Abstract
Intensive weight-supported treadmill training (WSTT)improves locomotor function following spinal cord injury. Because of a number of factors, undergoing intensive sessions of training may not be feasible. Whether reduced amounts of training are sufficient to enhance spinal plasticity to a level that is necessary for improving function is not known. The focus of the present study was to assess differences in recovery of locomotor function and spinal plasticity as a function of the amount of steps taken during WSTT in a rodent model of spinal cord injury. Rats were spinally transected at 5 days of age. When they reached 28 days of age, a robotic system was used to implement a weight-supported treadmill training program of either 100 or 1000 steps/training session daily for 4 weeks. Antibodies for brain-derived neurotrophic factor (BDNF), TrkB, and the pre-synaptic marker, synaptophysin, were used to examine the expression of these proteins in the ventral horn of the lumbar spinal cord. Rats that received weight-supported treadmill training performed better stepping relative to untrained rats, but only the rats that received 1000 steps/training session recovered locomotor function that resembled normal patterns. Only the rats that received 1000 steps/training session recovered normal levels of synaptophysin immunoreactivity around motor neurons. Weight-supported treadmill training consisting of either 100 or 1000 steps/training session increased BDNF immunoreactivity in the ventral horn of the lumbar spinal cord. TrkB expression in the ventral horn was not affected by spinal cord transection or weight-supported treadmill training. Synaptophysin expression, but not BDNF or TrkB expression was correlated with the recovery of stepping function. These findings suggested that a large amount of weight-supported treadmill training was necessary for restoring synaptic connections to motor neurons within the locomotor generating circuitry. Although a large amount of training was best for recovery, small amounts of training were associated with incremental gains in function and increased BDNF levels.
Key words: growth factors, rehabilitation, spinal cord injury
Introduction
Weight-supported treadmill training (WSTT) is a type of gait-training therapy that has been used successfully to improve walking following spinal cord injury (Wernig et al., 1995) and stroke (Sullivan et al., 2007). WSTT improved stepping by enhancing plasticity within neural circuits that control walking (Edgerton et al., 2004). Ideally, individuals would undergo an intensive WSTT regimen for optimal recovery, but several factors place practical limits on how much manual (therapist-based) WSTT therapy an individual may receive. For example, WSTT for individuals with complete spinal cord injury is labor intensive and physically demanding, often requiring the work of four therapists to assist leg and trunk movements. From the patient perspective, the cost and the time commitment associated with an intensive WSTT therapy may be too great and may prevent the patient from receiving therapy. For these reasons, it may be best to undergo a reduced amount of WSTT therapy particularly if the reduced amount was sufficient to trigger plasticity and achieve locomotor recovery. An important but unanswered question is “can any gains in function and plasticity be achieved in association with a reduced amount of WSTT?”
We previously compared the effects of imposing different amounts of WSTT in a rodent model of spinal cord injury. We used a robotic system to impose a low amount of WSTT (100 steps/day) versus a high amount of WSTT (1000 steps/day) in adult rats that were transected as neonates (Cha et al., 2007). The robotic system controlled weight bearing on the hindimbs and counted the number of steps that were generated in the hindlimbs during training (Cha et al., 2007). We found that functional recovery was greater in the rats that received the larger amount of WSTT. However, untrained rats were not included in the study. Therefore, it was unknown if the smaller amount of WSTT had any functional benefits relative to the absence of training. Moreover, biochemical and anatomical changes in the spinal cord were not examined in our previous study; therefore, the extent to which the different amounts of WSTT impacted the spinal cord circuitry was not known. A recent study has shown that the amount of synaptic inputs to motor neurons was greater in spinally transected (ST) rats that received daily treadmill training for 4 weeks (16–24 min/training session) relative to untrained ST rats (Macias et al., 2009). In addition, the ratio of excitatory to inhibitory synapses was increased in trained relative to untrained ST rats (Ichiyama et al., 2011). These findings suggested that synapses onto motor neurons were enhanced by treadmill training. However, no studies to date have investigated the effects of different amounts of step training on synaptic plasticity in the lumbar spinal cord. Therefore, it is unknown if small amounts of WSTT were as effective as large amounts of WSTT in enhancing synaptic plasticity within the spinal circuitry.
In the present study, we examined the effects of imposing different amounts of WSTT on locomotor recovery and spinal plasticity in rats that were spinally transected as neonates. To study plasticity, we examined the expression of the presynaptic marker, synaptophysin, the neurotrophin, brain-derived neurotrophic factor (BDNF), and its receptor, TrkB. WSTT has been shown to increase the expression of the presynaptic marker, synaptophysin, around large ventral horn motor neurons of ST rats and this was associated with improved stepping function (Macias et al., 2009). Hindlimb exercise also increased the expression of BDNF and TrkB, within the lumbar spinal cord after injury (Beaumont et al., 2008; Hutchinson et al., 2004; Ying et al., 2005). These findings suggested that hindlimb exercise promoted synaptic plasticity within the locomotor-generating circuitry and that this may have been triggered by the actions of BDNF. We hypothesized that imposing a greater amount of WSTT would have more beneficial effects on functional recovery and synaptic plasticity than imposing a lesser amount of WSTT. Our findings suggested that implementing a large amount of WSTT was critical for maximizing locomotor recovery and restoring normal amounts of synaptic connections to motor neurons following spinal cord injury. However, smaller gains in function and enhanced expression of BDNF were achieved by a small amount of WSTT. These findings have implications regarding the implementation of effective locomotor training therapies after spinal cord injury.
Methods
Research design
A total of 24 female Sprague Dawley rats received a complete mid-thoracic spinal cord transection at 5 days of age as previously described (Cha et al., 2007). Following surgery, the pups were returned to the mothers and weaned at 21 days of age. The bladders and colons of the rats were checked daily. After weaning (28 days old), baseline tests of stepping were performed using a robotic treadmill system (de Leon and Acosta, 2006). The ST rats were distributed into three experimental training groups that were balanced according to their locomotor performance during the baseline tests. One group (n=8) received daily WSTT that consisted of performing 100 steps/session whereas another group (n=8) performed 1000 steps/ training session. The third group was untrained (n=8). All training was performed 5 days/week for 4 weeks. Stepping in the ST rats was re-tested after the last day of training. Testing and data collection for a group of intact controls was also performed (n=8). The ST rats and intact controls underwent transcardiac perfusion, and the spinal cords removed and processed for immunhistochemical detection of BDNF, TrkB, and synaptophysin. All procedures with rats were performed in accordance with NIH guidelines and the protocols were approved by the Institutional Animal Care and Use Committee at California State University, Los Angeles.
