Abstract
Repair of double-strand breaks in chromosomal DNA is essential. Unfortunately, a paradigm central to most DNA repair pathways—damaged DNA is replaced by polymerases, by using an intact, undamaged complementary strand as a template—no longer works. The nonhomologous end joining (NHEJ) pathway nevertheless still uses DNA polymerases to help repair double-strand breaks. Bacteria use a member of the archaeo-eukaryal primase superfamily, whereas eukaryotes use multiple members of the polymerase X family. These polymerases can, depending on the biologic context, accurately replace break-associated damage, mitigate loss of flanking DNA, or diversify products of repair. Polymerases specifically implicated in NHEJ are uniquely effective in these roles: relative to canonic polymerases, NHEJ polymerases have been engineered to do more with less. Antioxid. Redox Signal. 14, 2509–2519.
More to It than Just Joining Ends
Chromosome double-strand breaks are generated directly by ionizing radiation and DNA-cleaving chemotherapeutic drugs or indirectly by replication failure and aborted repair (e.g., base excision repair; BER). Developmentally programmed chromosome breaks are also intermediates in recombinations required for meiosis and the adaptive immune response [V(D)J recombination or class switch recombination]. Relative to most other chromosome lesions, double-strand breaks are rare, and this is fortunate—the consequences of failed or aberrant repair of double-strand breaks are severe [reviewed in (75)].
Chromosome integrity can be restored by using homologous recombination, which replaces the broken region by using either the intact sister chromatid or homologue as a template. Eukaryotes and a subset of prokaryotic species also have a second option, where broken ends are simply re-joined by ligation [reviewed in (48)]. Unfortunately, the failure of end joining to reference an intact copy means that the terminal sequence can be lost, or worse, a given chromosome end can be joined to the wrong partner (translocations and chromosome fusions). End joining is thus typically viewed as the more error prone of the two strategies. Classically defined end joining (nonhomologous end joining, or NHEJ; see later) nevertheless plays a pivotal role in preserving genome stability. Defects in classically defined NHEJ result in severe radiosensitivity, immunodeficiency, blocked neurogenesis, premature cellular senescence, and cancer predisposition.
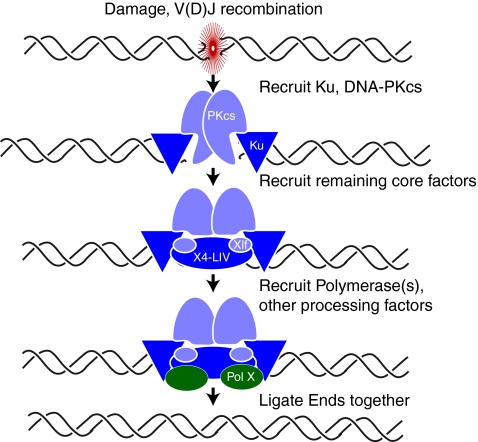
NHEJ is most simply defined by its use of the DNA end-binding scaffold Ku and an associated ATP-dependent DNA ligase (ligase IV/Dnl4 in eukaryotes). In most eukaryotes, the core NHEJ machine also includes XRCC4/Lif1, XLF/Cernunnos/Nej1, and the DNA-dependent protein kinase (DNA-PKcs) (Fig. 1). This machine is at least partly dispensable for repair of chromosome breaks due to alternate end-joining pathways (Alt-EJ) that join “sticky ends” (ends with >2 bp complementary overhangs) either directly, or after such ends are generated by extensive end resection [reviewed in (48, 66)]. By comparison, classically defined NHEJ (joining dependent on Ku and its associated ligase) uses end-processing activities that appear to be capable of making most ends, regardless of initial end structure, a good substrate for ligation without the need for extensive resection. Among processing activities used by NHEJ is the extension of DNA ends by a DNA polymerase (Fig. 1).
FIG. 1.
Pol X members form a complex with NHEJ core factors. pol X family members (green) require at least Ku and XRCC4-ligase IV (X4-LIV) (dark purple) to form a stable complex at DNA ends. Additional core factors not essential for stable pol X recruitment (light purple), but which may also participate, include XLF/Cernunnos (Xlf) and DNA-PKcs. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Polymerases Specifically Used by NHEJ
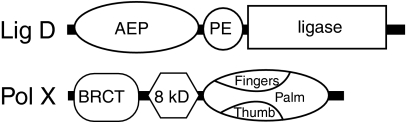
LigD polymerase domain
Bacterial genera with recognizable NHEJ machinery include Mycobacterium, Bacillus, Pseudomonas, and Agrobacterium sp. [reviewed in (81, 89)]. These species possess a gene for Ku, and this gene is often located near a gene encoding a member of a family of nucleotidyl transferases that include the primase catalytic subunit (archaeo-eukaryal primase superfamily, or AEP) (4, 32, 52). Strikingly, the AEP member is often a domain within a multidomain NHEJ “Swiss army knife” (29) (e.g., the ligD protein in mycobacteria; Fig. 2) that can also include a domain encoding another end-processing activity (phosphodiesterase), as well as a domain encoding the ATP-dependent ligase activity implicated in bacterial NHEJ. AEP members have been specifically implicated in NHEJ both biochemically (29) and through genetic analysis (3, 29).
FIG. 2.
Domain organization of NHEJ polymerases. Ligase D protein of Mycobacterium tuberculosis. AEP, archaeo-eukaryotic primase superfamily; PE, phosphodiesterase.
The pol X family
The pol X family are small (30–70 kDa) DNA polymerases found in most eukaryotes, with the notable exception of certain invertebrates (D. melanogaster, C. elegans) [reviewed in (67, 98)]. The most well-characterized members include the only pol X members in the fungi Saccharomyces cerevisiae and S. pombe, Pol 4, and four members of the pol X family found in vertebrates: pol β, pol λ, pol μ, and terminal deoxynucleotidyl transferase (TdT). Pol X members have a 30-kDa domain required for synthesis that has been likened to a hand, with palm, fingers, and thumb subdomains (67) (Fig. 2). The “hand” in pol X members is preceded by an 8-kDa domain that is typically active in binding of the downstream side of a gap, as well as excision of downstream 5′ terminal abasic sites [see later, and Fig. 5, and (67)]. They do not possess exonuclease activity. With the exception of pol β, these polymerases further possess an N-terminal BRCT (similar to BRCA1 C terminal protein–protein interaction) domain.
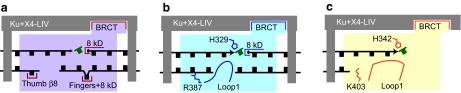
FIG. 5.
Structural elements proposed to promote activity of vertebrate pol X members during NHEJ. X4-LIV, the XRCC4-ligase IV complex. (a) pol λ; (b) pol μ; (c) TdT. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
The presence of BRCT domains in a pol X member is sufficient to define it as an “NHEJ polymerase” (see also later section), because the BRCT domains are essential for formation of a specific complex between the Pol X member and NHEJ core factors at DNA ends (Fig. 1) (60, 62, 71, 97). Ku and XRCC4-ligase IV are typically both essential for formation of a stable complex that includes the pol X member (62, 64, 71), and the core factor XLF further promotes pol X activity in vitro (1). However, specific protein–protein interaction interfaces between pol X members and NHEJ core factors have not yet been defined. Notably, BRCT domains often recognize phosphoserine/threonine-containing motifs (65, 102) and BRCT domain of TdT has higher affinity for a phosphoserine-containing peptide (102), but, as yet, no evidence exists for phosphorylation-dependent interactions of pol X members with core NHEJ factors. It is also not yet clear whether a defined order of assembly or a defined stoichiometry exists, or even if pol X recruitment to the complex occurs exclusive of, or in addition to, participation of other NHEJ factors (e.g., DNA-PKcs, other processing factors). The pol μ and TdT BRCT domains are ∼40% similar, and residues important for complex formation are conserved (30). Curiously, pol λ is much less similar with regard to primary sequence and structure, and this results in significant differences in how it interacts with NHEJ components (69). It is unclear whether the difference in interaction provides some advantage to NHEJ (e.g., by helping to determine which polymerase is used, or how). BRCT-domain–containing polymerases also help to increase the stability with which XRCC4-ligase IV is recruited to Ku-bound ends (64) and can generally promote ligase activity independent of a role for the polymerase (97).
