Abstract
The present work tested the hypothesis that short-term (S-T) dietary deficiency of magnesium (Mg) (21 days) in rats would: 1) result in reduction in serum(s) sphingomyelin (SM) and changes in several blood lipids, HDL-cholesterol (HDL-C) and phosphatidylcholine (PC) concomitant with elevations in s cholesterol (chol), s LDL+VLDL and trigycerides (TG), as well as reduction in the PC/cholesterol ratio; 2) lead to oxidative stress, characterized by reductions in glutathione (glut) content in the various chambers of the heart and activation of e-NOS and n-NOS in the atria, ventricles and aortic smooth muscle (ASM); 3) produce early cardiac damage characterized by leakage of creatine kinase (CK) and lactic dehydrogenase (LDH); and 4) demonstrate that these pathophysiological changes are a result of profound reductions in s ionized Mg (Mg2+) and activation of the SM-ceramide pathway. In addition, we hypothesized that: 1) exposure of primary cultured vascular smooth muscle cells (VSMCs) to low extracellular Mg2+ would lead to de novo synthesis of ceramide and activation of NO synthase with reduction in glut, both of which would be attenuated by inhibition of sphingomyelinase (SMase) and serine palmitoyl CoA transferase (SPT); and 2) low levels of Mg2+added to the drinking water would either prevent or ameliorate these manifestations. Our data indicate that S-T Mg deficiency resulted in reductions in s Mg2+, SM, PC, HDL-C and the PC/chol ratio concomitant with decreases in tissue levels of glut, leakage of cardiac CK and LDH, as well as activation of e-NOS and n-NOS in all chambers of the heart and ASM. The greater the reduction in s Mg2+, the greater the effects on all parameters analyzed; very significant correlations to levels of s SM and Mg2+ were found with all of the serum and tissue biochemical -molecular analytes measured. Our experiments also showed that VSMCs exposed to low Mg2+resulted in activation of NO synthase, loss of glut and de novo synthesis of ceramide which were attenuated by inhibitors of SMase and SPT. Low levels of drinking water Mg2+(e.g., 15 ppm) were cardio- and vascular protective. We believe these new findings support our concept of an important role for the SM-ceramide pathway in the manifestations of Mg deficiency and atherogenesis.
Keywords: sphingomyelin, lipids, NO synthases, glutathione, cardiac enzymes, vascular muscle
Introduction
Two of us have demonstrated that variation in free magnesium ions (Mg2+), in primary cultured cerebral and peripheral vascular smooth muscle cells, causes sustained changes in membrane phospholipids and second messengers, membrane oxidation, decreases in fatty acid chain length and double bonds; the latter resulting in truncation of the fatty acids [1-5]. Decrease in extracellular Mg2+ ([Mg2+]0) produced a fall in phosphatidylethanolamine, phosphatylinositol and phosphatidylcholine (PC), and expression of nuclear-factor K - B as well as the proto-oncogenes c-fos and c-jun [1-4] with concomitant rises in intracellular free calcium ions (Ca2+), a breakdown of membrane sphingomye-lin(SM) with a concomitant release of ceramide and apoptosis[2,6]. With respect to the latter, we noted formation of free radicals and frag-mentation of DNA [6]. Recent experiments revealed that short-term (S-T) Mg deficiency (21 days) in intact rats resulted in upregulation of serine palmitoyl-CoA transferase (SPT)(which catalyzes the first step in the biosynthesis of sphingolipids, including ceramide) and sphingo-myelin synthase (SMS) in left and right ventricular and atrial muscle as well as aortic VSMCs [5,7]. In addition, we demonstrated that reduction in serum [Mg2+]0, in S-T Mg deficiency (MgD), resulted in a reduction in serum SM [6], and presumably, ceramides, thus similar to our studies in the primary cultured vascular cells. Ceramides have been shown to produce apoptosis in numerous types of mammalian cells [8,9],including cerebral and peripheral vascular muscle cells in culture, placed in contact with C2 -,C8-, and C16-ceramides, as observed in our laboratory [10, unpublished data].
More than 25 years ago, it was suggested that, in humans, a decrease in the plasma PC/free cholesterol ratio connotes a higher correlation with ischemic vascular disease than either plasma cholesterol, itself, or high density lipo-protein cholesterol (HDL-C) [11]. PC is important in the lymphatic absorption of cholesterol and is the major phospholipid in HDL-cholesterol [12]. This phospholipid is also known to play an important role in cholesterol esterification, and thus is a potential risk factor for atherogenesis, cardiovascular disease and cerebral vascular accidents [for review, see 12]. We have recently reported that S-T Mg deficiency in rats results in a marked reduction in serum PC [7]. Whether such Mg deficiency results in a decrease in the PC/cholesterol ratio is not known.
Considerable evidence has accumulated over the past four decades to indicate that cardiovascular diseases are the leading cause of mortality in Western countries, caused mainly by atherosclerosis. Both animal and human studies [4, 13-20] have shown a strong link between dietary intake of Mg and atherosclerosis. Mg deficiency in a carefully-controlled rabbit model was shown to result in accelerated atherogenesis concomitant with increased numbers of lipid-laden macrophages [14). These latter in-vivo studies showed an inverse relationship between the serum level of Mg and cholesterol as well as triglycerides and numbers of macrophages; the lower the serum level of Mg, the higher the serum cholesterol, triglycerides and number of lipid-laden macrophages or vice-versa. However, the extent of the lesions was poorly correlated to the serum cholesterol levels in these rabbit studies. Older studies, using mature rats also indicates that as the plasma/serum level of Mg decreases, the plasma/serum level of cholesterol appears to rise [for review, see 13]. However, more recent studies using weanling male rats on very severely deficient Mg diets for only 8 days suggest that the plasma cholesterol levels either do not change or change minimally) [17,18,21] whereas mature male rats maintained on a somewhat severe Mg deficient diet for 2-10 weeks do appear to demonstrate elevations in serum cholesterol [22] supporting the earlier studies in mature rats. Even though the total cholesterol (TC): high-density lipoprotein-cholesterol (HDL-C) ratio is a well -known risk factor for coronary artery disease, atherogenesis and stroke, it is not known whether this important risk factor is elevated in S-T Mg deficiency.
Low-density lipoprotein cholesterol (LDL-C), a major component of TC, is recognized as one of the most important risk factors for atherogenesis, coronary arterial disease and peripheral vascular disease [23]. However, evidence is growing for a major role for the triglyceride fraction [24]. It would appear from the experiments in rabbits on a moderately Mg deficient diet [14], reviewed above, and experiments on both immature and mature rats on severely deficient diets [18,21,22], that the serum triglycerides are relatively affected more than the total serum cholesterol. However, these experiments did not monitor the ionized Mg levels.
Hypercholesterolemia, hypertriglyceridemia, hypertension, diabetes mellitus, immune injury, end-stage renal diseases,renal dialysis and obesity are widely accepted risk factors for atherosclerosis. No common link(s), however, has been identified that forms a rational basis to these disorders and atherogenesis. Until recently, it was not known how Mg deficiency might promote cardiovascular damage and oxidation of lipoproteins (a known important step in etiology of atherogenesis). During the past 15 years, reports from several laboratories have suggested that Mg deficiency can lead to formation of several types of oxygen-free radicals, reactive nitrogen species, superoxide, reduced levels of glutathione and inflammatory cytokines [3,4,6,21,23-27]. None of these studies, however, were performed either on intact blood vessels, the cardiac atria or ventricles. A very recent study from our laboratory, using S-T (21 days) Mg deficiency in rats, does suggest that lipid peroxidation is induced in intact blood vessels and the cardiac chambers [6]. Whether or not MgD results in reduced levels of glutathione in these different cardiovascular tissues or whether these alterations in molecular analytes are related to formation of ceramides and/or hydrolysis of SM in cardiovascular tissues, is not known. Since depletion of glutathione levels are thought to be important in the release of mito-chondrial cyto c and induction of programmed cell death [28,29], these are important questions which should be addressed, particularly as our recent studies in S-T Mg deficiency demonstrated release of mitochondrial cyto c and apoptosis [6,7].
More than 25 years ago, it was discovered that the endothelium releases a relaxant factor, which plays an active role in the regulation of blood flow [30,31]. This relaxant factor was later identified as nitric oxide (NO). In 1987, two of us, using coronary arteries, found that removal of extracellular Mg2+ inhibited these blood vessels from undergoing relaxation when challenged with acetylcholine, a standard test for this relaxant factor [32]. Since these studies, NO and nitric oxide synthases have been shown to play major and diverse roles in regulation of cardiac functions, peripheral and cerebral blood flow regulation as well as inflammation, vessel remodeling and lipid metabolism [for review, see 33]. Several isozymes of NO synthase are now known to be present in different cells and subcellular compartments. Although Mg has been demonstrated to modulate release of NO in intact animals [4], it is not known if endothelial-derived NO synthase (e- NOS) and neuronal - derived NO synthase (n-NOS) both play roles in responses of diverse cardiovascular tissues to changes in (Mg2+)0 in the intact animal and whether these are related to ceramide formation and/or hydrolysis of SM.