Robotic treadmill training and testing
A commercially-available robotic device (Rodent Robot 3000, Robomedica Inc.) was used to train and test treadmill stepping in the rats as previously described (Cha et al., 2007). Two robotic arms were attached to the ankles while a third arm raised the rat's body above the treadmill, thereby controlling the amount of weight on the hindlimbs (90–85% body weight support). The robotic device did not assist movements in the hindlimbs and only counted the number of steps during training (Cha et al., 2007). A training session was considered completed when the total number of steps performed by both hindlimbs was 100 or 1000 steps. Typically, the duration of training episodes was 5 min and 20 min for the 100 and 1000 step groups, respectively. During testing, unassisted ankle movements were recorded by the robotic device for 30 sec (8 cm/sc, 85% weight support) and the data were stored on a computer for subsequent analysis (Cha et al., 2007). Briefly, the start and end for each step cycle were identified based on horizontal and vertical velocities of the robotic arm. Step height was defined as the distance between the maximum and minimum vertical positions of the ankle during a step. Step length was defined as the horizontal distance traveled during forward ankle movement. Swing and stance duration were the intervals of time during forward and backward movement, respectively. Measurement of the height of the ankle during swing was used to distinguish weight-bearing steps from non–weight-bearing (i.e., dragging) steps as previously described (Heng and de Leon, 2009). Steps in which the ankle was raised >10 mm were defined as weight-bearing steps. The percentage of weight-bearing steps was calculated by dividing the number of weight-bearing steps by the total number of step cycles detected by the robotic arms (× 100%).
Immunohistochemical experiments
One day after the final training session, the ST rats and a group of intact controls (n=8) were anesthetized with isofluorane and then underwent transcardiac perfusion (4% paraformaldehyde) and the spinal cords were dissected, blocked and cut into 30-μm sections as previously described (Tillakaratne et al., 2002). Four immunohistochemical experiments were performed. One experiment was performed with BDNF antibody only and another experiment was performed with TrkB antibody only. A third experiment was performed with two antibodies, BDNF and NeuN (a neuronal marker). A fourth experiment involved two antibodies, one for synaptophysin and one for HSP27. HSP27 was used to label motor neurons in the ventral horn (Plumier et al., 1997). For each experiment, the tissue sections from all groups were processed simultaneously in a single experiment. The conditions for all procedures were identical for all the sections in every group. For the experiments with two antibodies, the spinal cord sections (L2–L5; 3–5 sections/rat) were washed, then transferred to a blocking solution of 3% normal donkey serum (NDS). Spinal cord sections were then transferred into 96-well plates and incubated overnight with one primary antibody (i.e., mouse synaptophysin antibody 1:300 or mouse BDNF antibody 1:500) followed by an overnight incubation with the other primary antibody ( goat HSP27 antibody 1:500 or goat NeuN antibody 1:500). Afterwards, the spinal sections were washed, blocked in 3% NDS then incubated for 1 h in anti-goat secondary antibody (Rhodamine, 1:200) and anti-mouse secondary antibody (FITC 1:200). Single labeling experiments with BDNF and TrkB antibodies followed the same protocol (BDNF 1:500; TrkB 1:300; FITC 1:200) but only one primary and secondary antibody were used. The spinal cord sections were transferred to glass slides, air dried, and coverslipped with VECTASHIELD® with DAPI staining.
Microscopic imaging and analyses
For analyses of synaptophysin labeling, microscopic images were acquired using an Olympus FluoView™ FV500 confocal laser scanning microscope (100×magnification). All the images were acquired on 1 day under the same conditions and settings. Images of HSP27-positive motor neurons in lamina IX were acquired. Five motor neurons for each rat were chosen based on size, a well-defined cell boundary, and a clearly visible nucleus. Six optical slices (5-μm thick) were scanned through each motor neuron. We determined from pilot experiments that 5-μm thickness was ideal. Synaptophysin labeling was found primarily on the perimeter of motor neurons. Simple PCI 6 software (Compix, Inc. PA) was used to analyze synaptophysin labeling based on a technique developed by Macias and associates for analyzing synaptophysin around motor neurons (Macias et al., 2009). Using the elliptical object drawing tool, an outline was generated that extended just beyond the perimeter of HSP27-labeled motor neurons. The area of synaptophysin labeling 2 μm beyond and 2 μm inside the perimeter of the motor neuron was measured after background signal was eliminated (based on a control section with secondary antibody only). The total area (summation of areas from each optical slice) was then calculated.
For BDNF and TrkB measurements in the ventral horn, microscopic images were acquired using C-Imaging under Leica DMLA microscope equipped with a Hamamatsu Digital color camera. Images were acquired on a single day under the same conditions and settings. All analyses were performed using the Simple PCI 6 software (Compix, Inc. PA). An elliptical region of interest was generated around lamina IX. The area of BDNF or TrkB expression was measured within this region of interest after background signal was eliminated. The same region of interest size was maintained for all rats in all groups. To measure BDNF labeling in neurons only, the software was used to detect cells with NeuN labeling within the region of interest in lamina IX. The intensity of BDNF label within all NeuN positive objects was then measured. Because the area of labeled objects was defined by the NeuN labeling, measurement of the area of BDNF labeling could not be performed for this analysis.
Statistical analysis
A two sample Kolmogorov-Smirnov test was used to determine if the distribution of weight- bearing steps was different among groups. One-way ANOVA was used to determine significant differences in kinematic characteristics (i.e., step length, height, swing duration, stance duration) and differences in BDNF, synaptophysin, and TrkB expression among groups. Tukey post-hoc test was used to determine significant differences between pairs. Statistical analyses of stepping data was performed in all rats, n=8 per group. Because of tissue damage occurring during the immunohistochemical procedures, the tissue from some rats could not be included in the analyses for BDNF, TrkB and synaptophysin. For the BDNF experiment, the number of rats from the 0, 100, and 1000 step groups were 5, 6, and 5 rats respectively. For the TrkB experiment, the number of rats from the 0, 100, and 1000 step groups was 5, 5, and 6 rats respectively. For the synaptophysin experiment, the number of rats from the 0, 100, and 1000 step groups was 5 for each group. For the BDNF and NeuN double-labeling experiment, the number of rats from the 0, 100, and 1000 step groups was 3, 3, and 4 respectively. Tissue from 5 intact rats was used in all experiments. To analyze the relationships between the stepping and immunohistochemical data, a Pearson's correlation coefficient was calculated. The number of weight-bearing steps performed during the final test for each rat in the 0, 100, and 1000 step group was correlated with their levels of synaptophysin label aound motor neurons and the expression of BDNF and TrkB label in the ventral horn. A second correlation was performed between the number of steps imposed during the 4 weeks of training and the expression of synaptophysin, BDNF, and TrkB label. For this analyses, 0 steps was used for each rat in the 0 step group whereas the number of steps in the trained rats was based on 100 or 1000 steps/training session for 20 training sessions. All statistics were performed using SPSS 17.0 for Windows software.
