Abstract
Specific receptors for pituitary adenylate cyclase-activating polypeptide (PACAP), a novel peptide with neuroregulatory and neurotrophic functions, have been identified recently in different brain regions, including the hippocampus. In this study, we examined the effects of PACAP-38 on the excitatory postsynaptic field potentials (fEPSPs) evoked at the Schaffer collateral-CA1 synapses. Brief bath application of PACAP-38 (0.05 nM) induced a long-lasting facilitation of the basal transmission. Enhancement of this response was occluded in part by previous high-frequency-induced long-term potentiation (LTP). PACAP-38 did not significantly alter the paired-pulse facilitation (PPF). PACAP-38 has been shown to have a presynaptic effect on the septohippocampal cholinergic terminals, which results in an increase in basal acetylcholine (ACh) release. To assess whether the PACAP-38 enhancement of CA1 synapses was related to the activation of the cholinergic system we examined the effect of this peptide in the presence of atropine, a muscarinic receptor antagonist. The enhancement of the fEPSPs by PACAP-38 was blocked by bath application of atropine. These results show that PACAP-38 induces facilitation of hippocampal synaptic transmission through activation of the cholinergic system via the muscarinic receptors.
Pituitary adenylate cyclase-activating polypeptide (PACAP) is a new member of a neuropeptide family that includes vasoactive intestinal peptide (VIP), glucagon, secretin, and growth hormone, and whose members are thought to play an important role in neuronal function (Miyata et al. 1989; Arimura 1998). PACAP has been isolated from the ovine hypothalamus on the basis of its ability to stimulate adenylate cyclase in rat anterior pituitary cells (Miyata et al. 1989) and it is present in the central nervous system (CNS) and in a variety of peripheral tissues (Masuo et al. 1992; Arimura and Shioda 1995; Shioda et al. 1997). Two distinct forms of this neuropeptide, PACAP-38 and PACAP-27, with 38 or 27 amino acids, respectively, have been characterized (Arimura and Shioda 1995). PACAP-38 and PACAP-27 possess similar biological activity (Miyata et al. 1990). PACAP-38 is the prevailing form in mammalian tissues (Arimura 1992). The amino-acid sequence of PACAP-38 is well-preserved in different species and is identical in sheep, rats, and humans (Arimura 1992). Two types of high-affinity PACAP receptors have been identified: The PACAP type-I receptor (PACAPRI), which specifically binds PACAP-38 and PACAP-27 with higher affinity than it binds VIP; and the PACAP type-II receptor (PACAPRII), which has high homology with the VIP receptor and binds PACAP-38, PACAP-27, and VIP with the same high affinity (Arimura 1992; Sokolov and Kleschevnikov 1995). PACAPRI is mainly distributed in the CNS, including the septum, hippocampus, cerebellar cortex, amygdala, nucleus accumbens, hypothalamus, and the entorhinal cortices (Masuo et al. 1992,1993) and it is positively coupled to adenylate cyclase and phospholipase C, whereas PACAPRII is highly expressed in thalamus, hippocampus, and in various hypothalamic nuclei (Masuo et al. 1992,1993) and is linked only to adenylate cyclase (Arimura 1992; Spengler et al. 1993; Leech et al. 1995). These second messenger pathways have been shown to underlie certain forms of synaptic plasticity (Greengard et al. 1991; Siegelbaum and Kandel 1991; Wang et al. 1991; Malenka and Nicoll 1999), suggesting that PACAP-38 may regulate long-term synaptic changes. It is interesting that PACAP is homologous with a peptide encoded by the Drosophila memory gene amnesiac, which has been shown to be involved in synaptic transmission and memory storage (Feany and Quinn 1995; Zhong and Pena 1995). Masuo et al. (1993) have shown that PACAP-38, applied via a microinjection cannula, enhances in a dose-dependent manner, the spontaneous release of acetylcholine (ACh) from the septal cholinergic fibers in the dorsal hippocampus in a dose-dependent manner. They reported that PACAP-38 was more potent than PACAP-27 in stimulating the release of ACh (Masuo et al. 1993). This release of ACh was highly calcium dependent, suggesting that PACAP-38 works via a presynaptic mechanism (Matthews et al. 1987; Margiotta and Pardi 1995). PACAP-38, acting through type-I receptors, enhances the ACh sensitivity of ciliary ganglionic neurons by a cAMP-dependent mechanism (Matthews et al. 1987). The hippocampus receives abundant extrinsic cholinergic innervation from the medial septal area (Frotscher and Léranth 1985; Matthews et al. 1987), and the septohippocampal cholinergic pathway has been suggested to have an important role in learning and memory (Valentino and Dingledine 1981; Krnjevic and Ropert 1982; Hepler et. al. 1985; Madison et al. 1987; Brandner and Schenk 1998). Together, these data suggest that PACAP-38 may influence synaptic plasticity through its actions on the cholinergic system in the hippocampus. Long-term potentiation (LTP), which is a long-lasting, use-dependent increase in the efficacy of synaptic transmission, is thought to underlie the fundamental properties of memory (Bliss and Lomo 1973; Chen and Tonegawa 1997; Abel and Kandel 1998). It has been shown that muscarinic receptor stimulation can induce LTP at the Schaffer collateral-CA1 synapses. This muscarinic-induced LTP (LTPm) resembles LTP produced by tetanic stimulation (Marchi and Raitieri 1989; Blitzer et al. 1990; Markram and Segal 1990; Shimoshige et al. 1997). With this background, the aim of this study was to investigate the effect of PACAP-38 in the hippocampus, the brain region that has been long known to be essential for learning and memory (Abel and Kandel 1998). In particular, we studied the effect of PACAP-38 on rat basal- CA1 hippocampal synaptic transmission and on activity-dependent changes of synaptic strength during LTP to determine if these effects are mediated through actions on ACh release.
