Abstract
Quality of life (QoL) refers to an individual's sense of overall well-being encompassing physical, psychological, emotional, social, and spiritual dimensions. Although genetics healthcare providers strive to promote patient well-being, and the term QoL is often invoked to refer to this outcome, there is lack of clarity as to what actually constitutes QoL from the patient's perspective. This systematic literature review aims to summarize and integrate research findings to help elucidate how healthcare providers can more effectively enhance the QoL of patients affected with rare genetic conditions. Eligible studies were those that measured QoL as a primary outcome variable using a validated, multi-dimensional scale. Detailed criteria were used to rate quality of design, methodology, and analytic rigor. Fifty-eight studies were selected for inclusion in the review, and a narrative synthesis of the data was performed. A central theme emerging from the literature is that, although genetic conditions have the potential to have significant negative consequences for individuals' lives, having a genetic condition does not necessarily entail poor QoL. Evidence demonstrates that factors beyond the physical manifestations of the disease, such as psychological well -being, coping, and illness perceptions, influence QoL and may serve as potent targets for intervention. The field of research on QoL in rare genetic conditions will be advanced by uniting around a clear conceptualization of QoL and using more rigorous methodology with comprehensive measures of global QoL.†
Keywords: quality of life, genetic diseases, genetic counseling, psychological adaptation
Introduction
The experience of living with a rare genetic condition is vastly more complex than its medical features. Any aspect of an individual's life may be affected. Quality of life (QoL) refers to an individual's sense of overall well-being encompassing physical, psychological, emotional, social, and spiritual dimensions. Although genetics healthcare providers strive to promote patient well-being, and the term QoL is often invoked to refer to this outcome, there is a lack of clarity as to what actually contributes to QoL from the patient's perspective. Historically, research into genetic conditions has been limited to natural history and descriptions of clinical features. In recent years, QoL has increasingly been studied in genetic conditions. Findings from this research have begun to illuminate the subjective experience of living with a genetic condition and the complex, often profound effects on individuals' QoL. However, progress in this emerging area of research has been hindered by conceptual and methodological issues, and the medical literature remains limited in fully representing the perspective of those affected with these conditions.
The goal of the next frontier in healthcare for individuals living with rare genetic conditions is to improve QoL, not only by advancements in medical treatment, but with interventions aimed at modifying psychosocial and contextual factors that influence QoL. Research thus far has revealed promising opportunities; yet improvements in overall well-being resulting from targeted QoL interventions are far from being realized.
Objectives
A systematic review of the literature of studies examining QoL in rare genetic conditions was conducted. The aims of this paper are to:
Explicate the concept of QoL.
Evaluate approaches to measurement and strategies in QoL research.
Summarize and integrate research findings to help elucidate how healthcare providers can more effectively enhance the QoL of patients living with rare genetic conditions.
Discuss clinical implications and directions for future research on QoL in rare genetic conditions.
Background
QoL has been defined as “a person's sense of wellbeing that stems from satisfaction or dissatisfaction with areas of life that are important to him/her” [Ferrans and Powers, 1992]. QoL encompasses all major aspects of an individual's life, including physical health and functioning, social, psychological, emotional, spiritual, and family dimensions. Importantly, QoL is not a representation of health per se. Although one's health may influence QoL, and indeed the impact of a health condition is often reflected in poorer QoL, the concept in its truest sense represents the global picture of well-being [Ferrans and Powers, 1992; Leventhal, 1997].
Theoretical Framework
Theories of adaptation are useful to framing an understanding of QoL. In the context of chronic illness and disability, adaptation is “the process of coming to terms with the implications of a health threat and the observable outcomes of that process” [Biesecker and Erby, 2008]. QoL can be conceptualized as an outcome of the adaptation process [Leventhal, 1997; Stanton et al., 2001]. Theoretical models based on stress and coping theory are especially well-suited for QoL research, because they feature concepts that clinicians can influence [Biesecker and Erby, 2008]. Stress and coping models postulate that in response to a stressor, such as having a genetic condition, individuals make cognitive and emotional appraisals of the stressor. These appraisals include perceptions of “the personal significance of the stressor, including susceptibility to the stressor and its causes, severity, and relevance to their lives,” as well as perceptions of “one's ability to cope with the problems and emotions generated by the stressor” [Lazarus and Folkman, 1984]. These appraisals direct coping behaviors, and the coping process leads to adaptation. As individuals adapt to living with the genetic condition, they gradually attain or restore optimal QoL.
Yet despite the availability of useful theoretical models in which to frame research, most QoL studies ignore theory, making it difficult to interpret the relationship of QoL to other highly correlated variables. Lack of a theoretical foundation leaves the literature without a coherent framework within which to integrate existing data into our understanding of QoL. Moreover, factors that contribute to positive QoL and greater adaptation to living with a genetic condition are largely unexamined. Facilitating adaptation to the medical, psychological, and familial implications of the condition is a fundamental goal of genetic counseling [Resta et al., 2006].
Historical Challenges in QoL Research
The lack of conceptual clarity in QoL research poses a major hurdle towards advancement of the field. Throughout the literature, the term QoL has been inexactly used to refer to a variety of related but conceptually distinct constructs, including functional health status, level of physical disability, clinical symptoms, psychological well-being, and mood [Anderson and Burckhardt, 1999]. The introduction of the term “health-related quality of life,” intended to distinguish QoL in health status from QoL in the broader context, has only served to exacerbate this confusion. The conceptualization of QoL-as-health is problematic, because it implies that “people make distinctions between some part of life that is influenced by health, and some other parts of life that are not so influenced” [Anderson and Burckhardt, 1999] and that QoL is merely the absence of pathology. This also assumes that individuals' perceived QoL correlates neatly with their objective or clinical health status. Objective assessments often do not accurately reflect subjective perceptions of health and well-being; therefore, it is important to capture QoL from the individual's perspective and employ patient-centered outcome measures to do so [Stevenson and Carey, 2009].
A related problem in the literature occurs when authors do not explicitly define QoL, but rather imply its meaning by the constructs measured. These omissions have led to inconsistencies and imprecision in QoL conceptualization and research. See a commentary by Anderson and Burckhardt [1999] for more in-depth discussion of the historical development and pitfalls of the conceptualization of QoL in health research.
Measurement of QoL
An ongoing challenge in the field of QoL research has been its measurement. There is debate about how to accurately assess QoL. The approach to measurement and selection of a particular instrument stem largely from the way one defines QoL. A wealth of distinct and discrepant scales have been created to measure QoL. Most consist of several subscales that encompass, at a minimum, the physical, psychological, emotional, and social domains. Although the particular subscales vary among measures, they can be grouped into physical and psychosocial domains. Broadly, there are two main approaches to measuring QoL: generic scales and disease-specific scales.
Generic Scales
Generic scales are designed to study to any health condition, and accordingly, they are most useful for making comparisons across populations. Thanks to the widespread use of these scales, normative data on healthy populations are readily available, thereby allowing comparison of affected patients to unaffected individuals. Generic scales can also be used to compare to other illness populations, assess within-group differences, and examine associations with other variables. An example of a generic QoL scale is the Medical Outcomes Study Short Form (SF-36) [Ware and Sherbourne, 1992]. This 36-item scale has eight domains that encompass physical and psychosocial components of QoL. Physical functioning, role-physical, bodily pain, and general health subscales make up the physical component; mental health, role-emotional, social functioning, and vitality make up the mental (psychosocial) component.
Although widely used, generic QoL scales are biased for two reasons. First, most generic scales measure status (i.e., level of impairment or satisfaction) in the various domains of QoL, without assessing importance of each domain. This is a crucial weakness because it overlooks the relative meaning of the various components of QoL to each individual. In effect, by asking only about status, this imposes an objective standard of ideal QoL. This notion was articulated by Ferrans [1996], who stated that “individuals personally define what QoL is for them;” therefore, because “different people value different things… there is no single QoL for all people with the same life condition.” The second weakness is that most generic scales specifically assess the impact of one's health condition on the aspects of one's life primarily related to physical functioning. This limited approach to measurement is incongruent with the definition of QoL as a global construct that includes psychological, spiritual, and social well-being.
A newer generation of generic QoL scales address these issues by incorporating importance ratings along with satisfaction ratings and by moving away from the health-related focus. An example of such a scale is the Ferrans and Powers Quality of Life Index (QLI), which consists of four domains: health and functioning, social and economic, psychological and spiritual, and family [Ferrans and Powers, 1992]. The 32 paired items assess these aspects of life in general, not specifically oriented to a health condition. For each item, respondents rate their degree of satisfaction and the level of importance, and the scale is scored weighting corresponding items against one another.
Disease-Specific Scales
More recently, disease-specific QoL scales have been developed for a number of health conditions, including a few genetic conditions, such as cystic fibrosis and sickle cell anemia. These scales resemble generic QoL scales in that they assess multiple domains. In addition, some or all of the questions relate to potential direct effects of the particular condition. For example, cystic fibrosis scales ask about the impact of pulmonary symptoms on the major domains of QoL. By their very nature, these are health-related scales, and thus do not measure QoL as a global construct. Disease-specific QoL scales may be useful for making within-group assessments but not normative comparisons. These scales also may be useful in evaluating outcomes of clinical trials specific to the condition.
Measuring QoL in Pediatric Populations
There are numerous challenges around measuring QoL in children and adolescents. Although the concept of QoL is the same regardless of age, the domains that constitute QoL differ across the lifespan. Therefore, it is necessary to employ either a pediatric QoL instrument or a QoL instrument with age-specific versions. In either case, the instrument must be validated in the age group. A controversial issue is reporting method. The underlying assumption is that the child is the best judge of his or her QoL, so a self-report scale is ideal. Indeed, a number of pediatric QoL scales are available for children as young as 5 years of age. However, self-report may not be feasible for some children, particularly those who are very young or cognitively impaired, so the parent or primary caregiver serves as a proxy. There is debate as to whether parents can “accurately” assess their child's QoL. Studies yield discrepant results between parents and children even within the same cohort. This begs the question: who is (more) correct? Do parents tend to overestimate the impact of disease on their children, and/or do children underestimate the impact? We refer the reader to a comprehensive review and commentary on the subject of QoL studies in pediatric populations by De Civita et al. [2005].