Results
Recovery of locomotor function was dependent upon the amount of stepping imposed during WSTT
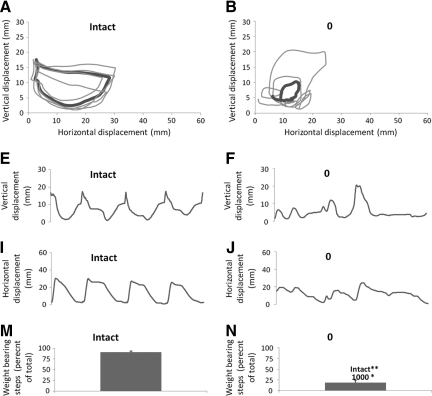
Figure 1 compares ankle movements recorded by the robotic system during stepping on the treadmill in representative Intact and ST rats. The Intact rats performed consistent, weight-bearing steps evident by the consistent shape of the ankle trajectories (Fig. 1A) and regular pattern of vertical and horizontal movements in the ankle (Fig. 1 E, I). In contrast, stepping in the untrained group of rats (will be referred to as 0 rats) was erratic and the ankle trajectories were irregular (Fig. 1B). Most of the vertical and horizontal movements of the ankle were small in the 0 rats (Fig. 1 F, J) which is an indication of non–weight-bearing steps and dragging of the hindpaws on the treadmill belt.
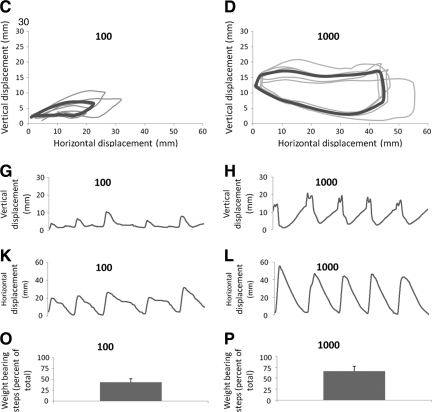
FIG. 1.
Stepping characteristics in intact and ST rats. Ankle movements during 4–5 steps recorded by the robotic device are shown for representative rats from the intact (A), 0 (B), 100 (C) and 1000 (D) groups. The thin lines are individual steps and the thick line is the average of the individual steps. Vertical and horizontal displacement of the ankle during the same steps is shown below the trajectory plots (E–L). Bar graphs of the percentage of weight-bearing steps are shown for the intact (M), 0 (N), 100 (O), and 1000 (P) rats. The bars represent the average for each group (n=8/group). Error bars are standard errors of the mean. Significant differences between pairs are indicated by group names on the bars. * and ** denote significantly different at p<0.05 and p<0.01 respectively.
The ST rats that received only 100 steps/training session (100 rats) performed steps that had more consistent ankle trajectories than the trajectories in the 0 rats (compare Fig. 1B and C). Horizontal movements in the 100 rats appeared larger than in the 0 rats, although the vertical movement amplitudes were similar between the groups (compare Fig. 1 F, J with G, K). The best stepping patterns among the ST rats were observed in the rats that had received 1000 steps/training session (1000 rats). The 1000 rats performed consistent, weight-bearing stepping evident by the regular ankle trajectory shape and large vertical and horizontal movements (Fig. 1D, H, L).
To quantify locomotor performance during testing, the total number of step cycles (weight-bearing + non–weight-bearing, dragging steps) was counted and the percentage of weight-bearing steps relative to the total number of step cycles was calculated. On average, 91% of the step cycles in the Intact rats were weight-bearing steps (Fig. 1M; intact rats occasionally resisted testing in the robotic device and this accounted for a small percentage of non–weight-bearing, dragging steps). In contrast, only 17% of the step cycles in the 0 rats were weight bearing (Fig. 1N). The 100 rats performed more (43%) weight-bearing steps, than the 0 rats (Fig. 1O). The best stepping in the ST rats was observed in the 1000 rats in which 66% of the step cycles were weight bearing (Fig. 1P). Statistical analyses showed that the mean percentage of weight-bearing steps among the groups was significantly different (one-way ANOVA, p<0.001). Pairwise comparison revealed significant differences between the 0 and intact rats and between the 0 and the 1000 rats (Fig. 1N).
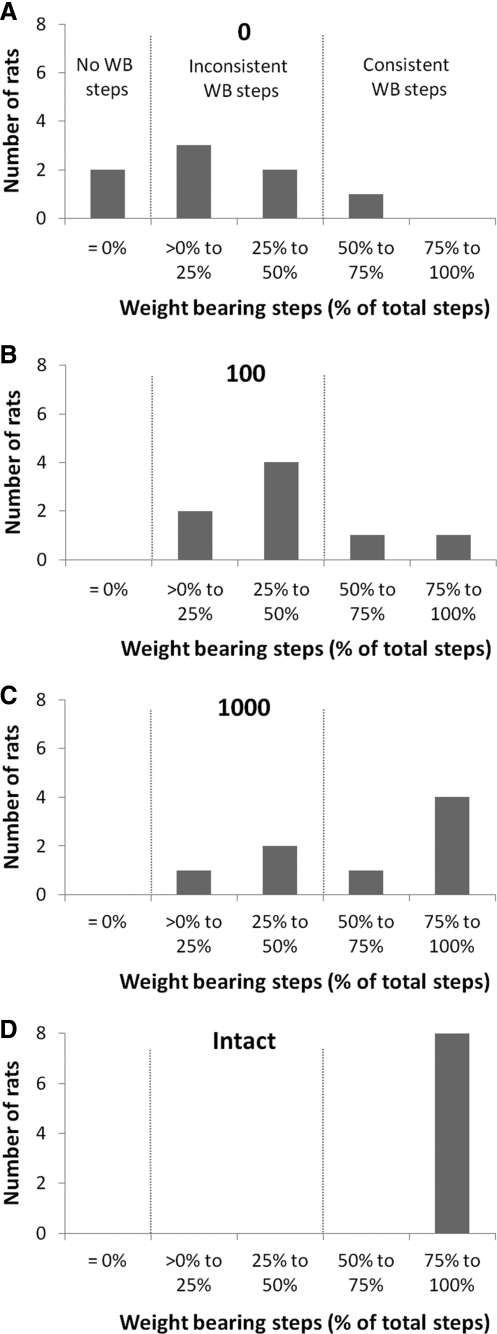
Figure 2 compares the distribution of weight-bearing steps among the groups. Inconsistent weight-bearing stepping (i.e., defined as <50% of all step cycles being weight bearing) was observed in most of the 0 rats, and two of the 0 rats failed to generate any weight-bearing steps at all (0% of all step cycles were weight bearing; Fig. 2A). Similarly, most of the 100 rats (six of the eight) could only perform inconsistent weight-bearing stepping (Fig. 2B). Distributions in the 1000 and intact rats indicated that more consistent stepping occurred in these rats. Five of the eight 1000 rats and all eight intact rats generated consistent weight- bearing steps (i.e., >50% of all step cycles were weight bearing; Fig. 2C, D). Statistical analyses revealed that the distributions for the 0 and 100 rats were significantly different than the distribution for the intact rats (two sample Kolmogorov-Smirnov test, p<0.001 for 0 vs intact rats and p<0.01 for the 100 vs the intact rats). Only the distribution of weight-bearing steps in the 1000 rats was not significantly different from the distribution in the intact rats.