Interestingly, it has been suggested that activation of the SM-ceramide pathway might: 1) regulate NO synthesis [34]; and 2) lead to a reduction in glutathione levels in certain cells [8,9]. Whether or not the SM-ceramide pathway is closely tied to lipoprotein metabolism, NO synthesis and glutathione levels in S-T MgD in the cardiovascular system is not known. Such relationships (if proven) could underlie the role of Mg in the immune-inflammatory system pathway in atherogenesis.
At present, the average dietary intake of Mg has declined from about 450-485 mg/day in 1900 to about 185-235 mg/day for large segments of the North American population [4,20,35,36]. Both animal and human studies have shown an inverse relationship between dietary intake of Mg and atherosclerosis [4, 14, 19, 20, 37-42]. The myocardial level of Mg has been observed to be lower in subjects dying from ischemic heart disease and sudden cardiac death in soft -water areas than those living in hard-water areas [38, 39, 43, 44]. Low Mg content in drinking water found in areas of soft-water and Mg-poor soil is associated with high incidences of ischemic heart disease, coronary vasospasm, atherosclerosis, and sudden cardiac death [4,38-40,43-46].
We designed experiments to determine whether S-T Mg deficiency in rats would: 1) lead to elevations in serum cholesterol, serum LDL + VLDL and triglycerides (TG), the TC:HDL-C ratio as well as reductions in HDL-C, and the PC/cholesterol ratio with a relatively greater effect on serum TG compared to serum cholesterol; 2) lead to reductions in glutathione content in the various chambers of the myocardium and in vascular smooth muscle; 3) result in activation of both e-NOS and n-NOS in the atria, ventricles and blood vessels; 4) result in early cardiac muscle damage in the intact animal, characterized by leakage of creatine kinase and lactic dehydrogenase; and 5) demonstrate, statistically, an association between serum SM levels, serum ionized Mg, lipoprotein metabolism, tissue levels of e-NOS, n-NOS, and glutathione levels. In addition, we designed experiments, using primary cultured aortic VSMCs, exposed to low [Mg2+]0, to determine if the latter maneuver would result in activation of NO synthase with concomitant de novo synthesis of ceramide and loss of cellular glutathione, and whether inhibition of SMase and/or SPT, here, would result in a decrease in de novo production of ceramide, a concomitant decrease in activation of NO synthase, and losses in cellular glutathione. Lastly, we determined whether imbibing low levels of a water-soluble Mg salt in drinking water would inhibit or reverse these effects of dietary Mg deficiency.
Materials and methods
Animals, diets, sera and organ-tissue collections
Mature male and female Wistar rats (200 +/- 65 gm) were utilized for all experiments. All experiments were approved by the Animal Use and Care Committee at SUNY Downstate Medical Center. Equal numbers of paired male and female animals were utilized for all experiments. Control (600 ppm Mg) and Mg deficient (MgD, 60 ppm Mg) synthetic pellet (semi-purified) diets were obtained from DYETS, Inc (Bethlehem, PA) (AIN 93G diets) and were utilized. All animals were given their respective diets for 21 days. The MgD animals were allowed to drink triply distilled water (Mg2+ = <10-6M) containing one of four different levels of magnesium (in the form of Mg aspartate HCl) (0, 15, 40 or 100 mg/L ;Verla Pharm, Tutzing, Germany ). All control animals received a normal Mg - containing diet (600 ppm Mg) and the triply-distilled water to drink. On the 22nd day, sera and organ- tissues (left and right ventricles, left and right atria, and abdominal aorta -between superior mesenteric and renal arteries) were collected quickly after anesthesia and sacrifice (sodium pentobarbital, 45 mg/kg i.m.). The tissues were stored rapidly under liquid nitrogen (-85°C) until use. It should be noted that no attempt was made to denude the endo-thelial layers from either the various chambers of the heart or from the abdominal aortae. Fasting whole blood was collected under anaerobic conditions in red-stoppered tubes (no anticoagulant present), allowed to clot under anaerobic conditions, then centrifuged in capped vacutainer tubes. The sera were then collected into additional red-stoppered vacutainer tubes under anerobic conditions for processing shortly thereafter [47,48]. Serum samples for total Mg were analyzed within two hours after collection by standard techniques in our laboratory ( Kodak DT-60 Analyzer, Ektachem colorimetric Instrument, Rochester, NY) [47,48]. This method compares favorably with atomic absorption techniques for total Mg [47, 48]. A Mg2+ ion-selective electrode (ISE) with a neutral carrier-based membrane (NOVA Biomedical Instruments, Waltham, MA) was used to measure the free divalent cations in the sera [47,48].
Serum total cholesterol, triglycerides, HDL-C, phosphatidylcholine, SM, creatine kinase, and lactic acid dehydrogenase
Serum total cholesterol (Infinity™ Cholesterol,TR 13421) and triglycerides (Infinity™ Triglyceride, TR 22421) were measured using commercial kits (Thermo Trace, Melbourne, Australia). Cholesterol and triglycerides in HDL were measured after the precipitation of apoB lipoproteins with sodium phosphotungstate and magnesium chloride [49]. In some experiments, Fast Phase Liquid Chromatography (FPLC) was utilized for separation and quantification of lipids.
Serum PC concentrations were determined by subtracting SM from total phospholipid concentration [50]. Serum measurements of SM levels were carried out by a 4-step enzymatic procedure detailed elsewhere [50]. Serum creatine kinase (CK) was measured using the method described by Moss et al [51] while serum lactate dehydrogenase was measured, quantitatively, using the spectrophotometric method described by Moss et al [51].
Glutathione (GSH) tissue analyses
The tissues were measured, quantitatively, for total GSH after the methodologies of Cotgreave and Moldeus for thiol compounds [52]. Briefly, tissues were homogenized in 0.5-1 ml of 5% sulfosalicyclic acid, and then centrifuged at 8,000 x g for 10 min. An enzyme working solution was then added (with lyophilyzed co-enzyme) to the homogenized extracts. The microplates were next incubated at 37°C for 10 min. Twenty microliters of the working enzyme solution was then added to the homogenized samples in the warmed microplates and incubated at room temperature for 5-10 min; the absorbances of the incubated mixtures were then read with a microplate reader at 405 nm. Glutathione (GSH -lyophilized) standards were run and a standard curve was made and utilized to measure the homogenized-incubated tissue samples.
Western -blot analyses for e-NOS and n-NOS
Our methods were adapted from Smith and Titheradge [53]. Left and right ventricles and atria as well as abdominal aortae were homogenized in a lysis buffer mixture consisting of: 0.01 M TRIS-HCL, pH 7.5;0.5% NP 40; 0.15 NaCl;0.01 M EDTA;1% NaN3;and 10 mM PMSF all at 4°C for 30 min at low speed (4,000 rpm) centrifugation. The resultant supernatants were then re-centrifuged at high speed (105,000 x g) for 30 min, Total protein concentrations were determined using protein assay kits (Pierce, Rockford, IL). 50 micrograms of total protein from each lysate was electrophoresed through 15% SDS-poyacrylamide gels at room temperature. One lane, each time, in each 15% poly-acrylamide PAGE gel was loaded with prestained Biorad MW standards. Proteins were electrotransferred to enhanced chemolumines-cence (ECL) nitrocellulose membranes (Pharmacia-Amersham, Piscataway, NJ) overnight at 6°C using a Towbin transfer buffer in a transblot electrophoresis transfer cell (Biorad apparatus). All of the remaining steps were carried out at room temperature. After the transfer of the proteins, membranes were blocked with a 5% nonfat milk (Carnation Products) in TBS-T (consisting of 20 mM TRIS-HCL, pH7.6; 137 mM NaCl; 0.1% Tween 20 Sigma Chemicals, St.Louis, MO) for 1 hr. After washing 3 -times (15 min the 1st time and 5 min each for the next two times) with TBS-T, the membranes were incubated for 1 hr with primary e-NOS or n-NOS polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) diluted in TBS-T for 1 hr. After 3-washes with TBS-T, the membranes were re-incubated with diluted (1:2,000 in TBS-T) horseradish peroxidase conjugated secondary antibodies (goat anti-rabbit and anti-mouse IgG+ peroxidase) for 1 hr. After 5-washes (one for 15 min, the other four for 5 min each) with TBS-T, the membranes were developed using ECL kits (ECL plus; Pharmacia-Amersham) and radiographed using X-Omat technology.
Isolation of vascular muscle and primary culture of aortic VSMCs
Rat aortic vascular smooth muscle cells were isolated according to established methods in our laboratory [54] (n=10-12 animals/group) and cultured in DMEM containing 1.2 mmol/l [Mg2+]0, FCS, and antibiotics at 37°C in a humidified atmosphere composed of 95% air-5% CO2 [54]. After confluence had been reached VSMCs were placed in media containing either 0.3, 0.6, or 1,2 mmol/l [Mg2+]0 for 18 or 72 h.
