Abstract
Background
Manganese is both an essential element and a known neurotoxicant to children. High manganese exposures have been associated with negative reproductive outcomes in animals, but few epidemiologic studies have examined the effects of human fetal manganese exposure.
Methods
We studied the association between maternal and umbilical cord blood manganese levels and birth weight in a cohort of 470 mother-infant pairs born at term (≥37 weeks gestation) in Ottawa County, Oklahoma. Nonlinear spline and quadratic regression models were used to test the hypothesis of an inverted U-shaped relationship between manganese levels and birth weight.
Results
Mean (standard deviation) concentration of manganese was 2.4 (0.95) μg/dL in the maternal blood and 4.2 (1.6) μg/dL in the cord blood. Umbilical cord manganese was not associated with birth weight. A nonlinear relationship was observed between maternal manganese and birth weight after adjusting for potential confounders. Birth weight increased with manganese levels up to 3.1 μg/L, and then a slight reduction in weight was observed at higher levels. Compared with the 3.1-μg/L point of inflection, birth weight estimates at the 5th (1.3 μg/L) and 95th (4.0 μg/L) percentiles of exposure were −160 g (95% confidence interval = −286 to −33) and −46 g (−38 to 131), respectively.
Conclusions
Maternal blood manganese levels during pregnancy are associated with birth weight in a nonlinear pattern in full-term infants. These findings suggest that manganese may affect fetal growth. Possible detrimental effects of elevated manganese levels on the fetus should be further examined in more highly exposed populations.
Manganese is an essential nutrient for humans and animals, and plays a role in bone formation, protein and energy metabolism, metabolic regulation, and functions as a cofactor in a number of enzymatic reactions.1 Overt manganese deficiency (characterized by bone abnormalities, connective tissue defects, and alterations in carbohydrate and lipid metabolism) is rare.2 Subclinical effects of manganese deficiency have not been well studied, but recent evidence suggests that this syndrome may include osteoporosis.3,4
The main source of manganese to the general population is through diet, but human exposure to manganese can occur through the environment because manganese is abundant in the earth’s crust and commonly found in the air, water, and soil.5 The most frequently reported health effect of high manganese exposure is neurotoxicity, typically among workers in occupational settings.6,7 Increasingly, there is an interest in the effects of manganese on other health endpoints and other population subgroups, notably children. In humans, manganese blood levels increase through pregnancy,8 and manganese crosses the placenta via active transport mechanisms.9 Postnatally, neonates and young infants exhibit increased gastrointestinal absorption of ingested manganese10 and decreased elimination mechanisms,11,12 possibly increasing their susceptibility to high manganese exposure. Most studies on manganese in children have focused on nervous system outcomes,13–16 and 1 recent study reported an increase in infant mortality among a population highly exposed to manganese through drinking water.17
Manganese-related embryotoxic and fetotoxic effects have been observed in animal studies, including decreased fetal size and weight in pregnant mice exposed intravenously or subcutaneously.18,19 Few epidemiologic data are available on the effects of in utero manganese exposure on pregnancy outcomes including birth weight, a predictor of survival and developmental outcomes during childhood.20–23 Unlike other toxic metals such as lead and mercury, which have no beneficial use in the human body and have been shown to affect adversely pregnancy outcomes,24–26 the effects of manganese are likely to be more complex because it is both an essential nutrient and a potential toxicant, depending on the amount of exposure.
We conducted a study of metal exposures and children’s health and development in a mother-infant cohort residing near a lead and zinc mining–related Superfund site in northeastern Oklahoma. The objective of the present analysis was to examine the relationship between in utero manganese exposure and birth weight. We hypothesized that the relationship between biomarkers of fetal manganese exposure and infant birth weight would be in the form of an inverse U-shaped curve and most appropriately modeled with nonlinear models.
METHODS
Study Subjects
Subjects were participants in a prospective birth cohort study of biologic markers of fetal and early childhood exposure to metals mixtures, psychosocial stress, and their impact on neurocognitive development. This ongoing research is being conducted in the area of the Tar Creek Superfund site in Ottawa County, Oklahoma, as a collaborative effort between the Harvard School of Public Health, Local Environmental Action Demanded Agency, a community-based nonprofit organization, and Integris Baptist Medical Center. Participant mothers received a detailed explanation of the study procedures before consenting to participate. The research protocol was approved by the Human Subjects Committees of Harvard School of Public Health and the Integris Baptist Medical Center.
