Abstract
Background
Although basic research has implicated abnormal glucose metabolism in the pathogenesis of hypertension, epidemiologic studies are limited.
Methods
We assessed whether baseline hemoglobin A1c was prospectively associated with hypertension in the Women’s Health Study. We analyzed 19,858 women initially free of hypertension, diabetes, and cardiovascular disease with baseline blood samples. We considered quintiles and clinical cutpoints of hemoglobin A1c for the risk of hypertension, defined as either a new physician diagnosis, the initiation of antihypertensive treatment, or systolic blood pressure ≥ 140 or diastolic blood pressure ≥ 90 mmHg.
Results
During a median follow-up of 11.6 years, 9408 (47.5%) women developed hypertension. In models adjusted for traditional cardiovascular risk factors, the hazard ratios (HRs) from the lowest (<4.8 %, referent) to the highest (≥ 5.2%) quintile of hemoglobin A1c were 1.0 (referent), 0.99, 1.06, 1.08, and 1.21 (p, linear trend <.0001). However, additional adjustment for body mass index eliminated the relation (extreme quintile comparison HR 1.04; p, linear trend 0.10). For clinical cutpoints, a similar pattern emerged although a positive association between hemoglobin A1c and hypertension remained in the highest category.
Conclusion
Hemoglobin A1c in women without diabetes was associated with an increased risk of hypertension in models controlling for the majority of traditional hypertension and coronary risk factors, but this relation was no longer significant after adjustment for body mass index. These findings underscore the need for additional studies to delineate the important inter-relationships between glycemia and adiposity with the risk of hypertension in other study populations.
Keywords: epidemiology, diabetes, body mass index
Introduction
Given that almost 40 percent of patients with diabetes are already hypertensive at the time of their diabetes diagnosis,1 there has been considerable interest in a potential link between pre-diabetic states and the development of hypertension (HTN). This association has important public health implications given the known increase in both microvascular and macrovascular diabetic complications among hypertensive individuals.2 However, any relationship between dysglycemia and incident HTN must properly consider the influence of body weight given the inherent associations between body mass index (BMI), glucose metabolism and blood pressure.
Studies from basic and translational science support a relationship between both obesity, insulin resistance and arterial HTN3,4 with endothelial dysfunction being a potential intermediary factor in these relationships.4–7 The association between obesity and HTN has been borne out in multiple large epidemiological studies.8,9 However, despite interest in the connection between pre-diabetic states and the development of hypertension, data from large prospective cohorts are sparse. Chronic hyperglycemia as measured by HbA1c levels may reflect either a defect in either pancreatic beta-cell dysfunction and/or insulin resistance, both of which are known to occur in pre-diabetic states.10 Therefore, the current study prospectively evaluated whether HbA1c is associated with incident HTN in the Women’s Health Study, and considered the role of body weight in any such association given its known importance in this relationship.
Methods
Study Population
The present study analyzed data from the Women’s Health Study (WHS), a randomized clinical trial of low-dose aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer. A detailed description of WHS has been previously published.11 Between November 1992 and July 1995, a total of 39,876 US female health professionals aged 45 years and older without prior diagnosed cardiovascular disease or cancer (except non-melanoma skin cancer) were enrolled and randomized into the study. Of these women 11,805 were excluded due to pre-randomization diagnosed HTN (n=10,318), missing information on HTN status at baseline (n=597), missing information on body mass index (n=820), and pre-randomization diabetes (n=1160). Among the remaining 28, 071 women, 19,955 (71%) had provided baseline blood specimens that were stored in liquid nitrogen until laboratory analysis. Of the samples received from the group, we were able to measure HbA1c in 19,858 subjects. Of these subjects, 56 subjects had a HbA1c greater than or equal to 6.5% and were therefore considered to have baseline diabetes and excluded leaving a final sample size of 19, 802.
