Abstract
Psychostimulant abuse represents a psychiatric disorder and societal concern that has been largely unamenable to therapeutic interventions. We have previously demonstrated that the 5-HT3 antagonist ondansetron or non-selective 5-HT2A/2C antagonist ketanserin administered 3.5 hours following daily pergolide, a non-selective DA agonist, reverses previously established cocaine sensitization. The present study was conducted to evaluate whether the same treatments or delayed pairing of pergolide with the antidepressant mirtazapine can also reverse consolidated methamphetamine (METH) behavioral sensitization. Sprague-Dawley rats received METH infusion via osmotic minipumps (25 mg/kg/day, s.c.) for 7 days, with accompanying daily injections of escalating METH doses (0–6 mg/kg, s.c.). This regimen takes into account the faster elimination of METH in rats, and is designed to replicate plasma METH concentrations with superimposed peak drug levels as observed during METH binging episodes in humans. Following a 7-day METH withdrawal, ondansetron (0.2 mg/kg, s.c.), ketanserin (1.0 mg/kg, s.c.), or mirtazapine (10 mg/kg, i.p.) was administered 3.5 hours after pergolide injections (0.1 mg/kg, s.c., qd) for 7 days. Behavioral sensitization as a model of METH abuse was assessed 14 days after the combination treatment cessation (i.e., day 28 of METH withdrawal) through an acute challenge with METH (0.5 mg/kg, i.p.). Pergolide combined with ondansetron or ketanserin reversed METH behavioral sensitization, but pergolide-mirtazapine combination was ineffective. The role of reactivation of addiction “circuit” by a non-selective DA agonist, and subsequent reconsolidation blockade through 5-HT3 or 5-HT2 antagonism in reversal of METH sensitization and treatment of METH addiction is discussed.
Keywords: methamphetamine sensitization reversal, pergolide, ondansetron, ketanserin, mirtazapine, psychostimulant abuse
Psychostimulant abuse and dependence continues to exert profound socioeconomic, legal and medical problems throughout the world. According to a recent survey in the United States (US), an estimated 49.4 million or 21.2 % of individuals aged 12 years or older had used cocaine or methamphetamine (METH) during their lifetime, and 6.1 million and 2.7 million of these individuals used the two drugs in the previous year and month, respectively [1]. Treatment program admissions in 2007 for persons with primary cocaine or METH abuse accounted for 21 % of 1,817,517 total admissions [2], while psychostimulant-related emergency department visits accounted for 33.9 % of visits due to illicit drug use in the US [3]. Psychostimulant abuse as a detriment to society is highlighted by a report from the National Drug Court Institute indicating that 35 – 50 % of the “Drug Court” participants were primary psychostimulant abusers [4].
In spite of the above problems, there are no pharmacological treatments that have shown consistent efficacy against chronic cocaine or METH abuse in controlled clinical trials. The compounds tested in previous clinical trials include selective serotonin reuptake inhibitors (e.g., fluoxetine), DA agonists (e.g., d-amphetamine, pergolide), DA antagonists (e.g., risperidone), γamino butyric acid agonists (e.g., gabapentin and baclofen), the analeptic modafinil, an antidepressant with smoking cessation efficacy, bupropion, n-acetylcysteine, and the antiepileptic topiramate [5,6]. To summarize, while more than two dozen medications have been tested to date as monotherapies against psychostimulant abuse, only a few have shown limited treatment efficacy in controlled clinical trials, and none are FDA-approved for this indication.
We have previously demonstrated that behavioral sensitization to cocaine and associated neurobiological changes, established by 5–7 daily cocaine injections (40 mg/kg/day) and subsequent withdrawal period lasting 7–9 days, can be reversed by a 7-day combination treatment with the non-selective DA agonist pergolide and 5-HT3 antagonist ondansetron [7]. Interestingly, this combination is only effective when ondansetron is administered 3.5 hour after pergolide. Apart from pergolide, cocaine itself can also reverse cocaine sensitization if it is again followed 3.5 h later by ondansetron, ketanserin (5-HT2 antagonist), mianserin (5-HT2 antagonists), clozapine (an atypical antipsychotic with affinities to 5-HT2 and 5-HT3 receptors), or WIN51708 (neurokinin-1 antagonist) [8,9,10]. These findings indicate a temporal requirement for the efficacy of combination treatments using a DA agonist and a antagonist at selected neurotransmitter receptor subtypes.
The above agonist/antagonist combination treatment is based on a hypothesis that repeated induction of aversive responses during psychostimulant withdrawal (“crash”), rather than the positive drug rewarding effects, plays a key role in long-term maintenance of relapse vulnerability in chronic psychostimulant abuse (Koob and Le Moal, 2001;[7, 10, 12] Zhang et al 2007). Acute (“hours”) or short-term (“days”) psychostimulant withdrawal is often associated clinically with dysphoria, anhedonia, anergia and other depressive symptoms [18] Ellinwood and Lee, 1989; Foltin and Fishman 1997; Newton et al 2004), and the intensity of these symptoms is a strong predictor of poor treatment response (Kampman et al 2001). Adminsitration of the daily agonist (e.g., cocaine or pergolide as a “cocaine substitute”) in our combination treatment regimen is designed to “therapeutically” induce an acute agonist withdrawal state. A 5-HT3, 5-HT2 or NK1 antagonist given 3.5 h after the agonist is hypothesized to attenuate withdrawal-associated aversive responses, and consequently interfere with neurobiological processes underlying long-term maintenance of psychostimulant abuse [7, see ref. 9 for further discussion of the time interval between agonist and antagonist administration]. It is noted that pergolide is not only a direct agonist at D1/D2 receptors, but also a partial 5-HT2 agonist [11]. This pharmacological profile partially matches the mixed indirect DA/5-HT agonist profile of psychostimulants, and, thus, is likely to reproduce a “psychostimulant-like” neurochemical sequella with a subsequent pharmacologic acute withdrawal state. Based on these considerations, the present study assessed whether ondansetron, ketanserin, or the antidepressant mirtazapine (antagonist at both 5-HT3 and 5-HT2 receptors) administered 3.5 hours after pergolide can also reverse previously established METH behavioral sensitization.