RESULTS
PACAP-38-Induced Enhancement of CA1 Excitatory Postsynaptic Field Potentials (fEPSPs)
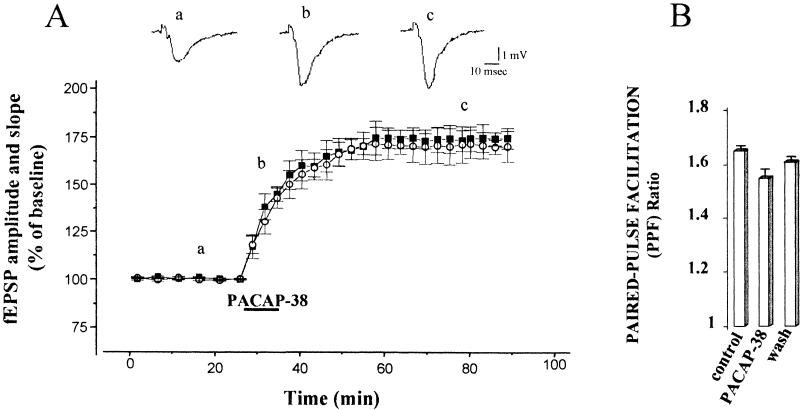
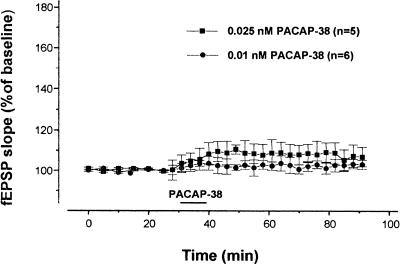
A test stimulus of Schaffer collateral fibers evoked a fEPSP in the stratum radiatum of the CA1 region. Bath application of 0.05 nM PACAP-38 for 10 min induced a long-lasting enhancement of this synaptic response. This potentiation of the fEPSP persisted following washout of PACAP-38 (Fig. 1A). In all slices analyzed (n = 8), after 10 min of PACAP-38 application, the mean fEPSP amplitude and the fEPSP slope increased significantly to 148.1 ± 13.1% and 150.0 ± 11.2%, respectively (P <0.001). The enhancement reached maximum levels 25 min after the washout of the peptide at which time the fEPSP amplitude and initial slope were 175.0 ± 11.1% and 174.2 ± 11.2%, respectively (P <0.001). In the graph, we show that the PACAP-38-induced potentiation lasted for 60 min, but the responses were potentiated for the entire recording period (180 min) after the washout of the peptide (not shown). In the concentration-response study, the application of lower concentrations of PACAP-38 (0.025 nM and 0.001 nM) did not cause any significant increase in the synaptic response (Fig. 2). To determine whether PACAP-38 action might affect short-term changes in synaptic strength, we studied the paired-pulse facilitation (PPF) before, during, and after PACAP-38 application. We determined the ratio of the slope of the second fEPSP to the slope of the first fEPSP (fEPSP2/fEPSP1 PPF ratio), averaged over all the slices and plotted at the same times as in Figure 1A (a,b,c; Fig. 1B). PACAP-38 increased the slope of the first and the second fEPSPs in parallel. Thus in the same slices there was no significant change in PPF as determined by fEPSP2/fEPSP1. The mean value of fEPSP2/fEPSP1 during the application of PACAP-38 was 1.55 ± 0.05, compared to the control value (1.65 ± 0.01, P >0.05).
Figure 1.
Pituitary adenylate cyclase–activating polypeptide-38 (PACAP-38) enhances excitatory postsynaptic field potentials (fEPSPs) in the CA1 region. (A) The graph shows data obtained from eight slices in which 0.05 nM PACAP-38, applied for 10 min, increased the mean fEPSP amplitude (solid square) and slope (open circle) from the baseline values to 148.1 ± 13.1% and 150.0 ± 11.2%, respectively (P <0.001). The enhancement of fEPSP amplitude and slope reached its maximum value during the washout (25 min, 175.0 ± 11.1% and 174.2 ± 11.2%, respectively) and persisted at that level for the remainder of the recording period. The mean ± the standard error of the mean of the fEPSP amplitude and slope are plotted as the percent change over the baseline values. The traces represent the CA1 synaptic responses to Schaffer collateral-commissural stimulation (average of five single sweeps) obtained from a slice recorded before (a), during (b), and after bath application of 0.05 nM PACAP-38 (c). (B) Paired stimuli using a 40-msec interpulse interval were delivered, the ratio of the second fEPSP slope to the first fEPSP slope (fEPSP2/fEPSP1) was determined, and the ratios were averaged and plotted. For clarity, the histograms show only the mean paired-pulse facilitation (PPF) ratio at the same times as those shown in graph (A): Baseline mean fEPSP2/fEPSP1 slope ratio measured before the PACAP-38 application (a, 1.65 ± 0.01), during the PACAP-38 bath application (b, 1.55 ± 0.05, n = 8; P >0.05), and during the washout (c, 1.61 ± 0.02).
Figure 2.
Minimal effective concentration of pituitary adenylate cyclase–activating polypeptide-38 (PACAP-38). Bath application of 0.025 nM PACAP-38 and 0.001 nM PACAP-38 did not alter the synaptic response significantly compared to the baseline values. The mean slopes of the excitatory postsynaptic field potentials (fEPSP) at 10 min after application of 0.025 nM PACAP-38 (solid square) and 0.001 nM PACAP-38 (solid circle) were 108.32 ± 5.8% and 104.32 ± 3.5%, respectively, neither of which was significantly different from the baseline value (P >0.05). The mean ± the standard error of the mean of the slope of the excitatory postsynaptic field potentials is plotted as the percent change over the baseline value.