Pregnant women were recruited during prenatal visits or at delivery from the Integris Baptist Medical Center in Miami, Oklahoma, the only hospital in Ottawa County. Eligibility criteria included: (1) giving birth at Integris Hospital; (2) intention to live within the study area for the next 2 years; (3) not currently enrolled in the study with another child; and (4) English-language proficiency sufficient to read, understand, and participate in the informed consent process. Information on gestational age, based on last menstrual period, ultrasound examinations, and clinical estimation, along with characteristics of the birth and newborn, were extracted from the medical records. We used interviewer-administered questionnaires to collect information on social and demographic characteristics and potential sources of metals exposure. Anthropometry measurements of the newborns were made by delivery room staff, using standard anthropometric procedures. The cohort consisted of 504 infants born between 2002 and 2007; however, for this analysis, we excluded preterm infants (<37 weeks gestation) (n = 29) and multiple births (n = 4). An additional infant for whom we did not have an umbilical cord blood sample was excluded, leaving a total of 470 mother-infant pairs in the analysis.
Blood Manganese Measurements
Umbilical cord and maternal venous whole blood samples were collected at delivery into 6-mL capacity Royal Blue Top Vacutainer (Becton Dickinson) tubes with EDTA as an anticoagulant (Ref. Number 368380, Becton Dickinson, Franklin Lakes, NJ). Samples were immediately refrigerated and shipped in batches to the Harvard School of Public Health Trace Metals Laboratory.
All samples were handled in a Class 10,000 clean room under a Class 100 clean hood. Blood samples were weighed (1 g) and digested in 2 mL of concentrated HNO3 acid for 24 hours and then diluted to 10 mL with deionized water after the addition 1 mL of 30% H2O2. Samples were analyzed using a dynamic reaction cell–inductively coupled plasma mass spectrometer (Elan 6100; Perkin Elmer, Norwark, CT). A 2-ng/mL solution of indium in 5% HNO3 was used as the internal standard. This solution was mixed with calibration standards and samples online, using a mixing Tee and a mixing coil. Samples were analyzed by the external calibration method by using 5 standard concentrations ranging from 0 to 50 ng/mL. Quality control measures included analysis of initial calibration verification standard, continuous calibration verification standard, procedural blanks, duplicate samples, spiked samples, and the National Institute of Standards and Technology Standard Reference Material for trace elements in water (NIST SRM 1643d). We used the certified reference material for human hair (GBW–07601) (Institute of Geophysical and Geochemical Exploration, Langfang, China) as our quality control standard for the blood manganese measurements because there is no standard reference material available for blood manganese. Daily variations were monitored using in-house pooled blood samples. Recovery of the quality control standard and the spiked sample by this procedure was 90% to 110% and less than 5% precision. Reported results were the average of 5 replicate measurements. Manganese blood sample concentrations in our study population were all above the limit of detection for this procedure (0.02 μg/dL).
Statistical Analysis
Univariate and bivariate summary statistics and distributional plots were examined for all variables. Blood manganese levels were positively skewed. Extreme outliers for maternal (n = 6) and cord (n = 1) manganese were identified by use of the letter value procedure27 (outer hinges) and excluded from multivariate analyses. There were no outliers identified for birth weight.
Multiple linear regression models were used to describe the relationships between birth weight, manganese exposure, and the main covariates of interest, which were determined a priori based on biologic and demographic characteristics of the infant (gestational age and sex) and mother (parity, race, education, smoking during pregnancy, age, term weight, and height); indicators of nutritional status (prenatal vitamin use and hemoglobin level at delivery); and using statistical considerations if covariates were significant (P < 0.10) in bivariate models. Nonlinear associations were initially examined with generalized additive models in R software,28 using penalized spline terms for the manganese biomarkers, and their difference from a linear term was assessed with a likelihood ratio test. If this indicated a potentially nonlinear association between manganese exposure and birth weight, it was then modeled with a quadratic polynomial regression function to obtain coefficients for the effect estimates using SAS version 9.1 (SAS Institute, Cary, NC). In order to avoid multicollinearity, we first subtracted the mean concentration from each participant’s manganese level and then squared each value. A similar approach was used to evaluate the suitability of higher order polynomial terms for gestational age, maternal age, maternal weight, maternal height, and maternal hemoglobin. Higher order polynomial terms were retained in the final model if they were significant at the 95% level of confidence.
We conducted regression diagnostics after model specifications to examine influential observations as possible sources of spurious associations. The final quadratic model was reevaluated using a robust regression method that uses maximum likelihood estimation to down-weight the influence of data points with large residuals.29
Last, we evaluated the effects of the exposure on the outcome by dividing the participants into 5 categories based on quintile distribution of each manganese biomarker among the study population included in the final model (n = 440). Trend tests were conducted by modeling categorical variables as ordinal variables in these models.