Male Sprague-Dawley rats, initially weighing 275–300 g (Charles River, Raleigh, NC), were acclimated to the vivarium on a 12 hour light/dark cycle (lights on 0700–1900 hrs) for 7 days prior to treatment. They were housed in pairs until the start of METH pretreatment (individual housing thereafter), and had free-access to food and water in accordance with the “Guide for Care and Use of Laboratory Animals”[13] All experimental procedures were conducted under a protocol approved by the Duke Institutional Animal Care and Use Committee.
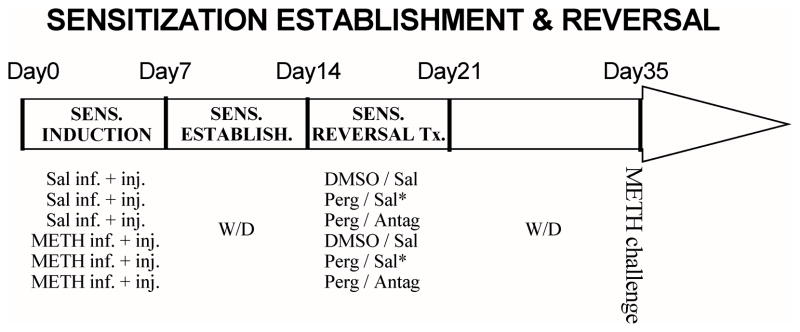
Fig. 1 presents the overview of the timeline for METH sensitization and combination treatments. Osmotic minipumps (model 2ML1; Alza Corporation, Palo Alto, CA) were filled with either saline (0.9 %) or (+)-METH hydrochloride dissolved in saline at concentrations to provide continuous 25 mg/kg/day dosing (based on the body weight at the time of surgery). The pumps were primed by warming in a water bath at 37 °C for 4 hours prior to implantation. Animals were shaved on the back and injected locally with 0.2 ml lidocaine at the dorsal midline incision site. They were then anesthetized with methoxyflurane or isoflurane, and implanted with a single primed minipump [14]. The incision site was closed with metal surgical autoclips.
Fig. 1. Timeline for METH sensitization and combination treatments.
Sprague-Dawley rats were treated with a 7-day saline or METH treatment regimens and withdrawn for 7 days to establish long-term METH senstization. Subsequently, they were adminstered ondansetron (0.2 mg/kg, s.c.), ketanserin (1.0 mg/kg, s.c.) or mirtazapine (10 mg/kg, i.p.) 3.5 hours after daily saline or pergolide (0.1 mg/kg, s.c.) injections for 7 days, followed by a second withdrawal period lasting 14 days (see Table 1 for treatment groups and their designations). On day 35 (i.e., METH withdrawal day 28), all animals were acutely challenged with METH (0.5 mg/kg, i.p.) in their home cages, and ambulatory and behavioral rating scores were quantified.
In addition to continuous infusion, animals in the METH treatment group were also injected daily with escalating doses of METH (0, 1, 2, 4, 6, 6 and 6 mg/kg, s.c. on METH infusion days 1–7), while those in the saline infusion group were injected daily with saline (1 ml/kg, s.c.). Importantly, METH has a much shorter half-life in rats than in humans (15–70 min vs. 6–24 hrs; [15]. The METH miniPump + Escalating Dosing (MPED) regimen used in this study was designed to replicate in rats the sustained METH plasma concentrations and superimposed drug concentration spiking in human METH abusers in binging episodes. Pumps were removed using the same surgical procedures after 7 days, and the residual volume of METH was measured to verify the total drug amount delivered through infusion. Animals were subsequently withdrawn for 7 days to allow for the establishment of METH sensitization in MPED-pretreated animals.
After 7 days of withdrawal, saline and MPED groups were divided into three combination treatment groups (Fig. 1). For ondansetron or ketanserin experiments, groups were given daily injections for 7 days of dimethyl sulfoxide (DMSO)/saline (D/S), pergolide/saline (P/S), or pergolide/antagonist (P/O or P/K) combinations. For the mirtazapine treatment experiment, DMSO/mirtazapine (D/m) combination was used instead of pergolide/saline treatment to directly compare the effects of mirtazapine monotherapy and pergolide/mirtazapine (P/m) combination (see Table 1 for treatment groups and group designations). For all reversal treatments, DMSO (1 ml/kg, s.c.) or pergolide (0.1 mg/kg, s.c.; Sigma, St.Louis, MO) was injected 3.5 hours before saline or antagonist administration (ondansetron, 0.2 mg/kg, s.c.; ketanserin, 1.0 mg/kg, s.c.; mirtazapine, 4 mg/ml suspension in saline, 10 mg/kg, i.p.). Ondansetron or ketanserin monotherapy was not used in the present study as we previously found that these antagonists exerted minimal efficacy in reversing previously established cocaine sensitization or progressive-ratio self-administration. [9].
Table 1.