FIG. 2.
Distribution of weight-bearing steps in 0 (A), 100 (B), 1000 (C), and intact (D) rats. The number of weight-bearing steps was counted in each rat (see Methods) and then expressed as a percentage of total step cycles recorded by the robotic device during locomotor testing. Consistent stepping is defined as >50% of all step cycles being weight bearing, inconsistent stepping is defined as >0% to 50% of all step cycles being weight bearing and no weight-bearing steps is defined as 0% of all step cycles being weight bearing.
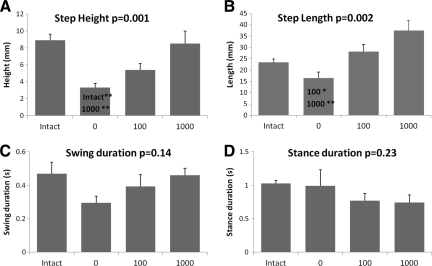
Generally, the size of the step cycles was smallest in the 0 rats, followed by the 100 rats, and the greatest step cycle sizes were found in the 1000 rats (see Fig. 1B, C, D). Kinematic analyses of the step cycle trajectories recorded by the robotic device confirmed these observations. For example, the lowest values for mean step height were found in the 0 rats, followed by the 100 rats (Fig. 3A). The 1000 rats had the greatest step height values of the ST rats and this was similar to the Intact step height values (Fig. 3A). A similar pattern, i.e., lowest values in 0 rats, followed by 100 and 1000 rats, was observed for step length and swing duration (Fig. 3B, C). Statistical analyses revealed that the average step length and step height were significantly different among the groups (one-way ANOVA, p<0.01 and p<0.01 respectively, Fig. 3A, B). Pairwise comparison showed that the 0 rats had step height values that were significantly lower than those of the Intact and 1000 rats (Fig. 3A) and that the 0 rats had significantly shorter steps than 100 and 1000 rats (Fig. 3B). No significant differences in swing and stance duration were found among the groups (Fig. 3D).
FIG. 3.
Bar graphs of mean step height (A), step length (B), swing duration (C), and stance duration (D) are shown. The bars represent the average for each group (n=8/group). Error bars are standard errors of the mean. The p values from one-way ANOVA are shown next to the plot titles. Significant differences between pairs are indicated by group names on the bars. * and ** denote significantly different at p<0.05 and p<0.01 respectively.
Synaptophysin expression around motor neurons was restored only in ST rats that received 1000 steps/training session
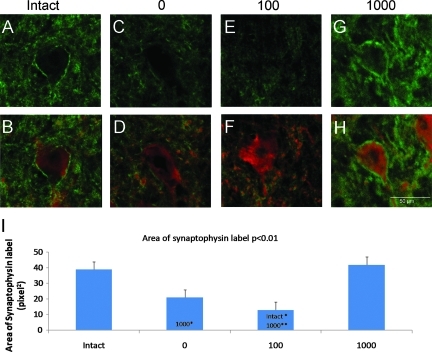
Figure 4 shows synaptophysin labeling around HSP27-labeled motor neurons in representative intact and ST rats. In intact rats, strong synaptophysin label was observed on the periphery of the motor neurons (Fig. 4A, B). Strong synaptophysin labeling was also observed around the periphery of motor neurons in the 1000 rats and this was similar to the labeling pattern observed in the Intact rats (compare Fig. 4G,H to A,B). In contrast, less synaptophysin labeling was found around the motor neurons in the 0 and 100 rats (Fig. 4C, D; E, F).
FIG. 4.
Synaptophysin expression around HSP27 labeled motor neurons in representative intact (A,B), 0 step (C,D), 100 steps (E,F), and 1000 steps (G,H) rats. The top rows show synaptophysin (green) labeling only (A, C, E, G) whereas the bottom row shows synaptophysin (green) + HSP27 labeling (red) (B,D,F,H). The images are from single optical slices acquired via confocal microscope. The plot shows area of synaptophysin labeling (I). The bars represent the average for each group (n=5/group). Error bars are standard errors of the mean. The p value from one-way ANOVA is shown next to the plot title. Significant differences between pairs are indicated by group names on the bars. * and ** denote significantly different at p<0.05 and p<0.01 respectively.
Analyses of the area of synaptophysin labeling around motor neurons revealed significant differences among the four groups of rats (one-way ANOVA, p<0.01; Fig. 4I). Pairwise comparisons showed no significant difference in the area of synaptophysin label between intact and 1000 rats (compare intact and 1000 in Fig. 4I). The area of synaptophysin label in the intact group was significantly greater than in the 100 group (compare intact and 100 in Fig. 4I) and there was a trend toward a significant difference between the intact and 0 group (p=0.09; compare intact and 0 in Fig. 4I). The area of synaptophysin in the 1000 group was significantly greater than in both the 0 and 100 groups (compare 1000 with 0, 100 in Fig. 4I).
BDNF but not TrkB expression in the ventral horn was enhanced in rats that received either 100 or 1000 steps/training session
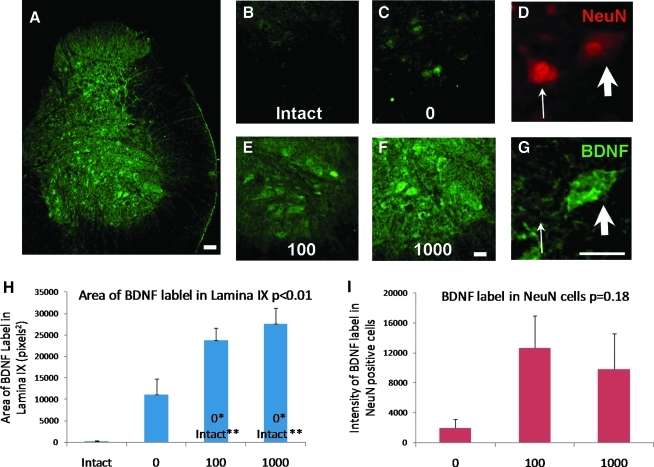
We performed immunohistochemical experiments to determine if WSTT affected BDNF and TrkB protein expression in the lumbar spinal cord of the ST rats. BDNF immunoreactivity was observed throughout the gray matter (Fig. 5A) which is consistent with previous reports (Macias et al., 2007). We focused on BDNF expression in the ventral horn based on the findings from previous studies that indicated treadmill training selectively increased BDNF expression in ventral horn cells (Macias et al., 2009). The lowest levels of BDNF immunoreactivity were observed in the ventral horns of intact rats and after correcting for background staining, little BDNF immunoreactivity was observed (Fig. 5B). In the 0 rats, BDNF immunoreactivity was observed, mostly in cells scattered throughout the ventral horn (Fig. 5C). Greater BDNF immunoreactivity was observed in the 100 and 1000 rats. BDNF immunoreactivity was found in large ventral horn cells in addition to diffuse staining in the gray matter (Fig. 5E, F). Double labeling of BDNF and the neuronal marker, NeuN, indicate that BDNF immunoreactivity was present in some large neurons (see arrowheads in Fig. 5D,G). However, not all neurons in the ventral horn expressed BDNF (see arrows in Fig. 5D,G). Statistical analyses revealed that the area of BDNF labeling in lamina IX was significantly different among the groups (one-way ANOVA, p<0.01, Fig. 5H). Pairwise comparisons showed that BDNF immunoreactivity was significantly greater in the 100 and 1000 rats than in the intact and 0 rats (Fig. 5H). A subset of animals in the 0, 100, and 1000 step groups was used to analyze neuronal expression of BDNF in the ventral horn. When BDNF was measured in NeuN-positive cells only, a greater amount of BDNF labeling was found in the 100 and 1000 rats relative to the 0 rats (Fig. 5I). However, this difference was not significant (one-way ANOVA, p=0.18).