Influence of [Mg2+]0 on ceramide levels in primary cultures of VSMCs
Cells were exposed for either 18 or 72 h in normal Krebs -Ringer solutions(composition, see ref. 5} with 95% air-5 %CO2 containing different concentrations of [Mg2+]0 (either 1.2, 0.3,or 0.6 mmol/l). The lipids were extracted as previously reported [1,2,5]. The ceramide was next converted into ceramide-1-[32P] phosphate by Esh-erichia coli DAG kinase in the presence of [gamma-32]ATP [5], and the lipids separated on high-performance TLC plates in a solvent system consisting of a chloroform-acetate-methanol-acetic acid-water [(50:20:15:10:5) (vol/vol/vol/vol.vol)] mixture. After autoradiography, spots corresponding to ceramide-1-phosphate were carefully scraped into vials, and the radioactivity was then counted in a scintillation counter (LS-6500), Beckman). Quantitation of ceramide was based on standard curves of known amounts of authentic ceramide [5]. The results were expressed as picomoles per 108 cells.
Influence of [Mg2+]0 on NO synthase (NOS) levels
VSMCs levels of NOS activity was measured either at 0, 18 or 72 h after exposure of the cultured cells to either 0.3, 0.6, or 1.2 mmol/l [Mg2+]0 utilizing the Greiss method, similar to what we reported previously [55]. The concentration of NO was estimated in the samples from standard curves using NaNO2 and absorb-ance at 540 nm. Experiments were performed in duplicate.
Influence of inhibitors of SPT and N-SMase on the content and de novo synthesis of ceramide and/or NOS and GSH content
Before the cells were radiolabeled (as a measure of de novo synthesis of ceramide), VSMCs were treated with an SPT inhibitor, myriocin (75nM, Sigma-Aldrich ) [5], for 3 h in NKRB solution [composed of(in mmol/l) [118 NaCl, 4.7 KCl, 1.2 KHPO4, 1.2 MgSO4, 2.5 CaCl2, 10 glucose, and 25 NaHCO3] [54]. Precise concentrations of free Mg2+ were determined with our specific ion-selective electrodes for Mg2+. After the treatment of VSMCs with the SPT inhibitor, cells were labeled with [3H]palmitic acid (4-20 uCi/ml) at 37°C for either 18 or 72 h, rinsed with fresh NKRB solution, and transferred to NKRB solutions (with the inhibitor) containing either 0.3,0.6,or 1.2 mmol/l [Mg2+]0, similar to previously described methods [5]. In addition, other cells were exposed to the N-SMase inhibitor, scyphostatin (75uM, Sigma-Aldrich) for either 18 or 72h. NOS levels were measured by the Greiss method, as above. Total glutathione (GSH) contents in the VSMCs were measured, where appropriate, spectrophotometrically using the GSH reductase-linked 5, 5'-dithiobis (2-nitrobenzoic acid) (DTNB) recycling assay. Here, we used a mixture of 100 mM KPO4(pH 7.0), 1 mM EDTA, 100uM DTNB, 300 uM NADPH, and 0.2 U/ml GSH reductase. For these GSH assays, cells were removed from the cultures after stopping with ice-cold PBS, then incubating them at 4°C for 30 min, followed by centrifugation for 10 min at 6,000g. The acid extracts were neutralized with 4 mM KOH with 0.6 M MOPS; the potassium perchlorate precipitate was removed by centrifugation. Results were quantified using standard curves employing known concentrations of GSH. The acid insoluble pellets were assayed for protein by the Lowry method,and results expressed, here, as total GSH(nmoles)/mg protein.
Statistical analyses
Where appropriate, means and S.E.M.'s were calculated. Differences between means were assessed for statistical significance by Student's t-tests and ANOVA followed by a Newman -Keuls test. Where appropriate, linear regression analyses and correlation coefficients were calculated. A p-value less than 0.05 was considered significant.
Results
Influence of diet on water consumption, food intake and overall physiological condition
As shown recently, using an identical dietary regimen of Mg in controls and Mg deficient animals [5-7], there were no significant differences in either water consumption or food intake between the diverse subgroups of rats (i.e., controls=600 ppm Mg, Mg deficient-MgD, MgD + 15 mg/L Mg/day, MgD + 40 mg/L Mg/day, or 100 mg/L Mg/day). All of the MgD subgroups (n=18-30 animals per group ), irrespective of the amount of Mg in the diets or in the drinking water, showed no loss in gait or any other outward signs of pathology or behavior.
Serum total and ionized Mg levels
Feeding the animals the synthetic AIN-93G MgD pellet diet (n=18-30/group) resulted in a total serum Mg level of approximately 1.00 mM/L, whereas the animals receiving the MgD diet exhibited a serum total Mg level of about 0.4 mM/L (p< 0.01). The serum level of ionized Mg in the normal, control group was approximately 0.60 mM/L whereas in the MgD group the serum ionized level was reduced to about 0.3 mM/L (p<0.01).
Feeding the MgD animals various levels of Mg in their drinking water (as seen previously, ref. 5,7) resulted in concentration-dependent rises in both the total and ionized levels of serum Mg. 100 mg/L/day of Mg2+ elevated the total Mg level to normal, i.e., approximately 1.0 mM/L, whereas feeding 15 and 40 mg/L/day of Mg in the drinking water raised the total Mg levels to 67 and 83%, respectively, of normal (n=18-28, p< 0.05). With respect to the serum ionized levels, feeding the animals 100 mg/L/day of Mg restored the level of ionized Mg to normal while feeding 15 and 40 mg/L/day of Mg to the rats raised the serum ionized levels to 60 and 65%, respectively, of normal (n=18-28, p <0.05).
Serum phosphatidylcholine and sphingomyelin levels: relationship to serum ionized Mg levels
Table 1 indicates that feeding animals MgD diets for 21 days produced, approximately, a 25% reduction in serum PC, similar to that reported recently [6]. Addition of low levels of Mg2+ to the drinking water (i.e., only 15 ppm) prevented the fall in serum PC observed in the MgD rats. Although not shown, linear regression analyses demonstrated a correlation between the reduction in serum ionized Mg and PC, that is, the lower the serum Mg2+, the lower the serum PC (r= 0.54, p<0.05).
Table 1.
Serum phosphatidylcholine(PC) and sphingomyelin(SM) in normal and Mg-deficient rats with and without Mg2+ added to the drinking water
| Group | PC | SM |
|---|---|---|
| Controls | 171.6 +/- 9.6 | 79.0 +/- 6.0 |
| MgD | 135.8 +/- 7.6* | 60.5 +/- 3.3* |
| MgD+15 | 153.5 +/- 8.4 | 69.5 +/- 4.2 |
| MgD+40 | 158.6 +/- 18.9 | 67.9 +/- 4.2 |
| MgD+100 | 174.5 +/- 10.2 | 78.3 +/- 8.6 |
Values are given as mg/dl (means+/- SE). Asterisks represent mean values which are significantly different from controls and all other groups (ANOVA, p<0.01). Concentrations of Mg2+ were added to the drinking water as follows: MgD+15= 15mg/L; MgD+40=40mg/L;MgD+100=100mg/L. N = 12-18/group.
With respect to serum SM levels, feeding animals the MgD for 21 days resulted in an approximate 25% reduction in SM, similar to that reported previously [7], whereas addition of only 15 ppm Mg2+ to the drinking water prevented this fall in serum SM (Table 1). Although not shown, linear regression analyses demonstrated a correlation between the reduction in serum ionized Mg and SM, i.e., the lower the serum Mg2+, the more the reduction in serum SM (r= 0.59, p<0.05).
Serum lipid fractions and the relationship to serum ionized Mg and serum sphingomyelin
Table 2 indicates that feeding animals a MgD for 21 days resulted in a significant (p<0.05) 12% rise in serum TC, a 70 % increase in TG, and a 31% increase in VLDL +LDL concomitant with almost a 20% fall in serum HDL levels. Addition of even low levels of Mg2+ to the drinking water prevented the elevations in TC, VLDL+LDL and TG levels; the fall in serum HDL was also ameliorated by addition of Mg2+ to the drinking water (Table 2). Although not shown, use of FPLC clearly validated these biochemical observations. Although not shown, linear regression analyses indicate that the elevations in serum TC, VLDL+LDL and TG are correlated negatively with the levels of serum Mg2+, i.e., the greater the rise in these lipid levels, the lower the level of serum Mg2+ (r= -0.37-0.55, p<0.05). Likewise, linear regression analyses indicate that the greater the rise in TC, VLDL+LDL, and TG, the lower the serum level of SM (r=-0.48-0.65, p<0.05).
Table 2.
Serum lipid fractions in normal and MgD rats with and without Mg2+ added to the drinking water
| Group | Total Cholesterol | HDL-C | VLDL+LDL-C | TG |
|---|---|---|---|---|
| Controls | 86.6 +/- 4.8 | 75.3 +/- 3.2 | 11.4 +/- 2.7 | 133+/-11.7 |
| MgD | 100.6 +/- 7.3* | 68.1 +/- 2.1* | 27.7 +/- 2.9* | 228+/-17.4* |
| MgD+15 | 91.9 +/- 3.4 | 82.3 +/- 2.8* | 9.6 +/- 1.3 | 119+/-14.1 |
| MgD+40 | 88.2 +/- 4.6 | 76.9 +/- 5.0 | 9.3 +/- 1.5 | 138+/-20.2 |
| MgD+100 | 69.4 +/- 2.8** | 62.8 +/- 3.2 | 7.6 +/- 1.1** | 140+/-17 |
Values are given as mean mg/dl +/- SE. N= 10-16 animals/group.