Reversal Treatment Designations for Experimental Groups
| Saline groups | Reversal treatmenta | MPED groups | Reversal treatmenta |
|---|---|---|---|
| S-D/S | DMSO + saline | M-D/S | DMSO + saline |
| S-P/S | Pergolide + saline | M-P/S | Pergolide + saline |
| S-P/Ob | Pergolide + ondansetron | M-P/O | Pergolide + ondansetron |
| S-P/Kc | Pergolide + ketanserin | M-P/K | Pergolide + ketanserin |
|
| |||
| S-D/S | DMSO + saline | M-D/S | DMSO + saline |
| S-D/m | DMSO + mirtazapine | M-D/m | DMSO + mirtazapine |
| S-P/m | Pergolide + mirtazapine | M-P/m | Pergolide + mirtazapine |
The second drug injection was given 3.5 hours after the first one.
Combination treatment group for the ondansetron and ketanserin experiments, respectively. DMSO, Dimethylsulfoxide
On day 28 of METH withdrawal (i.e., 14 days after reversal treatment), behavioral responses to acute METH challenge were determined in the home cages (28 × 18 × 12 cm). Prior to behavioral assessment, rats were acclimated overnight in the test room under normal lighting conditions. The rat cages were placed in Opto-Varimex photo-beam monitors (8 × 8 beams; Columbus Instruments, Columbus, OH), and baseline activity was monitored for 15 min. Animals were subsequently injected with 0.5 mg/kg METH (i.p.) and the number of photo-beam breaks (locomotor activity) was recorded every 5 min. For behavioral ratings, behaviors were evaluated by a blind observer at 5-min intervals using the Ellinwood and Balster rating scale [16].
The ondansetron and ketanserin experiments were conducted in parallel; hence, results for the S-D/S, S-P/S, M-D/S and M-P/S groups in these studies were combined with their respective treatment groups (Table 1). The resulting locomotor and behavioral rating data sets (8 experimental groups) consisting of the above 4 groups and S-P/O, M-P/O, S-P/K and M-P/K groups were collapsed over the 60-min after METH injection (i.e., cumulative scores). The cumulative locomotor data were analyzed using one-way analysis of variance (ANOVA), followed by individual group comparisons using post-hoc Newman-Keul’s test (Sigmastat, Systat Software, San Jose, CA). Since the Ellinwood and Balster rating scores are of the ordinal-scale type, the cumulative scores were analyzed with non-parametric Kruskal-Wallis test, followed by Dunn’s pair-wise test. Results from the mirtazapine experiment underwent similar analyses. The locomotor data were presented as means and standard errors of the mean, while the behavioral rating scores were presented as medians and 25–75 % confidence intervals.
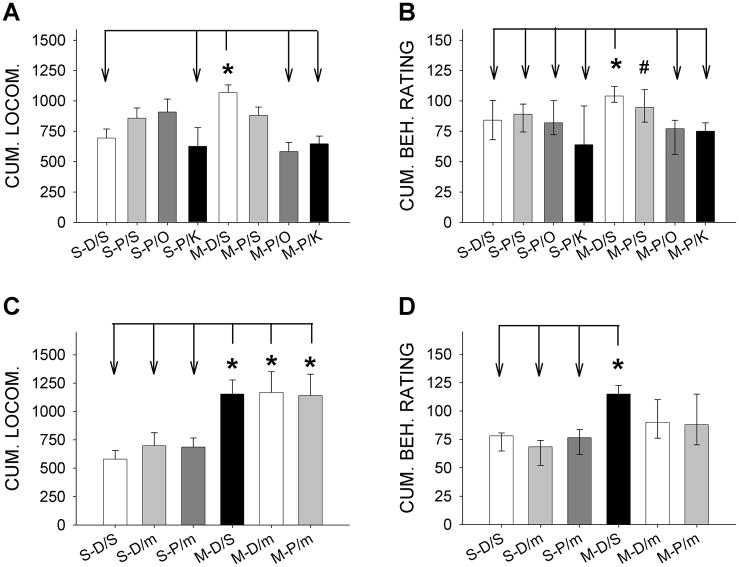
Similar to our previous results [e.g., 7, 9], no group differences were observed during the 15-min recordings of baseline locomotor activity or rating scores (data not shown). This finding indicates minimal residual effects of the earlier combination treatments on these behavioral measures. A one-way ANOVA on locomotor activity revealed significant treatment effects [F(7,136) = 4.57, p < 0.001; Fig. 2A]. Newman-Keul’s pair-wise comparisons for the main treatment effects revealed that M-D/S rats had significantly higher locomotor activity levels over the 60-min period following acute METH challenge than S-D/S group (*p < 0.008), indicating locomotor sensitization on day 28 of withdrawal from MPED. Combined pergolide/antagonists treatment reversed locomotor sensitization as the M-P/O and M-P/K groups showed significantly reduced sensitivity to acute METH challenge compared to M-D/S group (*p < 0.001 and *p < 0.003, respectively), and were not statistically different from the S-D/S control group. In contrast to combination treatment groups, locomotor activity levels in the pergolide monotherapy (M-P/S) group were not significantly different from either the sensitized M-D/S or any other experimental groups, suggesting lack of efficacy of pergolide monotherapy. These results indicate that established METH locomotor sensitization can be reversed by combinations of pergolide with a 5-HT3 or 5-HT2 antagonist (ondansetron or ketanserin, respectively) but not with pergolide alone.
Fig. 2. Effects of combined pergolide/ondansetron, pergolide/ketanserin and pergolide/mirtazapine treatment on established behavioral sensitization.
Cumulative locomotor data are of a “ratio-scale” data type, and, therefore, were analyzed using parametric one-way analysis of variance (ANOVA) and post-hoc Newman-Keul’s test. The behavioral rating scores, on the other hand, are of a “ordinal-scale” type, and were analyzed with non-parametric Kruskal-Wallis test, followed by Dunn’s pair-wise test.
