SUMMARY
Inflammasomes are cytoplasmic sensors of foreign molecules, including pathogens, and function to induce caspase-1 activation and IL-1β cytokine maturation. Whether such a mechanism exists in the nucleus and is effective against nuclear replicating pathogens is unknown. Nuclear replicating herpesvirus KSHV is associated with Kaposi Sarcoma, an angioproliferative tumor characterized by an inflammatory microenvironment including IL-1β. We demonstrate that during KSHV infection of endothelial cells, interferon gamma-inducible protein 16 (IFI16) interacts with the adaptor molecule ASC and procaspase-1 to form a functional inflammasome. This complex was initially detected in the nucleus and subsequently in the peri-nuclear area. KSHV gene expression and/or latent KSHV genome is required for inflammasome activation and IFI16 colocalizes with the KSHV genome in the infected cell nucleus. Caspase-1 activation by KSHV was reduced by IFI16 and ASC silencing. Our studies reveal IFI16 as a nuclear pathogen sensor and demonstrate that the inflammasome also functions in the nucleus.
INTRODUCTION
Kaposi sarcoma associated herpesvirus (KSHV) has been etiologically linked to Kaposi Sarcoma (KS), an angioproliferative tumor of the skin. KS lesions, induced by chronic inflammation, are characterized by endothelial spindle cells latently infected with KSHV, inflammatory cells, inflammatory cytokines (IC), growth factors (GF) and angiogenic factors (AF) (Ganem, 2007). Studies suggest that autocrine and paracrine effects of inflammatory microenvironments drive KS pathogenesis (Ganem, 2007). KS development is associated with elevated levels of ICs such as TNF-α, IFN-γ, IL-6 and IL-1 β. Also elevated levels of IL-1 β, TNF-α and IFN-γ were detected in KS lesions and in most KS patient sera (Samaniego et al., 1997). In vivo studies have demonstrated the ability of these ICs to promote the development of KS-like lesions (Samaniego et al., 1997). An array of germ line-encoded pattern recognition receptors (PRR), such as Toll like receptors (TLRs) and nucleotide binding domain leucine rich repeat containing receptors (NLRs) play key roles in initiating and orchestrating host defenses by regulating the production of pro-inflammatory cytokines. However, persistent activation of these systems might result in a non-resolving chronic inflammation such as the one seen in KS.
IL-1β, a potent multifunctional mediator of the inflammatory response, is one of the cytokine molecules that is secreted in elevated levels in de novo KSHV infected endothelial cells (Sadagopan et al., 2007; Sharma-Walia et al., 2010). IL-1β affects a variety of cells and regulates a wide range of pro-inflammatory responses. To control the inflammatory activity of IL-1β, its synthesis and secretion is highly regulated. IL-1β is synthesized as a 31-kDa precursor protein that is cleaved to form a 17-kDa biologically active mature cytokine by activated caspase-1. However, the caspase-1 activation itself is regulated by a multi-protein complex known as the inflammasome. The inflammasome is a caspase-1 activating molecular platform formed by interaction of three proteins, i) a “sensor protein” recognizing the trigger, ii) an adaptor molecule known as the apoptosis-associated speck-like protein containing CARD (ASC) and iii) procaspase-1. This platform provides the molecular scaffolds required for the proteolytic processing of inactive procaspase-1 in to active caspase-1.
Based on the identity of the sensor protein involved, four types of inflammasomes have been described; NLRP1, NLRC4, NLRP3 and AIM2 inflammasomes. Since inflammatory and angiogenic cytokines contribute to KS pathogenesis, a detailed analysis of how the inflammatory cytokines are controlled in response to latent KSHV infection is crucial for designing therapeutic strategies. However, how KSHV induces the caspase-1 inflammasome required for maturation and secretion of the biologically active IL-1β is not understood.
Studies have shown that the inflammasome acts as a sensor for diverse classes of molecules in the cytoplasm including bacteria, bacterial products, DNA, RNA and viruses replicating in the cytoplasm such as RNA viruses and DNA containing pox virus (Muruve et al., 2008; Rathinam et al., 2010; Schroder and Tschopp, 2010). Sensor molecules such as NLRP3 and AIM2 recognize cytosolic DNA and interact with ASC via a pyrin domain (PYD) to induce caspase-1 activation. However, it is not understood whether such PRRs exist in the nucleus against nuclear replicating DNA viruses including KSHV. Our studies demonstrate that KSHV infection induces caspase-1 activation via an inflammasome pathway involving IFI16 and ASC. IFI16 is a PYD containing HIN-200 protein expressed in the nuclei of endothelial cells. Interestingly, IFI16 mediated inflammasome induction by KSHV was associated with sub-cellular redistribution of ASC, caspase-1 and IFI16 from the nucleus to the cytoplasm. These studies demonstrate the involvement of a “nucleus associated inflammasome sensor component” involving IFI16 in the induction of caspase-1 and IL-1β. Our findings indicate that pathogen sensing functions of the inflammasome extend into the nucleus and IFI16 might have evolved to detect nuclear pathogens.
RESULTS
KSHV infection induces IL-1β and inflammatory cytokines
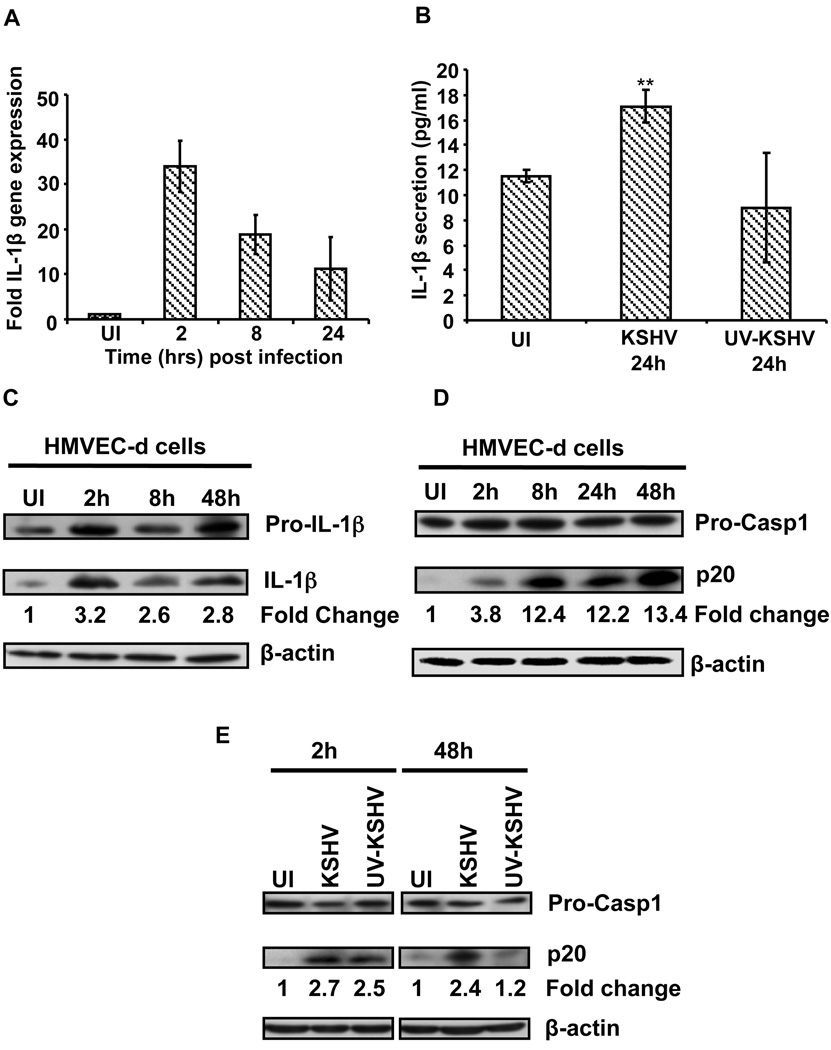
HMVEC-d (endothelial) cells and the human monocytic THP1 cells were infected with KSHV and at different times post infection (p.i.), gene expression and secretion of ICs and GFs were analyzed using PCR and antibody arrays, respectively. We observed the up-regulation of a spectrum of inflammatory cytokines including IL-1β (supplementary information Tables 1A, 1B and 1C). IL-1β precursor gene expression was up-regulated 34, 19 and 11-fold in HMVEC-d cells at 2, 8 and 24h p.i. (Fig. 1A), and 15, 14 and 9-fold in THP1 cells, respectively (Fig. S1A). Compared to uninfected HMVEC-d cells (11 pg/ml), a significant increase in IL-1β secretion (18 pg/ml) was observed in KSHV infected cells (Fig. 1B). Similarly, KSHV infected THP1 cells secreted 5-fold more IL-1β (45 pg/ml) compared to uninfected cells (9 pg/ml) (Fig. S1D).