Other Approaches to Measurement
Although this systematic review focused on quantitative research, qualitative studies into QoL can yield rich data. Interview-based studies are often important as the first step in understanding affected individuals' perspectives on QoL, and, more importantly, specific factors that they consider critical to defining their QoL. Qualitative data are also important to supplement quantitative findings by aiding in the interpretation of results.
Due to the above-described limitations, many of the published studies yield incomplete data for designing interventions aimed at enhancing the QoL of patients with rare genetic conditions. There are, however, a number of excellent, valuable studies that serve as models. This review aims to summarize and integrate available high-quality research on QoL in rare genetic conditions, in order to provide a launching point for future research that will advance our understanding of QoL, both as a concept and as an important clinical outcome.
Methods
Search Strategy
Reports of original research studies on the QoL of individuals affected with rare genetic conditions were identified. Specifically, a “quality of life study” was defined as a study in which QoL, consistent with the previously described conceptual definition of the construct, was measured as a primary outcome variable using a validated, multi-dimensional scale. For the purposes of this review, a “rare genetic condition” was defined as a single-gene disorder (i.e., a condition characterized by a Mendelian pattern of inheritance) with a general population frequency of less than 1 in 2,000. This frequency threshold was selected based on the National Organization for Rare Disorders definition of a rare disease as one that affects fewer than 200,000 individuals in the United States.
Articles published through January 1, 2009 in peer-reviewed journals were identified by searches of PubMed, Scopus, and Web of Science. Database searches were conducted using the keyword combinations including “quality of life,” “genetic disease,” “congenital disease,” and related MeSH terms. Additional reports were identified by hand-searching the references of the retrieved articles.
Selection Criteria
Detailed selection criteria were defined by the authors prior to embarking on the systematic review. The criteria, which comprised basic inclusion criteria as well as quality criteria, were developed based on a preliminary review of a random subset of relevant QoL studies and informed by theoretical and conceptual literature.
The three basic inclusion criteria are summarized in Table I. First, the population under study was individuals affected with rare genetic conditions. Familial cancer syndromes (e.g., hereditary non-polyposis colorectal cancer, familial adenomatous polyposis, and hereditary breast and ovarian cancer) were excluded from this review, as there is an extensive body of literature on QoL among cancer patients. Studies with mixed populations including genetic and non-genetic conditions that did not report subgroup analyses (i.e., a subgroup consisting only of individuals affected with the rare genetic condition) were also excluded. Studies located in developing countries, as designated by the International Monetary Fund, were excluded, because it is reasonable to expect that the inherent disparities in overall population health would preclude accurate comparison and generalizability with studies from developed countries. Second, only articles reporting original research studies were included. The third inclusion criterion related to the purpose of the study; at least one primary aim was to describe QoL and/or predictor variables or factors associated with QoL for rare genetic conditions. Clinical trials of a drug, surgery, or medical intervention on a clinical outcome were excluded from this review, because those studies were designed to evaluate the effectiveness of the medical intervention, rather than to understand QoL.
TABLE I. Basic Inclusion Criteria.
| Inclusion criteria | Studies excluded |
|---|---|
| Target population: affected with rare genetic condition | Non-rare, non-genetic, and/or multifactorial conditions |
| Familial cancer syndromes | |
| Unaffected family members or caregivers | |
| Located in developing country | |
| Article type: original research | Non-research publications, such as: commentaries, essays, consensus statements, reviews, case reports, economic analyses, and articles dealing with ethical or legal issues |
| Articles not available in English | |
| Study purpose: at least one primary aim was to describe QoL and/or predictors | Clinical trials or studies evaluating the effectiveness of a drug, surgery, or medical intervention on a clinical outcome |
| Studies about the development/validation of a QoL instrument | |
| Do not measure QoL, use a proxy variable instead |
Articles that met basic inclusion criteria were further evaluated for quality based on criteria summarized in Table II. Each article was evaluated for the quality of the study itself (design, methodology, and analytic rigor), as well as for specificity in reporting the research methods and results.
TABLE II. Quality Criteria.
| Adequate |
| Conceptualization of QoL |
| Comprehensive strategy for recruiting participants |
| Use of validated, multidimensional QoL instrument |
| Analytically rigorous, including attainment of sufficient sample size and use of appropriate control/comparison group |
| Reported |
| Specific objectives and hypotheses |
| Clear description of study design, recruitment source, inclusion and exclusion criteria |
| Sample characteristics and demographics |
| Clearly defined key variables and measures |
| Comprehensive presentation of QoL data on all domains of instrument |
| Detailed description of analyses performed and statistical significance of results |
Study Selection
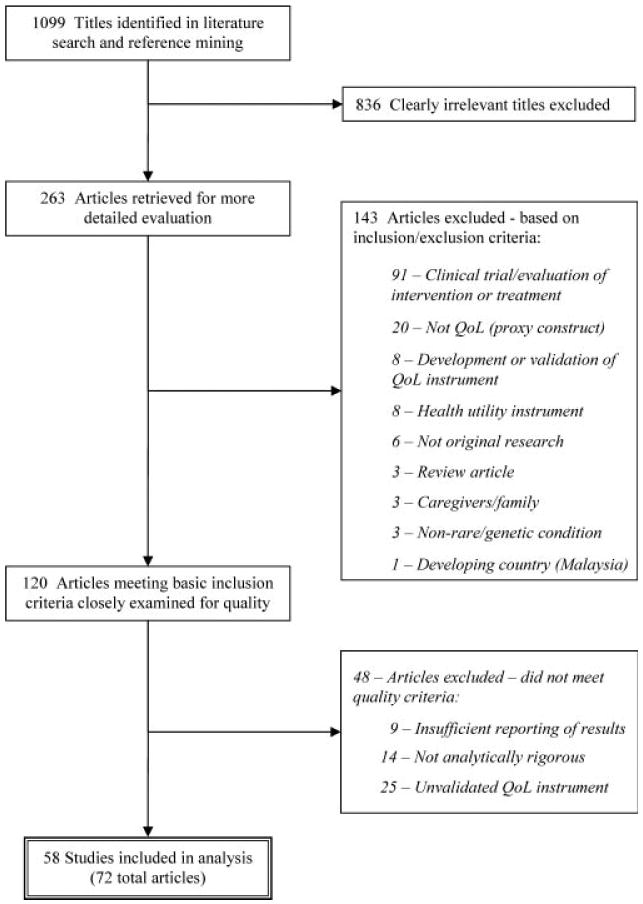
The literature searches and reference mining yielded a total of 1,099 titles. The authors independently sorted and evaluated the articles based on standardized selection criteria, with disagreements resolved by discussion. The selection process consisted of three rounds of examination, culminating in the final body of literature selected for inclusion. The flow of literature through the selection process is depicted in Figure 1.
FIG. 1. Flow diagram of study selection process.
Data Extraction
The first author extracted data from the selected studies. Information was extracted on: study population, recruitment source, study design, QoL instrument, key predictor variables, and measures. Each study was analyzed for the primary outcome variable of QoL. Topics of interest were: statistical comparisons with healthy controls/population norms, statistical comparisons with other disease populations, and analysis of associations with key disease-related and psychosocial predictor variables. Frequencies of data were tabulated. Articles were grouped by condition and by topic categories. Summary and evidence tables were created. Because the studies were too heterogeneous to permit statistical pooling, a narrative synthesis of the findings was performed, taking into account methodological quality and analytic rigor in the examination and reporting of findings.
Results
Fifty-eight QoL studies of 30 distinct rare genetic conditions were selected for inclusion in the review and analysis. Because several of the studies were reported in more than one article, the body of literature covered in this review consisted of 72 articles in total. Details of each study and selected findings are presented in Table III.
TABLE III. Summary of QoL Studies and Selected Findings1.