A) Locomotor activity data for pergolide/ketanserin and pergolide/ondansetron treatments: METH-pretreated rats given DMSO/saline treatment (M-D/S) showed significantly higher locomotor levels than S-D/S (*, p < 0.008) and S-P/K (*, p < 0.015) groups. Treatment with combined pergolide/ondansetron (M-P/O) or pergolide/ketanserin (M-P/K) reversed locomotor sensitization (*, p < 0.001 and p < 0.003, respectively). Pergolide monotherapy (M-P/S) group was not significantly different from either the sensitized M-D/S or any other experimental groups. S-P/S and S-P/O groups tended to show increases in locomotion compared to S-D/S group, and were not different from the sensitized M-D/S group. Data presented as means ± SEM. Number of animals: S-D/S (15), S-P/S (21), S-P/O (13), S-P/K (10), M-D/S (27), M-P/S (26), M-P/O (17) and M-P/K (15).
B) Behavioral Ratings for pergolide/ketanserin and pergolide/ondansetron treatments: METH pretreated rats given DMSO/saline treatment (M-D/S) exhibited significantly higher scores than those in the saline-pretreated groups (S-D/S, S-P/S, S-P/O and S-P/K; *p < 0.01 - 0.05, Dunn’s test). Pergolide/ondansetron (M-P/O) or pergolide/ketanserin (M-P/K) treatment reversed locomotor sensitization (*p < 0.01 for both comparisons vs. M-D/S group). In contrast, M-P/S group was statistically indistinguishable from the sensitized M-D/S group and exhibited higher behavioral rating scores than M-P/K group (#, p < 0.01). Data presented as medians with range from 25–75%. Number of animals: S-D/S (17), S-P/S (21), S-P/O (16), S-P/K (10), M-D/S (27), M-P/S (26), M-P/O (19) and M-P/K (15).
C) Locomotor activity data for pergolide/mirtazapine treatment: Newman-Keul’s test revealed that all three MPED-pretreated groups (M-D/S, M-D/m, and M-P/m) had higher activity levels than the S-D/S, S-D/m and S-P/m groups with no differences among the three saline or METH pretreated groups (*p < 0.05 for all indicated comparisons). Data presented as means ±SEM. Number of animals: S-D/S (10), S-D/m (9), S-P/m (9), D-D/S (12), M-D/m (10) and M-P/m (12).
D) Behavioral Ratings for pergolide/mirtazapine treatment: M-D/S animals were significantly different from S-D/S, S-D/m and S-P/m animals (*p < 0.05) but not from M-D/m or M-P/m animals. The M-D/M and M-P/m group were also not different from any of the three saline-pretreated groups. Data presented as medians with range from 25–75%. Number of animals: S-D/S (10), S-D/m (10), S-P/m (10), D-D/S (12), M-D/m (10) and M-P/m (12).
While pergolide monotherapy did not produce a robust reversal of behavioral sensitization, the 7-day pergolide treatment in saline-pretreated animals (i.e., S-P/S group) tended to increase the sensitivity to subsequent acute METH challenge (Fig. 2A). Thus, cumulative 60-min locomotor levels in this treatment group were not significantly different from those in either the sensitized M-D/S or control S-D/S group. Delayed blockade of 5-HT2 receptors with ketanserin given 3.5 hours after pergolide eliminated this tendency, as S-P/K group had significantly reduced METH sensitivity as compared to M-D/S group (*p < 0.015). In contrast, similar blockade of 5-HT3 receptors with ondansetron (S-P/O) exerted minimal effects on the sensitizing tendency of pergolide; S-P/O and M-D/S groups were statistically indistinguishable. Although further studies are needed, these data together suggest that daily pergolide injections alone may have a tendency to induce locomotor sensitization in saline-pretreated (“naive”) animals, a trend which may be mediated by a 5-HT2-dependent, but 5-HT3-independent mechanism.
Psychostimulant exposure can serve to restrict the behavioral repertoire of animals in the open field, such that locomotor activity is replaced by stereotypy [16,17]. Therefore, in addition to automated locomotor activity measurements, effects of METH-pretreatment and combination reversal treatments were assessed with a behavioral rating scale [16]. A non-parametric Kruskal-Wallis analysis behavioral ratings revealed significant effects of reversal treatment [H(7) = 45.07, p < 0.001; Fig. 2B]. METH pretreated rats given DMSO/saline treatment (M-D/S) exhibited significantly higher scores than those in the saline-pretreated groups (S-D/S, S-P/S, S-P/O and S-P/K; *p < 0.01 - 0.05 by Dunn’s test). This behavioral sensitization was reversed by either pergolide/ondansetron or pergolide/ketanserin treatments as indicated by significantly-reduced behavioral ratings in M-P/O and M-P/K groups compared to M-D/S group (*p < 0.01 for both comparisons). In contrast to those in the combination treatments, METH-pretreated animals given pergolide alone (M-P/S) were statistically indistinguishable from the sensitized M-D/S group and exhibited higher behavioral rating scores than the M-P/K group (#p < 0.01). Furthermore, pergolide monotherapy in “naive” animals (i.e., the S-P/S group) did not tend to increase the behavioral rating scores in contrast to locomotor results (as noted above). The present results demonstrate that established METH sensitization, as assessed by both locomotor activity and behavioral ratings, can be consistently reversed when the animals are treated with a combination of pergolide with either a 5-HT3 or 5-HT2 antagonist, but not with pergolide alone.
