Abstract
17α-Ethynyl-androst-5ene-3β, 7β, 17β-triol (HE3286) is an orally bioavailable analogue of androst-5-ene-3β,7β,17β-triol, a non-glucocorticoid anti-inflammatory metabolite of the adrenal steroid, dehydroepiandrosterone. The pharmacology of HE3286 was characterized in preparation for clinical trials in type 2 diabetes mellitus and other diseases of inflammation. Interactions with nuclear hormone receptors and P450 enzymes were measured in vitro. Drug metabolism was studied preclinically in mice, rats, dogs, and monkeys. Neurological and cardiopulmonary safety and dose-ranging and chronic toxicity studies were conducted in rats and dogs in accordance with FDA guidelines. Pharmacokinetics and metabolites were measured in Phase I clinical trials. HE3286 was differentially metabolized between species. HE3286 and metabolites did not bind or transactivate steroid binding nuclear hormone receptors or inhibit P450 enzymes. There were no adverse effects in safety pharmacology and canine toxicology studies. Although HE3286 did not elicit systemic toxicity in rats, mild estrogenic effects were observed, but without apparent association to hormonal changes. Safety margins were greater than 20-fold in rats and dogs with respect to the most commonly used clinical dose of 10 mg/day. The terminal half-life in humans was 8 hours in males and 5.5 hours in females. HE3286 is the first derivative of the DHEA metabolome to undergo a comprehensive pharmacological and safety evaluation. The results of these investigations have shown that HE3286 has a low potential for toxicity and possesses pharmacological properties generally suitable for use in human medicine. The favorable profile of HE3286 warrants further exploration of this new class of anti-inflammatory agents.
Keywords: Toxicology, metabolism, pharmacokinetics, pharmacology, androstene, HE3286
Introduction
Inflammation in the context of a protective immune response is a vital function of homeosta-sis, but many diseases are either potentiated by or have chronic inflammation as a major component of their pathophysiology [1-8]. The prevalence of inflammatory diseases speaks to both the importance of their management in health care as well as to the difficulty in identifying suitable anti-inflammatory treatments. The current arsenal of anti-inflammatory pharmaceuti-cals includes cyclo-oxygenase inhibitors, which prevent the formation of pro-inflammatory ara-chadonic acid metabolites; glucocorticoids, which engage the cognate nuclear hormone receptor initiating a complex sequence of events that ultimately result in the inhibition of inflammatory cytokine expression, and the new generation of biological agents that antagonize various inflammatory signal transduction pathways. Although these agents are generally regarded as effective, they all have significant limitations, which variously include anergy to prolonged treatment, immune suppression, bone loss, gastrointestinal bleeding, and high cost [8-11].
For many years now the scientific literature has indicated new prospects for anti-inflammatory agents may come from the dehydroepiandrosterone (DHEA) metabolome. DHEA and its sul-fate conjugate are major products of the human adrenal gland, but neither is present in rodent circulation in significant amounts. Exogenous DHEA exhibits striking anti-inflammatory activity in rodents [12], but not in humans. Several publications indicate that a significant portion of the anti-inflammatory activity of DHEA might be attributed to its poly-hydroxylated metabolites [13-17], which are readily formed only in rodents [18, 19]. Other likely factors contributing to DHEA's poor performance in humans are low oral bioavailability [20], metabolism into the sex steroid pathways [21], and secondary metabolism (sulfation) [22-24]. Although DHEA has been widely studied, there are few reports of nonclinical toxicity in the literature other than references to peroxisome proliferation.
Androst-5-ene-3β, 7β, 17β-triol (βAET), a metabolite of DHEA, has immune regulating and anti-inflammatory activity [13, 16, 17]. We have observed βAET to be similarly non-toxic, although C-7 hydroxylation abolishes peroxisome proliferator effects [25], and unlike DHEA, βAET is not metabolized into sex hormones [26]. However βAET, like other natural androstenes, has poor oral bioavailability (Ahlem, unpublished) [27], and the 17β-hydroxyl function is susceptible to inactivation by ubiquitous oxidative forms of 17β-hydroxysteroid dehydrogenase (17β-HSD) [28-30], reducing βAET's pharmaceutical utility. C-7 hydroxylated products may also inhibit 11β-hydroxysteroid dehydrogenase mediated inter-conversion of cortisone and cortisol, contributing to glucocorticoid opposing effects [31, 32].
17α-Ethynyl-androst-5-ene-3β, 7β, 17β-triol (HE3286) is a synthetic analogue of β-AET. 17β-Ethynylation prevents oxidation of the 17β-hydroxyl function by 17β-HSD and confers significant oral bioavailability. Considering the unpredictable effects of 17β substitution on sex steroid interactions with nuclear receptors, al-kynylation, fortuitously, does not perturb the anti-inflammatory activity of HE3286. HE3286 does not bind any of the steroid-binding nuclear hormone receptors (thus far tested, which excludes primarily the pregnane X and constitutive androstane receptors)[33], but is active in rodent models of type 2 diabetes mellitus [33, 34] and inflammatory disease [15, 35-39], independent of estrogen receptor ligation as indicated by activity in the presence of the estrogen antagonist, ICI182,170 [36]. Because of its broad spectrum of immune modulating activity, HE3286 and was selected for safety testing associated with pharmaceutical development prior to clinical trials. Our safety and pharmacokinetic evaluations found HE3286 to have a low potential for direct or indirect toxicity, and although extensively metabolized, to have suitable pharmacokinetics for clinical development.
Materials and methods
Reagents and chemicals
HE3286, 17α-ethynyl-5-androstene-3β,7β,17β-triol (C21H30O3, MW=330.46), and metabolites HE3291 (17α-ethynyl-5-androstene-3β,7α,17β-triol), HE3393 (17α-ethynyl-7-keto-5-androstene -3β,17β-diol), HE3545 (17α-ethynyl-5-androstene-3α,7β,17β-triol), HE3553 (17α-ethynyl-5-androstene-3β,7β,16α,17β-tetrol), HE3633 (17α-ethynyl-3-keto-5-androst-4,6-dien-17β-ol) were synthesized by Harbor BioSciences, Inc. All other steroids were purchased from Steraloids (Newport, RI). Recombinant rat CYPC19 (aromatase) was purchased from (BD Bioscience, San Jose, CA). Rosiglitazone and [4-[3-(4-acetyl-3-hydroxy-2-propylphenoxy)propoxy] phenoxy]acetic acid (L165041) were purchased from Tocris Bioscience (Ellisville, MO). Cyclodextrin formulations of HE3286 were prepared with 30% suflobutlyether-b-cyclodextrin (Cydex, Lenexa, KS). Except as noted, all other reagents and chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
In vitro biological systems
Rat and human liver microsomes and cryopre-served human hepatocytes and hepatocyte growth medium were purchased from In Vitro Technologies, Baltimore, MD. Cell line MDA-kb2 (American Type Culture Collection CRL-2713) harboring a mouse mammary tumor virus/ luciferase cassette and cell line T47D-kBluc (American Type Culture Collection 2865) stably transfected with a synthetic plasmid containing three copies of an estrogen response element fused upstream of a luciferase gene were used for nuclear hormone receptor transactivation assays.
Bioanalytical
HE3286 and metabolites in plasma or serum were identified by GC-MS, GC-MS/MS or LC-MS/MS and/or quantified by LC-MS/MS as indicated in the descriptions of specific experiments. GC-MS or GC-MS/MS was performed on samples extracted with 5 mL MTBE, and dried under nitrogen. The dried residue was derivatized with N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) and analyzed on a Varian CP-Sil 5 CB column with a Varian CP-3800 gas chromatograph and Varian 1200L mass spectrometer (Varian, Palo Alto, CA) in either full scan (150 to 800 amu) or MS/MS mode. LC-MS/MS was performed with either a Synergi 4μ POLAR-RP 80 column (150x2 mm, Phenomonex, Torrance CA), and a Varian 1200 LC-MS/MS spectrometer in ES+ mode (Varian, Palo Alto, CA), or samples were analyzed on an Xterra C18 column (2.1×250 mm, 5mm, Waters, Beverly, MA), eluted with a mobile phase gradient of 30-85% acetonitrile in water, with 0.1% formic acid, with the column temperature maintained at 40 °C. Analytes were detected with a tandem quadrupole mass spectrometer in ES+ mode (Waters, Beverly, MA).
Estradiol and estrone were measured as dansylated derivatives: plasma samples with 2H5-estradiol internal standard (C/D/N Isotopes, Inc., Pointe-Claire, Quebec, Canada) were extracted with MTBE, dried, dissolved in 1 mg/mL dansyl chloride in 1 M aqueous sodium bicarbonate, and incubated for 1 hour at 60°C. The resulting dansylated estradiol and estrone derivatives were extracted with MTBE, dried, and reconstituted in 200μL of water/acetonitrile (75:25, v:v), and analyzed by LC/MS-MS with the Waters instrument. DHEA, testosterone, androstenedione, and progesterone were quantified as oxime derivatives: plasma samples with 2H5-testosterone (C/D/N Isotopes, Inc., Pointe-Claire, Quebec, Canada) internal standard were extracted and dried as above, then dissolved in 1 M hydroxylamine hydrochloride and incubated at 60"C for 1 hr. The resulting steroid-oxime derivatives were extracted with MTBE and analyzed by LC/MS-MS with the Waters instrument.