Figure 1. KSHV infection induces IL-1β maturation and caspase-1 activation.
(A) HMVEC-d cells were infected with KSHV (30 DNA copies/cell) and IL-1β gene expression was examined by real-time PCR. Each bar represents the fold increase in gene expression ± SD of three independent experiments. These fold changes were calculated after normalizing with expression of the 18s rRNA gene (B) HMVEC-d cells were infected with KSHV (50 DNA copies/cell) and culture supernatants from infected and uninfected (UI) cells were used to quantitate the secreted IL-1β by ELISA. ** indicate p<0.05. (c and d) KSHV infected (2h) HMVEC-d cells were washed to remove uninternalized virus and cells were either harvested immediately (2h) or incubated for different time points. Proteins were analyzed by immunoblotting for pro- and mature-IL-1β (C), and for pro-caspase-1 and activated caspase-1 (p20) (D). The bands were analyzed and the fold changes in the mature IL-1β and activated caspase-1 levels were calculated considering uninfected as 1 with β-actin as loading control. (E) HMVEC-d cells were uninfected or infected with either live KSHV or UV inactivated KSHV (UV-KSHV) for 2h, washed, harvested immediately (2h) or incubated for 48h. Caspase-1 activation was analyzed as described above.
KSHV infection induces IL-1β maturation and caspase-1 activation
Analysis of IL-1β processing by Western blotting demonstrated a significant increase in mature IL-1β in lysates of KSHV infected HMVEC-d (Fig. 1C) and THP1 cells (Fig. S1B). Since KSHV infection of HMVEC-d and THP1 cells induced IL-1β processing and secretion, we next examined whether KSHV also induces the caspase-1 inflammasome. Uninfected and KSHV infected HMVEC-d and THP1 cells were examined for caspase-1 activation at different times p.i. As shown in Figure 1D and Figure S1C, KSHV induced a robust activation of caspase-1 as evidenced by the presence of a ∼20-kDa activated caspase-1 (p20) in infected HMVEC-d and THP1 cells. During de novo infection of target cells, KSHV does not undergo a productive lytic cycle; instead KSHV enters into latency with expression of only a few proteins from a non-integrated episomal genome in the infected cell nucleus (Ganem, 2007). Hence, though the cells were incubated with KSHV for only two hours, sustained caspase-1 activation throughout the 48h of observation suggested that KSHV that had entered and established a latent infection was responsible for the observed induction.
KSHV gene expression and/or latent KSHV genome is required for the induction of inflammasome activation
We have shown previously that UV-inactivated KSHV entered target cells but it failed to establish infection, as viral genes were not expressed (Sharma-Walia et al., 2005). At 2h p.i., both UV-inactivated and live-KSHV induced caspase-1 activation (Fig. 1E). However, at 48h p.i., UV-KSHV failed to induce caspase-1 activation, whereas live-KSHV could still strongly induce caspase-1 activation (Fig. 1E). Caspase-1 activation by UV-KSHV at an early time point (2h p.i.) suggested recognition of internalized virion/viral components/DNA by one of the inflammasome-forming NLRs during virus entry by endocytosis and/or nuclear delivery. These findings suggested that viral gene expression and/or latent KSHV genome is crucial for inducing caspase-1 activation and that the latent KSHV infection induces sustained inflammasome assembly leading to caspase-1 activation and maturation of IL-1β.
ASC and caspase-1 colocalize as nuclear and peri-nuclear aggregates upon KSHV infection
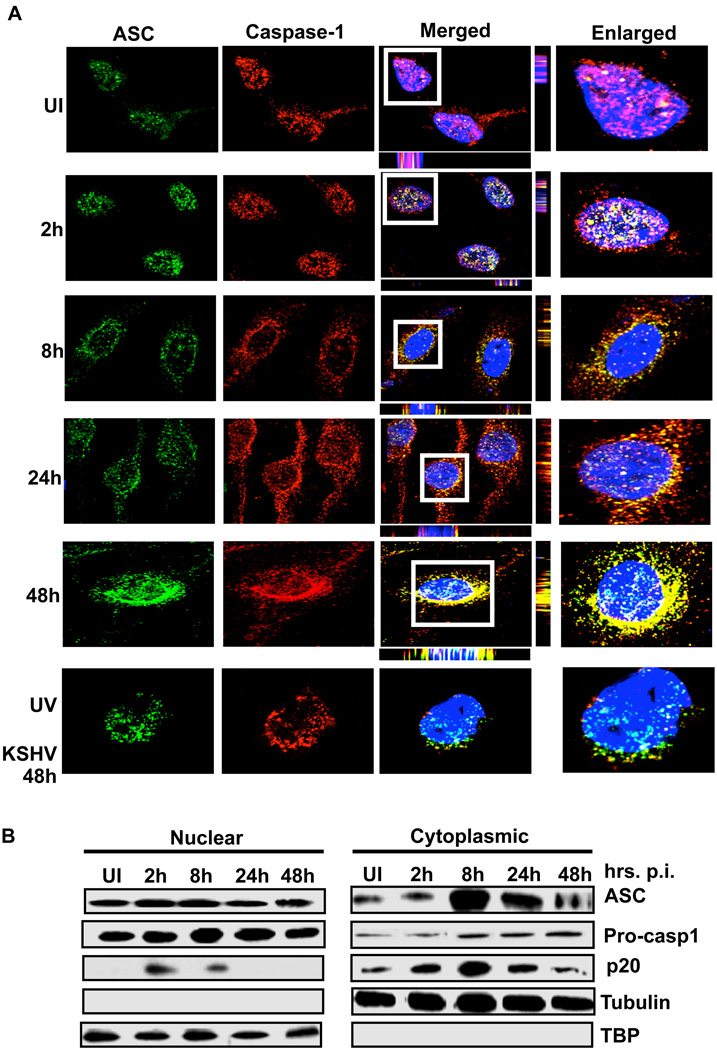
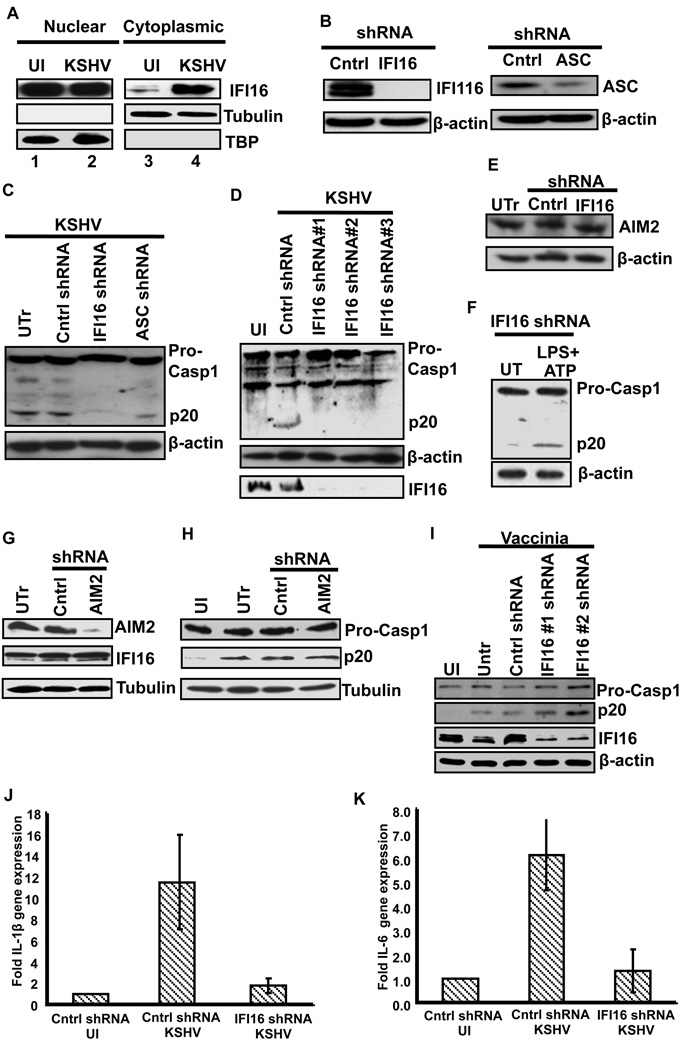
When infected HMVEC-d cells were analyzed by immunofluorescence microscopy, compared to uninfected cells, KSHV infected cells showed a distinct pattern of ASC and caspase-1 staining. In uninfected cells, ASC and caspase-1 showed a diffused pattern of staining which was predominantly concentrated within the nuclei with little distribution in the cytoplasm (Fig. 2A, UI). We also observed a low level of ASC and caspase-1 co-localization within the nuclei (Fig. 2A, UI, white spots). However, upon infection, we observed a speckled pattern of ASC and caspase-1 staining (Fig 2A). Compared to uninfected cells, a much higher level of ASC and caspase-1 colocalization in the infected cell nuclei was observed at 2h p.i. (Fig 2A, 2h, white spots). Subsequently, in addition to nuclear colocalizations, we also observed speckled ASC and caspase-1 aggregates in the peri-nuclear area (Fig. 2A, 8h, 24h, 48h, yellow spots). At 2h p.i., the majority of the cells showed nuclear staining of ASC. However, few cells show sub-cellular redistribution to the peri-nuclear area similar to the one observed at 8 h p.i. The timing of these events is probably dictated by a variety of factors such as the number of virus particles entering the cells, or the timing of virus entry in one cell compared to neighboring cells.
