Abstract
Background
The impact of alcohol consumption on depressive symptoms over time among patients who do not meet criteria for alcohol abuse or dependence is not known.
Objective
To evaluate the impact of varying levels of alcohol consumption on depressive symptoms over time in patients with and without HIV infection.
Design
We used data from the Veterans Aging Cohort Study (VACS). We used generalized estimating equations models to assess the association of alcohol-related categories, as a fixed effect, on the time-varying outcome of depressive symptoms.
Participants
VACS is a prospectively enrolled cohort study of HIV-infected patients and age-, race- and site-matched HIV uninfected patients.
Main Measures
Hazardous, binge drinking, alcohol abuse and alcohol dependence were defined using standard criteria. Depressive symptoms were measured by the Patient Health Questionnaire (PHQ-9).
Key Results
Among the 2446 patients, 19% reported past but not current alcohol use, 50% non-hazardous drinking, 8% hazardous drinking, 14% binge drinking, and 10% met criteria for alcohol or dependence. At baseline, depressive symptoms were higher in hazardous and binge drinkers than in past and non-hazardous drinkers (OR=2.65; CI=1.50/4.69; p<.001) and similar to those with abuse or dependence. There was no difference in the association between alcohol-related category and depressive symptoms by HIV status (OR=0.99; CI=.83/1.18; p=.88). Hazardous drinkers were 2.53 (95% CI = 1.34/4.81) times and binge drinkers were 2.14 (95% CI = 1.49/3.07) times more likely to meet criteria for depression when compared to non-hazardous drinkers. The associations between alcohol consumption and depressive symptoms persisted over three years and were responsive to changes in alcohol-related categories.
Conclusions
HIV-infected and HIV-uninfected hazardous and binge drinkers have depressive symptoms that are more severe than non-hazardous and non-drinkers and similar to those with alcohol abuse or dependence. Patients who switch to a higher or lower level of drinking experience a similar alteration in their depressive symptoms. Interventions to decrease unhealthy alcohol consumption may improve depressive symptoms.
Keywords: Alcohol drinking, Alcoholism, Depression, Depressive disorder, HIV, Acquired Immunodeficiency Syndrome
1. INTRODUCTION
High levels of alcohol consumption, alcohol abuse and alcohol dependence are associated with higher levels of depressive symptoms (Sullivan et al., 2005). A recent study examined the association between alcohol abuse or dependence and major depression (Fergusson et al., 2009). Using data from a 25-year longitudinal study of a birth cohort this study determined that there was a significant association between alcohol abuse or dependence and major depression at all ages and for both genders. Those with alcohol abuse or dependence were 1.9 times more likely to also have major depression. Using a series of structural equation models (Muthen et al., 1984) including one that assumed a reciprocal association, one that assumed a unidirectional association with major depression leading to alcohol problems and one that assumed a unidirectional association with alcohol problems leading to major depression, their results suggested a unidirectional causal association in which alcohol problems lead to an increased risk of major depression. There was no evidence of a reverse effect with major depression leading to alcohol abuse or dependence.
Patients with alcohol problems are routinely cared for in primary care. Less severe alcohol problems, such as at-risk drinking, is identified in close to 20% of patients seen in primary care (Fleming et al., 1998). In addition, the prevalence of depression ranges from 5% to 9% in primary care settings (1993; Simon and VonKorff, 1995) with between 40% and 60% of depression treatment occurring in this setting (Harman et al., 2006; Katon et al., 1996; Katon et al., 1995; Katz et al., 1998; Kessler et al., 2003; Pincus et al., 1998).
Patients with HIV are now commonly viewed as having a chronic medical condition and the majority of them receive their care from general internists. Data from the HIV Costs and Service Utilization Study, a study of a probability sample of HIV-infected patients in the U.S., revealed in one survey that of 379 physicians providing care to HIV-infected patients, 56% of them were general internists (Landon et al., 2002). As is true of other patients cared for by primary care physicians, alcohol use is common in patients with HIV and it, along with depression, adversely impacts outcomes in these patients (Cook et al., 2001; Palepu et al., 2004; Samet et al., 2004). The prevalence of alcohol problems and alcohol use disorders in patients with HIV is estimated to range from 22% to 60%(Cook et al., 2001; Lefevre et al., 1995; Petry, 1999; Phillips et al., 2001). Alcohol can lead to enhanced HIV viral replication (Bagasra et al., 1989; Cook et al., 1997), immunosuppression, high-risk sexual behaviors, impaired adherence to antiretroviral medication (Cook et al., 2001; Fabris et al., 2000; Galvan et al., 2002) decreased CD4-lymphocyte counts and higher HIV RNA levels (Samet et al., 2003). Depression is associated with immunosuppression, and poor medication adherence in patients with HIV (Cruess et al., 2003; Evans et al., 2002; Leserman, 2003; Turner et al., 2003).
Given the high rates of both alcohol consumption and depressive symptoms in HIV-infected and uninfected patients seen in primary care, and the spectrum of adverse consequences, it is critical to evaluate the impact of alcohol consumption on depressive symptoms in patients who do not meet criteria for alcohol abuse or dependence and to examine the potential differential effect based on HIV status. Therefore, the current study is designed to determine the impact of varying levels of alcohol consumption and alcohol-related categories on depressive symptoms over time in patients with and without HIV infection.