Women provided self-reports on mailed questionnaires of baseline risk factors including age, smoking, parental history of MI before age 60 years, vigorous exercise, alcohol use, blood pressure, history of high cholesterol (treatment, diagnosis, or total cholesterol ≥ 240 mg/dL), diabetes, and postmenopausal hormone use. BMI, in kilograms per meter squared, was calculated from height and weight. At baseline, participants completed a 131-item validated semiquantitative food frequency questionnaire (SFFQ). Details of the SFFQ and its use in the Women’s Health Study have been previously reported12,13,14
On the baseline questionnaire in WHS, we also collected information on self-reported systolic and diastolic blood pressure, treatment for high blood pressure (never, past, current), and history of a diagnosis of HTN (no, yes). From these data, those with baseline hypertension included past or current antihypertensive treatment, a prior HTN diagnosis, systolic blood pressure (SBP) ≥140 mmHg, or diastolic blood pressure (DPB) ≥90 mmHg at study entry. A previous report demonstrates a high correlation between a single measure of self-reported blood pressure in health professionals and measured SBP (r=0.72) and DBP (r=0.60).15 All participants gave written informed consent, and the study protocol was approved by the institutional review board at Brigham and Women’s Hospital, Boston, Massachusetts.
Outcome Ascertainment
To be considered an incident case of HTN during follow-up, participants must meet at least 1 of the following 4 criteria: (1) self-report of a new physician diagnosis on follow-up questionnaires at years 1, 3, or annually thereafter; (2) self-report of newly initiated antihypertensive treatment at years 1, 3, or 4; (3) self-reported SBP ≥ 140 mmHg; or (4) self-reported DBP ≥ 90 mmHg. We have previously validated self-reported HTN in WHS.16 Women provided the month and year of physician-diagnosed HTN, and if this information was not provided or the diagnosis of HTN was made by another method, then the month and year of diagnosis was assigned a random date between the current and previous questionnaire. If women developed CVD before HTN, they were censored at that point as their CVD diagnosis would likely influence subsequent blood pressure levels during follow-up.
Laboratory Analysis
HbA1c was estimated using the Tina-Quant turbidemtric inhibition immunoassay (Roche Diagnostics, Indianapolis, Ind) on a Hitachi 911 autoanalyzer using packed red blood cells. The assay is specific for HbA1c, standardized against the approved International Federation of Clinical Chemists reference method, and traceable to the Diabetes Control and Complications Trial by use of a conversion factor. Values of HbA1c presented in this study are Diabetes Control and Complications Trial aligned. The coefficient of variation for HbA1c computed from blinded simultaneously analyzed quality controls was 7.2%.
Statistical Analysis
Baseline characteristics were compared across quintiles of HbA1c. For each quintile of HbA1c, we computed the incidence rate of HTN by dividing the number of cases by their corresponding person-time. We used Cox proportional hazards models to compute multivariable-adjusted hazard ratios (HRs) with corresponding 95% confidence intervals (CIs). Assumptions for the proportional hazards models were tested by including main effects and product terms of covariates and time factor. These assumptions were met as all p values were >0.05. The initial model adjusted only for age (continuous). The multivariable model additionally controlled for smoking (never, former, <15 cigarettes per day, ≥ 15 cigarettes per day), alcohol use (rarely/never, 1–3 drinks/month, 1–6 drinks/week, and ≥ 1 drink/day), physical activity (rarely/never, <1 time/week, 1–3 times/week, or ≥4 times/week), hormone replacement therapy (never, past, current), history of elevated cholesterol ≥ 240 mg/dl or use of cholesterol lowering medication, and family history of myocardial infarction before age 60. We then ran a final multivariable model adding BMI as a continuous variable, given the known importance of body weight in the relationship between HbA1c and HTN. Tests of linear trends were computed using median values within each quintile. We then assessed the potential confounding effects of adding baseline SBP, DBP, dietary factors and C-reactive protein into the model. We performed a separate analysis to look at the impact of a diagnosis of prevalent diabetes on the risk of HTN. Women with self reported diabetes at study entry or a HbA1c ≥ 6.5 were included as a separate category and their risk was compared to those subjects without diabetes in the lowest quintile of HbA1c. We also re-examined the relationship between HbA1c and the risk of HTN using the clinical cutpoints of <5%, 5–5.5%, 5.5–6%, and >6%.
Stratified analyses were also conducted after dividing the population by baseline BMI using the World Health Organization (WHO) criteria (normal (<25 kg/m2), overweight (25–29 kg/m2), and obese (≥30 kg/m2) individuals). A test for interaction was performed between quintile of HbA1c (ordinal variable) and BMI category (ordinal variable). We also conducted stratified analyses according to dichotomized baseline SBP levels (<120, ≥ 120 mmHg). A test for interaction was performed between quintile of HbA1c (ordinal variable) and SBP category. All analyses were completed using SAS version 9.2 (SAS Institute, Cary, NC). The significance level was set at 0.05. All authors had full access to the data and take responsibility for its integrity.
