Abstract
Urinary tract morphogenesis requires subdivision of the ureteric bud (UB) into the intra-renal collecting system and the extra-renal ureter, by responding to signals in its surrounding mesenchyme. BMP4 is a mesenchymal regulator promoting ureter development, while GREM1 is necessary to negatively regulate BMP4 activity to induce UB branching. However, the mechanisms that regulate the GREM1-BMP4 signaling are unknown. Previous studies have shown that Six1-deficient mice lack kidneys, but form ureters. Here, we show that the tip cells of Six1−/− UB fail to form an ampulla for branching. Instead, the UB elongates within Tbx18- and Bmp4-expressing mesenchyme. We find that the expression of Grem1 in the metanpehric mesenchyme (MM) is Six1-dependent. Treatment of Six1−/− kidney rudiments with GREM1 protein restores ampulla formation and branching morphogenesis. Furthermore, we demonstrate that genetic reduction of BMP4 levels in Six1−/− (Six1−/−;Bmp4+/−) embryos restores urinary tract morphogenesis and kidney formation. This study uncovers an essential function for Six1 in the MM as an upstream regulator of Grem1 in initiating branching morphogenesis.
Keywords: SIX1, Gremlin1, BMP4, ureteric bud, ampulla
Introduction
Mesenchymal-epithelial interactions are known to be critical for the growth and patterning of many organs in the human body, including the renal system. The intra-renal collecting system and the extra-renal ureter are derived from the ureteric bud (UB). The UB formation is induced by GDNF (Glial cell line-derived neurotrophic factor), which is secreted by the metanephric mesenchyme (MM) and acts as a ligand that binds to the Ret receptor tyrosine kinase and the Gfrα1 coreceptor expressed in the Wolffian duct (WD), at the level of hindlimb at around E10.5 in mice (Costantini, 2006; Davies and Fisher, 2002; Saxen and Sariola, 1987; Vainio and Lin, 2002). The initial outgrowth of the UB is a critical step in the development of the urinary tract and if it fails to occur, the ureter and the kidney will not develop.
Soon after the UB forms, it grows out and develops into tip- and trunk-specific domains. The trunk region normally elongates and differentiates into the ureter, while the tip region swells to form an ampulla, a prerequisite for and the first evidence of UB branching (al-Awqati and Goldberg, 1998). Abnormal regulation of UB morphogenesis can cause a spectrum of congenital birth defects such as urinary tract obstruction (Airik and Kispert, 2007). Depending on whether it is metanephric (around the tip) or more posterior tailbud-derived (around the stalk), the mesenchyme surrounding the ureteric epithelium plays an essential role in promoting epithelial differentiation. BMP4, a paracrinefactor of the TGF-β family, mediates the activity of the tailbud-derived mesenchyme, directs the UB development towards ureter fate, and inhibits branching in explant cultures (Brenner-Anantharam et al., 2007; Miyazaki et al., 2000). The BMP antagonist gremlin1 (Grem1), a member of the CAN domain family that preferentially binds to BMP2/4 in vitro (Hsu et al., 1998), is expressed by the MM (Michos et al., 2004). A recent study has shown that GREM1-mediated reduction of BMP4 activity in the mesenchyme around the nascent UB is essential to initiate UB outgrowth and invasion of the MM by establishing WNT11/GDNF feedback signaling (Michos et al., 2007). However, how GREM1 activity is regulated in the MM and how it acts to locally restrict BMP4 signaling to induce UB tip domain formation and its invasion into the MM are essentially unknown.
We have previously reported that a lack of Six1 in mice leads to renal agenesis and mutations in the human SIX1 cause branchio-oto-renal (BOR) syndrome (Ruf et al., 2004; Xu et al., 2003). Six1 belongs to the murine homeobox Six gene family, which is homologous to the Drosophila sine oculis (so). In the developing kidney, Six1 is expressed in the MM before the initiation of UB branching morphogenesis, and in Six1−/− mutants, UB grows out normally and elongates to differentiate into ureter but fails to form collecting system (Bush et al., 2006; Li et al., 2003; Nie et al.; Xu et al., 2003). In the absence of UB branching, the MM is eliminated by apoptosis after E11.0 (Xu et al., 2003). Despite these observations, the mechanism by which SIX1 induces epithelial branching to form the collecting duct system remains unclear.
Here, we have specifically investigated the role of Six1 in the initiation of UB branching morphogenesis and have identified Grem1 as a crucial target for upregulation in the MM by SIX1. We provide strong evidence that the extra-cellular BMP antagonist GREM1 is the key player in the Six1-regulatory pathway to regulate UB ampulla formation and its subsequent branching morphogenesis, as GREM1-soaked beads when implanted into Six1−/− kidney primordia in culture were sufficient to promote branching morphogenesis. Furthermore, we show that inactivation of only one copy of the Bmp4 gene in Six1−/− embryos restored UB patterning and kidney organogenesis. In addition, our results show that the balance between the levels of BMP4 activity in the mesenchyme surrounding the nascent UB tip and GDNF production in the MM appears to be crucial for UB patterning. These results demonstrate that Six1 is specifically required for upregulating the expression of Grem1 in the MM to locally restrict BMP4 activity in the mesenchyme to initiate ampulla formation and branching morphogenesis during urinary tract morphogenesis.
Materials and methods
Animal and genotyping
Six1+/− mutant mice in 129/SvEv strain were used for this study. The Bmp4 gene was inactivated in the germline by intercrossing the floxed Bmp4loxP-lacZ allele (Kulessa and Hogan, 2002) with the Cre deleter mouse strain (Schwenk et al., 1995). The resulting Bmp4–lacZ allele was used to generate Six1;Bmp4 compound mutant mice for analysis in a mixed 129/C57BL6 background. The compound mutants carrying the Hoxb7-GFP transgene (Srinivas et al., 1999) in a mixed C57BL6/CBA/129 background to mark the ureteric epithelium were used for analysis. PCR genotyping of mice and embryos was done as described previously (Kulessa and Hogan, 2002; Srinivas et al., 1999).
Phenotype analysis, in situ hybridization and immunohistochemistry
Embryos for histology and in situ hybridization were dissected out in PBS, fixed with 4% PFA overnight at 4°C and processed using standard procedures.
Whole-mount and section in situ hybridization were carried out with digoxigenin-labeled riboprobes or with 35S-radioisotope labeled Bmp4 probe. We used 5 embryos for each genotype at each stage for each probe, and the staining was consistent in each embryo.
Anti-pSMAD1/5/8 (Cell Signaling), HRP-conjugated secondary antibody and DAB was used for detection. After staining, the slides were counter-stained with diluted hematoxylin.
Antibody staining for SMA was done as described previously (Nie et al., 2010).
Organ cultures
Metanephric kidney primordia of E10.5–E10.75 were isolated from wild-type and mutant embryos and laid flat onto the top of a Transwell filter (0.4 μm pore size) for in vitro culture. GREM1 (R&D systems) were added at 2–6 μg/ml into culture medium according to Michos et al. (Michos et al., 2007) and changed every 48 hours. GREM1-soaked beads (1.5 μg/μl) implantation experiments were performed as described (Sajithlal et al., 2005). After culture, the kidney primordia were fixed in 4% paraformaldehyde (PFA) and processed for whole-mount in situ hybridization.