2. METHODS
2.1 Study Sample
The Veterans Aging Cohort Study (VACS) is a prospectively enrolled cohort study of HIV-infected patients attending Infectious Disease (ID) clinics and age-, race- and site-matched HIV uninfected patients in General Medicine (GM) clinics. The VACS is a multi-site, multiwave study being conducted at eight Veterans Health Administration (VA) healthcare facilities: Atlanta, GA; Baltimore, MD; Bronx, NY; Houston, TX; Los Angeles, CA, New York City, NY; Pittsburgh, PA; and Washington, DC. The study is Institutional Review Board-approved at the VACS coordinating center at the VA Connecticut Healthcare System, at Yale University, and each of the local sites. All study participants provide written informed consent. Patients from two sites (Baltimore, MD and Washington, DC) were not enrolled until after the first wave of follow-up assessments so are not included in the current analysis. Of 3192 potential subjects, 708 were excluded because they had fewer than two follow-up assessments, 11 were excluded because they were missing one or more depression assessments, and 27 were excluded because they indicated that they never had a drink of alcohol in their life. This left the final analytic sample of 2446 patients. The patients excluded from the analyses did not differ significantly from those included on gender, race, marital status, employment status, hepatitis C status, drug, alcohol, or alcohol strata. General medicine patients were more likely to be excluded, as were younger patients; while those on selective serotonin reuptake inhibitors (SSRIs) were more likely to be included.
2.2 Data Collection
Data was collected between 2002 and 2008 using self-administered questionnaires, administrative, pharmacy and laboratory records. The self-report questionnaire was administered at baseline and after one, two, and three years. International Classification of Diseases, Ninth Revision (ICD-9) (1978) diagnostic codes were extracted from the VA administrative data (Justice et al., 2006b). CD4 lymphocyte counts, HIV log10 RNA, and hepatitis antibody status were extracted from laboratory files. Information on use and adherence to highly active antiretroviral (HAART) medication and SSRIs was extracted from the VA pharmacy database. Pharmacy data was reviewed for all prescription medications dispensed. HAART adherence was based on cumulative days exposure to HAART prior to each survey date with less than 90% adherence considered non-adherent (Braithwaite et al., 2007). Alcohol consumption was measured using the Alcohol Use Disorders Identification Test (AUDIT) (Saunders et al., 1993). Depressive symptoms were assessed using the Patient Health Questionnaire (PHQ-9) (Kroenke et al., 2001).
2.3 Alcohol-Related Categories and Depressive Symptoms Measures
All patients were categorized into alcohol-related categories in a sequential and hierarchical fashion based upon their baseline survey AUDIT responses and ICD-9 information. If a patient had been treated for an alcohol use disorder or related diagnosis in the year prior to or up to six months after the survey date based on ICD-9 codes, they were categorized as meeting criteria for alcohol abuse or dependence. Among the remaining patients, those who reported consuming 6 or more drinks on one occasion 3 or more times during the past year were categorized as binge drinkers (Gual et al., 2002). Patients who had an AUDIT score of 5 (females) or 7 (males) or more were categorized as hazardous drinkers (U.S. Department of Health and Human Services (U.S. Department of Health and Human Services, 1995). Those who reported they had consumed alcohol in the past year but did not meet criteria for the above categories were considered to be non-hazardous drinkers. Patients missing an AUDIT assessment score but who drank in the past year were considered to be non-hazardous drinkers. Patients who ever had a drink, but had not had a drink in over one year, were categorized as past drinkers. “Unhealthy” drinkers were those that fit into the category of hazardous drinking, binge drinking or alcohol abuse or dependence (Saitz, 2005). Low-risk drinkers included those with non-hazardous or no use. A PHQ-9 score of greater than nine has an 88% sensitivity and 88% specificity for a diagnosis of major depression (Kroenke et al., 2001).
2.4 Statistical Analyses
Analyses of demographic and clinical characteristics at baseline included descriptive statistics, chi-square, and t-tests. We conducted longitudinal models to evaluate the association between AUDIT and PHQ-9 scores over a 3 year period of time. In these models, using repeated PHQ-9 scores as the outcome, we examined alcohol*HIV*time interaction. Because the primary outcome was binary (depressed or not depressed), we used a generalized estimating equation (GEE) model to assess the association of alcohol-related category, as a fixed (or time-invariant) effect on the time-varying outcome of PHQ depression, with an unstructured covariance structure between the repeated measures. Finally, in patients with all four waves of data (n=1634), we also documented a change from baseline alcohol-related category to alcohol-related category in a subsequent wave and examined their median PHQ-9 scores. “Unhealthy to Unhealthy” were those with unhealthy drinking at all four time points; “Unhealthy to Low-risk” were those with unhealthy drinking at baseline but one subsequent categorization as a low-risk drinker; “Low-risk to Low-risk” were those with Low-risk drinking at all four time points; and “Low-risk to Unhealthy” were those with low-risk drinking at baseline but one subsequent categorization as an unhealthy drinker. All statistical analyses were performed using SAS version 9.1.3 (SAS, Inc., Cary, North Carolina).
3. RESULTS
3.1 Characteristics
At baseline, 19% (464) reported past but not current alcohol use, 49% (1213) non-hazardous use, 8% (191) hazardous, 14% (338) binge, and 10% (240) abuse or dependence. The mean (SD) AUDIT score at baseline was 4.3 (4.2). One thousand six hundred and sixty-seven (68%) were identified as low-risk drinkers, 32% (769) were identified as unhealthy drinkers. The mean (SD) PHQ-9 score at baseline was 5.5 (6.1). Twenty-one percent (501) had a PHQ-9 greater than 9 indicating that they met our criteria for major depression. The baseline demographic, clinical and substance use characteristics of all of the patients, those identified as low-risk drinkers and those identified as unhealthy drinkers are provided in Tables 1 and 2.
Table 1.