In a separate series of experiments, efficacy of combined pergolide/mirtazapine (P/m) treatment in reversing established METH-sensitization was determined. Instead of pergolide, mirtazapine alone served as the monotherapy control. Significant treatment group differences were observed for locomotor activity following acute METH challenge on day 28 of withdrawal [F(5,56) = 3.710, p < 0.006; Fig. 2C], Newman-Keul’s test revealed that all three METH-pretreated groups (M-D/S, M-D/m and M-P/m) had significantly higher activity levels than the three saline pretreated groups (*p < 0.05 for all comparisons). Locomotor activity levels within the three S-D/S, S-D/m, and S-P/m groups or those within the M-D/S, M-D/m, and M-P/m groups were not different.
Similar to locomotor activity, results from a Kruskal-Wallis test revealed overall group differences for the behavioral rating scores [H(5) = 23.102, p < 0.001; Fig. 2D]. The M-D/S group again exhibited significantly greater rating scores than the S-D/S, S-D/m and S-P/m groups (i.e., sensitization; *p < 0.05, Dunn’s test). METH-pretreated animals given mirtazapine alone or pergolide/mirtazapine combination (M-D/m or M-P/m) were not statistically distinguishable from the three saline-pretreated or M-D/S animals. These results demonstrate that, in contrast to combinations of pergolide with ondansetron or ketanserin, the antidepressant mirtazapine, either alone or in combination with pergolide, fails to exert robust and consistent reversal efficacy on established METH sensitization.
Behavioral sensitization is progressive and enduring augmentation of locomotor and stereotyped behaviors upon repeated intermittent administration of a psychostimulant (e.g., METH) is considered as a model of progressive intensification of psychostimulant craving observed in human abusers [18,19]. As noted in the introduction, acute withdrawal from such psychostimulant exposure in human abusers is often associated with symptoms of anhedonia, anergia, depression, and anxiety [20,21]. The intensity of these aversive withdrawal symptoms is a strong predictor of poor treatment response and is also correlated with the increased subjective “high” induced by subsequent psychostimulant challenge [22,23]. In animal studies, a spectrum of analogous behavioral and biochemical stress measures (e.g., ultrasound vocalization and elevated corticosteroid levels, respectively) have been reported for cocaine withdrawal [24,25,26,27,28]. Koob and Le Moal [12] have similarly suggested that aversive “allosteric dysregulation” during repeated acute cocaine withdrawal may contribute to the maintenance of long-term cocaine sensitization and abuse.
The present study demonstrates that combinations of the DA agonist pergolide with either the 5-HT3 antagonist ondansetron or 5-HT2 antagonist ketanserin given 3.5 hours later normalize the behavioral responsivity to METH challenge in previously-sensitized animals. Since all experimental groups exhibited similar baseline locomotor activity levels or behavioral rating scores before METH challenge, the normal METH responsivity in the M-P/O or M-P/K groups is likely to represent a true reversal of previously-established sensitization, rather than non-specific behavioral inhibition (e.g., lethargy) induced by earlier combination treatment . In our earlier studies, we also showed that combined pergolide/ondansetron treatment also reverses cocaine sensitization [7] and attenuates METH-induced reinstatement of METH self-administration [29]. 5-HT3-dependent neural processes have been implicated in various aversive responses such as anxiety, psychosis, nociception, and cognitive function [30]. Ondansetron and other 5-HT3 antagonists are clinically used as antiemetic agents for “anti-aversive” effects [31]. With respect to drugs of abuse, ondansetron has been used to treat alcohol withdrawal with some success [32]. Also, drugs with 5-HT2 antagonistic efficacy have been used in the treatment of anxiety disorders, obsessive compulsive disorder and major depression [33,34,35]. As stressful aversive symptoms occur during acute agonist withdrawal, appropriately-timed administration of a 5-HT3 or 5-HT2 antagonist (e.g., 3.5 hours after agonist) may provide a means to attenuate or eliminate these aversive responses, thereby providing a way to disassociate previously-established relationships between long-term sensitization/abuse dynamics and the acute aversive withdrawal effects of DA agonists. While the localization of the pharmacological action of the combination treatment awaits additional investigation, the prefrontal cortex (PFC) may be a major site of action. Thus, this brain area plays a key modulatory role in consolidated psychostimulant sensitization and self-administration as well as various aversive responses [7,29,36,37], and distinct distribution of 5-HT2A and 5-HT3 receptors on the prefrontal pyramidal and interneurons have been demonstrated [38].
In contrast to ondansetron or ketanserin, the antidepressant mirtazapine was not effective in reversing established METH sensitization when used alone or in a combination with pergolide. Similar to the other two antagonists used in the present study, main reasons for choosing mirtazapine were its clinical availability and its combined 5-HT3/5-HT2 antagonistic activity. Therefore, the lack of effect of the pergolide-mirtazapine combination in reversing behavioral models of addiction herein was unexpected. There could be several reasons for the minimal reversal efficacy. For example, mirtazapine increases extracellular DA concentrations in the prefrontal cortex (PFC), especially within the dose range used in the present study (e.g., 10 mg/kg, i.p.) [39,40]. Mirtazapine is a potent antagonist at both α2 adrenergic and 5-HT2C receptors [41], and the increases in the prefrontal DA concentrations have been attributed to the blockade of these two receptor subtypes: (1) indirect enhancement of local, 5-HT1A-dependent DA release via blockade of α2 heteroreceptors in the PFC [39]; and (2) increases in the activity levels of the mesocortical DA neurons due to blockade of 5-HT2C receptors in the ventral tegmental area [40]. When mirtazapine is given 3.5 hours after the DA agonist pergolide, subsequent increases in DA efflux may supplant acute pergolide (DA agonist) withdrawal and thus prevent timely activation of “aversive circuit” that could be subsequently targeted by 5-HT3 or 5-HT2 blockade. Whether or not a combination treatment with lower doses of mirtazapine, which are not associated with increased prefrontal DA efflux, can provide intended sensitization-reversing efficacy is to be investigated in future studies. It is noted that McDaid et al [42] recently reported that mirtazapine monotherapy (5 mg/kg, i.p. × 15 days, starting 3 days after chronic administration) could reduce behavioral sensitization following low-dose METH injections (2.5 mg/kg, s.c., once a day × 5 days). While further studies are needed to elucidate experimental factors that could account for this discrepancy, examples of such factors include the METH dosing regimen (MPED vs. low-dose intermittent injections) and the duration of METH withdrawal before commencement of mirtazapine treatment (7 days vs. 3 days) as well as the mirtazapine doses (10 mg/kg, i.p. vs. 5 mg/kg, i.p.).