FIG. 5.
BDNF expression in the lumbar spinal cord of a 1000 rat (A) and in the ventral horn of the lumbar spinal cord in representative rats from the intact (B), 0 (C), 100 (E), and 1000 (F) groups. BDNF staining in the images was corrected for background staining. NeuN positive cells in the ventral horn are shown in (D). BDNF labeling in these same cells is shown in (G). The arrowheads and arrows depict corresponding cells in (D) and (G). Plot of the area of BDNF labeling is shown (H). The bars represent the average for each group (n=5, 6, and 5 for the 0, 100, and 1000 groups respectively). Error bars are standard errors of the mean. The p value from one-way ANOVA is shown next to the plot title. Significant differences between pairs are indicated by group names on the bars. * and ** denote significantly different at p<0.0.5 and p<0.01 respectively. Plot of the intensity of BDNF labeling in NeuN positive cells is shown in (I). The bars represent the average for each group (n=3, 3, and 4 for the 0, 100, and 1000 groups respectively). Error bars are standard errors of the mean. The p value from one-way ANOVA is shown next to the plot title.
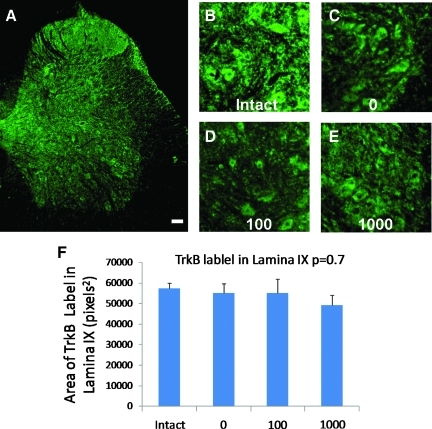
TrkB immunoreactivity was found throughout the gray matter but also in some areas of the white matter and this was consistent with previous reports (Macias et al., 2007). TrkB immunoreactivity was found in ventral horn cells of the intact and ST rats (Fig. 6B–E). Statistical analyses revealed no significant differences in TrkB expression in the ventral horn among the groups (Fig. 6F).
FIG. 6.
TrkB expression in the lumbar spinal cord of a 100 rat (A) and in the ventral horn of the lumbar spinal cord in representative rats from the intact (B), 0 (C), 100 (D), and 1000 (E) groups. TrkB staining in the images were corrected for background staining. Plot of the area of TrkB labeling is shown (F). The bars represent the average for each group (n=5, 5, and 6 for the 0, 100, and 1000 groups respectively). Error bars are standard errors of the mean. The p value from one-way ANOVA is shown next to the plot title. Significant differences between pairs are indicated by group names on the bars.
Synpatophysin and BDNF expression were correlated with stepping recovery and number of steps during training
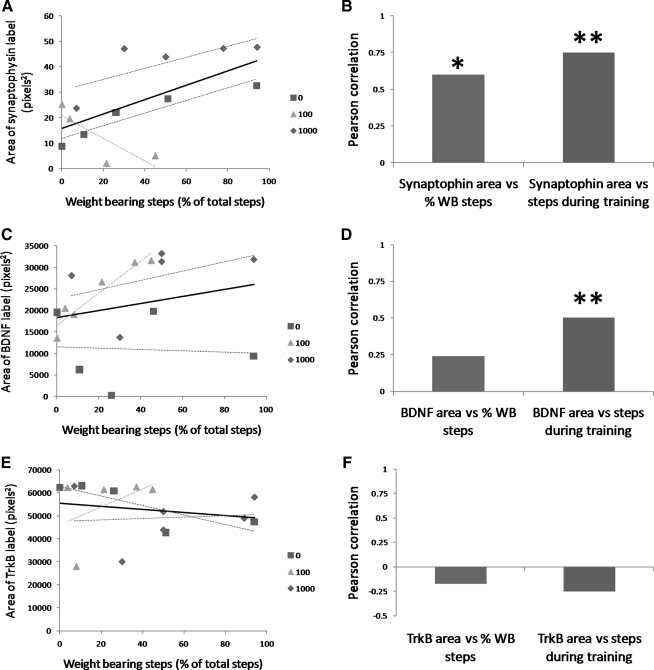
We next performed an analysis to examine how the levels of synaptophysin, BDNF, and TrkB expression were correlated with their recovery of weight-bearing stepping in each of the ST rats. Figure 7 shows the relationship between immunolabel around motor neurons and the percentage of weight-bearing steps. Linear regressions are shown for all of the ST rats (i.e., 0, 100, and 1000 rats; see black line in Fig. 7A, C, E) and for each group (see red, green, and blue dashed lines for the 0, 100, and 1000 groups respectively in Fig. 7A, C, E). When the data from all rats were combined, the area of synaptophysin label around motor neurons was positively correlated with the recovery of weight-bearing stepping and this correlation was statistically significant (see synaptophysin area versus % weight-bearing steps in Fig. 7B). The area of BDNF label in the ventral horn was also positively correlated with the recovery of weight-bearing stepping (Fig. 7C), but this correlation was not significant (see BDNF area versus % weight-bearing steps in Fig. 7D). There was slight negative correlation between TrkB label in the ventral horn and stepping recovery (Fig. 7E) but this was not significant (see TrkB area versus % weight-bearing steps in Fig. 7F). When correlations within each group were examined, only the correlations in the 100 rats between synaptophysin label and the percentage of weight-bearing steps and between BDNF label and the percentage of weight-bearing steps was significant (see green dashed line in Fig. 7A, C).
FIG. 7.