Because maternal smoking is a major determinant of low birth weight, we examined potential synergistic effects with manganese exposure in 2 ways by: (1) including an interaction term for smoking and manganese exposure in the final model and (2) stratifying by smoking status. We also created interaction terms with manganese and other important sociodemographic predictors of birth weight (race and education). Because information on prepregnancy weight was missing for about 10% of the cohort, mother’s prepregnancy body mass index (BMI) was not included in the final multivariate model but was replaced by mother’s term delivery weight (kilograms) and mother’s height (centimeters) as surrogates for maternal size, which is an important determinant of infant birth weight. To assess the efficacy of this approach, prepregnancy BMI (kg/m2) was later included in the final multivariate model as a sensitivity analysis among the subset of individuals with this measure.
RESULTS
Mean (±standard deviation [SD]) and median blood manganese levels for women at delivery were 2.4 (0.95) and 2.2 μg/dL, respectively. Mean (SD) and median umbilical cord blood manganese levels were 4.2 (1.6) and 4.0 μg/dL, respectively (higher than maternal levels). Maternal and umbilical cord blood levels were correlated (Spearman correlation = 0.38). Manganese levels observed in this cohort were similar to those in other published studies of mother-infant cohorts in North America and Europe (Table 1).14,30,31
TABLE 1.
Comparison of Maternal and Umbilical Cord Manganese Concentrations (μg/dL) by Study Site
| Location | Author (yrs) | Blood Biomarker | No. | Percentiles
|
||
|---|---|---|---|---|---|---|
| 5th | 50th | 95th | ||||
| Ottawa County, OK | Zota et al (current study) | Maternal (delivery) Umbilical cord |
470 470 |
1.3 1.8 |
2.2 4.0 |
4.1 6.8 |
| Southwest Quebec, Canada | Takser et al (2004) | Maternal (delivery) Umbilical cord |
101 91 |
1.0 1.9 |
1.6b 83.2b |
2.6 6.4 |
| Paris, France | Takser et al (2003)a | Maternal (delivery) Umbilical cord |
222 222 |
1.1 1.9 |
2.0b 3.9b |
4.0 7.1 |
| Montreal, Canada | Smargiassi et al (2002) | Maternal (delivery) Umbilical cord |
160 160 |
0.6 1.6 |
2.3c 4.5c |
5.2 8.5 |
| Paris, France | Smargiassi et al (2002)a | Maternal (delivery) Umbilical cord |
206 206 |
1.2 2.5 |
2.3c 4.2c |
4.0 6.8 |
Indicates overlapping cohort populations in these studies.
Geometric mean.
Arithmetic mean.
Characteristics of mother-infant pairs and their association with birth weight are presented in Table 2. Mean (SD) birth weight was 3434 g (465 g) and only 11 infants (2%) weighed less than 2500 g at birth. The mean age of women in this cohort was 24.5 (5.5) years, and 8% were 18 years of age or younger. Approximately 75% graduated from high school, and the majority of the study cohort (80%) received at least some public assistance with health insurance through Sooner-Care, the Oklahoma State Medicaid program. More than one-third reported smoking cigarettes at some time during their pregnancy and 57% were overweight or obese. Two-thirds (67%) of the population was white and 24% were of Native American ancestry. Maternal characteristics associated with smaller infant size were nonwhite race, lower education, public health insurance, marital status unmarried, primiparity, not using prenatal vitamins during pregnancy, and smoking during pregnancy. In addition, maternal height and weight at delivery, along with prepregnancy BMI, were positively associated with infant size. Maternal hemoglobin at delivery was negatively associated with infant size. Girls were smaller at birth than boys and, as would be expected, a shorter gestational duration was associated with lower birth weight.
TABLE 2.