Baseline Demographic Characteristics (All Patients and Based on Alcohol-related Category)
| Characteristic | All Patients (N= 2446) |
Low-risk Drinkers (n=1677) |
Unhealthy Drinkers(n= 769) |
P-value |
|---|---|---|---|---|
| Age, mean (SD) (range) | 50.2 (9.7) (22–87) | 49.8 (10.4) (22–87) | 50.9 (8) (28–82) | 0.003 |
| Male, % (n) | 95.2% (2330) | 94.2% (1580) | 97.5% (750) | <0.001 |
| Race/Ethnicity, % (n) | 0.114 | |||
| Black | 57.3% (1401) | 56.2% (942) | 59.7% (459) | |
| White | 32.3% (790) | 33.6% (564) | 29.3% (226) | |
| Other | 10.4%(255) | 10.2% (171) | 10.9% (84) | |
| Marital/Partner Status, %, (n) | 0.018 | |||
| Married | 23.4% (572) | 24.4% (409) | 21.2% (163) | |
| Divorced | 26.4% (645) | 25.6% (430) | 27.9% (215) | |
| Separated | 8.8% (217) | 8.0% (135) | 10.7% (82) | |
| Widowed | 3.8% (94) | 3.3% (55) | 5.1% (39) | |
| Never Married | 27.9% (682) | 28.7% (481) | 26.1% (201) | |
| Living with Partner | 8.9% (217) | 9.2% (154) | 8.2% (63) | |
| Employment, %, (n) | 28.2% (691) | 30.9% (518) | 22.5% (173) | <0.001 |
| High school education or greater, %, (n) | 92.1% (2253) | 93.5% (1569) | 88.9% (684) | <0.001 |
| Homelessness, % (n) | 10.7% (263) | 7.7 (130) | 17.3% (133) | <0.001 |
| HIV-infected, % (n) | 54.7% (1339) | 55.9% (938) | 52.1% (401) | 0.081 |
| Hepatitis C infection, % (n) | 24.5% (600) | 18.0% (302) | 38.7% (298) | <0.001 |
Table 2.
Baseline Clinical and Substance Use Characteristics (All Patients and Based on Alcohol-related Category)
| Characteristic | All Patients (N= 2446) |
Low-risk Drinkers (n=1677) |
Unhealthy Drinkers (n= 769) |
P-value |
|---|---|---|---|---|
| Current illicit drug use, % (n) | 18.67% (457) | 11.0% (184) | 35.5% (273) | <0.001 |
| Alcohol abuse or dependence, % (n) | 9.8% (240) | 0% (0) | 31.2% (240) | <0.001 |
| AUDIT score, mean (SD) (range) | 4.3 (4.2) (0–16) | 2 (1.8) (0–6) | 9.2 (3.8) (0–16) | <0.001 |
| Depression % (n) | <0.001 | |||
| None | 56.1% (1372) | 60.7% (1018) | 46.0% (354) | |
| Mild | 23.4% (573) | 22.1% (370) | 26.4% (573) | |
| Moderate | 9.8% (239) | 7.9% (132) | 13.9% (107) | |
| Moderate-severe | 6.5% (159) | 5.6% (94) | 8.4% (65) | |
| Severe | 4.2% (103) | 3.7% (63) | 5.2% (40) | |
| PHQ-9 score, mean (SD) (range) | 5.5 (6.1) (0–27) | 5 (5.9) (0–27) | 6.7 (6.5) (0–27) | <0.001 |
| Taking SSRI, %, (n) | 19.5% (478) | 16.1% (270) | 27.0% (208) | <0.001 |
| Clinic | 0.081 | |||
| General Medicine, % (n) | 45.2% (1107) | 44.1% (739) | 47.8% (368) | |
| Infectious Disease(HIV), % (n) | 54.7% (1339) | 55.9% (938) | 52.11% (401) | |
| Antiretroviral medication adherence, % (n) (for HIV+ patients) | 0.13 | |||
| Adherent | 21.7% (532) | 23.0% (384) | 19.2% (148) | |
| Not adherent | 21.2% (520) | 21.8% (366) | 20.0% (154) | |
| Not on medication | 11.7% (287) | 11.2% (188) | 12.9% (368) | |
| CD4 count, mean (SD) (range) (for HIV+ patients) | 415.9 (269.8) (2–1900) | 414.4 (269.3) (1–1900) | 419.7 (271.3) (3–1467) | 0.585 |
| HIV log RNA, mean (SD) (range) (for HIV+ patients) | 3.1 (1.3) (1.7–5.9) | 3.1 (1.3) (1.7–5.9) | 3.1 (1.3) (1.7–5.9) | 0.889 |
A greater proportion of those classified as unhealthy drinkers were older (p=.003), male (p<.001), unemployed (p<.001), had evidence of Hepatitis C infection by antibody (p<.001), were current illicit drug users (p<.001), were more likely to meet criteria for depression (p<.001), had higher PHQ-9 scores (p<.001), and were taking an SSRI (p<.001).
Among the 1339 HIV-infected patients, 79% (1058) were on HAART and of those, 51% (539) were considered to have adherence greater than 90%. The mean CD4 was 416, and the mean log10 HIV RNA was 3.1. Thirty percent (401) met criteria for unhealthy drinking and 47% (630) met criteria for depression. Among HIV-infected patients there were no differences in antiretroviral medication adherence, CD4 counts or HIV log RNA levels based on their alcohol-related category.