Scatterplots show the relationship between weight-bearing stepping recovery and synaptophysin labeling around motor neurons (A), BDNF labeling in the ventral horn (C), and TrkB labeling in the ventral horn (E). The square, triangle, and diamond symbols represent data from individual rats in the 0, 100, and 1000 groups respectively. The dashed lines are for the data points for the 0, 100, and 1000 groups. The solid black line is for the combined data points from all groups. Stepping recovery was measured as the percentage of steps that were weight bearing during 30 sec of testing. The Pearson correlation coefficients are shown for the relationships between stepping and synaptophysin label (B), BDNF label (D), and TrkB label (F). In these plots, the bar on the left is the correlation between synaptophysin, BDNF, or TrkB label and weight-bearing steps performed during testing. The bar on the right is the correlation between label and total number of steps imposed during 4 weeks of training. Combined data fromall on the right groups were used in these plots. * and ** indicate significant relationships at the p<0.05 and p<0.01 levels.
A second correlation analysis was performed to examine how the expression of synaptophysin, BDNF, and TrkB was correlated with the number of steps imposed during the 4 weeks of training. For this analyses, 0 steps was used for each rat in the 0 group whereas the number of steps in the trained rats was based on 100 or 1000 steps/training session for 20 training sessions. Both the area of synaptophysin around motor neurons and BDNF label in the ventral horn were positively correlated with the number of steps imposed during training and these correlations were highly significant (see synaptophysin and BDNF area vs steps during training in Fig. 7B, D). In contrast, TrkB label in the ventral horn and number of training steps were not significantly correlated (see TrkB area vs steps during training in Fig. 7F).
Discussion
In the present study, we used a robotic system to impose different amounts of WSTT on the hindlimbs of rats that received spinal cord transections as neonates. We found that a large amount of WSTT (1000 steps/training session) improved weight-bearing stepping on the treadmill, restored normal levels of synaptophysin immunoreactivity around motor neurons, and increased BDNF immunoreactivity in the ventral horn. A small amount of WSTT (100 steps/training session) also improved stepping and increased BDNF immunoreactivity, but failed to restore synaptophysin immunoreactivity.
WSTT and locomotor recovery in ST animals
WSTT improves locomotor recovery in animals following complete spinal cord injury and one of the main effects of WSTT was to increase the number of weight-bearing steps performed in the hindlimbs (Belanger et al., 1996; de Leon et al., 1998; Lovely et al., 1986). In the present study, a fourfold increase in weight-bearing steps was observed in the rats that received the large amount of WSTT relative to untrained rats, and this improvement was in line with previous studies of WSTT in neonatal ST rats (Cha et al., 2007; Kubasak et al., 2005; Petruska et al., 2007). Imposing a small amount of WSTT resulted in a twofold increase in the number of weight-bearing steps, but fewer of these rats were capable of consistent weight-bearing stepping compared to rats that received the higher number of steps/training session. Thus, some gains in function were achieved with the small amount of WSTT. However, the chance of recovering weight-bearing stepping was increased with more training. These findings were consistent with the hypothesis that the recovery of stepping after complete spinal cord injury was an activity-dependent process (Edgerton et al., 1997). WSTT stimulated activity that induced plasticity within the locomotor-generating circuitry. Based on the present findings, the levels of activity imposed on the lumbar spinal cord circuitry were crucial. If an insufficient amount of activity was generated during WSTT, the likelihood that the lumbar spinal cord circuitry would acquire the ability to generate consistent weight-bearing stepping was reduced.
One of the interesting findings in the present study was that different amounts of WSTT produced incremental changes in step cycle size. Step cycles were smallest in the untrained rats, followed by the 100 rats, and the largest step cycles were in the in the 1000 rats. A number of studies have shown that WSTT enhanced step cycle size in ST animals, and the result was a stepping pattern that had more normal kinematic characteristics (Belanger et al., 1996; de Leon et al., 1998; Lovely et al., 1990). It was evident from the present findings that the quality of the step cycles was dependent upon how much WSTT the ST rats received. More WSTT resulted in the generation of stepping patterns that were closer to the normal stepping patterns. Some aspects of stepping (trajectory shape, step length), however, remained abnormal even in the rats receiving the greatest amount of WSTT. The inability of WSTT to completely restore normal stepping patterns may be related to a ceiling effect rather than to an inadequate amount of training. This was supported by previous reports that deficits in stepping persisted in ST animals even when WSTT was performed over an extended period of time (Barbeau and Rossignol, 1987; Hodgson et al., 1994). These findings suggested that there were fundamental differences in spinally-mediated locomotion versus locomotion that was generated by the intact nervous system.
In order to facilitate WSTT, neonatal ST rats were used in the present study. Neonatal ST rats recover reflexive hindlimb function better than rats that received spinal transections as adults (Weber and Stelzner, 1977). Approximately 25% of neonatally transected rats spontaneously recover weight-bearing stepping (Giszter et al., 1998). This may explain why some rats in the 0 group were capable of weight-bearing stepping. The use of the neonatal ST rats also raised the possibility that reflexive hindlimb movements in the cage influenced locomotor recovery. Cage activity was not monitored and therefore it was unclear how much this factor contributed to overall functional recovery. However, we believe that the likelihood that this hindlimb activity enhanced the recovery of weight-bearing stepping was low, for two reasons. First, untrained rats in the present study recovered the poorest locomotion function and this finding was consistent with other studies of WSTT in neonatal ST rats (Kubasak et al., 2005; Petruska et al., 2007). Second, effective WSTT requires the generation of precisely timed patterns of proprioceptive and mechanoreceptive stimulation from the limbs (Behrman and Harkema, 2000). It was unlikely that in-cage, reflexive hindlimb activity would provide the correct patterns of sensory stimuli that would improve the generation of stepping by the lumbar spinal circuitry.
WSTT enhanced synaptic plasticity in the lumbar spinal cord circuitry
There is an increasing amount of evidence that hindlimb exercise enhanced synaptic connections within the lumbar spinal cord circuitry following a spinal cord injury. The levels of synapsin in the lumbar spinal cord were increased by wheel running exercise in spinally hemisected rats (Ying et al., 2005) and spinally contused rats (Beaumont et al., 2008). Similarly, daily swimming or stand training exercise increased synapsin levels in the lumbar spinal cord of spinally contused rats (Hutchinson et al., 2004). Until recently, it was unknown which spinal synapses were modulated by hindlimb exercise. However, there is now evidence that synaptic connections to motor neurons were increased. Daily WSTT resulted in increased synaptophysin expression around ventral horn motor neurons in ST rats and furthermore, these changes were correlated with improved stepping function (Macias et al., 2009). The present findings expand on these findings and indicate that a large amount of WSTT was required in order to restore normal amounts of synaptic connections to motor neurons. A small amount of WSTT failed to enhance synaptic plasticity and was essentially the same as no training at all. Given the parallel changes in stepping recovery, these findings suggested that there was a threshold level of activity that must be generated by WSTT for enhancing synaptic plasticity and improving functional recovery. Based on synaptophysin expression alone, one cannot determine if WSTT increased the number of terminals on motor neurons or if existing terminals were expanded (Macias et al., 2009). However, recent evidence suggests that the number of synapses onto α motor neurons was unchanged by spinal cord transection and WSTT (Ichiyama et al., 2011).
