999–2003. Zurich: University of Zurich Institute for Social and Preventive Medicine; 2005. [Google Scholar]
- 11.StataCorp. Stata®: Version 10.1. College Station (TX): StataCorp; 2010. [Google Scholar]
- 12.Anderson RM, May RM. Immunisation and herd immunity. Lancet. 1990;335:641–5. doi: 10.1016/0140-6736(90)90420-a. [DOI] [PubMed] [Google Scholar]
- 13.Muscat M, Bang H, Wohlfahrt J, Glismann S, Molbak K EUVAC.NET group. Measles in Europe: an epidemiological assessment. Lancet. 2009;373:383–9. doi: 10.1016/S0140-6736(08)61849-8. [DOI] [PubMed] [Google Scholar]
- 14.Swiss Federal Office of Public Health. [Measles epidemic: status as of March 2009 and recommendations] [press release] 2009 Mar 3; German. [Google Scholar]
- 15.Swiss Federal Office of Public Health. [Elimination of measles as focus during the European Immunization Week] [press release] 2004 Apr 20; German. [Google Scholar]
- 16.Swiss Federal Office of Public Health. [Elimination of measles: a realistic national goal] Bull BAG/OFSP 2010;14:416-9. German. [Google Scholar]
- 17.Swiss Federal Office of Public Health. [European Immunization Week: a world without measles is possible. Do your part] Bull BAG/OFSP 2010;16:456-7. German. [Google Scholar]
- 18.Swiss Federal Office of Public Health. [National Immunization Program] Bern (Switzerland): SFOPH; 2000. German. [Google Scholar]
- 19.Heininger U, Cherry JD. Pertussis immunisation in adolescents and adults—Bordetella pertussis epidemiology should guide vaccination recommendations. Expert Opin Biol Ther. 2006;6:685–97. doi: 10.1517/14712598.6.7.685. [DOI] [PubMed] [Google Scholar]
- 20.de Greeff SC, Mooi FR, Westerhof A, Verbakel JM, Peeters MF, Heuvelman CJ, et al. Pertussis disease burden in the household: how to protect young infants. Clin Infect Dis. 2010;50:1339–45. doi: 10.1086/652281. [DOI] [PubMed] [Google Scholar]
- 21.Wendelboe AM, Njamkepo E, Bourillon A, Floret DD, Gaudelus J, Gerber M, et al. Transmission of Bordetella pertussis to young infants. Pediatr Infect Dis J. 2007;26:293–9. doi: 10.1097/01.inf.0000258699.64164.6d. [DOI] [PubMed] [Google Scholar]
- 22.Hallander HO, Gustafsson L. Efficacy and effectiveness of acellular pertussis vaccines: a 20-year Swedish experience. Expert Rev Vaccines. 2009;8:1303–7. doi: 10.1586/erv.09.88. [DOI] [PubMed] [Google Scholar]
- 23.Rockenbach M. [Unvaccinated girl dies from pertussis infection in the Bruderholz's Children's Hospital]. Basler Zeitung 2009 Feb 17 [cited 2011 Mar 22] Available from: URL: http://bazonline.ch/basel/land/story/15491688.
- 24.Downey L, Tyree PT, Huebner CE, Lafferty WE. Pediatric vaccination and vaccine-preventable disease acquisition: associations with care by complementary and alternative medicine providers. Matern Child Health J. 2010;14:922–30. doi: 10.1007/s10995-009-0519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wilson K, Mills E, Boon H, Tomlinson G, Ritvo P. A survey of attitudes toward paediatric vaccinations amongst Canadian naturopathic students. Vaccine. 2004;22:329–34. doi: 10.1016/j.vaccine.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 26.Zuzak TJ, Zuzak-Siegrist I, Rist L, Staubli G, Simoes-Wüst AP. Attitudes towards vaccination: users of complementary and alternative medicine versus non-users. Swiss Med Wkly. 2008;138:713–8. doi: 10.4414/smw.2009.12681. [DOI] [PubMed] [Google Scholar]
- 27.Lindley MC, Boyer-Chu L, Fischbein DB, Kolasa M, Middlemann AB, Wilson T, et al. The role of schools in strengthening delivery of new adolescent vaccinations. Pediatrics. 2008;121(Suppl 1):S46–54. doi: 10.1542/peds.2007-1115F. [DOI] [PubMed] [Google Scholar]
- 28.Averhoff F, Linton L, Peddecord M, Edwards C, Wang W, Fishbein D. A middle school immunization law rapidly and substantially increases immunization coverage among adolescents. Am J Public Health. 2004;94:978–84. doi: 10.2105/ajph.94.6.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danis K, Georgakopoulou T, Stavrou T, Laggas D, Panagiotopoulos T. Socioeconomic factors play a more important role in childhood vaccination coverage than parental perceptions: a cross-sectional study in Greece. Vaccine. 2010;28:1861–9. doi: 10.1016/j.vaccine.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 30.Mikolajczyk RT, Akmatov MK, Stich H, Krämer A, Kretzschmar M. Association between acculturation and childhood vaccination coverage in migrant populations: a population based study from a rural region in Bavaria, Germany. Int J Public Health. 2008;53:180–7. doi: 10.1007/s00038-008-8002-4. [DOI] [PubMed] [Google Scholar]