Distribution of Maternal and Infant Characteristics, and Their Univariate Associations With Birth Weight in Infants Born in Ottawa County, Oklahoma, 2002–2007 (n = 470)a
| Descriptive Measureb | Birth Weight Difference in Grams (95% CI) | |
|---|---|---|
| Maternal characteristics | ||
| Age (y), % | ||
| 14–18 | 8 | −73 (−234 to 88) |
| 19–24c | 52 | 0 |
| 25–35 | 35 | 45 (−47 to 138) |
| ≥36 | 5 | 29 (−167 to 224) |
| Education, % | ||
| <12th grade | 26 | −103 (−198 to −7) |
| ≥12th gradec | 74 | 0 |
| Insurance status, % | ||
| State Medicaid | 80 | −62 (−167 to 43) |
| Privatec | 20 | 0 |
| Marital status, % | ||
| Married or living with partnerc | 61 | 0 |
| Never married/ separated/divorced | 39 | −64 (−152 to 23) |
| Race/Ethnicity, % | ||
| Whitec | 67 | 0 |
| Native American | 24 | −71 (−173 to 30) |
| Hispanic | 5 | −32 (−222 to 159) |
| Other | 4 | −5 (−233 to 233) |
| Parity, % | ||
| 1c | 42 | 0 |
| 2 | 50 | 93 (5 to 181) |
| ≥3 | 8 | 73 (−89 to 234) |
| Height (cm), mean ± SD | 164 ± 7.2 | 11 (5 to 16) |
| Term weight (kg), mean ± SD | 86.5 ± 17.5 | 7 (5 to 9) |
| Prepregnancy BMI (kg/m2), % | ||
| <25c | 43 | 0 |
| 25–30 | 30 | 67 (−41 to 175) |
| ≥30 | 27 | 91 (−20 to 203) |
| Smoked during pregnancy, % | ||
| Yes | 37 | −108 (−195 to −21) |
| Noc | 63 | 0 |
| Prenatal vitamin use, % | ||
| Yesc | 69 | 0 |
| No | 31 | −43 (−134 to 48) |
| Hemoglobin at delivery (g/dL), mean ± SD | 11.8 ± 1.4 | −33 (−63 to −3) |
| Blood manganese (μg/dL), % | ||
| 0.87 ≤ 1.7c | 20 | 0 |
| 1.8–2.0 | 20 | 89 (−44 to 221) |
| 2.1–2.3 | 20 | 59 (−74 to 192) |
| 2.4–2.8 | 20 | 156 (22 to 289) |
| 2.9–11.7 | 20 | 120 (−13 to 253) |
| Infant characteristics | ||
| Gestational age (wk), mean ± SD | 39.4 ± 1.1 | 112 (73 to 150) |
| Sex, % | ||
| Malec | 55 | 0 |
| Female | 45 | −162 (−245 to −78) |
| Umbilical cord manganese (μg/dL), % | ||
| 0.85 ≤ 2.8c | 20 | 0 |
| 2.8–3.6 | 20 | −81 (−214 to 53) |
| 3.7–4.3 | 20 | −63 (−197 to 70) |
| 4.4–5.2 | 20 | −29 (−163 to 104) |
| 5.3—10.5 | 20 | −67 (−200 to 67) |
Data were missing for insurance status (n = 2), marital status (n = 11), race/ethnicity (n = 13), prepregnancy BMI (n = 58), and maternal hemoglobin (n = 8).
Mean ± SD or percentage as indicated.
Reference category.
Table 2 also shows unadjusted associations between birth weight and manganese exposure. Birth weight increased with increasing maternal blood manganese up to the fourth quintile (midpoint, 2.6 μg/dL) and then decreased. Similarly, unadjusted smoothed plots suggest a nonlinear relationship between maternal blood manganese and birth weight (P = 0.08). Umbilical cord blood manganese was not associated with birth weight in quintile analyses (Table 2). Likewise, when umbilical cord manganese was modeled as a continuous variable, the estimated dose-response curve was consistent with a horizontal line at zero (no effect).
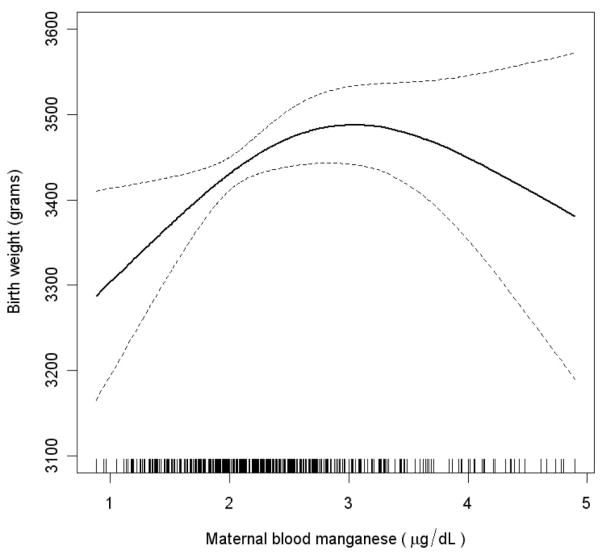
Using penalized splines, a nonlinear association was observed between maternal blood manganese and birth weight (P = 0.04) after adjusting for gestational age, gestational age squared, infant sex, and maternal age, race, parity, education, height, height squared, term weight, hemoglobin, prenatal vitamin use, and smoking (Fig. 1). This model was marginally a better fit than the model that included maternal blood manganese as a linear term only (likelihood ratio test P = 0.04). Infant birth weight increased linearly with maternal blood manganese up to about 3 μg/dL and, at higher blood manganese levels, a modest inverse relationship was observed between maternal manganese and birth weight. However, because of the small number of subjects in the higher range of exposures, the effect estimates were imprecise. The inverted U shape of the dose-response curve suggests that the use of a quadratic term to model manganese exposure may be most appropriate.
FIGURE 1.
