Summary
Background
Many species of mosquitoes, including the major malaria vector Anopheles gambiae, utilize carbon dioxide (CO2) and 1-octen-3-ol as olfactory cues in host-seeking behaviors that underlie their vectorial capacity. However, the molecular and cellular basis of such olfactory responses remains largely unknown.
Results
Here, we use molecular and physiological approaches coupled with systematic functional analyses to define the complete olfactory sensory map of the An. gambiae maxillary palp, an olfactory appendage that mediates the detection of these compounds. In doing so, we identify three olfactory receptor neurons (ORNs) that are organized in stereotyped triads within the maxillary-palp capitate-peg-sensillum population. One ORN is CO2-responsive and characterized by the coexpression of three receptors that confer CO2 responses, whereas the other ORNs express characteristic odorant receptors (AgORs) that are responsible for their in vivo olfactory responses.
Conclusions
Our results describe a complete and highly concordant map of both the molecular and cellular olfactory components on the maxillary palp of the adult female An. gambiae mosquito. These results also facilitate the understanding of how An. gambiae mosquitoes sense olfactory cues that might be exploited to compromise their ability to transmit malaria.
Introduction
Because of its role in transmitting malaria, the Afrotropical vector mosquito Anopheles gambiae represents one of the most significant threats to global health. As is the case for other mosquitoes, An. gambiae uses a large and divergent population of olfactory receptor neurons (ORNs) to respond to a myriad set of chemical cues with which it carries out odor-mediated behaviors, such as host seeking, nectar feeding, and oviposition [1-3]. An. gambiae and other mosquitoes have three olfactory appendages, the antenna, the proboscis, and the maxillary palp, and all are populated by ORN-containing porous sensilla [4, 5]. In contrast to the antenna, which contains the largest quantity and variety of olfactory sensilla, the maxillary palp is much less complex, harboring a single morphological type of chemosensory sensillum, the capitate peg [6]. Ultrastructural studies have revealed that each capitate-peg sensillum in An. gambiae is invariably innervated by three ORNs [6].
Carbon dioxide (CO2) is emitted by all potential vertebrate hosts and serves as a universal attractant to many mosquito species [1, 7]. It has been reported that CO2 stimulation synergizes with host body odor and induces take-off and sustained-flight behaviors in host-seeking anopheline mosquitoes [7-9]. In addition to CO2, 1-octen-3-ol is another small molecule that emanates from large herbivores as well as from humans [10, 11] and has been identified as a behavioral attractant to tsetse flies [10].
The maxillary palp is the primary CO2-sensitive organ of mosquitoes, and early studies observed a specific loss of response to CO2 in palpectomized female Culex mosquitoes [12]. Moreover, in the yellow-fever vector mosquito, Aedes aegypti, two palpal ORNs in the capitate-peg sensillum were shown to exhibit high sensitivity to both CO2 and 1-octen-3-ol in electrophysiological studies [13, 14]. In contrast, relatively little is known about olfactory responses of the maxillary palp in An. gambiae, which uses a different spectrum of odor cues than does Ae. aegypti in mediating host selection and location [1].
At the molecular level, odor coding in insects is thought to rely on the activation of a large family of highly divergent seven-transmembrane-domain odorant-receptor proteins (ORs) [15, 16]. Insect ORNs typically express one highly conserved and broadly expressed nonconventional DmOR83b-like OR together with one or two conventional odorant-binding ORs [16-19]. In An. gambiae, 79 putative odorant receptor (AgOR) genes have been identified [20], and, thus far, two of them have been demonstrated to encode functional ORs [21]. The anopheline DmOR83b ortholog, AgOR7, is widely expressed in olfactory organs [4].
Here we confirm that An. gambiae palpal ORNs respond to CO2 and 1-octen-3-ol with high sensitivity. We characterize palpal chemosensory receptors and map them to ORN triads that are invariably compartmentalized within each capitate-peg sensillum. The three receptors that were previously classified as gustatory receptors (AgGRs) [20] are coexpressed within the CO2-sensitive ORN, and the two AgORs are coexpressed with AgOR7 in their respective ORNs. We demonstrate that AgOR8 and AgOR28 genes encode functional receptors that confer odorant-induced responses when heterologously expressed in Xenopus oocytes. AgOR8 mediates specific and sensitive responses to 1-octen-3-ol, whereas AgOR28 is tuned to a broad panel of odorants. Lastly, we show that the coexpression of the AgGRs confers CO2 responses in the “empty neuron” in vivo expression system of Drosophila. Taken together, these data elucidate the complete molecular and cellular basis of odor coding in the maxillary palp, which An. gambiae uses for detecting olfactory cues that are crucial in establishing its vectorial capacity.
Results
Responses to CO2 and 1-Octen-3-ol in the Maxillary Palp
The maxillary palp of female An. gambiae is comprised of five segments, all of which are densely covered with flattened scales on their dorsal side (Figures 1A and 1B). Capitate pegs, the single type of chemosensory sensilla in this appendage, are distributed on the ventral side of palpal segments two, three and four [6] (Figures 1A and 1B). Transmission electron microscopic (TEM) studies confirmed that one neuron within An. gambiae capitate pegs had a uniquely lamellate dendritic structure (Figure 1C) that is characteristic of insect CO2-responsive ORNs [6, 22].
Figure 1. The Maxillary Palp in Female An. gambiae Mosquitoes.
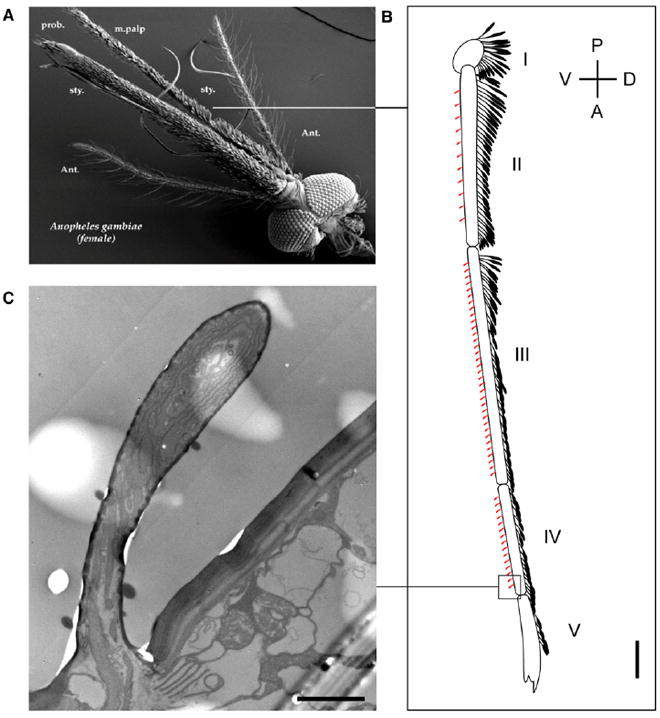
(A) Scanning electron micrograph showing the olfactory appendages of a female An. gambiae mosquito: the antenna, the maxillary palp, and the proboscis.
(B) A schematic drawing based on a bright field photograph showing that the female maxillary palp is comprised of five segments that are densely covered with scales on their dorsal side. Capitate pegs (red) are distributed on the ventral side of palpal segments two, three, and four. The scale bar represents 100 μm.