Results
Six1−/− UB tip cells fail to form an ampulla
To address what specific roles SIX1 may play to regulate branching morphogenesis, we performed a detailed analysis to specifically investigate whether Six1 acts to control the initiation of UB patterning into trunk and tip domains and to induce cells at the UB tip to form an ampulla. The UB is normally formed from the caudal segment of the WD at E10.5, and it grows out and divides into trunk and tip domains, as labeled by differential c-Ret expression (Fig. 1A). The trunk elongates to form the ureter, while the tip grows to form an ampulla showing strong c-Ret expression at around E11.0 (Fig. 1C). The UB elongates further to invade the mesenchyme and by E11.5, it undergoes branching morphogenesis to form the first T-shaped bud. In Six1−/− embryos, the UB grows out normally at E10.5, and its tip domain is initially formed as revealed by the expression of c-Ret (Fig. 1B). However, the mutant tip fails to swell and form an ampulla for branching morphogenesis (Fig. 1D,F), and the level of c-Ret expression in the tip region becomes largely reduced when compared to wild-type controls. In contrast, the distal trunk region continues to elongate in Six1−/− embryos (Fig. 1B,D,F,H). The lack of ampulla formation was further confirmed by staining with Emx2 and Gata3 at E10.75–11.25 (Fig. 1G,H and data not shown) and by tracing the ureteric epithelium expressing Hoxb7-GFP transgene (Srinivas et al., 1999) when the metanephric primordia were cultured in vitro (Fig. 1I). Thus, Six1 appears to have a specific role in inducing ampulla formation at the UB tip.
Fig. 1.
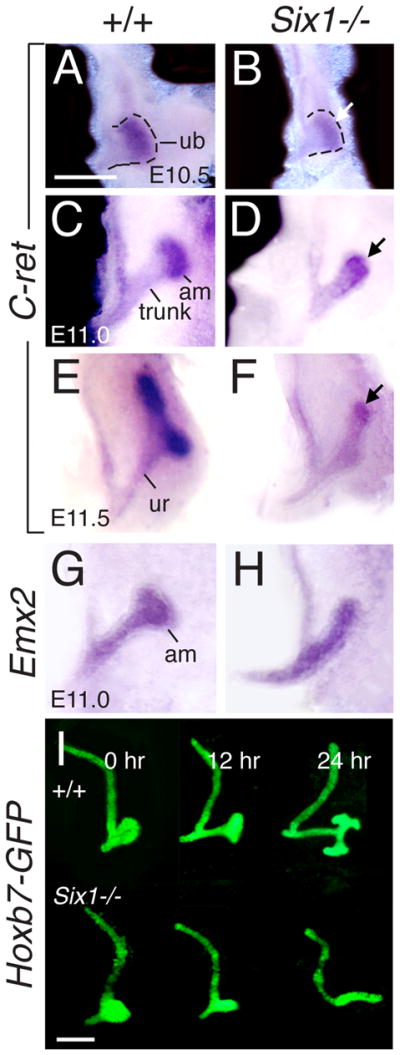
Six1−/− UB tip fails to form ampulla. (A–H) The metanephric region was dissected out from E10.5 (A,B), E11.0 (C,D,G,H) and E11.5 (E,F) embryos and stained for c-Ret or Emx2 by whole-mount in situ hybridization. (A) Normal UB outgrowth and the formation of tip domain as labeled by c-Ret expression. (C,E) The UB tip domain swells to form an ampulla, which undergo branching to the T-bud stage at E11.5 (E), while the trunk region elongates to form the ureter. (G) E11.0 metanephric region showing Emx2 expression in ureteric stalk and ampulla. (B, D, F, H) In Six1−/− embryos, the UB outgrowth, its subdivision into the trunk and tip domains and elongation appear to occur normally, but its tip region is not dilated for the formation of ampulla. (I) Kidney rudiments expressing Hoxb7-GFP transgene were dissected from E10.5 wild-type and Six1−/− embryos and cultured in vitro for 0, 12 or 24 hour, further confirming no ampulla formation in the mutant. Abb.: am, ampulla, ub, ureteric bud, ur, ureter. Scale bars: 100 μm in A–H; 50 μm in I.
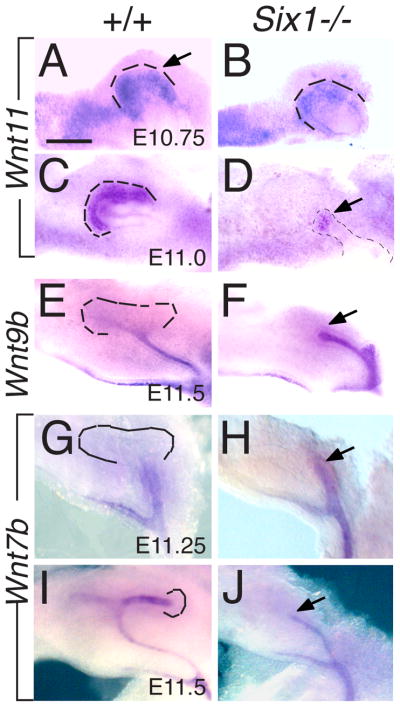
To further investigate this phenotype, we examined several other UB tip-specific markers. The Wnt family member Wnt11 is specifically expressed in the UB tips at all stages of ureteric development (Fig. 2A,C) and reciprocal interaction between Wnt11 and Gdnf/Ret regulates UB branching (Kispert et al., 1996; Majumdar et al., 2003). However, in Six1−/− embryos, Wnt11 expression in the tip region is reduced at E10.75 (Fig. 2B) and markedly reduced at E11.0 (Fig. 2D). We then tested whether in the absence of ampulla formation, the cells at the tip region gradually lose its tip-specificity by examining the expression of trunk-specific markers such as Wnt9b and Wnt7b. Wnt9b is expressed in the UB and its expression gradually becomes excluded from the tip domain (Fig. 2E) (Carroll et al., 2005). By contrast, in Six1−/− embryos, Wnt9b expression was detected throughout the ureteric epithelium at E11.25-11.5 (Fig. 2F and data not shown). At E11.25-11.5, Wnt7b is specifically expressed in the trunk region in normal embryos (Fig. 2G,I). However in Six1−/− embryos, Wnt7b expression was detected throughout the developing ureteric epithelium (Fig. 2H,J). Thus, our results indicate that in the absence of ampulla formation, the mutant UB tip eventually loses its tip-specificity as revealed by reduction of tip-specific gene expression but presence of trunk-specific gene expression at E11.25-11.5.
Fig. 2. Altered Wnt11 expression in Six1−/− UB tips.
The metanephric region was dissected out from E10.5–11.5 embryos and stained for Wnt11, Wnt9b or Wnt7b by whole-mount in situ hybridization. (A–D) Wnt11 expression in the most proximal tip domain at E10.75 (A,B) and E11.0 (C,D) in wild-type embryos and Six1−/− embryos. (E,F) Wnt9b expression in the WD and the ureteric stalk in controls and Six1−/− embryos at E11.25-11.5. (G–J) Wnt7b expression in WD and UB trunk region at E11.25 (I,J) and E11.5 (K,L) in control and Six1−/− embryos. Arrows in J and L point to the proximal end of ureteric epithelium. Dashed lines outline the UB tip regions. Scale bar: 50 μm in A–D; 80 μm in E–J.