Other polymerases (i.e., those without BRCT domains) also contribute to NHEJ. For example, human Pol β (24) and even S. cerevisiae pol δ (3, 19) have both been argued to substitute partly for Pol 4 during NHEJ in S. cerevisiae, and other polymerases can act in the absence of the LigD polymerase function (3). However, their activity is not efficiently coupled to the other events in NHEJ, especially end alignment and ligation, and consequently it appears restricted to a limited class of substrates (mostly 3′ recessed ends). Other polymerases may thus serve as serviceable substitutes in very specific contexts, and possibly even then only when an NHEJ polymerase is unavailable.
Biologic Roles of NHEJ Polymerases
Genetic analysis in the mycobacterium M. smegmatis indicates that the polymerase domain of LigD is required for NHEJ: deletion of the domain has an impact on NHEJ equivalent to deletion of Ku, even though the LigD ligase domain remains active. However, this requirement for the polymerase domain is independent of its synthesis activity (3). Inactivation of the polymerase domain catalytic activity primarily affects junction structure, rather than joining efficiency.
Eukaryotic NHEJ polymerase activity also is not essential for all NHEJ reactions. For example, vertebrate cells deficient in pol μ, pol λ, or both have been characterized as having no radiosensitivity (8, 9, 57), or are at best weakly radiosensitive (61) relative to equivalent cells deficient in Ku or ligase IV. Mice deficient in pol μ (alone or in combination with pol λ) are also immunodeficient (8, 9), but again, this phenotype is less severe than mice deficient in Ku or ligase IV. Gross phenotypes of polymerase-deficient cells/mice are thus significant, but generally milder than those associated with cells or mice deficient in core factors.
It has been difficult to determine how various NHEJ polymerases contribute to these phenotypes. This is partly because NHEJ polymerases make important contributions to other pathways: specific examples so far include Pol λ in base excision repair (13, 93), and Pol 4 in alternate (i.e., Ku-independent) end joining (27, 30). Additionally, the roles of NHEJ polymerases within NHEJ appear to overlap with each other as well as with polymerases not typically linked to NHEJ, depending on the substrate and cell type (discussed in detail later). Consistent with this idea, overexpression of catalytically inactive pol λ (17), or even a partly defective, cancer-associated pol λ mutant (94) have fairly severe effects on both the efficiency and accuracy of NHEJ that probably would not have been predicted from analysis of pol λ–deficient mice (9, 57).
Specifically how do NHEJ polymerases contribute to cellular repair, and in organisms with multiple NHEJ polymerases, to what extent are their roles overlapping?
Accurate repair
Short-patch BER at double-strand breaks
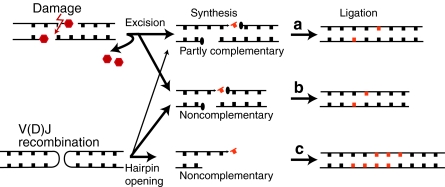
Damage-induced double-strand breaks are often “staggered” (strand breaks are offset on opposing strands), and termini possess flanking nucleotide damage (99). Experiments both with cell extracts (20) and in cells (25) indicate that polymerases active in NHEJ contribute to a type of short-patch BER that accurately repairs damage-associated chromosome breaks (Fig. 3a). NHEJ shares with BER/single-strand break repair some of the same enzymes [reviewed in (16)] that excise oxidative nucleotide damage expected near damaged induced breaks. Alignment-based gap fill-in, a long appreciated property of NHEJ (59, 78, 85, 95), is then sufficient to replace excised nucleotides accurately, as long as aligned ends still retain at least one to two complementary nucleotides (Fig. 3). The polymerase domain of bacterial ligase D (29), Pol4 (24, 97, 101), pol μ (72), and pol λ (60) can all in principle contribute to accurate alignment-based gap fill-in (Figs. 3 and 4).
FIG. 3.
Biologic source, end structure, and NHEJ products. Chromosome breaks generated by damage (e.g., ionizing radiation) or V(D)J recombination have complementary or noncomplementary ends, and this determines whether repair products require (a) gap fill-in directed by partially complementary overhangs, (b) gap fill-in after alignment of noncomplementary overhangs, or (c) template-independent extension of noncomplementary overhangs. Strand break 3′OH and 5′PO4 termini are denoted by arrows and ellipses, respectively. Damaged nucleotides are in red, whereas orange identifies incoming nucleotide triphosphate in substrate and products of synthesis in junctions. (To see this illustration in color the reader is referred to theweb version of this article at www.liebertonline.com/ars).
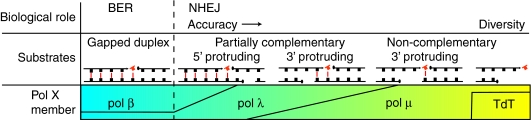
FIG. 4.
A gradient of template strand dependence for pol X family members. Different biologic roles (top row), substrates with decreasing base-pairing interactions between primer or incoming dNTP (orange), and template strand (highlighted in red; middle row), and the proposed vertebrate pol X members active in these roles and on these substrates (bottom row) are correlated. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
This specialized short patch BER has the ability to restore the original chromosome sequence accurately, even when break-associated damage is sufficient initially to block ligation (20). Does polymerase activity during NHEJ suppress damage-induced mutation in vivo? Deletion of Pol4 increases the mutation rate both spontaneously and after exposure to ionizing radiation (50) or methyl methanesulfonate (MMS), and additionally increases sensitivity to MMS (92). Moreover, increased mutation and MMS sensitivity due to Pol4 deficiency are at least partly suppressed by additional deletion of a core NHEJ component (Dnl4 or yKu70). This implies both that these affects of Pol4 are mediated mostly through repair by NHEJ, and that complete ablation of NHEJ allows the damage to be efficiently repaired by a different repair pathway that is at least as accurate (probably homologous recombination).
Synthesis at noncomplementary ends: mitigating the need for resection
Partially complementary overhangs and alignment-based fill-in allow the accurate resolution of damage-induced chromosome breaks, but a significant fraction of breaks will presumably possess fully noncomplementary sequence (Fig. 3b). Noncomplementary overhangs are also frequently expected during NHEJ-dependent resolution of V(D)J recombination intermediates. One alternative is to use a nuclease to resect chromosome ends until a complementary sequence is found (microhomologies). This is the primary pathway for Alt-EJ of noncomplementary ends [reviewed in (66)], and Mre11 may be well engineered for this purpose (100). However, classically defined NHEJ can use polymerases (alone, or together with limited resection) as a more conservative means for matching ends. The 3′ recessed ends can be filled in (85) [and this probably does not need a specific polymerase (3, 24, 101)], whereas the NHEJ polymerases pol μ (71) and Pol 4 (76) have been surprisingly implicated in a variation of alignment-based gap fill-in with 3′ protruding ends that possess no complementary sequence (Fig. 3b). Ends are extended by limited synthesis, usually template instructed, to generate complementary overhangs de novo (“end-bridging” synthesis). In contrast to synthesis that takes advantage of a complementary sequence present after initial alignment of ends, end-bridging synthesis is less likely to restore the original chromosome sequence accurately. End-bridging synthesis may nevertheless help to minimize deletion associated with resolving noncomplementary ends, relative to resolutions reliant entirely on end resection.
At the same time, the ability to match ends promiscuously can be a threat to genome stability. Rap1 deletion in S. cerevisiae results in NHEJ-dependent fusion of chromosomes at telomeres, and although Pol 4 is dispensable for many NHEJ reactions (24, 101), it is essential for telomere fusion (76). The ability of Pol 4 to bypass a need for partially complementary ends thus apparently removes an important block to aberrant chromosome fusion or translocation.
Distinct roles for pol μ and pol λ?
Most vertebrates possess three pol X members, and two (Pol μ and pol λ) are both widely expressed and possess overlapping in vitro activities. Nevertheless, levels of expression of the two polymerases vary according to cell type [e.g. (9)], and each polymerase has substrates on which it is uniquely active (Fig. 4, and later section). Are they functionally redundant in vivo?