Figure 2. Inflammasome activation and sub-cellular redistribution of ASC and caspase-1.
(A) HMVEC-d cells were uninfected (UI) or infected with either live KSHV or UV-KSHV for 2 h, washed, incubated at 37°C for the indicated time, washed, fixed in 2% paraformaldehyde for 10 min, permeabilized with 0.2% Triton X-100 for 5 min and blocked with signal enhancer. Cells were reacted with anti-ASC and anti-caspase-1 antibodies, washed and incubated with Alexa-488 (green) and Alexa-594 (red) secondary antibodies, respectively. Cell nuclei were visualized by DAPI (blue). The boxed areas were enlarged and shown in the far right panel. (B) HMVEC-d cells were infected with KSHV (30 DNA copies/cell) for 2h, washed and incubated at 37°C for the indicated time. Nuclear and cytoplasmic extracts were examined by immunoblotting with anti-ASC and anti-caspase-1 antibodies. These membranes were stripped and immunoblotted with anti-tubulin and anti-TATA binding protein (TBP) antibodies to check the purity of cytoplasmic and nuclear lysates, respectively and to confirm equal loading.
At 48h p.i., UV inactivated KSHV did not induce any significant ASC and caspase-1 colocalization (Fig. 2A UV-KSHV). This suggested that live viral genome/gene expression is critical for this sub-cellular redistribution.
To further confirm these observations, nuclear and cytoplasmic fractions from uninfected and KSHV infected HMVEC-d cells were analyzed by Western blotting for the sub-cellular distribution of ASC and caspase-1. As seen in Fig. 2B, ASC and procaspase-1 showed steady levels in the nuclear fractions. However, compared to uninfected cells, infected HMVEC-d cells showed higher levels of both ASC and procaspase-1 in the cytoplasmic fractions. These findings suggested that ASC and procaspase-1 undergo sub-cellular redistribution from the nucleus to the cytoplasm upon KSHV infection.
Analysis of the nuclear and cytoplasmic fractions revealed the presence of active caspase-1 (p20) in the nuclear fractions of infected HMVEC-d cells at 2 and 8h p.i. (Fig. 2B) suggesting that inflammasome assembly also occurred in the nucleus of infected cells. Since colocalization of ASC and caspase-1 was observed in the nucleus at 2h p.i. (Fig. 2A), these observations corroborated the strong band of active caspase-1 in the Western blot analysis of nuclear fractions at 2h p.i. (Fig. 2B). Detection of active caspase-1 in infected cells suggested that the inflammasome is activated upon sensing KSHV in the nucleus.
KSHV infection induces formation of an IFI16, ASC and caspase-1 protein complex
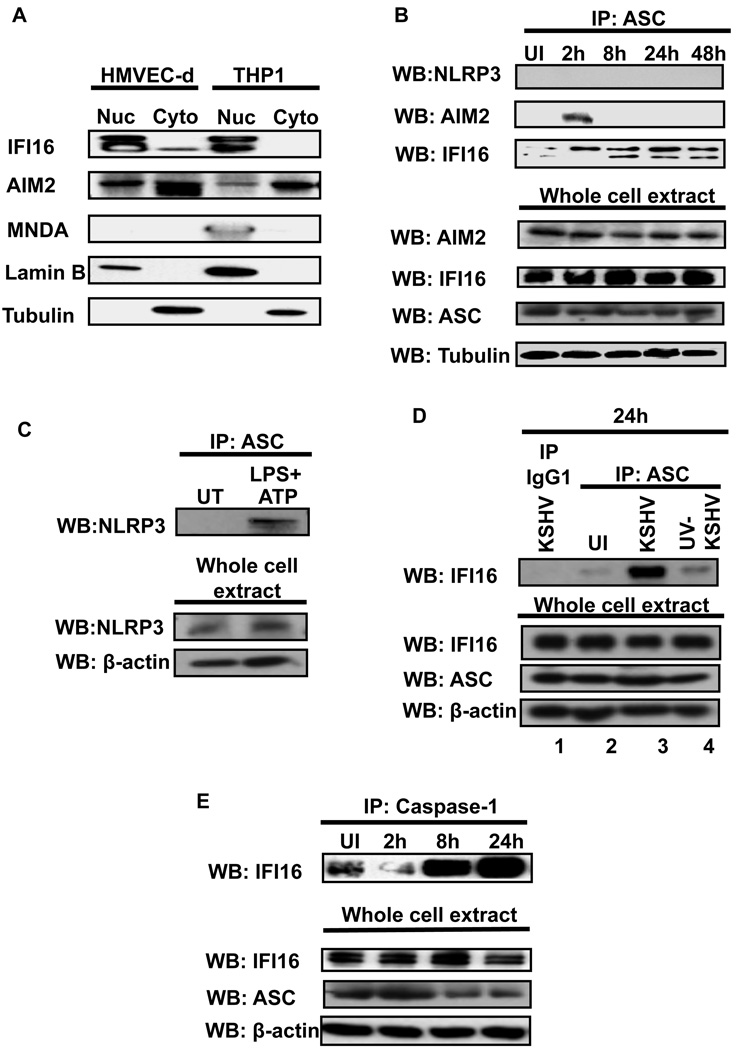
ASC functions as an adaptor molecule recruiting and interacting with “sensor” proteins such as NLRP3 or AIM2 (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009; Martinon et al., 2002). Hence, we next sought to identify the ASC interacting “sensor” protein involved in inflammasome activation during KSHV infection. We considered NLRP1, NLRC4, NLRP3, AIM2, MNDA, IFI16 and IFIX as potential ASC interacting proteins. We ruled out the possibility of NLRC4 and NLRP1 participation as ASC is dispensable for the NLRP1 and NLRC4 inflammasomes (Case et al., 2009; Hsu et al., 2008). We excluded MNDA as this protein was not expressed in HMVEC-d cells (Fig. 3A) and excluded IFIX as its gene expression could not be detected in HMVEC-d cells (data not shown). We selected NLRP3, AIM2 and IFI16 as the potential ASC interacting proteins and examined their expression in uninfected cells by Western blots and real-time RT-PCR (Fig. 3A and Fig. S2). IFI16 was detected predominantly in the nucleus of HMVEC-d and THP1 cells. It was not detected in the cytoplasm of THP1 cells and only very low levels were observed in the cytoplasm of HMVEC-d cells (Fig. 3A). In contrast, AIM2 was detected in the nucleus as well as the cytoplasm of both HMVEC-d and THP1 cells with more quantity in the cytoplasm (Fig. 3A). MNDA was not detected in HMVEC-d cells and was detected only in the THP1 nucleus (Fig. 3A).
Figure 3. IFI16 interacts with ASC and caspase-1 in KSHV infected HMVEC-d cells.