3.2 Association Between Alcohol-Related Categories and Depressive Symptoms
The association between alcohol-related category and depression is strong and consistent (Figure 1). Hazardous and binge drinkers have depressive symptoms similar to those with alcohol abuse or dependence and significantly higher than past drinkers and non-hazardous drinkers (OR=2.65; CI=1.50/4.69; p<.001) and this association persists over time. There is no difference in depressive symptoms between the groups with hazardous drinking versus abuse/dependence (OR=.87; CI=.63/1.22; p.43), between the groups with binge versus hazardous drinking (OR=1.11; CI=.81/1.52; p=.53) or between the groups with past drinking versus non-hazardous drinking (OR=1.15; CI=.93/1.42; p=.21). After adjusting for correlated outcome data, gender, race, and age, patients with hazardous drinking were 2.53 (95% CI = 1.34/4.81) times and those with binge drinking were 2.14 (95% CI = 1.49/3.07) times more likely to meet criteria for depression when compared to those with non-hazardous levels of drinking (Table 3). Patients with alcohol abuse or dependence were 2.29 (95% CI = 1.44/3.63) times more likely to meet criteria for depression when compared to those with non-hazardous levels of drinking.
Figure 1.
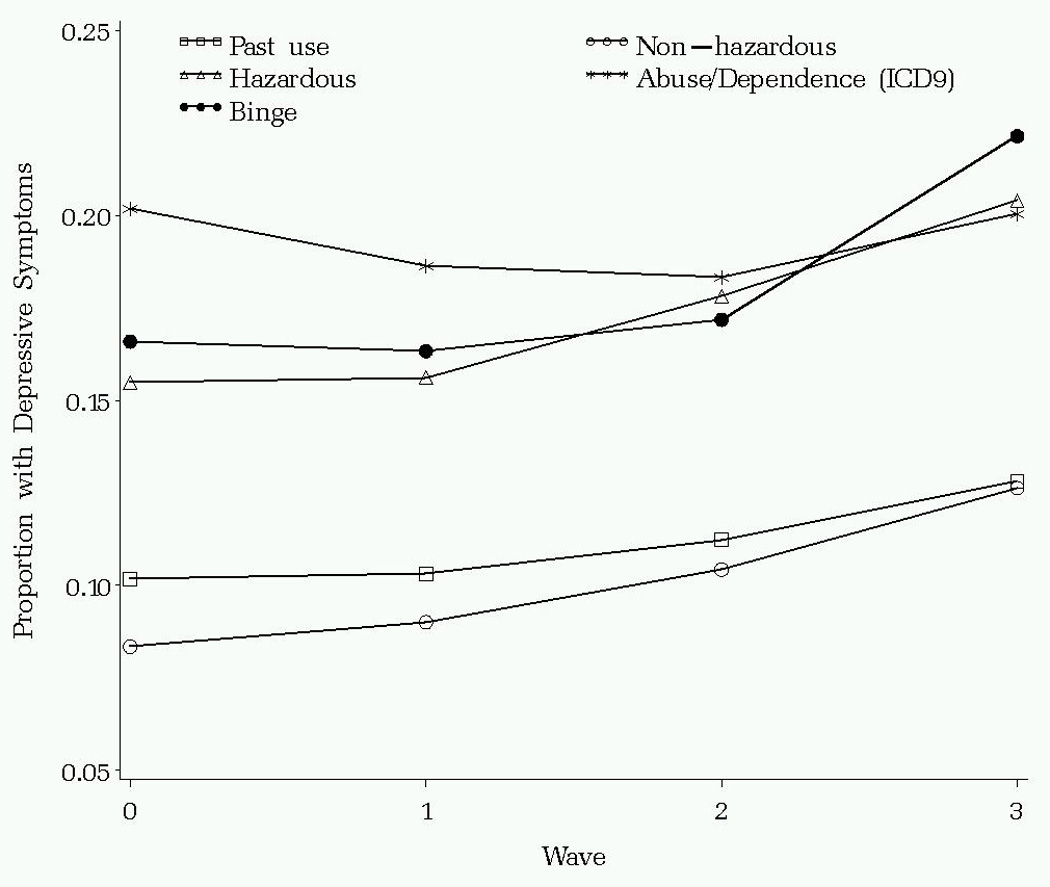
Results of Longitudinal Models of the Association of Alcohol-related Categories and Depressive Symptoms Over Time
Table 3.