In vivo evidence for a unique role for pol μ in NHEJ comes from studies of the impact of pol μ on V(D)J recombination. During V(D)J recombination, NHEJ must resolve chromosome breaks with 3′ overhangs of diverse sequence (88); aligned ends thus frequently possess little or no complementarity (Fig. 3). Pol μ facilitates more-accurate joining of these ends, at least during recombination at the immunoglobulin kappa (Igκ) locus: deficiency in polymerase μ results in increased deletion (8), and overexpression of pol μ has the opposite effect (71). When compared with structures of Igκ ends (88), the sequences of Igκ junctions indicated both that pol μ reduces deletion by promoting retention of overhang sequence, and that overhang retention is not reliant on partially complementary overhangs generated by chance (71). Comparably overexpressed pol λ or a catalytically inactive pol μ did not similarly promote overhang retention, confirming that this role is a specific consequence of the catalytic activity of pol μ, and is unique to pol μ. Finally, mutants of pol μ that are defective only in the unique activity of pol μ—its ability to add complementary nucleotides after bridging noncomplementary ends—were also relatively unable to promote overhang retention (26, 71). Reasonably accurate Igκ recombination thus relies on the ability of pol μ to extend from noncomplementary overhangs. Possibly consistent with a more-general role for this activity in NHEJ, other cell types (mouse embryo fibroblasts, bone marrow, and splenocytes) deficient in pol μ can be radiosensitive and show increased numbers of radiation-dependent γH2AX foci and chromosome aberrations, relative to wild-type controls (61). Overexpression of catalytically inactive pol μ also promotes radiation-dependent chromosome aberrations in Chinese hamster ovary cells (18).
Surprisingly, Pol λ deficiency also results in increased deletion during V(D)J recombination. In contrast to pol μ deficiency, though, pol λ deficiency affects only recombination at the immunoglobulin heavy chain (IgH) locus (9). Does this mean that pol λ has activity equivalent to pol μ on noncomplementary ends? And if their biochemical activities entirely overlap, why are the biologic roles of these two polymerases not overlapping? The pol μ is expressed at very low levels in cells active in IgH recombination, probably explaining why pol μ is unable to compensate for pol λ deficiency at this stage in B-cell development (9). It is less clear why pol λ cannot compensate for pol μ deficiency during Igκ recombination: the expression of pol λ remains relatively constant through B-cell development (9), and it has little impact on Igκ recombination, even when overexpressed (71). However, when we factor in the number of recombinations involved (two for IgH; one for Igκ), the ability of pol λ to mitigate deletion is significantly less than that of pol μ (8, 9). IgH recombination additionally samples a much wider variety of possible end structures. Therefore, the pol λ significant but reduced ability to mitigate deletion during NHEJ of V(D)J recombination intermediates might be consistent with restriction of the pol λ activity to those ends that, after alignment, possess by chance 1–2 bp of complementary sequence.
Conclusive determination of the extent of overlap between pol μ and pol λ function in cells will require experiments in which substrate end structure and expression level are systematically varied. At present, though, it appears that pol λ could be the primary NHEJ polymerase in most cell types. It efficiently and accurately performs the last step of a specialized base excision repair, using ends with partially complementary sequence (Figs. 3a and 4). Pol μ, conversely, is probably capable of performing most of the same functions but also allows a more “creative” solution when ends of unrelated sequence are aligned (Figs. 3b and 4). This activity is critical in mitigating resection associated with joining of noncomplementary ends in pre-B cells active in Igκ light-chain recombination, and probably plays a significant role in other cell types as well. However, the pol μ contribution may be accompanied with some risk, as suggested both by the ability of the similar activity of Pol 4 to promote telomere fusion, and the possible utilization by pol μ of ribonucleotides during synthesis (see later section).
Diversification
NHEJ polymerases can also contribute to diversity in NHEJ junctions (Fig. 3c). TdT is the third pol X member present in most vertebrates, but is expressed only in cells active in V(D)J recombination, and even then, expression is absent during V(D)J recombination early in ontogeny (mouse fetal live) and during immunoglobulin light-chain recombination (7). TdT thus does not significantly contribute to NHEJ beyond the role of NHEJ in resolution of intermediates in V(D)J recombination. TdT is required for the majority of template-independent additions introduced during resolution of V(D)J recombination intermediates (42, 58) (Figs. 3c and 4). Mice without TdT thus have reduced diversity in antigen-specific receptors, and this reduced diversity limits the effectiveness of adaptive immune responses (46, 56, 70).
In Mycobaterium smegmatis, the polymerase activity of Lig D often introduces a single, template-independent addition into junctions made by using blunt-ended substrates (3, 43). A polymerase-deficient LigD mutant directs less mutagenic NHEJ, but joining in this context is also threefold less efficient (3). Increased efficiency of joining might be reason enough to support this mutagenic process. However, mutagenic repair may also be beneficial in the sense that increased mutation in cells under specific stress, including stationary phase or starvation, may promote escape from antibiotics or otherwise improve fitness or virulence.
Distinguishing Features in Function and Structure
DNA polymerases typically need two things: a primer and annealed template, and a deoxynucleotide triphosphate complementary to the template. Neither need is met in most end-joining contexts, making it difficult for canonic polymerases to function (Figs. 3 and 4).
End bridging and variable dependence on template
Activity on a 5′ overhang typically does not necessarily require end bridging and is readily supported by canonic polymerases (3, 24, 101). The ability to use partly complementary 5′ overhangs and perform alignment-based fill in (rather than simply “blunting” the end) nevertheless still affords some advantage to NHEJ (Fig. 4). This requires coupling of synthesis to ligation, or at least gap recognition, and available evidence suggests that it is the primary pathway for resolution of such ends in vertebrates (78, 85, 95).
However, the biggest challenge facing NHEJ polymerases is sustaining activity on a 3′-protruding primer, as it is cannot be stably aligned with template by base pairing (Fig. 4). Accurate template-dependent repair requires either that the polymerase align the end itself, or that the polymerase work within a complex of NHEJ core factors that aligns primer and template for the polymerase. Alternatively, the NHEJ polymerase could add nucleotides to ends independent of the template overhang sequence, either in hope of randomly generating complementary overhangs, or to promote genome diversification, as described earlier [e.g., during V(D)J recombination].
All of these strategies are used; which strategy is used depends on the polymerase and substrate (see earlier; Fig. 4). Here we address how the polymerases differ in regard to how they sustain activity, as well as describe progress in determining the structural basis for these differences.
Lig D
The polymerase domain of the Mycobacterium tuberculosis ligD gene is a striking example of a polymerase that is intrinsically sufficient to align ends and perform alignment-based fill-in synthesis on partly complementary 3′ protruding ends (14). A crystal structure captured a dimer of polymerase domains aligning two ends (14). Key features identified in this structure were a β hairpin loop from each monomer that both constitutes the protein dimer interface and cradles the interacting DNA ends, and the recognition by both monomers of the downstream 5′ PO4 (14, 82, 108).
Pol X
Deletion of Pol 4 in yeast primarily affects joining of ends that require synthesis (24, 101). Similarly, the phenotype of mice, even with both pol μ and pol λ deleted, is mild, at least relative to mice missing a core NHEJ component (8, 9, 57, 61). Moreover, pol X polymerases possess intrinsic ability to perform synthesis over a double-strand break only in relaxed contexts (extensive complementary sequence and reduced ionic strength) (2, 37, 54). Therefore, relative to bacterial NHEJ, eukaryotic NHEJ uses polymerases primarily for synthesis, and their synthesis activity in this context is mostly dependent on end bridging provided extrinsically, through a complex formed with the core NHEJ components (26). Nevertheless, NHEJ-associated polymerases possess additional characteristics that make them less reliant than canonic polymerases on extensive primer-template base pairing for activity (80, 104). Strikingly, the four vertebrate pol X family members—pol β, pol λ, pol μ, and TdT—differ incrementally in this regard, possessing a gradient of independence on template (71). Their differences in dependence on template help dictate their biologic role (Fig. 4).
Elements required for partly complementary 3′ overhangs
Pol λ (60), pol μ (72), and pol 4 (24) are active in NHEJ when the primer terminus and template can be aligned with at least one to two terminal complementary nucleotides (Figs. 3a and 4). This activity requires a BRCT domain: deletion (37, 60, 62, 71, 97) or substitutions of key residues (30, 69) in the BRCT domains have no significant impact on intrinsic catalytic activity, but correlate alignment-based gap fill-in activity during NHEJ with the ability to form a complex between the polymerase, Ku, and XRCC4-ligase IV at DNA ends.
The ability of these polymerases to act during NHEJ thus relies on end-bridging interactions supplied by core NHEJ factors. Nevertheless, pol X members are generally known for gap recognition: simultaneous recognition of both the primer by the catalytic domain and downstream 5′ PO4 by the 8-kDa domain (67). This will also promote end bridging (Fig. 5a and b). At least for pol μ, gap recognition has been shown both to contribute to the ability of pol μ to act during NHEJ (26) and to remain primarily template instructed (2, 26) (see also later).