Nonlinear association between birth weight and maternal blood manganese concentration adjusted for gestational age, gestational age squared, infant sex, and maternal age, race, parity, education, height, height squared, term weight, hemoglobin, prenatal vitamin use, and smoking (n = 440). The estimate is indicated by the solid line and the 95% confidence intervals by the dashed lines. Maternal blood manganese concentrations for all individual subjects are indicated by short vertical lines on the abscissa.
Table 3 presents data on maternal blood manganese and birth weight when modeled with a quadratic polynomial function in a multivariate regression model. After adjusting for important biologic and socioeconomic predictors of birth weight (detailed previously), both the linear (effect estimate (±standard error [SE]) = 76 [32], P = 0.02) and quadratic (effect estimate [SE] = −53 [24], P = 0.03) term for maternal blood manganese were statistically significant (Table 3). Consistent with the nonlinear spline model, birth weight increased with manganese levels up to 3.1 μg/L, and a slight reduction in weight was observed when levels exceeded this point (n = 69). Compared with the 3.1-μg/L point of inflection, birth weight estimates at the 5th (1.3 μg/L) and 95th (4.0 μg/L) percentiles of exposure were −160 g (95% CI = −286 to −33) and −46 g (−38 to 131), respectively.
TABLE 3.
Quadratic Association of Maternal Manganese on Birth Weight (n = 440)
| Effect Estimate | SE | 95% CI | |
|---|---|---|---|
| Maternal Mn (μg/dL) | 76.0 | 32 | (12.8 to 139.3) |
| Maternal Mn2 | −53 | 24 | (−100.3 to −5.1) |
| Gestational age (wk) | 75 | 22 | (32.3 to 118.2) |
| Gestational age2 | −38 | 16 | (−69.2 to −7.1) |
| Male sex | 128 | 41 | (48.6 to 207.7) |
| Maternal age (y) | 3 | 4 | (−4.8 to 11.3) |
| Primiparous | −102 | 45 | (−190.5 to −13.5) |
| Less than high school | −77 | 48 | (−171.2 to 17.0) |
| Smoked during pregnancy | −87 | 43 | (−170.6 to −2.8) |
| Race/Ethnicity (White)a | |||
| Native American | −86 | 48 | (−180.5 to 8.9) |
| Other | 34 | 71 | (−105.7 to 173.1) |
| Maternal height (cm) | 7 | 3 | (0.5 to 13.5) |
| Maternal height2 | −1 | 0.3 | (−1.3 to −0.02) |
| Maternal term weight (kg) | 5 | 1 | (2.9 to 8.0) |
| No prenatal vitamin use | −9 | 44 | (−78.6 to 96.0) |
| Maternal hemoglobin (g/dL) | −33 | 15 | (−61.4 to −4.0) |
Reference group.
SE indicates standard error; 95% CI, 95% confidence interval.
Residuals from this quadratic model were normally distributed and below the absolute value of 4. As a sensitivity analysis, influential observations with large residuals were down-weighted using robust regression techniques. Effect estimates and level of statistical significance from the robust regression were generally similar to those in Table 3. However, the linear term for maternal manganese slightly decreased (effect estimate [SE] = 73 [32], P = 0.02), whereas the magnitude of the quadratic term (effect estimate [SE] = −59 [24], P = 0.02) slightly increased.
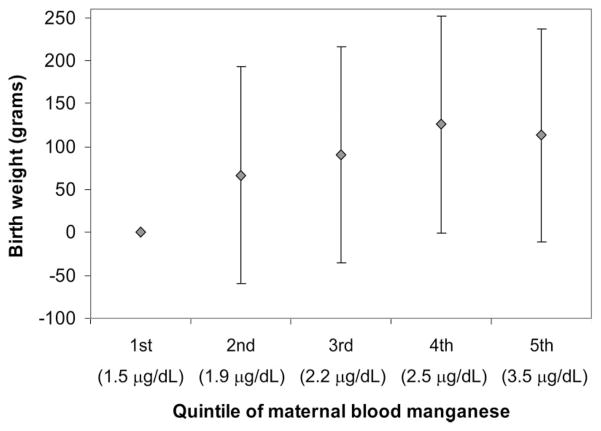
To evaluate the robustness of our findings, we also examined the association between maternal blood manganese and birth weight with maternal blood manganese ranked into quintiles after adjusting for other important covariates. Generally, birth weight increased with increasing quintiles of maternal blood manganese (Fig. 2). The corresponding increase in birth weight with an increase in 1 quintile of maternal blood manganese was 28 g (range, 1–56 g) (P = 0.04). However, consistent with the nonlinear models, the dose-response curve began leveling off at higher manganese levels such that the estimate for the fifth quintile was lower than that of the fourth quintile (Fig. 2), albeit not significantly (P = 0.75). Infants born to women in the lowest quintile of manganese exposure weighed on average 129 g less (−3 to −255, P = 0.04) than infants born to women in the fourth quintile.