| Genetic condition | References | Study population (N, age, recruit source) | Study Design | QoL Scale2 | Selected Findings |
|---|---|---|---|---|---|
| Achondroplasia | Gollust et al. 2003a,b (USA) | 189 adults from support org. | XS | QLI | Individuals with achondroplasia had globally poorer QoL than their unaffected first-degree relatives |
| In multivariate regression, affected status was only modestly signif. for total QoL (P= 0.039) and physical QoL (P= 0.024), and NS for the other three QoL domains | |||||
| When controlling for demographics and affected status, greater perceived seriousness and lower self-esteem were strongly associated with poorer QoL in all domains | |||||
| Charcot-Marie-Tooth disease | Padua et al. 2008a,b (Italy) | 98 adults/teens (14 y+) with CMT1A, clinic patients | L(2 y) | SF-36 | Globally poorer QoL compared to pop. norm |
| QoL scores at baseline and 2 y follow-up were NS different | |||||
| Clinical/neurophysiological features (e.g., ability to toe-walk, nerve conduction) associated with QoL scores in some physical health domains, few correlations with psychosocial QoL | |||||
| Redmond et al. 2008 (Australia) | 295 adults with CMT1A and CMT2, from support org. | XS | SF-36 | Globally poorer QoL compared to pop. norm, NS differences in QoL between CMT types | |
| Physical symptoms (e.g., leg weakness, cramps) associated with lower QoL in some domains | |||||
| Vinci et al. 2005 (Italy) | 121 adults/teens (15 y+), clinic patients | XS | SF-36 | Globally poorer QoL compared to norm | |
| Disease duration negatively associated with physical QoL (PCS) but not psychosocial QoL (MCS) | |||||
| Teunissen et al. 2003 (The Netherlands) | 43 adults/teens with CMT2, clinic patients | L (5 y) | SF-36 | Signif. poorer QoL in most domains compared to pop. norm | |
| In the longitudinal cohort (n = 27), NS change in any QoL domains over the 5-year period, although clinical status/disability had worsened | |||||
| Congenital adrenal hyperplasia | Jaaskelainen and Voutilainen 2000 (Finland) | 32 adult clinic patients | XS | SF-36 | Greater QoL in physical and psychosocial domains compared to population norms |
| Cystic fibrosis (CF) | Gee et al. 2003, 2005, Abbott et al. 2007, 2008 (UK) | 223 adult clinic patients | XS | CFQoL | Lung function signif. associated with QoL in most domains |
| Coping styles: higher optimism signif. associated with higher QoL, higher distraction signif. associated with lower QoL, hopefulness and avoidance NS associated with QoL | |||||
| Britto et al. 2002, 2004, Arrington-Sanders et al. 2006 (USA) | 162 adults and children (5 y+), clinic patients | XS | SF-36 CHQ | Adults (n=48) had signif. poorer physical QoL compared to pop. norm, but NS differences in psychosocial QoL domains | |
| Among children/adolescents (n = 114), parent-rated QoL was signif. poorer in all physical domains compared to norms, but only signif. difference in psychosocial domains was for self-esteem | |||||
| Lung function NS correlated with QoL, but frequency of pulmonary exacerbations was signif. associated with poorer physical QoL | |||||
| Parent-rated QoL was signif. poorer than child/adolescent self-rated QoL in physical domains, but NS different for psychosocial domains | |||||
| Havermans et al. 2008 (Belgium) | 57 adult clinic patients | XS | CFQ | Lung function signif. negatively associated with QoL in some physical health domains | |
| After controlling for lung function, anxiety and depression signif. associated with 6/12 psychosocial and 3/12 physical QoL domains | |||||
| Palermo et al. 2006 (USA) | 46 children/adolescents (8–17 y), clinic patients | XS | CFQ | Pain signif. associated with QoL in physical health domains, NS for emotional or social domains | |
| Riekert et al. 2007 (USA) | 76 adult clinic patients | XS | CFQ | Depression signif. associated with poorer QoL in all domains | |
| Szyndler et al. 2005 (Australia) | 52 adolescents (12–18 y), clinic patients | XS | CFQ | Higher levels of psychopathology and lower optimism for the future signif. associated with poorer QoL in most domains | |
| Family functioning characteristics signif. associated with QoL in some domains | |||||
| Thomas et al. 2006 (Australia) | 162 children/adolescents (2–19 y), clinic patients | XS | PedsQL CFQ | Globally poorer QoL compared to norm | |
| Lung function signif. negatively associated with some CFQ domains, NS with any PedsQL | |||||
| Darier's and Hailey–Hailey diseases | Harris et al. 1996 (UK) | 201 adults/teens (13 y+), clinic patients | XS | DLQI | QoL was most negatively affected in the symptoms/feelings domain (highest score of all the domains); however, mean DLQI scores were within “small” to “moderate” effect range, indicating that the disease did not have a major negative impact on patients' QoL |
| NS correlation between clinical severity and QoL; NS difference in QoL between disease groups (DD vs. HHD), despite differences in symptoms | |||||
| Ehlers–Danlos syndrome | Berglund and Nordstrom 2001, Berglund et al. 2003 (Sweden) | 77 adults from support org. | XS | SIP | Globally poorer QoL compared to pop. norm |
| Greater acceptance of disability and sense of coherence signif. associated with better QoL | |||||
| Fabry disease | Miners et al. 2002 (UK) | 38 male adult clinic patients | XS | SF-36 | Globally poorer QoL compared to male pop. norm |
| Compared to patients with severe hemophilia [Miners et al., 1999], Fabry patients had signif. poorer psychosocial QoL (MCS), but physical QoL (PCS) NS different | |||||
| Ries et al. 2005 (USA) | 25 male children and adolescents (6–18 y), clinic patients | XS | CHQ | Among children (n = 9), parent-rated QoL was poorer than norms in all domains, but differences were statistically signif. for two domains | |
| Teens (n = 15, self-report) had signif. more pain (lower QoL) and better QoL in behavior, social, and emotional domains than norm | |||||
| Street et al. 2006 (USA) | 202 adult female heterozygotes, from support org. and clinic | XS | SF-36 | Globally poorer QoL compared to female pop. norm | |
| Familial dysautonomia | Sands et al. 2006 (USA) | 145 adults and children (4 y+), clinic patients | XS | SF-36 CHQ | Among adults (n = 74), NS differences in QoL than pop. norm |
| Among children (n = 71), parent-rated QoL was signif. poorer in all physical domains and 2/4 psychosocial domains compared to norm; physical and psychosocial summary scores were also significantly poorer than children with various other chronic medical conditions | |||||
| Friedrich Ataxia | Epstein et al. 2008 (USA) | 130 adult clinic patients | XS | SF-36 | Compared to age/sex-matched control group and to pop. norm, patients had signif. poorer QoL in all domains except RE and MCS (NS differences) |
| Disease duration and clinical severity (neurological impairment) signif. associated only with PF domain; disability signif. associated with PF and GH domains | |||||
| Wilson et al. 2007 (Australia) | 63 adult clinic patients | XS | SF-36 | Globally poorer QoL compared to pop. norm, physical worse than psychosocial QoL | |
| Clinical severity (neurological impairment) signif. associated with only PF domain | |||||
| After controlling for severity and disease duration, age of onset was signif. associated with QoL in some domains, with adult-onset patients having lower QoL than patients whose disease began <18 y | |||||
| In multivariate regression, age of onset and severity were strongest predictors of PCS, whereas disease duration was the only factor signif. associated with MCS | |||||
| Galactosemia | Bosch et al. 2004 (The Netherlands) | 63 adults and children (1 y+), from support org. | XS | TAPQoL TACQoL TAAQoL | For all age groups, trend towards poorer QoL in most domains compared to healthy norms, but differences were statistically significant only in some domains (small sample sizes: n = 17 adults, n = 25 children/adolescents, n = 22 young children) |
| Gaucher disease | Damiano et al. 1998 (USA) | 212 adults/teens (14 y+) on enzyme replacement therapy, clinic patients | XS | SF-36 | Signif. poorer QoL in physical domains compared to norm, NS differences for psychosocial domains |
| Ageing and clinical status (e.g., joint replacement, splenectomy) signif. associated with poorer QoL in some domains | |||||
| Glycogen storage disease type 1 | Storch et al. 2008 (USA) | 29 children/adolescents (6–18 y), clinic patients | XS | PedsQL | Compared to healthy control group, patients had signif. lower QoL in physical and social domains, NS differences in emotional and school domains |
| Compared to sample of children with various chronic medical conditions, NS differences in QoL | |||||
| Hemophilia and coagulation disorders | Miners et al. 1999 (UK) | 164 male adult clinic patients | XS | SF-36 | Hemophilia patients had signif. worse QoL than pop. norm in physical domains, but NS differences in psychosocial domains |
| Compared to patients with mild/moderate disease, patients with severe hemophilia had signif. poorer physical QoL, NS differences in psychosocial domains | |||||
| Tusell et al. 2002 (Spain) | 70 male adults with severe hemophilia, clinic patients | XS | SF-36 | Signif. poorer QoL than pop. norm in all domains except mental health and emotional role-functioning | |
| Walsh et al. 2008 (Canada) | 47 male adults with mild hemophilia A from same kindred, identified through population survey | XS | SF-36 | Compared to control cohort of unaffected age-matched male relatives, affected males had significantly poorer QoL in GH and RE domains, trend towards poorer QoL in all other domains | |
| In multivariate regression analysis, clinical status/symptoms (heart disease and joint damage) were signif. predictors of PCS, but affected status was NS (suggests that the difference in physical QoL between hemophilia and control is largely explained by heart disease and joint damage, rather than hemophilia itself) | |||||
| Solovieva 2001, Solovieva et al. 2004 (Finland) | 164 adults with hemophilia, von Willebrand disease, and Factor XIII deficiency, clinic patients | L(3 y) | SF-36 | Signif. poorer physical QoL and greater mental QoL than healthy control group | |
| NS change in QoL in most domains between baseline and 3-year follow-up | |||||
| In multivariate regression analysis, patients with severe disease and/or whose disease severity increased were more likely to have reduction in QoL over time | |||||
| Hereditary hemorrhagic telangiectasia | Geisthoff et al. 2007 (Germany) | 77 adults/teens (13 y+), clinic patients | XS | SF-36 | Signif. poorer QoL in all domains except pain compared to pop. norm |
| Clinical symptoms (e.g., epistaxis) signif. correlated with some QoL domains | |||||
| Greater perceived consequences (strain on profession, private life, and psyche) and worries about HHT signif. associated with lower QoL in all domains (P < 0.05) | |||||
| Pasculli et al. 2004 (Italy) | 50 adult clinic patients | XS | SF-36 | Poorer QoL in all domains except pain than pop. norm | |
| Clinical symptoms (e.g., epistaxis) signif. associated with PCS but not MCS | |||||
| Huntington disease | Helder et al. 2001, 2002 (The Netherlands) | 77 adults recruited from clinic and support org. | XS | SF-36 SIP | As assessed by the SIP: QoL was globally poorer than pop. norm Psychosocial aspects impacted to a greater extent than physical aspects Cognitive/motor functioning and disease duration predicted a signif. amount of variance in physical SIP but not psychosocial SIP |