Several structural elements probably unique to Pol λ (i.e., beyond its BRCT domain and ability to recognize gaps, both of which are found in other pol X members) have been associated with activity of pol λ in NHEJ. Interactions between the Pol λ β8 strand of its “thumb” subdomain and an unpaired nucleotide upstream in the template promotes activity on misaligned (or minimally aligned) primer termini (38) (Fig. 5a). A pocket formed by the pol λ finger subdomain and its 8-kDa domain binds a yet-to-be copied or downstream template nucleotide and promotes processive synthesis on longer gaps by “scrunching” (40) (Fig. 5a).
Elements required for activity on noncomplementary 3′overhangs
The pol λ is largely inactive if the terminal nucleotide of the primer is unable to pair with template sequence (noncomplementary 3′ overhangs; Figs. 3b and c, and 4) (71). Pol μ (33), TdT (55) [and possibly Pol4 as well (76)) retain activity, in part because of structural elements they share, but which are absent from other pol X family members (Fig. 4b and c). A loop between beta strands 3 and 4 (loop1) of the TdT palm subdomain is more than 13 aa longer than is that of pol λ and is found in place of a template in a structure of TdT with a ssDNA primer (28). This position of the TdT loop1 suggests an explanation for the TdT affinity and activity for ssDNA and 3′ overhang-containing substrates. The pol μ loop1 is of similar length and probably also interacts with a 3′ overhang (Fig. 4b): as with TdT (84), deletion of the pol μ loop both reduces its activity on 3′ overhangs and stimulates its activity on recessed primers (54, 71). Histidines in both pol μ (H329) and TdT (H342) have also been proposed to help position the incoming dNTP near the primer terminus in the absence of coordinating primer/template base pairs (Fig. 4b and c) (68).
Pol μ thus remains active in the absence of base-pairing between primer and template; it is less “dependent” on template, at least when compared with polymerase λ or pol β. However, the contribution of Pol μ to NHEJ appears primarily template dependent in other respects. Pol μ is template dependent in the sense that it is most active, both in vitro and in cells, when it has access to a template through end-bridging interactions provided by both NHEJ core factors and pol μ itself (Fig. 4b) (26). More important, pol μ is efficient at adding nucleotides under these conditions only when they are complementary to template (26, 71). Pol μ nevertheless is probably more prone to template-independent additions than are most other polymerases (excluding TdT) (21, 33, 34, 54, 62), and consequently may direct the typically rare template-independent additions in NHEJ junctions that are observed when TdT is not expressed (8, 42, 45, 58, 71).
Elements required for template independence
TdT is >40% identical to pol μ and possesses both of the elements previously linked to activity on unpaired 3′ overhangs (an extended “loop1,” as well as the active-site histidine; H342 in TdT; Fig. 4b and c). Relative to pol μ, though, TdT is both less able to make template-dependent additions and more active in making template-independent additions. The TdT loop1 motif is mostly responsible for blocking template-dependent additions: mutation of the loop, or replacement of the TdT loop with that of pol μ is sufficient to allow template-dependent additions (84). Conversely, the reciprocal chimera (pol μ with the TdT loop1) is less inclined to perform template-dependent additions (54). The TdT loop1 may be more rigid than is that of pol μ and obligatorily excludes template near the primer terminus (Fig. 4c). By comparison, the pol μ loop is not resolved in a crystal structure of pol μ bound to a gapped duplex DNA (68), possibly because the loop is displaced by template.
TdT also has a much higher capacity for template-independent additions than does pol μ (54, 68, 84), especially when Mg2+ is used (84). Strikingly, a large part of this latter difference may be associated with a single residue in pol μ (R387) that inhibits template-independent activity (Fig. 4b and c) (2). Substitution of this residue in pol μ to agree with the identity of the analogous residue in TdT (pol μ R387K) is sufficient to increase the pol μ template-independent activity from 10-fold to 100-fold, with little apparent impact on the pol μ template-dependent activity. Finally, comparison of TdT with other pol X members indicates little structural evidence for a pocket to bind downstream 5′ PO4 (67). TdT probably has the least intrinsic ability to promote end bridging, and consequently, template-strand interactions, when comparing pol X members.
Excision or bypass of damaged nucleotides
Damage-induced strand breaks are typically associated with a wide variety of oxidized nucleotides (abasic sites and oxidized bases) that flank the strand breaks (99). This damage can hinder the ligation step in NHEJ (20, 31, 83).
In BER/single-strand break repair, Pol β first excises 5′ terminal abasic sites by using its 5′dRP lyase activity before its synthesis activity is used to fill in the resulting gap (90, 91). The pol β 5′dRP lyase activity is at least as important as its synthesis activity in certain contexts (90). Both fungal Pol 4 (5, 44) and vertebrate pol λ (39) possess 5′dRP lyase activity that could similarly excise 5′ terminal abasic sites. However, Pol4 lyase activity is dispensable for excision of 5′ terminal abasic sites in NHEJ in S. cerevisiae (25). Pol λ is also relatively inactive on DSB proximal abasic sites in vitro, and mammalian NHEJ primarily uses Ku for this function instead (83).
NHEJ can alternatively use a polymerase that can bypass damaged nucleotides (translesion synthesis activity) or extend from primer/template alignments with damaged nucleotides. This will make termini sufficiently “clean” so that they can now participate in ligation (22, 105), although the damaged nucleotide will still be embedded in the NHEJ product; presumably it eventually is excised by canonic base excision repair. NHEJ polymerases are unusually effective in sustaining activity with primers that are poorly paired with template [(80, 104) and discussed earlier]. This distinguishing characteristic probably explains why NHEJ polymerases pol μ (21, 34, 49, 103) and pol λ (6, 11, 23, 63, 79, 105) sustain significant activity either when the templating nucleotide is damaged or when base pairs near the primer terminus include damaged nucleotides. Pol λ is also surprisingly accurate when using oxidized nucleotides as template (23, 63).
Reduced sugar selectivity
For seemingly obvious reasons, polymerases active in replication and repair of DNA genomes typically have a strong preference for adding deoxynucleotides over ribonucleotides (>1,000-fold) (53, 73). Most of the NHEJ polymerases are exceptions to this rule. The LigD polymerase domain has a >20-fold preference for adding ribonucleotides (29, 82, 106), possibly consistent with its origin as a primase. Pol μ (72, 86), TdT (12, 55), and fungal Pol 4 (5, 44) all modestly prefer deoxynucleotides, with sugar selectivities varying between 1 and 50, depending on the polymerase and the nucleotide. The related pol β excludes ribonucleotides by using a tyrosine (77), and reduced sugar selectivity in other pol X members has been attributed to substitution of this tyrosine with glycine (aa 433 in pol μ) or histidine (86). Importantly, ribonucleotides are typically 10- to 100-fold more abundant than deoxynucleotides in cells [reviewed in (96)], suggesting that these polymerases will incorporate RNA primarily during repair of DNA genomes in vivo (72).
RNA incorporated during NHEJ will interfere with subsequent replication (73) over the junction, and possibly transcription as well. Does an advantage exist to using RNA?
Ribonucleotide use can directly affect the ligation step of NHEJ. Terminal ribonucleotides strongly stimulate ligase activities of LigD and LigC (107) and modestly stimulate the activity of eukaryotic ligase IV (72). The ability of ligD polymerase domains (106) and pol μ (72, 86) or TdT (12) to extend with ribonucleotides is also dramatically reduced with each successive ribonucleotide added, effectively limiting synthesis tracts to fewer than five nucleotides. This may be helpful in curtailing template-independent activity of these enzymes, because long noncomplementary tails would be difficult to align and ligate. As support for the idea that restriction of tail length is important, many bacterial ligD genes also possess a 3′ phosphodiesterase domain implicated in trimming 3′ polynucleotide tails to a single terminal ribonucleotide (109).
The ability to use ribonucleotides might help polymerases remain active when deoxynucleotide pools are low [e.g., in G1/G0 cells (10, 47)]. In this regard, it is interesting that pol λ is the sole NHEJ-associated polymerase unable to use ribonucleotides effectively; nevertheless, it has unusually high affinity for deoxynucleotides (>30-fold better than pol β), and this may still allow it to retain activity when deoxynucleotide pools are low but without resorting to the use of ribonucleotides (41). Both eukaryotes and bacteria are more reliant on NHEJ for DSBR, relative to homologous recombination (HR), when cells are not active in DNA synthesis (i.e., G1/G0 animal cells, stationary phase or sporulating bacteria, and metabolically starved fungi) (51). This is typically linked to the absence in G1/G0 cells of a sister chromatid that HR prefers to use as template [reviewed in (51)]; however, limiting amounts of the other key substrate for HR, deoxynucleotides, may be equally restrictive (15).