FIGURE 2.
Association [β(95% CI)] between birth weight and quintiles of maternal blood manganese (reference group = first quintile; midpoint of each quintile shown in parentheses) adjusted for gestational age, gestational age squared, infant sex, and maternal age, race, parity, education, height, height squared, term weight, hemoglobin, prenatal vitamin use, and smoking (n = 440).
Other predictors of lower birth weight were shorter gestational age, female infant sex, primiparity, smoking during pregnancy, higher maternal hemoglobin, lower maternal height, and lower maternal term weight. Women who were less educated or were of Native American descent tended to have infants of lower birth weight. There was no evidence of effect modification by smoking or sociodemographic factors. No association was observed between umbilical cord blood manganese and birth weight in the fully adjusted regression models. None of these findings was materially affected by replacing maternal term weight and height with prepregnancy BMI in the subset of participants with BMI data available (n = 388).
DISCUSSION
In this cross-sectional community study, there was an inverted U–shaped association between maternal blood manganese levels at delivery and birth weight in full-term infants. This suggests that both lower and higher manganese exposures are associated with lower birth weight, although the association of higher manganese with lower weight was rather weak and imprecise. This is the first epidemiologic study to provide clear evidence of a nonlinear association between maternal manganese exposure and birth weight. Takser et al31 also examined this hypothesis; however, their analysis on 91 mother-infant pairs did not support it, possibly because of insufficient statistical power. Another recent study found that infant birth size was positively associated with maternal blood manganese and negatively associated with umbilical cord blood manganese.32 However, the latter study did not consider nonlinear models, and the maternal manganese levels in that study (mean, 1.91 μg/dL) were lower than those in our study.
Although observed relationships seem consistent with our a priori hypothesis, the cross-sectional design of our study prevents an inference of causality. We did control for a host of possible confounders including gestational age, sex, and maternal age, parity, hemoglobin, weight and height, smoking, prenatal vitamin use, education, and race, without substantially altering the observed association.
Several biologically plausible mechanisms might explain the lower birth weight with decreased maternal blood manganese. Manganese is a critical component of the bone matrix and an important cofactor for enzymes necessary for bone metabolism.4 Manganese may be necessary for bone formation and growth in the developing fetus. Impaired growth and bone abnormalities have been observed in animals suffering from severe manganese deficiency.33 Impaired maternal insulin metabolism could also contribute to the observed findings, in that dietary deficiency of manganese has been associated with impaired insulin synthesis in animal studies.2 None of our study participants had a blood manganese level below the lower limit of the reference range (0.8–1.2 μg/dL) for healthy adults.34 However, there are no health-based guidelines for blood manganese levels during pregnancy. Women’s blood manganese levels greatly increase during pregnancy,31 with an average 3-fold increase between nonpregnant and pregnant women at the end of pregnancy. This may reflect increased requirements for fetal development.35
With respect to the lower birth weights at blood manganese levels above 3.1 μg/dL, this could either reflect a flattening of the dose-response relationship at higher blood manganese levels or a true inverse relationship. The precision of the quadratic term for blood manganese and the better fit of the model including such an exponential term suggest that a parabolic curve fits the data most closely. One possible explanation for this effect would be oxidative stress caused by high manganese levels, leading to impairment of cellular function and growth. Manganese, like iron, is a transitional metal and can catalyze oxidative cellular reactions.36 Exposure to high levels of iron, a metal with overlapping chemical properties to manganese, has been associated with low birth weight.37
Similar to other studies, we found higher manganese levels in the umbilical cord compared with the levels in the mother’s blood.23,31,32 We did not observe an association between umbilical cord blood manganese and birth weight, suggesting that the underlying mechanism is related to placental factors regulating fetal growth, rather than the cellular mechanisms directly regulating growth within the fetus. Further research is needed to elucidate placental transfer and regulation of manganese during pregnancy.
The present study has several limitations. The effect estimate associated with higher levels of blood manganese and decreased birth weight was imprecise because of the small number of observations in the high range of blood manganese levels. In addition, the blood manganese biomarkers measured in this study may not be perfect surrogates for maternal manganese load or fetal exposure. Despite the inclusion of important indicators and predictors of maternal nutritional status, including maternal hemoglobin, smoking status, and prenatal vitamin use, there could be residual confounding from nutritional and dietary factors that were not accounted for in the present study. Given the cross-sectional nature of our study, it is possible that higher blood manganese levels occur in response to, or otherwise serve as a proxy for, another unmeasured causative agent. A prospective study of blood manganese concentrations collected at multiple points during pregnancy may help to sort out the direction of this relationship. However, the parabolic relationship in our study is consistent with expectations by other authors.31 A similar parabolic dose-response relationship between maternal blood manganese levels and fetal enzyme activity was observed in a study of manganese exposure.38
In conclusion, our cross-sectional study found that maternal manganese exposure, as measured by blood manganese concentration at delivery, was nonlinearly associated with infant birth weight. This study supports previous research suggesting that manganese is important to fetal growth, and could indicate possible detrimental effects of higher manganese levels.