Values which are significantly different from controls (p<0.05).
Values which are significantly different from all other paired values (p<0.05, ANOVA).
Glutathione levels in cardiac and vascular muscles obtained from Mg-deficient animals: relationships to serum Mg and SM
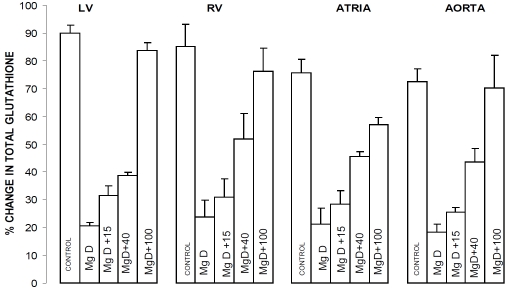
Figure 1 indicates that placing rats on diets of 10% magnesium intake for 21 days results in almost a 60% fall in tissue levels of total glutathione in left and right ventricular muscle, right and left atrial muscle and aortic smooth muscle. All of the levels of Mg2+ added to the drinking water inhibited, to different degrees, the falls in glutathione. Use of 100 ppm Mg2+, in the drinking water, completely prevented any loss of glutathione (Figure 1). Although not shown, linear regression analyses demonstrated a correlation between the percent reduction in glutathione content in the ventricular, atrial and aortic smooth muscles and the level of serum Mg2+, i.e., the greater the fall in serum Mg2+, the greater the fall in percent glutathione content (r=0.69-0.94, p<0.05). In addition, we found, using linear regression analyses, a correlation between the percent reduction in glutathione content in the ventricular, atrial and aortic smooth muscles and the serum level of SM, i.e., the greater the fall in serum SM, the more the percent reductions in glutathione content in all of the tissues examined (r=0.49-0.66, p<0.05).
Figure 1.
Glutathione levels in left ventricular (LV) muscle, right ventricular (RV) muscle, atria, and abdominal aortic smooth muscle in normal and MgD rats with and without Mg2+ added to the drinking water (DW). N = 16-22 animals per group. Mean values for MgD animals are significantly different from all other groups (p< 0.01, ANOVA).
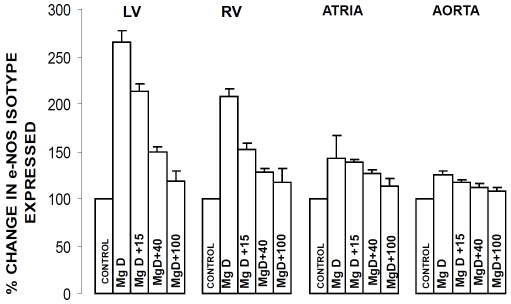
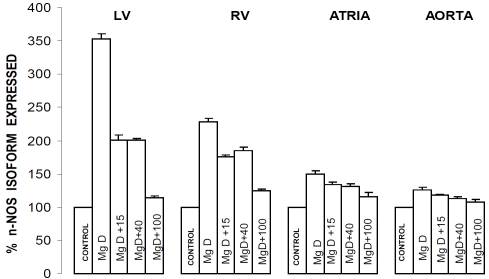
eNOS and nNOS levels in cardiac and vascular muscle obtained from Mg-deficient animals: relationships to serum Mg2+ and SM
Figures 2 and 3 indicate that dietary deficiency of Mg for 21 days resulted in very significant (p<0.001) 35-250% increases in the expression of both eNOS and nNOS in the ventricular, atrial and abdominal aortic smooth muscles. All of the levels of Mg2+ added to the drinking water inhibited, to different degrees, the increase in expression of both eNOS and nNOS. Although not shown, linear regression analyses demonstrated inverse correlations between the percent rises in expression of eNOS and nNOS in the cardiac and vascular muscles and serum Mg2+ (and SM) (r=-0.43-66, p<0.05); i.e., the greater the percent increase in expression of eNOS or nNOS, the lower the serum Mg2+ and level of SM.
Figure 2.
e-NOS levels in LV muscle, RV muscle, atrial muscle and abdominal aortic smooth muscle in normal and MgD rats with and without Mg2+ added to the DW. Designations and numbers of animals per group are identical to those in Figure1. Mean values for MgD animals are significantly different from all other groups (p< 0.01, ANOVA).
Figure 3.
n-NOS levels in LV muscle, RV muscle, atrial muscle and abdominal aortic smooth muscle in normal and MgD rats with and without Mg2+ added to the DW. Designations and numbers of animals per group are identical to those in Figure1. Mean values for MgD animals are significantly different from all other groups (p<0.01, ANOVA).
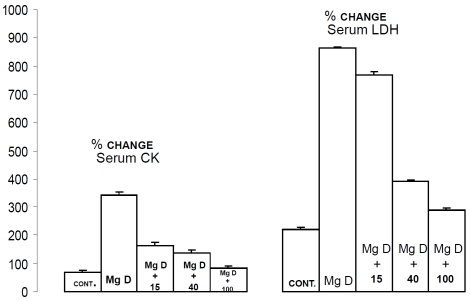
Serum creatine kinase (CK) and lactic dehydrogenase (LDH) levels: relationships to serum Mg2+ and SM
Figure 4 indicates that feeding rats the MgD diet for 21 days resulted in significant 5-15-fold rises (p<0.01) in the serum levels of CK and LDH, whereas addition of Mg2+ to the drinking water resulted in a dose-dependent inhibition of the rises in the serum levels of both enzymes in the animals given the MgD diet. Although not shown, linear regression analyses demonstrated an inverse correlation between the rises in CK and LDH and the serum level of both Mg2+ and SM (r=-0.47-0.86, p<0.01).
Figure 4.
Serum creatine kinase and lactic dehydrogenase levels in normal and MgD rats with and without Mg2+ added to the DW. All values are means +/- SE. N = 16-22 animals per group. All mean values are significantly different from controls (p<0.01, ANOVA).
Serum phosphatidylcholine/cholesterol ratio: relationship to serum Mg2+
Table 3 indicates that the serum PC/cholesterol ratio rose, significantly, in the MgD animals, i.e., approximately 33%,whereas feeding animals as little as 15 ppm Mg2+/day ameliorated, greatly, this fall in the serum PC/cholesterol ratio. Although not shown, linear regression analyses demonstrated a correlation between the PC/cholesterol ratio and the serum level of Mg2+ (r=0.58, p<0.05).
Table 3.
Serum phosphatidylcholine/cholesterol (PC/Chol) ratios in normal and MgD rats with and without Mg2+ added to the drinking water
Values are given as means. Asterisks represent values which are significantly different from controls (p<0.01).
Influence of low [Mg2+]0 with and without an SMase inhibitor on content of ceramide, NOS and GSH in primary aortic VSMCs
The results shown in Table 4 demonstrate that treatment of aortic VSMCs with low [Mg2+]0 produced both concentration- and time-dependent increases in the cellular content of ceramide; the lower the [Mg2+]0, the greater the increase in cellular content of ceramide. Likewise, the data shown in Table 4 indicate that the lower the [Mg2+]0, the greater the activation of NOS. Moreover, these experiments show a marked attenuation of the cellular content of glutathione.
Table 4.
Influence of low [Mg2+]O with and without an N- SMase inhibitor on content of ceramide, NOS and glutathione in primary aortic VSMCs
| Group, [Mg2+]o | Ceramide | NOS activity | Total GSH |
|---|---|---|---|
| Time, h | (pmol/10 to the 8th power cells) | (mM/mg protein) | (nM/mg protein) |
| Controls, 1.2mM | 38+/-3.2 | 7.2+/-0.65 | 24+/-1.6 |
| 0.3 mM Mg2+, 18h | 92+/-5.8* | 17.5-/-1.2* | 14/-0.8* |
| 72h | 115+/-7.5* | 24+/-1.8* | 10.2+/-0.6* |
| 0.6mM Mg2+, 18h | 64+/-5.6* | 14.3+/-1.6* | 17.6+/-0.8* |
| With Scy-Mg2+ | |||
| 0.3 mM, 18h | 72 +/-4.6** | 13.2+/-1.6** | 19+/-1.2** |
| 72h | 90+/-5.1** | 19.8+/-1.8** | 16.2+/-0.6** |
| 0.6mM, 18h | 52+/-4.2** | 11+/-1.3** | 21+/- 1.1** |
| 72h | 68+/-4.6** | 14+/-1.4** | 18+/-1.0** |
Values are means +/-SEM. Scy=scyphostatin(75 uM). Single asterisk represents mean values which are significantly different from controls (p<0.05). Double asterisk indicates paired values which are significantly different from experimental values (in 0.3 or 0.6 mM Mg2+) which are significantly from values without scyphostatin.
Treatment of aortic VSMCs (in low [Mg2+]0 with the N-SMase inhibitor resulted in an attenuation in both the measured level of ceramide and activation of NOS (Table 4). Incubation with the N-SMase inhibitor prevented some of the drop in cellular glutathione in the presence of low [Mg2+]0.