Modeled Association of Alcohol-related Categories and Depressive Symptoms Over Time
| Variable | Estimate (Standard Error) |
Odds Ratio |
95% Confidence Interval |
p-value |
|---|---|---|---|---|
| Alcohol abuse or dependence* | 0.83 (0.24) | 2.29 | 1.44–3.63 | <0.001 |
| Binge drinking * | 0.76 (0.19) | 2.14 | 1.49–3.07 | <0.001 |
| Hazardous drinking | 0.93 (0.33) | 2.53 | 1.34–4.81 | <0.001 |
| Past alcohol use | 0.26 (0.21) | 1.30 | 0.86–1.96 | 0.21 |
| HIV-infected ^ | 0.21 (0.16) | 1.23 | 0.90–1.69 | 0.19 |
| Alcohol abuse or dependence X HIV-infected | 0.18 (0.31) | 1.20 | 0.66–2.19 | 0.55 |
| Binge drinking X HIV-infected | −0.46 (0.25) | 0.63 | .38–1.03 | 0.07 |
| Hazardous drinking X HIV-infected | −0.20 (0.45) | 0.82 | 0.34–1.97 | 0.66 |
| Past use X HIV-infected | −0.11 (0.27) | 0.90 | 0.52–1.53 | 0.68 |
| Male † | −0.11 (0.19) | 0.90 | 0.62–1.29 | 0.56 |
| Black ** | −0.22 (0.06) | 0.80 | 0.72–.89 | <0.001 |
| Other/Unknown race | 0.19 (0.08) | 1.21 | 1.03–1.41 | 0.02 |
| Age (years) | −0.02 (0.00) | 0.98 | 0.98–0.99 | <0.001 |
| Time X HIV-infected | −0.10 (0.07) | 0.90 | 0.8–1.03 | 0.14 |
| Time X Alcohol abuse or dependence X HIV-infected | 0.02 (0.14) | 1.02 | 0.91–1.36 | 0.89 |
| Time X Binge drinking X HIV-infected | 0.11 (0.10) | 1.12 | 0.91–1.36 | 0.28 |
| Time X Hazardous drinking X HIV-infected | 0.04 (0.17) | 1.04 | 0.74–1.46 | 0.81 |
| Time X Past alcohol use X HIV-infected | 0.02 (0.12) | 1.02 | 0.82–1.29 | 0.83 |
| Time X time | 0.04 (0.02) | 1.04 | 1.00–1.08 | 0.05 |
y* Reference group is non-hazardous drinkers
Reference group is white race
Reference group is HIV uninfected
Reference group is female
3.3 Association Between Change in Alcohol-Related Categories and Depressive Symptoms over Time
There is an impact of changing from one drinking category to another on depressive symptoms (Table 4). Those patients who remained in the unhealthy drinking category had the highest level of depressive symptoms and those who remained in the low-risk category had the lowest level of depressive symptoms, while those who switched from low-risk to unhealthy had higher levels than those who switched from unhealthy to low-risk drinking categories. Finally, there was no significant difference in the association of alcohol-related category with depressive symptoms by HIV status (3-way interaction was not significant) (OR=0.99; CI=.83/1.18; p=.88).
Table 4.
Impact of Change in Drinking Category on Depressive Symptoms
| Group PHQ-9 Score Over all 4 time points | ||
|---|---|---|
| Change from baseline | N | Median |
| Unhealthy Drinking to Unhealthy Drinking (Unhealthy drinking at all 4 time points) | 66 | 6.5 |
| Unhealthy to Low-risk (Unhealthy drinking at baseline but one categorization of low-risk drinking) | 312 | 5 |
| Low-risk Drinking to Low-risk (Low-risk drinking at all 4 time points) | 743 | 3 |
| Low-risk to Unhealthy (Low-risk drinking at baseline but one categorization of unhealthy drinking) | 126 | 4 |
4. DISCUSSION
This study is one of the first to examine the impact of lower levels of alcohol consumption (not alcohol abuse or dependence) on depressive symptoms in both HIV-infected and HIV-uninfected patients over time. After demonstrating a high prevalence of unhealthy drinking and depressive symptoms in the overall sample (32% identified as unhealthy drinkers and 44% with depressive symptoms – 21% of whom were likely to have greater than mild depression) and among HIV-infected patients (30% identified as unhealthy drinkers and 47% with depressive symptoms-21% of whom were likely to have greater than mild depression), we found that for both HIV-infected and HIV-uninfected individuals, those with lower levels of drinking (hazardous, binge) had depressive symptoms similar to those with alcohol abuse or dependence and that this association persisted over the three year study period.
Hazardous and binge drinking, along with alcohol abuse or dependence were all independently associated with depression after adjusting for potential confounding variables. Perhaps most notably, patients who switched to either a higher or lower level of alcohol-related category experienced a similar alteration in their level of depressive symptoms. Finally, the current study demonstrated that these findings were the same, regardless of HIV status.
Our study has important strengths including a clinical sample, a longitudinal design, demographically similar HIV infected and HIV-uninfected comparators, a large multi-racial, multi-ethnic sample, widely dispersed geographic sites, and long term follow-up. Our sample reflects the racial and ethnic distribution of HIV in the United States and anticipates the aging of the HIV epidemic in that the median age is approximately 10 years older than that reported by the CDC nationally (Justice et al., 2006a) . Nonetheless, Veterans in care may not represent non- Veterans in care more generally.
A recent study examined the impact of alcohol use on depressive symptoms in 400 individuals with HIV and a documented history of alcohol-related diagnoses (Sullivan et al., 2008). This study found that alcohol dependence and heavy alcohol use were significantly associated with higher depression scores in unadjusted models but in adjusted analyses, the association of current alcohol dependence persisted (p<.0001), however, the effect of heavy drinking was no longer noted (p=.11). Therefore, this study, conducted in an exclusively HIV-infected cohort with prior or current alcohol related diagnoses, found in contrast to the current study, that lower levels of alcohol consumption did not have a similar impact as alcohol dependence on depressive symptoms in HIV-infected patients. This finding may be in part due to the fact that the study cohort had a considerable burden of depressive symptoms at baseline and therefore may be masking some of the effect that varying levels of alcohol had on these symptoms.
Our study reveals the interesting finding that the patients with lower levels of drinking in the form of hazardous or binge drinking behaved similarly in terms of their depressive symptoms as those with a diagnosis of alcohol abuse or dependence. These findings highlight the importance of screening for alcohol consumption in this at-risk group (32% of this clinical population were unhealthy drinkers). Although patients with hazardous and binge drinking do not meet criteria for alcohol abuse or dependence, the consequences with respect to depressive symptoms may be very similar. Interestingly, a recent study examining the characteristics of hazardous drinkers found that they were more similar in their socio-demographic characteristics to individuals with alcohol abuse or dependence than they were to those with moderate drinking (Halme et al., 2008). These data prompt the question as to whether these lower levels of drinking, hazardous and binge, should be viewed similar to alcohol abuse or dependence in terms of the evaluation and treatment of these patients?