PACAP-38 Effect on CA1 fEPSPs Following LTP Saturation by Repeated Tetani
The PACAP-38-induced enhancement of the synaptic strength at the Schaffer collateral-CA1 synapses was long- lasting (Fig. 1A). This long-lasting increase in the efficacy of synaptic transmission suggested that common mechanisms lie downstream from both the PACAP-38 action and the expression of LTP. To verify these putative similarities, we examined the effect of PACAP-38 following tetanic LTP saturation by sequential deliveries of high frequency stimulation (HFS). LTP expression was detected by measuring the fEPSP slope, which increased significantly to 157.0 ± 6.9% of the pretetanus value (n = 8, P <0.001) 20 min after the delivery of the last HFS and immediately before the application of PACAP-38 (Fig. 3A). PACAP-38, further, significantly enhanced the fEPSP slope to 203.7 ± 13.1% (n = 8, P<0.001). Under this condition of LTP saturation, the PACAP-38-induced enhancement which lasted for a shorter period. However, within 30 min after washout of the peptide, the PACAP-38-induced enhancement of the fEPSP slope returned to preapplication levels (157.4 ± 7.2%; Fig. 3A). Figure 3B shows that LTP was accompanied by a significant decrease in the average PPF ratio. The baseline PPF ratio over the control period before the HFS was 1.98 ± 0.06 (n = 8). It declined to 1.83 ± 0.01 (P <0.05) after the final HFS and remained at that level throughout the recording period before the application of PACAP-38. PACAP-38 induced a slight decline in the PPF ratio to 1.74 ± 0.08 (P >0.05), with recovery on washout (1.82 ± 0.06).
Figure 3.
Further pituitary adenylate cyclase–activating polypeptide-38 (PACAP-38)-induced enhancement of CA1 excitatory postsynaptic field potentials (fEPSPs) following long-term potentiation (LTP) saturation by repeated tetani. (A) The graphs represent pooled data from eight slices. The tetanic LTP was saturated by successive trains of high-frequency stimulation (HFS; 100 Hz, for 1 sec , three trains at 20 min intervals); the arrows denote the points at which the HFS was delivered. After LTP saturation, the mean fEPSP slope was 157 ± 6.9% of pretetanus value (n = 8, P <0.001). Bath application of 0.05 nM PACAP-38 for 10 min further enhanced the fEPSP slope to 203.7 ± 13.1% (n = 8, P <0.001). Potentiation caused by PACAP-38 in this condition was not long lasting and the mean fEPSP slope returned to the preapplication value on washout (157.4 ± 7.2%). Each point plotted is the average of five traces, except for the first point after each HFS, which represents PTP and is the average of a single trace from each experiment. Insets: Representative records of fEPSP obtained from a slice at the times marked on the graph (baseline fEPSP [a]; after tetanic LTP saturation and immediately before the PACAP-38 bath application [b]; during [c], and after [d] the PACAP-38 perfusion). (B) mean paired-pulse facilitation (PPF) ratio taken in the same slices as in A at the same times marked in the graph (a, b, c, d). After LTP was saturated, 0.05 nM PACAP-38 did not significantly alter the mean PPF ratio (1.83 ± 0.05 vs. 1.74 ± 0.08, P >0.05).
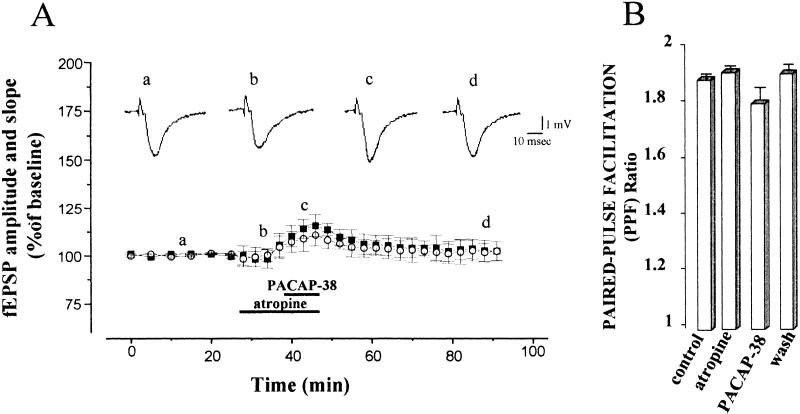
Atropine Block of PACAP-38-Induced Enhancement of CA1 fEPSPs
It has been shown that low doses of PACAP-38 increase the spontaneous basal release of ACh from the septal cholinergic terminals (Masuo et al. 1993). In addition, it has been shown that bath application of submicromolar concentrations of carbachol (CCh) produced a gradually developing, long-lasting increase in the CA1 fEPSP, called muscarinic long-term potentiation (LTPm), that is blocked by atropine (Madison et al. 1987; Miyata et al. 1989; Blitzer et al. 1990; Auerbach and Segal 1994; Liu and Madsen 1997; Shimoshine et al. 1997). Therefore, to assess the contribution of cholinergic receptors to the facilitation induced by PACAP-38 of the fEPSP described above, we repeated the experiments in the presence of atropine. Atropine (1 μM) alone caused a small but nonsignificant reduction of fEPSP amplitude and initial slope (not shown) during 20 min of application, with recovery on washout. Atropine markedly blocked the fEPSP increase produced by 0.05 nM PACAP-38 in all nine of the slices that were tested (148.1 ± 13.1% and 150.0 ± 11.2% fEPSP amplitude and slope facilitation, respectively, induced by PACAP-38 alone, and 115.7 ± 5.7 % and 110.0 ± 5.5% fEPSP amplitude and slope facilitation, respectively, induced by PACAP-38 in the presence of atropine, P <0.05; Fig. 4A). Figure 4B shows the mean PPF ratio at the same times marked in Fig. 4A. PPF did not change during atropine application. Mean baseline PPF ratio of fEPSP slope was 1.88 ± 0.02 and the PPF ratio of fEPSP measured after 15 min of atropine perfusion was 1.9 ± 0.03, respectively (P >0.05). The mean PPF ratio decreased slightly to 1.78 ± 0.05 during the application of PACAP-38 (P >0.05; Fig. 4B).