- 31.Chu SY, Barker LE, Smith PJ. Racial/ethnic disparities in preschool immunizations: United States, 1996–2001. Am J Public Health. 2004;94:973–7. doi: 10.2105/ajph.94.6.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Van der Wal MF, Diepenmaat AC, Pel JM, Hirasing RA. Vaccination rates in a multicultural population. Arch Dis Child. 2005;90:36–40. doi: 10.1136/adc.2003.047126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vandermeulen C, Roelants M, Theeten H, Van Damme P, Hoppenbrouwers K. Vaccination coverage and sociodemographic determinants of measles-mumps-rubella vaccination in three different age groups. Eur J Pediatr. 2008;167:1161–8. doi: 10.1007/s00431-007-0652-3. [DOI] [PubMed] [Google Scholar]
- 34.Mixer RE, Jamrozik K, Newsom D. Ethnicity as a correlate of the uptake of the first dose of mumps, measles and rubella vaccine. J Epidemiol Community Health. 2007;61:797–801. doi: 10.1136/jech.2005.045633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith PJ, Stevenson J. Racial/ethnic disparities in vaccination coverage by 19 months of age: an evaluation of the impact of missing data resulting from record scattering. Stat Med. 2008;27:4107–18. doi: 10.1002/sim.3223. [DOI] [PubMed] [Google Scholar]
- 36.Vandermeulen C, Roelants M, Theeten H, Depoorter AM, Van Damme P, Hoppenbrouwers K. Vaccination coverage in 14-year-old adolescents: documentation, timeliness, and sociodemographic determinants. Pediatrics. 2008;121:e428–34. doi: 10.1542/peds.2007-1415. [DOI] [PubMed] [Google Scholar]
- 37.Bonanni P, Bergamini M. Factors influencing vaccine uptake in Italy. Vaccine. 2001;20(Suppl 1):S8–12. doi: 10.1016/s0264-410x(01)00284-5. [DOI] [PubMed] [Google Scholar]
- 38.Stampi S, Ricci R, Ruffilli I, Zanetti F. Compulsory and recommended vaccination in Italy: evaluation of coverage and non-compliance between 1998–2002 in Northern Italy. BMC Public Health. 2005;5:42. doi: 10.1186/1471-2458-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Topuzoglu A, Ozaydin GA, Cali S, Cebeci D, Kalaca S, Harmanci H. Assessment of sociodemographic factors and socio-economic status affecting the coverage of compulsory and private immunization services in Istanbul, Turkey. Public Health. 2005;119:862–9. doi: 10.1016/j.puhe.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 40.Working Group for Differentiated Vaccinations. [Tips on planning an individualized vaccination schedule] [cited 2010 May 15] Available from: URL: http://www.impfo.ch/htm-dokumente/hinweis-indivimpfplan.htm.
- 41.Lehrke P, Nuebling M, Hofmann F, Stoessel U. Attitudes of homeopathic physicians towards vaccination. Vaccine. 2001;19:4859–64. doi: 10.1016/s0264-410x(01)00180-3. [DOI] [PubMed] [Google Scholar]
- 42.Heininger U, Zuberbühler M. Immunization rates and timely administration in pre-school and school-aged children. Eur J Pediatr. 2006;165:124–9. doi: 10.1007/s00431-005-0014-y. [DOI] [PubMed] [Google Scholar]
- 43.Luman ET, McCauley MM, Stokley S, Chu SY, Pickering LK. Timeliness of childhood immunizations. Pediatrics. 2002;110:935–9. doi: 10.1542/peds.110.5.935. [DOI] [PubMed] [Google Scholar]
- 44.Stokley S, Maurice E, Smith PJ, Klevens RM. Evaluation of invalid vaccine doses. Am J Prev Med. 2004;26:34–40. doi: 10.1016/j.amepre.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Hull BP, McIntyre PB. Timeliness of childhood immunisation in Australia. Vaccine. 2006;24:4403–8. doi: 10.1016/j.vaccine.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 46.Dannetun E, Tegnell A, Hermansson G, Törner A, Giesecke J. Timeliness of MMR vaccination—influence on vaccination coverage. Vaccine. 2004;22:4228–32. doi: 10.1016/j.vaccine.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 47.Szilagyi PG, Bordley C, Vann JC, Chelminski A, Kraus RM, Margolis PA, et al. Effect of patient reminder/recall interventions on immunization rates: a review. JAMA. 2000;284:1820–7. doi: 10.1001/jama.284.14.1820. [DOI] [PubMed] [Google Scholar]
- 48.Kellerman RD, Allred CT, Frisch LE. Enhancing influenza immunization. Postcard and telephone reminders and the challenge of immunization site shift. Arch Fam Med. 2000;9:368–72. doi: 10.1001/archfami.9.4.368. [DOI] [PubMed] [Google Scholar]
- 49.Brambilla DJ, McKinlay SM. A comparison of responses to mailed questionnaires and telephone interviews in a mixed mode health survey. Am J Epidemiol. 1987;126:962–71. doi: 10.1093/oxfordjournals.aje.a114734. [DOI] [PubMed] [Google Scholar]
- 50.Fowler FJ, Jr, Gallagher PM, Stringfellow VL, Zaslavsky AM, Thompson JW, Cleary PD. Using telephone interviews to reduce nonresponse bias to mail surveys of health plan members. Med Care. 2002;40:190–200. doi: 10.1097/00005650-200203000-00003. [DOI] [PubMed] [Google Scholar]