Experimental design
All experimental procedures in animals were conducted with the approval of institutional animal care and use committees (IACUC), and in accordance with local, state, and federal animal welfare regulations.
Interspecies comparative metabolism (in vivo)
Metabolites were characterized in male CD-1 mice following oral, subcutaneous, and intraperitoneal administration. Plasma samples were collected under CO2 anesthesia by cardiac puncture near the time of maximum HE3286 concentration (0.25 hr), and stored frozen until derivatized with N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) and analyzed by GC-MS/MS. Steroid conjugates were isolated by solid phase extraction and hydrolyzed by sequential treatment with glucuronidase and sul-fatase and analyzed by GC-MS/MS. Plasma metabolites (free steroids) were also characterized by LC/MS-MS in humans, dogs, Sprague-Dawley rats [Crl:CD® (SD)], and CD-1 mice (male only) following oral administration of HE3286.
Nuclear hormone receptor binding and transactivation
Nuclear hormone receptor interactions for HE3286 metabolites were measured as previously described for HE3286 [33]. Assessment of nuclear hormone receptor binding for the androgen receptor (AR), estrogen receptor alpha (ERα), estrogen receptor beta (ERβ), and the glucocorticoid receptor (GR) was performed with the PolarScreen fluorescence polarization system (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. Transactivation assays for AR and GR and ERα and ERβ were performed in cell lines MDA-kb2 and T47D-kBluc respectively. Human mineralocorticoid receptor (MR) and peroxisome proliferator-activated receptor (PPAR-γ and PPAR-δ) transactivation were measured with the Gene Blazer® β-lactamase assay system (Invitrogen, Carlsbad, CA) following the manufacturer's instructions.
P450 inhibition and identification of metabolizing enzyme studies
HE3286 reactions with human microsomal enzymes CYP1A2, 2C9, 2C19, 2D6, and 3A4 used probe substrates and assays conforming to FDA guidance for drug interaction studies. Metabolizing enzymes were identified by a decreased rate of HE3286 metabolism in the presence of an inhibitor compared to a control reaction using LC-MS/MS to measure the concentration of enzyme reaction products.
In vitro induction of CYP1A2 and CYP3A4 by HE3286
Human hepatocytes were cultured with HE3286 to measure the potential for HE3286 to induce the two major inducible P450 enzymes, CYP1A2 and CYP3A4. Pooled cryopreserved human hepatocytes and hepatocyte growth medium were prepared for P450 induction studies for CYP1A2 and CYP3A4 according to the manufacturer's instructions using 50 μM omeprazole for CYP1A2 positive control; 25μM rifampicin for CYP3A4 positive control, and HE3286 (1, 10, and 100 mM).
Interactions with CYPC19 (aromatase)
Inhibition of CYPC19 (aromatase) was measured in vitro with recombinant rat enzyme in 1 mL of phosphate-buffered saline containing 3.3 mM magnesium chloride, 1 mM NADPH, and 100 ng/mL (approximately 300 nM) of the test compound, HE3286 (17α-ethynyl-5-androstene-3β,7β,17β-triol), HE3291 (17α-ethynyl-5-androstene-3β,7α,17β-triol), HE3393 (17α-ethynyl-7-keto-5-androstene-3β,17β-diol), HE3545 (17α-ethynyl-5-androstene-3α,7β,17β-triol), HE3553 (17α-ethynyl-5-androstene-3β,7β,16α,17β-tetrol), HE3633 (17α-ethynyl-3-keto-5-androst-4,6-dien-17β-ol), or testosterone (positive control), and 5 pmol recombinant human aromatase. The reaction products were derivatized with MSTFA and analyzed by gas chromatography/mass spectrometry (GC-MS) in full scan mode from 150 to 800 amu.
Interactions with CYP7B and 11β-hydroxysteroid dehydrogenase
Inhibition of CYP7B and 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) was measured in vitro in triplicate assays with rat liver micro-somes, by measuring the conversion of 3β-hydroxy-androst-5-en-17-one (DHEA) to 7α-hydroxy and 7β-hydroxy DHEA derivatives (products of CYP7B and 11β-HSD1 respectively) in the presence of 100 ng/mL HE3286 or metabolites HE3291, HE3393, HE3545, HE3553, and HE3633 in 2 mL phosphate-buffered saline containing 10 ng/mL DHEA, 1 mM NADPH, and 0.1 mg/mL rat liver microsomes. Reaction products were derivatized with MSTFA, and analyzed by gas chromatography/mass spectrometry (GC-MS/MS).
Nonclinical pharmacokinetics
Plasma or serum samples for pharmacokinetics (PK) were collected from a peripheral vein and stored frozen until analyzed by LC-MS/MS.
Pharmacokinetics in rats
Pharmacokinetics was measured in toxicology studies in male and female Sprague-Dawley rats following 1 and 28 daily oral gavage doses ranging from 3 to 200 mg/kg HE3286, formulated in cyclodextrin, and 1, 90 and 181 daily oral gavage doses ranging from 3 to 200 mg/kg HE3286 formulated as an aqueous microsus-pension. Plasma (lithium heparin) was collected from a peripheral vein from three animals per gender at each time point for each dose. Cohorts of three animals were used for each gender per time point period (0, 0.5, 1, 2, 4, 8, 12, and 24 hr), with each cohort sampled only twice in the 24-hour period to limit the impact of blood loss. Plasma samples were stored frozen prior to LC-MS/MS analysis with the Waters system. PK parameters for HE3286 and metabolites were determined for the observed maximum plasma concentration (Cmax), time of the observed maximum plasma concentration (Tmax), and integrated plasma drug concentration for a given period of time [AUC(0-t)] and calculated with WinNonlin 5.2 (Pharsight, St. Louis, Missouri) using the mean values for each collection time.
Pharmacokinetics in pregnant rabbits
Four groups of three time-mated female New Zealand White Hra:(NZW)SPF rabbits received a daily oral gavage of 13, 40, 130, or 400 mg/kg HE3286 as an aqueous suspension on gestation days (GD) 6 through 18. Plasma (lithum heparin) was collected for PK and metabolite profiling 0, 1, 2, 4, 8, and 24 hours post dose on GD 6 and 18, stored frozen and analyzed by LC-MS/MS for HE3286 and metabolites as described for rats.
Pharmacokinetics in dogs
Pharmacokinetics was measured in toxicology studies in groups of 5-8 male and female Beagle dogs after administration of a single or 28 daily oral gavage doses ranging from 3 to 66 mg/kg of HE3286 formulated in cyclodextrin. Pharmacokinetics for longer exposure intervals was measured with HE3286 formulated in capsules in doses ranging from 1 to 15 mg/kg. Plasma (lithium heparin) was collected from a peripheral vein prior to dosing and at 0.25, 0.5, 1, 2, 3, 4, 8, 12, and 24 hr after administration and stored frozen. HE3286 and metabolites were quantified and pharmacokinetic parameters calculated for individual animals using LC/ MS-MS on the Waters system and WinNonlin.
Pharmacokinetics in monkeys
The absolute oral bioavailability of HE3286 was measured in groups of non-naïve fasted rhesus monkeys (one male, two female). One group received a single IV dose of 5 mg/kg HE3286, and a second a single oral-gastric dose of 20 mg/kg. Blood (serum) was collected prior to dosing and 0.05, 0.25, 0.5, 0.75, 1, 2, 4, 8, and 24 hours after administration for the IV group, and 0, 0.25, 0.5, 0.75, 1, 2, 3, 4, 8, and 24 hours after administration for the oral group. Samples were stored frozen and analyzed using LC/MS-MS and WinNonlin as described for dogs.
The absolute oral bioavailability (% F) was expressed as the dose mass adjusted ratio of the integrated drug plasma concentration [AUC(0-infinity)] for the oral dose ratioed to the IV dose.
Safety and toxicity studies
HE3286 was evaluated for genotoxic effects in GLP assays in accordance with FDA guidelines for testing new chemical entities in support of clinical trials [40].
The genotoxic potential was assessed in vitro using a bacterial reverse mutation assay and a cytogenetic chromosomal aberration assay that evaluates damage in metaphase cells. An in vivo test for the occurrence of chemically induced micronucleated polychromatic erythrocytes in bone marrow cells was conducted in mice.
Bacterial reverse mutation (Ames) study
The S. typhimurium and E. coli reverse mutation assay, which measures the reversion frequency of frame-shift and base substitution mutants from auxotrophic to prototrophic phenotypes was conducted essentially as described by B. N. Ames [41], D. M. Maron and B.N. Ames [42], and M. H. L. Green and W. J. Muriel [43]. Four strains of S. typhimurium (TA98, TA100, TA1535, TA1537) and one strain of E. coli (WP2 [uvrA]) were used. HE3286 dissolved in DMSO was tested with and without rat liver S9 activation at concentrations of 5-5000 μg per plate (in triplicate with viability and strain specific positive controls) for formation of spontaneous revertant colonies.