Figure 4.
Blockade of atropine of pituitary adenylate cyclase–activating polypeptide-38 (PACAP-38)-induced enhancement of CA1 excitatory postsynaptic field potentials (fEPSP). (A) Atropine (1 μM), a muscarinic cholinergic receptor antagonist, was applied to the slices 10–15 min before and 10 min simultaneously with 0.05 nM PACAP-38 to examine whether muscarinic receptors mediate the PACAP-38-induced enhancement of excitatory post-synaptic field potentials (fEPSP). Atropine significantly blocked the PACAP-38-induced enhancement of fEPSP amplitude and slope. PACAP-38 induced only a small and transient facilitation of the fEPSP. At 10 min after PACAP-38 bath application, the percentage change in fEPSP amplitude and initial slope was 110.78 ± 6.30% and 107.42 ± 3.0%, respectively, (n = 9, P >0.05) in the slices treated with atropine plus PACAP-38, which was smaller than the percentage change in the slices treated with PACAP-38 alone (148.10 ± 13.14% and 150.0 ± 11.2%, respectively, n = 8, P <0.001). The traces shown in the top part of the figure represent the fEPSP (average of five single sweeps) obtained from a slice recorded during the control period (a), during the application of atropine (b), during coapplication of atropine and PACAP-38 (c >) and on washout (d). (B) The mean control paired-pulse facilitation (PPF) ratio of the fEPSP slope was 1.88 ± 0.02 and the mean PPF ratio measured after 15 min of atropine perfusion was 1.9 ± 0.03 (P >0.05). The PPF ratio value decreased to 1.78 ± 0.05 during the application of PACAP-38 (P >0.05) and recovered completely during washout (1.9 ± 0.02).
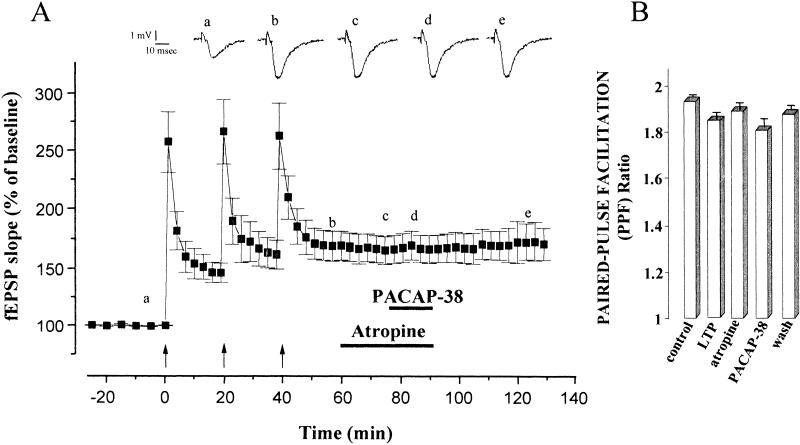
Atropine Block of PACAP-38-Induced Enhancement of CA1 fEPSPs Following LTP Saturation by Repeated Tetani
To assess whether the muscarinic receptors were also involved in the additive enhancing effects of PACAP-38 and LTP of fEPSP, we performed a series of experiments using atropine. In all the slices analyzed (n = 12), atropine, applied after LTP saturation by repeated tetani, completely blocked the enhancement induced by PACAP-38. Figure 5A shows that the increase in the fEPSP slope after LTP saturation was 168.3 ± 12.4%. The application of atropine did not induce a significant change in the magnitude of the LTP expression (at 15 min after atropine perfusion, the mean fEPSP slope was 165.4 ± 11.6%, P >0.05), but it prevented the PACAP-38 facilitation (at 10 min of coapplication the mean fEPSP slope was 166.4 ± 11.2%, P >0.05). Atropine did not alter the PPF ratio of the fEPSP slope (Fig. 4B). Similarly, PACAP-38 induced a slight decrease of the PPF ratio from 1.89 ± 0.05 to 1.81 ± 0.08 (P >0.05) with recovery during washout of the peptide (Fig. 5B).
Figure 5.
Blockade by atropine of pituitary adenylate cyclase–activating polypeptide-38 (PACAP-38)-induced enhancement of CA1 excitatory postsynaptic field potentials (fEPSP) following long-term potentiation (LTP) saturation by repeated tetani. (A) The potentiation of the mean fEPSP slope recorded after the series of high frequency stimulation (HFS) was 168.3 ± 12.4% (n = 12, P <0.001). 1 μM atropine applied after LTP saturation had no effect on fEPSP slope (mean fEPSP slope 165.4 ± 11.6, P >0.05) but when applied with 0.05 nM PACAP-38, atropine completely blocked the PACAP-38-induced enhancement (at 10 min after coapplication of atropine and PACAP-38 the mean fEPSP slope was 166.4 ± 11.2, n = 12, compared with PACAP-38-induced enhancement of fEPSP slope 203.7 ± 13.1%, n = 8). The traces show the fEPSP (average of five single sweeps) recorded from a representative slice at the times marked on the graph: At the control period (a); after tetanic LTP saturation (b); after atropine application (c); during coapplication of atropine and PACAP-38 (d); and on washout. (B) mean paired-pulse facilitation (PPF) ratio at the time points marked in A (a, b, c, d, e). After LTP saturation, atropine did not alter the PPF ratio of the fEPSP slope. PACAP-38 induced a slight decrease of the PPF ratio from 1.89 ± 0.05 to 1.81 ± 0.08 (P >0.05) and during washout of the peptide the mean PPF ratio returned to 1.87 ± 0.06.