Influence of low [Mg2+]0 with and without an SPT inhibitor on de novo synthesis of ceramide and activation of NOS and GSH content
The data shown in Table 5 demonstrate that treatment of VSMCs, exposed to low [Mg2+]0, resulted in concentration-dependent increases in the de novo synthesis of ceramide; the lower the concentration of [Mg2+]0,the greater the increase in the de novo synthesis of ceramide. Treatment of the VSMCs with the SPT inhibitor, myriocin, resulted in an attenuation of the de novo synthesis of ceramide as well as reduction in the activation of NOS; likewise the reduction in glutathione observed in low Mg2+ was attenuated.
Table 5.
Influence of low [Mg2+]o with and without an SPT inhibitor on de novo synthesis of ceramide and activation of NOS and glutathione content
| Group, [Mg2+]o | Ceramide | NOS activity | Total GSH |
|---|---|---|---|
| Time, h | (cpm ×10 to the 3rd power/mg wet wt) | (mM/mg protein) | (nM/mg protein) |
| Controls, 1.2 mM | 0.58+/-0.04 | 6.3+/-0.51 | 22+/-1.4 |
| 0.3 mM Mg2+, 18h | 1.22+/-0.10* | 15.8+/-1.2* | 12+/-0.6* |
| 72h | 1.44+/-0.12 ** | 21+/-1.6** | 9.8+/-0.4** |
| With Myriocin | |||
| 0.3 mM Mg2+,18h | 0.90+/-0.08 *** | 10.8+/-0.8*** | 17+/-0.9*** |
| 72h | 1.12+/-0.10 *** | 16+/-1.2 *** | 12.5+/-0.8*** |
Values are means +/-SEM. Single asterisk represents mean value which are significantly different from controls (p<0.01). Double asterisk represents mean values which are significantly different from controls and in low Mg2+ (p<0.01,ANOVA). Triple asterisk represents mean values which are significantly different from controls and paired values without myriocin(p<0.01).
Discussion
Hypercholesterolemia, hypertrigyceridemia, hypertension, diabetes mellitus, immune injury, end-stage renal disease, renal dialysis, inflammatory-vascular injury, oxidative stress, and prolonged systemic stress are all now accepted risk factors for atherogenesis. No common link has been identified that forms a rational basis for these disorders and atherogenesis. Nor is it clear, how these diverse risk factors lead to ischemic heart disease (IHD) and strokes. Over the past three decades, we and others have provided evidence, both clinical and experimental, to suggest that Mg deficiency may be the common link (for recent review, see 4). Although extra- and intracellular deficits in Mg2+ have been shown to turn-on a number of signaling pathways (Ca2+, protein kinases, tyrosine kinases, mitogen-activated protein kinases [MAPK], MAPK kinases, P-I-3 kinases, proto-oncogenes, nuclear factor N-kB, cytokines, among others) (for recent review, see 4 ), it has not been clear as to how these are linked to the cardiovascular risk factors and atherogenesis. Approximately, 12 years ago, two of us performed experiments on different types of primary vascular muscle cells,in culture, which indicated that reduction in the extracellular level of Mg2+ activated the SM-ceramide pathway and, thus, released sizable quantities of cera-mides as well as altered the cellular levels of other lipid second messengers such as 1,2-diacylglycerol, phosphatidylinositol and PC [2]. Recently, we reported that 21 days of MgD, in rats, resulted in significant reductions in serum SM (with, presumably, release of ceramides) and PC, as reproduced herein, concomitant with lipid peroxidation, fragmentation of DNA, and activation of caspase-3 (and thus apoptosis)as well as activation of SPT and CS [5-7] in cardiac tissues and vascular smooth muscle with reduction of serum free, ionized calcium [5-7].
Ceramides are now thought to play important roles in fundamental processes such as cell proliferation, membrane-receptor functions, oxidative stress, angiogenesis, diverse microcir-culatory functions, immune inflammatory functions, cell adhesion, activation of eNOS, alterations in membrane lipid domains, and programmed cell death, all characteristics found in, and required for, atherogenesis [9,20,57-62]. We confirm, herein, in an intact animal model of short-term MgD, that the fall in serum SM (which would lead to ceramide production) [2,5] is a direct function of the fall in extracellular free Mg ions. We, therefore, conclude that the present work lends further support to our hypothesis, that dietary deficiency of Mg produces an activation of sphingomyelinase (SMase), which would lead to reduction in serum SM reported, herein, thus leading to increased plasma and cellular levels of ceramide.
Our present experiments, employing primary cultured aortic VSMCs, confirm and support recent observations which demonstrate that exposure of these cells to low [Mg2+]0 do, indeed, result in de novo synthesis of ceramide [5]. In addition, as predicted, use of a specific N-SMase inhibitor attenuates the production of ceramide in the VSMCs, thus adding support to our hypothesis that the sphingomyelin-ceramide pathway can be activated by low [Mg2+]0 environments [2,5-7].
We also demonstrate, herein, for the first time that the decreases in the natural, cellular anti-oxidant glutathione, in the four chambers of the heart (as well as vascular smooth muscle) concomitant with rises in ventricular, atrial and vascular muscle eNOS (and nNOS) levels are directly- and highly- correlated to the falls in the level of serum Mg2+ and SM. These findings support the idea that rises in cellular ceramides, most likely, trigger important alterations in cellular signaling molecules [9,33,60], and lend further support to our hypothesis that deficiency in cellular Mg2+ can be an underlying trigger to initiate the above cellular and molecular events by causing activation of N-SMase. With respect to our findings of glutathione depletion in the MgD cardiovascular tissues, and it being related to the level of serum SM, the present results could be used to support previous ideas of others who suggested that SMase activities are intimately associated with glutathione levels [61, 62]. We believe most of the cellular alterations seen here (i.e., fall in glutathione levels, loss of cardiac CK and LDH) are suggestive of oxidative stress and inflammatory-vascular disease processes, factors known to be important in atherogenesis, hypertensive-vascular diseases, and cardiac insufficient states.
Our new findings which demonstrate that short-term Mg deficiency, in intact rats, results in very significant (p<0.001) elevations in expression of n-NOS and e-NOS in all four chambers of the heart as well as in abdominal aortic smooth muscle deserve some comments. These results may aid in explaining, in part, why perfused working rat hearts, obtained from MgD animals [25] and in vitro studies from our laboratory on perfused working rat hearts [63], demonstrate that even short-term MgD results in reductions in a variety of hemodynamic functions (i.e., cardiac output, coronary flows, stroke volume, developed pressures, and cellular high-energy phosphates). NO produced by either neuronal or endothelial cells in the heart is known to be a regulator of cardiac functions (for review, see 64) and to inhibit sympathetic beta-adrenergic activity in the myocardium [65,66], which would result in reductions in cardiac output, coronary flows, stroke volume, and developed pressures seen in the MgD hearts [25,63]. Such a compromise of cardiac hemodynamics could well-form a milieu for activation of N-SMase and increased plasma/cellular levels of ceramides and the programmed cell death which we reported previously in this animal model of short-term MgD [6,7].
The increased expression of the e-NOS and n-NOS in the ventricles and atria of the MgD animals may help to explain the inflammatory lesions noted by Weglicki and co-workers in cardiac muscle of rats exposed to MgD diets [23,25]. The programmed cell death in all chambers of the heart [6],supported by fragmentation of DNA, activation of caspase-3,and the release of cyto c, noted recently [7], as well as the formation of free radicals [6, 24,67] may also aid in explaining the high frequency of heart failure observed in patients medicated with several types of Mg-wasting diuretics [68,69].
Atherosclerosis is now classified as an inflammatory disease (for review, see [70]) that is, primarily, caused by interactions between macrophages, T -lymphocytes, lipoproteins, Ca2, free radicals, and the vascular endothelial and smooth muscle cells in the blood vessel walls. Animals placed on MgD diets exhibit increased numbers of lipid-laden macrophages, increased T-lymphocytes, increased plasma levels of TC, TG and VLDL+ LDL-C, as well as increased plasma levels of Ca2+ (14;unpublished findings], high blood pressure and increased vascular tone [71]. The increased expression of e-NOS and n-NOS, found in the abdominal aortic vascular wall, herein, could help to explain the increased lesions observed in the arterial walls of the MgD animals [14]. NO has been demonstrated in several tissues-organs to induce a number of pathophysiologic changes found in certain immunopathologic syndromes [27, 55, 64, 72].