Results
Among the 19,802 women comprising the baseline population of women free of HTN and diabetes, the mean age was 53.8 ± 6.6 years. Table 1 presents baseline characteristics of the study participants according to increasing quintiles of HbA1c. Significant associations between HbA1c quintile were noted with age, smoking status, BMI, high cholesterol, physical activity, alcohol intake, hormone use, baseline SBP, baseline DBP, and intake of saturated fat (p values all <.0001). During a median follow-up of 11.6 years, 9,408 new cases of HTN developed.
Table 1.
Baseline characteristics of 19, 802 US women according to quintile of hemoglobin A1c
| Hemoglobin A1c Quintiles | |||||
|---|---|---|---|---|---|
| 1 n = 3960 |
2 n = 3961 |
3 n = 3960 |
4 n = 3961 |
5 n=3960 |
|
| Age (mean, SD) | 52.2 (5.7) | 53.1 (6.1) | 53.8 (6.5) | 54.4 (6.8) | 55.6 (7.3) |
| BMI (mean, SD) | 24.1 (3.7) | 24.6 (3.9) | 24.8 (4.0) | 25.3 (4.4) | 26.4 (5.1) |
| Fruit and Vegetables, servings/day | 6.1 (3.8) | 6.0 (3.2) | 6.2 (3.6) | 6.2 (3.5) | 6.1 (3.6) |
| Dietary Saturated Fat, g/day | 19.0 (4.8) | 19.4 (4.8) | 19.4 (4.7) | 19.6 (4.7) | 19.9 (4.8) |
| High Cholesterol (%) | 19.0 | 22.4 | 25.8 | 26.0 | 31.0 |
| Baseline Systolic BP (%) | |||||
| < 110 mm Hg | 24.0 | 23.2 | 20.6 | 19.5 | 16.7 |
| 110–119 mm Hg | 40.8 | 38.6 | 39.2 | 38.1 | 34.0 |
| 120–129 mmHg | 25.5 | 27.8 | 28.3 | 29.4 | 31.2 |
| 130–139 mmHg | 9.7 | 10.4 | 11.8 | 13.1 | 18.1 |
| Baseline Diastolic BP (%) | |||||
| < 65 mm Hg | 14.4 | 13.6 | 12.6 | 11.7 | 10.5 |
| 65–74 mm Hg | 43.9 | 42.9 | 42.9 | 41.7 | 37.1 |
| 75–84 mmHg | 35.6 | 36.2 | 37.2 | 38.6 | 42.2 |
| 85–89 mmHg | 6.1 | 7.3 | 7.3 | 7.7 | 10.3 |
| Smoking (%) | |||||
| Never | 50.1 | 51.7 | 51.6 | 52.7 | 51.9 |
| Former | 40.8 | 37.0 | 35.9 | 34.6 | 35.2 |
| < 15 cigarettes/day | 3.7 | 4.3 | 5.1 | 4.7 | 4.9 |
| > 15 cigarettes/day | 5.4 | 7.1 | 7.5 | 7.9 | 8.0 |
| Alcohol (%) | |||||
| Rarely/Never | 35.7 | 39.9 | 41.2 | 44.1 | 48.1 |
| 1–3 drinks/month | 13.0 | 13.3 | 13.7 | 13.4 | 14.1 |
| 1–6 drinks/week | 36.9 | 35.3 | 35.7 | 33.0 | 30.4 |
| 1+ drinks/day | 14.4 | 11.5 | 9.3 | 9.6 | 7.4 |
| Exercise (%) | |||||
| Rarely/Never | 32.4 | 33.6 | 35.1 | 34.9 | 39.3 |
| < 1 time/week | 19.1 | 19.8 | 19.8 | 20.1 | 20.7 |
| 1–3 times/week | 34.9 | 34.8 | 32.9 | 32.9 | 30.3 |
| 4 or > times/week | 13.5 | 11.8 | 12.3 | 12.1 | 9.7 |
| Hormone Replacement (%) | |||||
| Never | 50.9 | 48.0 | 47.8 | 48.9 | 51.5 |
| Former | 5.1 | 6.3 | 7.7 | 8.1 | 12.0 |
| Current | 44.0 | 45.7 | 44.6 | 43.0 | 36.5 |
In the age-adjusted and initial multivariable models, a significantly graded increase in risk of HTN was observed across the quintiles of HbA1c. HRs of HTN were 1.00 (reference), 1.00 (0.93–1.07), 1.08 (1.01–1.16), 1.13 (1.06–1.20), 1.26 (1.18–1.34) (p for trend, <.0001) for the age-adjusted model with little attenuation upon adjustment for usual cardiovascular risk factors except BMI (extreme quintile comparison HR 1.21 (1.13–1.30; p for trend, <.0001). However, when BMI was added to the multivariable model, the HRs were substantially diminished, with no residual association across increasing quintiles of HbA1c (extreme quintile comparison HR1.04 (0.97–1.12; p for trend, 0.10) (Table 2). Additional adjustment for dietary factors, C-reactive protein, baseline SBP and DBP did not significantly alter these findings.