Discussion
Studies in the last few years indicate that neuropeptides can modulate a wide variety of synapses. The main finding of this study is that the peptide, PACAP-38 (0.05 nM), enhanced excitatory synaptic responses in the CA1 region during baseline synaptic transmission and during activity-dependent alteration of synaptic transmission (i.e., LTP). We also found evidence that atropine blocked the PACAP-38-induced enhancement of the CA1 fEPSP responses, which indicates that PACAP-38 action is mediated by the cholinergic system. In contrast to our results, Kondo et al. (1997) previously reported that a higher concentration of PACAP-38 (1 μM) induced a long-lasting depression, rather than a potentiation, of transmission at hippocampal CA1 synapses. LTP is induced by low-frequency-induced long-term depression (LTD), as it could be elicited in the presence of 2-amino-5-phosphonovalerate (APV) or in the presence of inhibitors of cAMP- or Ca2+-dependent protein kinases (Kondo et al. 1997). Our preliminary data confirmed that 1 μM PACAP-38 induces LTD of fEPSPs (not shown). In addition, we showed that atropine did not block 1 μM PACAP-38-induced depression (not shown). These differential concentration-dependent PACAP-38 effects suggest that the mechanism involved in PACAP-38-induced enhancement of CA1 fEPSP responses is distinct from those underlying PACAP-38-induced depression and that these responses might be mediated by different sites of action of this peptide. Our primary finding is that a lower dose of PACAP-38 (0.05 nM) induced a long-lasting enhancement of Schaffer collateral-commissural CA1 synaptic strength, which was similar to the long-term facilitation of hippocampal synaptic transmission that has been observed in LTP. LTP is a long-lasting increase in the CA1 postsynaptic responses that results from a brief train of tetanic stimulation of afferent fibers, and it is considered to be a prolonged form of activity-dependent synaptic plasticity related to learning and memory (Bliss and Lomo 1973; Chen and Tonegawa 1997; Abel and Kandel 1998). We therefore investigated the possibility of a common mechanism downstream from the PACAP-38-induced enhancement and the tetanus-induced LTP. If the mechanisms were separate phenomena, it might be expected that they should be additive. In these experiments, PACAP-38 application caused only a transient increase in the evoked responses after the LTP mechanism was saturated by three successive trains of HFS. This different time course of PACAP-38 action after the saturation of LTP compared with the baseline evoked activity indicates that PACAP-38 and afferent tetanic stimulation share, in part, a common mechanism for induction of enhancement of the CA1 synaptic responses. Masuo et al. (1993) showed that PACAP-38 (12 pM, 120 pM) increases the spontaneous release of ACh from the cholinergic terminals in the rat dorsal hippocampus in a dose-dependent manner. The hippocampus receives massive cholinergic innervation from the medial septum–diagonal band (Valentino and Dingledine 1981; Krnjevic and Ropert 1982; Frotscher and Léranth 1985; Hepler et al. 1985; Madison et al. 1987). ACh is known to evoke multiple effects in hippocampal neurons and the cholinergic influences on the evoked responses are strongly dependent on the sites of application (Bernardo and Prince 1982; Cherubini et al. 1982; Frotscher and Léranth 1985; Madison et al. 1987; Benson et al. 1988; Chen and Tonegawa 1997). Several lines of evidence show that the cholinergic system plays a role in facilitating plastic changes at Schaffer collateral-commissural-CA1 synapses in accordance with the well-known memory-facilitating action of ACh (Markram and Segal 1990; Auerbach and Segal 1994). Specifically, ACh has an excitatory postsynaptic muscarinic effect on the CA1 pyramidal neurons by inducing a long-lasting enhancement of evoked responses (Marchi and Raitieri 1989; Markram and Segal 1990; Auerbach and Segal 1994; Shimoshine et al. 1997). Recently, it has been shown that this ACh-induced, long-lasting enhancement (LTPm) is prevented by atropine, a muscarinic receptor antagonist (Marchi and Raitieri 1989; Blitzer et al. 1990; Markram and Segal 1990; Shimoshine et al. 1997). The molecular mechanism of this long-lasting facilitation of EPSPs is still under debate. One possibility is that LTPm is correlated with an increase in postsynaptic excitability (Krnjevic et al. 1981; Bernardo and Prince 1982; Krnjevic and Ropert 1982; Madison et al. 1987; Benson et al. 1988) associated with an increase in membrane resistance that is mediated by a selective inactivation of the K+ channel (Madison et al. 1987; Markram and Segal 1990). Alternatively, LTPm could be attributable to an increase in the Ca2+ component of the N-methyl-d-aspartate (NMDA) response or by the activation of second messenger systems, which regulate intracellular Ca2+, which, in turn, modifies the NMDA response (Madison et al. 1987; Auerbach and Segal 1994; Markram and Segal 1990; Cormier et al. 1993; Huang and Malenka 1993). In either event, it has been shown that cholinergic agonist CCh had no effect when administered after the LTP mechanism had been saturated by repeated tetani. This evidence indicates that tetanus-induced potentiation and LTPm share a common mechanism and provide a direct link between ACh and mechanisms of synaptic plasticity (Auerbach and Segal 1994,1996). To investigate the possible involvement of the cholinergic system in PACAP-38 action, we tested the effects of atropine. It is interesting that atropine blocked the facilitator effects on the fEPSP produced by PACAP-38 application on basal synaptic transmission and after LTP saturation. These data suggest that fEPSP facilitation induced by PACAP-38 might be correlated with an increase in ACh release from the septohippocampal terminals through the activation of muscarinic receptors. This might lead to postsynaptic regulation of NMDA responses, which are mediated by muscarinic receptors. We cannot exclude the possibility that the facilitator action of PACAP-38 might be a synergistic effect that is attributable to the cross-talk among the complex biochemical pathways by the combined activation of PACAP receptors and muscarinic receptors. In addition, this may explain the different time course of PACAP-38-induced enhancement during baseline condition and during activity-dependent LTP. In fact, electrophysiological studies have recently shown a dose-dependent potentiation of NMDA currents by PACAP-38 (10–30 nM) through a cAMP intracellular messenger (Wu and Dun 1997). Liu and Madsen (1997) provided the first detailed description of PACAP-38 (0.5–2 nM) acting as a direct modulator of NMDA receptors through the glycine coagonist site. In addition, stimulatory glutamatergic effects of PACAP-38 and other members of the peptide family also have been reported (Martin et al. 1995, Stella and Magistretti 1996; Wu and Dun 1997). In future experiments, to investigate the involvement of NMDA-receptor activation in the PACAP-38 actions, we will test the effect of APV on the PACAP-38-induced potentiation. In conclusion, this work has clearly shown that PACAP-38 enhances the CA1 synaptic strength and that this effect is mediated by the muscarinic cholinergic receptors. This PACAP-38 action might represent an interesting mechanism for potentiating the processes of learning and memory in the mammalian nervous system.