Failure of the UB invasion into the MM in Six1−/− mice results in its elongation within Tbx18- and Bmp4-expressing mesenchyme
As the mesenchyme surrounding the different regions of the UB plays an instructive role in promoting epithelial differentiation (Brenner-Anantharam et al., 2007), we wished to investigate further if the lack of UB ampulla formation correlated with alterations in the cellular composition and activity of the mesenchyme surrounding the proximal UB tips. We therefore examined the expression of the mesenchymal markers that are important for ureter differentiation. Tbx18 is specifically expressed in the ureteral mesenchymal progenitors at around E11.25 and in the absence of Tbx18, ureteral mesenchymal progenitors fail to differentiate into ureter smooth muscle cells (SMCs) (Airik et al., 2006). Consistent with previous observations (Airik et al., 2006), in wild-type embryos, Tbx18 is strongly expressed in the ureteral mesenchyme at E11.25-E12.5 but is excluded from the MM at E11.25-11.5 (Fig. 3A,C,E). The UB normally invades the MM (Fig. 1A,C), which is located dorsally to the Tbx18-expressing mesenchyme, and undergoes branching morphogenesis to form the collecting duct system (Fig. 3A,C,E). However, in Six1−/− embryos, the UB tip is embedded in Tbx18-expressing mesenchyme and fails to invade the MM (Fig. 3B). Instead, the UB elongates within the Tbx18-expressing mesenchyme (Fig. 3D,F).
Fig. 3. Six1−/− UB elongates within Tbx18- and Bmp4-expressing mesenchyme.
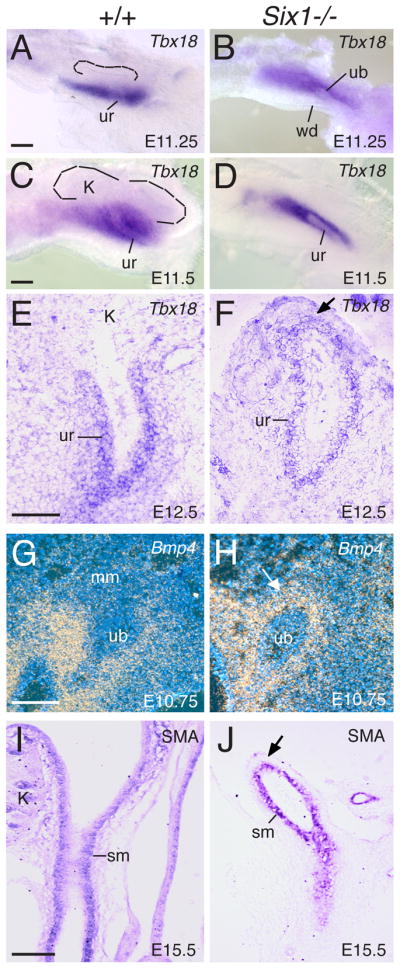
(A,C) Whole mount in situ hybridization of the metanephric region showing the strong Tbx18 expression domain surrounding the UB trunk region, which is ventral to the MM. Dashed lines point to UB branching within the MM. (B,D) In Six1−/− embryos, the elongating ureter is embedded within the Tbx18-expressing mesenchyme. (E,F) Section in situ at E12.5 showing Tbx18 expression surrounding the ureteral mesenchyme in control and Six1−/− embryos. Arrow points to the proximal end of the ureter in the mutant. (G,H) Radioisotope section in situ hybridization showing normal Bmp4 expression in the mesenchyme surrounding the ureteric stalk and cloaca but is absent in the metanephric mesenchyme in wild-type control (G), whereas in Six1−/− embryos, the UB tip is wrapped up by Bmp4-expressing mesenchyme (arrow). (I,J) Longitudinal sections showing SMA-positive cells surrounding the ureter in control and Six1−/− embryos at E15.5. Abb.: k, kidney, mm, metanephric mesenchyme, sm, smooth muscle, ub, ureteric bud, ur, ureter, wd, Wolffian duct. Scale bars: 50 μm in A-D; 100 μm in E–J.
In addition to Tbx18, BMP4 signaling has been shown to promote ureteric stalk elongation and inhibit branching morphogenesis (Brenner-Anantharam et al., 2007; Miyazaki et al., 2000). Bmp4 requires Tbx18 function to maintain its normal expression in the ureteral mesenchyme (Airik et al., 2006). At earlier stages, it is expressed in the mesenchyme surrounding the WD, nascent UB and ureteric stalk (Brenner-Anantharam et al., 2007; Michos et al., 2007). After UB outgrowth, Bmp4 is not expressed in the MM surrounding the UB tip (Fig. 3G). However, the tips of Six1−/− UBs at E10.5-11.5 during which the ampulla formation and its first branching event normally take place are surrounded by Bmp4-expressing mesenchyme (Fig. 3H). We also investigated Bmp4 expression at E12.0–15.5 and found that Bmp4-positive cells also surrounded the proximal end of the mutant ureters at these stages (data not shown). Ureteral smooth muscle cells (SMCs) in the mutant ureters were also detected at E15.5 by staining with anti-α-smooth muscle actin (SMA) antibody (Fig. 3I,J), an early marker for differentiating SMCs. Therefore, it appears that in the absence of ampulla formation in Six1−/− embryos, the UB tip cells are induced to differentiate into ureter by responding to signaling from the Tbx18- and Bmp4-expressing mesenchyme.
Grem1 expression in the MM is Six1-dependent
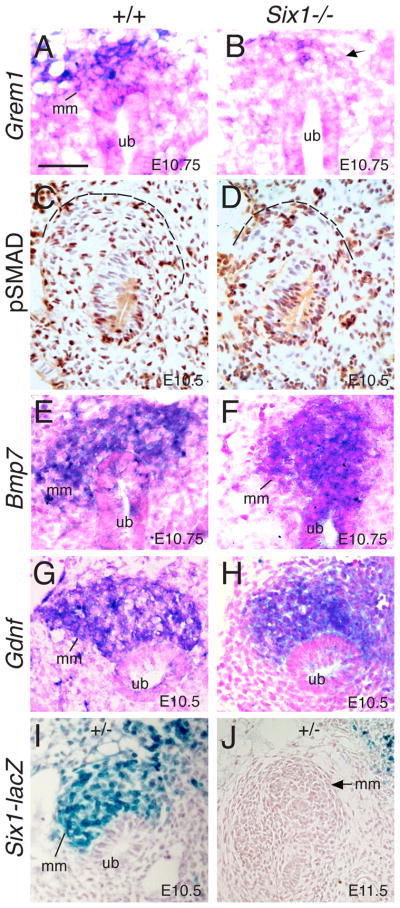
It was previously proposed that GREM1 in the MM antagonizes BMP4 activity to initiate branching by establishing an autoregulatory GDNF/WNT11 feedback signaling loop (Michos et al., 2007). We therefore set out to test whether there is an alteration in Grem1 expression in the MM of Six1−/− embryos. High level of Grem1 expression was detected in the mesenchyme surrounding the nascent UB tip at around E10.5–10.75 (Fig. 4A) (Michos et al., 2007). By contrast, in Six1−/− embryos, Grem1 expression was markedly reduced at E10.75 (Fig. 4B) and became undetectable at around E11.0 (data not shown). This observation raises the possibility that downregulation of Grem1 expression in the MM of Six1−/− embryos may lead to increase of mesenchymal BMP4 activity.
Fig. 4. Grem1 expression in the mesenchyme surrounding the UB tips is Six1-dependent.