Incorporation of RNA during NHEJ has not been detected in products of cellular NHEJ, making it difficult to evaluate the significance of the reduced in vitro sugar selectivities. RNA embedded in NHEJ junctions may be rapidly excised and replaced with DNA (35, 36, 87). Alternatively, deoxynucleotide pools may be locally inflated near DSB sites (74), making ribonucleotide incorporation less frequent in cells than in vitro experiments with whole cell pool estimates would suggest.
Doing More with Less
The consequences of NHEJ polymerase deficiency are not very impressive by a routinely used assay—colony formation after exposure to ionizing radiation—probably because NHEJ does not absolutely need to use a polymerase to resolve a double-strand break. As described earlier, though, closer examination reveals that NHEJ polymerases have critical roles in determining the quality of repair by NHEJ, with important physiological consequences. NHEJ polymerases are required for these roles because they still work when most polymerases will not: they have been engineered to remain active with broken and damaged template, and without abundant dNTPs. This engineering also allows different polymerases to do different things with these substrates, according to the needs imposed by different biologic contexts. NHEJ polymerases thus do not typically determine whether we can do NHEJ, but they have a big impact on how well we do it.
Abbreviations Used
- AEP
archaeo-eukaryal primase superfamily
- Alt-EJ
alternate end joining
- BER
base excision repair
- Bp
base pair
- BRCT
similar to BRCA1 C terminal protein–protein interaction domain
- DNA-PKcs
DNA-dependent protein kinase catalytic subunit
- HR
homologous recombination
- kDa
kilodalton
- Lig D
ligase D
- MMS
methyl methanesulfonate
- NHEJ
nonhomologous end joining
- Pol
polymerase
- TdT
terminal deoxynucleotidyl transferase
Acknowledgments
I thank Stephanie A. Nick McElhinny (Ph.D.), Dr. Bryan J. Davis (Ph.D.), and Jody M. Havener for performing a significant fraction of the work referred to earlier. I also thank the other members of the Ramsden laboratory, the Kunkel laboratory, the Pedersen laboratory, and Drs. L. Povirk and L. Blanco for helpful discussion and more. This work was supported by Public Health Service (PHS) grant CA 97096 and a Leukemia and Lymphoma Society scholar award to D.A.R.
References
- 1.Akopiants K. Zhou RZ. Mohapatra S. Valerie K. Lees-Miller SP. Lee KJ. Chen DJ. Revy P. de Villartay JP. Povirk LF. Requirement for XLF/Cernunnos in alignment-based gap filling by DNA polymerases lambda and mu for nonhomologous end joining in human whole-cell extracts. Nucleic Acids Res. 2009;37:4055–4062. doi: 10.1093/nar/gkp283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade P. Martin MJ. Juarez R. Lopez de Saro F. Blanco L. Limited terminal transferase in human DNA polymerase mu defines the required balance between accuracy and efficiency in NHEJ. Proc Natl Acad Sci U S A. 2009;106:16203–16208. doi: 10.1073/pnas.0908492106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aniukwu J. Glickman MS. Shuman S. The pathways and outcomes of mycobacterial NHEJ depend on the structure of the broken DNA ends. Genes Dev. 2008;22:512–527. doi: 10.1101/gad.1631908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aravind L. Koonin EV. Prokaryotic homologs of the eukaryotic DNA-end-binding protein Ku, novel domains in the Ku protein and prediction of a prokaryotic double-strand break repair system. Genome Res. 2001;11:1365–1374. doi: 10.1101/gr.181001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bebenek K. Garcia-Diaz M. Patishall SR. Kunkel TA. Biochemical properties of Saccharomyces cerevisiae DNA polymerase IV. J Biol Chem. 2005;280:20051–20058. doi: 10.1074/jbc.M501981200. [DOI] [PubMed] [Google Scholar]
- 6.Belousova EA. Maga G. Fan Y. Kubareva EA. Romanova EA. Lebedeva NA. Oretskaya TS. Lavrik OI. DNA polymerases beta and lambda bypass thymine glycol in gapped DNA structures. Biochemistry. 2010;49:4695–4704. doi: 10.1021/bi901792c. [DOI] [PubMed] [Google Scholar]
- 7.Benedict CL. Gilfillan S. Thai TH. Kearney JF. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev. 2000;175:150–157. [PubMed] [Google Scholar]
- 8.Bertocci B. De Smet A. Berek C. Weill JC. Reynaud CA. Immunoglobulin kappa light chain gene rearrangement is impaired in mice deficient for DNA polymerase mu. Immunity. 2003;19:203–211. doi: 10.1016/s1074-7613(03)00203-6. [DOI] [PubMed] [Google Scholar]
- 9.Bertocci B. De Smet A. Weill JC. Reynaud CA. Nonoverlapping functions of DNA polymerases mu, lambda, and terminal deoxynucleotidyltransferase during immunoglobulin V(D)J recombination in vivo. Immunity. 2006;25:31–41. doi: 10.1016/j.immuni.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Bjursell G. Skoog L. Control of nucleotide pools in mammalian cells. Antibiot Chemother. 1980;28:78–85. doi: 10.1159/000386063. [DOI] [PubMed] [Google Scholar]
- 11.Blanca G. Villani G. Shevelev I. Ramadan K. Spadari S. Hubscher U. Maga G. Human DNA polymerases lambda and beta show different efficiencies of translesion DNA synthesis past abasic sites and alternative mechanisms for frameshift generation. Biochemistry. 2004;43:11605–11615. doi: 10.1021/bi049050x. [DOI] [PubMed] [Google Scholar]
- 12.Boule JB. Rougeon F. Papanicolaou C. Terminal deoxynucleotidyl transferase indiscriminately incorporates ribonucleotides and deoxyribonucleotides. J Biol Chem. 2001;276:31388–31393. doi: 10.1074/jbc.M105272200. [DOI] [PubMed] [Google Scholar]
- 13.Braithwaite EK. Prasad R. Shock DD. Hou EW. Beard WA. Wilson SH. DNA polymerase lambda mediates a back-up base excision repair activity in extracts of mouse embryonic fibroblasts. J Biol Chem. 2005;280:18469–18475. doi: 10.1074/jbc.M411864200. [DOI] [PubMed] [Google Scholar]
- 14.Brissett NC. Pitcher RS. Juarez R. Picher AJ. Green AJ. Dafforn TR. Fox GC. Blanco L. Doherty AJ. Structure of a NHEJ polymerase-mediated DNA synaptic complex. Science. 2007;318:456–459. doi: 10.1126/science.1145112. [DOI] [PubMed] [Google Scholar]
- 15.Burkhalter MD. Roberts SA. Havener JM. Ramsden DA. Activity of ribonucleotide reductase helps determine how cells repair DNA double strand breaks. DNA Repair (Amst) 2009;8:1258–1263. doi: 10.1016/j.dnarep.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 17.Capp JP. Boudsocq F. Bertrand P. Laroche-Clary A. Pourquier P. Lopez BS. Cazaux C. Hoffmann JS. Canitrot Y. The DNA polymerase lambda is required for the repair of non-compatible DNA double strand breaks by NHEJ in mammalian cells. Nucleic Acids Res. 2006;34:2998–3007. doi: 10.1093/nar/gkl380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capp JP. Boudsocq F. Besnard AG. Lopez BS. Cazaux C. Hoffmann JS. Canitrot Y. Involvement of DNA polymerase mu in the repair of a specific subset of DNA double-strand breaks in mammalian cells. Nucleic Acids Res. 2007;35:3551–3560. doi: 10.1093/nar/gkm243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan CY. Galli A. Schiestl RH. Pol3 is involved in nonhomologous end-joining in Saccharomyces cerevisiae. DNA Repair (Amst) 2008;7:1531–1541. doi: 10.1016/j.dnarep.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Chen S. Inamdar KV. Pfeiffer P. Feldmann E. Hannah MF. Yu Y. Lee JW. Zhou T. Lees-Miller SP. Povirk LF. Accurate in vitro end joining of a DNA double strand break with partially cohesive 3′-overhangs and 3′-phosphoglycolate termini: effect of Ku on repair fidelity. J Biol Chem. 2001;276:24323–24330. doi: 10.1074/jbc.M010544200. [DOI] [PubMed] [Google Scholar]