MATERIALS AND METHODS
The experiments were performed on transverse hippocampal slices (400 μm) obtained from Wistar rats (100–180 g). We prepared hippocampal slices as described previously (Berretta et al. 1990). Briefly, the animals were anesthetized with halothane (3% in air) and then decapitated. The brain was rapidly excised and cut using the vibroslicer (Campden Instruments Ltd.). Slices were stored at room temperature for at least 60 min in artificial cerebrospinal fluid (ACSF) of the following composition: NaCl, 130 mM; KCl, 3.5 mM; NaH2PO4, 1.25 nM; MgSO4, 1.5 nM; CaCl2, 2 nM; NaHCO3, 24 nM; glucose, 10 nM. The rats were then gassed with a 95% O2 –5% CO2 gas mixture, kept constantly at pH 7.4. The slices were then transferred to a submersion recording chamber and perfused with ACSF at a rate of 2 ml/min at 30–32°C. fEPSPs were recorded extracellularly from the apical dendritic layer of CA1 (stratum radiatum) with a glass micropipette (2–5MΩ) filled with ACSF. The signals were amplified and filtered (DC-3KHz) using an amplifier (Extracellular Amplifier BM 622). The data were stored on a PC for analysis using software developed with LABVIEW (National Instruments). Bipolar electrodes (SNEX-200, Rhodes Medical Instruments) were used for stimulation of Schaffer collateral-commissural afferent fibers. The intensity of the test stimulus, delivered at 0.033 Hz, was adjusted for each slice so that the fEPSP was equal to approximately 50% of the maximum amplitude of the fEPSP. The saturation of LTP was induced by successive trains of high frequency stimulation (HFS; 100 Hz for 1 sec, three trains at 20 min intervals) at the same intensity as the test stimulus until no additional potentiation was elicited. PPF was examined in each slice using a 40-msec interpulse interval as previously described (Dobrunz and Stevens 1997). PPF is a transient increase in synaptic efficacy that is caused by the accumulation of residual Ca2+ within the presynaptic terminals following the initial stimulus pulse of a two-stimulation protocol, when the second stimulus follows shortly (20–200 msec) after the first stimulus (Voronin and Kuhnt1990; Andreasen and Hablitz 1994; Dobrunz and Stevens 1997; Schlulz 1997). Only the slices in which the elicited fEPSPs were constant for 20–30 min were used in this study. Atropine, when present, was applied in the bath for 10–15 min before the application of PACAP-38 in the same solution, and then both were removed. PACAP-38 was dissolved in 5% Acetic acid (AcOH) then added to ACSF and applied for 10 min (we observed no effects of this AcOH concentration on synaptic transmission). Atropine was dissolved directly in ACSF. We averaged the evoked responses from five sweeps and measured the amplitude and slope of fEPSP. Paired-pulse data were normalized to determine the relative amount of facilitation for each slice. This was done by expressing the data as the ratio of the second response with respect to the first response. Compiled data were expressed as mean ± standard error of the mean (S.E.M.). Statistical analysis was performed using a one-factor, one-way analysis of variance (ANOVA) with repeated measures; when appropriate, this was followed by the Fisher's post hoc test. Statistical significance was set at the P <0.05 level.
Acknowledgments
We thank Dr. Thomas Nelson and Dr. Jeff Netzeband for valuable discussions, and Dr. Maurizio Cammalleri for graphics assistance. This research was supported in part by a 60% Ministero dell'Universita e della Ricerca Scientifica Technologica (MURST) grant.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL marbru@dfb.unipi.it; FAX 39-50-552183.
Article and publication are at www.learnmem.org/cgi/doi/10.1101/lm.34200.