In vitro chromosomal aberration assay
The Chinese hamster ovary cell (CHO, clone WBL) chromosome aberration test was performed essentially as described by S. M. Galloway [44]. HE3286 in DMSO at concentrations of 10-400 μg/mL (50- 200 μg/mL for rat S-9 activation) was added to replicate cultures of 1×105 cells/mL that had been conditioned at 37°C for 24 hours. The cultures were incubated for 18 hr (1.5 cell cycles for control cultures), with addition of 0.1 μg/mL Colcemid for the final two hours to facilitate karyotyping. Cell cultures (HE3235, vehicle control, and positive controls [0.8 μg/mL mitomycin C and 7.5 μg/mL cyclophosphamide]) were collected by centrifugation, fixed and stained with 5% Geimsa, and examined microscopically for the relative mitotic index, chromosome aberrations, ploidy, and frequency of endoreduplication. The Chi-squared test determined the statistical significance of the results.
Micronucleus assay in mice
The mouse micronucleus test was performed in 42-day old CD-1 male and female mice essentially as described by J. A. Heddle [45], W. Schmid [46], and M. Hayashi [47]. Briefly, a single dose of HE3286 aqueous microsuspen-sion was administered by intraperitoneal injection (IP) to five animals per sex at doses of 250, 500, and 1000 mg/kg (20 mL/kg). Control groups of ten per sex received vehicle IP (20 mL/kg) and cyclophosphamide by oral gavage (positive control, 80 mg/kg, 10 mL/kg). Two parallel groups were treated for sacrifice and bone marrow collection at 24 and 48 hours. Femoral bone marrow was harvested, fixed and stained on microscope slides for enumeration of the micronucleated polychromatic erythrocyte, polychromatic erythrocyte and normochromatic erythrocyte frequency. The one-tailed student t-test determined the statistical significance of the results.
Systemic toxicology and safety
Safety pharmacology and systemic toxicity of HE3286 were evaluated in GLP compliant studies at MPI Research (Matawan, MI). Systemic toxicity studies were conducted in Sprague-Dawley rats (4, 13 and 26 weeks) and Beagle dogs (4, 13 and 37 weeks).
Rats
Groups of 10-15 rats per sex (10 terminal sacrifice, 5 recovery) received daily oral gavage doses ranging from 100 to 400 mg/kg HE3286 formulated as a solution in cyclodextrin in 4-week studies, and 3 to 200 mg/kg HE3286 formulated as an aqueous microsuspension in 13- and 26-week studies that included ophthalmic examinations. Recovery animals were evaluated 28 days after the end of HE3286 treatment. A neurological assessment (functional observation battery) was performed as part of a 4-week study, with daily doses ranging from 100-400 mg/kg HE3286 using methods described by Moser [48], Meyer [49], Edwards and Parker [50], and Ankier [51]. Toxi-cokinetics (TK) was measured in satellite animals periodically throughout each study.
Dogs
Groups of 5 dogs/sex in 4-week studies (3 terminal, 2 recovery) received daily oral gavage doses ranging from 13 to 66 mg/kg HE3286 formulated as a solution in cyclodextrin, and 8 dogs per sex (5 terminal sacrifice, 3 recovery) received 90 and 271 daily doses of HE3286 formulated in capsules in doses ranging from 1 to 15 mg/kg. Electrocardiographic exams (ten-lead measuring RR, PR, and QTc intervals, and QRS duration) were performed on all animals prestudy and after dosing on the first day and during the last week. TK samples were collected from all animals periodically throughout each study.
Cardiopulmonary safety
Electrocardiographic and pulomonary effects of HE3286 were evaluated in beagle dogs. Three males and three females instrumented with transmitters for physiological monitoring and arterial vascular access ports were randomized (Latin square) to receive a once weekly single oral dose of vehicle or 16.5, 33, or 66 mg/kg HE3286 formulated as a solution in 30% sulfobutylether-β-cyclodextrin. Arterial blood pressure, heart rate, and ECG were monitored continuously for 2 hours prior to dosing and 22 hours after dosing. Pulmonary and arterial blood gas measurements were taken prior to dosing and 1, 4, and 21 hours post-dose in sling confined animals using a nose and mouth mask and pneumotach spirometer. TK samples were collected at 0.5 and 4 hours post-dose.
Measurement of sex steroids in HE3236 treated animals
Plasma concentrations of estradiol, estrone, testosterone, DHEA, androstenedione, and progesterone were measured during 13-week (rat and dog), 26 week (rat), and 39-week (dog) toxicology studies. Plasma (lithium heparin) was collected at approximately the same time of day prior to dosing on day 1 and after various intervals of dosing, and stored frozen until analyzed by LC-MS/MS.
Pitiutary hormones in HE3236 treated animals
Plasma concentrations of follicle stimulating hormone (FSH), luteinizing hormone (LH), and prolactin were quantified in 13- and 26-week rat systemic toxicology studies with ELISA kits from USCN-Life (Wuhan, China; FSH and LH) and Alpco, (Salem, NH; prolactin) according to the manufacturer's instructions.
The circulating concentrations of adrenocorticotropic hormone (ACTH) in dogs were measured by ELISA (Calbiotech, Inc., Spring Valley, CA) in EDTA plasma collected prestudy and after 13 weeks of HE3286 treatment.
Metabolite HE3545 toxicology in mice
The toxicity and estrogenic potential of the major metabolite of HE3286 in rodents, 17α-ethynyl-androst-5-en-3α,7β,17β-triol (HE3545) were evaluated in female CD-1 mice at MPI Research. Groups of 10 mice received cyclodextrin vehicle (10 mL/kg BID), 400 mg/kg HE3545 (200 mg/kg BID), or 400 mg/kg (200 mg/kg BID) HE3286 by oral gavage for 14 days. The animals were observed twice daily for signs of toxicity. Body weight and food consumption were measured weekly. The mice were sacrificed on day 15. Clinical pathology was evaluated by hematology and an abbreviated panel of clinical chemistry analytes that emphasized liver function. The adrenal glands, liver, and uterus from each animal were weighed and examined for histopathology.
Clinical pharmacokinetics
Clinical studies were approved by RCRC Institutional Review Board (IRB), Austin, Texas and Pennington Biomedical Research Center IRB, Baton Rouge, LA, and conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization/WHO Good Clinical Practice standards. All subjects/patients provided written informed consent prior to participating in the studies.
Two Phase I clinical studies were conducted to evaluate safety, pharmacokinetics, and drug metabolism. In one study, two groups of four male and four female normal subjects received sequential escalating once weekly oral doses of 10, 25, 50, and 100 mg QD, or 10, 25, and 50 mg BID. A subsequent double blind placebo controlled study was conducted in three cohorts of pre-diabetic subjects. Ten to twenty subjects per cohort (2:1 active: placebo) received 5 mg QD, 5 mg BID, or 10 mg BID or placebo for 28 days. Eight diabetic subjects received 5 mg BID (open label) for 28 days. Blood and urine were collected for pharmacokinetics and metabolite profiling on day 1 and 28. HE3286 and metabolite concentrations were measured with LC-MS/ MS.
Data analysis and statistical procedures
PK parameters were calculated with WinNonlin 5.2 (Pharsight, St. Louis, MO). The statistical significance of HE3286 mediated changes in hormone concentrations in toxicity studies was calculated with Student's t-test or the Mann-Whitney test using GraphPad Prism software (GraphPad Software Inc., San Diego, CA). Several methods of statistical analysis were used for other toxicological endpoints: Levene's test [52] was used to assess homogeneity of group variances. When Levene's test was not significant (p>0.01), a pooled estimate of the variance (Mean Square Error or MSE) was computed from a one-way analysis of variance (ANOVA), followed by a Dunnett's comparison [53] of each treatment group with the control group. When Levene's test was significant (p<0.01), comparisons with the control group were made using Welch's t-test [54] with a Bon-ferroni correction. Leukocyte counts (total and differential) were analyzed after log transformation, and urinalysis results were analyzed after rank transformation. All endpoints were analyzed using two-tailed tests, with significance ascribed to p < 0.05.
Results
HE3286 metabolites
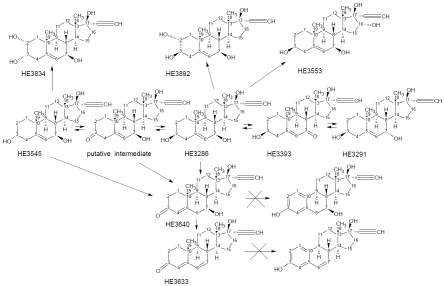
In CD-1 mice, GC-MS/MS was used to identify possible metabolites of HE3286, monitoring masses from 542 to 548 (corresponding to a 17-ethynyl androstene with 3 oxygen atoms and 0-3 double bonds), 630-636 (corresponding to a 17-ethynyl androstene with 4 oxygen atoms and 0-4 double bonds), 718-724 (corresponding to a 17-ethynyl androstene with 5 oxygen atoms and 0-5 double bonds), and 526-528 (corresponding to aromatic analogs of HE3286). Only peaks with appropriate mass transitions and spectra containing characteristic androstene fragments (e.g. M-15, M-90, M-105, M-180, M-195, etc.) were considered as potential metabolites. In mice, HE3286 was converted to its 7α-hydroxy and 7-keto analogs (i.e. HE3291 and HE3393 respectively), and several triol, tetrol, and diol-keto analogs of the parent compound. All these metabolites were sulfated and, to a much less extent, glucuronidated. The metabolic tree for HE3286 metabolites is shown in Figure 1. Using LC/MS-MS, quantitative differences in the HE3286 metabolite profiles were observed between species (Tables 1A and 1B), but there were no apparent gender associated differences. In humans and rabbits (only female rabbits were studied), HE3286 was the major unconjugated molecular species, and HE3291, HE3892, and HE3633 were the major metabolites; but in dogs, HE3291 was dominant and appreciable amounts of HE3393 were also formed. Only rodents formed appreciable amounts of HE3545. In mice, the AUCs for HE3286, HE3291, HE3393, and HE3545 were similar. The 16α-hydroxy tetrol, HE3553, was found only in mice. In rats, HE3286 was metabolized almost exclusively into HE3545, which was the dominant unconjugated molecular species. There was no evidence that enzyme induction altered metabolite profiles after multiple doses in any species.