The current study has limitations. The cohort was comprised of U.S. Veterans and the vast majority were men. Therefore these findings may not be completely generalizable to other populations. In addition, while the data on alcohol consumption and depressive symptoms were collected via self-report raising the concern for some potential bias, the assessments used for this data collection are well-established and validated instruments (Brown et al., 1992; Hesselbrock et al., 1983). We used an eighteen month window around the baseline survey to detect evidence of alcohol abuse or dependence and some individuals with a lifetime history of an alcohol use disorder, and increased risk for depression, may have been misclassified. Most individuals with alcohol use disorders are not diagnosed nor do they receive treatment for their disorder (Hasin et al., 2007) and there is evidence that a major reason is that these individuals do not perceive a need for treatment (Edlund et al, 2009). Given this finding, there is a possibility that individuals in our study who would have met criteria for an alcohol use disorder but who did not receive treatment were misclassified and included in the undiagnosed group in our analysis. This would bias our results toward not finding a difference in depressive symptoms unless the majority of those misclassified were included in the hazardous or binge drinking groups. We believe our use of ICD-9 codes from a variety of settings, both alcohol treatment and non-treatment settings, self-report data on alcohol consumption, and hierarchical assignment to risk categories has increased the specificity of our alcohol classifications and helped to reduce the potential impact of misclassification bias. The PHQ-9 as a measurement of depressive symptoms may be subject to interference by symptoms of the alcohol withdrawal syndrome. There is strong data indicating that alcohol withdrawal symptoms may mimic depressive symptoms (Hasin et al., 2002, Schuckit et al., 1997, Martinotti et al., 2008), therefore some of the subjects in our sample may have been experiencing alcohol withdrawal that was reflected as depressive symptoms on the PHQ-9. The fact that these assessments were conducted during routine clinical care and not during periods of acute alcohol detoxification should diminish the impact of withdrawal on the assessment of depressive symptoms. Nonetheless, alcohol withdrawal occurs over a prolonged period of time and with reductions in alcohol consumption as well as abstinence, therefore future research with more detailed assessments of alcohol withdrawal and specific depression measures could help parse out the overlap between these syndromes. Finally, we did not include in our analyses whether a given subject was currently receiving medication therapy for depression at a given wave which could potentially impact on their level of depressive symptoms.
There are important clinical and research implications of our findings. Patients with lower levels of alcohol consumption (hazardous and binge drinking) who did not meet criteria for alcohol abuse or dependence, had depressive symptoms that were similar to those with abuse or dependence. The association between alcohol-related categories and depressive symptoms persisted over time. Moreover, changing alcohol-related categories, from unhealthy to low-risk drinking was associated with improvement in depressive symptoms. These findings raise the possibility that clinicians caring for these patients may consider alcohol treatments, such as brief interventions, which may decrease alcohol consumption in those with hazardous and binge drinking, to improve depressive symptoms. It also provides a potential rationale to evaluate a more broad application of treatments traditionally reserved for patients meeting criteria for alcohol abuse or dependence such as pharmacotherapies that may in fact be effective in reducing both their unhealthy drinking and depressive symptoms (Anton et al., 2006; Kranzler, 2000; Srisurapanont and Jarusuraisin, 2006). Finally, the current study demonstrated that the association between alcohol-related categories and depressive symptoms were the same, regardless of HIV status.
All these findings warrant further investigation. The similarities between patients with hazardous and binge drinking and alcohol abuse or dependence, both in HIV-infected and HIV-uninfected patients provide critical information regarding treatment options for these groups of unhealthy drinkers. More successful treatments for these patients may not only reduce their levels of depressive symptoms but also provide a more stable foundation on which to provide treatment for their depression. In addition, further exploration into the commonalities between hazardous and binge drinking and alcohol abuse or dependence might shed some light on whether and at what point hazardous and binge drinkers potentially progress to a level of abuse or dependence such that specific interventions can be created to interfere with this transformation, thereby reducing the development of the concomitant health-related consequences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Commission on Professional and Hospital Activities The International Classification of Diseases, 9th Revision, Clinical Modification, (ICD-9-CM) Ann Arbor, MI: 1978. [Google Scholar]
- Depression in primary care: Volume 1. Detection and diagnosis. Rockville, MD: U.S. Department of Health and Human Services; Clinical practice guideline no 5 Depression guideline panel. 1993