Table 2.
Incidence rates and hazard ratios (95% confidence intervals) of hypertension according to hemoglobin A1c quintile
| A1c Quintile | Cases | Crude Incidence Rate (cases/10,000 person-years) | Age Adjusted | Multivariable Model* | Multivariable Model + BMI |
|---|---|---|---|---|---|
| 1 (< 4.78%) | 1734 | 435.9 | 1.0 | 1.0 | 1.0 |
| 2 (4.78–4.92%) | 1765 | 447.0 | 1.00 (0.93–1.07) | 0.99 (0.92–1.06) | 0.96 (0.89–1.03) |
| 3 (4.92–5.04%) | 1867 | 490.4 | 1.08 (1.01–1.16) | 1.06 (0.99–1.13) | 1.01 (0.95–1.09) |
| 4 (5.04–5.19%) | 1939 | 519.1 | 1.13 (1.06–1.20) | 1.08 (1.01–1.16) | 1.00 (0.93–1.07) |
| 5 (≥ 5.19%) | 2103 | 600.2 | 1.26 (1.18–1.34) | 1.21 (1.13–1.30) | 1.04 (0.97–1.12) |
| P for trend | <.0001 | <.0001 | 0.10 |
Adjusted for age, smoking, alcohol consumption, physical activity, hormone use, high cholesterol, and parental history of myocardial infarction <60 years.
In our analysis that included subjects with baseline prevalent diabetes (defined by self-report or a baseline HbA1c ≥ 6.5%) as a separate category, the HR for incident HTN among subjects with diabetes was 1.55 (1.36–1.77) in the multivariable plus BMI-adjusted model compared to non-diabetic individuals with HbA1c values in the lowest quintile.
In analyses considering the relationship between HbA1c and incident HTN using clinical cutpoints of HbA1c, our findings were largely similar to our primary analyses using HbA1c quintiles (Table 3). A graded relationship between HbA1c and HTN was seen in both the age-adjusted and multivariate models (HR HbA1c > 6.0 vs. < 5.0; 1.88 (1.42–2.50), p for trend <.0001), and addition of BMI to the multivariate model led to a diminution of the HR’s [HR HbA1c >6.0 vs. < 5.0: 1.44 (1.08, 1.90)]. However, as opposed to the results with HbA1c quintiles, the linear trend across categories in this latter BMI-adjusted persisted (p for trend =0.02) with this finding driven by subjects having HbA1c values > 5.5%.
Table 3.
Incidence rates and hazard ratios (95% confidence intervals) of hypertension according to hemoglobin A1c clinical cutpoints
| Hemoglobin A1c (%) | Cases | Crude Incidence Rate (cases/10,000 person-years) | Age Adjusted | Multivariable Model* | Multivariable Model + BMI |
|---|---|---|---|---|---|
| <5.0 | 4813 | 453 | 1.0 | 1.0 | 1.0 |
| 5–5.5 | 4144 | 537 | 1.13 (1.09–1.18) | 1.10 (1.05–1.15) | 1.02 (0.97, 1.06) |
| 5.5–6.0 | 400 | 684 | 1.36 (1.23–1.51) | 1.36 (1.22–1.51) | 1.11 (0.99, 1.24) |
| ≥6 | 51 | 932 | 1.96 (1.49–2.58) | 1.88 (1.42–2.50) | 1.44 (1.08, 1.90) |
| p value for trend | <.0001 | <.0001 | p=0.02 |
Adjusted for age, smoking, alcohol consumption, physical activity, hormone use, high cholesterol, and parental history of myocardial infarction <60 years.