Figure 1.
HE3286 metabolites. HE3286 is extensively metabolized in all species (refer to Table 1). Neither HE3640 nor HE3633 are aromatized by CYP19.
Table 1A.
HE3286 metabolites
| Abundance1,2,3 |
||||
|---|---|---|---|---|
| Structure* | Code | Transformation | Animals | Humans |
| 3β,7β,17β-triol | HE3286 | None | Major: mouse, dog, monkey. Minor: rats | Major |
| 3β,7α,17β-triol | HE3291 | C-7 inversion | Major: mouse, dog. Minor: rats | Major |
| 3β,17β-diol-7-one | HE3393 | C-7 oxidation | Major: mouse, dog. Minor: rats | Minor |
| androst-4,6-dien-3-one | HE3633 | C-3 oxidation and dehydration | Major: all | Major |
| 3α,7β,17β-triol | HE3545 | C-3 inversion | Major: mouse, rat. Minor: dog, rabbit | Minor |
| 3β,7β,16α,17β-tetrol | HE3553 | C-16 hydroxlation | Detected only in mice | ND5 |
| 2α,3β,7β,17β-tetrol | HE3892 | C-2 hydroxylation | Major: all (rat4) | Major |
*derivatives of 17α-ethynyl-androst-5-ene, except for metabolite HE3633 HE3633 (17α-ethynyl-17β-hydroxy-androst-4,6-dien-3-one);
metabolites were not characterized in monkeys;
considered minor in animals if the daily AUC was less than 5% relative to HE3286;
considered minor in humans if usually undetected from 20 mg HE3286 daily dose;
present as 2α,3α,7β,17β-tetrol in rats, compound code HE3834;
not detected
Table 1B.
Relative abundance (%) of HE3286 and major known metabolites in plasma
| Species | Gender | HE3286 | HE3291 | HE3393 | HE3545 | HE3553 | HE3633 |
|---|---|---|---|---|---|---|---|
| Human | Male | 100 | 23 | 0.4 | 3 | <0.1 | 46 |
| Female | 100 | 49 | <0.1 | 1 | <0.1 | 80 | |
| Dog | Male | 100 | 479 | 16 | <0.1 | <0.1 | 66 |
| Female | 100 | 473 | 13 | <0.1 | <0.1 | 54 | |
| Rabbit | Female | 100 | 60 | 8 | 1 | <0.1 | 9 |
| Rat | Male | 100 | 3 | 0.1 | 401 | <0.1 | 9 |
| Female | 100 | <0.1 | 0.2 | 760 | <0.1 | 8 | |
| Mouse | Male | 100 | 57 | 49 | 59 | 10 | 8 |
Nuclear hormone receptor interactions
Nuclear hormone receptor binding and transactivation
The absence of HE3286 interactions with many nuclear hormone receptors has been published [33], although interactions with the pregnane X or constitutive androstane receptors have not been studied. Binding activity is also absent for metabolites HE3291 and HE3393 (Ki > 10,000 nM for AR, ERα, ERβ, PR, and GR). Although there was no evidence of nuclear receptor binding, metabolites HE3291, HE3393, and HE3545 weakly transactivated ERα and/or ERβ relative to estradiol (Table 2). HE3286 metabolites did not transactivate AR, GR, MR, and PPAR. The transactivation potential of metabolite HE3633 was only tested for ERα/ERβ, and was negative.
Table 2.
Nuclear hormone receptor transactivation by HE3286 metabolites
| Ligand | AR/GRa | ERα/ERβb | ERβc | MR | PPARy | PPARδ |
|---|---|---|---|---|---|---|
| DHT | 0.06±0.03 (9) | 112 | 408±224 (6) | -d | -d | -d |
| Estradiol | 987±293 (5) | 0.002±0.002(10) | 0.04±0.04 (17) | -d | -d | -d |
| Dexamethasone | 14.1±9.3 (5) | -d | -d | -d | -d | -d |
| Aldosterone | -d | -d | -d | 0.018±3.4 | -d | -d |
| Rosiglitazone | -d | -d | -d | -d | 1.3±0.6 (3) | -d |
| L165041e | -d | -d | -d | -d | -d | 1.1±0.1(2) |
| HE3286f | AQLg | 268±25 | 3,648±540 | -d | AQLg | AQLg |
| HE3291 | AQLg | 162 | 1,129 | AQLg | AQLg | AQLg |
| HE3545 | AQLg | 1010 | NDd | AQLg | AQLg | AQLg |
| HE3393 | AQLg | 9.1 | 120 | AQLg | AQLg | AQLg |
| HE3633 | -d | AQLg | -d | -d | -d | -d |
Results are expressed as the mean EC50 (nM) ± SEM, with independent replicates other than one (n).
MDA-kb2 cells are stably transfected with a promoter/reporter construct sensitive to sex steroid receptor stimulation (MMTV promoter) fused upstream of a luciferase reporter gene, and endogenously express both AR and GR.
T47D-kBluc cells are stably transfected with a synthetic promoter/reporter construct sensitive to estrogenic stimulation, consisting of 3 copies of the estrogen response element (ERE) fused upstream of a luciferase reporter gene, and endogenously express both ERα and ERβ.
ERβ-HEK293 cells are HEK293 fibroblasts transiently co-transfected with an ERE/luciferase promoter/reporter construct and a cDNA expression vector encoding the full-length human ERβ, with virtually undetectable levels of endogenous sex steroid receptors.
not determined.
[4-[3-(4-Acetyl-3-hydroxy-2-propylphenoxy)propoxy]phenoxy]acetic acid.
(Wang eta l., 2010).
gabove quantifiable limit of 10,000 nM.
P450 interactions
The ability of HE3286 and metabolites HE3291 and HE3393 to inhibit or induce the major P450 enzymes was measured in vitro. HE3286 and HE3393 did not inhibit CYP1A2, 2C9, 2C19, 2D6, or 3A4 (HE3286 IC50 >100 mM; HE3393 IC50 >40 mM [HE3393 maximum concentration limited by solubility]). HE3291 inhibited CYP3A4 approximately 50% and CYP 2C9 40% at suprapharmacological concentrations (100 mM). HE3286 and HE3393 did not produce time dependent inhibition, but HE3291 inhibited CYP3A4 approximately 70% after metabolic preactivation. Metabolites HE3633 and HE3892 were not studied, but HE3892 was readily formed by microsomal CYP3A4 in vitro, and was thus present in HE3286 microsomal incubates, and apparently not a potent P450 inhibitor. HE3286 was primarily metabolized by CYP3A4 in vitro.
CYP7B and 11β-HSD-1 interactions
CYP7B hydroxylates androstenes to form 7α-hydroxylated products [55, 56] [57] [58] that can be epimerized to the 7β- configuration by 11β-HSD1 [59] [31]. HE3286 and metabolites HE3291, HE3393, HE3545, HE3553, and HE3633 were incubated with DHEA, a CYP7B substrate, and rat liver microsomes that contain both CYP7B and 11β-HSD1. The mean concentrations of C-7 hydroxylated products, 7α- and 7β-hydroxy-DHEA, varied less than 10% from control values in the presence and absence of HE3286 and metabolites, indicating that the HE3286 metabolome did not inhibit either enzymatic activity.
Pharmacokinetics
Clinical pharmacokinetics
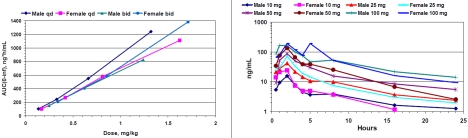
In humans, HE3286 was orally bioavailable, and exposure was dose proportional from 5 to 100mg (Figure 2). Less than 1% of the administered dose of HE3286 was found as free steroid in the urine. The terminal half-life was slightly longer in males than females (T½ 8.2 and 5.4 hours in males and females respectively). Drug plasma concentration curves for 10 mg BID are shown in Figure 3. Modest accumulation consistent with this half-life was observed with BID dosing schedules. Mean HE3286 plasma concentration vs. time profiles for 10 mg BID for day 1 and day 28 HE3286 are shown in Figure 3. Pharmacokinetics was similar in normal and pre-diabetic subjects, affected only by body mass differences between the two groups.
Figure 2.
HE3286 exposure and half-life in normal subjects. HE3286 is orally bioavailable. Mean drug exposure was dose proportional for QD and BID administration schedules in humans (left panel). In most instances HE3286 was found in plasma for the entire 24-hour period following a single dose of 10 to 100 mg. The terminal half-life of elimination was approximately 8 hours in males and 5.4 hours in females (right panel).
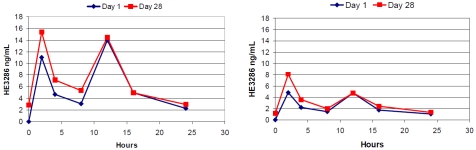
Figure 3.