| As assessed by the SF-36, QoL was signif. poorer in most physical health domains, but NS differences for psychosocial domains; After controlling for demographics and illness-related variables, coping and illness perceptions predicted signif. amount of variance in QoL | |||||
| Coping: “acceptance” positively associated with mental health QoL domain; venting of emotions, behavioral and mental disengagement were negatively associated with QoL | |||||
| Illness identity and “cure” perceptions associated with some QoL domains | |||||
| Hyperimmunoglobulinemia type D | van der Hilst et al. 2008 (International) | 28 adult clinic patients | XS | SF-36 | Poorer QoL in some domains than pop. norm Symptoms (frequency of attacks) signif. associated with physical QoL, NS psychosocial QoL |
| Marfan syndrome | Peters et al. 2002 (USA) | 174 adults recruited from clinic and support org. | XS | QLI | Individuals with Marfan syndrome had signif. poorer QoL in psychological/spiritual domain than patients with cardiovascular disease, NS difference in physical health/functioning domain |
| Muscular dystrophies | Ahlstrom et al. 1994, Ahlstrom and Gunnarsson 1996, Ahlstrom and Sjoden Ahlstrom and Sjoden, 1996a, Natterlund et al. 2000, Bostrom et al. 2005 (Sweden) | 57 adults with various MD types, identified through general population survey | L (10 y) | SIP | Globally poorer QoL compared to pop. norm, greater impact (worst QoL) in physical dimension |
| NS difference in QoL between types of MD, despite differences in physical disability | |||||
| Disability signif. negatively associated with physical QoL and, to a lesser extent, psychosocial QoL | |||||
| Coping strategies signif. associated with psychosocial and overall QoL, NS for physical QoL | |||||
| Psychosocial well-being NS correlated with QoL | |||||
| QoL signif. deteriorated over the 10-year period, physical QoL to a greater extent than psychosocial QoL | |||||
| Disability signif. increased over time, whereas NS change in psychosocial well-being | |||||
| Piccininni et al. 2004 (Italy) | 45 adults with various MD types, clinic patients | XS | SIP | Globally poor QoL (SIP scores in “clinically-significant impairment” range), QoL in physical dimension worse than psychosocial dimension | |
| Disability signif. negatively associated with QoL | |||||
| Higher psychological well-being signif. associated with better QoL | |||||
| Anxiety and depression signif. negatively correlated with QoL | |||||
| Grootenhuis et al. 2007 (The Netherlands) | 107 adults and children (8 y+) with various MD types, clinic patients | XS | TAAQoL TACQoL | Children (n = 18) and adolescents (n = 22) with MD had signif. poorer QoL on some domains, but signif. better QoL in the physical functioning domains compared to healthy norms for the same age group | |
| Adults with MD (n = 67) had signif. poorer QoL on 8/12 domains compared to healthy norms | |||||
| Clinical severity signif. negatively associated with fine motor functioning and social functioning domains of QoL among adults | |||||
| Antonini et al. 2006 (Italy) | 20 adults with myotonic dystrophy, clinic patients | XS | SF-36 | Globally poorer QoL compared to healthy matched controls and pop. norm | |
| Clinical severity signif. associated with poorer QoL in physical domains, NS for psychosocial QoL | |||||
| Anxiety and depressive symptoms signif. associated with poorer QoL in RP and MH domains | |||||
| Ford et al. 2006 (New Zealand) | 36 adults with various MD types, clinic patients | XS | SF-36 | QoL signif. poorer in physical health domains as compared to pop. norm, NS for psychosocial QoL | |
| NS differences between patients with myotonic dystrophy versus other MD types | |||||
| Neurofibromatosis type 1 | Graf et al. 2006 (Switzerland) | 46 children and adolescents (7–16 y), clinic patients | XS | TACQoL | Self-reported and proxy-reported QoL were signif. poorer than norm in the majority of domains |
| Clinical severity and visibility signif. associated with poorer QoL in emotional domains | |||||
| Family functioning: greater cohesion and lower conflict signif. associated with better QoL when rated by parents, NS relationships with child self-reported QoL | |||||
| Family history: parents with NF1 rated their children's emotional QoL lower than did parents without NF1; family history was NS associated with children's self-reported QoL | |||||
| Kodra et al. 2009 (Italy) | 129 adults clinic patients | XS | SF-36 Skindex | Significantly poorer QoL in all SF-36 domains than pop. norm | |
| Impact of NF1 on QoL was greater for psychosocial aspects than physical health aspects | |||||
| Visibility signif. associated with poorer skin-specific QoL (Skindex) on all domains, but NS associated with SF-36 scores | |||||
| Krab et al. 2009 (The Netherlands) | 58 children and adolescents (7–17 y), clinic patients | XS | CHQ | Parent-rated QoL was signif. poorer than pop. norm in 6/8 domains | |
| Adolescents (n = 43 self-report) had signif. better QoL in behavior domain than norm | |||||
| Severity signif. associated with some QoL domains; visibility NS associated with QoL | |||||
| Oostenbrink et al. 2007 (The Netherlands) | 34 young children (1–6 y), clinic patients | XS | ITQoL | Signif. poorer QoL in some domains compared to healthy reference sample | |
| Visibility signif. negatively associated with health perceptions domain of QoL | |||||
| Page et al. 2006 (USA) | 166 adults recruited from clinic and support organization | XS | SF-36 SkinDex | Signif. poorer QoL in all SF-36 domains than population norms; | |
| Impact of NF1 on QoL was greater for psychosocial aspects than physical health aspects | |||||
| Visibility signif. associated with poorer skin-specific QoL (Skindex) on all domains, but NS associated with SF-36 scores | |||||
| Clinical severity signif. associated with poorer QoL in all SF-36 domains and 2/3 Skindex domains (functioning and physical symptoms, NS emotional symptoms) | |||||
| Wolkenstein et al. 2001 (France) | 128 adult clinic patients | XS | SF-36 SkinDex | Signif. poorer QoL in all SF-36 domains than population norms | |
| Impact of NF1 on QoL was greater for psychosocial aspects than physical health aspects | |||||
| Visibility signif. associated with poorer QoL on all Skindex domains and most SF-36 domains | |||||
| Clinical severity signif. associated with poorer QoL in some SF-36 domains, but NS associated with Skindex scores | |||||
| Wolkenstein et al. 2009 (France) | 79 children and adolescents (8–16 y), clinic patients | XS | DISABKIDS CDLQI | Using DISABKIDS, impact on total QoL was greater (i.e., worse QoL) for NF1 than for asthma | |
| Using the CDLQI, impact on QoL was lower for NF1 (i.e., better QoL) than for other skin diseases (psoriasis, eczema, acne) | |||||
| Disease complications/symptoms signif. associated with greater impact (lower QoL) | |||||
| Osteogenesis imperfecta | Widmann et al. 2002 (USA) | 30 adult clinic patients | XS | SF-36 | QoL signif. poorer in most physical health domains than norm, NS differences in psychosocial QoL |
| Phenylketonuria | Landolt et al. 2002 (Switzerland) | 37 children/adolescents (3–18 y) on treatment, clinic patients | XS | TACQoL | As rated by parents, children/adolescents with PKU had signif. poorer QoL than norms in one psychosocial domain (positive emotional functioning), but NS differences in any other QoL domains |
| Simon et al. 2008 (Germany) | 67 adult clinic patients on treatment | XS | PLC | No signif. differences in any QoL domains compared to pop. norm | |
| Pompe disease | Hagemans et al. 2004 (International) | 210 adults from support org. | L (1 y) | SF-36 | Disability signif. associated with lower QoL in PF, SF, and RE domains |
| Longer disease duration signif. associated with lower PF scores, but higher RP and MH scores | |||||
| Mean QoL scores for the Dutch subgroup (n = 51) were signif. lower than population norms for 3/4 physical health and 2/4 mental health domains; NS differences for BP, RE, and MH | |||||
| In the Dutch cohort who were followed longitudinally (n = 38), no signif. change in QoL over the 1 y follow-up period, even among those who reported physical deterioration | |||||
| Porphyria | Holme et al. 2006 (UK) | 220 adults and children (5 y+) with erythropoietic protoporphyria, clinic patients | XS | DLQI CDLQI | For both adults and children, QoL was markedly impaired (mean DLQI/CDLQI scores were within the “very large effect” range) |
| Clinical severity signif. associated with QoL | |||||
| Millward et al. 2001 (UK) | 81 adults with acute porphyrias, clinic patients | XS | MOS | Globally poorer QoL compared to pop. norm | |
| Patients manifesting symptoms had signif. lower QoL than patients without symptoms (latent) | |||||
| Patients with acute intermittent porphyria had more pain and poorer social functioning than patients with other types (variegate porphyria and hereditary coproporphyria) | |||||
| Prader–Willi syndrome | Caliandro et al. 2007 (Italy) | 29 adults and children (5 y+), clinic patients | XS | SF-36 CHQ | Compared to pop. norm, adults and children had signif. poorer QoL in most domains |
| Sickle cell disease | McClish et al. 2005 (USA) | 308 adult clinic patients | XS | SF-36 | Patients with sickle cell had signif. poorer QoL in all domains except mental health, as compared to population norms and patients with cystic fibrosis |
| Pain signif. negatively associated with QoL in all domains except mental health | |||||
| Palermo et al. 2002 (USA) | 58 children/adolescents (5–18 y), clinic patients | XS | CHQ | Globally poorer QoL than healthy controls | |
| Disease complications negatively | |||||
| associated with physical QoL, but NS with psychosocial QoL | |||||
| Panepinto et al. 2005 (USA) | 99 children and adolescents (5–18 y), clinic patients | XS | CHQ | Parent-rated QoL was signif. poorer than norm in physical and psychosocial domains | |
| Adolescents (n = 53) self-rated their QoL as signif. poorer than norms in physical domains, but NS different in psychosocial domains | |||||
| QoL as rated by parents tended to be lower than adolescents' self-reported QoL | |||||
| Disease complications (number of crises) was signif. negatively correlated with physical QoL | |||||
| Turner syndrome | Carel et al. 2005 (France) | 568 adult females treated with growth hormone, clinic patients | XS | SF-36 | NS differences in any QoL domains compared to reference sample |
| Psychological distress signif. correlated with lower QoL (women with symptoms of psychological distress had signif. poorer QoL in all domains than those without symptoms) | |||||
| Height and other treatment-related variables NS associated with QoL | |||||
| X-linked agammaglobulinemia | Howard et al. 2006 (USA) | 41 male adult clinic patients | XS | SF-12 | NS differences in QoL between patients and pop norms, except GH domain |
| Lung disease signif. associated with lower MCS scores, but NS difference in PCS | |||||
| Winkelstein et al. 2008 (USA) | 25 male adult clinic patients | XS | SF-12 | Trend towards lower QoL among patients than pop norms, but NS |
Abbreviations used in Table III: support org., support organization; signif., significant; NS, not significant; y, years; XS, cross-sectional; L, longitudinal; pop. norm, population norm; PCS, physical component score; MCS, mental component score; PF, physical functioning; RP, role physical; BP, bodily pain; VT, vitality; MH, mental health; GH, general health perceptions; RE, role emotional; SF, social functioning.