Acknowledgments
The authors acknowledge the active involvement and continued cooperation of our community partners: Local Environmental Action Demanded (L.E.A.D.) Agency and Integris Baptist Regional Health Center, Miami, Oklahoma. We thank Bethany Hedt and Eric Tchetgen for statistical consultation.
Supported by the National Institute of Environmental Health Sciences (NIEHS) Superfund Basic Research Program Grant P42-ES05947; NIEHS Center Grant 2 P30-ES 00002; NIEHS K23 ES000381; NIEHS Children’s Center Grant 1 P01-ES012874; and US Environmental Protection Agency (EPA) STAR Research Assistance Agreement No. RD-83172501.
Footnotes
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS, NIH, or EPA.
References
- 1.Wedler F. Biochemical and nutritional role of manganese: an overview. In: Klimis-Tavantzis DJ, editor. Manganese in Health and Disease. Boca Raton, FL: CRC Press, Inc; 1994. pp. 2–37. [Google Scholar]
- 2.Keen CL, Ensunsa JL, Watson MH, et al. Nutritional aspects of manganese from experimental studies. Neurotoxicology. 1999;20:213–223. [PubMed] [Google Scholar]
- 3.Okano T. Effects of essential trace elements on bone turnover–in relation to the osteoporosis. Nippon rinsho. 1996;54:148–154. [PubMed] [Google Scholar]
- 4.Palacios C. The role of nutrients in bone health, from A to Z. Crit Rev Food Sci Nutr. 2006;46:621–628. doi: 10.1080/10408390500466174. [DOI] [PubMed] [Google Scholar]
- 5.ATSDR. Toxicological Profile for Manganese. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 2000. [PubMed] [Google Scholar]
- 6.Iregren A. Manganese neurotoxicity in industrial exposures: proof of effects, critical exposure level, and sensitive tests. Neurotoxicology. 1999;20:315–323. [PubMed] [Google Scholar]
- 7.Levy BS, Nassetta WJ. Neurologic effects of manganese in humans: a review. Int J Occup Environ Health. 2003;9:153–163. doi: 10.1179/oeh.2003.9.2.153. [DOI] [PubMed] [Google Scholar]
- 8.Tholin K, Palm R, Hallmans G, et al. Manganese status during pregnancy. Ann NY Acad Sci. 1993;678:359–360. doi: 10.1111/j.1749-6632.1993.tb26146.x. [DOI] [PubMed] [Google Scholar]
- 9.Krachler M, Rossipal E, Micetic-Turk D. Trace element transfer from the mother to the newborn–investigations on triplets of colostrum, maternal and umbilical cord sera. Eur J Clin Nutr. 1999;53:486–494. doi: 10.1038/sj.ejcn.1600781. [DOI] [PubMed] [Google Scholar]
- 10.Dorner K, Dziadzka S, Hohn A, et al. Longitudinal manganese and copper balances in young infants and preterm infants fed on breast-milk and adapted cow’s milk formulas. Br J Nutr. 1989;61:559–572. doi: 10.1079/bjn19890143. [DOI] [PubMed] [Google Scholar]
- 11.Miller ST, Cotzias GC, Evert HA. Control of tissue manganese: initial absence and sudden emergence of excretion in the neonatal mouse. Am J Physiol. 1975;229:1080–1084. doi: 10.1152/ajplegacy.1975.229.4.1080. [DOI] [PubMed] [Google Scholar]
- 12.Hatano S, Nishi Y, Usui T. Erythrocyte manganese concentration in healthy Japanese children, adults, and the elderly, and in cord blood. Am J Clin Nutr. 1983;37:457–460. doi: 10.1093/ajcn/37.3.457. [DOI] [PubMed] [Google Scholar]
- 13.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115:122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24:667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman GA, Liu X, Parvez F, et al. Water manganese exposure and children’s intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114:124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 17.Hafeman D, Factor-Litvak P, Cheng Z, van Geen A, Ahsan H. Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environ Health Perspect. 2007;115:1107–1112. doi: 10.1289/ehp.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colomina MT, Domingo JL, Llobet JM, Corbella J. Effect of day of exposure on the developmental toxicity of manganese in mice. Vet Hum Toxicol. 1996;38:7–9. [PubMed] [Google Scholar]
- 19.Sanchez DJ, Domingo JL, Llobet JM, Keen CL. Maternal and developmental toxicity of manganese in the mouse. Toxicol Lett. 1993;69:45–52. doi: 10.1016/0378-4274(93)90144-m. [DOI] [PubMed] [Google Scholar]