HE3286 mean plasma drug concentration profiles in pre-diabetic and diabetic subjects. HE3286 is detectable throughout the day with BID dose schedules for daily doses of 10 or 20 mg. Pre-diabetic subjects received 10 mg BID for 28 days (left panel). Diabetic subjects received 5 mg BID for 28 days (right panel).
Pharmacokinetics in monkeys
The absolute oral bioavailability of HE3286 was 11 % in monkeys. The terminal half-life appeared to be highly variable ranging from 2.1 to 7.4 hours.
Pharmacokinetics in pregnant rabbits
HE3286 was orally bioavailable in pregnant rabbits. The metabolite profile was similar to (normal) humans, and quantitatively dissimilar to rodents with regard to HE3291, HE3393, and HE3545 abundance. In the 130 and 400 mg/kg groups, the AUC increased approximately 3-fold from GD 6 to 18 (17,800 rising to 48,500 ng*h/mL for the 400 mg/kg group). The increased exposure appeared to be caused by decreased drug clearance, which resulted in significant plasma concentrations up to 24 hours following administration. The terminal half-life of elimination was approximately 4 and 7 hours for 13 and 40 mg/kg respectively on both Day 6 and Day 18, and significant dose building would not be expected with these half-lives. In the two highest dose groups the T1/2 could not be extrapolated, but the 3-fold increase in AUC suggests the T1/2 was approximately 48 hours, establishing steady state conditions on approximately GD 14 [Rac = 1/(1-e-k) for QD dosing]. The increase in T1/2 with increased dose mass is consistent with saturation of a clearance pathway, and suggested a possible role of secondary metabolism as the limiting pathway since the major primary metabolites (HE3291 and HE3393) were formed in approximately constant ratios relative to parent drug at all doses. However, the subsequent analysis of conjugates indicated that the abundance of the conjugates paralleled unconjugated drug, leaving the source of the increased AUC as an open question.
Safety studies
Genotoxicity
HE3286 was negative for genotoxic effects in the reverse mutation, CHO chromosome aberration, and mouse micronucleus assays.
Systemic toxicity studies in rats
Toxicology in rats was evaluated in GLP oral studies up to 6 months in duration. There was no systemic or neurotoxicity at daily doses up 400 mg/kg in 4-week studies, and no toxicity at doses of 200 mg/kg and 30 mg/kg in 13- and 26-week studies respectively. In all studies, HE3286 treated males consumed approximately 30% less food than vehicle controls, and gained proportionally less weight, but without a correlating pathology or apparent effect on animal health. Dose dependent, generally mild, estrogenic effects were observed in both sexes. In relation to potential interactions with pregnane X and constitutive androstane receptors, which were not studied in vitro, there was no evidence of hepatocellular hypertrophy, although hypertrophy was observed in CD-1 mice (described below). In 4-week studies, Cmax values greater than 6,670 ng/mL were achieved in males and 7,310 ng/mL in females, with daily HE3286 exposures above 21,000 ng*h/mL in males and 28,000 ng*h/mL in females. The mean daily HE3286 exposure in 13-week studies for the highest dose was 7,620 and 11,700 ng*h/mL in males and females respectively, and the corresponding values in 26-weeks studies were 1,936 and 2,818 ng*h/mL. Metabolite exposure was significant only for HE3545 and HE3633, with mean daily exposures of approximately 39,000 ng*h/mL and 730 ng*h/mL, respectively for males and females combined in the 13-week study, and corresponding values of 10,300 and 213 ng*h/mL in the 26-week study.
Hormonal effects in rats
Estrogenic effects resulting from HE3286 administration included dose-dependent atrophy/degeneration of androgen responsive tissues and breast feminization in males, and follicular atresia in females, and mild mammary hyperplasia in both sexes.
In the 26-week study, with the exception of male breast feminization, estrogenic effects in males were similar to studies of shorter duration, but estrogenic effects in females were generally milder in the chronic study, or if present, indistinguishable from the naturally occurring hormonal changes in 8-month-old rats.
In male rats, hormonal effects included a dose dependent decrease in prostate weights (11% to 60%) that correlated microscopically to atrophy and/or decreased secretory product. Group mean seminal vesicle weights were decreased 30% to 59% for all HE3286-treated male groups, with a microscopic correlate of diffuse bilateral atrophy. The absolute weights of the epididymides were also decreased, and although this effect may have been primarily due to decreased body weight, there were still microscopic observations of oligospermia and atrophy. Testicular weights were not decreased, but there were microscopic observations of Leydig cell depletion. Breast feminization was observed in all treated males. Minimal to mild mammary gland lobular hyperplasia and galac-tocele were observed in a minority.
In long-term studies, hormonal effects in female rats were limited to a few observations of minimal to mild mammary gland lobular hyperplasia, and occasional observations of galactocele. Rats at 7-8 months of age generally have altered estrous cycles due to ovarian senescence, and exhibit histological characteristics of persistent estrus. Microscopic changes in the ovaries that are associated with senescence include atrophy, absence of corpora lutea, persistent corpora lutea, and follicular cysts. No HE3286 treatment related-effects on the altered estrous cycle of senescence were observed.
In studies of shorter duration, observations in females included minimal to mild mammary hyperplasia and increased follicular atresia, and minimally to severely decreased corpora lutea. The latter two effects would be difficult to distinguish from ovarian senescence in 8-month old rats. Chronic dosing did not increase the incidence and/or severity of mammary hyperplasia.
Interestingly, pituitary weights were increased in both sexes (1.3-fold in females, 1.7-fold in males), but without abnormal histopathology, and without measurable effects on plasma concentrations of FSH, LH, and prolactin.
Longitudinal and group-wise terminal measurements of pituitary hormones (FSH, LH, and prolactin) and sex steroids (estradiol, estrone, progesterone, testosterone, androstenedione, and DHEA) were not useful in identifying either a molecular or physiological mechanism through which HE3286 exerts its estrogenic effects in rats. Absolute concentrations and patterns of pituitary hormone fluctuations were similar in vehicle and HE3286 treated rats. Prolactin was not increased in response to the estrogenic influence of HE3286 treatment.
Twenty-six weeks of HE3286 treatment had variable effects on serum concentrations of sex steroids. HE3286 decreased estradiol approximately 75% in females at all dose levels, and estradiol concentrations were less than 5 pg/ mL in males, treated and untreated, and thus estrogenic effects in either sex did not appear to be the result of increased endogenous estradiol. Other sex steroids were stable or decreasing over the course of the study in both sexes.
Systemic toxicity studies in dogs
HE3286 was well tolerated in canine oral toxicology studies of up to 9 months duration. The no-observable-adverse-effect-level for the 13-and 37-week studies corresponded to the highest doses evaluated, 15 and 10 mg/kg respectively. There were no gender specific or sex hormone effects. Specifically absent were electro-cardiographic abnormalities and effects on circulating adrenocorticotropic hormone (ACTH). In 13-week studies, toxicokinetic measurements demonstrated a mean daily HE3286 exposure for males of 6,780 ng*h/mL and 6,280 ng*h/ mL in females, with corresponding values in 39-week studies of 4,054 and 2,210 ng*h/mL. By way of comparison, HE3286 exposure in humans is typically less than 100 ng*h/mL with the most commonly investigated dose of 10 mg per day.
Metabolite (HE3545) toxicology in mice
HE3545 is the major HE3286 metabolite in rats, and as a free steroid is generally present at 3-6 times the concentration of HE3286. The primary objective of the study was to determine if HE3545 was estrogenic in rodents. HE3545 did not significantly increase uterine weight, while HE3286 increased uterine weight over 90%. Both HE3286 and HE3545 increased liver weight and caused panlobular hepatocyte hypertrophy. No other effects were remarkable. The failure of HE3545 (and metabolites) to increase uterine weight indicated that HE3545 and metabolites were not the source of estrogenic effects observed with HE3286 in rodents.
Discussion
HE3286 is active in inflammatory disease and T2D rodent models [33-35] [15, 33-39], but without the side effects of glucocorticoids. HE3286 counters dexamethasone induced bone loss (Harbor BioSciences, unpublished) and the natural analog of HE3286, βAET, abrogates glucocorticoid mediated bone resorption [60], and was reported to counter the immuno-suppressive effects of cortisol [17]. HE3286 itself is not immunosuppressive and does not interfere with the anti-inflammatory activity of exogenous dexamethasone [38]. Mechanistically, the anti-inflammatory and anti-diabetic activity of HE3286 stem from regulation of the MAPK and NF-kB signal transduction pathways [33, 34], and the molecular target has recently been identified (manuscript in preparation).
In our nonclinical studies, HE3286 was found to have low potential for systemic toxicity and negligible interactions with the HPA and HPG axis, but decreased weight gain and estrogenicity were found as rodent specific treatment effects. These effects raised concerns for estrogenic potential in humans and led to longer-term studies to characterize the biochemical changes associated with HE3286 treatment. In addition to estrogenicity in rats, HE3286 was also estrogenic in mice, where it produced uterine hypertrophy (only female mice were studied), an effect not found in rats. Interestingly, ERα activation has reported to decrease weight gain and food intake in females rats [61, 62]. Although we did not find publications on the anorexigenic activity of estradiol in males, the magnitude of estadiol's effect in ovariectomzed female rats was similar to our results with HE3286 in males [63]. We do not believe decreased weight gain was related to the induction of thermogenesis as reported for DHEA [64], because DHEA thermogenesis did not affect caloric intake whereas HE3286 treatment reduced food intake. Anorexigenic activity may also be strain specific, since it was absent in Zucker diabetic fatty rats (CDF-Leprfa/Crl) [34], and db/db (BKS.Cg-m +/+ Leprdb/J) mice [33].