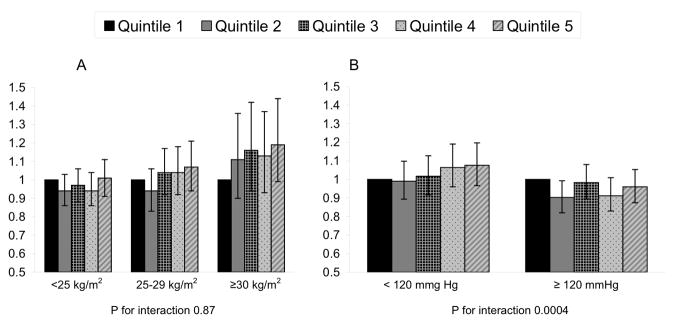
In analyses stratified by WHO BMI category, the risk of HTN was similar in subjects regardless of BMI category in the multivariable plus BMI-adjusted models (p for interaction, .87) (Figure 1). In analyses stratified by baseline SBP, the risk of HTN was significantly different in subjects with baseline systolic blood pressure < 120 mmHg compared with ≥ 120 mmHg in the multivariable plus BMI-adjusted model (p for interaction, .0004). For each HbA1c quintile, HRs for incident HTN were higher in subjects with baseline systolic blood pressure <120 mmHg (Figure 1).
Figure 1.
Hazard ratios of hypertension according to hemoglobin A1c quintile stratified by (a) body mass index and (b) baseline systolic blood pressure
*adjusted for age, smoking, alcohol consumption, physical activity, hormone use, high cholesterol, parental history of myocardial infarction <60 years, and BMI.
Discussion
In this large, long-term prospective cohort study, we found a positive association between increasing HbA1c quintile and increased risk of developing HTN in both age-adjusted models and models controlling for the majority of traditional hypertension and coronary risk factors. However, after addition of BMI to our multivariable models, the relation between HbA1c and HTN was no longer statistically significant. In contrast, using clinical cutpoints of HbA1c, a significantly increased risk of HTN was present in non-diabetics with HbA1c greater than 6% even in multivariable models which included BMI. These findings emphasize both the powerful influence of BMI on the development of HTN as well as the strong relationship between adiposity and metabolic factors, including HbA1c.
Despite evidence in the basic and translational sciences of an association between either glycemia or insulin resistance with HTN, the corresponding epidemiological studies investigating these potential link have been comparatively limited and provide conflicting results. A series of studies have looked at the association between glycemia, as opposed to insulin resistance, and the association with HTN. Overall, these studies tended to find an association between either fasting or post-load glycemia and incident HTN although the findings somewhat differed by gender17,18,19. Given the interest in a link between insulin resistance, as opposed to purely glycemia, and hypertension, other studies have also examined additional markers of insulin resistance and sensitivity and their relationship with hypertension. In addition to examining the relation between glycemia and HTN, the Paris Prospective Study examined the association between insulin levels, as a more specific marker of insulin resistance, and incident HTN19. These findings demonstrated that after adjustment for BMI, insulin levels were only associated with HTN in the presence of a family history of HTN. In contrast, both a cross sectional study in Iran using the homeostatic model of insulin resistance and a prospective analysis in the Insulin Resistance Atherosclerosis Study examining insulin sensitivity,20,21 suggest an association between insulin resistance and HTN. A slightly different cross sectional analysis by the Framingham Heart Study investigators using echocardiographic markers of left ventricular mass, as opposed to clinical HTN, as the outcome did show a significant association between glucose tolerance and left ventricular mass. However, insulin resistance, as measured by the homeostasis model, was only associated with left ventricular mass in women, and this relationship lost statistical significance after adjustment for BMI.