- 20.Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-age outcomes in children with birth weights under 750 g. N Engl J Med. 1994;331:753–759. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- 21.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312:82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 22.Read JS, Clemens JD, Klebanoff MA. Moderate low-birth-weight and infectious-disease mortality during infancy and childhood. Am J Epidemiol. 1994;140:721–733. doi: 10.1093/oxfordjournals.aje.a117320. [DOI] [PubMed] [Google Scholar]
- 23.Sorensen HT, Sabroe S, Olsen J, Rothman KJ, Gillman MW, Fischer P. Birth weight and cognitive function in young adult life: historical cohort study. Br Med J. 1997;315:401–403. doi: 10.1136/bmj.315.7105.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gonzalez-Cossio T, Peterson KE, Sanin LH, et al. Decrease in birth weight in relation to maternal bone-lead burden. Pediatrics. 1997;100:856–862. doi: 10.1542/peds.100.5.856. [DOI] [PubMed] [Google Scholar]
- 25.Hernandez-Avila M, Peterson KE, Gonzalez-Cossio T, et al. Effect of maternal bone lead on length and head circumference of newborns and 1-month-old infants. Arch Environ Health. 2002;57:482–488. doi: 10.1080/00039890209601441. [DOI] [PubMed] [Google Scholar]
- 26.Xue F, Holzman C, Rahbar MH, Trosko K, Fischer L. Maternal fish consumption, mercury levels, and risk of preterm delivery. Environ Health Perspect. 2007;115:42–47. doi: 10.1289/ehp.9329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tukey JW. Exploratory Data Analysis. Reading, MA: Addison-Wesley Publishing Co; 1977. [Google Scholar]
- 28.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2004. [Google Scholar]
- 29.Ludbrook J. Outlying observations and missing values: how should they be handled? Clin Exp Pharmacol Physiol. 2008;35:670–678. doi: 10.1111/j.1440-1681.2007.04860.x. [DOI] [PubMed] [Google Scholar]
- 30.Smargiassi A, Takser L, Masse A, et al. A comparative study of manganese and lead levels in human umbilical cords and maternal blood from two urban centers exposed to different gasoline additives. Sci Total Environ. 2002;290:157–164. doi: 10.1016/s0048-9697(01)01071-3. [DOI] [PubMed] [Google Scholar]
- 31.Takser L, Lafond J, Bouchard M, St-Amour G, Mergler D. Manganese levels during pregnancy and at birth: relation to environmental factors and smoking in a Southwest Quebec population. Environ Res. 2004;95:119–125. doi: 10.1016/j.envres.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Vigeh M, Yokoyama K, Ramezanzadeh F, et al. Blood manganese concentrations and intrauterine growth restriction. Reprod Toxicol. 2008;25:219–223. doi: 10.1016/j.reprotox.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 33.Bolze MS, Reeves RD, Lindbeck FE, Kemp SF, Elders MJ. Influence of manganese on growth, somatomedin and glycosaminoglycan metabolism. J Nutr. 1985;115:352–358. doi: 10.1093/jn/115.3.352. [DOI] [PubMed] [Google Scholar]
- 34.Hardy IJ, Gillanders L, Hardy G. Is manganese an essential supplement for parenteral nutrition? Curr Opin Clin Nutr Metab Care. 2008;11:289–296. doi: 10.1097/MCO.0b013e3282f9e889. [DOI] [PubMed] [Google Scholar]
- 35.Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 36.HaMai D, Bondy SC. Oxidative basis of manganese neurotoxicity. Redox-Active Metals Neurol Disord. 2004;1012:129–141. doi: 10.1196/annals.1306.010. [DOI] [PubMed] [Google Scholar]
- 37.Fellman V, Rapola J, Pihko H, Varilo T, Raivio KO. Iron-overload disease in infants involving fetal growth retardation, lactic acidosis, liver haemosiderosis, and aminoaciduria. Lancet. 1998;351:490–493. doi: 10.1016/S0140-6736(97)09272-6. [DOI] [PubMed] [Google Scholar]
- 38.Yazbeck C, Moreau T, Sahuquillo J, Takser L, Huel G. Effect of maternal manganese blood levels on erythrocyte calcium-pump activity in newborns. Sci Total Environ. 2006;354:28–34. doi: 10.1016/j.scitotenv.2004.12.012. [DOI] [PubMed] [Google Scholar]