Abstract
Background
Previous studies have shown that women younger than 55 years have higher hospital mortality rates after acute myocardial infarction (MI) than age-matched men. We examined whether such mortality differences have decreased in recent years.
Methods
We investigated temporal trends in the hospital case-fatality rates of MI by sex and age from June 1, 1994, through December 31, 2006. The study population included 916 380 patients from the National Registry of Myocardial Infarction with a confirmed diagnosis of MI.
Results
In-hospital mortality decreased markedly between 1994 and 2006 in all patients but more so in women than men. The mortality reduction in 2006 relative to 1994 was largest in women younger than 55 years (52.9%) and lowest in men younger than 55 years (33.3%). In patients younger than 55 years, the absolute decrease in mortality was 3 times larger in women than men (2.7% vs 0.9%). As a result, the excess mortality in younger women (<55 years) compared with men was less pronounced in 2004-2006 (unadjusted odds ratio, 1.32; 95% confidence interval, 1.07-1.67) than it was in 1994-1995 (unadjusted odds ratio, 1.93; 95% confidence interval, 1.67-2.24). The sex difference in mortality decrease was lower in older patients (P=.004 for the interaction among sex, age, and year). Changes in comorbidity and clinical severity features at admission accounted for more than 90% of these mortality trends.
Conclusions
In recent years, women, particularly younger ones, experienced larger improvements in hospital mortality after MI than men. The narrowing of the mortality gap between younger women and men is largely attributable to temporal changes in risk profiles.
Although myocardial infarction (MI) in women occurs predominantly in old age (greater than 65 years), it is not uniquely a disease of elderly women. In the United States, more than 100 000 women younger than 65 years are diagnosed as having acute MI each year, which represents 21% of all acute MI cases in women.1 Although less common, an MI that occurs at a younger age is associated with a substantial risk of morbidity and mortality in women. In a series of studies2-4 beginning in the late 1990s, we reported that younger, but not older, women with MI have higher mortality rates than men of similar age. The risk among women relative to men increases linearly with decreasing age and is not fully explained by differences in MI severity, comorbidity, or treatment.4 These findings, which since then have been confirmed by many authors,5-10 have raised the important question of why younger women, a group traditionally considered at low risk, have a higher risk of adverse events and mortality after MI than age-matched men.11,12
Many of the reports that examine mortality rates after MI by sex and age were based on patient populations enrolled decades ago. It is possible that the excess mortality in younger women has decreased in recent years because of increasing awareness of heart disease in women, which may have translated into better diagnosis, treatment, and outcomes.13 On the other hand, recent mortality trends for coronary heart disease have been less favorable for younger than older Americans.14 These mortality statistics could potentially reflect an increased case-fatality rate that disproportionately affects younger women.
The main purpose of this study was to describe temporal trends in the case-fatality rates of MI according to sex and age during a period of approximately 12 years (June 1, 1994, through December 31, 2006). We sought to determine whether improvements in hospital mortality over time were comparable in younger and older patients and in women and men and whether sex differences in mortality after MI among younger patients have decreased in recent years.
Methods
Patients
The National Registry of Myocardial Infarction (NRMI) was a prospective, observational study of patients hospitalized for acute MI. Participating NRMI hospitals enrolled consecutive patients with acute MI, whether the MI was their first or a recurrent event, as previously described.15 Case ascertainment and clinical data were validated by comparison with the Cooperative Cardiovascular Project.16 This report includes 4 subsequent waves of NRMI data collection: 1994-1997, 1998-1999, 2000-2003, and 2004-2006. Data elements and definitions evolved slightly over time, but core content remained constant. To focus on patients who presented with acute myocardial ischemia, we included only patients whose admission diagnosis was acute coronary ischemia (MI, rule-out MI, or unstable angina) and excluded those who had myocardial injury secondary to surgery, hypotension, or other events after hospitalization. We also excluded patients who were transferred to another acute care hospital (because the outcome of these patients was unknown) and patients who were transferred from other acute care hospitals (because admission data were often missing). The patients transferred in or out were younger (64 vs 68 years), but the proportion of men and women transferred was fairly similar (48% vs 41%). This proportion did not substantially change over time. These exclusions resulted in a final sample of 916 380 patients.