To our knowledge, only cross-sectional epidemiological studies have specifically focused on the relationship between HbA1c and HTN. Similar to the studies of glycemia, the results of all of these studies highlight the importance of BMI in this relationship and present conflicting results in terms of the effect of gender. In a cross-sectional analysis of both diabetic and non-diabetic survivors of the original cohort of the Framingham Heart Study,22 there was a positive association between HbA1c and HTN. However, this was a univariate analyses and therefore did not adjust for obesity. A cross-sectional study of 746 men and 595 women in Taiwan examined the relationship between HbA1c as a continuous variable and SBP. In women, a univariate association was present, but after addition of age and BMI to the model, this relationship was no longer significant. In contrast, in men, the significant univariate association between HbA1c and SBP persisted after adjustment for both age and BMI. However, the regression coefficient diminished from 1.57 to 1.03.23 Importantly, this study did not exclude subjects with diabetes although only 2% of the population had an HbA1c above 7%. Finally, a cross-sectional analysis of 1846 non-diabetic subjects from the EPIC-Postdam cohort study24 examined the association between quintiles of HbA1c and HTN, stratifying the population by gender. This analysis found a univariate association between increasing HbA1c and HTN, but this relationship disappeared after adjustment for age and BMI.
Both the previous cross-sectional data and our prospective analysis are largely consistent, all supporting the existence of a relationship between HbA1c and HTN that is substantially diminished or abolished with additional adjustment for BMI, emphasizing the essential role of body weight in the relationship between glucose metabolism and hypertension. Our study also emphasizes the significant risk of HTN among non-diabetic subjects with a HbA1c above 6.0% in which the relationship between HbA1c and incident HTN remains significant despite adjustment for BMI.
Despite the important contribution of our findings to the knowledge of the interconnections between pre-diabetic states and incident hypertension, the potential pathophysiologic links between glycemia, insulin resistance, measures of adiposity, and HTN remain incompletely understood. Epidemiological studies have established that increasing BMI increases the risk of diabetes, and presumably insulin resistance,25 and there is strong scientific consensus that overweight and obesity are antecedents to the development of insulin resistance among other metabolic abnormalities. However, the effect of insulin resistance on adipocyte pathophysiology and clinical fat deposition remains an area of active investigation. Experimental evidence has demonstrated that obese individuals have a higher free fatty acid flux, and these circulating free fatty acids have been shown to cause peripheral insulin resistance. However, this relationship may not be unidirectional as these circulating free fatty acids associated with obesity not only cause insulin resistance, but are also deposited into non-adiposetissues, including both muscle and liver.26,27 Hepatocytes, when activated by factors including fat deposition, are known to have increased secretion of both inflammatory cytokines and angiotensin II28,29 which may contribute to the development of HTN. These findings emphasize the complex relationship between BMI and glucose metabolism and suggest that fat deposition may not only be a confounder of the relationship between HbA1c and HTN, but may also be a causal intermediate. Therefore, it may be difficult to completely untangle the effects of insulin resistance and BMI in terms of their influence on the development of HTN.
The major strengths of our study include the large number of cases of incident HTN accrued over a long period of follow-up, allowing for sufficient statistical power to detect more modest effects. In addition, the available data on the important confounders strengthened the multivariate analysis. Our study does have some potentially important limitations. The WHS population is confined to middle-aged and old women, limiting the generalizability of our findings to men and to younger women who may be at risk for hypertension. Furthermore, both hemoglobin A1c and lifestyle factors were measured at baseline, and our study does not take into account changes over the length of follow-up. Misclassification bias is possible given the self-reporting of the outcome. However, HTN has previously been validated in the WHS with good accuracy.16 Another possible limitation of the study is our focus on hemoglobin A1c, which is not a direct measure of insulin resistance. However, the use of the HOMA-IR is not currently used in clinical practice due to the lack of standardization in the insulin assay across laboratories30,31 whereas hemoglobin A1c is now standardized and widely available.
In conclusion, our findings suggest that increasing levels of HbA1c, even within the normal range of HbA1c < 6.5%, are associated with an increasing risk of incident HTN among non-diabetic women prior to, but not after adjustment for BMI. Our findings highlight the complex and important interconnections among metabolic factors, including hemoglobin A1c, body weight, and HTN, although the specific mechanisms underlying these likely links are still being elucidated. Additional research is needed to better understand the contributions of both adiposity and dysglycemia in the development of HTN in this setting.
Acknowledgments
Source of Funding
We thank the dedicated participants of the Women’sHealth Study. We also appreciate the critical contributions of the research staff.
This work was supported by research grants CA-47988, HL-43851, HL-080467 and HL-099355 from the National Institutes of Health, Bethesda, MD. Dr Ridker is a Donald W. Reynolds Investigator and receives additional support from the Doris Duke Charitable Foundation and the Leducq Foundation.