Hypercholesterolemia has been widely accepted as a high risk factor for development of atherosclerosis and IHD while increased levels of HDL-C are thought to attenuate or prevent these events from occurring [73,74]. Increased blood levels of lipoproteins are thought to eventually lead to endothelial injury or denudation with concomitant uptake of the former molecules (for recent review, see [70]), increased permeability to Ca2+, invasion of the arterial wall by macrophages [70], and phenotypically-altered vascular smooth muscle cells [70]. Increased plasma levels of TG are now also considered risk factors for atherogenesis [75], particularly as elevated TG levels are intimately associated with chylomicrons, VLDL remnant particles, and most importantly with the more dense atherogenic LDL particles [76]. It is, however, not clear as to exactly what factor(s) initiate and sustain the atherogenic process. The sphingolipid- ceramide pathway may be a key factor in atherogenesis [8, 50]. Our findings demonstrate that the alterations in the serum lipoproteins, TC and TG are direct functions of the serum levels of SM and Mg2+. To our knowledge, this is the first time that such a linkage has been shown in any animal model of MgD. The present findings complement our previous work in normal, healthy rabbits which showed that the level of plasma Mg regulates the plasma levels of TC, TG and several lipoproteins [14; unpublished findings]. Our new findings extend the work of Rayssiguier's group who showed that a very short-term (i.e., 8 days), but very severe MgD in weanling, immature rats also raised plasma levels of TC, TG, and several lipoproteins [18,21]. Using a severe model of MgD on a high carbohydrate diet, these investigators reported that the increased levels of TG were associated with TG-rich lipoproteins [17]. Interestingly, they found that the TG-rich lipoprotein fraction isolated from the severely MgD rats demonstrated an increased susceptibility to oxidation compared to control animals [18]. Such results, when viewed in light of our new findings which demonstrate increased expression of e-NOS and n-NOS concomitant with reduced levels of the natural antioxidant, glutathione, and the increased levels NO, together with the recently reported increased levels of the tumor-promoter p53 [5,7] and fragmentation of DNA (indicative of apoptosis) [6] in the vascular walls, could help to explain the increased atherosclerotic lesions and invasion of the arterial walls by lipid-laden macrophages, T-lymphocytes and Ca2+ which we have observed previously in animal models of MgD [14; unpublished results]. This scenario could also help to explain the increased permeability of arterial walls noted in atherogenesis. It is important, here, not to lose sight that the initiating factor is, most likely, the lowering of plasma ionized Mg which activates N-SMase, SPT and CS [5-7] to induce formation of ceramides.
Our new findings reported, herein, which demonstrate that inhibition of N-SMase in primary cultured cells, exposed to low [Mg2+]0, resulted in a marked attenuation of NOS activation are of interest. These new findings suggest that the well-documented observations, since 1987, of activation of NOS by low Mg2+ [32] may be a consequence of de novo synthesis of ceramide, at least in part.
Our observations on cellular depletion of the natural anti-oxidant glutathione (SH) in the presence of low[Mg2+]0 are of interest, particularly as the loss of this molecule is to, some extent, prevented by inhibition of N-SMase-hydrolysis of sphingomyelin(thus formation of ceramide) and prevented by inhibition of formation of de novo synthesis of ceramide (Table 5). If these results can be confirmed in the intact tissues-organs of the cardiovascular system, our new findings could point the way towards new avenues for therapeutic approaches to the possible treatment of numerous cardiovascular disorders.
In view of the present findings, and those reported recently on S-T Mg deficiency [5-7], it will be important to determine if the molecular-biochemical changes noted in the cardiovascular tissues from MgD animals are associated with physiologic-functional alterations in cardiac hemodynamics, blood pressure, and microcirculatory hemodynamics in vivo. We believe our findings merit such further studies into the roles of the sphingomyelin- ceramide pathway in MgD states and cardiovascular regulation.
More than 50 years ago, it was suggested in an epidemiologic study that where water hardness was elevated, the rate of death from cardiovascular diseases decreased [77]. In the intervening years to the present time, this has been borne out by numerous studies [for reviews, see 15,42,45]; that is, cardiovascular death rates are lower in hard-water areas than in soft-water areas. Although the hardness of water is due, primarily, to the concentrations of Ca and/or Mg, the overwhelming evidence, to date, supports the notion that it is the Mg content which is responsible for most of the protective effects of hard water [15,39,45]. Although it has been hypothesized on the basis of several epidemiologic human studies, from around the globe, that as little as 6-30 ppm Mg (or 6-30 mg/L ) in the drinking water should be cardioprotective and ameliorate vascular changes [6,15,39], the present study and our previous reports [5-7], are the very first ones to demonstrate that as little as 15 mg/L of water-borne, bioavailable Mg can either prevent or ameliorate early cardiovascular organ and tissue changes (indicated, herein, by rises in creatine kinase and LDH)induced by a short-term Mg-deficiency state in a well-controlled experimental animal model. This low concentration of water-borne Mg2+was effective in ameliorating the loss of CK, LDH, and glutathione in ventricular, atrial, and vascular smooth muscle as well as ameliorating the degree of activation of e-NOS and n-NOS in the cardiovascular tissues of the MgD animals. In addition, we clearly demonstrate that this low concentration of Mg2+ was quite effective in preventing the rises in serum levels of TC, TG and VLDL+LDL-C and the reduction in serum HDL-C and the fall in the PC/cholesterol ratio observed in the MgD animals.
Lastly, the present studies could be used to support the hypothesis, suggested more than 25 years ago,that water intake (i.e., from tap water, bottled waters, and beverages using tap water) in humans varying between 1 and 2 L/day, with Mg2+ intakes varying from 5 mg to higher than 100 mg/day, may represent an excellent way to overcome and control the marginal intakes of Mg obtained with many Western diets [4,15,20,37,39]. In addition, in view of the present results and those shown previously [5-7], it is probably propitious to suggest that all desalinated-purified waters, recovered/recycled waters, harvested rainwaters, tap waters, and all bottled waters (and probably bottled beverages) given to humans should be supplemented with bioavailable Mg2+ in order to ameliorate/prevent the induction of cardiovascular risk factors and cardiovascular disease processes worldwide.
Acknowledgments
We appreciate the gratis supply of magnesium aspartate-HCl that was provided to us by Dr.Angela Weigert of Verla Pharm (Tutzing, Germany). We appreciate the technical assistance of Gatha Shah. This study was supported, in part, by a research grant from Regalware Worldwide (To B.M. Altura) and NIH Research Grants to X-C. Jiang and M.M.Hussain
References
- 1.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane lipids in vascular smooth muscle: a link to atherogenesis. FEBS Lett. 1997;408:191–194. doi: 10.1016/s0014-5793(97)00420-1. [DOI] [PubMed] [Google Scholar]
- 2.Morrill GA, Gupta RK, Kostellow AB, Ma GY, Zhang A, Altura BT, Altura BM. Mg2+ modulates membrane sphingolipids and lipid second messenger levels in vascular smooth muscle cells. FEBS Lett. 1998;440:167–171. doi: 10.1016/s0014-5793(98)01446-x. [DOI] [PubMed] [Google Scholar]
- 3.Altura BM, Kostellow AB, Zhang A, Li W, Morrill GA, Gupta RK, Altura BT. Expression of nuclear factor-kB and the proto-oncogenes c-fos and c-jun are induced by low extra cellular Mg2+in aortic and cerebral vascular smooth muscle: possible links to hyperten sion, atherogenesis and stroke. Am J Hypertens. 2003:701–707. doi: 10.1016/s0895-7061(03)00987-7. [DOI] [PubMed] [Google Scholar]
- 4.Altura BM, Altura BT. Magnesium: forgotten mineral in cardiovascular biology and atherogenesis. In: Nishizawa N, Morii H, Durlach J, editors. New Perspectives in Magnesium Research. 2007. pp. 239–260. [Google Scholar]