REFERENCES
- Abel T, Kandel E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res Rev. 1998;26:360–378. doi: 10.1016/s0165-0173(97)00050-7. [DOI] [PubMed] [Google Scholar]
- Andreasen M, Hablitz JJ. Paired-pulse facilitation in the dentate gyrus: A patch-clamp study in rat hippocampus in vitro. J Neurophysiol. 1994;72:326–336. doi: 10.1152/jn.1994.72.1.326. [DOI] [PubMed] [Google Scholar]
- Arimura A. Receptors for pituitary adenylate cyclase-activating polypeptide. Comparison with vasoactive intestinal peptide receptors. Trends Endocrinol Metab. 1992;3:288–294. doi: 10.1016/1043-2760(92)90139-r. [DOI] [PubMed] [Google Scholar]
- ————— Perspectives on pituitary adenylate cyclase-activating polypeptide (PACAP) in the neuroendrocrine, endocrine, and nervous system. Jpn J Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- Arimura A, Shioda S. Pituitary adenylate cyclase-activating polypeptide (PACAP) and its receptors: Neuroendocrine and endocrine interactions. Front Neuroendocr. 1995;16:53–88. doi: 10.1006/frne.1995.1003. [DOI] [PubMed] [Google Scholar]
- Auerbach JM, Segal M. A novel cholinergic induction of long-term potentiation in rat hippocampus. J Neurophysiol. 1994;72:2034–2040. doi: 10.1152/jn.1994.72.4.2034. [DOI] [PubMed] [Google Scholar]
- ————— Muscarinic receptors mediating depression and long-term potentiation in rat hippocampus. J Neuropysiol. 1996;492:479–493. doi: 10.1113/jphysiol.1996.sp021323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson DM, Blitzer RL, Landau EM. An analysis of the depolarization produced in guinea-pig hippocampus by cholinergic receptor stimulation. J Physiol. 1988;404:479–496. doi: 10.1113/jphysiol.1988.sp017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo LS, Prince DA. Ionic mechanisms of cholinergic excitation in mammalian hippocampal pyramidal cells. Brain Res. 1982;249:333–344. doi: 10.1016/0006-8993(82)90067-1. [DOI] [PubMed] [Google Scholar]
- Berretta N, Berton F, Bianchi R, Capogna M, Francesconi W, Brunelli M. Effects of dopamine, D-1 and D-2 dopaminergic agonists on the excitability of hippocampal CA1 pyramidal cells in guinea pig. Exp Brain Res. 1990;83:124–130. doi: 10.1007/BF00232200. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of anaesthetised rabbit following perforant path stimulation. J Neurophysiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Gil O, Landau EM. Cholinergic stimulation enhances long-term potentiation in the CA1 region of rat hippocampus. Neurosci Lett. 1990;119:207–210. doi: 10.1016/0304-3940(90)90835-w. [DOI] [PubMed] [Google Scholar]
- Brandner C, Schenk F. Septal lesions impair the acquisition of cued place navigation task: Attentional or memory deficits? Neurobiol Learn Mem. 1998;69:106–125. doi: 10.1006/nlme.1997.3814. [DOI] [PubMed] [Google Scholar]
- Chen C, Tonegawa S. Molecular genetic analysis of synaptic plasticity, activity-dependent neural development, learning and memory in mammalian brain. Ann Rev Neurosci. 1997;20:157–184. doi: 10.1146/annurev.neuro.20.1.157. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Rovira C, Ben-Ari Y, Padjen A. Simultaneous recording of somatic and dendritic field potentials and combined microiontophoresis in the rat Ammon's horn in situ: Effects of GABA and acetylcholine. Neurosci Lett. 1982;31:19–24. doi: 10.1016/0304-3940(82)90047-7. [DOI] [PubMed] [Google Scholar]
- Cormier RJ, Mauk MD, Kelly PT. Glutamate iontophoresis induces long-term potentiation in the absence of evoked presynaptic activity. Neuron. 1993;10:907–919. doi: 10.1016/0896-6273(93)90206-7. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Feany MB, Quinn WG. A neuropeptide gene defined by the Drosophila memory mutant amnesiac. Science. 1995;268:869–873. doi: 10.1126/science.7754370. [DOI] [PubMed] [Google Scholar]
- Frotscher M, Léranth C. Cholinergic innervation of the rat hippocampus as revealed by choline actyltransferase immunocytochemistry: A combined light and electron microscopic study. J Comp Neurol. 1985;239:237–246. doi: 10.1002/cne.902390210. [DOI] [PubMed] [Google Scholar]
- Greengard P, Jen J, Nairn AC, Stevens CF. Enhancement of the glutamate response by cAMP-dependent protein kinase in hippocampal neurons. Science. 1991;253:1136–1138. doi: 10.1126/science.1716001. [DOI] [PubMed] [Google Scholar]
- Hepler DJ, Wenk BL, Cribbs BL, Olton DS, Coyle JT. Memory impairment following basal forebrain lesions. Brain Res. 1985;346:8–14. doi: 10.1016/0006-8993(85)91088-1. [DOI] [PubMed] [Google Scholar]
- Huang Y, Malenka RC. Examination of TEA-induced synaptic enhancement in area CA1 of the hippocampus: The role of voltage-dependent Ca2+ channels in the induction of LTP. J Neurosci. 1993;13:568–576. doi: 10.1523/JNEUROSCI.13-02-00568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T, Tominaga T, Ichikawa M, Iijima T. Differential alteration of hippocampal synaptic strength induced by pituitary adenylate cyclase activating polypeptide-38 (PACAP-38) Neurosci Lett. 1997;221:189–192. doi: 10.1016/s0304-3940(96)13323-1. [DOI] [PubMed] [Google Scholar]
- Krnjevic RP, Reiffenstein RJ, Ropert N. Disinhibitory action of acetylcholine in the rat hippocampus: Extracellular observations. Neuroscience. 1981;6:2465–2474. doi: 10.1016/0306-4522(81)90092-0. [DOI] [PubMed] [Google Scholar]
- Krnjevic RP, Ropert N. Electrophysiological and pharmacological characteristics of facilitation of hippocampal population spikes by stimulation of the medial septum. Neuroscience. 1982;7:2165–2183. doi: 10.1016/0306-4522(82)90128-2. [DOI] [PubMed] [Google Scholar]