Footnotes
Conflicts of Interest: None
Disclosures
The authors have no conflicts of interest to declare.
References
- 1.Hypertension in Diabetes Study (HDS) I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11(3):309–317. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Adler AI, Stratton IM, Neil HAW, Yudkin JS, Matthews DR, Cull CA, Wright AD, Turner RC, Holman RR. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–419. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sowers J. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004;286(5):H1597–1602. doi: 10.1152/ajpheart.00026.2004. [DOI] [PubMed] [Google Scholar]
- 4.Vecchione C, Colella S, Fratta L, Gentile MT, Selvetella G, Frati G, Trimarco B, Lembo G. Impaired Insulin-Like Growth Factor I Vasorelaxant Effects in Hypertension. Hypertension. 2001;37(6):1480–1485. doi: 10.1161/01.hyp.37.6.1480. [DOI] [PubMed] [Google Scholar]
- 5.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. The Journal of Clinical Investigation. 1996;97(11):2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al Suwaidi J, Higano ST, Holmes DR, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. Journal of the American College of Cardiology. 2001;37(6):1523–1528. doi: 10.1016/s0735-1097(01)01212-8. [DOI] [PubMed] [Google Scholar]
- 7.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-Sectional Relations of Digital Vascular Function to Cardiovascular Risk Factors in the Framingham Heart Study. Circulation. 2008;117(19):2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Z, Willett WC, Manson JE, Rosner B, Stampfer MJ, Speizer FE, Colditz GA. Body Weight, Weight Change, and Risk for Hypertension in Women. Ann Intern Med. 1998;128(2):81–88. doi: 10.7326/0003-4819-128-2-199801150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Gelber RP, Gaziano JM, Manson JE, Buring JE, Sesso HD. A Prospective Study of Body Mass Index and the Risk of Developing Hypertension in Men[ast] Am J Hypertens. 2007;20(4):370–377. doi: 10.1016/j.amjhyper.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faerch K, Vaag A, Holst JJ, Hansen T, Jorgensen T, Borch-Johnsen K. Natural History of Insulin Sensitivity and Insulin Secretion in the Progression From Normal Glucose Tolerance to Impaired Fasting Glycemia and Impaired Glucose Tolerance: The Inter99 Study. Diabetes Care. 2009;32(3):439–444. doi: 10.2337/dc08-1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. A Randomized Trial of Low-Dose Aspirin in the Primary Prevention of Cardiovascular Disease in Women. N Engl J Med. 2005;352(13):1293–1304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 12.Jones BR, Barrett-Connor E, Criqui MH, Holdbrook MJ. A community study of calorie and nutrient intake in drinkers and nondrinkers of alcohol. Am J Clin Nutr. 1982;35(1):135–139. doi: 10.1093/ajcn/35.1.135. [DOI] [PubMed] [Google Scholar]
- 13.Rissanen A, Heliovaara M, Knekt P, Reunanen A, Aromaa A. Determinants of weight gain and overweight in adult Finns. Eur J Clin Nutr. 1991;45(9):419–430. [PubMed] [Google Scholar]
- 14.Wannamethee SG, Shaper AG. Alcohol, body weight, and weight gain in middle-aged men. Am J Clin Nutr. 2003;77(5):1312–1317. doi: 10.1093/ajcn/77.5.1312. [DOI] [PubMed] [Google Scholar]
- 15.Klag MJHJ, Mead LA, Ford DE, Pearson TA, Levine DM. Validity of physicians’ self-reports of cardiovascular disease risk factors. Ann Epidemiol. 1993;3(4):442–447. doi: 10.1016/1047-2797(93)90074-e. [DOI] [PubMed] [Google Scholar]
- 16.Sesso HD, Cook NR, Buring JE, Manson JE, Gaziano JM. Alcohol Consumption and the Risk of Hypertension in Women and Men. Hypertension. 2008;51(4):1080–1087. doi: 10.1161/HYPERTENSIONAHA.107.104968. [DOI] [PubMed] [Google Scholar]