See Table V QoL scale abbreviations.
Genetic Conditions
The 30 rare genetic conditions represent a diverse spectrum of disorders (Table IV). Although it is difficult to categorize each condition precisely, the groupings include neuromuscular and neurologic, metabolic, dermatologic, chromosomal, connective tissue, blood and vascular disorders, and skeletal dysplasias. The conditions also vary in illness typology; that is, age of onset, disease course, morbidity and mortality. Cystic fibrosis and neurofibromatosis type 1 were the most frequently studied conditions, with seven studies each.
TABLE IV. List of Genetic Conditions by Clinical Category (With Number of Studies).
| Metabolic disorders |
| Phenylketonuria (2) |
| Galactosemia (1) |
| Congenital adrenal hyperplasia (1) |
| Porphyrias (2) |
| Glycogen storage disease type 1 (1) |
| Hyperimmunoglobulinemia D (1) |
| Lysosomal storage disorders |
| Fabry disease (3) |
| Gaucher disease (1) |
| Pompe disease (1) |
| Blood and vascular disorders |
| Hereditary hemorrhagic telangiectasia (2) |
| Sickle cell disease (3) |
| Coagulopathies |
| Hemophilia (4) |
| Factor XIII deficiency (1) |
| Von Willebrand disease (1) |
| Neuromuscular and neurologic disorders |
| Muscular dystrophies (5) |
| Charcot-Marie-Tooth disease (4) |
| Friedrich ataxia (2) |
| Familial dysautonomia (1) |
| Huntington disease (1) |
| Dermatologic disorders |
| Neurofibromatosis type 1 (7) |
| Darier's disease (1) |
| Hailey–Hailey disease (1) |
| Connective tissue disorders |
| Marfan syndrome (1) |
| Ehlers–Danlos syndrome (1) |
| Skeletal dysplasias |
| Achondroplasia (1) |
| Osteogenesis imperfecta (1) |
| Chromosomal disorders |
| Prader–Willi syndrome (1) |
| Turner syndrome (1) |
| Other |
| Cystic fibrosis (7) |
| X-linked agammaglobulinemia (2) |
Participant Characteristics
Forty studies focused on adults, whereas 12 studies focused exclusively on pediatric populations (children and adolescents) using a pediatric-specific QoL instrument or an instrument with age-appropriate versions. Six studies assessed QoL across the lifespan, with participants ranging in age from childhood through adulthood. In the eight studies that assessed children's QoL by both proxy- and self-report, there was some agreement between parents' assessments and their children's self-reported QoL; however, in several studies, the parents rated the QoL of their children significantly lower (poorer).
Recruitment
The majority of study populations were comprised of patients from clinical or hospital settings. Nine studies recruited participants from support organizations, such as the National Marfan Foundation [Peters et al., 2002]. These two recruitment methods each have their advantages and disadvantages regarding recruitment/selection bias. One could argue that the clinic-based studies oversampled more severely affected individuals, since they are more likely to seek medical care from a specialized clinic than less severely affected individuals. On the other hand, members of support organizations may differ from non-members in relevant ways; for example, individuals who are having difficulty coping with their disease may be more likely to seek support than individuals who are coping well. Interestingly, two studies perhaps overcame the threat of recruitment bias by identifying participants directly from the general population, thereby reaching a spectrum of affected individuals in the geographic region. Walsh attempted to include all males with hemophilia from a distinct Canadian kindred who share the same founder mutation [Walsh et al., 2008], and Ahlstrom et al. [1994] identified all eligible individuals with neuromuscular disorders in one county; both studies achieved high response rates.
Sample Size
Sample sizes varied widely, ranging from 20 to 568. Eighteen studies included less than 50 participants, 6 of these studies had less than 30 participants. Twenty-two studies had at least 100 participants. The average sample size was 108, median sample size was 77. In total, 6,270 subjects were reported. Most articles did not report power analyses; therefore, it was difficult to judge the whether these studies had sufficient power to detect statistically significant differences. Caution must be used in interpreting the findings of the smaller studies, likely underpowered to detect small or even moderate effects.
Study Design and Objectives
All of the studies were descriptive in nature. Broadly, the descriptive aims can be summarized as: quantification (what is the QoL of affected individuals?), comparison (how does the QoL of affected individuals compare to unaffected individuals and/or patients with other chronic health conditions?), and correlation (what factors are associated with QoL?). The studies were infrequently hypothesis-driven. The vast majority were cross-sectional in design. The five longitudinal studies had follow-up periods ranging from 1 to 10 years. Three studies utilized a case–control design, with healthy matched control individuals. In two of these studies, unaffected first-degree relatives served as the matched controls [Gollust et al., 2003a; Walsh et al., 2008]. Disappointingly, none of the 58 studies were of counseling interventions aimed at enhancing QoL.
QoL Instruments
An array of QoL scales were used (Table V). By far, the most popular instrument was the SF-36, which was employed in 30 studies. A number of pediatric QoL scales, such as the Child Health Questionnaire, were used in studies of children. Of the studies included in our review, cystic fibrosis was the only condition that had its own disease-specific QoL scale. Dermatologic-specific QoL scales (i.e., designed for use in any condition that affects the skin) were used in studies of neurofibromatosis type 1 and porphyria. Despite the variety of different QoL scales used, each with its own set of domains and scoring system, it is possible to make comparisons of the findings across studies that used different scales by grouping the QoL domains into two general categories: physical and psychosocial aspects of QoL.
TABLE V. List of QoL Scales (Alphabetical by Abbreviation, With Number of Studies).
| Abbreviations | Scale name | # |
|---|---|---|
| CDLQI | Children's Dermatology Life Quality Index | 2 |
| CFQ | Cystic Fibrosis Questionnaire | 5 |
| CFQoL | Cystic Fibrosis Quality of Life Questionnaire | 1 |
| CHQ | Child Health Questionnaire | 7 |
| DISABKIDS | DISABKIDS Questionnaire | 1 |
| DLQI | Dermatology Life Quality Index | 2 |
| ITQoL | Infant/Toddler Quality of Life Questionnaire | 1 |
| MOS | Medical Outcomes Study General Health Survey | 1 |
| PedsQL | Pediatric Quality of Life Inventory | 2 |
| PLC | Profile of Quality of Life in the Chronically Ill | 1 |
| QLI | Quality of Life Index | 2 |
| SF-12 | Medical Outcomes Study Short Form 12 | 2 |
| SF-36 | Medical Outcomes Study Short Form 36 | 30 |
| SIP | Sickness Impact Profile | 4 |
| Skindex | Skin Diseases Quality of Life Index | 3 |
| TAAQoL | TNO-AZL Adult Quality of Life | 2 |
| TACQoL | TNO-AZL Children's Quality of Life | 4 |
| TAPQoL | TNO-AZL Preschool Children Quality of Life | 1 |
Overall Synthesis of Findings
Genetic conditions have the potential to have major negative impact on individuals' lives. Many of the studies found that QoL of affected individuals was significantly poorer than their unaffected counterparts. An important pattern emerged: physical and psychosocial aspects are perceived differently. Some studies found no impairment in physical domains of QoL, but significantly poorer psychosocial QoL compared to unaffected individuals. This demonstrates that individuals who are objectively “healthy” may still experience lower QoL. Factors beyond the physical manifestations of the disease influence QoL. Overall, the findings highlight the subjective nature of individuals' perceptions of their QoL.
Importantly, several studies found that individuals with genetic conditions experienced higher QoL than their unaffected peers. For example, patients with hemophilia had significantly more positive psychosocial QoL than healthy controls [Solovieva, 2001; Solovieva et al., 2004]. Even more remarkable is the finding by Grootenhuis et al. [2007] on adolescents with muscular dystrophy, whose perceived QoL in the physical functioning domains was significantly better than that of their unaffected peers. The authors posited that living with a progressive disease changed patients' values in such a way that they respond differently than healthy children. In the health psychology literature, this concept of reframing to adjust expectations and thus satisfaction is referred to as response shift. It remains inadequately studied in living with rare genetic conditions.
Predictors of QoL: Disease-Related Factors
Of the 41 studies that investigated relationships between QoL and other variables in order to identify predictors or determinants of QoL, 38 examined disease-related factors, such as clinical severity, degree of disability or impairment, and frequency of medical complications. As expected, many of these studies found that disease-related factors have a negative impact on QoL among individuals with genetic conditions. Typically, these factors are strongly correlated only with physical dimensions of QoL. Clinical variables appear to have much less of an impact on psychosocial QoL, even among individuals with poor psychosocial QoL. Many studies found that clinical variables explained a relatively small or insignificant amount of the variance in psychosocial QoL. Furthermore, several studies found that clinical severity was not significantly related to any domains of QoL. Individuals with more severe disease do not necessarily have poor QoL. The converse is also true; individuals who are mildly affected physically may experience poor QoL. The data highlight the importance of studying non-clinical factors that affect QoL.
