Abstract
Acute on chronic kidney disease will be familiar to many nephrologists. In this issue of Kidney International, the risk of acute on chronic disease is quantified across the stages of pre-existing chronic kidney disease. This study demonstrates the valuable insights that large epidemiological studies can bring to the field of acute kidney injury.
Several studies have shown that the prevalence of chronic kidney disease (CKD) is increasing[1] using consensus definitions developed by the Kidney Disease Outcome Quality Initiative (K/DOQI). These definitions and staging of CKD allow researchers to compare disease prevalence across time and across populations and establish links between CKD and other diseases[2]. For example, it is well established that CKD is a risk factor for cardiovascular disease and this risk is significant even with mild impairment of kidney function[3]. In this issue of Kidney International, Hsu and colleagues explore the relationship between CKD stage and the risk of developing acute kidney injury (AKI)[4]. They demonstrate that even mild chronic impairment of kidney function significantly increases the risk of AKI.
Patients with ‘acute-on-chronic’ kidney disease should be familiar to most nephrologists. In terms of clinical practice, one of the strengths of the Hsu et al. study is the quantification of the relationship between CKD stage and risk of in-hospital, dialysis-requiring, AKI. The authors studied a large patient group, adults from a Kaiser Permanente cohort in Northern California. By definition, this population has health insurance and we hope future studies include patients without insurance. The staging of ‘baseline’ CKD was based on out-patient measurements of serum creatinine that predated the index episode of AKI, a significant advantage over inferring baseline creatinine from in-hospital measurements. This strategy allows for a more inclusive and perhaps more accurate view of the acute-on-chronic population. When the incidence of dialysis-requiring AKI was compared across the CKD stages the authors found that ‘the propensity to develop dialysis-requiring AKI is another complication of CKD whose risk markedly increases below an estimated GFR of 60 ml/min/1.73m2’ [4]. In addition, pre-existing diabetes, hypertension and proteinuria also significantly increased the risk of inhospital, dialysis-requiring AKI.
These findings may be the tip of the iceberg as the risk of non-dialysis requiring AKI (a disease with a significant morbidity and mortality[5]) remains undetermined. This increased risk of AKI across the stages of CKD warrants clear translation to the non-nephrology community as patients with CKD are often exposed to potentially nephrotoxic drugs, as well as surgical and septic insults, and it is important that all clinicians recognize the increased risk and significance of an acute deterioration in kidney function. Furthermore, nephrologists should continue to encourage the inclusion of CKD patients in clinical trials rather than their being excluded[6]. Similarly, given their increased risk, patients with CKD deserve to be included in future trials of AKI prevention or treatment. Trial inclusion will not only provide valuable data to guide clinical practice, but also allow the collection of biological samples for biomarker studies. The need for new biomarkers reflects the well-described limitations of serum creatinine[7], for example, in the study of Hsu et al. creatinine cannot easily distinguish between the natural progression of CKD and ‘acute on chronic’ disease. Biomarkers that distinguish AKI from chronic kidney dysfunction could be valuable in determining where ‘AKI starts and CKD finishes’ – a question that may have significant therapeutic implications. Which, if any, of the present candidates will prove to be clinically useful remains to be determined, but sample collection from large studies of well-characterized patients will be essential for biomarker development.