In naive (saline-pretreated) animals, pergolide alone tended to induce cross sensitization to METH following a 7-day treatment and 2 week withdrawal (i.e., S-P/S group). Notably, this effect was prevented by the 5-HT2 antagonist ketanserin, but not the 5-HT3 antagonist ondansetron. These results are consistent with a potential contribution of 5-HT2-dependent mechanisms in psychostimulant sensitization [43]. Inconsistent treatment outcome for pergolide monotherapy in clinical trials [44,45] could be partially due to the “mild psychostimulant property” of pergolide and its tendency to induce sensitization through DA/5-HT agonistic activity [11]. In a controlled clinical study, pergolide has been shown to induce cocaine “craving” in abstinent cocaine abusers [46]. Thus, it may appropriately serve as a psychostimulant “substitute” for induction of the acute psychostimulant withdrawal state.
Neurobiologically, established METH sensitization or abuse may be conceptualized as a form of stable “memory” that has been induced and consolidated following chronic drug administration and withdrawal. Similar to other forms of consolidated memory [47,48,49], the consolidated “METH sensitization circuit” may remain stable and unresponsive to disruption until it is reactivated by an exposure to METH (drug “tasting”), associated cues (e.g., craving upon seeing drug paraphernalia) or therapeutic delivery of a METH “substitute” such as pergolide. Once reactivated, the abuse circuit may become transiently labile, and consequently could be disrupted by treatments interfering with its reconsolidation. The time period of 3.5 hours after pergolide administration may represent such a temporal window for ondansetron- or ketanserin-mediated reconsolidation blockade. Notably, disruption of cue-induced psychostimulant self-administration or conditioned place preference by protein synthesis inhibition during a similar time window (3–4 hours after cue exposure) has been demonstrated [50]. Lastly, a recent human study [51] have reported that disruption of reconsolidation of fear-conditioned memory can be also best achieved when cues that interfere with reconsolidation is delivered ~3 hr after the reactivation cue. These findings provide support for the concept of temporally-mediated therapeutic erasure of dysfunctional memory. Taken together, these data provide a framework for continued evaluation of combinatory treatments towards cessation of psychostimulant craving with clinical studies currently ongoing.
Acknowledgments
This work was supported by grants from the National Institute of Drug Abuse (DA-14323 and DA-12768). We thank Mrs. Xueying Xiong for her technical support.
Footnotes
Conflict of Interest
No authors have conflict of interest to declare
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NSDUH Series H-36, HHS Publication No. SMA 09-4434. Rockville, MD: 2009. Results from the 2008 national survey on drug use and health: National findings. [Google Scholar]
- 2.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. National Admissions to Substance Abuse Treatment Services, DASIS Series: S-43, DHHS Publication No. SMA 08-4347. Rockville, MD: 2008. Treatment Episode Data Set (TEDS): 1996–2006. [Google Scholar]
- 3.Substance Abuse and Mental Health Services Administration, Office of Applied Studies. Drug abuse warning network, 2007: National estimates of drug-related emergency department visits. Rockville, MD: 2010. [Google Scholar]
- 4.Huddleston CW, Marlowe DB, Casebolt R. Painting the current picture: A national report card on drug courts and other problem-solving court programs in the United States. Washington DC: National Drug Court Institute; 2008. [Google Scholar]
- 5.Vocci FJ, Elkashef A. Pharmacotherapy and other treatments for cocaine abuse and dependence. Curr Opin Psychiatry. 2005;18:265–70. doi: 10.1097/01.yco.0000165596.98552.02. [DOI] [PubMed] [Google Scholar]
- 6.Acri JB, Chiang CN, McCann DJ, Shih ML, Vocci FJ. Summary of NIDA medications workshop: New opportunities for chemists and pharmacologists. Drug Alcohol Depend. 2008;92:307–11. doi: 10.1016/j.drugalcdep.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Zhang X, Lazarus C, Wetsel WC, Davidson C, Lee TH, Ellinwood EH. Reversal of cocaine-induced behavioral sensitization and associated phosphorylation of the NR2B and GluR1 subunits of the NMDA and AMPA receptors. Neuropsychopharmacol. 2007;32:377–87. doi: 10.1038/sj.npp.1301101. [DOI] [PubMed] [Google Scholar]
- 8.Davidson C, Lazarus C, Xiong X, Lee TH, Ellinwood EH. 5-HT2 antagonists given in the acute withdrawal from daily cocaine injections reverse established sensitization. European J of Pharmacol. 2002;453:255–63. doi: 10.1016/s0014-2999(02)02390-7. [DOI] [PubMed] [Google Scholar]
- 9.Davidson C, Lee TH, Xiong Z, Ellinwood EH. Ondansetron given in the acute withdrawal from a repeated cocaine sensitization dosing regimen reverses the expression of sensitization and inhibits self-administration. Neuropsychopharmacol. 2002;27:542–53. doi: 10.1016/S0893-133X(02)00336-6. [DOI] [PubMed] [Google Scholar]