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A, Group CSR. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Bagasra O, Kajdacsy-Balla A, Lischner HW. Effects of alcohol ingestion on in vitro susceptibility of peripheral blood mononuclear cells to infection with HIV and of selected T-cell functions. Alcohol. Clin. Exp. Res. 1989;13:636–643. doi: 10.1111/j.1530-0277.1989.tb00396.x. [DOI] [PubMed] [Google Scholar]
- Braithwaite RS, Kozal MJ, Chang CC, Roberts MS, Fultz SL, Goetz MB, Gibert C, Rodriguez-Barradas M, Mole L, Justice AC. Adherence, virological and immunological outcomes for HIV-infected veterans starting combination antiretroviral therapies. AIDS. 2007;21:1579–1589. doi: 10.1097/QAD.0b013e3281532b31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Kranzler HR, Del Boca FK. Self-reports by alcohol and drug abuse inpatients: factors affecting reliability and validity. Br. J. Addict. 1992;87:1013–1024. doi: 10.1111/j.1360-0443.1992.tb03118.x. [DOI] [PubMed] [Google Scholar]
- Conigliaro J, Gordon AJ, McGinnis KA, Rabeneck L, Justice AC Veterans Aging Cohort 3-Site Study. How harmful is hazardous alcohol use and abuse in HIV infection: do health care providers know who is at risk? J. Acquir. Immune Defic. Syndr. 2003;33(4):521–525. doi: 10.1097/00126334-200308010-00014. [DOI] [PubMed] [Google Scholar]
- Cook RL, Sereika SM, Hunt SC, Woodward WC, Erlen JA, Conigliaro J. Problem drinking and medication adherence among persons with HIV infection. J. Gen. Intern. Med. 2001;16:83–88. doi: 10.1111/j.1525-1497.2001.00122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook RT, Stapleton JT, Ballas ZK, Klinzman D. Effect of a single ethanol exposure on HIV replication in human lymphocytes. J. Investig. Med. 1997;45:265–271. [PubMed] [Google Scholar]
- Cruess DG, Petitto JM, Leserman J, Douglas SD, Gettes DR, Ten Have TR, Evans DL. Depression and HIV infection: impact on immune function and disease progression. CNS Spectr. 2003;8:52–58. doi: 10.1017/s1092852900023452. [DOI] [PubMed] [Google Scholar]
- Edlund MJ, Booth BM, Feldman ZL. Perceived need for treatment for alcohol use disorders: results from two national surveys. Psychiatr. Serv. 2009;60(12):1618–1628. doi: 10.1176/appi.ps.60.12.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans DL, Ten Have TR, Douglas SD, Gettes DR, Morrison M, Chiappini MS, Brinker-Spence P, Job C, Mercer DE, Wang YL, Cruess DG, Dube B, Dalen EA, Brown T, Bauer R, Pettito JM. Association of depression with viral load, CD8 T lymphocytes, and natural killer cells in women with HIV infection. Am. J. Psychiatry. 2002;159:1752–1759. doi: 10.1176/appi.ajp.159.10.1752. [DOI] [PubMed] [Google Scholar]
- Fabris P, Tositti G, Manfrin V, Giordani MT, Vaglia A, Cattelan AM, Carlotto A. Does alcohol intake affect highly active antiretroviral therapy (HAART) response in HIV-positive patients? J. Acquir. Immune Defic. Syndr. 2000;25:92–93. doi: 10.1097/00042560-200009010-00013. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Tests of causal links between alcohol abuse or dependence and major depression. Arch. Gen. Psychiatry. 2009;66:260–266. doi: 10.1001/archgenpsychiatry.2008.543. [DOI] [PubMed] [Google Scholar]
- Fleming MF, Manwell LB, Barry KL, Johnson K. At-risk drinking in an HMO primary care sample: prevalence and health policy implications. Am. J. Public Health. 1998;88:90–93. doi: 10.2105/ajph.88.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan FH, Bing EG, Fleishman JA, London AS, Caetano R, Burnam MA, Longshore D, Morton SC, Orlando M, Shapiro M. The prevalence of alcohol consumption and heavy drinking among people with HIV in the United States: results from the HIV Cost and Services Utilization Study. J. Stud. Alcohol. 2002;63:179–186. doi: 10.15288/jsa.2002.63.179. [DOI] [PubMed] [Google Scholar]
- Gual A, Segura L, Contel M, Heather N, Colom J. Audit-3 and audit-4: effectiveness of two short forms of the alcohol use disorders identification test. Alcohol Alcohol. 2002;37:591–596. doi: 10.1093/alcalc/37.6.591. [DOI] [PubMed] [Google Scholar]
- Halme JT, Seppa¨ K, Alho H, Pirkola S, Poikolainen K, Lo¨nnqvist J, Aalto M. Hazardous drinking: Prevalence and associations in the Finnish general population. Alcohol. Clin. Exp. Res. 2008;32:1615–1621. doi: 10.1111/j.1530-0277.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- Harman JS, Veazie PJ, Lyness JM. Primary care physician office visits for depression by older Americans. J. Gen. Intern. Med. 2006;21:926–930. doi: 10.1111/j.1525-1497.2006.00497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch. Gen. Psychiatry. 2007;64(7):830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Grant BF. Major depression in 6050 former drinkers association with past alcohol dependence. Arch. Gen. Psychiatry. 2002;59:794–800. doi: 10.1001/archpsyc.59.9.794. [DOI] [PubMed] [Google Scholar]
- Hesselbrock M, Babor TF, Hesselbrock V, Meyer RE, Workman K. “Never believe an alcoholic”? On the validity of self-report measures of alcohol dependence and related constructs. Int. J. Addict. 1983;18:593–609. doi: 10.3109/10826088309027359. [DOI] [PubMed] [Google Scholar]
- Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, Rodriguez-Barradas MC, Gibert CL, Oursler KAK, Brown S, Leaf DA, Goetz MB, Bryant K. Veterans Aging Cohort Study (VACS): overview and description. Med. Care. 2006a;44:S13–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice AC, Dombrowski E, Conigliaro J, Fultz SL, Gibson D, Madenwald T, Goulet J, Simberkoff M, Butt AA, Rimland D, Rodriguez-Barradas MC, Gibert CL, Oursler KK, Brown S, Leaf DA, Goetz MB, Bryant K. Veterans Aging Cohort Study (VACS): Overview and description. Med. Care. 2006b;44:S12–S24. doi: 10.1097/01.mlr.0000223741.02074.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katon W, Robinson P, Von Korff M, Lin E, Bush T, Ludman E, Simon G, Walker E. A multifaceted intervention to improve treatment of depression in primary care. Arch. Gen. Psychiatry. 1996;53:924–932. doi: 10.1001/archpsyc.1996.01830100072009. [DOI] [PubMed] [Google Scholar]