Predictors of QoL: Psychosocial Factors
Thirteen studies examined associations between psychosocial factors and QoL. These factors included: psychological well-being (6), coping (5), illness perceptions (3), family functioning (2), and self-esteem (1). Overall, these studies reveal strong correlations of each with QoL and demonstrate the importance of psychosocial factors in determining an individual's QoL.
All six studies that measured psychological well-being found strong negative associations with QoL [Piccininni et al., 2004; Carel et al., 2005; Szyndler et al., 2005; Antonini et al., 2006; Riekert et al., 2007; Havermans et al., 2008]. Individuals with symptoms of depression, anxiety, and psychological distress generally had poorer QoL in physical as well as psychosocial domains. Self-esteem and illness perceptions were also strong predictors of QoL [Helder et al., 2002; Gollust et al., 2003a; Geisthoff et al., 2007]. The matched case–control study on achondroplasia by Gollust [2003a] is particularly noteworthy, because it showed that self-esteem and perceived seriousness were the strongest predictors of QoL, independent of affected status. This supports the notion that it is an individual's feelings and beliefs that determine their QoL—more than having a genetic condition.
In the stress and coping theoretical framework, coping is posited to be the mediator between the stressor (having a genetic condition) and QoL. Indeed, coping strategies were found to be significant predictors of QoL [Ahlstrom and Sjoden, 1996; Natterlund et al., 2000; Helder et al., 2002; Berglund et al., 2003; Szyndler et al., 2005; Abbott et al., 2008]. Although a particular coping strategy is not inherently good or bad (i.e., adaptive or maladaptive), some strategies appear to be more effective than others in adapting to living with a genetic condition. The use of avoidance, distraction, and disengagement techniques were associated with lower QoL in some studies. Individuals with more fatalistic or helpless attitudes also tended to have lower QoL. On the other hand, acceptance, optimism, and hopefulness were associated with higher QoL. Results from the study on adults with Ehlers–Danlos syndrome [Berglund et al., 2003] are especially compelling. Using validated instruments, they explored “Acceptance of Disability,” a construct similar to psychological adaptation, and “Sense of Coherence,” which encompasses one's life orientation and ability to cope with stressors. Both constructs were found to be robust predictors of positive QoL.
Family functioning also appears to significantly influence QoL among children and adolescents with genetic conditions. In a study on neurofibromatosis type 1, affected children/adolescents from families with greater cohesion and lower conflict had significantly better QoL [Graf et al., 2006]. Szyndler [2005] found similar results regarding family cohesion and conflict for teens with cystic fibrosis; in addition, teens with greater independence within the family had significantly higher QoL on some domains than their less-independent peers.
Discussion
The large number of quality studies aimed at assessing QoL among individuals affected with rare genetic conditions is heartening. They help to enhance understanding of the broader experience of living with a genetic condition. Yet there remains in the literature an emphasis on the bio-medical model that fails to encompass a more comprehensive and subjective self-assessment. In the health psychology literature, comprehensive measures of QoL as described are used more routinely. Thus, studies of rare genetic conditions would benefit from following suit.
Of the studies that examined predictors of QoL, most focused on disease-related variables and resulted in mixed evidence regarding the importance of factors such as clinical severity in influencing QoL. Far fewer studies explored the impact of psychosocial factors, but those that did revealed strong correlations of these variables with QoL. They reveal promising opportunities to enhance QoL. Clearly, there is more to QoL than physical health and functioning. For the majority of genetic diseases, there is no cure and medical treatments are limited in their ability to ameliorate the physical effects and medical complications. Certainly we, as genetics healthcare providers and researchers, should still strive to find effective treatments, and QoL is an important outcome measure to include in clinical trials [Stevenson and Carey, 2009]. Yet, we must also attend to factors that are potentially and more readily modifiable: psychosocial factors.
Interventions aimed at enhancing QoL by adjusting psychosocial factors need to be designed and tested. For children and adults living with rare genetic conditions, the stress and coping theoretical framework can inform the research. Interventions might aim to adjust appraisals of the stress evoked by the threat of the condition. One approach might be to enhance feelings of control over the condition or its implications and/or to enhance self-efficacy (confidence in one's ability to do something about the condition or its implications). Another approach might be to enhance the effectiveness of the coping strategies used by patients. An example would be to assist patients in recognizing what aspects of their lives have been directly affected by the condition and what aspects have not. Past studies show that, in living with a chronic condition, it is common to perceive that many more aspects of one's life are negatively affected than truly are [Navon, 1999]. An exercise in distinguishing the two can help patients to see important areas of their lives untouched by disease. Re-engaging in these aspects of one's life can enhance QoL.
Scales that focus primarily on physical aspects will not sufficiently capture QoL and may fail to uncover potentially important associations with psychosocial factors. A more comprehensive measure of global QoL can be used to determine the impact of disease in a number of ways.
One resource for validated items that can be used to measure QoL is the Patient-Reported Outcomes Measurement Information System (PROMIS), an NIH Roadmap Initiative (http://www.nihpromis.org). One of the objectives of PROMIS is to standardize the measures that are used to assess outcomes such as QoL, while still allowing for tailoring of measures to a specific population. The measures can be used in a wide variety of chronic diseases and conditions. They should be re-validated in the new populations but will help to facilitate comparisons across conditions. The measures are publicly available on the PROMIS website for use in studies of genetic conditions.
The importance of conceptual clarity, rigorous methodology with appropriate QoL scales, and theoretically grounded research cannot be overemphasized. The field of research on QoL in rare genetic conditions will be advanced by uniting around a clear conceptualization of QoL and using more rigorous methodology. This research will yield more potent evidence for clinical applications and interventions to facilitate improvements in the healthcare and counseling for individuals living with rare genetic conditions, and, ultimately, to enhance patients' QoL.
Acknowledgments
The authors would like to acknowledge Anne White-Olson and Susannah Green for their assistance with the literature search.
This work was supported by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Footnotes
This article is a US Government work and, as such, is in the public domain in the United States of America.
References
- Abbott J, Morton AM, Musson H, Conway SP, Etherington C, Gee L, Fitzjohn J, Webb AK. Nutritional status, perceived body image and eating behaviours in adults with cystic fibrosis. Clin Nutr. 2007;26:91–99. doi: 10.1016/j.clnu.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Abbott J, Hart A, Morton A, Gee L, Conway S. Health-related quality of life in adults with cystic fibrosis: The role of coping. J Psychosom Res. 2008;64:149–157. doi: 10.1016/j.jpsychores.2007.08.017. [DOI] [PubMed] [Google Scholar]
- Ahlstrom G, Gunnarsson LG. Disability and quality of life in individuals with muscular dystrophy. Scand J Rehabil Med. 1996;28:147–157. [PubMed] [Google Scholar]
- Ahlstrom G, Sjoden PO. Coping with illness-related problems and quality of life in adult individuals with muscular dystrophy. J Psychosom Res. 1996;41:365–376. doi: 10.1016/s0022-3999(96)00191-2. [DOI] [PubMed] [Google Scholar]
- Ahlstrom G, Gunnarsson LG, Kihlgren A, Arvill A, Sjoden PO. Respiratory function, electrocardiography and quality of life in individuals with muscular dystrophy. Chest. 1994;106:173–179. doi: 10.1378/chest.106.1.173. [DOI] [PubMed] [Google Scholar]
- Anderson KL, Burckhardt CS. Conceptualization and measurement of quality of life as an outcome variable for health care intervention and research. J Adv Nurs. 1999;29:298–306. doi: 10.1046/j.1365-2648.1999.00889.x. [DOI] [PubMed] [Google Scholar]
- Antonini G, Soscia F, Giubilei F, De Carolis A, Gragnani F, Morino S, Ruberto A, Tatarelli R. Health-related quality of life in myotonic dystrophy type 1 and its relationship with cognitive and emotional functioning. J Rehabil Med. 2006;38:181–185. doi: 10.1080/16501970500477967. [DOI] [PubMed] [Google Scholar]