(C) Transmission electron micrograph showing the specialized lamellate dendritic structure of one neuron within the capitate-peg sensillum. The scale bar represents 2 μm.
In order to characterize the olfactory response profile of this appendage, we performed extensive single-sensillum electrophysiological recordings (SSRs) of 45 capitate-peg sensilla at various positions from all three segments of the maxillary palp. Based on response amplitudes and additional sorting criteria, action potentials in most recordings could be resolved into three distinct populations (Figures 2A–2C), indicating the presence of three ORNs (henceforth referred to as the capitate peg (cp) A, B, or C neuron) within each capitate-peg sensillum, which is in accord with previous ultrastructural studies [6]. The cpA neuron is characterized by the largest action potential amplitude, and the cpB and cpC neurons manifest intermediate and the smallest amplitudes, respectively (Figures 2A–2C).
Figure 2. Single-Sensillum Electrophysiological Recordings from Capitate-Peg Sensilla.
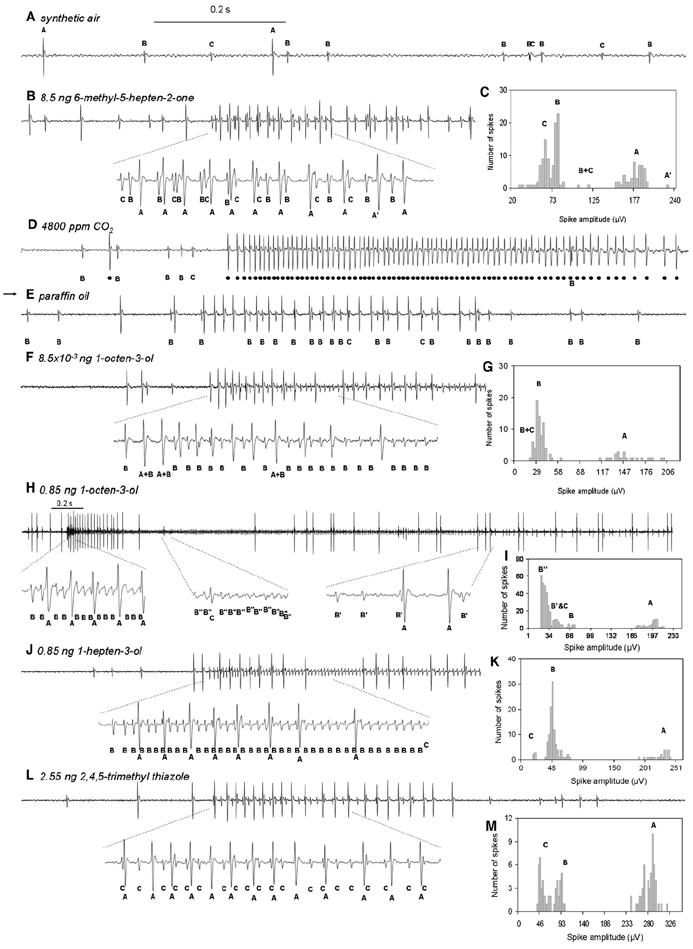
Representative extracellular recordings from capitate-peg sensilla in response to air, CO2, paraffin oil (arrow), and four other odorants. Individual action potentials (spikes) are labeled A, B, or C according to spike amplitude, spike shape, and interspike intervals. The time scales of all presented traces (except for H) are the same, and the 0.2 s odor stimulation for all is indicated above the recording in (A) (horizontal bar). For (H), a different time scale was adopted, and odor stimulation is indicated with a separate bar.
(A) Recording from a capitate-peg sensillum that is exposed to synthetic air.
(B) Both the cpB and cpC neurons are moderately activated by 8.5 ng of 6-methyl-5-hepten-2-one. One large spike marked as A′ represents the superposition of a cpA spike with a cpB or cpC spike.
(C) Spike amplitude distribution of 156 spikes from 2.5 s of the recording in (B).
(D) Large action potentials from the cpA neuron (dots) increase in frequency in response to 4800 ppm of CO2.
(E) The cpA neuron is also activated by paraffin oil (arrow).
(F, H, and J) The cpB neuron responds sensitively to the stimuli of 1-octen-3-ol and 1-hepten-3-ol. As shown in (H), the stimulus of 0.85 ng 1-octen-3-ol suppresses the spike amplitudes of the cpB neuron to a level (marked as B″) smaller than the cpC spikes before the cpB spike amplitudes recover (marked as B′).
(G) Spike amplitude distribution of 96 spikes from 1 s of the recording in (F).
(I) Spike amplitude distribution of 270 spikes from 5 s of the recording in (H).
(K) Spike amplitude distribution of 129 spikes from 1.8 s of the recording in (J).
(L) Response of the cpC neuron to its strongest ligand, 2,4,5-trimethylthiazole.
(M) Spike amplitude distribution of 102 spikes from 5 s of the recording in (L).
To test the response profiles of the ORN population in the maxillary palp, we adopted a broad panel of 97 compounds that included specific odorants implicated in mosquito behavior. All analyzed capitate pegs fell into a uniform functional class, each housing three distinct types of ORNs. Of the 97 compounds tested, we found that 36 were able to elicit responses from at least one of the three neurons (Figure 3A).
Figure 3. Response Spectra and Dose-Response Curves of the Three ORNs within the Capitate-Peg Sensillum.
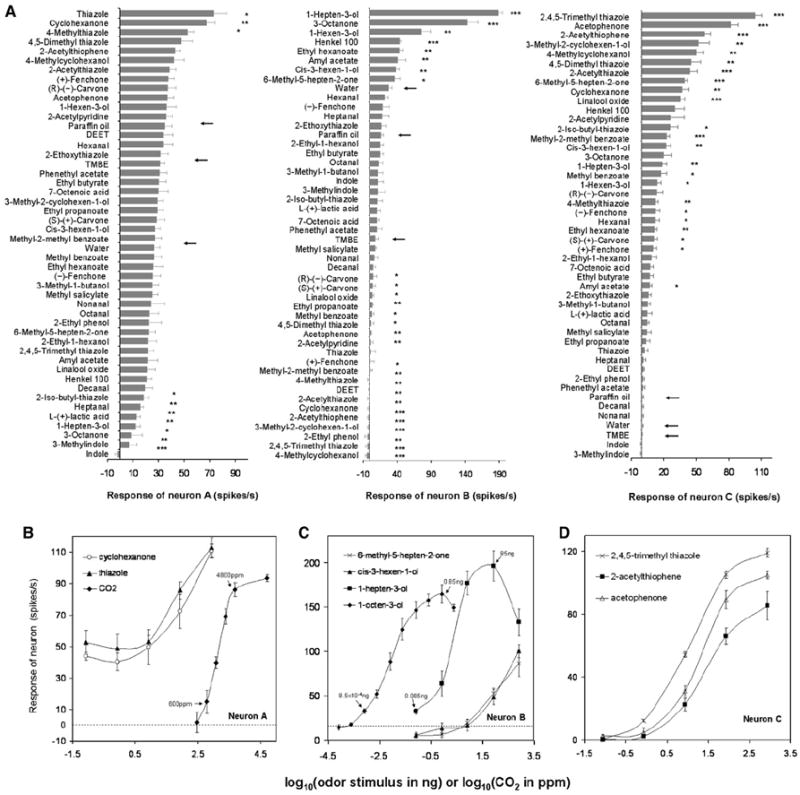
(A) Response spectra to 48 odorants are shown for the three neurons within the capitate-peg sensillum. The odorants are diluted 10 −2 (85 ng) in paraffin oil. Responses indicate increases or decreases over spontaneous frequency (see text) and are presented as spikes/s. Error bars represent the SEM (n = 8–12 recordings). Asterisks indicate a significant increase or decrease in spike frequency of the neuron in response to the odorant compared with the corresponding solvent control (t test, “*” indicates p < 0.05; “**” indicates p < 0.01; and “***” indicates p < 0.001). Responses to the three solvents are indicated by arrows.