Characterization of nuclear hormone receptor interactions with the HE3286 metabolome did not identify a source of the differential estrogenic effects observed between rodent and non-rodent species. HE3286 and several metabolites weakly transactivated ERα in vitro, although none were found to have appreciable interactions with the ligand-binding domain of nuclear receptors. Moreover, in vitro transactivation data for ERα did not correlate with the absence of estrogenicity observed in vivo in dogs.
Because of the structural similarity between the HE3286 metabolome and sex steroids, we measured the plasma concentrations of endogenous hormones that could be affected by the HPA or HPG axis. There was no apparent treatment effect on estradiol, estrone, testosterone, androstenedione, DHEA and progesterone that could explain the estrogenic effects in rats, noting that median concentrations of sex steroids were lower in HE3286 treated female rats than in corresponding vehicle controls, and estradiol was below the limit of detection (5 pg/ mL) in male rats. Mammary glands effects (lobular hyperplasia, galactocele, and male breast feminization), were consistent with an estrogenic effect that should have been accompanied by a large increase in serum prolactin [65, 66]. However, HE3286 did not alter serum levels of prolactin and other pituitary hormones. Male breast feminization was exacerbated by prolonged treatment, but testicular effects were not, suggesting equilibrium between HE3286 atrophic and endogenous regenerative influences on the testes, but none of the estrogenic effects in females were exacerbated by extended treatment. Additional experiments in male rats found that the estrogen receptor antagonist, ICI182,780, blocked HE3286 mediated atrophic testicular effects, confirming the estrogenic nature of the effect, rather than anti-androgenic anti-androgenic (Harbor BioSciences, unpublished). The contrast in chronic effects between males and females may suggest a dependence on the HPG axis, which is functional in males throughout life.
HE3286 was extensively metabolized in all species studied, and although HE3286 metabolism was quantitatively different between rats, dogs, and humans, our data indicated that the complementary set of metabolites in rats and dogs measured in toxicology studies completely encompassed and over represented all metabolites anticipated and subsequently quantified in human clinical studies. Because HE3286 elicited estrogenic effects in rodents, we were keenly interested in the potential for estrogenic metabolites, and because HE3286 was not estrogenic in dogs, we focused on identification of possible rodent specific metabolites. Our investigations revealed no metabolites bearing the aromatized A-ring of a classic estrogen. The HE3286 metabolite profile in rats was dominated by 17α-ethynyl-androst-5-ene-3α,7β,17β-triol (HE3545), a compound that after administration to rodents produced a plasma metabolite profile similar to HE3286, except that HE3286 was negligible. We administered large doses of HE3545 to mice without inducing estrogenic effects, suggesting that the HE3545 metabolome was not responsible for estrogenic effects.
An unexpected metabolite discovery, HE3633 (17α-ethynyl-17β-hydroxy-androst-4,6-dien-3-one), forms through spontaneous dehydration of an unstable 17α-ethynyl-7β,17β-dihydroxy-androst-5-en-3-one intermediate. The analogous metabolite of androst-5-ene-3β,7β,17β-triol (βAET),7β,17β-dihydroxy-androst-5-en-3-one, was also found after βAET administration to both rodents and humans (Harbor BioSciences, unpublished). To our knowledge the metabolic conversions of 7-hydroxy-5-androstenes to androst-4,6-dien-3-ones has not been previously described, indicating them to be newly discovered components of the metabolome. Both the natural and synthetic di-ene metabolites were potential substrates for CYP19 (aromatase) because of the similarity to testosterone (3-keto-4-ene), but neither were aromatized, nor were HE3286, HE3291, HE3393, and HE3545.
As 7-hydroxy androstenes are not metabolized to potent androgens and estrogens [26], we assume that estrogenic effects in rodents were caused by an as yet unidentified metabolite (not found in other species) or rodent specific transcription factors that permit ERα transactivation by a component of the HE3286 metabolme.
HE3286 and metabolites did not have clinically significant potential to interact with the major P450 enzymes, which is an important feature of a drug intended for use in combination with a wide variety of the co-medications currently used to manage chronic inflammatory conditions. The lack of CYP3A4, CYP7B and 11β-HSD1 interactions also has important implications regarding local regulation of inflammation in tissues. CYP3A4, CYP7B and 11β-HSD1 are involved with 7-hydroxylation reactions in the formation of βAET (anti-inflammatory) and its 17 -keto form (pro-inflammatory) [18, 67]. The transcription of these enzymes is regulated by inflammatory cytokines to form an interdependent regulatory network [31, 68-70], so that inhibition of either enzyme would have unpredictable consequences for clinical safety and efficacy.
HE3286 was orally bioavailable in all species. The oral bioavailability in monkeys was estimated to be approximately 11%. Although low in comparison to synthetic glucocorticoids [27], it is superior to DHEA [20], and adequate to achieve pharmacologically active exposures in humans with low doses. The margin of safety at these low doses is greater than 20-fold. The half -life of HE3286 in humans is approximately 8 hours in males and 5 hours in females. It is amenable to dosing either once daily with a high dose or twice daily with a lower dose, which was adopted for early phase clinical activity trials to reduce fluctuation of the plasma drug concentration.
Phase I/II clinical trials have been conducted for type 2 diabetes (manuscript submitted), ulcerative colitis, and rheumatoid arthritis. The breadth of potential clinical indications for HE3286 necessitates a safety profile that is free of clinically significant toxicities and side effects, and that can be used safely in combination with other medications. Long-term toxicology studies with HE3286 did not identify an adverse effect that would limit its clinical utility. Of particular interest for new type 2 diabetes medications is the potential to cause or exacerbate congestive heart failure, which has been recognized by the USFDA as a safety concern for several of the widely prescribed PPAR agonists. In contrast to PPAR agonists, cardiopulomonary edema was not found in HE3286 in nonclinical safety studies, and considering the reported anti-inflammatory activity, HE3286 might also be expected to decrease microvascular disease.
HE3286 is the first member of the DHEA metabolome to undergo extensive pharmacological and safety profiling. Although earlier studies demonstrated that HE3286 possessed potent anti-inflammatory activity, many questions remained regarding the potential for toxicity and untoward interactions with endogenous hormone signaling networks, especially with chronic exposure. The information gathered indicates that HE3286 should be safe for chronic use, and paves the way for clinical trials with this new class of immune modulating steroid and brings us one step closer to translating in humans the broad spectrum of immune modulating activity exhibited by DHEA in rodents.
Acknowledgments
The authors thank Dr. Sonia Villegas for her characterization of HE3286 metabolite nuclear hormone receptor interactions.
Glossary
Abbreviations
- βAET
androst-5-ene-3β,7β,17β-triol
- AR
androgen receptor
- DHEA
dehydroepiandroster-one
- ERα
estrogen receptor alpha
- ERβ
estrogen receptor beta
- HE3286
17α-ethynyl-androst-5ene-3β, 7β, 17β-triol
- HE3291
17α-ethynyl-5-androstene-3β, 7α,17β-triol
- HE3393
17α-ethynyl-7-keto-5-androstene-3β, 17β-diol
- HE3545
17α-ethynyl-5-androstene-3α, 7β, 17β-triol
- HE3553
17α-ethynyl-5-androstene-3β, 7β, 16α, 17β-tetrol
- HE3633
17α-ethynyl-3-keto-5-androst-4,6-dien-17β-ol
- GR
gluco-corticoid receptor
- HSD
hydroxysteroid dehydro-genase
- ICI182,170
7α,17β-[9-[(4,4,5,5,5-pentafluoropentyl)sulfinyl]nonyl]estra-1,3,5(10)-triene -3,17-diol
- L165041
[4-[3-(4-acetyl-3-hydroxy-2-propylphenoxy)propoxy]phenoxy]acetic acid
- MR
mineralocorticoid receptor
- PPAR
peroxisome prolif-erator activated receptor
- PR
progesterone receptor
- MSTFA
N-methyl-N-(trimethylsilyl)trifluoroacetamide
Statement of conflicts of interest
This work was sponsored by Harbor Biosciences, Inc. Harbor Biosciences holds patent positions on HE3286 and its pharmaceutical use. Authors Ahlem, Kennedy, Page, White, Reading, Stickney, and Frincke hold equity positions in Harbor Biosciences. Authors McKenzie and Cole declare no conflicts of interest.