(A,B) Section in situ with Grem1 probe showing its expression in the mesenchyme surrounding the UB tip in normal and Six1−/− embryos. (C,D) Immunohistochemistry for pSMAD on sections from kidney region of normal and Six1−/− embryos. pSMAD-positive nuclei (brown) are present in both the mesenchyme and the epithelium of wild-type and Six1−/− embryos. pSMAD-positive cells from five sections from each MM (three kidneys) were quantified as the ratio of the pSMAD-positive cells to the total number of metanephric mesenchymal cells in the control and mutant ureters. P-values (P=0.304) were calculated using StatView t-test. Dashed lines outline the peripheral boundary of the MM. (E,F) Section in situ showing Bmp7 expression in the MM and UB in normal and Six1−/− embryos at E10.75. (G,H) Section in situ showing Gdnf expression in the MM at E10.5 normal and Six1−/− embryos. Note that the mutant MM is not as well condensed as in controls. (I) A section stained by X-gal showing Six1 expression in the MM at E10.5, (J) but its expression in the MM disappears at E11.5. Abb.: mm, metanephric mesenchyme; ub, ureteric bud. Scale bars: 50 μm.
Since phosphorylated SMAD (pSMAD) proteins are established mediators of BMP signaling pathways (Massague et al., 2005), we tested whether the level of pSMAD expression in the mesenchyme is altered in the mutant by performing immunohistochemistry with an antibody that recognizes phosphorylated SMAD1/5/8. pSMAD-positive cells were apparently present in the UB and its surrounding mesenchyme including the MM in both wild-type and Six1−/− embryos at E10.5 (Fig. 4C,D and data not shown). We counted five sections from each kidney rudiment (n=3) and quantified the number of pSMAD-positive cells as the ratio of pSMAD-positive cells to the total number of metanephric mesenchymal cells in the control and mutant kidney primordial respectively. Although the total number of cells in the mutant MM is reduced to ~86% of wild-type mesenchymal cells, the percentage of pSMAD-positive cells vs total number of cells in the MM is comparable between wild-type (51.1±7) and mutant (52.2±9) embryos.
The presence of a fraction of pSMAD-positive cells in the MM of wild-type embryos indicates that those cells receive BMP signals. As Bmp7 is known to be expressed in both the MM and the UB (Fig. 4E), we analyzed its expression in Six1−/− embryos. At around E10.5–10.75, Bmp7 expression was detectable in the MM but the level of its expression appeared to be decreased in Six1−/− embryos (Fig. 4F) when compared to wild-type controls (Fig. 4E). However, as a recent report has shown that BMP7 acts through a SMAD-independent signaling mechanism in nephrogenic progenitor cells (Blank et al., 2009), reduction of BMP7 expression in Six1−/− MM may not affect the levels of pSMAD expression.
We found that Six1 is specifically expressed in the MM (Fig. 4I) (Xu et al., 2003), but its expression is transient and disappears soon after the first epithelial branching event (Fig. 4J). Therefore, Six1 may play a critical role in initiating branching morphogenesis by modulating Grem1 expression in the MM. To further evaluate the nature of the mutant MM, in addition to Bmp7, we analyzed a number of additional MM-specific markers such as Gdnf, Eya1 and Wt1. Consistent with our previous observations (Xu et al., 2003), we found that the levels of Eya1, Wt1 and Bmp7 expression were normal at E10.5 just before the initiation of UB outgrowth (data not shown). The levels of Gdnf expression also appeared to be normal at around E10.5, before UB outgrowth (Fig. 4G,H). It should be noted that the mutant MM appears smaller and not as well condensed as in wild-type controls at E10.5 and E10.75 (Fig. 4F,H, compare with 4E,G). It ultimately degenerates by apoptosis, which was observed from E11.5 (Xu et al., 2003). Thus, while the MM appears morphologically abnormal from E10.5, the clear reduction of Grem1 expression in Six1−/− MM indicates that it may act as a critical player in the Six1-regulatory pathway during the initiation of branching morphogenesis.
Exogenous GREM1 restores branching morphogenesis in Six1−/− kidney primordia
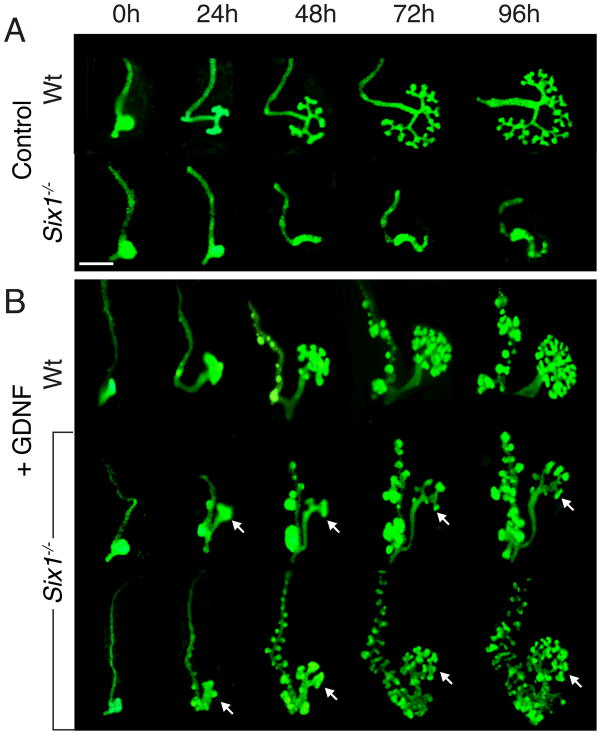
Since exogenous GDNF can rescue branching morphogenesis in Grem1−/− kidney rudiments (Michos et al., 2007), we first tested the responsiveness of the mutant ureteric epithelium to exogenous GDNF by treating Six1−/− kidney primordia at different stages with different concentrations of GNDF (100–250 ng/ml). Our results show that the efficiency of exogenous GNDF rescue varies depending upon the GDNF concentration and developmental stages. Addition of GDNF at a higher dose (~200 ng/ml) most efficiently restored branching morphogenesis as well as small kidney formation in Six1−/− kidney rudiments at E10.5 (n=15/15) (Fig. 5). Massive epithelial overgrowth and branching were induced along the entire WD in the mutant, similar to what occurred in wild-type (Michos et al., 2007; Shakya et al., 2005). However, GDNF failed to effectively induce UB branching and kidney formation of Six1−/− kidney rudiments after E11.25 (data not shown). Very few epithelial budding from the UB tips of the mutant rudiments at around E11.25-11.5 was only observed when GDNF concentration was increased to ~250 ng/ml (data not shown). No kidney formation was observed, and the MM degenerated completely (data not shown). However, as the epithelium of Six1−/− kidney rudiments at E10.5, before the onset of cell apoptosis that occurs in the MM after E11.0 (Xu et al., 2003), is able to respond to GDNF to undergo branching morphogenesis, the failure of branching morphogenesis in Six1−/− embryos is not caused by an intrinsic defect in the epithelium but rather by defects in mesenchyme-epithelial signaling.
Fig. 5. Excessive GDNF is sufficient to restore not only UB branching but also tubulogenesis of the mesenchyme in Six1-deficient kidney primordia in culture.
(A) Wild-type and Six1-deficient kidney primordia cultured in control medium showing normal UB outgrowth and branching and no branching in Six1−/− rudiments. (B) Control and Six1−/− kidney primordia at E10.5 were cultured in the presence of GDNF at ~200 ng/ml, many epithelial buds along the WD and overgrowth of the ureteric epithelium within 24 hours, which results in excessive branching in both wild-type and Six1-deficient kidney primordia. Note the branching and the small kidneys in GDNF-treated Six1-deficient kidney primordia. Scale bars: 100 μm.