(B–D) Dose-response curves of the three neurons in capitate-peg sensilla to their most-potent ligands. Concentrations of CO2 are plotted as log10ppm, and concentrations of other odorants are plotted as log10ng. Error bars represent the SEM (n = 6). For the dose-response curve of the cpA neuron to CO2, response threshold and saturation level are marked (small arrows) (B). Dose-response curves of the cpB neuron are shown in (C). The dashed line indicates the mean response of this neuron to paraffin oil. Small arrows indicate response thresholds and maximal response concentrations of the cpB neuron to 1-octen-3-ol and 1-hepten-3-ol.
The cpA neuron exhibited a spontaneous firing rate of 6.7 ± 63.1 spikes/s and responded strongly to stimulation by CO2 (Figure 2D). We demonstrated the cpA neuron to be a highly sensitive CO2 detector, with a dose-response curve exhibiting a steep slope of 90 spikes every 10log concentration and a detection threshold at around 600 ppm (Figure 3B). In addition to its CO2 detection, this neuron manifested significant excitatory responses to thiazole, 4-methylthiazole, and cyclohexanone (Figures 3A and 3B). We also note that the cpA neuron, as was observed in the case of an individual ORN within the Drosophila antennal basiconic type 1 (ab1) sensillum [23], was unusually activated by the paraffin oil solvent, accounting for much of its response to other odorants (Figures 2E and 3A, arrow). Finally, the cpA neuron was significantly inhibited by seven compounds, with indole and 3-methylindole inducing the strongest inhibition (Figure 3A).
We showed the cpB neuron, displaying a spontaneous firing rate of 3.2 ± 2.5 spikes/s, to be a highly specific and sensitive detector of 1-octen-3-ol, being able to detect 0.1 ppm or 0.85 pg of this odorant in our delivery system (Figures 2F, 2G, 3A, and 3C). The cpB neuron continued to increase its firing rate to 1-octen-3-ol at concentrations between 8.5 × 10 −4 ng and 0.85 ng until the response reached its maximum at 0.85 ng and started to decline at higher concentrations (Figure 3C). Moreover, we found that, at higher concentrations, stimulation with 1-octen-3-ol suppressed the amplitude of the cpB neuron to a level that was not distinguishable from background noise (Figures 2H and 2I), probably because of overstimulation; thus, the response could no longer be reliably quantified at concentrations higher than 2.55 ng. This neuron also responded strongly to 1-hepten-3-ol (Figures 2J and 2K), and, although the dose-response curve of 1-hepten-3-ol has a shape similar to that of 1-octen-3-ol, its response threshold is about 100 times higher (Figure 3C). Much weaker excitatory responses were evoked by 3-octanone and 1-hexen-3-ol and five other tested odorants (Figures 3A and 3C). Furthermore, the cpB neuron was significantly inhibited by 19 tested compounds, among which 4-methylcyclohexanol, 2,4,5-trimethylthiazole, and 2-ethylphenol elicited the strongest inhibitory effects (Figure 3A). Notably, the insect repellent DEET (N,N-diethyl-3-methylbenzamide) was one of the compounds that inhibited the firing of the cpB neuron (Figure 3A).
The cpC neuron displayed a low spontaneous frequency of 0.8 ± 0.8 spikes/s and was not significantly inhibited by any of the tested odorants (Figure 3A). In contrast to the other two neurons, the cpC neuron appeared to be a more broadly tuned ORN that was excited by 24 compounds of our odor panel; the strongest excitatory responses were found upon stimulation with 2,4,5-trimethylthiazole, acetophenone, and 2-acetylthiophene (Figures 2L, 2M, 3A, and 3D). A small number of compounds (e.g., 6-methyl-5-hepten-2-one) evoked excitatory responses in both cpB and cpC neurons (Figures 2B, 2C, and 3A).
Expression of AgOR8 and AgOR28 Genes in the Maxillary Palp
To search for conventional AgOR genes expressed in the maxillary palp, we initially carried out reverse-transcriptase polymerase chain reaction (RT-PCR) amplifications with primer pairs targeting all 79 AgOR genes, by using RNA extracted from either hand-dissected male or female whole maxillary palps. In analyses of three independently prepared RNAs from each sex, we consistently found amplification products of only AgOR7 and two conventional AgORs, AgOR8 and AgOR28 (Figure S1 in the Supplemental Data available online). Of these, AgOR8 appears to be palp specific because we have never detected it with similar assays in other mosquito olfactory appendages (M.R. and L.J.Z., unpublished data); AgOR28, however, is also expressed in the proboscis that acts as another accessory olfactory appendage in female An. gambiae [5]. We next investigated the localization of these two AgORs in female An. gambiae mosquitoes through the use of fluorescent in situ hybridization (FISH) studies (Figures 4A–4D and Figure S3).
Figure 4. Expression of AgOR and AgGR Genes in the Maxillary Palp.
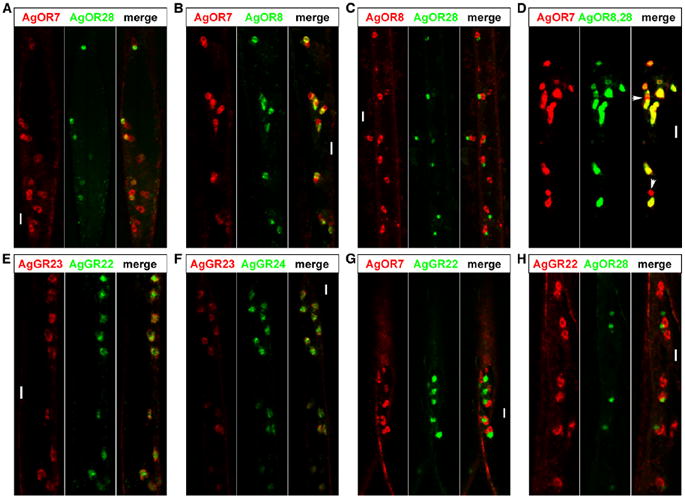
(A) FISH analyses reveal that two AgOR7-positive neurons are paired within the same sensillum, and the AgOR28 probe consistently labels one of the paired neurons.
(B) AgOR8 likewise labels one of the paired AgOR7-positive neurons.
(C) AgOR8 and AgOR28 are not coexpressed and are mapped to paired neurons.
(D) A mixture of AgOR8 and AgOR28 probes labels almost the entire population of AgOR7-positive neurons. However, two neurons in this section are not labeled (arrows).
(E) Probes for AgGR23 and AgGR22 label the same neurons.
(F) AgGR23 and AgGR24 also colabel the same neurons.
(G) AgGR22 is not coexpressed with AgOR7, and the AgGR22-positive neuron forms neuronal triads with the paired AgOR7-positive neurons.