- 21.Covo S. Blanco L. Livneh Z. Lesion bypass by human DNA polymerase mu reveals a template-dependent, sequence-independent nucleotidyl transferase activity. J Biol Chem. 2004;279:859–865. doi: 10.1074/jbc.M310447200. [DOI] [PubMed] [Google Scholar]
- 22.Covo S. de Villartay JP. Jeggo PA. Livneh Z. Translesion DNA synthesis-assisted non-homologous end-joining of complex double-strand breaks prevents loss of DNA sequences in mammalian cells. Nucleic Acids Res. 2009;37:6737–6745. doi: 10.1093/nar/gkp703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crespan E. Hubscher U. Maga G. Error-free bypass of 2-hydroxyadenine by human DNA polymerase lambda with proliferating cell nuclear antigen and replication protein A in different sequence contexts. Nucleic Acids Res. 2007;35:5173–5181. doi: 10.1093/nar/gkm568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daley JM. Laan RL. Suresh A. Wilson TE. DNA joint dependence of pol X family polymerase action in nonhomologous end joining. J Biol Chem. 2005;280:29030–29037. doi: 10.1074/jbc.M505277200. [DOI] [PubMed] [Google Scholar]
- 25.Daley JM. Wilson TE. Evidence that base stacking potential in annealed 3′ overhangs determines polymerase utilization in yeast nonhomologous end joining. DNA Repair (Amst) 2008;7:67–76. doi: 10.1016/j.dnarep.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davis BJ. Havener JM. Ramsden DA. End-bridging is required for pol mu to efficiently promote repair of noncomplementary ends by nonhomologous end joining. Nucleic Acids Res. 2008;36:3085–3094. doi: 10.1093/nar/gkn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Decottignies A. Microhomology-mediated end joining in fission yeast is repressed by pku70 and relies on genes involved in homologous recombination. Genetics. 2007;176:1403–1415. doi: 10.1534/genetics.107.071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delarue M. Boule JB. Lescar J. Expert-Bezancon N. Jourdan N. Sukumar N. Rougeon F. Papanicolaou C. Crystal structures of a template-independent DNA polymerase: murine terminal deoxynucleotidyltransferase. EMBO J. 2002;21:427–439. doi: 10.1093/emboj/21.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Della M. Palmbos PL. Tseng HM. Tonkin LM. Daley JM. Topper LM. Pitcher RS. Tomkinson AE. Wilson TE. Doherty AJ. Mycobacterial Ku and ligase proteins constitute a two-component NHEJ repair machine. Science. 2004;306:683–685. doi: 10.1126/science.1099824. [DOI] [PubMed] [Google Scholar]
- 30.DeRose EF. Clarkson MW. Gilmore SA. Galban CJ. Tripathy A. Havener JM. Mueller GA. Ramsden DA. London RE. Lee AL. Solution structure of polymerase mu's BRCT domain reveals an element essential for its role in nonhomologous end joining. Biochemistry. 2007;46:12100–12110. doi: 10.1021/bi7007728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dobbs TA. Palmer P. Maniou Z. Lomax ME. O'Neill P. Interplay of two major repair pathways in the processing of complex double-strand DNA breaks. DNA Repair (Amst) 2008;7:1372–1383. doi: 10.1016/j.dnarep.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Doherty AJ. Jackson SP. Weller GR. Identification of bacterial homologues of the Ku DNA repair proteins. FEBS Lett. 2001;500:186–188. doi: 10.1016/s0014-5793(01)02589-3. [DOI] [PubMed] [Google Scholar]
- 33.Dominguez O. Ruiz JF. Lain de Lera T. Garcia-Diaz M. Gonzalez MA. Kirchhoff T. Martinez AC. Bernad A. Blanco L. DNA polymerase mu (Pol mu), homologous to TdT, could act as a DNA mutator in eukaryotic cells. EMBO J. 2000;19:1731–1742. doi: 10.1093/emboj/19.7.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duvauchelle JB. Blanco L. Fuchs RP. Cordonnier AM. Human DNA polymerase mu (Pol mu) exhibits an unusual replication slippage ability at AAF lesion. Nucleic Acids Res. 2002;30:2061–2067. doi: 10.1093/nar/30.9.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eder PS. Walder JA. Ribonuclease H from K562 human erythroleukemia cells: purification, characterization, and substrate specificity. J Biol Chem. 1991;266:6472–6479. [PubMed] [Google Scholar]
- 36.Eder PS. Walder RY. Walder JA. Substrate specificity of human RNase H1 and its role in excision repair of ribose residues misincorporated in DNA. Biochimie. 1993;75:123–126. doi: 10.1016/0300-9084(93)90033-o. [DOI] [PubMed] [Google Scholar]
- 37.Fan W. Wu X. DNA polymerase lambda can elongate on DNA substrates mimicking non-homologous end joining and interact with XRCC4-ligase IV complex. Biochem Biophys Res Commun. 2004;323:1328–1333. doi: 10.1016/j.bbrc.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Diaz M. Bebenek K. Krahn JM. Pedersen LC. Kunkel TA. Structural analysis of strand misalignment during DNA synthesis by a human DNA polymerase. Cell. 2006;124:331–342. doi: 10.1016/j.cell.2005.10.039. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Diaz M. Bebenek K. Kunkel TA. Blanco L. Identification of an intrinsic 5′-deoxyribose-5-phosphate lyase activity in human DNA polymerase lambda: a possible role in base excision repair. J Biol Chem. 2001;276:34659–34663. doi: 10.1074/jbc.M106336200. [DOI] [PubMed] [Google Scholar]
- 40.Garcia-Diaz M. Bebenek K. Larrea AA. Havener JM. Perera L. Krahn JM. Pedersen LC. Ramsden DA. Kunkel TA. Template strand scrunching during DNA gap repair synthesis by human polymerase lambda. Nat Struct Mol Biol. 2009;16:967–972. doi: 10.1038/nsmb.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garcia-Diaz M. Bebenek K. Sabariegos R. Dominguez O. Rodriguez J. Kirchhoff T. Garcia-Palomero E. Picher AJ. Juarez R. Ruiz JF. Kunkel TA. Blanco L. DNA polymerase lambda, a novel DNA repair enzyme in human cells. J Biol Chem. 2002;277:13184–13191. doi: 10.1074/jbc.M111601200. [DOI] [PubMed] [Google Scholar]
- 42.Gilfillan S. Dierich A. Lemeur M. Benoist C. Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993;261:1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- 43.Gong C. Bongiorno P. Martins A. Stephanou NC. Zhu H. Shuman S. Glickman MS. Mechanism of nonhomologous end-joining in mycobacteria: a low-fidelity repair system driven by Ku, ligase D and ligase C. Nat Struct Mol Biol. 2005;12:304–312. doi: 10.1038/nsmb915. [DOI] [PubMed] [Google Scholar]
- 44.Gonzalez-Barrera S. Sanchez A. Ruiz JF. Juarez R. Picher AJ. Terrados G. Andrade P. Blanco L. Characterization of SpPol4, a unique X-family DNA polymerase in Schizosaccharomyces pombe. Nucleic Acids Res. 2005;33:4762–4774. doi: 10.1093/nar/gki780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gozalbo-Lopez B. Andrade P. Terrados G. de Andres B. Serrano N. Cortegano I. Palacios B. Bernad A. Blanco L. Marcos MA. Gaspar ML. A role for DNA polymerase mu in the emerging DJH rearrangements of the postgastrulation mouse embryo. Mol Cell Biol. 2009;29:1266–1275. doi: 10.1128/MCB.01518-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haeryfar SM. Hickman HD. Irvine KR. Tscharke DC. Bennink JR. Yewdell JW. Terminal deoxynucleotidyl transferase establishes and broadens antiviral CD8+ T cell immunodominance hierarchies. J Immunol. 2008;181:649–659. doi: 10.4049/jimmunol.181.1.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hakansson P. Hofer A. Thelander L. Regulation of mammalian ribonucleotide reduction and dNTP pools after DNA damage and in resting cells. J Biol Chem. 2006;281:7834–7841. doi: 10.1074/jbc.M512894200. [DOI] [PubMed] [Google Scholar]
- 48.Hartlerode AJ. Scully R. Mechanisms of double-strand break repair in somatic mammalian cells. Biochem J. 2009;423:157–168. doi: 10.1042/BJ20090942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Havener JM. Nick McElhinny SA. Bassett E. Gauger M. Ramsden DA. Chaney SG. Translesion synthesis past platinum DNA adducts by human DNA polymerase mu. Biochemistry. 2003;42:1777–1788. doi: 10.1021/bi0270079. [DOI] [PubMed] [Google Scholar]