We next performed kidney culture experiment to examine the ability of exogenous GREM1 in rescuing Six1−/− UB morphogenesis. E10.5 wild-type and Six1−/− kidney primordia expressing the Hoxb7-GFP transgene were cultured for up to 96 hours in the presence or absence of soluble recombinant GREM1 protein. The development of Six1−/− kidney primordia arrested at the UB stage and the UB elongated into ureter when cultured in medium without GREM1 protein (Fig. 1I, n=13/16). Previous studies have shown that GREM1 is able to induce ectopic bud formation but is less effective in wild-type kidney rudiments than in Grem1−/− samples (Michos et al., 2007). Consistent with the previous observation, we found that GREM1 (2–6 μg/ml) was less effective in inducing ectopic UB outgrowth than GDNF and the ectopic buds that underwent two or four branching events by 96 hours were only formed adjacent to the metanephric kidney region (data not shown). Ureteric epithelial tracings of the GREM1-treated Six1−/− kidney primordia revealed that the UB tip was dilated to form an ampulla (n=10/10) but only less than half of them proceeded to the first branching event (n=3/10; data not shown).
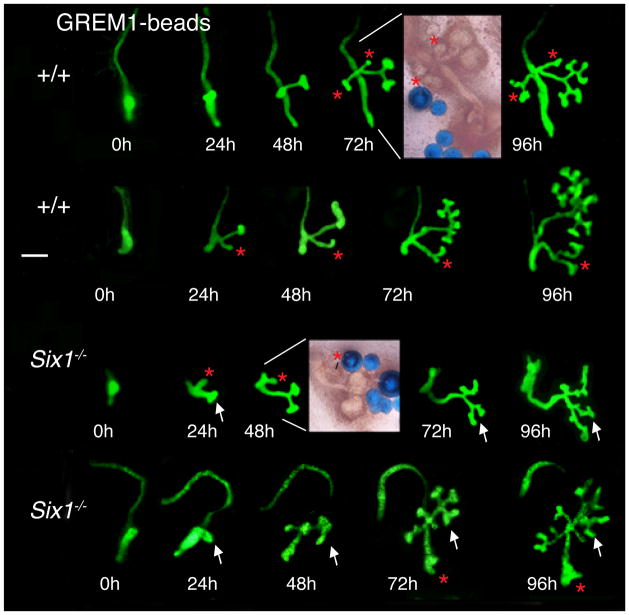
Our results raise the possibility that UB branching in Six1−/− embryos may require locally concentrated GREM1 or additional mesenchymal factors, which are downregulated or missing in Six1−/− MM. To address this, microdissected wild-type and E10.5 Six1−/− kidney rudiments were implanted with GREM1-soaked beads (1.5 μg/μl). Wild-type kidney rudiments showed ectopic bud formation (red stars, Fig. 6; n=12/12), and majority of them were able to undergo one or more branching (n=10/12). The ectopic buds that formed are all close to the metanephric kidney region. Interestingly, Six1−/− rudiments also showed the UB outgrowth from its normal position (white arrows, Fig. 6; n=6/6) and the formation of ectopic buds (red asterisks, Fig. 6; n=6/6). The UBs observed in Six1−/− rudiments showed ampulla formation at the tip of the bud that was able to undergo several branching events (arrows, Fig. 6), which was not observed when GREM1 was added into the culture medium (data not shown). No ectopic UB induction and branching were observed when Six1−/− rudiments were cultured with beads containing BSA (data not shown). This result establishes that recombinant GREM1 is able to restore epithelial branching morphogenesis in the absence of Six1.
Fig. 6. Recombinant GREM1 protein is able to induce ampulla formation and branching morphogenesis in Six1−/− kidney primordia.
Wild-type and Six1−/− kidney primordia expressing the Hoxb7-GFP transgene in their WD and ureteric epithelium were isolated from mouse embryos at E10.5–11.0, implanted with GREM1-soaked beads and cultured for up to 96 hours. Panels show from left to right: cultures at 0 hours, 24 hours, 48 hours, 72 hours and 96 hours (time: ±2–3 hours). White asterisks indicate ureteric buds, red asterisks indicate ectopic epithelial buds and ectopic branches. Note the branching and the small kidneys in Six1-deficient kidney primordia (white arrows). Scale bar: 100 μm.
We further analyzed the responsiveness of the epithelium and mesenchyme in Six1−/− kidney primordia by staining with Wnt11, Gdnf and Wt1. The expression of all three markers was detectable in the mutant samples (Fig. 7), although the kidney rudiments appeared to be smaller when compared with controls. This indicates that recombinant GREM1 reconstitutes mesenchyme-epithelial signaling necessary for branching morphogenesis and nephron differentiation. These results show that Grem1 is a critical mesenchymal regulator downstream of Six1 in inducing the initiation of UB branching morphogenesis.
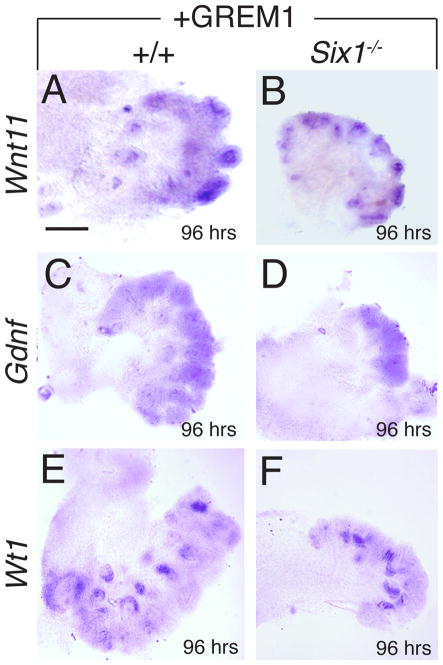
Fig. 7. Recombinant GREM1 is able to restore Wnt11 and Gdnf expression in Six1-deficient kidney rudiments in culture.
Mouse kidney primordia were isolated at E10. 5–11.0, cultured for 96 hours in the presence of recombinant GREM1-soaked beads and then whole-mount stained with Wnt11 (A,B), Gdnf (C,D) and Wt1 (E,F) probes. Obvious Wnt11, Gdnf, and Wt1 expression was observed in the mutant rudiments implanted with GREM1-soaked beads and culture for 96 hours. Scale bars: 25 μm.
Genetic reduction of BMP4 activity in Six1−/− embryos rescues kidney organogenesis
Since BMP4 promotes ureter differentiation in zones of the UB normally fated to differentiate into collecting tubules in kidney explant cultures (Brenner-Anantharam et al., 2007; Wang et al., 2009), and GREM1 acts as an antagonist to negatively regulate BMP4 activity in the mesenchyme to initiate UB outgrowth and invasion into the mesenchyme (Michos et al., 2007), we further tested the hypothesis that excessive mesenchymal BMP4 activity blocks the UB tip cells from forming an ampulla in Six1−/− embryos by examining Six1;Bmp4 compound mutant embryos. Because of early lethality of Bmp4−/− embryos (Winnier et al., 1995), we tested whether inactivation of one copy of the Bmp4 gene in Six1−/− (Six1−/−; Bmp4+/−) embryos restores ampulla formation and its branching morphogenesis. In Six1−/−;Bmp4+/− mutant embryos, ampulla formation and its branching were initiated normally at around E11.25-11.5 (Fig. 8A–D and data not shown). Kidneys did indeed form bilaterally in Six1−/−;Bmp4+/− embryos at E17.5 (6 out of 6) but were smaller (Fig. 8G,H) when compared with normal kidneys (Fig. 7E,F). In contrast, among Six1−/−;Bmp4+/+ littermate controls examined (n=9), ~78% (7 out of 9) had no kidneys, while ~22% (2 out of 9) showed unilateral rudimentary kidneys (data not shown). Histological and marker analyses of kidneys confirmed the presence of kidney structures in Six1−/−;Bmp4+/− embryos (Fig. 8I–L). Ureteric epithelial tracing of Hoxb7-GFP of Six1−/−;Bmp4+/− kidneys revealed that branching morphogenesis was restored (Fig. 8M,N). This demonstrates that the nephrogenic progenitors in the Six1−/−;Bmp4+/− mutant are able to induce UB branching and undergo nephrogenesis. In addition to the kidney, hydroureter phenotype was observed in Six1−/−;Bmp4+/− animals and the mice die at the end of gestation probably because of multiple defects in several organ systems, as Six1−/− mice also lack thymus, parathyroid, ear and olfactory epithelium as well as exhibiting severe defects in craniofacial, muscle and sensory nervous systems (Chen et al., 2009; Laclef et al., 2003a; Laclef et al., 2003b; Xu et al., 2003; Zheng et al., 2003). Taken together, these results demonstrate that the genetic reduction of BMP4 activity in Six1-deficient embryos restores UB patterning and metanephric kidney organogenesis (Fig. 8F,H), thus providing the first evidence for the requirement of Six1 in spatially restricting BMP4 signaling during UB morphogenesis.