(H) AgGR22 and AgOR28 label paired neurons.
The scale bar represents 10 μm.
We included antisense AgOR7 probes in our FISH experiments as a marker for ORNs and found AgOR7 labeling was consistently restricted to paired ORN cell bodies in palpal segments two to four (Figures 4A, 4B, and 4D and data not shown). As was the case in previous studies in Drosophila and the silkmoth Bombyx mori [17, 18, 24], the labeling of paired cell bodies strongly supports that two adjoining AgOR7-positive ORNs are present within a single capitate-peg sensillum.
FISH analyses of AgOR8 and AgOR28 indicated that both AgORs were colocalized in palpal ORNs along with AgOR7 (Figures 4A and 4B). Antisense AgOR8 and AgOR28 riboprobes labeled one of the paired AgOR7-positive cell bodies (Figures 4A and 4B), suggesting that these AgORs characteristically map to only one of the two ORNs. This was subsequently confirmed by AgOR8-AgOR28 double-labeling experiments in which colocalization was never observed (Figure 4C). Indeed, it appeared that expressions of AgOR8 and AgOR28 were mutually exclusive of each other in these paired maxillary-palp ORNs (Figure 4C). Consistent with this hypothesis, a mixture of AgOR8 and AgOR28 FISH probes labeled an almost entirely overlapping ORN population with the AgOR7 probe (Figure 4D).
A small fraction of AgOR7-positive ORNs (<10%), however, appeared to be devoid of AgOR8 or AgOR28 signals (Figure 4D, arrowheads). We cannot rule out the possibility that AgOR8 or AgOR28 might not be uniformly expressed across the entire ORN population such that, at a given time point, a subset of ORNs can express either AgOR at a level below the detection threshold of our FISH protocols. Alternatively, the minor fraction of unlabeled ORNs observed here might have expressed another chemosensory receptor gene.
AgOR8 and AgOR28 Both Encode Functional Odorant Receptors
Do AgOR8 and AgOR28 encode functional OR proteins, and, if so, do they respond to maxillary-palp stimuli such as CO2 or 1-octen-3-ol? To address these questions, we conducted two-electrode, voltage-clamp recordings in Xenopus oocytes coinjected with complementary RNAs (cRNAs) encoding AgOR8 or AgOR28, along with AgOR7 and a Gα15 subunit. Xenopus oocytes respond to odorant stimuli by the release of Ca2+ from internal stores to activate an endogenous Ca2+-activated Cl− channel. This system has been successfully employed in previous studies so that functions of insect odorant and pheromone receptors could be characterized [18, 25].
We used a panel of 82 odorants in the Xenopus oocyte system. When AgOR28 was coexpressed in oocytes with AgOR7 and Gα15, it conferred the strongest responses to 2,4,5-trimethylthiazole, acetophenone, and 2-acetylthiophene (Figures 5A–5C), as well as weaker responses to 16 other odorants (Figure 5B). Although AgOR28 appeared to be a broadly tuned receptor (Figure 5B, inset), it should be noted that three of its five strongest ligands were shown to be acetyl-containing compounds, one of which, acetophenone, elicits a large inward current of over 2000 nA at a 10−4 dilution (Figure 5B). Importantly, these data also demonstrated that odorant-induced increases in inward currents are consistently dose dependent (Figure 5C). The response spectrum of AgOR28 matches nicely that of the cpC neuron in the SSR analyses, indicating that AgOR28 is probably expressed in this ORN to determine its odorant response properties.
Figure 5. Functional Characterization of AgOR8 and AgOR28 Genes in Xenopus Oocytes.
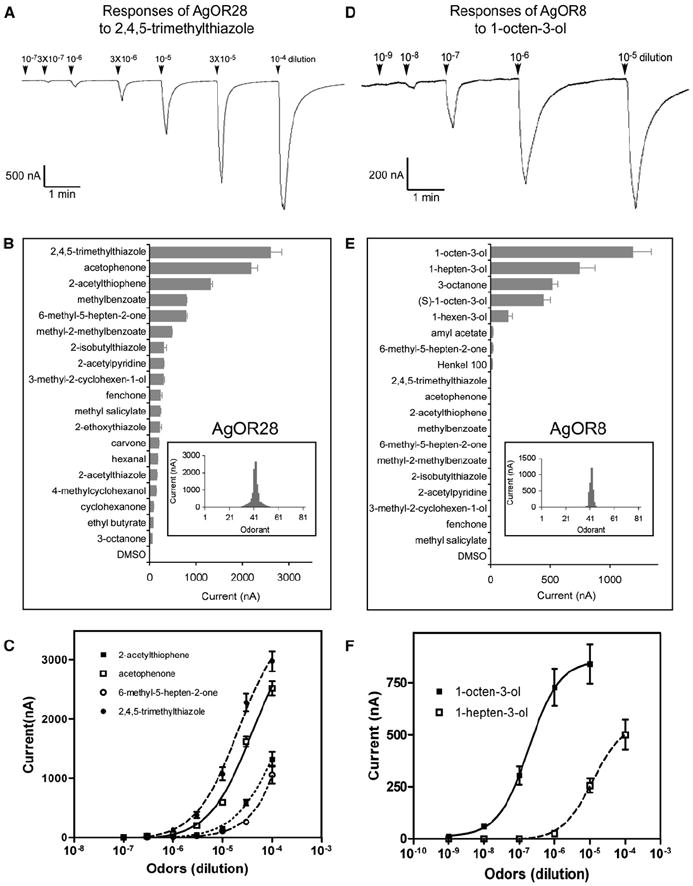
(A) Response trace of AgOR28 to the indicated concentrations of 2,4,5-trimethylthiazole. Odorants are applied for 10 s at the time point indicated by the arrows and induce inward currents in oocytes coinjected with cRNAs encoding AgOR28, AgOR7 and a Gα15 subunit.
(B) Odorant response spectrum of AgOR28 heterologously expressed in Xenopus oocytes. Response is measured as induced currents in nA. Error bars show the SEM (n = 5–6). The tuning curve for this receptor is placed in the inset. The 82 odorants are displayed along the x axis with the odorants eliciting the strongest responses being placed near the center and the odorants eliciting the weakest responses being placed near the edges.
(C) Dose-response curves of AgOR28 to four of its most-effective ligands. Every point represents the means (±SEM) of 5–6 independent oocytes.
(D) Response trace of AgOR8 to the indicated concentrations of 1-octen-3-ol.
(E) Odorant response spectrum of AgOR8 heterologously expressed in Xenopus oocytes. Error bars show the SEM (n = 5–6). The tuning curve for this receptor is placed in the inset.
(F) Dose-response curves of AgOR8 to two most effective ligands. Every point represents the means (±SEM) of 5–6 independent oocytes.