(A) Expression and sub-cellular distribution of IFI16, AIM2 and MNDA proteins was examined in nuclear and cytoplasmic fractions of uninfected HMVEC-d and THP1 cells by Western blotting. Lamin-B and β-actin show purity and equal loading of nuclear and cytoplasmic fractions, respectively. (B) HMVEC-d cells were infected with KSHV (30 DNA copies/cell) for 2h, washed, and incubated at 37°C for the indicated time. Protein lysates from uninfected (UI) and infected cells were immunoprecipitated with anti-ASC antibodies and Western blotted for NLRP3, AIM2 and IFI16 proteins. Whole cell extracts were blotted with AIM2, IFI16, ASC and tubulin antibodies. (C) HMVEC-d cells were either left untreated (UT) or treated with LPS (20ng/ml for 6h) followed by ATP (5mM for 20min). Protein lysates were immunoprecipitated with anti-ASC antibody and Western blotted for NLRP3. Whole cell extracts were blotted with NLRP3 and b-actin antibodies. (D) HMVEC-d cells were uninfected (UI) or infected with either live KSHV or UV-KSHV for 2h, washed and incubated for 24 h. Protein lysates were immunoprecipitated with anti-ASC antibodies or with irrelevant mouse IgG1 and immunoblotted with anti-IFI16 antibody. (E) Immunoprecipitates prepared by anti-caspase-1 antibodies from uninfected (UI) and KSHV infected HMVEC-d cells were examined for IFI16 by Western blotting. Whole cell extracts were immunoblotted with IFI16, ASC and β-actin antibodies.
When gene expression in uninfected and infected HMVEC-d cells was analyzed, we observed a significant increase in caspase-1 expression from 2 to 24h p.i. (Fig. S2A). Significant induction of the ASC gene was detected only at 24h p.i. and IFI16 gene expression slightly increased over the time of observation (Fig. S2 B and C). In contrast, no significant increase in NLRP-1, NLRP3 and AIM2 was observed (Fig. S2D, E and F). IFIX and MNDA gene expression was not detected in HMVEC-d cells.
Next, we infected HMVEC-d cells for 2 h, washed, incubated for 2, 8, 24 and 48h, immunoprecipitated ASC from each time point and examined the ASC interacting proteins by Western blotting. In these co-immunoprecipitation (co-IP) experiments, no interaction between ASC and NLRP3 was observed in the infected cells (Fig. 3B, top panel). However, in LPS+ATP stimulated (known inducer of NLRP3) HMVEC-d cells, we detected NLRP3 in ASC immunoprecipitates suggesting the existence of an NLRP3 inflammasome in HMVEC-d cells (Fig. 3C). Interaction between AIM2 and ASC was detected only at 2h p.i. and not at later time points (Fig. 3B, middle panel). Detection of AIM2 interaction with ASC at 2h p.i. and not at later time points suggested the possibility of exposure of AIM2 to virion protein and/or genome early during virus internalization and not during latency. In contrast, KSHV infection induced a strong interaction between ASC and IFI16 as shown by the co-IP reactions (Fig. 3B, bottom panel). Compared to uninfected HMVEC-d cells, an increasing amount of IFI16 was detected in ASC immunoprecipitates at all time points p.i. IP of KSHV infected HMVEC-d cells with irrelevant mouse IgG did not pull down IFI16 (Fig. 3D, lane 1). Compared to live KSHV, only a low level of ASC-IFI16 interaction was observed with UV-KSHV at 24 h p.i. (Fig. 3D, lane 4). We next examined whether the ASC-IFI16 protein complex also contained caspase-1. Compared to uninfected cells, infected HMVEC-d cells showed high levels of IFI16 in the caspase-1 immunoprecipitates (Fig. 3E, top panel) suggesting IFI16, ASC and caspase-1 were present in the same protein complex.
Taken together, these results strongly suggested that KSHV induces the formation of a protein complex involving IFI16, ASC and procaspase-1, thus providing evidence that IFI16 forms a hitherto unidentified inflammasome complex with ASC and caspase-1 in response to KSHV infection.
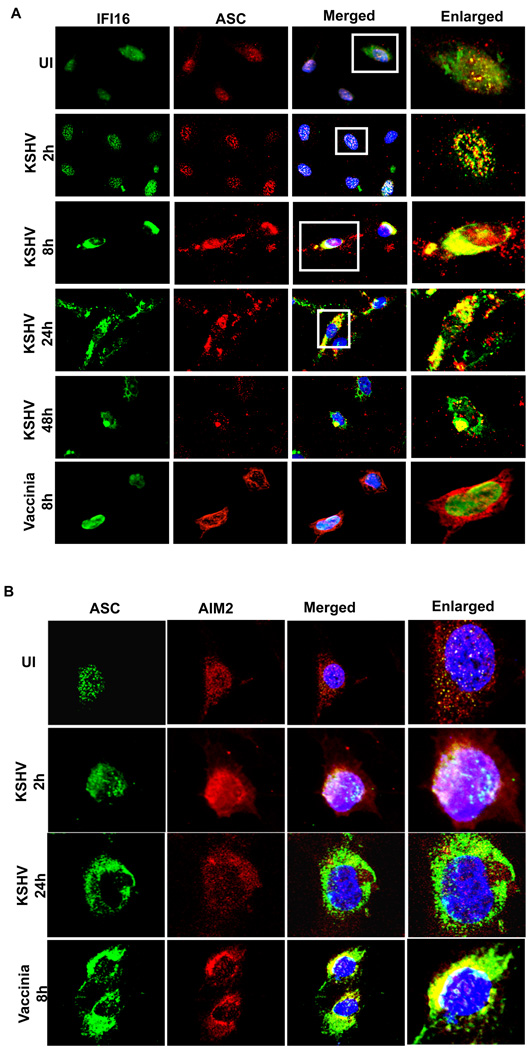
KSHV infection induces the colocalization of IFI16 and ASC
IFI16 was localized in the uninfected cell nuclei and showed a moderate level of colocalization with ASC (Fig. 4A, UI). In contrast, at 2h p.i., ASC staining started to become more speckled and colocalized with IFI16 appreciably within the nuclei of infected cells (Fig. 4A, 2h). At later time points, we also observed cytoplasmic aggregates of IFI16 and ASC (Fig. 4A, 8h, 24h, 48h). Not all IFI16, ASC and caspase-1 underwent redistribution to the cytoplasm as several colocalizing spots were also observed in the nucleus. In contrast, upon infection with vaccinia virus that replicates its DNA genome in the cytoplasm, IFI16 remained in the nucleus Fig. 4A, Vaccinia 8h). However, cytoplasmic redistribution of ASC without colocalizing with IFI16 was observed in vaccinia infected HMVEC-d cells (Fig. 4A, Vaccinia 8h).
Figure 4. Immunofluorescence microscopic analysis of ASC-IFI16 and ASC-AIM2 association in KSHV infected cells.
HMVEC-d cells were infected with KSHV (30 DNA copies per cell) or vaccinia virus (5 pfu) for 2h, washed, and incubated at 37°C. At the indicated time, cells were washed, fixed, permeabilized, blocked with signal enhancer, reacted with (A) anti-IFI16 and ASC antibodies and (B) anti-ASC and AIM2 antibodies, washed and incubated with Alexa-488 (green) and Alexa-594 (red) secondary antibodies. The images were merged with DAPI stained nuclei. The boxed areas in Fig. 4a were enlarged without DAPI for clarity (right most panels).
When we used IFA to examine whether KSHV infection induces ASC-AIM2 interaction, in support of our co-IP experimental observations (Fig. 3B), considerable colocalization of ASC and AIM2 was observed only at 2h p.i. but not at 24h p.i. (Fig. 4B). In contrast, vaccinia virus known to activate AIM2 inflammasome induced a profound cytoplasmic ASC-AIM2 colocalization at 8 h p.i. (Fig. 4B).
When we examined IFI16 distribution in the nuclear and cytoplasmic fractions at 48h p.i. with KSHV, as expected, KSHV infected HMVEC-d cells showed higher levels of IFI16 in the cytoplasmic fractions than seen in the uninfected cells (Fig. 5A, lanes 3 and 4). There was no significant change in the nuclear levels of IFI16 between uninfected and infected HMVEC-d cells (Fig. 5A, lanes 1 and 2).
Figure 5. IFI16 undergoes nuclear to cytoplasmic redistribution upon KSHV infection and IFI16 is required for inflammsome activation by KSHV.