References
- 1.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 2.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.McClain CJ, Mokshagundam SP, Barve SS, Song Z, Hill DB, Chen T, Deaciuc I. Mechanisms of non-alcoholic steatohepatitis. Alcohol. 2004;34:67–79. doi: 10.1016/j.alcohol.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Michelsen KS, Arditi M. Toll-like receptors and innate immunity in gut homeostasis and pathology. Curr Opin Hematol. 2007;14:48–54. doi: 10.1097/00062752-200701000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Shibata N, Glass CK. Regulation of macro-phage function in inflammation and atherosclerosis. J Lipid Res. 2009;(50 Suppl):S277–281. doi: 10.1194/jlr.R800063-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sinden NJ, Stockley RA. Systemic inflammation and comorbidity in COPD: a result of ‘overspill’ of inflammatory mediators from the lungs? Review of the evidence. Thorax. 2010;65:930–936. doi: 10.1136/thx.2009.130260. [DOI] [PubMed] [Google Scholar]
- 8.Schett G, Saag KG, Bijlsma JW. From bone biology to clinical outcome: state of the art and future perspectives. Ann Rheum Dis. 2010;69:1415–1419. doi: 10.1136/ard.2010.135061. [DOI] [PubMed] [Google Scholar]
- 9.Barnes PJ. Mechanisms and resistance in glu-cocorticoid control of inflammation. J Steroid Biochem Mol Biol. 2010;120:76–85. doi: 10.1016/j.jsbmb.2010.02.018. [DOI] [PubMed] [Google Scholar]
- 10.Callen JP. Complications and adverse reactions in the use of newer biologic agents. Semin Cutan Med Surg. 2007;26:6–14. doi: 10.1016/j.sder.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 11.PengS, Duggan A. Gastrointestinal adverse effects of non-steroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2005;4:157–169. doi: 10.1517/14740338.4.2.157. [DOI] [PubMed] [Google Scholar]
- 12.Dillon JS. Dehydroepiandrosterone, dehydroepi-androsterone sulfate and related steroids: their role in inflammatory, allergic and immunological disorders. Curr Drug Targets Inflamm Allergy. 2005;4:377–385. doi: 10.2174/1568010054022079. [DOI] [PubMed] [Google Scholar]
- 13.Marcu AC, Paccione KE, Barbee RW, Diegelmann RF, Ivatury RR, Ward KR, Loria RM. Androstenetriol immunomodulation improves survival in a severe trauma hemorrhage shock model. J Trauma. 2007;63:662–669. doi: 10.1097/TA.0b013e31802e70d9. [DOI] [PubMed] [Google Scholar]
- 14.Auci D, Nicoletti F, Mangano K, Pieters R, Nierkens S, Morgan L, Schraufstatter I, Frincke J, Reading C. Anti-inflammatory and immune regulatory properties of 5-androsten-3b,17b-diol (HE2100) and synthetic analogue HE3204; implications for treatment of autoimmune diseases. Autoimmun Rev. 2004;3:87–88. doi: 10.1196/annals.1361.117. [DOI] [PubMed] [Google Scholar]
- 15.Auci DL, Reading CL, Frincke JM. 7-Hydroxy androstene steroids and a novel synthetic analogue with reduced side effects as a potential agent to treat autoimmune diseases. Autoimmun Rev. 2009;8:369–372. doi: 10.1016/j.autrev.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Offner H, Zamora A, Drought H, Matejuk A, Auci DL, Morgan EE, Vandenbark AA, Reading CL. A synthetic androstene derivative and a natural androstene metabolite inhibit relapsing-remitting EAE. J Neuroimmunol. 2002;130:128–139. doi: 10.1016/s0165-5728(02)00214-x. [DOI] [PubMed] [Google Scholar]
- 17.Loria RM, Padgett DA. Control of the immune response by DHEA and its metabolites. Rinsho Byori. 1998;46:505–517. [PubMed] [Google Scholar]
- 18.Fitzpatrick JL, Ripp SL, Smith NB, Pierce WM, Jr, Prough RA. Metabolism of DHEA by cyto-chromes P450 in rat and human liver micro-somal fractions. Arch Biochem Biophys. 2001;389:278–287. doi: 10.1006/abbi.2001.2341. [DOI] [PubMed] [Google Scholar]
- 19.Marwah A, Marwah P, Lardy H. Ergosteroids. VI. Metabolism of dehydroepiandrosterone by rat liver in vitro: a liquid chromatographic-mass spectrometric study. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;767:285–299. doi: 10.1016/s1570-0232(01)00570-0. [DOI] [PubMed] [Google Scholar]
- 20.Leblanc M, Labrie C, Belanger A, Candas B, Labrie F. Bioavailability and pharmacokinetics of dehydroepiandrosterone in the cynomolgus monkey. J Clin Endocrinol Metab. 2003;88:4293–4302. doi: 10.1210/jc.2003-022012. [DOI] [PubMed] [Google Scholar]
- 21.Labrie F, Belanger A, Labrie C, Candas B, Cusan L, Gomez JL. Bioavailability and metabolism of oral and percutaneous dehydroepiandroster-one in postmenopausal women. J Steroid Bio-chem Mol Biol. 2007;107:57–69. doi: 10.1016/j.jsbmb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Saner KJ, Suzuki T, Sasano H, Pizzey J, Ho C, Strauss JF, 3rd, Carr BR, Rainey WE. Steroid sulfotransferase 2A1 gene transcription is regulated by steroidogenic factor 1 and GATA-6 in the human adrenal. Mol Endocrinol. 2005;19:184–197. doi: 10.1210/me.2003-0332. [DOI] [PubMed] [Google Scholar]
- 23.Meloche CA, Falany CN. Expression and characterization of the human 3 beta-hydroxysteroid sulfotransferases (SULT2B1a and SULT2B1b) J Steroid Biochem Mol Biol. 2001;77:261–269. doi: 10.1016/s0960-0760(01)00064-4. [DOI] [PubMed] [Google Scholar]
- 24.Tashiro A, Sasano H, Nishikawa T, Yabuki N, Muramatsu Y, Coughtrie MW, Nagura H, Hongo M. Expression and activity of dehy-droepiandrosterone sulfotransferase in human gastric mucosa. J Steroid Biochem Mol Biol. 2000;72:149–154. doi: 10.1016/s0960-0760(00)00020-0. [DOI] [PubMed] [Google Scholar]
- 25.Webb SJ, Geoghegan TE, Prough RA, Michael Miller KK. The biological actions of dehy-droepiandrosterone involves multiple receptors. Drug Metab Rev. 2006;38:89–116. doi: 10.1080/03602530600569877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lardy H, Partridge B, Kneer N, Wei Y. Ergos-teroids: induction of thermogenic enzymes in liver of rats treated with steroids derived from dehydroepiandrosterone. Proc Natl Acad Sci U S A. 1995;92:6617–6619. doi: 10.1073/pnas.92.14.6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sietsema WK. The absolute oral bioavailability of selected drugs. Int J Clin Pharmacol Ther Toxicol. 1989;27:179–211. [PubMed] [Google Scholar]
- 28.Labrie F, Belanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez JL, Candas B. DHEA and the intracrine formation of andro-gens and estrogens in peripheral target tissues: its role during aging. Steroids. 1998;63:322–328. doi: 10.1016/s0039-128x(98)00007-5. [DOI] [PubMed] [Google Scholar]
- 29.Sam KM, Auger S, Luu-The V, Poirier D. Steroidal spiro-gamma-lactones that inhibit 17 beta-hydroxysteroid dehydrogenase activity in human placental microsomes. J Med Chem. 1995;38:4518–4528. doi: 10.1021/jm00022a018. [DOI] [PubMed] [Google Scholar]
- 30.Puranen TJ, Kurkela RM, Lakkakorpi JT, Poutanen MH, Itaranta PV, Melis JP, Ghosh D, Vihko RK, Vihko PT. Characterization of molecular and catalytic properties of intact and truncated human 17beta-hydroxysteroid dehydrogenase type 2 enzymes: intracellular localization of the wild-type enzyme in the endoplasmic reticulum. Endocrinology. 1999;140:3334–3341. doi: 10.1210/endo.140.7.6861. [DOI] [PubMed] [Google Scholar]
- 31.Muller C, Pompon D, Urban P, Morfin R. Inter-conversion of 7alpha- and 7beta-hydroxy-dehydroepiandrosterone by the human 11beta-hydroxysteroid dehydrogenase type 1. J Steroid Biochem Mol Biol. 2006;99:215–222. doi: 10.1016/j.jsbmb.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Chalbot S, Morfin R. Dehydroepiandrosterone metabolites and their interactions in humans. Drug Metabol Drug Interact. 2006;22:1–23. doi: 10.1515/dmdi.2006.22.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Wang T, Villegas S, Huang Y, White SK, Ahlem C, Lu M, Olefsky JM, Reading C, Frincke JM, Alleva D, Flores-Riveros J. Amelioration of glucose intolerance by the synthetic androstene HE3286: link to inflammatory pathways. J Pharmacol Exp Ther. 2010;333:70–80. doi: 10.1124/jpet.109.161182. [DOI] [PubMed] [Google Scholar]
- 34.Lu M, Patsouris D, Li P, Flores-Riveros J, Frincke JM, Watkins S, Schenk S, Olefsky JM. A new antidiabetic compound attenuates inflammation and insulin resistance in Zucker diabetic fatty rats. Am J Physiol Endocrinol Metab. 2010;298:E1036–1048. doi: 10.1152/ajpendo.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Auci DL, Mangano K, Destiche D, White SK, Huang Y, Boyle D, Frincke J, Reading CL, Nicoletti F. Oral treatment with HE3286 ameliorates disease in rodent models of rheumatoid arthritis. Int J Mol Med. 2010;25:625–633. doi: 10.3892/ijmm_00000385. [DOI] [PubMed] [Google Scholar]