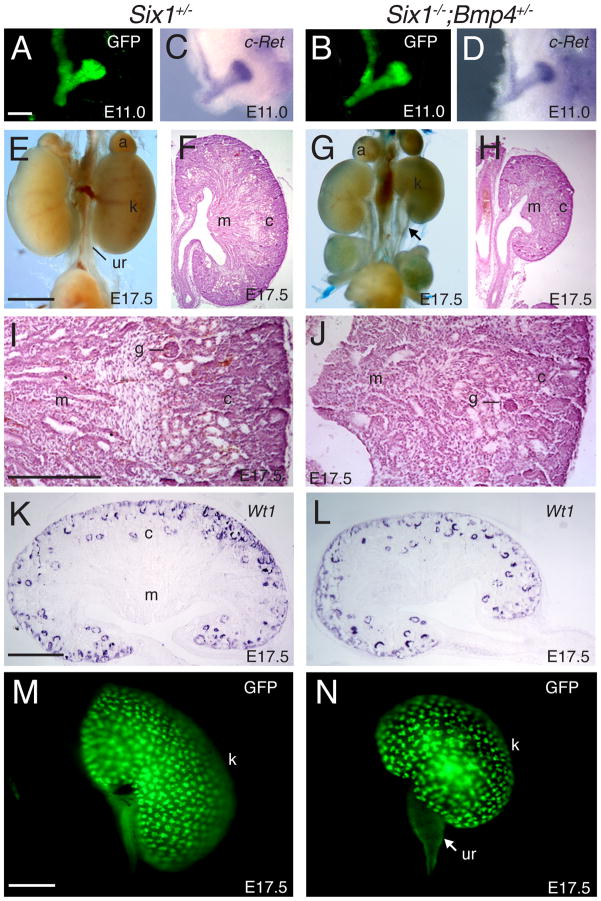
Fig. 8. Inactivation of one copy of the Bmp4 gene in Six1−/− embryos results in formation of two small kidneys.
(A,C) GFP reveals the morphology of the epithelium before fixation and (B,D) whole-mount in situ detection for the expression of the Ret receptor in control and Six1−/−;Bmp4+/− embryos at E11.25. (E,G) Gross morphology of urogenital system and (F,H) H&E staining of histological sections of kidneys of Six1+/− control (E,F) and Six1−/−;Bmp4+/− (G,H) embryos at E17.5. (I,J) Higher magnification of boxed area of F and H respectively. Developing kidney structures such as glomeruli, tubules and collecting ducts are present in the compound mutant (J). However, the patterning of the medulla (m) and cortex (c) appeared abnormal. (K,L) Kidney section of control and Six1−/−;Bmp4+/− kidney stained with Wt1 probe showing the developing nephrons. (M,N) Epithelial tracing of Hoxb7-GFP of E17.5 kidney in control and Six1−/−;Bmp4+/− animals. Arrow points to hydroureter phenotype in the compound mutant. Abb.: a, adrenal gland; c, cortex; g, glomeruli; k, kidney; m, medulla; ur, ureter. Scale bars: 50 μm in A–N; 25 μm in I,J.
Discussion
Although many mesenchymal genes have been implicated in regulating the formation of ampulla at the UB tip, the regulatory relationship among these genes and the cellular and molecular mechanisms controlling the initiation of branching morphogenesis remain largely unknown. A recent study has shown that GREM1 is necessary to negatively regulate BMP4 signaling for establishing mesenchyme-epithelial signaling during UB branching morphogenesis (Michos et al., 2007). However, how the GREM1-BMP4 signaling is regulated during the initiation of UB branching is currently unknown. This study is the first to demonstrate that Six1 functions as an upstream regulator of Grem1 to locally restrict and negatively regulate BMP4 activity in the mesenchyme during the initiation of branching morphogenesis. Moreover, we show that reduction of Bmp4 dosage in Six1−/− embryos restores nephron differentiation.
Previous studies have suggested that initiation of UB outgrowth and invasion of the mesenchyme requires antagonism of BMP4 by the extracellular BMP antagonist GREM1 in the mesenchyme (Michos et al., 2007; Michos et al., 2004). Our analyses show that the disruption of UB outgrowth in Six1−/− embryos appears less severe than that observed in Grem1−/− embryos. In Grem1−/− embryos, no proper ureteric tip domain is formed and the ureteric stalk does not elongate but degenerates soon after its outgrowth (Michos et al., 2007). However, in Six1−/− embryos, the UB tip domain is initially formed and the UB elongation also occurs normally. This agrees with the fact that Grem1 is initially expressed in Six1−/− kidneys. In contrast to the UB development, the expression of Gdnf in the MM is progressively lost, which is likely caused by gradual elimination of the MM because of lack of epithelial-mesenchymal interactions (Xu et al., 2003). This phenotype is similar to that observed in Grem1−/− embryos (Michos et al., 2007; Michos et al., 2004). UB invasion into the MM is blocked in both the Grem1- and Six1-deficient embryos (Figs 1–3 of the present study; (Michos et al., 2007). It has been shown that Grem1 is only expressed in the WD at E10.0, and its expression becomes evident in the mesenchyme surrounding the UB tip region at around E11.0 (Michos et al., 2007; Michos et al., 2004). Consistent with this observation, we detected Grem1 expression in the MM from around E10.75 (Fig. 4). In contrast, Six1 is expressed earlier than Grem1 in the MM but its expression disappears soon after the initiation of the first branching event at around E11.5 (Fig. 4). This expression pattern of Six1 further indicates that it may play a key role for the MM to induce initiation of epithelial branching morphogenesis. Our in vivo ChIP analysis performed on embryonic MM extracts has demonstrated direct binding of SIX1 to a region of the Grem1 promoter containing conserved MEF3 consensus sites, which are recognized by Six family protein (Giordani et al., 2007; Spitz et al., 1998) (Elaine Y-W Wong, Feng Wang and P-X. Xu, unpublished). This suggests that SIX1 exerts its action via a direct interaction with the Grem1 promoter to specifically upregulate its transcription in the metanephric mesenchymal cells at this critical early stage. This upregulation may be required to locally repress BMP4 activity in the mesenchyme to ensure that UB tip is induced for ampulla formation. This explains why exogenous GREM1 is able to restore ampulla formation and its subsequent branching events in Six1−/− kidney primordia. We are currently testing the requirement of each individual binding site for the upregulation of Grem1 promoter activity by Six1 in vitro.