- Katon W, Von Korff M, Lin E, Walker E, Simon GE, Bush T, Robinson P, Russo J. Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA. 1995;273:1026–1031. [PubMed] [Google Scholar]
- Katz SJ, Kessler RC, Lin E, Wells KB. Medication management of depression in the United States and Ontario. J. Gen. Intern. Med. 1998;13:77–85. doi: 10.1046/j.1525-1497.1998.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS National Comorbidity Survey, R. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Kranzler HR. Medications for alcohol dependence—new vistas. JAMA. 2000;284:1016–1017. doi: 10.1001/jama.284.8.1016. [DOI] [PubMed] [Google Scholar]
- Kroenke KR, Spitzer L, Williams JB. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landon BE, Wilson IB, Wenger NS, Cohn SE, Fichtenbaum CJ, Bozzette SA, Shapiro MF, Cleary PD. Specialty training and specialization among physicians who treat HIV/AIDS in the United States. J. Gen. Intern. Med. 2002;17:12–22. doi: 10.1046/j.1525-1497.2002.10401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre F, O'Leary B, Moran M, Mossar M, Yarnold PR, Martin GJ, Glassroth J. Alcohol consumption among HIV-infected patients. J. Gen. Intern. Med. 1995;10:458–460. doi: 10.1007/BF02599920. [DOI] [PubMed] [Google Scholar]
- Leserman J. HIV disease progression: depression, stress, and possible mechanisms. Biol. Psychiatry. 2003;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Nicola MD, Reina D, Andreoli S, Foca F, Cunniff A, Tonioni F, Bria P, Janiri L. Alcohol protracted withdrawal syndrome: the role of anhedonia. Subst. Use Misuse. 2008;43(3–4):271–284. doi: 10.1080/10826080701202429. [DOI] [PubMed] [Google Scholar]
- Muthen BO. A general structural equation model with dichotomous, ordered categorical, and continuous latent variable indicators. Psychometrika. 1984;49:115–132. [Google Scholar]
- Palepu A, Horton NJ, Tibbetts N, Meli S, Samet JH. Uptake and adherence to highly active antiretroviral therapy among HIV-infected people with alcohol and other substance use problems: the impact of substance abuse treatment. Addiction. 2004;99:361–368. doi: 10.1111/j.1360-0443.2003.00670.x. [DOI] [PubMed] [Google Scholar]
- Petry NM. Alcohol use in HIV patients: what we don't know may hurt us. Int. J. STD AIDS. 1999;10:561–570. doi: 10.1258/0956462991914654. [DOI] [PubMed] [Google Scholar]
- Phillips SJ, Freedberg KA, Traphagen ET, Horton NJ, Samet JH. Screening for alcohol problems in HIV-infected primary care patients. J. Gen. Intern. Med. 2001;16:165. [Google Scholar]
- Pincus HA, Tanielian TL, Marcus SC, Olfson M, Zarin DA, Thompson J, Magno Zito J. Prescribing trends in psychotropic medications: primary care, psychiatry, and other medical specialties. JAMA. 1998;279:526–531. doi: 10.1001/jama.279.7.526. [DOI] [PubMed] [Google Scholar]
- Saitz R. Clinical practice. Unhealthy alcohol use. New Engl. J. Med. 2005;352:596–607. doi: 10.1056/NEJMcp042262. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Meli S, Freedberg KA, Palepu A. Alcohol consumption and antiretroviral adherence among HIV-infected persons with alcohol problems. Alcohol. Clin. Exp. Res. 2004;28:572–577. doi: 10.1097/01.alc.0000122103.74491.78. [DOI] [PubMed] [Google Scholar]
- Samet JH, Horton NJ, Traphagen ET, Lyon SM, Freedberg KA. Alcohol consumption and HIV disease progression: are they related? Alcohol. Clin. Exp. Res. 2003;27:862–867. doi: 10.1097/01.ALC.0000065438.80967.56. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption — II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Tipp JE, Bucholz KK, Nurnberger JI, Hesselbrock CM, Crowe RR, Kramer J. The lifetime rates of 3 major mood disorders and 4 major anxiety disorders in alcoholics and controls. Addiction. 1997;92:1289–1304. [PubMed] [Google Scholar]
- Simon GE, VonKorff M. Recognition, management, and outcomes of depression in primary care. Arch. Fam. Med. 1995;4:99–105. doi: 10.1001/archfami.4.2.99. [DOI] [PubMed] [Google Scholar]
- Srisurapanont M, Jarusuraisin N. Opioid antagonists for alcohol dependence. Cochrane Database Syst. Rev. 2005:CD001867. doi: 10.1002/14651858.CD001867.pub2. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Fiellin DA, O'Connor PG. The prevalence and impact of alcohol problems in major depression: a systematic review. Am. J. Med. 2005;118:330–341. doi: 10.1016/j.amjmed.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Sullivan LE, Saitz R, Cheng DM, Libman H, Nunes D, Samet JH. The impact of alcohol use on depressive symptoms in HIV-infected patients. Addiction. 2008;103:1461–1467. doi: 10.1111/j.1360-0443.2008.02245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BJ, Laine C, Cosler L, Hauck WW. Relationship of gender, depression, and health care delivery with antiretroviral adherence in HIV-infected drug users. J. Gen. Intern. Med. 2003;18:248–257. doi: 10.1046/j.1525-1497.2003.20122.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Washington, DC: U.S. Department of Health and Human Services, P.H.S., National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism; The Physicians' Guide to Helping Patients with Alcohol Problems. 1995