In contrast to AgOR28, AgOR8 was more narrowly tuned when expressed in Xenopus oocytes, displaying strong responses to 1-octen-3-ol, 1-hepten-3-ol, 3-octanone, and 1-hexen-3-ol (Figure 5E, inset, and Figure 5F). Among these, 1-octen-3-ol was clearly the most effective ligand, with as low as a 10−8 dilution of this odorant eliciting a significant response (Figures 5D and 5F). Interestingly, despite the fact that both 1-hepten-3-ol and 1-hexen-3-ol are structurally related to 1-octen-3-ol, AgOR8 displayed strikingly lower sensitivity to these two odorants than to 1-octen-3-ol (Figure 5F). Because 1-octen-3-ol occurs as an optically active mixture of enantiomers in human and animal odors [14], we next tested whether AgOR8 responded to (S)-1-octen-3-ol and found that, at all concentrations, currents induced by (S)-1-octen-3-ol were considerably smaller than currents induced by the racemic mixture (Figure 5E and data not shown). These data closely reflect the response profiles of the cpB neuron of the An. gambiae maxillary palp, strongly suggesting that the expression of AgOR8 in this ORN mediates its highly sensitive response to 1-octen-3-ol.
A Third ORN in the Capitate Peg Expresses Mosquito CO2 Receptors
In Drosophila, one GR gene, DmGR21a, has been implicated in CO2 detection [26, 27] and, more recently, it has been shown that DmGR21a together with a second GR, DmGR63a, mediate CO2 sensitivity [28, 29]. Both An. gambiae and Ae. aegypti have three closely related homologs of DmGR21a and DmGR63a (Figure S2) (20, L.B. Kent and H.M. Robertson, personal communication). Although AgGR22 and AgGR24 are likely to be orthologs of DmGR21a and DmGR63a, with 68% and 65% identity, respectively [20], AgGR23 is less conserved, sharing 38% and 26% identity with DmGR21a and DmGR63a, respectively (Figure S2). Although Drosophila species do not have a close homolog of AgGR23, two non-Dipteran species, the silkworm Bombyx mori and the beetle Tribolium castaneum do (Figure S2), suggesting that AgGR23 is likely to be the ortholog of a DmGR that has been lost subsequent to the divergence of these two species.
To investigate whether these putative mosquito CO2 receptors are expressed in the maxillary palp, we carried out FISH experiments with riboprobes for all three AgGR genes (Figures 4E–4H and Figure S4). We reasoned that the CO2-sensitive ORN might not express AgOR7 and thus would not have been labeled by the AgOR7 probe in our previous FISH experiments. Interestingly, in the female maxillary palp, AgGR22, AgGR23, and AgGR24 were all coexpressed in the same AgOR7-negative neurons that are restricted to segments two to four (Figures 4E and 4F and data not shown). This extends recently published data showing the colocalization of DmGR21a and DmGR63a in Drosophila and AgGR22 and AgGR24 in An. gambiae [28]. Furthermore, AgOR7-AgGR22 double-labeling studies also showed that the AgGR22-positive cell always forms a triad with a set of paired AgOR7-positive ORN cell bodies in the maxillary palp (Figure 4G). This is further supported by the observation that AgGR22 and AgOR28 labeled paired cell bodies in the maxillary palp (Figure 4H).
Moreover, we note that the AgGR22-positive ORN has a much larger cell volume than either of the AgOR7-positive ORNs (Figures 4G and 4H). This is in accordance with its more voluminous dendrite that displays considerably more branching than do the other two dendrites within the capitate peg (Figure 1C) [6].
Coexpression of AgGR Genes Confers a CO2 Response
To test whether the AgGR genes are capable of conferring a response to CO2, we expressed them in an in vivo expression system, the empty neuron system [30]. This system is based on a mutant ORN of the Drosophila antenna, ab3A, which lacks its endogenous receptor and does not respond to odors.
When we coexpressed one copy of both AgGR22 and AgGR24 cDNAs in the empty neuron, we did not observe a response to CO2. However, upon increasing the dosage of both transgenes by 2-fold, we observed a marked response to CO2: 27 ± 5.6 spikes/s (standard error of the mean [SEM]; n = 8) over the spontaneous firing rate (Figures 6A and 6B). A steep dependence on gene dosage was also found for the Drosophila orthologs of these genes [29]. Cyclohexanone elicited only 4.7 ± 1.8 spikes/s (SEM; n = 6) (data not shown), suggesting the possibility that response to this odorant (Figures 3A and 3B) depended on AgGR23 or another receptor.
Figure 6. AgGR22, AgGR23, and AgGR24 Confer a Response to CO2.
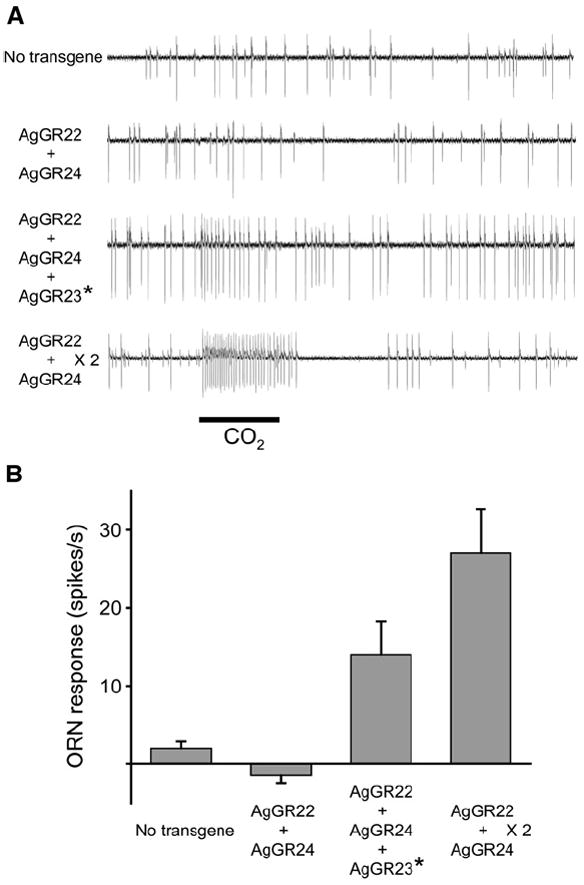
(A) Representative traces from a mutant, transgenic ab3 sensillum, which contains two ORNs, ab3A and ab3B. The large spikes represent the activity of the ab3A neuron and are counted; the small spikes represent the activity of the neighboring ab3B neuron [30]. Flies contain a DmOR22a promoter-GAL4 construct and a variable number of UAS-AgGR transgenes (from top: no transgenes, one copy each of AgGR22 and AgGR24, one copy each of AgGR22, AgGR23*, and AgGR24, and two copies each of AgGR22 and AgGR24). The scale bar represents a 0.5 s stimulus period of 100% CO2.
(B) Mean responses to 100% CO2 of the ab3A neuron expressing the indicated transgenes. The number of spikes in the 0.5 s prestimulus period was subtracted from the number in the 0.5 s CO2 stimulation period. Error bars represent the SEM; n = 8–20. All transgenes are cDNA clones except the AgGR23* transgene, which is marked by an asterisk to indicate that it is a myc-tagged genomic clone.
To investigate whether AgGR23 acts in detection of cyclohexanone or CO2, we added an AgGR23 transgene to the empty neuron containing a single copy of AgGR22 and AgGR24. We found that the addition of the AgGR23 transgene had no effect on response to cyclohexanone (3.0 ± 2.7 spikes/s; n = 8) (data not shown). However, addition of AgGR23 increased the CO2 response from − 1.4 ± 1.0 spikes/s (n = 10) to 14 ± 4.2 spikes/s (n = 8) (Figures 6A and 6B). Although the AgGR23 transgene was a myc-tagged genomic clone, and although we have not tested the response of a neuron containing two copies of all three transgenes, the results clearly implicate AgGR23 in the CO2 response.