- Leech CA, Holz GG, Habener JF. Pituitary adenylate cyclase-activating polypeptide induces the voltage-independent activation of inward membrane currents and elevation of intracellular calcium in HIT-T15 insulinoma cells. Endrocrinology. 1995;136:1530–1536. doi: 10.1210/endo.136.4.7895663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GJ, Madsen BW. PACAP-38 modulates activity of NMDA receptors in cultured chick cortical neurones. J Neurophysiol. 1997;78:2231–2234. doi: 10.1152/jn.1997.78.4.2231. [DOI] [PubMed] [Google Scholar]
- Madison DV, Lancaster B, Nicoll RA. Voltage clamp analysis of cholinergic action in the hippocampus. J of Neurosci. 1987;7:733–741. doi: 10.1523/JNEUROSCI.07-03-00733.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation- A decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Marchi M, Raitieri M. Interaction acetylcholine-glutamate in rat hippocampus: Involvement of two subtypes of M-2 muscarinic receptor. J Pharmacol Exp Ther. 1989;248:1255–1260. [PubMed] [Google Scholar]
- Margiotta JF, Pardi D. Pituitary Adenylate Cyclase-Activating Polypeptide type I receptors mediate cyclic AMP-dependent enhancement of neuronal acetylcholine sensitivity. Mol Pharmacol. 1995;48:63–71. [PubMed] [Google Scholar]
- Markram H, Segal M. Long-lasting facilitation of excitatory postsynaptic potentials in the rat hippocampus by acetylcholine. J Physiol. 1990;427:381–393. doi: 10.1113/jphysiol.1990.sp018177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J-L, Gasser D, Magistretti PJ. Vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide potentiate c-fos expression induced by glutamate in cultured cortical neurones. J Neurochem. 1995;65:1–9. doi: 10.1046/j.1471-4159.1995.65010001.x. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Matsumoto Y, Tokito F, Tsuda M, Fujino M. Effects of vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) on the spontaneous release of acetylcholine from hippocampus by brain microdialysis. Brain Res. 1993;611:207–215. doi: 10.1016/0006-8993(93)90504-g. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ohtaki T, Masuda Y, Fujino M. Binding sites for pituitary adenylate cyclase activating polypeptide (PACAP): Comparison with vasoactive intestinal polypeptide (VIP) binding site localization in rat brain. Brain Res. 1992;575:113–123. doi: 10.1016/0006-8993(92)90430-h. [DOI] [PubMed] [Google Scholar]
- Matthews DA, Salvaterra PM, Crawford GD, Houser CR, Vaughn JE. An immunocytochemical study of choline acetyltransferase containing neurones and axon terminals in normal and partially deafferented hippocampal formation. Brain Res. 1987;402:30–43. doi: 10.1016/0006-8993(87)91044-4. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38-residue hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminals 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP-38) Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Schlulz PE. Long term potentiation involves increase in the probability of neurotransmitter release. Proc Natl Acad Sci. 1997;94:5888–5893. doi: 10.1073/pnas.94.11.5888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoshige Y, Maeda T, Kaneko S, Akaike A, Satoh M. Involvement of M2 receptor in an enhancement of long-term potentiation by carbachol in Schaffer collateral-CA1 synapses of hippocampal slices. Neurosci Res. 1997;27:175–180. doi: 10.1016/s0168-0102(96)01147-9. [DOI] [PubMed] [Google Scholar]
- Shioda S, Shuto Y, Somogyvari-Vigh A, Legradi G, Onda H, Coy DH, Nakajo S, Arimura A. Localization and gene expression of the receptor for pituitary adenylate cyclase-activating polypeptide in the rat brain. Neurosci Res. 1997;28:345–354. doi: 10.1016/s0168-0102(97)00065-5. [DOI] [PubMed] [Google Scholar]
- Siegelbaum SA, Kandel ER. Learning-related synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1991;1:113–120. doi: 10.1016/0959-4388(91)90018-3. [DOI] [PubMed] [Google Scholar]
- Sokolov MV, Kleschevnikov AM. Atropine suppresses associative LTP in CA1 region of rat hippocampual slices. Brain Res. 1995;672:281–284. doi: 10.1016/0006-8993(94)01376-s. [DOI] [PubMed] [Google Scholar]
- Spengler D, Waeber C, Pantaloni C, Holsboer F, Bockaert J, Seeburg PH, Journot L. Differential signal transduction by five splice variants of the PACAP receptor. Nature. 1993;365:170–175. doi: 10.1038/365170a0. [DOI] [PubMed] [Google Scholar]
- Stella N, Magistretti PJ. Vasoactive intestinal polypeptide (VIP) and pituitary adenylate cyclase activating polypeptide (PACAP) potentiate the glutamate-evoked release of arachidonic acid from mouse cortical neurones. J Biol Chem. 1996;271:23705–23710. doi: 10.1074/jbc.271.39.23705. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Dingledine R. Presynaptic inhibitory effect of acetylcholine in the hippocampus. J Neurosci. 1981;1:784–792. doi: 10.1523/JNEUROSCI.01-07-00784.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronin LL, Kuhnt U. Long-term potentiation affects facilitation ratio of EPSPs recorded from CA1 pyramidal cells in the guinea pig hippocampal slice. Neurosci Res Comm. 1990;6:149–155. [Google Scholar]
- Wang L-Y, Salter MW, Macdonald JF. Regulation of kainate receptors by cAMP-dependent protein kinase and phosphatases. Science. 1991;253:1132–1135. doi: 10.1126/science.1653455. [DOI] [PubMed] [Google Scholar]
- Wu SY, Dun NJ. Potentiation of NMDA currents by pituitary adenylate cyclase-activating polypeptide in neonatal rat sympathetic preganglionic neurones. J Neurophysiol. 1997;78:1175–1179. doi: 10.1152/jn.1997.78.2.1175. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Pena LA. A novel synaptic transmission mediated by a PACAP-like neuropeptide in Drosophila. Neuron. 1995;14:527–536. doi: 10.1016/0896-6273(95)90309-7. [DOI] [PubMed] [Google Scholar]