Other factors than activity level may have contributed to the expression of synaptophysin. For example, stress that occurs as a result of restraining rats has been shown to increase synatophysin expression in the hippocampus (Gao et al., 2006). Given that the rats in the present study were restrained during robot testing, it is possible that restraint-related stress was induced and that this influenced synatophysin expression. This effect may have been strongest in the 0 rats who were not as accustomed to robotic device as the other groups. Such an effect may explain why synaptophysin expression in the 0 rats was greater (but not statistically different) than in the 100 rats.
At this point, the sources of these synaptic connections and their effects on motor neuron function (excitatory or inhibitory) are unknown. It seems likely that at least some of the changes were occurring in synapses from sensory and propriospinal pathways. Sensory feedback from the hindlimb may facilitate the activation of motor neuronal pools after descending input has been removed. WSTT strengthened excitatory synapses between sensory neurons (fibers stretch receptors) and lumbar spinal cord motor neurons in ST rats (Petruska et al., 2007). Similarly, excitatory synapses between motor neurons and propriospinal neurons traveling within the ventral lateral funiculus were enhanced in the trained ST rats (Petruska et al., 2007). These findings suggested that some of the enhanced input to motor neurons was excitatory in nature and may include sensory and proprioceptive neurons. However, there was also the possibility that the increased synaptic connections included inhibitory synapses. GABAergic and glycinergic terminals make up nearly half of all synaptic terminals on the membrane of motor neurons (Ichiyama et al., 2006). The balance between inhibitory and excitatory inputs to motor neurons within the lumbar spinal cord has recently been shown to be modulated by WSTT in ST rats (Ichiyama et al., 2011; Macias et al., 2009).
It is unknown how activity stimulates synaptic plasticity within the lumbar spinal cord. In the brain, BDNF synthesis and release is stimulated by exercise and the increased BDNF levels trigger pre-synaptic and post-synaptic changes that ultimately promote synaptic plasticity (Cotman et al., 2007). In the present study, BDNF expression was significantly correlated with the number of steps imposed during training but it was not significantly correlated with the recovery of weight-bearing stepping. Our interpretation of these findings is that BDNF presence within the lumbar spinal cord circuitry increased as a general response to exercise. One role of BDNF after spinal cord injury may be to activate pathways that ultimately strengthen synaptic connections within the locomotor-generating circuitry. This conclusion is supported by previous findings linking exercise-enhanced BDNF expression and increased synaptic plasticity in spinal cord injured animals (Ying et al., 2008).
Whether BDNF is effective in mediating changes in synapses will depend upon a number of factors including how much BDNF is stimulated by exercise. It was interesting that the small amount of WSTT provided a sufficient stimulus for BDNF expression. Previously, only three sessions of treadmill exercise were shown to increase BDNF levels in the lumbar spinal cord of intact rats (Gomez-Pinilla et al., 2001). These findings suggested that BDNF synthesis was quite sensitive to changes in hindlimb activity level. This may also explain why the lowest amounts of BDNF expression were found in the intact rats, a finding consistent with previous reports (Macias et al., 2009). The intact rats were not exercised at any time during the present study. In contrast, all of the ST rats including the untrained rats were handled daily (e.g., bladder expression). This provided opportunities for stimulating hindlimb activity, and this may have been sufficient for stimulating BDNF expression.
TrkB receptor availability will also be a factor. Contrary to previous reports (Macias et al., 2007; Skup et al., 2002; Ying et al., 2008), TrkB receptor expression in the present study was not modulated by exercise levels. Clearly, further studies are necessary to more closely examine the extent that BDNF contributed to neuroplasticity. In addition, it would be valuable to determine if the exercise-induced increases in BDNF levels improved the generation of stepping in other ways, such as promoting local sprouting or neuroprotection after spinal cord injury.
Clinical implications
Activity-based therapies such as WSTT are effective in enhancing functional recovery in individuals who have a spinal cord injury. Intensive therapies provide the best chances for functional recovery and the present findings are consistent with this notion. The more WSTT that can be provided to an individual with a spinal cord injury, the greater the chances are for impacting the lumbar spinal cord circuitry and recovering weight-bearing stepping. Unfortunately, there are practical considerations (i.e., costs, time commitment) that may limit how much therapy an individual can experience. In these instances, the amount of training may not be sufficient for maximizing the effects of WSTT. The present findings suggested that small amounts of WSTT produced incremental gains in function, stepping quality, and BDNF levels. Therefore, individuals with spinal cord injuries who are unable to receive intensive WSTT, can still derive some benefits from a reduced amount of WSTT. The amount of WSTT necessary for stimulating plasticity in the spinal cord will of course vary among individuals and will be determined by many factors (e.g., extent of the injury, the duration of time that therapy starts following the injury, baseline levels of functional recovery). The key to providing WSTT that is both effective and efficient will be to identify the optimal amount of WSTT and then ensure that this amount of activity is imposed throughout the therapy.
Acknowledgments
This work was supported by the National Institutes of Health (NS 42951-01S).
Author Disclosure Statement
No competing financial interests exist.