In summary, we have shown that coexpression of AgGR genes, orthologs of Drosophila CO2 receptors, is sufficient to confer responses to CO2 on CO2-insensitive ORNs. These results, together with our FISH experiments, show that the ORN coexpressing AgGR22, AgGR23, and AgGR24 is the CO2-sensitive ORN (the cpA neuron) identified in our electrophysiological studies.
Discussion
A Topographic Map of ORNs and Chemosensory Receptors in the Maxillary Palp
We have described a complete and highly concordant map of both the molecular and cellular olfactory components on the maxillary palp of the adult female An. gambiae mosquito, which is the principal Afrotropical vector for malaria. We have used in vivo SSR analyses to identify and physiologically characterize three functional types of ORNs within each capitate-peg sensillum, as well as RT-PCR, FISH, and heterologous expression to fully elucidate the expression patterns and functional characteristics of chemosensory genes throughout the maxillary palp. These studies suggest that a relatively small number of AgOR and AgGR genes are responsible for encoding the entire functional repertoire of chemo-sensory receptors in palpal ORNs.
In Drosophila, a discrete subset of DmOR genes is expressed in the maxillary palp as compared to those found in the antenna [17, 24], and this expression pattern might represent a form of organotypic segregation of sensory inputs across the peripheral olfactory apparatus. In An. gambiae, however, AgOR28 has been detected by RT-PCR amplifications in both the maxillary palp (this study) and the labelum of adult females [5]. This is consistent with observations that acetophenone, one of the strongest ligands for AgOR28, has also been shown to activate a labial sensillum associated with the expression of AgOR28 [5]. Furthermore, 1-octen-3-ol, the most effective ligand for the AgOR8-expressing ORN in the maxillary palp, also elicits responses from a subset of antennal ORNs [3, 31], albeit with much lower sensitivity (threshold at circa 0.25 mg versus 0.85 pg) and response intensity (maximal response at 10 spikes/s versus 165 spikes/s) [31]. These data support a model whereby differential expressions of AgORs across the three olfactory appendages of An. gambiae manifest a range of affinities for overlapping sets of odorant ligands, reflecting a topographic ordering of both high-sensitivity and low-sensitivity receptors. This, in turn, could afford the mosquito a more precise ability to locate and respond to odorant cues across biological space.
Recent anterograde labeling and three-dimensional reconstruction studies of the antennal lobe (AL) from female An. gambiae have revealed convergence of antennal and maxillary-palpal projections onto three dorsal-medial glomeruli [32]. This might represent a fundamental difference from Drosophila, in which sensory inputs of antennal and palpal ORNs fail to converge to common AL glomeruli and remain organotopically distinct [17, 33].
Odor Coding in the An. gambiae Maxillary Palp
We conducted physiological measurements of palpal ORNs against a broad panel of odorants and demonstrated that there were only three types of ORNs (cpA, cpB, and cpC) within the capitate-peg-sensillum repertoire exhibiting partly overlapping but nevertheless distinct response spectra. Of these, two types mediate the highly sensitive detection of two important mosquito kairomones, CO2 and 1-octen-3-ol. Within our panel of tested compounds, the CO2-sensitive cpA neuron was shown to be narrowly tuned to a few odorants. The cpB neuron was also narrowly tuned to a few odorants, including its best ligand 1-octen-3-ol, whereas the cpC neuron appeared to be more broadly tuned.
We successfully used Xenopus oocytes to recapitulate the response profiles of AgOR8 and AgOR28, the two conventional AgORs expressed on the maxillary palp of An. gambiae, and found that the response spectrum measured in oocytes largely corresponded to that of the native ORNs (Figures 3A, 5B, and 5E). These data foster the assignment of AgOR8 and AgOR28 to the cpB and cpC neurons in the maxillary palp, respectively, and are consistent with current paradigms suggesting that insect ORs provide the primary determinant of response spectra of ORNs [18, 34].
AgOR8 and AgOR28 were shown to vary sharply in their breadth of tuning: Although AgOR8 appeared to be a narrowly tuned receptor, AgOR28 responded to a broad panel of odorants, albeit most strongly to only a few ligands, such as 2,4,5-trimethylthiazole and acetophenone. Consistent with previous studies in which some Drosophila ORs show reduced promiscuity when tested with odorants at lower concentrations [34, 35], AgOR28 remained responsive to a smaller subset of odorants at lower concentrations. Furthermore, a substantial fraction of AgOR28’s ligands were demonstrated to be structurally related and fall into two categories: acetyl-containing compounds and thiazole derivatives. Of the two most effective AgOR28 ligands, 2,4,5-trimethylthiazole is a naturally occurring odorant, and acetophenone is a plant volatile that evokes physiological or behavioral responses in many insect species [36]. Because these odorants are not associated with human hosts, these data support a hypothesis that AgOR28 is not directly involved in the host-seeking behaviors of An. gambiae; instead, it might mediate responses to nectar-feeding or oviposition cues. It should, however, be noted that other formal possibilities exist because the odor panel employed here is hardly exhaustive, and AgOR28 might respond with high sensitivity to untested compounds of particular biological significance.
AgOR8 provided the molecular basis for the sensitive palpal response to 1-octen-3-ol. In addition, it was also strongly excited by 1-hepten-3-ol, and considerably weaker responses were elicited from 3-octanone and 1-hexen-3-ol. Furthermore, the native AgOR8-expressing cpB neuron as well as the CO2-sensitive cpA neuron exhibited strikingly prevalent inhibitory responses to a large number of tested odorants; they might use inhibition as a mechanism to suppress noise, as suggested of several Drosophila specialist ORNs [35]. The monounsaturated eight-carbon alcohol 1-octen-3-ol is a well-established odorant cue for mosquitoes [11, 37] and has been documented to synergize the maxillary-palp-based attractiveness of CO2 to several mosquito species [9]. Interestingly, Ae. aegypti also manifests a highly sensitive electrophysiological response to 1-octen-3-ol in its maxillary palp [14] and has a close ortholog of AgOR8 (AaOR8, with 70% identity) that is likewise exclusively expressed in the maxillary palp [38].
The recent data for their Drosophila homologs [28, 29], together with the SSR, FISH, and functional data presented here, strongly support the hypothesis that the palpal cpA neuron coexpressing AgGR22, AgGR23, and AgGR24 mediates CO2 detection in An. gambiae. This ORN reaches its maximal response level near 4000 ppm of CO2 (Figure 3B), about one-tenth the concentration measured in human breath [7]. This concentration corresponds to an apparently important behavioral response threshold because An. gambiae and other mosquitoes do not show increased responses to CO2 levels above 4000 ppm [39]. Fruit flies, however, use this odorant as an avoidance signal [26, 27], and, accordingly, the CO2-sensitive neuron continues to increase its firing rate at CO2 concentrations above 4% [28]. The An. gambiae cpA neuron, similar to the CO2-responsive ORN in Ae. aegypti [13], is a highly sensitive CO2 detector, exhibiting a steep slope of dose-response curves that facilitates detecting small increments in CO2 concentration. However, when compared with the Ae. aegypti neuron, the CO2-sensitive ORN in An. gambiae has a higher detection threshold (around 600 ppm), and the slope of its dose-response curve is not as steep, indicating that CO2-chemosensation is somewhat less acute in this mosquito. This is in agreement with behavioral studies in which this highly anthropophilic mosquito shows less dependency on CO2 in favor of a greater reliance on human-specific cues as a basis for host-seeking behaviors [1, 9].