Study Variables
Information with regard to study variables (Table 1 and Table 2) was abstracted from the medical records at each hospital.15,17 The degree of ventricular dysfunction was measured by the Killip classification18 and by left ventricular ejection fraction. The definition of ST-segment elevation MI (STEMI) was given as ST-segment elevation or left bundle branch block (new, unknown, or old) on the first or subsequent 12-lead electrocardiogram. Registry patients whose MI did not meet the definition for STEMI were classified as having nonSTEMI. The outcome of the study was hospital mortality.
Table 1. Demographic, Clinical, and Hospital Characteristics in Patients Younger Than 65 Years Classified According to Sex and Admission Yeara.
| Characteristics | Men, % (n=268230) |
Women, % (n=91 700) |
Risk Ratios | |||||
|---|---|---|---|---|---|---|---|---|
| 1994-1995 | 1996-1997 | 1998-1999 | 2000-2001 | 2002-2003 | 2004-2006 | |||
| Race other than white | 18.6 (49 681) | 23.9 (21 852) | 1.26 | 1.31 | 1.26 | 1.28 | 1.31 | 1.28 |
| Primary medical insuranceb | ||||||||
| PPO | 45.9 (123 045) | 39.5 (36 248) | 0.86 | 0.87 | 0.85 | 0.87 | 0.86 | 0.86 |
| HMO | 19.8 (53 048) | 18.9 (17 337) | 0.96 | 0.90 | 0.95 | 0.99 | 0.96 | 0.97 |
| Medicare | 9.5 (25 370) | 12.9 (11 855) | 1.30 | 1.28 | 1.32 | 1.36 | 1.46 | 1.53 |
| Medicaid | 5.5 (14 703) | 12.5 (11 454) | 2.33 | 2.43 | 2.44 | 2.20 | 2.14 | 2.09 |
| Self-pay | 12.7 (34 171) | 12.3 (11 253) | 0.99 | 1.02 | 0.94 | 0.92 | 0.93 | 0.96 |
| Medical history and risk factors | ||||||||
| Myocardial infarction | 21.8 (58 517) | 18.8 (17 220) | 0.84 | 0.84 | 0.87 | 0.87 | 0.87 | 0.90 |
| Angina | 11.7 (31 365) | 12.2 (11 229) | 1.09 | 1.05 | 1.08 | 1.02 | 1.02 | 1.01 |
| Heart failure | 4.7 (12 541) | 8.5 (7771) | 1.86 | 1.86 | 1.88 | 1.70 | 1.79 | 1.80 |
| PTCA | 13.7 (36 637) | 10.9 (9973) | 0.75 | 0.76 | 0.79 | 0.80 | 0.81 | 0.83 |
| CABG | 11.0 (29 395) | 8.4 (7657) | 0.65 | 0.73 | 0.78 | 0.77 | 0.81 | 0.85 |
| Stroke | 3.5 (9310) | 5.6 (5160) | 1.65 | 1.68 | 1.55 | 1.61 | 1.53 | 1.67 |
| Diabetes mellitus | 20.9 (56 066) | 33.1 (30 314) | 1.72 | 1.68 | 1.58 | 1.46 | 1.51 | 1.54 |
| Hypertension | 46.5 (124 614) | 55.9 (51 291) | 1.22 | 1.24 | 1.22 | 1.19 | 1.18 | 1.15 |
| Ever smoked | 48.7 (130 705) | 46.9 (42 970) | 0.96 | 0.96 | 0.95 | 0.96 | 0.94 | 1.00 |
| Clinical characteristics at admission | ||||||||
| Chest pain on presentation | 85.0 (227 914) | 81.5 (74 759) | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 | 0.96 |