- Arrington-Sanders R, Yi MS, Tsevat J, Wilmott RW, Mrus JM, Britto MT. Gender differences in health-related quality of life of adolescents with cystic fibrosis. Health Qual Life Outcomes. 2006;4:5. doi: 10.1186/1477-7525-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund B, Nordstrom G. Symptoms and functional health status of individuals with Ehlers-Danlos syndrome (EDS) J Clin Rheumatol. 2001;7:308–314. doi: 10.1097/00124743-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Berglund B, Mattiasson AC, Nordstrom G. Acceptance of disability and sense of coherence in individuals with Ehlers-Danlos syndrome. J Clin Nurs. 2003;12:770–777. doi: 10.1046/j.1365-2702.2003.00776.x. [DOI] [PubMed] [Google Scholar]
- Biesecker BB, Erby L. Adaptation to living with a genetic condition or risk: A mini-review. Clin Genet. 2008;74:401–407. doi: 10.1111/j.1399-0004.2008.01088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch AM, Grootenhuis MA, Bakker HD, Heijmans HS, Wijburg FA, Last BF. Living with classical galactosemia: Health-related quality of life consequences. Pediatrics. 2004;113:e423–e428. doi: 10.1542/peds.113.5.e423. [DOI] [PubMed] [Google Scholar]
- Bostrom K, Natterlund BS, Ahlstrom G. Sickness impact in people with muscular dystrophy: A longitudinal study over 10 years. Clin Rehabil. 2005;19:686–694. doi: 10.1191/0269215505cr866oa. [DOI] [PubMed] [Google Scholar]
- Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121:64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- Britto MT, Kotagal UR, Chenier T, Tsevat J, Atherton HD, Wilmott RW. Differences between adolescents' and parents' reports of health-related quality of life in cystic fibrosis. Pediatr Pulmonol. 2004;37:165–171. doi: 10.1002/ppul.10436. [DOI] [PubMed] [Google Scholar]
- Caliandro P, Grugni G, Padua L, Kodra Y, Tonali P, Gargantini L, Ragusa L, Crino A, Taruscio D. Quality of life assessment in a sample of patients affected by Prader-Willi syndrome. J Paediatr Child Health. 2007;43:826–830. doi: 10.1111/j.1440-1754.2007.01200.x. [DOI] [PubMed] [Google Scholar]
- Carel JC, Ecosse E, Bastie-Sigeac I, Cabrol S, Tauber M, Leger J, Nicolino M, Brauner R, Chaussain JL, Coste J. Quality of life determinants in young women with turner's syndrome after growth hormone treatment: Results of the StaTur population-based cohort study. J Clin Endocrinol Metab. 2005;90:1992–1997. doi: 10.1210/jc.2004-1395. [DOI] [PubMed] [Google Scholar]
- Damiano AM, Pastores GM, Ware JE., Jr The health-related quality of life of adults with Gaucher's disease receiving enzyme replacement therapy: Results from a retrospective study. Qual Life Res. 1998;7:373–386. doi: 10.1023/a:1008814105603. [DOI] [PubMed] [Google Scholar]
- De Civita M, Regier D, Alamgir AH, Anis AH, Fitzgerald MJ, Marra CA. Evaluating health-related quality-of-life studies in paediatric populations: Some conceptual, methodological and developmental considerations and recent applications. Pharmacoeconomics. 2005;23:659–685. doi: 10.2165/00019053-200523070-00003. [DOI] [PubMed] [Google Scholar]
- Epstein E, Farmer JM, Tsou A, Perlman S, Subramony SH, Gomez CM, Ashizawa T, Wilmot GR, Mathews K, Wilson RB, et al. Health related quality of life measures in Friedreich Ataxia. J Neurol Sci. 2008;272:123–128. doi: 10.1016/j.jns.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Ferrans CE. Development of a conceptual model of quality of life. Sch Inq Nurs Pract. 1996;10:293–304. [PubMed] [Google Scholar]
- Ferrans CE, Powers MJ. Psychometric assessment of the Quality of Life Index. Res Nurs Health. 1992;15:29–38. doi: 10.1002/nur.4770150106. [DOI] [PubMed] [Google Scholar]
- Ford C, Kidd A, Hammond-Tooke G. Myotonic dystrophy in Otago, New Zealand. N Z Med J. 2006;119:U2145. [PubMed] [Google Scholar]
- Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Quality of life in cystic fibrosis: The impact of gender, general health perceptions and disease severity. J Cyst Fibros. 2003;2:206–213. doi: 10.1016/S1569-1993(03)00093-6. [DOI] [PubMed] [Google Scholar]
- Gee L, Abbott J, Hart A, Conway SP, Etherington C, Webb AK. Associations between clinical variables and quality of life in adults with cystic fibrosis. J Cyst Fibros. 2005;4:59–66. doi: 10.1016/j.jcf.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Geisthoff UW, Heckmann K, D'Amelio R, Grunewald S, Knobber D, Falkai P, Konig J. Health-related quality of life in hereditary hemorrhagic telangiectasia. Otolaryngol Head Neck Surg. 2007;136:726–735. doi: 10.1016/j.otohns.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Gollust SE, Thompson RE, Gooding HC, Biesecker BB. Living with achondroplasia in an average-sized world: An assessment of quality of life. Am J Med Genet Part A. 2003a;120A:447–458. doi: 10.1002/ajmg.a.20127. [DOI] [PubMed] [Google Scholar]
- Gollust SE, Thompson RE, Gooding HC, Biesecker BB. Living with achondroplasia: Attitudes toward population screening and correlation with quality of life. Prenat Diagn. 2003b;23:1003–1008. doi: 10.1002/pd.743. [DOI] [PubMed] [Google Scholar]
- Graf A, Landolt MA, Mori AC, Boltshauser E. Quality of life and psychological adjustment in children and adolescents with neurofibromatosis type 1. J Pediatr. 2006;149:348–353. doi: 10.1016/j.jpeds.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Grootenhuis MA, de Boone J, van der Kooi AJ. Living with muscular dystrophy: Health related quality of life consequences for children and adults. Health Qual Life Outcomes. 2007;5:31. doi: 10.1186/1477-7525-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemans ML, Janssens AC, Winkel LP, Sieradzan KA, Reuser AJ, Van Doorn PA, Van der Ploeg AT. Late-onset Pompe disease primarily affects quality of life in physical health domains. Neurology. 2004;63:1688–1692. doi: 10.1212/01.wnl.0000142597.69707.78. [DOI] [PubMed] [Google Scholar]
- Harris A, Burge SM, Dykes PJ, Finlay AY. Handicap in Darier's disease and Hailey-Hailey disease. Br J Dermatol. 1996;135:959–963. doi: 10.1046/j.1365-2133.1996.d01-1102.x. [DOI] [PubMed] [Google Scholar]
- Havermans T, Colpaert K, Dupont LJ. Quality of life in patients with Cystic Fibrosis: Association with anxiety and depression. J Cyst Fibros. 2008;7:581–584. doi: 10.1016/j.jcf.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Helder DI, Kaptein AA, van Kempen GM, van Houwelingen JC, Roos RA. Impact of Huntington's disease on quality of life. Mov Disord. 2001;16:325–330. doi: 10.1002/mds.1056. [DOI] [PubMed] [Google Scholar]
- Helder DI, Kaptein AA, Van Kempen GM, Weinman J, Van Houwelingen JC, Roos RA. Living with Huntington's disease: Illness perceptions, coping mechanisms, and spouses' quality of life. Int J Behav Med. 2002;9:37–52. doi: 10.1207/s15327558ijbm0901_03. [DOI] [PubMed] [Google Scholar]
- Holme SA, Anstey AV, Finlay AY, Elder GH, Badminton MN. Erythropoietic protoporphyria in the UK: Clinical features and effect on quality of life. Br J Dermatol. 2006;155:574–581. doi: 10.1111/j.1365-2133.2006.07472.x. [DOI] [PubMed] [Google Scholar]
- Howard V, Greene JM, Pahwa S, Winkelstein JA, Boyle JM, Kocak M, Conley ME. The health status and quality of life of adults with X-linked agammaglobulinemia. Clin Immunol. 2006;118:201–208. doi: 10.1016/j.clim.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen J, Voutilainen R. Long-term outcome of classical 21-hydroxylase deficiency: Diagnosis, complications and quality of life. Acta Paediatr. 2000;89:183–187. doi: 10.1080/080352500750028807. [DOI] [PubMed] [Google Scholar]
- Kodra Y, Giustini S, Divona L, Porciello R, Calvieri S, Wolkenstein P, Taruscio D. Health-related quality of life in patients with neurofibromatosis type 1. A survey of 129 Italian patients. Dermatology. 2009;218:215–220. doi: 10.1159/000187594. [DOI] [PubMed] [Google Scholar]
- Krab LC, Oostenbrink R, de Goede-Bolder A, Aarsen FK, Elgersma Y, Moll HA. Health-related quality of life in children with neurofibromatosis type 1: Contribution of demographic factors, disease-related factors, and behavior. J Pediatr. 2009;154:420–425. doi: 10.1016/j.jpeds.2008.08.045. [DOI] [PubMed] [Google Scholar]
- Landolt MA, Nuoffer JM, Steinmann B, Superti-Furga A. Quality of life and psychologic adjustment in children and adolescents with early treated phenylketonuria can be normal. J Pediatr. 2002;140:516–521. doi: 10.1067/mpd.2002.123663. [DOI] [PubMed] [Google Scholar]
- Lazarus R, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- Leventhal H. Quality of Life: A Process Review. Psychol Health. 1997;12:753–767. [Google Scholar]
- McClish DK, Penberthy LT, Bovbjerg VE, Roberts JD, Aisiku IP, Levenson JL, Roseff SD, Smith WR. Health related quality of life in sickle cell patients: The PiSCES project. Health Qual Life Outcomes. 2005;3:50. doi: 10.1186/1477-7525-3-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward LM, Kelly P, Deacon A, Senior V, Peters TJ. Self-rated psychosocial consequences and quality of life in the acute porphyrias. J Inherit Metab Dis. 2001;24:733–747. doi: 10.1023/a:1012901607040. [DOI] [PubMed] [Google Scholar]
- Miners AH, Sabin CA, Tolley KH, Jenkinson C, Kind P, Lee CA. Assessing health-related quality-of-life in individuals with haemophilia. Haemophilia. 1999;5:378–385. doi: 10.1046/j.1365-2516.1999.00347.x. [DOI] [PubMed] [Google Scholar]
- Miners AH, Holmes A, Sherr L, Jenkinson C, MacDermot KD. Assessment of health-related quality-of-life in males with Anderson Fabry Disease before therapeutic intervention. Qual Life Res. 2002;11:127–133. doi: 10.1023/a:1015009210639. [DOI] [PubMed] [Google Scholar]