(A) HMVEC-d cells were infected with KSHV (30 DNA copies/cell) for 2h, washed and infected and uninfected (UI) cells incubated at 37°C for 48h. Nuclear and cytoplasmic extracts were examined by immunobotting with anti-IFI16 antibodies. The purity and equal loading were determined by immunoblotting for TBP and tubulin. (B) HMVEC-d cells were transduced with control shRNA, ASC or IFI16 specific shRNA lentivirus particles and selected with puromycin. Knockdown of IFI16 and ASC protein expression was examined by Western blotting with mouse anti-IFI16 and ASC monoclonal antibodies. (C) Untransduced (UTr), control shRNA (Cntrl) and IFI16 (IFI16 shRNA) and ASC (ASC shRNA) knockdown HMVEC-d cells were infected with KSHV (30 DNA copies/cell) for 2h, washed, incubated at 37°C for 24h, and lysates were analyzed by Western blotting to detect pro-caspase-1 and activated caspase-1 (p20). (D) HMVEC-d cells transduced with control shRNA (Cntrl) or three different IFI16 shRNA lentiviruses were infected with KSHV (30 DNA copies/cell) for 2h, washed, incubated for 24h and lysates were analyzed by Western blotting to detect pro-caspase-1, activated caspase-1 (p20) and IFI16. (E) Protein lysates from untransduced (UTr), control (Cntrl) and IFI16 shRNA transduced HMVEC-d cells were immunoblotted to examine the effect of IFI16 shRNA on AIM2 expression. (F) IFI16 knockdown HMVEC-d were either left untreated (UT) or treated with LPS (20ng/ml for 6h) followed by ATP (5mM for 20min). Protein lysates from untreated and treated cells were immunoblotted with caspase-1 antibody to examine caspase-1 activation. (G) HMVEC-d cells left untransduced (UTr) or transduced with control shRNA (Cntrl) or AIM2 specific shRNA lentivirus particles. The transduced cells were selected with puromycin and then AIM2 and IFI16 protein expression was examined by Western blotting with antibodies against AIM2 and IFI16. (H) Untransduced (UTr), control (Cntrl) and AIM2 knockdown HMVEC-d cells were infected with KSHV (30 DNA copies/cell) for 2h, washed and infected and uninfected (UI) cells further incubated for 24h and lysates were analyzed by Western blotting to detect pro-caspase-1 and activated caspase-1 (p20). (I) Caspase-1 activation was examined in IFI16 knockdown HMVEC-d cells infected with vaccinia virus (5 pfu for 8h) (j and k) Control (cntrl) and IFI16 knockdown cells were infected with KSHV (30 DNA copies/cell) for 2h, washed and infected and uninfected cells incubated for 24h. IL-1β (J) and IL-6 (K) gene expression was examined by real-time RT-PCR. Each bar represents the fold increase in gene expression (compared to uninfected) ± SD of three independent experiments. These fold changes were calculated after normalizing with expression of the18s rRNA gene
IFI16 and ASC knockdown inhibits KSHV infection induced inflammasome activation
To determine whether KSHV induced the formation of a functional inflammasome involving IFI16, ASC and procaspase-1, expression of ASC and IFI16 in HMVEC-d cells was knocked down using lentivirus delivered shRNAs specific for ASC and IFI16 (Fig. 5B). We verified the specificity of the knock downs as well as specificity of the antibodies by IFA (Fig. S3, A, B and C). HMVEC-d cells transduced with shRNA lentivirus were infected with KSHV and caspase-1 activation was examined. As shown in figure 5C, KSHV induced caspase-1 activation was reduced by 85% and 60% in IFI16 and ASC knockdown cells, respectively. The effect of IFI16 knockdown was also tested by using three different shRNA lentivirus particles targeting IFI16. As seen in figure 5D, all three shRNAs inhibited KSHV induced caspase-1 activation ruling out the possibility of off target effects and the effects of IFI16 knockdown was seen at the level of caspase-1 cleavage
Furthermore, knocking down IFI16 did not affect the expression of structurally similar AIM2 inflammasome protein (Fig. 5E) nor did it affect NLRP3 mediated inflammasome activation in response to LPS+ATP (Fig. 5F). Similarly, knocking down AIM2 did not affect IFI16 levels or inflammasome activation in response to KSHV infection (Fig. 5G and 5H). In addition, IFI16 knockdown did not affect caspase-1 activation by vaccinia virus (Fig. 5I. These results not only demonstrated the specificity of IFI16 knockdown but also confirmed that IFI16 and ASC play critical roles in inflammasome activation in response to KSHV infection.
Recently, Unterholzner et al (Unterholzner et al., 2010) have shown that in response to herpes simplex virus type 1 (HSV-1) infection, IFI16 mediates the induction of transcription factors IRF3 and NF-κB as well as their target genes. Hence, we examined whether IFI16 also mediates IL-1β and IL-6 gene expression during KSHV infection of HMVEC-d cells. As shown in figures 5J and 5K, KSHV induced IL-1β and IL-6 gene expression which was significantly reduced by IFI16 knockdown.
Reconstitution of IFI16 inflammasome in HEK293T cells
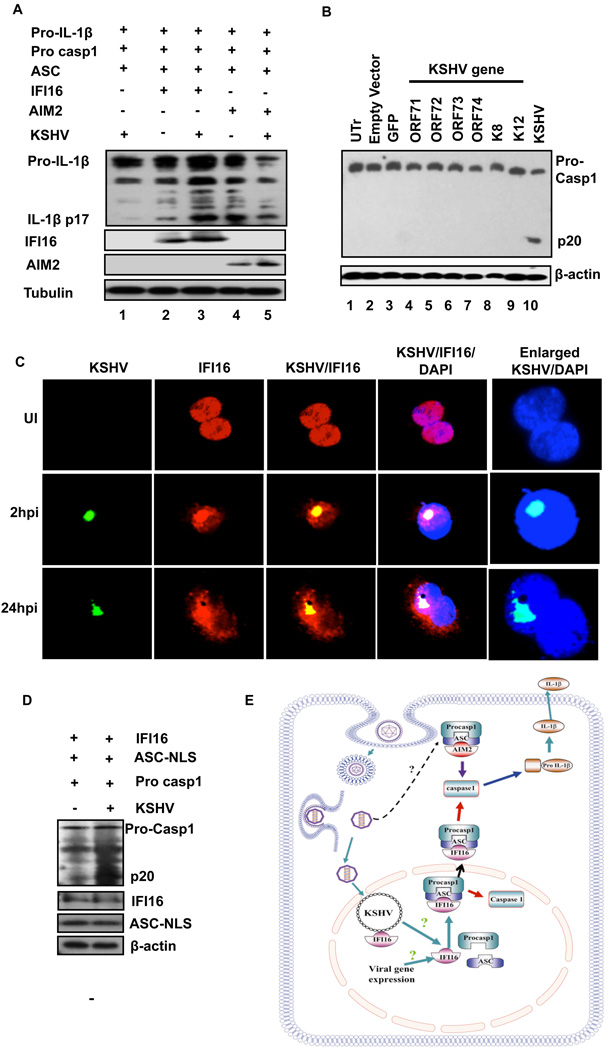
Plasmids encoding full-length pro-IL-1β, Caspase-1, ASC and IFI16 or AIM2 proteins were transiently transfected into 293T cells that are known to have minimal endogenous levels of these proteins. Expression of these proteins was verified by western blotting (Fig. 6A) and by IFA (Fig. S3D). At 24h post-transfection, cells were infected with KSHV for 2h, washed, incubated further for 24h and protein lysates examined for IL-1β maturation. Expression of IFI16 with other inflammasome components in 293T cells exhibited a low level of mature IL-1β (Fig. 6A, lane 2, IL-1β p17). However, upon KSHV infection of these cells, we observed an elevated level of IL-1β maturation (Fig. 6A, lane 3). As shown by others (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009), overexpression of AIM2 alone with other inflammasome components lead to inflammasome activation (Fig. 6A, lane 4). In contrast, KSHV infection did not have any added effect on IL-1β maturation in these cells (Fig. 6A, lane 5).
Figure 6.