- 50.Heidenreich E. Eisler H. Non-homologous end joining dependency of gamma-irradiation-induced adaptive frameshift mutation formation in cell cycle-arrested yeast cells. Mutat Res. 2004;556:201–208. doi: 10.1016/j.mrfmmm.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Huertas P. DNA resection in eukaryotes: deciding how to fix the break. Nat Struct Mol Biol. 2010;17:11–16. doi: 10.1038/nsmb.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iyer LM. Koonin EV. Leipe DD. Aravind L. Origin and evolution of the archaeo-eukaryotic primase superfamily and related palm-domain proteins: structural insights and new members. Nucleic Acids Res. 2005;33:3875–3896. doi: 10.1093/nar/gki702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joyce CM. Choosing the right sugar: how polymerases select a nucleotide substrate. Proc Natl Acad Sci U S A. 1997;94:1619–1622. doi: 10.1073/pnas.94.5.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Juarez R. Ruiz JF. Nick McElhinny SA. Ramsden D. Blanco L. A specific loop in human DNA polymerase mu allows switching between creative and DNA-instructed synthesis. Nucleic Acids Res. 2006;34:4572–4582. doi: 10.1093/nar/gkl457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kato KI. Goncalves JM. Houts GE. Bollum FJ. Deoxynucleotide-polymerizing enzymes of calf thymus gland, II: properties of the terminal deoxynucleotidyltransferase. J Biol Chem. 1967;242:2780–2789. [PubMed] [Google Scholar]
- 56.Kedzierska K. Thomas PG. Venturi V. Davenport MP. Doherty PC. Turner SJ. La Gruta NL. Terminal deoxynucleotidyltransferase is required for the establishment of private virus-specific CD8+ TCR repertoires and facilitates optimal CTL responses. J Immunol. 2008;181:2556–2562. doi: 10.4049/jimmunol.181.4.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kobayashi Y. Watanabe M. Okada Y. Sawa H. Takai H. Nakanishi M. Kawase Y. Suzuki H. Nagashima K. Ikeda K. Motoyama N. Hydrocephalus, situs inversus, chronic sinusitis, and male infertility in DNA polymerase lambda-deficient mice: possible implication for the pathogenesis of immotile cilia syndrome. Mol Cell Biol. 2002;22:2769–2776. doi: 10.1128/MCB.22.8.2769-2776.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Komori T. Okada A. Stewart V. Alt FW. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993;261:1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- 59.Kramer KM. Brock JA. Bloom K. Moore JK. Haber JE. Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, nonhomologous recombination events. Mol Cell Biol. 1994;14:1293–1301. doi: 10.1128/mcb.14.2.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee JW. Blanco L. Zhou T. Garcia-Diaz M. Bebenek K. Kunkel TA. Wang Z. Povirk LF. Implication of DNA polymerase lambda in alignment-based gap filling for nonhomologous DNA end joining in human nuclear extracts. J Biol Chem. 2004;279:805–811. doi: 10.1074/jbc.M307913200. [DOI] [PubMed] [Google Scholar]
- 61.Lucas D. Escudero B. Ligos JM. Segovia JC. Estrada JC. Terrados G. Blanco L. Samper E. Bernad A. Altered hematopoiesis in mice lacking DNA polymerase mu is due to inefficient double-strand break repair. PLoS Genet. 2009;5:e1000389. doi: 10.1371/journal.pgen.1000389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ma Y. Lu H. Tippin B. Goodman MF. Shimazaki N. Koiwai O. Hsieh CL. Schwarz K. Lieber MR. A biochemically defined system for mammalian nonhomologous DNA end joining. Mol Cell. 2004;16:701–713. doi: 10.1016/j.molcel.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 63.Maga G. Villani G. Crespan E. Wimmer U. Ferrari E. Bertocci B. Hubscher U. 8-Oxo-guanine bypass by human DNA polymerases in the presence of auxiliary proteins. Nature. 2007;447:606–608. doi: 10.1038/nature05843. [DOI] [PubMed] [Google Scholar]
- 64.Mahajan KN. Nick McElhinny SA. Mitchell BS. Ramsden DA. Association of DNA polymerase mu (pol mu) with Ku and ligase IV: role for pol mu in end-joining double-strand break repair. Mol Cell Biol. 2002;22:5194–5202. doi: 10.1128/MCB.22.14.5194-5202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Manke IA. Lowery DM. Nguyen A. Yaffe MB. BRCT repeats as phosphopeptide-binding modules involved in protein targeting. Science. 2003;302:636–639. doi: 10.1126/science.1088877. [DOI] [PubMed] [Google Scholar]
- 66.McVey M. Lee SE. MMEJ repair of double-strand breaks (director's cut): deleted sequences and alternative endings. Trends Genet. 2008;24:529–538. doi: 10.1016/j.tig.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moon AF. Garcia-Diaz M. Batra VK. Beard WA. Bebenek K. Kunkel TA. Wilson SH. Pedersen LC. The X family portrait: structural insights into biological functions of X family polymerases. DNA Repair (Amst) 2007;6:1709–1725. doi: 10.1016/j.dnarep.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moon AF. Garcia-Diaz M. Bebenek K. Davis BJ. Zhong X. Ramsden DA. Kunkel TA. Pedersen LC. Structural insight into the substrate specificity of DNA polymerase mu. Nat Struct Mol Biol. 2007;14:45–53. doi: 10.1038/nsmb1180. [DOI] [PubMed] [Google Scholar]
- 69.Mueller GA. Moon AF. Derose EF. Havener JM. Ramsden DA. Pedersen LC. London RE. A comparison of BRCT domains involved in nonhomologous end-joining: introducing the solution structure of the BRCT domain of polymerase lambda. DNA Repair (Amst) 2008;7:1340–1351. doi: 10.1016/j.dnarep.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nguyen HH. Zemlin M. Ivanov II. Andrasi J. Zemlin C. Vu HL. Schelonka R. Schroeder HW., Jr Mestecky J. Heterosubtypic immunity to influenza A virus infection requires a properly diversified antibody repertoire. J Virol. 2007;81:9331–9338. doi: 10.1128/JVI.00751-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nick McElhinny SA. Havener JM. Garcia-Diaz M. Juarez R. Bebenek K. Kee BL. Blanco L. Kunkel TA. Ramsden DA. A gradient of template dependence defines distinct biological roles for family X polymerases in nonhomologous end joining. Mol Cell. 2005;19:357–366. doi: 10.1016/j.molcel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 72.Nick McElhinny SA. Ramsden DA. Polymerase mu is a DNA-directed DNA/RNA polymerase. Mol Cell Biol. 2003;23:2309–2315. doi: 10.1128/MCB.23.7.2309-2315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nick McElhinny SA. Watts BE. Kumar D. Watt DL. Lundstrom EB. Burgers PM. Johansson E. Chabes A. Kunkel TA. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proc Natl Acad Sci U S A. 2010;107:4949–4954. doi: 10.1073/pnas.0914857107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Niida H. Katsuno Y. Sengoku M. Shimada M. Yukawa M. Ikura M. Ikura T. Kohno K. Shima H. Suzuki H. Tashiro S. Nakanishi M. Essential role of Tip60-dependent recruitment of ribonucleotide reductase at DNA damage sites in DNA repair during G1 phase. Genes Dev. 2010;24:333–338. doi: 10.1101/gad.1863810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pardo B. Gomez-Gonzalez B. Aguilera A. DNA repair in mammalian cells: DNA double-strand break repair: how to fix a broken relationship. Cell Mol Life Sci. 2009;66:1039–1056. doi: 10.1007/s00018-009-8740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pardo B. Ma E. Marcand S. Mismatch tolerance by DNA polymerase Pol4 in the course of nonhomologous end joining in Saccharomyces cerevisiae. Genetics. 2006;172:2689–2694. doi: 10.1534/genetics.105.053512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pelletier H. Sawaya MR. Kumar A. Wilson SH. Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]