- 5.Altura BM, Shah NC, Li Z, Jiang X-C, Zhang A, Li W, Zheng T, Perez-Albela JL, Altura BT. Short-term magnesium deficiency upregulates sphingomyelin synthase and p53 in card-iovascular tissues and cells: relevance to de novo synthesis of ceramide. Am J Physiol Heart Circ Physiol. 2010;299:H2046–2055. doi: 10.1152/ajpheart.00671.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altura BM, Shah NC, Jiang X-C, Li Z, Perez-Albela JL, Sica AC, Altura BT. Short-term magnesium deficiency results in decreased levels of serum sphingomyelin, lipid peroxdation, and apoptosis in cardiovascular tissues. Am J Physiol Heart and Circ Physiol. 2009;297:H86–H92. doi: 10.1152/ajpheart.01154.2008. [DOI] [PubMed] [Google Scholar]
- 7.Altura BM, Shah NC, Li Z, Jiang X-C, Perez-Albela JL, Altura BT. Magnesium deficiency upregulates serine palmitoyltransferase (SPT 1 and SPT 2) in cardiovascular tissues: relationship to serum ionized Mg and cytochromec. Am J Physiol Heart and Circ Physiol. 2010;299:H932–H938. doi: 10.1152/ajpheart.01076.2009. [DOI] [PubMed] [Google Scholar]
- 8.Futerman AH, editor. New York: Kluwer Academic Pub; 2002. Ceramide Signaling. [Google Scholar]
- 9.Gulbins E, Li PL. Physiological and pathophysiological aspects of ceramide. Am J Physiol Reg Integr Comp Physiol. 2006;290:R11–R26. doi: 10.1152/ajpregu.00416.2005. [DOI] [PubMed] [Google Scholar]
- 10.Li JF, Li W, Altura BT, Altura BM. Peroxynitrite induces apoptosis and decline of intracellular free Mg with concomitant elevation in [Ca2+]I in rat aortic smooth muscle cells: possible roles of extracellular and intracellular magnesium ions in peroxynitrite induced cell death. Drug Metab Lett. 2007;1:85–89. doi: 10.2174/187231207780363651. [DOI] [PubMed] [Google Scholar]
- 11.Kuksis A, Roberts A, Thompson JS, Myher JJ, Geher K. Plasma phosphatidylcholine free cholesterol ratio as an indicator for atherosclerosis. Arteriosclerosis. 1983;3:389–397. doi: 10.1161/01.atv.3.4.389. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan LA, Pesce AJ, Kazmierczak SC. Theory, Analysis and Correlation. 4th Ed. St. Louis: Mosby; 2003. Clinical Chemistry. [Google Scholar]
- 13.Altura BM. Magnesium and regulation of contractility of vascular smooth muscle. Advances in Microcirculation. 1982;11:77–113. [Google Scholar]
- 14.Altura BT, Brust M, Barbour RL, Stempak J, Bloom S, Altura BM. Magnesium dietary intake modulates blood lipid levels and atherogenesis. Proc Natl Acad Sci USA. 1990;87:1840–1844. doi: 10.1073/pnas.87.5.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durlach J. London: John Libbey; 1998. Magnesium in Clinical Practice. [Google Scholar]
- 16.Djurhuus S, Henriksen JE, Klitgaard NAH, Blaaberg O, Thye-Ron P, Altura BM, Altura BT, Beck-Neilsen H. Effect of moderate improvement in metabolic control on magnesium and lipid concentrations in patients with type 1 diabetes. Diabetes Care. 1999;22:546–554. doi: 10.2337/diacare.22.4.546. [DOI] [PubMed] [Google Scholar]
- 17.Rayssiguier Y, Gueux E, Bussiere L, Durlach J, Mazur A. Effect of magnesium deficiency on lipid metabolism in rats fed a high carbohydrate diet. J Nutr. 1981;111:1876–1883. doi: 10.1093/jn/111.11.1876. [DOI] [PubMed] [Google Scholar]
- 18.Rayssiguier Y, Mazur A, Cardot P, Gueux E. Effects of magnesium on lipid meta-bolism and cardiovascular disease. In: Itokawa Y, Durlach J, editors. Magnesium in Health and Disease. London: John Libbey; 1989. pp. 199–207. [Google Scholar]
- 19.Singh RB, Niaz MA, Mashiri M, Zheng G. Magnesium status and risk of coronary artery disease in rural and urban populations with variable magnesium consumption Magnes Res. 1997;1:205–213. [PubMed] [Google Scholar]
- 20.Altura BM, Altura BT. Magnesium and cardiovascular biology: an important link between cardiovascular risk factors and atherogenesis. Cell Mol Biol Res. 1995;41:347–359. [PubMed] [Google Scholar]
- 21.Rayssiguier Y, Gueux E, Bussiere L, Durlach J, Mazur A. Dietary magnesium affects susceptibility of lipoproteins to peroxidation in rats. J Am Coll Nutr. 1993;12:133–137. doi: 10.1080/07315724.1993.10718293. [DOI] [PubMed] [Google Scholar]
- 22.Luthringer C, Rayssiguier Y, Gueux E, Berthelot A. Effect of moderate magnesium deficiency on serum lipids, blood pressure and cardiovascular reactivity in normotensive rats. Br J Nutr. 1988;59:243–250. doi: 10.1079/bjn19880031. [DOI] [PubMed] [Google Scholar]
- 23.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JL, Hasani M, Volkova E, Kazmi K, Yusuf S. Lipids, lipoproteins and apoplipoproteins as risk markers of myocardial infarction in 52 countries(The INTERHEART Study): a case—control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 24.Wu F, Altura BT, Gao J, Barbour RL, Altura BM. Ferrylmyoglobin formation induced by acute magnesium deficiency in perfused rat heart causes cardiac failure. Biochim Biophys Acta. 1994;1225:158–164. doi: 10.1016/0925-4439(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 25.Weglicki WB, Mak IT, Dickens BF, Stafford RE, Komarov AM, Gibson B, Cassidy MM, Phillips TM, Kramer JH. Neuropeptides, free radical stress and antioxidants in models of Mgdeficient cardiomyopathy. In: Theophanides T, Anastassopolou J, editors. Magnesium: Current Status and New Developments. London: Kluwer Academic; 1997. pp. 169–178. [Google Scholar]
- 26.Bussiere FI, Gueux E, Rock E, Girardeau J-P, Tridon A, Mazur A, Rayssiguier Y. Increased phagocytosis and production of reactive oxygen species by neutrophils during magnesium deficiency in rats and inhibition by high magnesium concentration. Br J Nutr. 2002;87:107–113. doi: 10.1079/BJN2001498. [DOI] [PubMed] [Google Scholar]
- 27.Maier J, Malpuech-Brugere C, Zimowska W, Rayssiguier Y, Mazur A. Low magnesium promotes endothelial cell dysfunction : implications for atherosclerosis, inflammation and thrombosis. Biochim Biophys Acta. 2004;1689:13–21. doi: 10.1016/j.bbadis.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 28.Ghibelli L, Coppola S, Fanelli C, Rotillo G, Civitareale P, Scovassi AI, Ciriolo MR. Glutathione depletion causes cytochrome c release even in the absence of cell commitment to apoptosis. FASEB J. 1999;13:2031–2036. doi: 10.1096/fasebj.13.14.2031. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM, Knox SJ. Role of glutathione and reactive species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differen. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- 30.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature(London) 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 31.Chand N, Altura BM. Acetylcholine and bradykinin relax intrapulmonary arteries by acting on endothelial cells: role in lung diseases. Science. 1981;213:1367–1369. doi: 10.1126/science.7268440. [DOI] [PubMed] [Google Scholar]
- 32.Altura BT, Altura BM. Endotheliimdependent relaxation in coronary arteries requires magnesium ions. Br J Pharmacol. 1987;91:449–451. doi: 10.1111/j.1476-5381.1987.tb11235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chikani G, Zhu W, Smart EJ. Lipids: potential regulators of nitric oxide generation. Am J Physiol Endocrine Metab. 2004;287:E386–E389. doi: 10.1152/ajpendo.00106.2004. [DOI] [PubMed] [Google Scholar]
- 34.Barsacchi R, Perotta C, Sestili P, Cantoni O, Moncada S, Clementi E. Cyclic GMP-dependent inhibition of acid sphingomyelinase by nitric oxide: an early step in protection against apoptosis. Cell Death Differen. 2002;9:252–263. doi: 10.1038/sj.cdd.4401095. [DOI] [PubMed] [Google Scholar]
- 35.Seelig MS, Rosanoff A. New York: Penguin Group Inc; 2003. The Magnesium Factor. [Google Scholar]
- 36.Moshfegh A, Goldman J, Ahuja J, Rhodes D, LaComb R. U.S. Department of Agriculture, Agricultural Research Service; 2009. What We Eat in America. NHANES 2005-2006: Usual Intakes from Food and Water Compared to 1997 Dietary Reference Intakes for Vitamin D, Calcium, Phosphorus, and Magnesium. [Google Scholar]
- 37.Durlach J, Bara M, Guiet-Bara A. Magnesium level in drinking water and cardiovascular risk factor: a hypothesis. Magnesium. 1985;4:5–15. [PubMed] [Google Scholar]
- 38.Eisenberg MJ. Magnesium deficiency and sudden death. Am Heart J. 1992;124:544–549. doi: 10.1016/0002-8703(92)90633-7. [DOI] [PubMed] [Google Scholar]
- 39.Marier J, Neri LC. Quantifying the role of magnesium in the interrelationship between human mortality/morbidity and water hardness. Magnesium. 1985;4:53–59. [PubMed] [Google Scholar]
- 40.Leary WP. Content of magnesium in drinking water and deaths from ischemic heart disease in White South Africans. Magnesium. 1986;5:150–153. [PubMed] [Google Scholar]