(A) Reconstitution of the IFI16 inflammasome. HEK293T cells were cotransfected with 0.5µg of pro-IL-1β, pro-caspase-1, ASC and IFI16 or AIM2 expressing plasmids as indicated. 24h post-transfection, the cells were infected with KSHV (30 DNA copies/cell) for 2h, washed and incubated for 24h. Protein lysates were examined for IL-1β maturation by immunoblotting. Tubulin was used as loading control. (B) Effect of KSHV genes on inflammasome induction. Empty vector, GFP or indicated KSHV genes were expressed in HMVEC-d cells via a lentivirus gene delivery system. Cells were also infected with KSHV for 2 h, washed and kept for 24h. Untransduced (UTr), transduced or KSHV infected cell lysates were examined for caspase-1 activation by immunoblotting with anti-caspase-1 antibodies. Actin was used as loading control. (C) FISH showing IFI16 and KSHV genome interaction. HMVEC-d cells were infected with KSHV (30 DNA copies/cell) for 2h, washed and incubated for the indicated time points. The cells were fixed, permeabilized and immuno-stained with mouse anti-IFI16 antibodies followed by donkey anti-mouse Alexa Fluor 594 secondary antibodies. The cells were then subjected to in situ hybridization with a spectrum green labeled whole KSHV genome probe. (D) HEK293T cells were cotransfected with 0.5mg pro-caspase-1, IFI16 and ASC-NLS expressing plasmids as indicated. 24h post-transfection, the cells were infected with KSHV (30 DNA copies/ cell) for 2h, washed, incubated for 24h and lysates were examined for caspase-1 activation by immunoblotting. (E) Schematic depicting a model for IFI16 mediated inflammasome activation in response to KSHV infection of endothelial cells. In uninfected endothelial cells, IFI16, ASC and caspase-1 are predominantly distributed in the nucleus. During de novo infection leading to the establishment of latency, IFI16, ASC and procaspase-1 form the multi-protein inflammasome complex in the nucleus leading to activation of caspase-1. The IFI16 containing inflammasome complex also migrates from the nucleus to the cytoplasm and forms peri-nuclear aggregates. Early during de novo infection, KSHV also induces the interaction between ASC and AIM2 possibly suggesting that the AIM2 inflammasome might be involved in sensing during the virus internalization process.
KSHV latent proteins are not involved in inflammasome induction
Our studies with UV inactivated KSHV suggested that KSHV gene expression and/or the persistence of latent genome is crucial for inducing the inflammasome. To determine whether KSHV latency gene products mediate inflammasome induction, HMVEC-d cells were transduced with lentivirus vectors carrying latent ORF71, ORF72, ORF73 and K12 genes, lytic K8 and ORF74 genes, and GFP control gene. Real-time RT-PCR measurements confirmed the expression of these genes (data not shown). As shown in figure 6B (lanes 1–9), none of the KSHV genes tested were able to induce caspase-1 activation, although caspase-1 activation was observed with live KSHV de novo infection (Fig. 6B, lane 10). This suggested that these KSHV genes individually do not play a role in KSHV induced inflammasome activation.
IFI16 colocalizes with the KSHV genome in the infected cell nucleus
Studies have demonstrated that AIM2 can directly bind to cytosolic DNA to induce inflammasome activation (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009). Since IFI16 is structurally related to AIM2, and has the DNA binding HIN-200 domains, we hypothesized that binding of IFI16 to the KSHV genome might be responsible for inflammasome activation in response to KSHV infection. To test this hypothesis, HMVEC-d cells were infected with KSHV for different time points and IFI16 was stained with anti-IFI16 antibodies and the KSHV genome was visualized with a spectrum green labeled KSHV genome FISH probe. As shown in figure 6C, IFI16 and the KSHV genome colocalized in the nuclei of infected HMVEC-d cells. In contrast, no such colocalization was observed in uninfected cells. These observations suggest that similar to AIM2’s role in detecting cytosolic DNA, IFI16 is probably involved in the innate sensing of foreign DNA in the cell nucleus.
ASC-NLS expresses ASC with three nuclear localization signal (NLS) sequences derived from SV40 large T antigen. Due to this, though ACS-NLS is functionally active, it remains in the nucleus even after inflammatory stimulation (Bryan et al., 2009). Therefore, to further confirm that inflammasome activation can indeed occur in the nucleus, we reconstituted the IFI16 inflammasome with plasmids encoding full-length caspase-1, IFI16 and ASC-NLS in 293T cells. Caspase-1 activation by KSHV was observed even in the HEK293T cells expressing ASC-NLS (Fig. 6.D, lanes 1 and 2). Taken together these findings suggest that IFI16 can form a functional inflammasome in KSHV infected cell nucleus.
DISCUSSION
Our studies suggest that KSHV induces the inflammasome via a previously unknown pathway and IFI16 possesses, hitherto not identified, properties of interaction with ASC to form a caspase-1 activating molecular platform. IFI16, a resident nuclear protein, is known for its role in cell cycle regulation and DNA damage responses (Aglipay et al., 2003; Fujiuchi et al., 2004).
Studies by Bryan et al., (Bryan et al., 2009) demonstrated that inflammasome activation in THP1 cells by bacterial RNA results in the redistribution of ASC from the nucleus to the cytoplasm and colocalization with caspase-1 and NLRP3 in the peri-nuclear area. Our demonstration of active caspase-1 in the nuclear fractions of infected HMVEC-d cells at 2h and 8h p.i. (Fig. 2B) as well as activation of caspase-1 in the ASC-NLS containing IFI16 inflammasome in HEK293T cells (Fig. 6D) suggest that inflammasome assembly can also occur in the nucleus. However, at later times of KSHV infection, the majority of activated caspase-1 was observed in the cytoplasmic fractions. This shift in the caspase-1 activation from nucleus to cytoplasm coupled with nuclear to cytoplasmic redistribution of ASC, caspase-1 and IFI16 indicate a higher order of complexity involved in the regulation of caspase-1 activation (Fig. 6E). Such mechanism may help preventing excessive caspase-1 activation and/or to avert unwanted caspase-1 activity in the nucleus.
Cytosolic sensors like AIM2 and NLRP3 have been shown to activate inflammasomes in response to infection by bacteria, DNA and RNA viruses (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009; Kim et al., 2010; Muruve et al., 2008; Rathinam et al., 2010; Schroder et al., 2009). During in vitro infection of endothelial cells, KSHV does not undergo a productive cycle, instead it enters into a latency program and the genome persists in the nuclei as an episome tethered to host chromatin. Interestingly, UV-inactivated KSHV did not to induce significant caspase-1 activation at 48h p.i. (Fig. 1E) prompting us to surmise that establishment of latency is critical for continued inflammasome induction. However, none of the KSHV latency associated genes were able to induce caspase-1 activation suggesting that the KSHV latency genes are not directly involved in inflammasome activation. Additionally, colocalization of IFI16 with the KSHV genome in the infected cell nuclei (Fig. 6C) suggests that IFI16 functions as a sensor of pathogen associated molecular pattern (PAMP) within the nucleus. Our studies showed that silencing IFI16 inhibited inflammasome activation without affecting AIM2 or NLRP3 pathways. Furthermore, IFA staining and co-IP studies showed ASC, IFI16 and cascase-1 interaction. Additionally, IFI16 did not mediate caspase-1 activation during vaccinia virus infection that replicates in the cytoplasm. Taken together, these observations demonstrate that IFI16 along with ASC and caspase-1 forms a functional inflammasome in response to KSHV infection in the nucleus.
Earlier studies did not detect interaction between ASC and IFI16 (Burckstummer et al., 2009; Fernandes-Alnemri et al., 2009; Hornung et al., 2009). These studies were aimed at identifying a protein that can detect cytoplasmic DNA and used transfected cytoplasmic DNA as a trigger to examine ASC interaction with IFI16 and other HIN-200 members. Therefore, nuclear IFI16 or other nuclear HIN-200 proteins may not have any role in this context. ASC-IFI16 interaction becomes detectable in the presence of latent KSHV infection with a little constitutive interaction in uninfected cells which suggests the requirement for an appropriate stimulus in the nucleus. Hence, the lack of an appropriate trigger, such as nuclear viral DNA in the above studies, could be one of the reasons for not detecting IFI16 in inflammasome activation. This is very well supported by our inflammasome reconstitution studies in HEK293T cells. When IFI16 was expressed along with other inflammasome components, elevated IL-1β maturation was observed only upon infection with KSHV (Fig. 6A, lane 3) whereas over expression without infection induced substantially lower levels of IL-1β maturation (Fig. 6B, lane 2). Differential inflammasome activation in response to adenoviral DNA also supports the above cited possibilities (Muruve et al., 2008). The inflammasome is activated via NLRP3 when adenovirus DNA is delivered by infection or transduction, while NLRP3 is not required in response to adenovirus DNA delivered via lipid-mediated transfection suggesting that virus infection is sensed in a different way than transfected DNA.