Like their orthologs in Drosophila, AgGR22 and AgGR24 encode functional receptors and produce a significant response to CO2 when coexpressed in an “empty” olfactory neuron. This result indicates that the two AgGRs are capable of being functionally expressed in a nonnative ORN. Furthermore, as was the case for DmGR21a and DmGR63a [29], the level of response depends on the dosage of AgGR22 and AgGR24 genes. Two copies of both transgenes induced CO2 sensitivity in the ab3A neuron, whereas one copy of both transgenes was insufficient. Interestingly, the ectopic CO2 response in the ab3A neuron is lower than that measured in the An. gambiae cpA neuron. This might be accounted for by lower levels of gene expression achieved with the GAL4-UAS system, by lack of the uniquely lamellate dendritic structure that characterizes the cpA neuron and might facilitate CO2 chemosensation [6, 22], or by lack of additional components. In this regard, we have shown that the third AgGR gene, AgGR23, which is also a close homolog of Drosophila CO2 receptors and is coexpressed with AgGR22 and AgGR24 within the mosquito CO2-sensitive ORN, enhances the response conferred by AgGR22 and AgGR24.
Both FISH and SSR analyses reveal a stereotyped triad of ORNs within the capitate-peg-sensillum population of the maxillary palp: Two AgOR7-positive neurons always pair with a third ORN coexpressing AgGR22, AgGR23, and AgGR24 (Figure 7). One possible biological implication for this stereotyped pairing of the CO2-sensitive ORN and the 1-octen-3-ol-sensitive ORN is that the An. gambiae mosquito might use a complex host odor blend as a crucial host-seeking cue, of which CO2 and 1-octen-3-ol are two components. As a consequence of having the two ORNs responsive to these two components linked together in the same sensilla, the mosquito might ultimately be able to perceive the ratio between these two compounds within the host odor blend with higher fidelity and spatial resolution [40]. This is remarkably similar to several cases of insect pheromone-sensing ORNs, in which neurons responsive to individual components of the pheromone blend are compartmentalized within the same sensillum [18, 41].
Figure 7. Spatial Organization of the Three Neurons within the Capitate-Peg Sensillum.
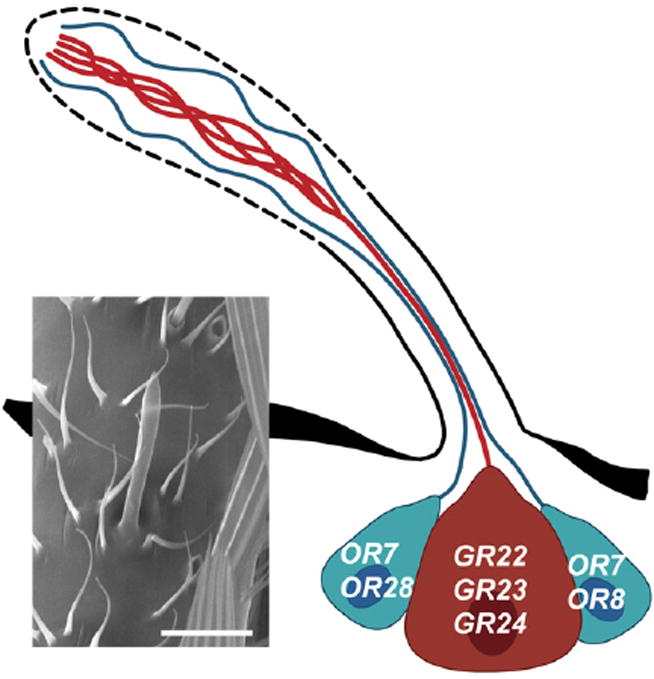
Schematic drawing of three ORNs in a capitate peg as opposed to a scanning electron micrograph of the sensillum (left). Three ORNs form stereotyped triads within each capitate-peg sensillum. The neuron coexpressing AgGR22, AgGR23, and AgGR24 (red) responds to CO2. The other two ORNs (green) express AgOR7 along with AgOR8 or AgOR28, respectively. The scale bar represents 10 μm.
The Maxillary Palp as an Accessory Olfactory Organ
The An. gambiae maxillary palp is an olfactory organ with a relatively simple spatial and functional organization. It has but a single type of chemosensory sensillum, the capitate peg, which is distributed along the three middle segments and invariably innervated by three physiologically and molecularly distinct ORNs. Remarkably, two of these ORNs respond with extreme sensitivity to two crucial signal molecules for mosquitoes, CO2 and 1-octen-3-ol. The stereotyped organization of three ORNs within the capitate-peg sensillum, as defined through our studies, is reminiscent of the Drosophila maxillary palp, in which six functional classes of ORNs are housed in stereotyped pairs within three sensillum types [24, 42]. Although no apparent functional segregation of antennal and palpal ORNs has been observed in Drosophila [23, 42], one type of sensilla, the trichoid sensilla, is exclusively associated with pheromone detection in this insect [43-45]. Our results suggest that the An. gambiae capitate-peg sensillum is another type of functionally specialized olfactory sensillum in insects. The lower sensitivity of AgOR28-positive ORNs in this sensillum might reflect the relative abundance of plant or bacterial odors in the atmosphere and a correspondingly low level of noise from other irrelevant cues. In contrast, host-seeking cues such as CO2 and 1-octen-3-ol are likely to be encountered at much higher noise levels, and the mosquito ORNs responsive to these odorants would be required to detect them at extremely low concentrations that are naturally mixed with a myriad of irrelevant cues. This chemosensory organization might be crucial in enabling the host-seeking An. gambiae mosquito to detect and recognize CO2 and 1-octen-3-ol among a complex odor blend. Perception of CO2 activates mosquitoes and elicits oriented flight behavior, during the course of which they will detect additional cues, including more host-specific odorants, and integrate these signals to drive sustained host-seeking flight [1, 9].
Supplementary Material
Acknowledgments
We thank J. Bohbot for creating the illustrations of Figures 1B and 7; Z. Li, P.L. Russell, L. Sun, F. van Aggelen, A.J. Gidding, and L. Koopman for mosquito rearing and technical assistance; A.L. Goldman, H.P. Yan, and C.E. Carter for valuable advice and help during the development of the FISH technique; M. Birkett, T. Gerke, G. Lepperdinger, D.A. Melton, A.L. George, and H. Hatt for reagents; L.B. Kent and H.M. Robertson for communicating unpublished data; A.M. McAinsh for editorial assistance; and P.X. Xu and members of the Zwiebel, Takken, and Carlson labs for discussions and comments on the manuscript. This work was funded in part by grants from the Foundation for the National Institutes of Health (NIH) through the Grand Challenges in Global Health Initiative to L.J.Z. and from the NIH to J.R.C. and L.J.Z.
Footnotes
Note Added in Proof The data referred to throughout as “L.B. Kent and H.M. Robertson, personal communication” are now in press: Kent, L.B., Walden, K.K.O., and Robertson, H.M. (2007). The Gr family of candidate gustatory and olfactory receptors in the yellow fever mosquito Aedes aegypti. Chem. Senses, in press.