Our observation that the UB tip of Six1−/− embryos is wrapped up by the Tbx18- and Bmp4-expressing mesenchyme suggests that the block of UB ampulla formation at the UB tip may be caused by responding to the signaling in the Tbx18- and Bmp4-expressing mesenchyme, which are cellular sources for ureteral smooth muscle (Airik et al., 2006; Wang et al., 2009). Our recent observation that reduction of one-copy of the Tbx18 gene dosage in Six1−/− (Tbx18+/−;Six1−/−) embryos, which show reduced Bmp4 expression in the ureteral mesenchyme, also rescues UB branching morphogenesis and kidney formation (Nie et al., 2010) further indicates that reducing BMP4 activity in the Tbx18-expressing mesenchyme surrounding the UB tip of Six1−/− embryos reconstitutes mesenchyme-epithelial signaling crosstalk to enable branching morphogenesis and nephron formation. As previous lineage-tracing study has shown that Bmp4+ cells are derived from tail-bud mesenchyme (Brenner-Anantharam et al., 2007) and there is no evidence that Grem1 is expressed in the same cell lineage as Bmp4, GREM1 is less likely to directly repress BMP4 activity in the same cells. Although lineage tracing of Grem1-expressing cells and colocalization studies are important to address this issue, GREM1 may act as an extracellular signaling molecule to locally antagonize BMP4 activity in the Bmp4-expressing mesenchyme (Fig. 9), which would normally act on the UB epithelium to induce its differentiation into the ureter (Brenner-Anantharam et al., 2007). It is possible that the BMP4 signaling may repress Wnt11 expression in the UB tip, which normally signals back to the mesenchyme either directly or indirectly to upregulate the expression of Gdnf in the MM. Nonetheless, GREM1 is likely to play an indirect role in establishing the WNT11-GDNF signaling to an optimal level for branching morphogenesis to occur in Six1−/− kidney primordia.
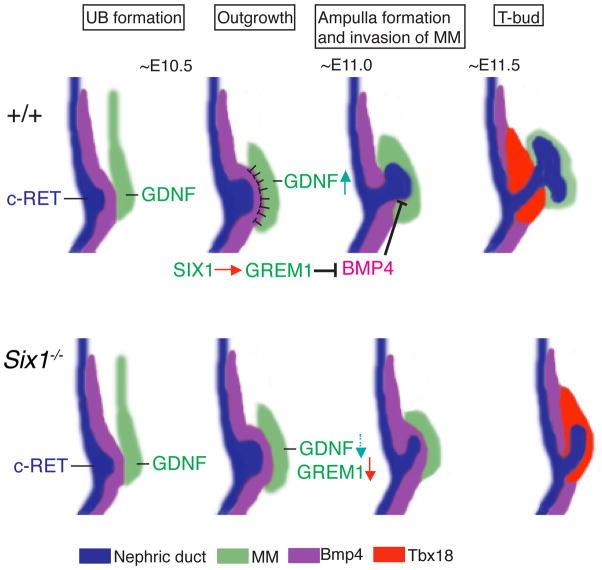
Fig. 9. Upregulation of Grem1 by Six1 in the MM is essential to locally restrict BMP4 signaling for the initiation of branching morphogenesis.
In mice, the UB formation and outgrowth is induced by GDNF-RET signaling, which is initially expressed at normal levels in Six1−/− embryos. During this inductive period, Six1 is specifically expressed in the MM (green). Bmp4 (purple) is expressed by the mesenchyme enveloping the WD (blue) and nascent UB (blue). Grem1 transcripts are detected in the MM around the tip region of the UB, thereby locally antagonizing BMP4 in the Bmp4-expressing mesenchyme (purple) (around E10.75-11.0). This reduction of BMP4 activity by GREM1 establishes epithelial-mesenchymal interactions to enable ampulla formation of the UB and its invasion into the MM. Gdnf expression is also upregulated during this period to stimulate ampulla formation and establish WNT11-GDNF signaling for branching of the ampulla. Our results indicate that Six1 plays an essential role in lowering BMP4 activity by upregulating Grem1 expression.
In Six1−/− embryos, the UB outgrowth is normal but the tip cells fail to be induced for ampulla. Instead, the UB tip is wrapped up by Tbx18- and Bmp4-expressing mesenchyme, which is the cellular source for ureteral mesenchyme (Airik et al., 2006). By responding to the signaling in the Tbx18- and Bmp4-positive mesenchyme, the tip is induced for ureter differentiation. Our results show that the balance between the levels of BMP4 activity in the Tbx18+ and Bmp4+ mesenchyme and GDNF production in the MM may also be critical for UB patterning, as excessive GDNF can restore branching as well as kidney formation in Six1−/− kidney rudiments in culture and lowering BMP4 activity in vivo can rescue branching morphogenesis and nephron formation.
Recent studies have suggested that BMP4 in the mesenchyme may utilize ActRIIA and its co-receptor Dragon, both are highly expressed in the ureteric stalk, for signaling (Samad et al., 2005; Xia et al.); however the downstream pathway that mediates the function of BMP4/Dragon/ActRIIA remains to be determined. In Grem1−/− embryos, BMP4 signaling transduction in the MM appears to be increased because the levels of pSMAD expression are increased in the MM of Grem1−/− embryos (Michos et al., 2007). However, this does not appear to occur in Six1−/− MM, as we failed to detect obvious changes in the levels of pSMAD expression (Fig. 4). Since BMPs are known to activate both SMAD1/5/8 and mitogen-activated protein kinase (MAPK) p38, JNKs and Erk1/2 pathways (Derynck and Zhang, 2003; Yu et al., 2008), it is possible that BMP4 may utilize SMAD-dependent and SMAD-independent pathways as recently demonstrated for BMP7 in the nephron progenitor cells (Blank et al., 2009).
Our analysis also shows that the balance between the levels of BMP4 activity in the Bmp4-expressing mesenchyme surrounding the nascent UB tip and GDNF production specifically in the MM appears to be crucial for UB patterning. When the BMP4 activity is lowered in the Tbx18- or Bmp4-expressing mesenchyme enveloping the UB tip of Six1−/− embryos, the level of GDNF produced in the MM becomes sufficient to induce the UB tip cells to proliferate and initiate branching (Fig. 9). Alternatively, when excessive GDNF is added in culture medium, it becomes sufficient to restore branching morphogenesis in Grem1−/− (Michos et al., 2007) or Six1−/− kidney rudiments at earlier stages (Fig. 5). Thus, BMP4 and GDNF may antagonize each other’s effect in inducing ampulla formation. In support of this, BMP4 has been shown to suppress the effects of GDNF in inducing ectopic buds in vitro (Brophy et al., 2001). As Gdnf expression appears to be normal in rescued Six1−/− kidney primordia, Six1 is unlikely to play a direct role in modulating Gdnf expression in the MM during early epithelial branching.
In summary, our results presented here identify Six1 as an upstream regulator of Grem1 in the MM, which locally restricts BMP4 signaling in the mesenchyme surrounding the nascent UB (Fig. 9). Our findings that genetically lowering BMP4 activity in Six1−/− mice restores UB branching and kidney organogenesis in vivo provide strong evidence that Six1 is indeed required for spatially restricting BMP4 activity to ensure ampulla formation and its invasion into the MM during kidney development. After the initiation of branching morphogenesis, Six1 is no longer expressed in the MM (Fig. 4), it therefore seems to be only required for the initiation of branching morphogenesis, but not for repeated branching events to occur and for the maintenance of Grem1 expression in the MM.