Sub-cellular distribution of ectopically expressed caspase-1 depends on the cell type. Ectopically expressed caspase-1 was predominantly cytoplasmic with little nuclear distribution in HEK293 and Jurkat cells, whereas in HeLa cells the expression was predominantly nuclear (Shikama et al., 2001). Similarly, ASC isoforms vary in their sub-cellular distribution as well as in their ability to form functional inflammasomes (Bryan et al., 2010). Hence, it is possible that differences might exist between endothelial cells, macrophages and THP1 cells with respect to inflammasome activation.
The large amount of viral genome in the infected cell nuclei is often sensed as abnormal extra-chromosomal DNA leading to activation of DNA damage response (DDR) signaling pathways (Schwartz et al., 2009; Weitzman et al., 2004). IFI16 has been shown to be a part of the large multi-protein BRCA1-associated genome surveillance complex (BASC) that is involved in the DDR (Aglipay et al., 2003; Wang et al., 2000). Notably, IFI16 undergoes nuclear to cytoplasmic redistribution following exposure to ultraviolet-B (UVB) radiation (Costa et al., 2011). Similar sub-cellular redistribution of IFI16 following KSHV infection suggests that the latent KSHV genome in the infected cell nucleus is recognized as DNA damage similar to UVB exposure. Furthermore, no such redistribution of IFI16 was observed upon infection by cytoplasimc vaccinia virus. Thus, IFI16 might function as a double-edged sword sensing the KSHV genome in the nucleus as ‘damage’ and inducing the inflammasome. IFI16 with its dual potential to bind DNA (via HIN-200) and ASC (via PYD) may be strategically localized in the cell nucleus to detect abnormal/non-self genetic material and respond by inducing the inflammasome.
In uninfected cells, IFI16, ASC and caspase-1 proteins are predominantly localized to the nucleus. During early time of KSHV infection (2h), these proteins formed a complex in the nucleus, and at later time points these proteins colocalized in the perinuclear area. It is possible that IFI16 directly or indirectly through the involvement of some other proteins is sensing the KSHV genome in the nucleus. This sensing might result in the activation of signaling pathways to induce the nuclear export of ASC/Caspase-1/IFI16 as complex or individual proteins, which assemble into functional inflammasome complex in the perinuclear area (Fig. 6E). Further studies are required to understand the reason for sub-cellular redistribution upon sensing the stimulus.
The de novo infected endothelial cells as well as in KS lesions show signs of persistent DNA damage response as demonstrated by the presence of activated ATM– Chk2 signaling pathways, γ-H2AX and p53 binding protein-1 (p53BP1) (Koopal et al., 2007). If the extent of DNA damage is irreparable as seen in the latent KSHV infection (Koopal et al., 2007), the cells may maintain a chronic, low level DDR. This persistent DDR is known to induce the robust secretion of inflammatory cytokines called Senescence-Associated Secretory Phenotype (SASP) (Coppe et al., 2010; Rodier et al., 2009). Knockdown of upstream components of the DDR such as ATM, NBS1, or CHK2, has been shown to prevent the induction of inflammatory cytokines (Rodier et al., 2009). Additional studies are required to study whether IFI16 directly recognizes KSHV genome or acts as downstream link in the DDR response initiated by Mre/Rad50/NBS1 and ATM.
Although the IFI16 inflammasome can be viewed as a host intrinsic response to KSHV infection, it is possible that KSHV has evolved to usurp this mechanism for establishing latency since IL-1β has been shown to up-regulate KSHV latent gene expression (Yu et al., 1999). However, the levels of IL-1β secretion from KSHV infected endothelial cells were low compared IL-1β secretion from THP1 cells. Hence, further studies are required to establish the biological significance of IL-1β secretion from KSHV infected endothelial cells as well as whether IFI16 is involved in inflammasome induction during primary as well as latent infection of other cell types including THP1 cells and lymphoma cells. A recent study has demonstrated that KSHV tegument protein ORF63 is an NLR homolog which can inhibit inflammasome activation by binding to NLRP1 and NLRP3 (Gregory et al., 2011). This study also demonstrated that inflammasome activation suppresses KSHV reactivation from latency suggesting that inflammasome activation and IL-1β mediated signaling facilitates KSHV latency. During primary KSHV infection and internalization, KSHV tegument ORF63 protein might be binding to NLRP3 and NLRP1 to prevent the detrimental effects of inflammasome activation. This could also be a potential reason for the absence of NLRP3 in our IP reactions (Fig. 3B). Thus KSHV might have evolved to hijack ORF63 mediated phenomenon to establish latency during primary infection. However, on the other hand, IFI16 mediated inflammasome activation after delivering the viral genome to the nucleus could be aiding the virus to establish latency. Further studies are needed to understand the role of IFI16 in KSHV induced inflammasome in other cell types including monocytes and KSHV and EBV transformed B cell lymphoma cell lines.
Studies demonstrated that IFI16 is critical for the interferon-β response upon exposure to intracellular cytoplasmic DNA and HSV-1 infection (Unterholzner et al., 2010). IFI16 was directly associated with IFN-β-inducing viral DNA motifs and STING involved in IFN-β responses to DNA. These authors thus identified IFI16 as a PYD-HIN protein involved in IFN-β induction and labeled IFI16 and AIM2 as a new family of innate DNA sensors “'AIM2-like receptors' (ALRs)”. The involvement of IFI16 in inflammasome induction was not examined in this study. Our findings demonstrate that IFI16 induces inflammasome activation in response to KSHV infection and identifies IFI16 as a nuclear pathogen sensing molecule (receptor) analogous to cytoplasmic PRR inflammasomes. It is possible that NLRP1, NLRP3, NLRC4 and AIM2 might have evolved to detect cytoplasmic pathogen cues, while IFI16 might have evolved to detect nuclear pathogens. Additional studies are required to decipher whether IFI16 mediated caspase-1 activation is unique to KSHV or a common mechanism against nuclear replicating DNA viruses with episomal genomes.
EXPERIMENTAL PROCEDURES
Cells and viruses
Primary HMVEC-d cells (Clonetics, Walkersville, MD) and THP1 cells (ATCC, Manassas, VA) were grown as per procedures described before (Kerur et al., 2010; Sadagopan et al., 2007). Purification of KSHV from BCBL-1 cells and determination of viral copy numbers was carried out as described previously (Krishnan et al., 2004; Sharma-Walia et al., 2005).
Plasmids and constructs
Full length procaspase-1 and ASC-NLS expression plasmids were a gift from Dr. Christian Stehlik, Northwestern University, Chicago (Bryan et al., 2009). Full-length expression plasmids IL-1β, ASC, IFI16 and AIM2 were from Origene Technologies, Rockville, MD.
Virus infection
HMVEC-d and THP1 cells were incubated with 20–30 KSHV DNA genome copies/cell for 2h, washed to remove uninternalized virus and incubated for different time points. HMVEC-d cells were infected with 5 pfu of vaccinia virus.
Antibodies and reagents
Details of the antibodies and other reagents used in the studies are given in the supplementary information
Gene expression profile of cytokines
1×106 THP1 cells were serum starved for 8h, infected with KSHV (30 DNA copies/cell) for 2, 12 and 24h and gene expression of inflammatory molecules was determined using a qPCR based PCR array (cat# PAHS-011, SABiosciences, Frederick, MD).
Inflammatory cytokine secretion
A human protein cytokine array kit from Ray Biotech (Norcross, GA) was used. 1×106 cells were serum starved for 8h, infected with KSHV (30 DNA copies/cell) and conditioned medium was collected at different times p.i. from infected and uninfected cells. The change in cytokine secretion was represented as fold change compared to uninfected cells
Quantitative real-time RT-PCR
Gene expression of IL-1β, IL-6, caspase-1, ASC, NLRP3, NLRP1, AIM2, MNDA, IFI16 and IFIX was by real-time RT-PCR using a SYBR green detection system. The expression levels of these genes were normalized to GAPDH/18s rRNA gene expression. The final mRNA levels of the genes studied were normalized using the comparative cycle threshold method.
Nuclear and cytoplasmic-extract preparation
Nuclear and cytoplasmic extracts were prepared using a Nuclear Extract Kit (Active Motif Corp, Carlsbad, CA). The purity of nuclear and cytoplasmic extracts was assessed by immunoblotting with anti-TBP/lamin B and anti-tubulin antibodies, respectively.