In comparison with CKD, if we turn our attention towards the definition and staging of AKI then we are on the cusp of significant progress. Not unreasonably, Hsu et al. defined AKI ‘as peak inpatient serum creatinine greater than last observed pre-admission outpatient serum creatinine by 50% and receipt of dialysis in hospital’. Among nephrologists there is no consensus regarding timing of initiation of dialysis in AKI and this is a continuing problem if dialysis is used as an end-point[8]. Other studies have defined AKI in multiple other ways making cross-study comparisons difficult[9]. Despite this heterogeneity, it is clear that AKI is an important disease as the incidence is increasing and the development of AKI significantly increases mortality[5]. The impact of AKI on the long-term risk of developing CKD and cardiovascular disease is uncertain and is a research priority identified by a recent interdisciplinary Delphi process[8]. To promote research consistency, the Acute Kidney Injury Network (AKIN) has described common standards for diagnosis and classification of AKI[10] and the adoption of consistent staging in future epidemiological studies has the potential to galvanize research. However, an inherent risk of establishing staging criteria is that when new biomarkers are established, or when new data compel the reclassification of staging boundaries, further changes can create confusion, and can serve as a disincentive to conduct longitudinal studies for fear of obsolescence. This creates a bit of a Catch-22 situation, where data from large patient populations are needed to establish consensus staging criteria, yet staging criteria are needed to analyze the data in a standardized manner, especially in a longitudinal study. A balance needs to be maintained between these competing parameters as we iteratively adjust staging criteria and evaluate patient outcomes. For example, data and biological sample collection should be as inclusive as possible to allow re-analysis of the data as staging criteria change.
The study by Hsu et al. provides valuable information on the relationship between CKD and AKI. Future large-scale longitudinal studies employing and challenging the proposed staging criteria will allow even more accurate understanding of risk, predict outcomes, and ultimately guide decision making and develop new therapies.
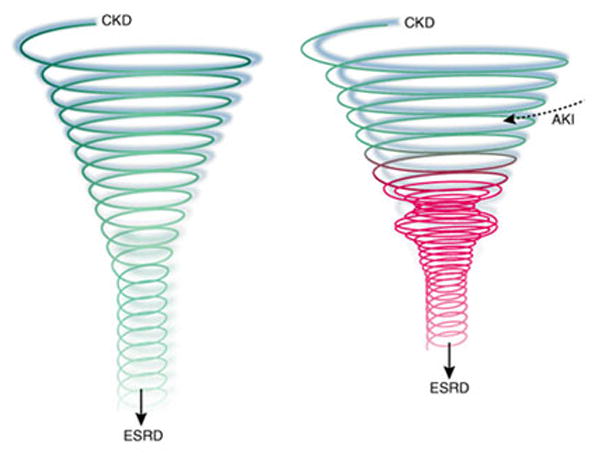
Figure 1.
References
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation. K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, Classification, and Stratification. 2000 http://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm.
- 3.Schiffrin EL, Lipman ML, Mann JFE. Chronic Kidney Disease. Effects on the Cardiovascular System. Circulation. 2007;116:85–97. doi: 10.1161/CIRCULATIONAHA.106.678342. [DOI] [PubMed] [Google Scholar]
- 4.Hsu C, Ordonez J, Chertow GM, et al. Chronic kidney disease and the risk of acute renal failure. Kidney Int. 2008;xxx:xxx–xxx. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waikar SS, Liu KD, Chertow GM. The incidence and prognostic significance of acute kidney injury. Curr Opin Nephrol Hypertens. 2007;16:227–236. doi: 10.1097/MNH.0b013e3280dd8c35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coca SG, Krumholz HM, Garg AX, et al. Underrepresentation of Renal Disease in Randomized Controlled Trials of Cardiovascular Disease. JAMA. 2006;296:1377–1384. doi: 10.1001/jama.296.11.1377. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt SM, Dear J, Star RA. Discovery of protein biomarkers for renal diseases. J Am Soc Nephrol. 2004;15:1677–1689. doi: 10.1097/01.asn.0000129114.92265.32. [DOI] [PubMed] [Google Scholar]
- 8.Kellum JA, Mehta RL, Levin A, et al. Development of a Clinical Research Agenda for Acute Kidney Injury Using an International, Interdisciplinary, Three-Step Modified Delphi Process. Clin J Am Soc Nephrol. 2008 doi: 10.2215/CJN.04891107. [DOI] [PubMed] [Google Scholar]
- 9.Star RA. Treatment of acute renal failure. Kidney Int. 1998;54:1817–1831. doi: 10.1046/j.1523-1755.1998.00210.x. [DOI] [PubMed] [Google Scholar]
- 10.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]