Acknowledgments
We thank F. Costantini at Columbia University for kindly letting us use the Hoxb7-GFP mutant mice and technical assistance in setting up kidney organ culture, B. Hogan at Duke University for providing the Bmp4loxp-lacZ mice, and A. Zuniga at University of Basel Medical School for the Grem1 in situ probe. This work was supported by NIH RO1 DK64640 (P-X. X.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Airik R, Bussen M, Singh MK, Petry M, Kispert A. Tbx18 regulates the development of the ureteral mesenchyme. J Clin Invest. 2006;116:663–74. doi: 10.1172/JCI26027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airik R, Kispert A. Down the tube of obstructive nephropathies: the importance of tissue interactions during ureter development. Kidney Int. 2007;72:1459–67. doi: 10.1038/sj.ki.5002589. [DOI] [PubMed] [Google Scholar]
- al-Awqati Q, Goldberg MR. Architectural patterns in branching morphogenesis in the kidney. Kidney Int. 1998;54:1832–42. doi: 10.1046/j.1523-1755.1998.00196.x. [DOI] [PubMed] [Google Scholar]
- Blank U, Brown A, Adams DC, Karolak MJ, Oxburgh L. BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development. 2009;136:3557–66. doi: 10.1242/dev.036335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner-Anantharam A, Cebrian C, Guillaume R, Hurtado R, Sun TT, Herzlinger D. Tailbud-derived mesenchyme promotes urinary tract segmentation via BMP4 signaling. Development. 2007;134:1967–75. doi: 10.1242/dev.004234. [DOI] [PubMed] [Google Scholar]
- Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development. 2001;128:4747–56. doi: 10.1242/dev.128.23.4747. [DOI] [PubMed] [Google Scholar]
- Bush KT, Vaughn DA, Li X, Rosenfeld MG, Rose DW, Mendoza SA, Nigam SK. Development and differentiation of the ureteric bud into the ureter in the absence of a kidney collecting system. Dev Biol. 2006;298:571–84. doi: 10.1016/j.ydbio.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Carroll TJ, Park JS, Hayashi S, Majumdar A, McMahon AP. Wnt9b plays a central role in the regulation of mesenchymal to epithelial transitions underlying organogenesis of the mammalian urogenital system. Dev Cell. 2005;9:283–92. doi: 10.1016/j.devcel.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Chen B, Kim EH, Xu PX. Initiation of olfactory placode development and neurogenesis is blocked in mice lacking both Six1 and Six4. Dev Biol. 2009;326:75–85. doi: 10.1016/j.ydbio.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation. 2006;74:402–21. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- Davies JA, Fisher CE. Genes and proteins in renal development. Exp Nephrol. 2002;10:102–13. doi: 10.1159/000049905. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- Giordani J, Bajard L, Demignon J, Daubas P, Buckingham M, Maire P. Six proteins regulate the activation of Myf5 expression in embryonic mouse limbs. Proc Natl Acad Sci U S A. 2007;104:11310–5. doi: 10.1073/pnas.0611299104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu DR, Economides AN, Wang X, Eimon PM, Harland RM. The Xenopus dorsalizing factor Gremlin identifies a novel family of secreted proteins that antagonize BMP activities. Mol Cell. 1998;1:673–83. doi: 10.1016/s1097-2765(00)80067-2. [DOI] [PubMed] [Google Scholar]
- Kispert A, Vainio S, Shen L, Rowitch DH, McMahon AP. Proteoglycans are required for maintenance of Wnt-11 expression in the ureter tips. Development. 1996;122:3627–37. doi: 10.1242/dev.122.11.3627. [DOI] [PubMed] [Google Scholar]
- Kulessa H, Hogan BL. Generation of a loxP flanked bmp4loxP-lacZ allele marked by conditional lacZ expression. Genesis. 2002;32:66–8. doi: 10.1002/gene.10032.abs. [DOI] [PubMed] [Google Scholar]
- Laclef C, Hamard G, Demignon J, Souil E, Houbron C, Maire P. Altered myogenesis in Six1-deficient mice. Development. 2003a;130:2239–52. doi: 10.1242/dev.00440. [DOI] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six 1 deficient mice. Mech Dev. 2003b;120:669–79. doi: 10.1016/s0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–54. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Vainio S, Kispert A, McMahon J, McMahon AP. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development. 2003;130:3175–85. doi: 10.1242/dev.00520. [DOI] [PubMed] [Google Scholar]
- Massague J, Seoane J, Wotton D. Smad transcription factors. Genes Dev. 2005;19:2783–810. doi: 10.1101/gad.1350705. [DOI] [PubMed] [Google Scholar]
- Michos O, Goncalves A, Lopez-Rios J, Tiecke E, Naillat F, Beier K, Galli A, Vainio S, Zeller R. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development. 2007;134:2397–405. doi: 10.1242/dev.02861. [DOI] [PubMed] [Google Scholar]
- Michos O, Panman L, Vintersten K, Beier K, Zeller R, Zuniga A. Gremlin-mediated BMP antagonism induces the epithelial-mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development. 2004;131:3401–10. doi: 10.1242/dev.01251. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–73. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie X, Sun J, Gordon RE, Cai CL, Xu PX. SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation. Development. doi: 10.1242/dev.045757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM, Jr, Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, Hildebrandt F. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101:8090–5. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajithlal G, Zou D, Silvius D, Xu PX. Eya 1 acts as a critical regulator for specifying the metanephric mesenchyme. Dev Biol. 2005;284:323–36. doi: 10.1016/j.ydbio.2005.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad TA, Rebbapragada A, Bell E, Zhang Y, Sidis Y, Jeong SJ, Campagna JA, Perusini S, Fabrizio DA, Schneyer AL, Lin HY, Brivanlou AH, Attisano L, Woolf CJ. DRAGON, a bone morphogenetic protein co-receptor. J Biol Chem. 2005;280:14122–9. doi: 10.1074/jbc.M410034200. [DOI] [PubMed] [Google Scholar]
- Saxen L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol. 1987;1:385–92. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–1. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya R, Watanabe T, Costantini F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev Cell. 2005;8:65–74. doi: 10.1016/j.devcel.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, Maire P. Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci U S A. 1998;95:14220–5. doi: 10.1073/pnas.95.24.14220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Goldberg MR, Watanabe T, D’Agati V, al-Awqati Q, Costantini F. Expression of green fluorescent protein in the ureteric bud of transgenic mice: a new tool for the analysis of ureteric bud morphogenesis. Dev Genet. 1999;24:241–51. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<241::AID-DVG7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Vainio S, Lin Y. Coordinating early kidney development: lessons from gene targeting. Nat Rev Genet. 2002;3:533–43. doi: 10.1038/nrg842. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Brenner-Anantharam A, Vaughan ED, Herzlinger D. Antagonism of BMP4 signaling disrupts smooth muscle investment of the ureter and ureteropelvic junction. J Urol. 2009;181:401–7. doi: 10.1016/j.juro.2008.08.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–16. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Xia Y, Babitt JL, Bouley R, Zhang Y, Da Silva N, Chen S, Zhuang Z, Samad TA, Brenner GJ, Anderson JL, Hong CC, Schneyer AL, Brown D, Lin HY. Dragon enhances BMP signaling and increases transepithelial resistance in kidney epithelial cells. J Am Soc Nephrol. 21:666–77. doi: 10.1681/ASN.2009050511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development. 2003;130:3085–94. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]