- 17.Kristiansson KSN, Sigvaldason H, Thorgeirsson G. Glucose tolerancea nd blood pressure in a population-based cohort study of males and females: the Reykjavik Study. J Hypertens. 1995;13(6):581–586. doi: 10.1097/00004872-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Haffner SMVR, Morales PA, Mitchell BD, Hazuda HP, Stern MP. Greater effect of glycemia on incidence of hypertension in women than in men. Diabetes Care. 1992;15(10):1277–1284. doi: 10.2337/diacare.15.10.1277. [DOI] [PubMed] [Google Scholar]
- 19.Fagot-Campagna A, Balkau B, Simon D, Ducimetiere P, Eschwege E. Is insulin an independent risk factor for hypertension? The Paris Prospective Study. Int J Epidemiol. 1997;26(3):542–550. doi: 10.1093/ije/26.3.542. [DOI] [PubMed] [Google Scholar]
- 20.Esteghamati A, Khalilzadeh O, Abbasi M, Nakhjavani M, Novin L, Esteghamati AR. HOMA-Estimated Insulin Resistance Is Associated with Hypertension in Iranian Diabetic and Non-Diabetic Subjects. Clinical and Experimental Hypertension. 2008;30(5):297 – 307. doi: 10.1080/10641960802269919. [DOI] [PubMed] [Google Scholar]
- 21.Goff DCZD, Haffner SM, Saad MF. Insulin sensitivity and the risk of incident hypertension: insight from the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2003;26(3):805–809. doi: 10.2337/diacare.26.3.805. [DOI] [PubMed] [Google Scholar]
- 22.Singer DEND, Anderson KM, Wilson PW, Evans JC. Association of HbA1c with prevalent cardiovascular disease in the original cohort of the Framingham Heart Study. Diabetes. 1992;41(2):202–208. doi: 10.2337/diab.41.2.202. [DOI] [PubMed] [Google Scholar]
- 23.Chu N-F, Lee MM-S, Wang D-J, Chen L-M, Shieh S-M. The reappraisal of the association of glycosylated hemoglobin Alc (HbAlc) and blood pressure: A hypertension and diabetes study in a Taiwan rural area. Journal of Clinical Epidemiology. 1993;46(2):173–179. doi: 10.1016/0895-4356(93)90055-6. [DOI] [PubMed] [Google Scholar]
- 24.Kroke A, Liese AD, Keil U, Boeing H. Arterial hypertension and glycemia in non-diabetic subjects: is there an association independent of obesity? Diabetes/Metabolism Research and Reviews. 1999;15(2):99–105. doi: 10.1002/(sici)1520-7560(199903/04)15:2<99::aid-dmrr22>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Colditz GA, Willett WC, Stampfer MJ, Manson JE, Hennekens CH, Arky RA, Speizer FE. WEIGHT AS A RISK FACTOR FOR CLINICAL DIABETES IN WOMEN. Am J Epidemiol. 1990;132(3):501–513. doi: 10.1093/oxfordjournals.aje.a115686. [DOI] [PubMed] [Google Scholar]
- 26.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu Y-H, Ginsberg HN. Adipocyte Signaling and Lipid Homeostasis: Sequelae of Insulin-Resistant Adipose Tissue. Circ Res. 2005;96(10):1042–1052. doi: 10.1161/01.RES.0000165803.47776.38. [DOI] [PubMed] [Google Scholar]
- 28.Edens MA, Kuipers F, Stolk RP. Non-alcoholic fatty liver disease is associated with cardiovascular disease risk markers. Obesity Reviews. 2009;10(4):412–419. doi: 10.1111/j.1467-789X.2009.00594.x. [DOI] [PubMed] [Google Scholar]
- 29.Bataller R, Sancho-bru P, Ginès P, Lora JM, Al-garawi A, Solé M, Colmenero J, Nicolás JM, Jiménez W, Weich N, Gutiérrez-ramos J-c, Arroyo V, Rodés J. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125(1):117–125. doi: 10.1016/s0016-5085(03)00695-4. [DOI] [PubMed] [Google Scholar]
- 30.Bonora ETG, Alberiche M, Bonadonna RC, Saggiani F, Zenere M, Monauni T, Muggeo M. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity. Diabetes Care. 2000;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 31.Robbins DCAL, Bowsher R, Chance R, Dinesen B, Frank B, Gingerich R, Goldstein D, Widemeyer HM, Haffner S, Hales CN, Jarett L, Polonsky K, Porte D, Skyler J, Webb G, Gallagher K. Report of the American Diabetes Association’s Task Force on standardization of the insulin assay. Diabetes. 1996;45(2):242–256. doi: 10.2337/diab.45.2.242. [DOI] [PubMed] [Google Scholar]