- 10.Davidson C, Lee TH, Ellinwood EH. The NK(1) receptor antagonist WIN51708 reduces sensitization after chronic cocaine. Eur J Pharmacol. 2004;499:355–56. doi: 10.1016/j.ejphar.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Newman-Tancredi A, Cussac D, Quentric Y, Touzard M, Verriele L, Carpenter N, Millan MJ. Differential actions of anti-Parkinson’s agents at multiple classes of monoaminergic receptor III agonist and antagonist properties at serotonin. J Pharmacol and Exp Ther. 2002;303:815–22. doi: 10.1124/jpet.102.039883. [DOI] [PubMed] [Google Scholar]
- 12.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis [Review] Neuropsychopharmacol. 2001;24:97–29. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 13.Guide for Care and Use of Laboratory Animals. Washington DC: The National Academies Press; 1996. [PubMed] [Google Scholar]
- 14.King GR, Ellinwood EH, Jr, Silvia C, Joyner CM, Xue Z, Caron MG, Lee TH. Withdrawal from continuous or intermittent cocaine administration: changes in D2 receptor function. J Pharmacol Exp Ther. 1994;269:743–49. [PubMed] [Google Scholar]
- 15.Cho AK, Melega WP, Kuczensk R, Segal DS. Relevance of pharmacokinetic parameters in animal models of methamphetamine abuse. Synapse. 2001;39:161–66. doi: 10.1002/1098-2396(200102)39:2<161::AID-SYN7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Ellinwood EH, Balster RL. Rating the behavioral effects of amphetamine. Eur J Pharmacol. 1974;28:35–41. doi: 10.1016/0014-2999(74)90109-5. [DOI] [PubMed] [Google Scholar]
- 17.Lyon M, Randrup A. The dose–response effect of amphetamine upon avoidance behaviour in the rat seen as a function of increasing stereotypy. Psychopharmacologia. 1972;23:334–47. doi: 10.1007/BF00406736. [DOI] [PubMed] [Google Scholar]
- 18.Gawin FH, Ellinwood EH. Cocaine and other stimulants: Actions, abuse and treatment. New Eng J Med. 1988;318:1173–81. doi: 10.1056/NEJM198805053181806. [DOI] [PubMed] [Google Scholar]
- 19.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–91. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 20.Mulvaney FD, Alterman AI, Boardman CR, Kampman K. Cocaine abstinence symptomatology and treatment attrition. J Sub Abuse Treatment. 1999;16:129–35. doi: 10.1016/s0740-5472(98)00017-8. [DOI] [PubMed] [Google Scholar]
- 21.Kampman KM, Alterman AI, Volpicelli VR, Maany I, Muller ES, Luce DD, et al. Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict. 2001;15:52–59. doi: 10.1037/0893-164x.15.1.52. [DOI] [PubMed] [Google Scholar]
- 22.Newton TF, Kalechstein AD, Tervo KE, Ling W. Irritability following abstinence from cocaine predicts euphoric effects of cocaine administration. Addict Behav. 2003;28:817–21. doi: 10.1016/s0306-4603(01)00273-8. [DOI] [PubMed] [Google Scholar]
- 23.Sofuoglu M, Dudish-Poulsen S, Brown SB, Hatsukami DK. Association of cocaine withdrawal symptoms with more severe dependence and enhanced subjective response to cocaine. Drug and Alcohol Dependence. 2003;69:273–82. doi: 10.1016/s0376-8716(02)00328-9. [DOI] [PubMed] [Google Scholar]
- 24.Barros HM, Miczek KA. Withdrawal from oral cocaine in rate: ultrasonic vocalizations and tactile startle. Psychopharmacology. 1996;125:379–84. doi: 10.1007/BF02246021. [DOI] [PubMed] [Google Scholar]
- 25.Costall B, Jones BJ, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB. Ondansetron inhibits a behavioral consequence of withdrawing from drugs of abuse. Pharmacol Biochem Beh. 1990;6:339–44. doi: 10.1016/0091-3057(90)90414-d. [DOI] [PubMed] [Google Scholar]
- 26.Fung YK, Richard LA. Behavioural consequences of cocaine withdrawal in rats. J of Pharm and Pharmacol. 1994;46:150–52. doi: 10.1111/j.2042-7158.1994.tb03761.x. [DOI] [PubMed] [Google Scholar]
- 27.Koeltzow TE, White FJ. Behavioral depression during cocaine withdrawal is associated with decreased spontaneous activity of ventral tegmental area dopamine neurons. Behavioral Neurosci. 2003;117:860–65. doi: 10.1037/0735-7044.117.4.860. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Spangler R, Ho A, Kreek MJ. Increased CRH mRNA levels in the rat amygdale during short-term withdrawal from cocaine “binge” cocaine. Br Res Mol Br Res. 2003;114:73–79. doi: 10.1016/s0169-328x(03)00139-6. [DOI] [PubMed] [Google Scholar]
- 29.Davidson C, Gopalan R, Ahn C, Chen Q, Mannelli P, Patkar AA, Weese GD, Lee TH, Ellinwood EH. Reduction in methamphetamine induced sensitization and reinstatement after combined pergolide plus ondansetron treatment during withdrawal. Eur J Pharmacol. 2007;565:113–18. doi: 10.1016/j.ejphar.2007.02.056. [DOI] [PubMed] [Google Scholar]
- 30.Grant KA. The role of 5-HT3 receptors in drug dependence. Drug Alcohol Depend. 1995;38:155–71. doi: 10.1016/0376-8716(95)01120-n. [DOI] [PubMed] [Google Scholar]