- 78.Pfeiffer P. Thode S. Hancke J. Vielmetter W. Mechanisms of overlap formation in nonhomologous DNA end joining. Mol Cell Biol. 1994;14:888–895. doi: 10.1128/mcb.14.2.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Picher AJ. Blanco L. Human DNA polymerase lambda is a proficient extender of primer ends paired to 7,8-dihydro-8-oxoguanine. DNA Repair (Amst) 2007;6:1749–1756. doi: 10.1016/j.dnarep.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Picher AJ. Garcia-Diaz M. Bebenek K. Pedersen LC. Kunkel TA. Blanco L. Promiscuous mismatch extension by human DNA polymerase lambda. Nucleic Acids Res. 2006;34:3259–3266. doi: 10.1093/nar/gkl377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pitcher RS. Brissett NC. Doherty AJ. Nonhomologous end-joining in bacteria: a microbial perspective. Annu Rev Microbiol. 2007;61:259–282. doi: 10.1146/annurev.micro.61.080706.093354. [DOI] [PubMed] [Google Scholar]
- 82.Pitcher RS. Brissett NC. Picher AJ. Andrade P. Juarez R. Thompson D. Fox GC. Blanco L. Doherty AJ. Structure and function of a mycobacterial NHEJ DNA repair polymerase. J Mol Biol. 2007;366:391–405. doi: 10.1016/j.jmb.2006.10.046. [DOI] [PubMed] [Google Scholar]
- 83.Roberts SA. Strande N. Burkhalter MD. Strom C. Havener JM. Hasty P. Ramsden DA. Ku is a 5′-dRP/AP lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214–1217. doi: 10.1038/nature08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romain F. Barbosa I. Gouge J. Rougeon F. Delarue M. Conferring a template-dependent polymerase activity to terminal deoxynucleotidyltransferase by mutations in the Loop1 region. Nucleic Acids Res. 2009;37:4642–4656. doi: 10.1093/nar/gkp460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Roth DB. Wilson JH. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986;6:4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ruiz JF. Juarez R. Garcia-Diaz M. Terrados G. Picher AJ. Gonzalez-Barrera S. Fernandez de Henestrosa AR. Blanco L. Lack of sugar discrimination by human Pol mu requires a single glycine residue. Nucleic Acids Res. 2003;31:4441–4449. doi: 10.1093/nar/gkg637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rydberg B. Game J. Excision of misincorporated ribonucleotides in DNA by RNase H (type 2) and FEN-1 in cell-free extracts. Proc Natl Acad Sci U S A. 2002;99:16654–16659. doi: 10.1073/pnas.262591699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schlissel MS. Structure of nonhairpin coding-end DNA breaks in cells undergoing V(D)J recombination. Mol Cell Biol. 1998;18:2029–2037. doi: 10.1128/mcb.18.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shuman S. Glickman MS. Bacterial DNA repair by non-homologous end joining. Nat Rev Microbiol. 2007;5:852–861. doi: 10.1038/nrmicro1768. [DOI] [PubMed] [Google Scholar]
- 90.Sobol RW. Prasad R. Evenski A. Baker A. Yang XP. Horton JK. Wilson SH. The lyase activity of the DNA repair protein beta-polymerase protects from DNA-damage-induced cytotoxicity. Nature. 2000;405:807–810. doi: 10.1038/35015598. [DOI] [PubMed] [Google Scholar]
- 91.Srivastava DK. Berg BJ. Prasad R. Molina JT. Beard WA. Tomkinson AE. Wilson SH. Mammalian abasic site base excision repair: identification of the reaction sequence and rate-determining steps. J Biol Chem. 1998;273:21203–21209. doi: 10.1074/jbc.273.33.21203. [DOI] [PubMed] [Google Scholar]
- 92.Sterling CH. Sweasy JB. DNA polymerase 4 of Saccharomyces cerevisiae is important for accurate repair of methyl-methanesulfonate-induced DNA damage. Genetics. 2006;172:89–98. doi: 10.1534/genetics.105.049254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tano K. Nakamura J. Asagoshi K. Arakawa H. Sonoda E. Braithwaite EK. Prasad R. Buerstedde JM. Takeda S. Watanabe M. Wilson SH. Interplay between DNA polymerases beta and lambda in repair of oxidation DNA damage in chicken DT40 cells. DNA Repair (Amst) 2007;6:869–875. doi: 10.1016/j.dnarep.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Terrados G. Capp JP. Canitrot Y. Garcia-Diaz M. Bebenek K. Kirchhoff T. Villanueva A. Boudsocq F. Bergoglio V. Cazaux C. Kunkel TA. Hoffmann JS. Blanco L. Characterization of a natural mutator variant of human DNA polymerase lambda which promotes chromosomal instability by compromising NHEJ. PLoS One. 2009;4:e7290. doi: 10.1371/journal.pone.0007290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Thode S. Schafer A. Pfeiffer P. Vielmetter W. A novel pathway of DNA end-to-end joining. Cell. 1990;60:921–928. doi: 10.1016/0092-8674(90)90340-k. [DOI] [PubMed] [Google Scholar]
- 96.Traut TW. Physiological concentrations of purines and pyrimidines. Mol Cell Biochem. 1994;140:1–22. doi: 10.1007/BF00928361. [DOI] [PubMed] [Google Scholar]
- 97.Tseng HM. Tomkinson AE. A physical and functional interaction between yeast Pol4 and Dnl4-Lif1 links DNA synthesis and ligation in nonhomologous end joining. J Biol Chem. 2002;277:45630–45637. doi: 10.1074/jbc.M206861200. [DOI] [PubMed] [Google Scholar]
- 98.Uchiyama Y. Takeuchi R. Kodera H. Sakaguchi K. Distribution and roles of X-family DNA polymerases in eukaryotes. Biochimie. 2009;91:165–170. doi: 10.1016/j.biochi.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Ward JF. Some biochemical consequences of the spatial distribution of ionizing radiation-produced free radicals. Radiat Res. 1981;86:185–195. [PubMed] [Google Scholar]
- 100.Williams RS. Moncalian G. Williams JS. Yamada Y. Limbo O. Shin DS. Groocock LM. Cahill D. Hitomi C. Guenther G. Moiani D. Carney JP. Russell P. Tainer JA. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell. 2008;135:97–109. doi: 10.1016/j.cell.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wilson TE. Lieber MR. Efficient processing of DNA ends during yeast nonhomologous end joining: evidence for a DNA polymerase beta (Pol4)-dependent pathway. J Biol Chem. 1999;274:23599–23609. doi: 10.1074/jbc.274.33.23599. [DOI] [PubMed] [Google Scholar]
- 102.Yu X. Chini CC. He M. Mer G. Chen J. The BRCT domain is a phospho-protein binding domain. Science. 2003;302:639–642. doi: 10.1126/science.1088753. [DOI] [PubMed] [Google Scholar]
- 103.Zhang Y. Wu X. Guo D. Rechkoblit O. Taylor JS. Geacintov NE. Wang Z. Lesion bypass activities of human DNA polymerase mu. J Biol Chem. 2002;277:44582–44587. doi: 10.1074/jbc.M207297200. [DOI] [PubMed] [Google Scholar]
- 104.Zhang Y. Wu X. Yuan F. Xie Z. Wang Z. Highly frequent frameshift DNA synthesis by human DNA polymerase mu. Mol Cell Biol. 2001;21:7995–8006. doi: 10.1128/MCB.21.23.7995-8006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhou RZ. Blanco L. Garcia-Diaz M. Bebenek K. Kunkel TA. Povirk LF. Tolerance for 8-oxoguanine but not thymine glycol in alignment-based gap filling of partially complementary double-strand break ends by DNA polymerase lambda in human nuclear extracts. Nucleic Acids Res. 2008;36:2895–2905. doi: 10.1093/nar/gkn126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhu H. Shuman S. A primer-dependent polymerase function of Pseudomonas aeruginosa ATP-dependent DNA ligase (LigD) J Biol Chem. 2005;280:418–427. doi: 10.1074/jbc.M410110200. [DOI] [PubMed] [Google Scholar]
- 107.Zhu H. Shuman S. Bacterial nonhomologous end joining ligases preferentially seal breaks with a 3′-OH monoribonucleotide. J Biol Chem. 2008;283:8331–8339. doi: 10.1074/jbc.M705476200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu H. Shuman S. Gap filling activities of Pseudomonas DNA ligase D (LigD) polymerase and functional interactions of LigD with the DNA end-binding Ku protein. J Biol Chem. 2010;285:4815–4825. doi: 10.1074/jbc.M109.073874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhu H. Shuman S. Substrate specificity and structure-function analysis of the 3′-phosphoesterase component of the bacterial NHEJ protein, DNA ligase D. J Biol Chem. 2006;281:13873–13881. doi: 10.1074/jbc.M600055200. [DOI] [PubMed] [Google Scholar]