- Natterlund B, Gunnarsson LG, Ahlstrom G. Disability, coping and quality of life in individuals with muscular dystrophy: A prospective study over five years. Disabil Rehabil. 2000;22:776–785. doi: 10.1080/09638280050200278. [DOI] [PubMed] [Google Scholar]
- Navon S. The non-illness intervention model: Psychotherapy for physically ill patients and their families. Am J Family Therapy. 1999;27:251–270. [Google Scholar]
- Oostenbrink R, Spong K, de Goede-Bolder A, Landgraf JM, Raat H, Moll HA. Parental reports of health-related quality of life in young children with neurofibromatosis type 1: Influence of condition specific determinants. J Pediatr. 2007;151:182–186. doi: 10.1016/j.jpeds.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Padua L, Pareyson D, Aprile I, Cavallaro T, Quattrone A, Rizzuto N, Vita G, Tonali P, Schenone A. Natural history of CMT1A including QoL: A 2-year prospective study. Neuromuscul Disord. 2008a;18:199–203. doi: 10.1016/j.nmd.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Padua L, Shy ME, Aprile I, Cavallaro T, Pareyson D, Quattrone A, Rizzuto N, Vita G, Tonali P, Schenone A. Correlation between clinical/neurophysiological findings and quality of life in Charcot-Marie-Tooth type 1A. J Peripher Nerv Syst. 2008b;13:64–70. doi: 10.1111/j.1529-8027.2008.00159.x. [DOI] [PubMed] [Google Scholar]
- Page PZ, Page GP, Ecosse E, Korf BR, Leplege A, Wolkenstein P. Impact of neurofibromatosis 1 on Quality of Life: A cross-sectional study of 176 American cases. Am J Med Genet Part A. 2006;140A:1893–1898. doi: 10.1002/ajmg.a.31422. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Schwartz L, Drotar D, McGowan K. Parental report of health-related quality of life in children with sickle cell disease. J Behav Med. 2002;25:269–283. doi: 10.1023/a:1015332828213. [DOI] [PubMed] [Google Scholar]
- Palermo TM, Harrison D, Koh JL. Effect of disease-related pain on the health-related quality of life of children and adolescents with cystic fibrosis. Clin J Pain. 2006;22:532–537. doi: 10.1097/01.ajp.0000210996.45459.76. [DOI] [PubMed] [Google Scholar]
- Panepinto JA, O'Mahar KM, DeBaun MR, Loberiza FR, Scott JP. Health-related quality of life in children with sickle cell disease: Child and parent perception. Br J Haematol. 2005;130:437–444. doi: 10.1111/j.1365-2141.2005.05622.x. [DOI] [PubMed] [Google Scholar]
- Pasculli G, Resta F, Guastamacchia E, Di Gennaro L, Suppressa P, Sabba C. Health-related quality of life in a rare disease: Hereditary hemorrhagic telangiectasia (HHT) or Rendu-Osler-Weber disease. Qual Life Res. 2004;13:1715–1723. doi: 10.1007/s11136-004-7865-y. [DOI] [PubMed] [Google Scholar]
- Peters KF, Kong F, Hanslo M, Biesecker BB. Living with Marfan syndrome III. Quality of life and reproductive planning. Clin Genet. 2002;62:110–120. doi: 10.1034/j.1399-0004.2002.620203.x. [DOI] [PubMed] [Google Scholar]
- Piccininni M, Falsini C, Pizzi A. Quality of life in hereditary neuromuscular diseases. Acta Neurol Scand. 2004;109:113–119. doi: 10.1046/j.1600-0404.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- Redmond AC, Burns J, Ouvrier RA. Factors that influence health-related quality of life in Australian adults with Charcot-Marie-Tooth disease. Neuromuscul Disord. 2008;18:619–625. doi: 10.1016/j.nmd.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Resta R, Biesecker BB, Bennett RL, Blum S, Hahn SE, Strecker MN, Williams JL. A new definition of genetic counseling: National Society of Genetic Counselors' Task Force Report. J Genet Couns. 2006;15:77–83. doi: 10.1007/s10897-005-9014-3. [DOI] [PubMed] [Google Scholar]
- Riekert KA, Bartlett SJ, Boyle MP, Krishnan JA, Rand CS. The association between depression, lung function, and health-related quality of life among adults with cystic fibrosis. Chest. 2007;132:231–237. doi: 10.1378/chest.06-2474. [DOI] [PubMed] [Google Scholar]
- Ries M, Gupta S, Moore DF, Sachdev V, Quirk JM, Murray GJ, Rosing DR, Robinson C, Schaefer E, Gal A, et al. Pediatric Fabry disease. Pediatrics. 2005;115:e344–e355. doi: 10.1542/peds.2004-1678. [DOI] [PubMed] [Google Scholar]
- Sands SA, Giarraffa P, Jacobson CM, Axelrod FB. Familial dysautonomia's impact on quality of life in childhood, adolescence, and adulthood. Acta Paediatr. 2006;95:457–462. doi: 10.1080/08035250500440386. [DOI] [PubMed] [Google Scholar]
- Simon E, Schwarz M, Roos J, Dragano N, Geraedts M, Siegrist J, Kamp G, Wendel U. Evaluation of quality of life and description of the sociodemographic state in adolescent and young adult patients with phenylketonuria (PKU) Health Qual Life Outcomes. 2008;6:25. doi: 10.1186/1477-7525-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovieva S. Clinical severity of disease, functional disability and health-related quality of life. Three-year follow-up study of 150 Finnish patients with coagulation disorders. Haemophilia. 2001;7:53–63. doi: 10.1046/j.1365-2516.2001.00476.x. [DOI] [PubMed] [Google Scholar]
- Solovieva S, Santavirta N, Santavirta S, Konttinen YT. Assessing quality of life in individuals with hereditary blood coagulation disorders. Qual Life Res. 2004;13:987–1000. doi: 10.1023/B:QURE.0000025587.97216.8a. [DOI] [PubMed] [Google Scholar]
- Stanton AL, Collins CA, Sworowski LA. Adjustment to chronic illness: Theory and research. In: Baum A, Revenson TA, Singer JE, editors. Handbook of health psychology. Lawrence Erlbaum Associates; 2001. pp. 387–403. [Google Scholar]
- Stevenson DA, Carey JC. Health-related quality of life measures in genetic disorders: An outcome variable for consideration in clinical trials. Am J Med Genet Part C. 2009;151C:255–260. doi: 10.1002/ajmg.c.30217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch E, Keeley M, Merlo L, Jacob M, Correia C, Weinstein D. Psychosocial functioning in youth with glycogen storage disease type I. J Pediatr Psychol. 2008;33:728–738. doi: 10.1093/jpepsy/jsn017. [DOI] [PubMed] [Google Scholar]
- Street NJ, Yi MS, Bailey LA, Hopkin RJ. Comparison of health-related quality of life between heterozygous women with Fabry disease, a healthy control population, and patients with other chronic disease. Genet Med. 2006;8:346–353. doi: 10.1097/01.gim.0000223545.63012.5a. [DOI] [PubMed] [Google Scholar]
- Szyndler JE, Towns SJ, van Asperen PP, McKay KO. Psychological and family functioning and quality of life in adolescents with cystic fibrosis. J Cyst Fibros. 2005;4:135–144. doi: 10.1016/j.jcf.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Teunissen LL, Notermans NC, Franssen H, Van Engelen BG, Baas F, Wokke JH. Disease course of Charcot-Marie-Tooth disease type 2: A 5-year follow-up study. Arch Neurol. 2003;60:823–828. doi: 10.1001/archneur.60.6.823. [DOI] [PubMed] [Google Scholar]
- Thomas C, Mitchell P, O'Rourke P, Wainwright C. Quality-of-life in children and adolescents with cystic fibrosis managed in both regional outreach and cystic fibrosis center settings in Queensland. J Pediatr. 2006;148:508–516. doi: 10.1016/j.jpeds.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Tusell JM, Aznar JA, Querol F, Quintana M, Moreno M, Gorina E. Results of an orthopaedic survey in young patients with severe haemophilia in Spain. Haemophilia. 2002;8:38–42. doi: 10.1046/j.1351-8216.2001.00119.x. [DOI] [PubMed] [Google Scholar]
- van der Hilst JC, Bodar EJ, Barron KS, Frenkel J, Drenth JP, van der Meer JW, Simon A. Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine (Baltimore) 2008;87:301–310. doi: 10.1097/MD.0b013e318190cfb7. [DOI] [PubMed] [Google Scholar]
- Vinci P, Serrao M, Millul A, Deidda A, De Santis F, Capici S, Martini D, Pierelli F, Santilli V. Quality of life in patients with Charcot-Marie-Tooth disease. Neurology. 2005;65:922–924. doi: 10.1212/01.wnl.0000176062.44360.49. [DOI] [PubMed] [Google Scholar]
- Walsh M, Macgregor D, Stuckless S, Barrett B, Kawaja M, Scully MF. Health-related quality of life in a cohort of adult patients with mild hemophilia A. J Thromb Haemost. 2008;6:755–761. doi: 10.1111/j.1538-7836.2008.02929.x. [DOI] [PubMed] [Google Scholar]
- Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- Widmann RF, Laplaza FJ, Bitan FD, Brooks CE, Root L. Quality of life in osteogenesis imperfecta. Int Orthop. 2002;26:3–6. doi: 10.1007/s002640100292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CL, Fahey MC, Corben LA, Collins VR, Churchyard AJ, Lamont PJ, Delatycki MB. Quality of life in Friedreich ataxia: What clinical, social and demographic factors are important? Eur J Neurol. 2007;14:1040–1047. doi: 10.1111/j.1468-1331.2007.01881.x. [DOI] [PubMed] [Google Scholar]
- Winkelstein JA, Conley ME, James C, Howard V, Boyle J. Adults with X-linked agammaglobulinemia: Impact of disease on daily lives, quality of life, educational and socioeconomic status, knowledge of inheritance, and reproductive attitudes. Medicine (Baltimore) 2008;87:253–258. doi: 10.1097/MD.0b013e318187ed81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkenstein P, Zeller J, Revuz J, Ecosse E, Leplege A. Quality-of-life impairment in neurofibromatosis type 1: A cross-sectional study of 128 cases. Arch Dermatol. 2001;137:1421–1425. doi: 10.1001/archderm.137.11.1421. [DOI] [PubMed] [Google Scholar]
- Wolkenstein P, Rodriguez D, Ferkal S, Gravier H, Buret V, Algans N, Simeoni MC, Bastuji-Garin S. Impact of neurofibromatosis 1 upon quality of life in childhood: A cross-sectional study of 79 cases. Br J Dermatol. 2009;160:844–848. doi: 10.1111/j.1365-2133.2008.08949.x. [DOI] [PubMed] [Google Scholar]