- 31.Barnes JM, Costall B, Coughlan J, Domeney AM, Gerrard PA, Kelly ME, Naylor RJ, Onaivi ES, Tomkins DM, Tyers MB. The effects of ondansetron, a 5-HT3 receptor antagonist, on cognition in rodents and primates. Pharmacol Biochem Behav. 1990;35:955–62. doi: 10.1016/0091-3057(90)90385-u. [DOI] [PubMed] [Google Scholar]
- 32.Johnson BA, Roache JD, Ait-Daoud N, Javors MA, Harrison JM, Elkashef A, Mojsiak J, Li SH, Bloch DA. A preliminary randomized, double-blind, placebo-controlled study of the safety and efficacy of ondansetron in the treatment of cocaine dependence. Drug & Alcohol Depend. 2006;84:256–63. doi: 10.1016/j.drugalcdep.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 33.de Leeuw AS, Westenberg HG. Hypersensitivity of 5-HT2 receptors in OCD patients. An increased prolactin response after a challenge with meta-chlorophenylpiperazine and pre-treatment with ritanserin and placebo. J Psychiatr Res. 2008;42:894–01. doi: 10.1016/j.jpsychires.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Landén M, Thase ME. A model to explain the therapeutic effects of serotonin reuptake inhibitors: the role of 5-HT2 receptors. Psychopharmacol Bull. 2006;39:147–66. [PubMed] [Google Scholar]
- 35.Kent JM. SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet. 2000;355:911–18. doi: 10.1016/S0140-6736(99)11381-3. [DOI] [PubMed] [Google Scholar]
- 36.Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- 37.Berretta S. Cortico-amygdala circuits: role in the conditioned stress response. Stress. 2005;8:221–32. doi: 10.1080/10253890500489395. [DOI] [PubMed] [Google Scholar]
- 38.Jakab RL, Goldman-Rakic PS. Segregation of serotonin 5-HT2A and 5-HT3 receptors in inhibitory circuits of the primate cerebral cortex. J Comp Neurol. 2000;417:337–348. doi: 10.1002/(sici)1096-9861(20000214)417:3<337::aid-cne7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama K, Sakurai T, Katsu H. Mirtazapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Brain Res Bull. 2004;63:237–41. doi: 10.1016/j.brainresbull.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 40.Millan MJ, Gobert A, Rivet JM, Adhumeau-Auclair A, Cussac D, Newman-Tancredi A, Dekeyne A, Nicolas JP, Lejeune F. Mirtazapine enhances frontocortical dopaminergic and corticolimbic adrenergic, but not serotonergic, transmission by blockade of alpha2-adrenergic and serotonin2C receptors: a comparison with citalopram. Eur J Neurosci. 2000;12:1079–95. doi: 10.1046/j.1460-9568.2000.00982.x. [DOI] [PubMed] [Google Scholar]
- 41.de Boer TH, Maura G, Raiteri M, de Vos CJ, Wieringa J, Pinder RM. Neurochemical and autonomic pharmacological profiles of the 6-aza-analogue of mianserin, Org 3770 and its enantiomers. Neuropharmacol. 1988;27:399–08. doi: 10.1016/0028-3908(88)90149-9. [DOI] [PubMed] [Google Scholar]
- 42.McDaid J, Tedford CE, Mackie AR, Dallimore JE, Mickiewicz AL, Shen F, Angle JM, Napier TC. Nullifying drug-induced sensitization: behavioral and electrophysiological evaluations of dopaminergic and serotonergic ligands in methamphetamine-sensitized rats. Drug Alcohol Depend. 2007;86:55–66. doi: 10.1016/j.drugalcdep.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Lanteri C, Salomon L, Torrens Y, Glowinski J, Tassin JP. Drugs of abuse specifically sensitize noradrenergic and serotonergic neurons via a non-dopaminergic mechanism. Neuropsychopharmacol. 2008;33:1724–34. doi: 10.1038/sj.npp.1301548. [DOI] [PubMed] [Google Scholar]
- 44.Malcolm R, Kajdasz DK, Herron J, Anton RF, Brady KT. A double-blind, placebo-controlled outpatient trial of pergolide for cocaine dependence. Drug Alcohol Depend. 2000;60:161–8. doi: 10.1016/s0376-8716(99)00151-9. [DOI] [PubMed] [Google Scholar]
- 45.Levin FR, McDowell D, Evans SM, Brooks D, Spano C, Nunes EV. Pergolide mesylate for cocaine abuse: a controlled preliminary trial. Am J Addict. 1999;8:120–7. doi: 10.1080/105504999305929. [DOI] [PubMed] [Google Scholar]
- 46.Haney M, Foltin RW, Fischman MW. Effects of pergolide on intravenous cocaine self-administration in men and women. Psychopharmacol (Berl) 1998;137:15–24. doi: 10.1007/s002130050588. [DOI] [PubMed] [Google Scholar]
- 47.Nader K. Memory traces unbound. Trends Neurosci. 2003;26:65–72. doi: 10.1016/S0166-2236(02)00042-5. [DOI] [PubMed] [Google Scholar]
- 48.Centonze D, Siracusano A, Calabresi P, Bernardi G. Removing Pathogenic Memories: A Neurobiology of Psychotherapy. Mol Neurobiol. 2005;32:123–132. doi: 10.1385/MN:32:2:123. [DOI] [PubMed] [Google Scholar]
- 49.Lee JL, Everitt BJ, Thomas KL. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science. 2004;304:839–43. doi: 10.1126/science.1095760. [DOI] [PubMed] [Google Scholar]
- 50.Lee JL, Di Ciano P, Thomas KL, Everitt BJ. Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron. 2006;47:795–01. doi: 10.1016/j.neuron.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Schiller D, Monfils MH, Raio CM, Johnson DC, Ledoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463:49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]