References
- Barbeau H. Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Beaumont E. Kaloustian S. Rousseau G. Cormery B. Training improves the electrophysiological properties of lumbar neurons and locomotion after thoracic spinal cord injury in rats. Neurosci. Res. 2008;62:147–154. doi: 10.1016/j.neures.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Behrman A.L. Harkema S.J. Locomotor training after human spinal cord injury: a series of case studies. Phys. Ther. 2000;80:688–700. [PubMed] [Google Scholar]
- Belanger M. Drew T. Provencher J. Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. J. Neurophysiol. 1996;76:471–491. doi: 10.1152/jn.1996.76.1.471. [DOI] [PubMed] [Google Scholar]
- Cha J. Heng C. Reinkensmeyer D.J. Roy R.R. Edgerton V.R. de Leon R.D. Locomotor ability in spinal rats is dependent on the amount of activity imposed on the hindlimbs during treadmill training. J. Neurotrauma. 2007;24:1000–1012. doi: 10.1089/neu.2006.0233. [DOI] [PubMed] [Google Scholar]
- Cotman C.W. Berchtold N.C. Christie L.A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- de Leon R.D. Acosta C.A. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J. Neurotrauma. 2006;23:1147–1163. doi: 10.1089/neu.2006.23.1147. [DOI] [PubMed] [Google Scholar]
- de Leon R.D. Hodgson J.A. Roy R.R. Edgerton V.R. Locomotor capacity attributable to step training versus spontaneous recovery after spinalization in adult cats. J. Neurophysiol. 1998;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- Edgerton V. R. de Leon R.D. Tillakaratne N. Recktenwald M.R. Hodgson J.A. Roy R.R. Use-dependent plasticity in spinal stepping and standing. Adv. Neurol. 1997;72:233–247. [PubMed] [Google Scholar]
- Edgerton V.R. Tillakaratne N.J. Bigbee A.J. de Leon R.D. Roy R.R. Plasticity of the spinal neural circuitry after injury. Annu. Rev. Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- Gao Y. Bezchlibnyk Y.B. Sun X. Wang J.F. McEwen B.S. Young L.T. Effects of restraint stress on the expression of proteins involved in synaptic vesicle exocytosis in the hippocampus. Neuroscience. 2006;141:1139–1148. doi: 10.1016/j.neuroscience.2006.04.066. [DOI] [PubMed] [Google Scholar]
- Giszter S.F. Kargo W.J. Davies M. Shibayama M. Fetal transplants rescue axial muscle representations in M1 cortex of neonatally transected rats that develop weight support. J. Neurophysiol. 1998;80:3021–3030. doi: 10.1152/jn.1998.80.6.3021. [DOI] [PubMed] [Google Scholar]
- Gomez–Pinilla F. Ying Z. Opazo P. Roy R.R. Edgerton V.R. Differential regulation by exercise of BDNF and NT-3 in rat spinal cord and skeletal muscle. Eur. J. Neurosci. 2001;13:1078–1084. doi: 10.1046/j.0953-816x.2001.01484.x. [DOI] [PubMed] [Google Scholar]
- Heng C. de Leon R.D. Treadmill training enhances the recovery of normal stepping patterns in spinal cord contused rats. Exp. Neurol. 2009;216:139–147. doi: 10.1016/j.expneurol.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson J.A. Roy R.R. de Leon R. Dobkin B. Edgerton V.R. Can the mammalian lumbar spinal cord learn a motor task? Med. Sci. Sports Exerc. 1994;26:1491–1497. [PubMed] [Google Scholar]
- Hutchinson K.J. Gomez–Pinilla F. Crowe M.J. Ying Z. Basso D.M. Three exercise paradigms differentially improve sensory recovery after spinal cord contusion in rats. Brain. 2004;127:1403–1414. doi: 10.1093/brain/awh160. [DOI] [PubMed] [Google Scholar]
- Ichiyama R.M. Broman J. Edgerton V.R. Havton L.A. Ultrastructural synaptic features differ between alpha- and gamma-motoneurons innervating the tibialis anterior muscle in the rat. J. Comp. Neurol. 2006;499:306–315. doi: 10.1002/cne.21110. [DOI] [PubMed] [Google Scholar]
- Ichiyama R.M. Broman J. Roy R.R. Zhong H. Edgerton V.R. Havton L.A. Locomotor training maintains normal inhibitory influence on both alpha- and gamma-motoneurons after neonatal spinal cord transection. J. Neurosci. 2011;31:26–33. doi: 10.1523/JNEUROSCI.6433-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubasak M.D. Hedlund E. Roy R.R. Carpenter E.M. Edgerton V.R. Phelps P.E. L1 CAM expression is increased surrounding the lesion site in rats with complete spinal cord transection as neonates. Exp. Neurol. 2005;194:363–375. doi: 10.1016/j.expneurol.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Lovely R.G. Gregor R.J. Roy R.R. Edgerton V.R. Effects of training on the recovery of full-weight-bearing stepping in the adult spinal cat. Exp. Neurol. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Lovely R.G. Gregor R.J. Roy R.R. Edgerton V.R. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Res. 1990;514:206–218. doi: 10.1016/0006-8993(90)91417-f. [DOI] [PubMed] [Google Scholar]
- Macias M. Dwornik A. Ziemlinska E. Fehr S. Schachner M. Czarkowska–Bauch J. Skup M. Locomotor exercise alters expression of pro-brain-derived neurotrophic factor, brain-derived neurotrophic factor and its receptor TrkB in the spinal cord of adult rats. Eur. J. Neurosci. 2007;25:2425–2444. doi: 10.1111/j.1460-9568.2007.05498.x. [DOI] [PubMed] [Google Scholar]
- Macias M. Nowicka D. Czupryn A. Sulejczak D. Skup M. Skangiel–Kramska J. Czarkowska–Bauch J. Exercise-induced motor improvement after complete spinal cord transection and its relation to expression of brain-derived neurotrophic factor and presynaptic markers. BMC Neurosci. 2009;10:144. doi: 10.1186/1471-2202-10-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruska J.C. Ichiyama R.M. Jindrich D.L. Crown E.D. Tansey K.E. Roy R.R. Edgerton V.R. Mendell L.M. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J. Neurosci. 2007;27:4460–4471. doi: 10.1523/JNEUROSCI.2302-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumier J.C. Hopkins D.A. Robertson H.A. Currie R.W. Constitutive expression of the 27-kDa heat shock protein (Hsp27) in sensory and motor neurons of the rat nervous system. J. Comp. Neurol. 1997;384:409–428. [PubMed] [Google Scholar]
- Skup M. Dwornik A. Macias M. Sulejczak D. Wiater M. Czarkowska–Bauch J. Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp. Neurol. 2002;176:289–307. doi: 10.1006/exnr.2002.7943. [DOI] [PubMed] [Google Scholar]
- Sullivan K.J. Brown D.A. Klassen T. Mulroy S. Ge T. Azen S.P. Winstein C.J. Effects of task–specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomized clinical trial. Phys. Ther. 2007;87:1580–1602. doi: 10.2522/ptj.20060310. [DOI] [PubMed] [Google Scholar]
- Tillakaratne N.J. de Leon R.D. Hoang T.X. Roy R.R. Edgerton V.R. Tobin A.J. Use-dependent modulation of inhibitory capacity in the feline lumbar spinal cord. J. Neurosci. 2002;22:3130–3143. doi: 10.1523/JNEUROSCI.22-08-03130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E.D. Stelzner D.J. Behavioral effects of spinal cord transection in the developing rat. Brain Res. 1977;125:241–255. doi: 10.1016/0006-8993(77)90618-7. [DOI] [PubMed] [Google Scholar]
- Wernig A. Muller S. Nanassy A. Cagol E. Laufband therapy based on 'rules of spinal locomotion' is effective in spinal cord injured persons. Eur. J. Neurosci. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Ying Z. Roy R.R. Edgerton V.R. Gomez–Pinilla F. Exercise restores levels of neurotrophins and synaptic plasticity following spinal cord injury. Exp. Neurol. 2005;193:411–419. doi: 10.1016/j.expneurol.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Ying Z. Roy R.R. Zhong H. Zdunowski S. Edgerton V.R. Gomez–Pinilla F. BDNF-exercise interactions in the recovery of symmetrical stepping after a cervical hemisection in rats. Neuroscience. 2008;155:1070–1078. doi: 10.1016/j.neuroscience.2008.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]