- 41.Schroeder HA. Relations between hardness of drinking water and deaths from certain chronic and degenerative diseases in the United States. J Chronic Dis. 1960;12:586–591. doi: 10.1016/0021-9681(60)90002-3. [DOI] [PubMed] [Google Scholar]
- 42.Ford ES, Mokdad AH. Dietary magnesium intake in a national sample of US adults. J Nutr. 2003;133:2879–2882. doi: 10.1093/jn/133.9.2879. [DOI] [PubMed] [Google Scholar]
- 43.Chipperfield B, Chipperfield JR. Relation of myocardial metal concentration to water hardness and death from ischemic heart disease. Lancet. 2:709–712. doi: 10.1016/s0140-6736(79)90641-x. [DOI] [PubMed] [Google Scholar]
- 44.Marx A, Neutra RR. Magnesium in drinking water and deaths from ischemic heart disease. Epidemiol Rev. 1997;19:258–272. doi: 10.1093/oxfordjournals.epirev.a017957. [DOI] [PubMed] [Google Scholar]
- 45.Rubenowitz E, Molin I, Axelsson G, Rylander R. Magnesium in drinking water in relation to morbidity and mortality from acute myocardial infarction. Epidemiol. 2003;11:416–421. doi: 10.1097/00001648-200007000-00009. [DOI] [PubMed] [Google Scholar]
- 46.Turlapty PDMV, Altura BM. Magnesium deficiency produces spasms of coronary arteries: relationship to etiology of sudden death ischemic heart disease. Science. 1980:198–200. doi: 10.1126/science.7361117. [DOI] [PubMed] [Google Scholar]
- 47.Altura BT, Altura BM. Measurement of ionized magnesium in whole blood, plasma and serum with a new ion-selective electrode in healthy and diseased human subjects. Magnes Trace Elem. 1991;10:90–98. [PubMed] [Google Scholar]
- 48.Altura BT, Shirey TL, Young CC, Dell'Orfano K, Hiti J, Welsh R, Yeh Q, Barbour RL, Altura BM. Characterization of a new ion selective electrode for ionized magnesium In whole blood, plasma, serum and aqueous samples. Scand J Clin Lab Invest. 1994;54(Suppl 217):21–36. doi: 10.3109/00365519409095208. [DOI] [PubMed] [Google Scholar]
- 49.Seigler L, Wu WT. Separation of serum high-density lipoprotein for cholesterol determination: ultracentrifugation vs precipitation wth sodium phosphotungstate and magnesium chloride. Clin Chem. 1981;27:838–841. [PubMed] [Google Scholar]
- 50.Jiang XC, Pautre F, Pearson TA, Reed RG, Francis CK, Lin Berglund L, Tall AR. Plasma Sphingomyelin level as a risk factor for coronary artery disease. Arterioscler Thromb Vasc Biol. 2000;20:2614–2818. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 51.Moss DW, Henderson AR. Enzymes. In: Burtis CA, Ashwood AR, editors. Tietz Textbook of Clinical Chemistry. second ed. Philadelphia: Saunders; 1994. pp. 735–896. [Google Scholar]
- 52.Cotgreave IA, Moldeus P. Methodologies for the application of monobromobimane to the simultaneous analysis of soluble proteinthiol compounds of biological systems. J Biochem Biophys Methods. 1986;13:231–249. doi: 10.1016/0165-022x(86)90102-8. [DOI] [PubMed] [Google Scholar]
- 53.Smith FS, Titheradge MA. Methods in Molecular Biology, vol. 100: Nitric Oxide Protocols. New York: Springer; 1997. Detection of NOS isoforms by Westernblot analysis; pp. 171–180. [DOI] [PubMed] [Google Scholar]
- 54.Zhang A, Cheng TPO, Altura BM. Magnesium regulates intracellular free ionized calcium concentration and cell geometry in vascular smooth muscle cells. Biochim Biophys Acta. 1992;1134:25–29. doi: 10.1016/0167-4889(92)90024-6. [DOI] [PubMed] [Google Scholar]
- 55.Yang ZW, Gebrewold A, Nowakowski M, Altura BT, Altura BM. Mg2+ -induced endo-thelium-dependent relaxation of blood vessels and blood pressure lowering role of NO. Am J Physiol Regulatory Comp Physiol. 2000;278:R628–R639. doi: 10.1152/ajpregu.2000.278.3.R628. [DOI] [PubMed] [Google Scholar]
- 56.Kolesnick R. Signal transduction through the sphingomyelin pathway. Mol Chem Neuropathol. 1994;21:287–297. doi: 10.1007/BF02815356. [DOI] [PubMed] [Google Scholar]
- 57.Hannun YA. Functions of ceramide in coordinating responses to stress. Science. 1996;274:1855–1859. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 58.Quillet-Quintans J, Kilkus J, McShan CI, Gottschalk AR, Dawson G. Ceramide mediates the apoptotic response of WEHI 231 cells to anti-immunoglobulin, corticosteroids and irradiation. Biochim Biophys Res Comm. 1994;202:710–714. doi: 10.1006/bbrc.1994.1988. [DOI] [PubMed] [Google Scholar]
- 59.Vandrager AB, Houweling M. Effect of ceramide on phospholipid biosynthesis and its implications for apoptosis. In: Quinn PJ, Kagan VE, editors. Phospholipid Metabolism in Apoptosis. Kluwer Academic; 2002. pp. 207–227. [DOI] [PubMed] [Google Scholar]
- 60.Wyman MP, Schneiter R. Lipid signaling in disease. Nat Rev Mol Cell Biol. 2008;9:162–176. doi: 10.1038/nrm2335. [DOI] [PubMed] [Google Scholar]
- 61.Altura BM, Gebrewold A, Zheng T, Altura BT. Sphingomyelinase and ceramide analogs induce vasoconstriction and leukocyteendothelial interactions in cerebral venules in the intact brainsight into mechanisms and possible relation to brain injury. Brain Res Bull. 2002;58:273–278. doi: 10.1016/s0361-9230(02)00772-4. [DOI] [PubMed] [Google Scholar]
- 62.Zheng T, Li W, Wang J, Altura BT, Altura BM. Sphingomyelinase and ceramide analogs induce contraction and rises in [Ca2+]I in canine cerebral vascular muscle. Am J Physiol Heart and Circ Physiol. 2000;278:H1421–H1428. doi: 10.1152/ajpheart.2000.278.5.H1421. [DOI] [PubMed] [Google Scholar]
- 63.Altura BM, Barbour RL, Dowd TL, Wu F, Altura BT, Gupta Low extracellular magnesum induces intracellular Mg deficits, depletion of high-energy phosphates and cardiac failure in intact working rat hearts: a 31P-NMR study. Biochim Biophys Acta. 1993;1182:329–332. doi: 10.1016/0925-4439(93)90077-e. [DOI] [PubMed] [Google Scholar]
- 64.Casadei B. The emerging role of neuronal nitric oxide synthase in the regulation of myocardial function. Exp Physiol. 2006;91:943–955. doi: 10.1113/expphysiol.2006.035493. [DOI] [PubMed] [Google Scholar]
- 65.Shah A, MacCarthy PA. Paracrine and autocrine effects of nitric oxide on myocardial function. Pharmacol Rev. 2000;86:45–86. doi: 10.1016/s0163-7258(99)00072-8. [DOI] [PubMed] [Google Scholar]
- 66.Paton JF, Kasparov S, Paterson DJ. Nitric oxide and autonomic control of heart rate: a question of specificity. Trends Neurosci. 2002;25:626–631. doi: 10.1016/s0166-2236(02)02261-0. [DOI] [PubMed] [Google Scholar]
- 67.Tejero-Taido MI, Chmielinska JJ, Weglicki WB. Chronic dietary Mg2+ deficiency induces cardiac apoptosis in the rat heart. Magnes Res. 2007;20:208–212. [PubMed] [Google Scholar]
- 68.Douban S, Brodsky MA, Whang DD, Whang R. Significance of magnesium in heart failure. Am Heart J. 1996;132:664–671. doi: 10.1016/s0002-8703(96)90253-7. [DOI] [PubMed] [Google Scholar]
- 69.Lassere B. Magnesium and heart failure: an update. Where do we stand? In: Smetana R, editor. Advances in Magnesium Research. 1. Cardiology. London: John Libbey; 1997. pp. 12–20. [Google Scholar]
- 70.Gotlieb AI. Blood vessels. In: Rubin R, Strayer DS, editors. Rubin's Pathology: Clinicopathologic Foundations of Medicine. Philadelphia: Walters Kluwer/Lippincott Williams and Wilkins; 2009. pp. 387–426. [Google Scholar]
- 71.Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. Magnesium deficiency and hypertension: a correlation between magnesium deficient diets and microcirculatory changes in situ. Science. 1984;223:1315–1317. doi: 10.1126/science.6701524. [DOI] [PubMed] [Google Scholar]
- 72.Kauser K, Da Cunha V, Fitch R, Mallari C, Rubanyi GM. Role of endogenous nitric oxide in progression of atherosclerosis in apoprotein E-deficient mice. Am J Physiol Heart and Circ Physiol. 2000;278:H1679–H1685. doi: 10.1152/ajpheart.2000.278.5.H1679. [DOI] [PubMed] [Google Scholar]
- 73.Brown MS, Goldstein JL. A receptormediated pathway for cholesterol homeostasis. Science. 1986;232:34. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- 74.Lancet Prospective Studies Collaboration. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 75.Tirosh A, Rudich A, Shochat T, Tekes-Manova D, Isrraeli E, Henkin Y, Kochba I, Shai I. Changes in triglyceride levels and risk for coronary heart disease in young men. Ann Inter Med. 2007;147:377–385. doi: 10.7326/0003-4819-147-6-200709180-00007. [DOI] [PubMed] [Google Scholar]
- 76.Geurian K, Pinson JB, Weart CW. The triglyceride connection in atherosclerosis. Ann Pharmacother. 1992;26:1109–1117. doi: 10.1177/106002809202600913. [DOI] [PubMed] [Google Scholar]
- 77.Kobayashi J. On geographical relationship between the chemical nature of river water and death from apoplexy. Berichte des Ohara Inst fur Landwirtsch Biol. 1957;11:12–21. [Google Scholar]
- 78.Liu B, Hannun YA. Inhibition of the neutral magnesium dependent sphingomyelinase by glutathione. J Biol Chem. 1997;272:16281–16287. doi: 10.1074/jbc.272.26.16281. [DOI] [PubMed] [Google Scholar]