- 36.Ahlem C, Auci D, Mangano K, Reading C, Frincke J, Stickney D, Nicoletti F. HE3286: a novel synthetic steroid as an oral treatment for autoimmune disease. Ann N Y Acad Sci. 2009;1173:781–790. doi: 10.1111/j.1749-6632.2009.04798.x. [DOI] [PubMed] [Google Scholar]
- 37.Auci D, Kaler L, Subramanian S, Huang Y, Frincke J, Reading C, Offner H. A new orally bioavailable synthetic androstene inhibits collagen-induced arthritis in the mouse: androstene hormones as regulators of regulatory T cells. Ann N Y Acad Sci. 2007;1110:630–640. doi: 10.1196/annals.1423.066. [DOI] [PubMed] [Google Scholar]
- 38.Offner H, Firestein GS, Boyle DL, Pieters R, Frincke JM, Garsd A, White SK, Reading CL, Auci DL. An orally bioavailable synthetic analog of an active dehydroepiandrosterone metabolite reduces established disease in rodent models of rheumatoid arthritis. J Pharmacol Exp Ther. 2009;329:1100–1109. doi: 10.1124/jpet.108.145086. [DOI] [PubMed] [Google Scholar]
- 39.Offner H, Zamora A, Subramanian S, Polanczyk M, Krogstad A, Auci DL, Morgan EE, Reading CL. A synthetic androstene analogue inhibits collagen-induced arthritis in the mouse. Clin Immunol. 2004;110:181–190. doi: 10.1016/j.clim.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 40. FDA Guidance for Industry. Genotoxicity Testing and Data Intrepretation for Pharmaceuticals Intended for Human Use Rockville, MD.: U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), ICH,
- 41.Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res. 1975;31:347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 42.Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 43.Green MH, Muriel WJ. Mutagen testing using TRP+ reversion in Escherichia coli. Mutat Res. 1976;38:3–32. doi: 10.1016/0165-1161(76)90076-5. [DOI] [PubMed] [Google Scholar]
- 44.Galloway SM, Bloom AD, Resnick M, Margolin BH, Nakamura F, Archer P, Zeiger E. Development of a standard protocol for in vitro cyto-genetic testing with Chinese hamster ovary cells: comparison of results for 22 compounds in two laboratories. Environ Mutagen. 1985;7:1–51. doi: 10.1002/em.2860070102. [DOI] [PubMed] [Google Scholar]
- 45.Heddle JA. A rapid in vivo test for chromosomal damage. Mutat Res. 1973;18:187–190. doi: 10.1016/0027-5107(73)90035-3. [DOI] [PubMed] [Google Scholar]
- 46.Schmid W. The micronucleus test. Mutat Res. 1975;31:9–15. doi: 10.1016/0165-1161(75)90058-8. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi M, Tice RR, MacGregor JT, Anderson D, Blakey DH, Kirsh-Volders M, Oleson FB, Jr, Pacchierotti F, Romagna F, Shimada H, et al. In vivo rodent erythrocyte micronucleus assay. Mutat Res. 1994;312:293–304. doi: 10.1016/0165-1161(94)90039-6. [DOI] [PubMed] [Google Scholar]
- 48.Moser VC, McCormick JP, Creason JP, MacPhail RC. Comparison of chlordimeform and carbaryl using a functional observational battery. Fundam Appl Toxicol. 1988;11:189–206. doi: 10.1016/0272-0590(88)90144-3. [DOI] [PubMed] [Google Scholar]
- 49.Meyer OA, Tilson HA, Byrd WC, Riley MT. A method for the routine assessment of fore- and hindlimb grip strength of rats and mice. Neuro-behav Toxicol. 1979;1:233–236. [PubMed] [Google Scholar]
- 50.Edwards PM, Parker VH. A simple, sensitive, and objective method for early assessment of acrylamide neuropathy in rats. Toxicol Appl Pharmacol. 1977;40:589–591. doi: 10.1016/0041-008x(77)90083-7. [DOI] [PubMed] [Google Scholar]
- 51.Ankier SI. New hot plate tests to quantify anti-nociceptive and narcotic antagonist activities. Eur J Pharmacol. 1974;27:1–4. doi: 10.1016/0014-2999(74)90195-2. [DOI] [PubMed] [Google Scholar]
- 52.Milliken GA JD. London: Chapman and Hall; 1992. Analysis of messy data. [Google Scholar]
- 53.Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- 54.Welch BL. The significance of difference between two means when the population variances are unequal. Biometrika. 1937;29:350–362. [Google Scholar]
- 55.Doostzadeh J, Cotillon AC, Benalycherif A, Morfin R. Inhibition studies of dehydroepian-drosterone 7 alpha- and 7 beta- hydroxylation in mouse liver microsomes. Steroids. 1998;63:608–614. doi: 10.1016/s0039-128x(98)00071-3. [DOI] [PubMed] [Google Scholar]
- 56.Doostzadeh J, Cotillon AC, Morfin R. Hydroxylation of pregnenolone at the 7 alpha- and 7 beta- positions by mouse liver microsomes. Effects of cytochrome p450 inhibitors and structure-specific inhibition by steroid hormones. Steroids. 1998;63:383–392. doi: 10.1016/s0039-128x(98)00012-9. [DOI] [PubMed] [Google Scholar]
- 57.Kim SB, Chalbot S, Pompon D, Jo DH, Morfin R. The human cytochrome P4507B1: catalytic activity studies. J Steroid Biochem Mol Biol. 2004;92:383–389. doi: 10.1016/j.jsbmb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Morfin R, Lafaye P, Cotillon AC, Nato F, Chmielewski V, Pompon D. 7 alpha-hydroxy-dehydroepiandrosterone and immune response. Ann N Y Acad Sci. 2000;917:971–982. doi: 10.1111/j.1749-6632.2000.tb05464.x. [DOI] [PubMed] [Google Scholar]
- 59.Chalbot S, Morfin R. Human liver S9 fractions: metabolism of dehydroepiandrosterone, epiandrosterone, and related 7-hydroxylated derivatives. Drug Metab Dispos. 2005;33:563–569. doi: 10.1124/dmd.104.003004. [DOI] [PubMed] [Google Scholar]
- 60.Malik AK, Khaldoyanidi, Sophia, Auci, Dominick L, Miller, Scott C, Ahlem, Clarence N, Reading, Christopher L, Page, Theodore, Frincke, James M. 5-Androstene-3b,7b,17b-triol (b-AET) Slows Thermal Injury Induced Osteopenia in Mice: Relation to Aging and Osteoporosis. PLoS ONE. 2010;5:e13566. doi: 10.1371/journal.pone.0013566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santollo J, Wiley MD, Eckel LA. Acute activation of ER alpha decreases food intake, meal size, and body weight in ovariectomized rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2194–2201. doi: 10.1152/ajpregu.00385.2007. [DOI] [PubMed] [Google Scholar]
- 62.Santollo J, Katzenellenbogen BS, Katzenellenbogen JA, Eckel LA. Activation of ERalpha is necessary for estradiol's anorexigenic effect in female rats. Horm Behav. 2010;58:872–877. doi: 10.1016/j.yhbeh.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Santollo J, Eckel LA. Effect of a putative ERalpha antagonist, MPP, on food intake in cycling and ovariectomized rats. Physiol Behav. 2009;97:193–198. doi: 10.1016/j.physbeh.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cleary MP. The antiobesity effect of dehydroepiandrosterone in rats. Proc Soc Exp Biol Med. 1991;196:8–16. doi: 10.3181/00379727-196-43158b. [DOI] [PubMed] [Google Scholar]
- 65.Lucas JN, Rudmann DG, Credille KM, Irizarry AR, Peter A, Snyder PW. The rat mammary gland: morphologic changes as an indicator of systemic hormonal perturbations induced by xenobiotics. Toxicol Pathol. 2007;35:199–207. doi: 10.1080/01926230601156260. [DOI] [PubMed] [Google Scholar]
- 66.Nolan LA, Levy A. The trophic effects of oestrogen on male rat anterior pituitary lacto-trophs. J Neuroendocrinol. 2009;21:457–464. doi: 10.1111/j.1365-2826.2009.01864.x. [DOI] [PubMed] [Google Scholar]
- 67.Miller KK, Cai J, Ripp SL, Pierce WM, Jr, Rushmore TH, Prough RA. Stereo- and regiose-lectivity account for the diversity of dehydroepiandrosterone (DHEA) metabolites produced by liver microsomal cytochromes P450. Drug Metab Dispos. 2004;32:305–313. doi: 10.1124/dmd.32.3.305. [DOI] [PubMed] [Google Scholar]
- 68.Dulos J, van der Vleuten MA, Kavelaars A, Heijnen CJ, Boots AM. CYP7B expression and activity in fibroblast-like synoviocytes from patients with rheumatoid arthritis: regulation by proinflammatory cytokines. Arthritis Rheum. 2005;52:770–778. doi: 10.1002/art.20950. [DOI] [PubMed] [Google Scholar]
- 69.Dulos J, Boots AH. DHEA metabolism in arthritis: a role for the p450 enzyme Cyp7b at the immune-endocrine crossroad. Ann N Y Acad Sci. 2006;1069:401–413. doi: 10.1196/annals.1351.038. [DOI] [PubMed] [Google Scholar]
- 70.Muller C, Hennebert O, Morfin R. The native anti-glucocorticoid paradigm. J Steroid Biochem Mol Biol. 2006;100:95–105. doi: 10.1016/j.jsbmb.2006.03.001. [DOI] [PubMed] [Google Scholar]