Western blot analysis
Equal amount of proteins from cells lysed in RIPA lysis buffer were subjected to Western blotting. Processing of caspase-1 and IL-1β was examined by antibodies detecting both the pro-form and mature-form of each protein. Sub-cellular redistribution of ASC, caspase-1 and IFI16 was examined by immunoblotting nuclear and cytoplasmic extracts with ASC, caspase-1 and IFI16 specific antibodies.
Co-Immunoprecipitation
Uninfected or HMVEC-d cells infected with KSHV (20 DNA copies/cell) for different times were washed, lysed in RIPA buffer, clarified by centrifugation for 15 min at 4°C and normalized to equal amounts of total protein. The lysate was incubated with immunoprecipitating antibody (anti-ASC or anti-caspase-1 antibody) or control antibody for 2h at 4°C, and immune complexes were captured using 10 µl of protein G-Sepharose. They were washed three times with PBS, boiled with sample buffer, resolved by SDS-10% PAGE, and subjected to Western blotting.
HIGHLIGHTS.
KSHV infection induces formation of a nuclear IFI16, ASC and caspase-1 inflammasome complex
Inflammasome activation requires KSHV gene expression and/or latent 7 KSHV genome
IFI16 colocalizes with the KSHV genome in the infected cell nucleus
IFI16 and ASC knockdown inhibits KSHV infection induced inflammasome activation
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported in part by Public Health Service grants CA 099925 and AI 057349 as well as the RFUMS H. M. Bligh Cancer Research Fund to B.C. We thank Dr. Christian Stehlik, NWU, Chicago for providing the full length procaspase-1 and ASC-NLS expression plasmids. We thank Dr. Alice Gilman-Sachs, Dr. Michael Fennewald and Keith Philibert for critically reading the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Additional methods for immunofluorescence microscopy (IFA), fluorescence in situ hybridization, transfection, lentivirus vector production and lentivirus delivered shRNA system are described in supplementary information.
REFERENCES
- Aglipay JA, Lee SW, Okada S, Fujiuchi N, Ohtsuka T, Kwak JC, Wang Y, Johnstone RW, Deng C, Qin J, et al. A member of the Pyrin family, IFI16, is a novel BRCA1-associated protein involved in the p53-mediated apoptosis pathway. Oncogene. 2003;22:8931–8938. doi: 10.1038/sj.onc.1207057. [DOI] [PubMed] [Google Scholar]
- Bryan NB, Dorfleutner A, Kramer SJ, Yun C, Rojanasakul Y, Stehlik C. Differential splicing of the apoptosis-associated speck like protein containing a caspase recruitment domain (ASC) regulates inflammasomes. J Inflamm (Lond) 2010;7:23. doi: 10.1186/1476-9255-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol. 2009;182:3173–3182. doi: 10.4049/jimmunol.0802367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, Planyavsky M, Bilban M, Colinge J, Bennett KL, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- Case CL, Shin S, Roy CR. Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun. 2009;77:1981–1991. doi: 10.1128/IAI.01382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa S, Borgogna C, Mondini M, Deandrea M, Meroni PL, Berti E, Gariglio M, Landolfo S. Redistribution of the nuclear protein IFI16 into the cytoplasm of UVB-exposed keratinocytes as a mechanism of autoantigen processing. Br J Dermatol. 2011 doi: 10.1111/j.1365-2133.2010.10097.x. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiuchi N, Aglipay JA, Ohtsuka T, Maehara N, Sahin F, Su GH, Lee SW, Ouchi T. Requirement of IFI16 for the maximal activation of p53 induced by ionizing radiation. J Biol Chem. 2004;279:20339–20344. doi: 10.1074/jbc.M400344200. [DOI] [PubMed] [Google Scholar]
- Ganem D. Kaposi's sarcoma-associated herpesvius, Vol. 5th edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S, Reed JC, Ting JP, Damania B. Discovery of a viral NLR homolog that inhibits the inflammasome. Science. 2011;331:330–334. doi: 10.1126/science.1199478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, Eckmann L, Powell JJ, Nizet V, Dixit VM, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci U S A. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerur N, Veettil MV, Sharma-Walia N, Sadagopan S, Bottero V, Paul AG, Chandran B. Characterization of entry and infection of monocytic THP-1 cells by Kaposi's sarcoma associated herpesvirus (KSHV): role of heparan sulfate, DC-SIGN, integrins and signaling. Virology. 2010;406:103–116. doi: 10.1016/j.virol.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Bauernfeind F, Ablasser A, Hartmann G, Fitzgerald KA, Latz E, Hornung V. Listeria monocytogenes is sensed by the NLRP3 and AIM2 inflammasome. Eur J Immunol. 2010;40:1545–1551. doi: 10.1002/eji.201040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopal S, Furuhjelm JH, Jarviluoma A, Jaamaa S, Pyakurel P, Pussinen C, Wirzenius M, Biberfeld P, Alitalo K, Laiho M, et al. Viral oncogene-induced DNA damage response is activated in Kaposi sarcoma tumorigenesis. PLoS Pathog. 2007;3:1348–1360. doi: 10.1371/journal.ppat.0030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan HH, Naranatt PP, Smith MS, Zeng L, Bloomer C, Chandran B. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J Virol. 2004;78:3601–3620. doi: 10.1128/JVI.78.7.3601-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Muruve DA, Petrilli V, Zaiss AK, White LR, Clark SA, Ross PJ, Parks RJ, Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, Vanaja SK, Monks BG, Ganesan S, Latz E, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Coppe JP, Patil CK, Hoeijmakers WA, Munoz DP, Raza SR, Freund A, Campeau E, Davalos AR, Campisi J. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagopan S, Sharma-Walia N, Veettil MV, Raghu H, Sivakumar R, Bottero V, Chandran B. Kaposi's sarcoma-associated herpesvirus induces sustained NF-kappaB activation during de novo infection of primary human dermal microvascular endothelial cells that is essential for viral gene expression. J Virol. 2007;81:3949–3968. doi: 10.1128/JVI.02333-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaniego F, Markham PD, Gendelman R, Gallo RC, Ensoli B. Inflammatory cytokines induce endothelial cells to produce and release basic fibroblast growth factor and to promote Kaposi's sarcoma-like lesions in nude mice. J Immunol. 1997;158:1887–1894. [PubMed] [Google Scholar]
- Schroder K, Muruve DA, Tschopp J. Innate immunity: cytoplasmic DNA sensing by the AIM2 inflammasome. Curr Biol. 2009;19:R262–R265. doi: 10.1016/j.cub.2009.02.011. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Schwartz RA, Carson CT, Schuberth C, Weitzman MD. Adeno-associated virus replication induces a DNA damage response coordinated by DNA-dependent protein kinase. J Virol. 2009;83:6269–6278. doi: 10.1128/JVI.00318-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Krishnan HH, Naranatt PP, Zeng L, Smith MS, Chandran B. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J Virol. 2005;79:10308–10329. doi: 10.1128/JVI.79.16.10308-10329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma-Walia N, Paul AG, Bottero V, Sadagopan S, Veettil MV, Kerur N, Chandran B. Kaposi's sarcoma associated herpes virus (KSHV) induced COX-2: a key factor in latency, inflammation, angiogenesis, cell survival and invasion. PLoS Pathog. 2010;6:e1000777. doi: 10.1371/journal.ppat.1000777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikama Y, U M, Miyashita T, Yamada M. Comprehensive studies on subcellular localizations and cell death-inducing activities of eight GFP-tagged apoptosis-related caspases. Exp Cell Res. 2001;264:315–325. doi: 10.1006/excr.2000.5153. [DOI] [PubMed] [Google Scholar]
- Unterholzner L, Keating SE, Baran M, Horan KA, Jensen SB, Sharma S, Sirois CM, Jin T, Latz E, Xiao TS, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11:997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- Weitzman MD, Carson CT, Schwartz RA, Lilley CE. Interactions of viruses with the cellular DNA repair machinery. DNA Repair (Amst) 2004;3:1165–1173. doi: 10.1016/j.dnarep.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Yu Y, Roan F, Offermann MK. Interleukin 1beta induces expression of human herpesvirus 8 encoded genes in BCBL-1 cells. Aids. 1999;13:2178–2180. doi: 10.1097/00002030-199910220-00028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.