Supplemental Data Experimental Procedures and four figures are available at http://www.current-biology.com/cgi/content/full/17/18/bxs/DC1/.
References
- 1.Takken W, Knols BG. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- 2.Zwiebel LJ, Takken W. Olfactory regulation of mosquito-host interactions. Insect Biochem Mol Biol. 2004;34:645–652. doi: 10.1016/j.ibmb.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu YT, van Loon JJA, Takken W, Meijerink J, Smid HM. Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chem Senses. 2006;31:845–863. doi: 10.1093/chemse/bjl027. [DOI] [PubMed] [Google Scholar]
- 4.Pitts RJ, Fox AN, Zwiebel LJ. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:5058–5063. doi: 10.1073/pnas.0308146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwon HW, Lu T, Rutzler M, Zwiebel LJ. Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2006;103:13526–13531. doi: 10.1073/pnas.0601107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIver S, Siemicki R. Palpal sensilla of selected anopheline mosquitoes. J Parasitol. 1975;61:535–538. [Google Scholar]
- 7.Gillies MT. The role of carbon dioxide in host-finding in mosquitoes (Diptera: Culicidae): a review. Bull Entomol Res. 1980;70:525–532. [Google Scholar]
- 8.Dekker T, Takken W, Carde RT. Structure of host-odour plumes influences catch of Anopheles gambiae s.s. and Aedes aegypti in a dual-choice olfactometer. Physiol Entomol. 2001;26:124–134. [Google Scholar]
- 9.Mboera LEG, Takken W. Carbon dioxide chemotropism in mosquitoes (Diptera: Culicidae) and its potential in vector surveillance and management programmes. Review of Medical Veterinary Entomology. 1997;85:355–368. [Google Scholar]
- 10.Hall DR, Beevor PS, Cork A, Nesbitt BF, Vale GA. 1-octen-3-ol, a potent olfactory stimulant and attractant for tsetse isolated from cattle odours. Insect Sci Appl. 1984;5:335–339. [Google Scholar]
- 11.Cork A, Park KC. Identification of electrophysiologically-active compounds for the malaria mosquito, Anopheles gambiae, in human sweat extracts. Med Vet Entomol. 1996;10:269–276. doi: 10.1111/j.1365-2915.1996.tb00742.x. [DOI] [PubMed] [Google Scholar]
- 12.Omer SM, Gillies MT. Loss of response to carbon dioxide in palpectomized female mosquitoes. Entomology for Experimental Applications. 1971;14:251–252. [Google Scholar]
- 13.Grant AJ, Wigton BE, Aghajanian JG, O’Connell RJ. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J Comp Physiol [A] 1995;177:389–396. doi: 10.1007/BF00187475. [DOI] [PubMed] [Google Scholar]
- 14.Grant AJ, O’Connell RJ. Electrophysiological responses from receptor neurons in mosquito maxillary palp sensilla. Ciba Found Symp. 1996;200:233–248. doi: 10.1002/9780470514948.ch17. discussion 248–253, 281–284. [DOI] [PubMed] [Google Scholar]
- 15.Rutzler M, Zwiebel L. Molecular biology of insect olfaction: Recent progress and conceptual models. J Comp Physiol [A] 2005;191:777–790. doi: 10.1007/s00359-005-0044-y. [DOI] [PubMed] [Google Scholar]
- 16.Hallem EA, Dahanukar A, Carlson JR. Insect odor and taste receptors. Annu Rev Entomol. 2006;51:113–135. doi: 10.1146/annurev.ento.51.051705.113646. [DOI] [PubMed] [Google Scholar]
- 17.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307:1638–1642. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- 19.Larsson MC, Domingos AI, Jones WD, Chiappe ME, Amrein H, Vosshall LB. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Hill CA, Fox AN, Pitts RJ, Kent LB, Tan PL, Chrystal MA, Cravchik A, Collins FH, Robertson HM, Zwiebel LJ. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- 21.Hallem EA, Fox AN, Zwiebel LJ, Carlson JR. Olfaction: Mosquito receptor for human-sweat odorant. Nature. 2004;427:212–213. doi: 10.1038/427212a. [DOI] [PubMed] [Google Scholar]
- 22.McIver SB. Fine structure of pegs on the palps of female culicine mosquitoes. Can J Zool. 1972;50:571–576. doi: 10.1139/z72-078. [DOI] [PubMed] [Google Scholar]
- 23.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 24.Goldman AL, Van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–666. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Wetzel CH, Behrendt HJ, Gisselmann G, Stortkuhl KF, Hovemann B, Hatt H. Functional expression and characterization of a Drosophila odorant receptor in a heterologous cell system. Proc Natl Acad Sci USA. 2001;98:9377–9380. doi: 10.1073/pnas.151103998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209:2739–2748. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- 27.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 28.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 29.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobritsa AA, Van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 31.van den Broek IV, den Otter CJ. Olfactory sensitivities of mosquitoes with different host preferences (Anopheles gambiae s.s., An. arabiensis, An. quadriannulatus, An. m. atroparvus) to synthetic host odours. J Insect Physiol. 1999;45:1001–1010. doi: 10.1016/s0022-1910(99)00081-5. [DOI] [PubMed] [Google Scholar]
- 32.Ghaninia M, Hansson BS, Ignell R. The antennal lobe of the African malaria mosquito, Anopheles gambiae – innervation and three-dimensional reconstruction. Arthropod Structure and Development. 2007;36:23–39. doi: 10.1016/j.asd.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 33.Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 34.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 35.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 36.Wright GA, Lutmerding A, Dudareva N, Smith BH. Intensity and the ratios of compounds in the scent of snapdragon flowers affect scent discrimination by honeybees (Apis mellifera) J Comp Physiol [A] 2005;191:105–114. doi: 10.1007/s00359-004-0576-6. [DOI] [PubMed] [Google Scholar]
- 37.Mboera LE, Takken W, Sambu EZ. The response of Culex quinquefasciatus (Diptera: Culicidae) to traps baited with carbon dioxide, 1-octen-3-ol, acetone, butyric acid and human foot odour in Tanzania. Bull Entomol Res. 2000;90:155–159. doi: 10.1017/s0007485300000262. [DOI] [PubMed] [Google Scholar]
- 38.Bohbot J, Pitts RJ, Kwon HW, Rützler M, Robertson HM, Zwiebel LJ. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol Biol. 2007 doi: 10.1111/j.1365-2583.2007.00748.x. Published online July 17, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costantini C, Gibson G, Sagnon N, Della Torre A, Brady J, Coluzzi M. Mosquito responses to carbon dioxide in a west African Sudan savanna village. Med Vet Entomol. 1996;10:220–227. doi: 10.1111/j.1365-2915.1996.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 40.Baker TC, Fadamiro HY, Cosse AA. Moth uses fine tuning for odour resolution. Nature. 1998;393:530. [Google Scholar]
- 41.Wojtasek H, Hansson BS, Leal WS. Attracted or repelled?–a matter of two neurons, one pheromone binding protein, and a chiral center. Biochem Biophys Res Commun. 1998;250:217–222. doi: 10.1006/bbrc.1998.9278. [DOI] [PubMed] [Google Scholar]
- 42.de Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: The Drosophila maxillary palp. J Neurosci. 1999;19:4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu P, Atkinson R, Jones DN, Smith DP. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45:193–200. doi: 10.1016/j.neuron.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 45.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