| STEMI | 60.8 (163 049) | 55.0 (50 436) | 0.98 | 0.95 | 0.90 | 0.87 | 0.84 | 0.84 |
| Killip class | ||||||||
| 1. No heart failure | 90.1 (241 673) | 85.8 (78 650) | 0.94 | 0.94 | 0.95 | 0.95 | 0.96 | 0.97 |
| 2. Heart failure | 6.5 (17 332) | 9.0 (8282) | 1.40 | 1.38 | 1.39 | 1.42 | 1.41 | 1.45 |
| 3. Pulmonary edema | 2.3 (6222) | 3.7 (3396) | 1.65 | 1.77 | 1.52 | 1.56 | 1.58 | 1.50 |
| 4. Cardiogenic shock | 1.1 (3000) | 1.5 (1372) | 1.42 | 1.70 | 1.27 | 1.45 | 1.08 | 0.92 |
| CK or CK-MB ≥2 times reference range, % | 76.0 (203 768) | 71.7 (65 762) | 0.97 | 0.97 | 0.95 | 0.92 | 0.92 | 0.94 |
| Ejection fraction, % | ||||||||
| ≥40% | 59.6 (159 784) | 59.4 (54 430) | 0.98 | 0.99 | 1.00 | 0.99 | 1.00 | 1.01 |
| <40% | 14.6 (39 096) | 14.0 (12 857) | 0.95 | 0.96 | 0.94 | 0.99 | 0.98 | 0.92 |
| Not assessed | 25.9 (69 350) | 26.6 (24 413) | 1.04 | 1.03 | 1.04 | 1.06 | 1.01 | 1.04 |
| Treatments in first 24 hours | ||||||||
| Aspirin | 91.0 (244 162) | 88.0 (80 704) | 0.94 | 0.96 | 0.97 | 0.97 | 0.97 | 0.98 |
| β-Blockers | 67.6 (181 334) | 63.5 (58 244) | 0.88 | 0.92 | 0.93 | 0.94 | 0.96 | 0.97 |
Abbreviations: CABG, coronary artery bypass graft; CK, creatine kinase; CK-MB, creatine kinase MB; HMO, health maintenance organization; PPO, preferred provider organization; PTCA, percutaneous transluminal coronary angioplasty; STEMI, ST-segment elevation myocardial infarction.
Percentages do not total 100% because of missing values.
The numbers in parentheses do not equal the totals for “Men” and “Women” because a small category, “Other Insurance” was omitted from the Table.
Table 2. Demographic, Clinical, and Hospital Characteristics in Patients 65 Years or Older Classified According to Sex and Admission Yeara.
| Characteristics | Men, % (n=285 735) |
Women, % (n=270 715) |
Risk Ratios | |||||
|---|---|---|---|---|---|---|---|---|
| 1994-1995 | 1996-1997 | 1998-1999 | 2000-2001 | 2002-2003 | 2004-2006 | |||
| Race other than white | 12.7 (36 200) | 13.6 (36 609) | 1.06 | 1.05 | 1.06 | 1.09 | 1.06 | 1.10 |
| Primary medical insuranceb | ||||||||
| PPO | 22.5 (64 182) | 20.1 (54 545) | 0.64 | 0.63 | 0.92 | 0.91 | 0.93 | 0.90 |
| HMO | 14.0 (40 076) | 12.0 (32 496) | 0.71 | 0.77 | 0.84 | 0.87 | 0.90 | 0.93 |
| Medicare | 80.9 (231 256) | 84.7 (229 287) | 1.05 | 1.06 | 1.05 | 1.04 | 1.04 | 1.04 |
| Medicaid | 3.5 (10 040) | 6.7 (18 205) | 1.25 | 1.40 | 2.00 | 2.06 | 1.90 | 1.88 |
| Self-pay | 1.6 (4499) | 1.3 (3488) | 0.78 | 0.70 | 0.80 | 0.86 | 0.81 | 0.82 |
| Medical history and risk factors | ||||||||
| Myocardial infarction | 31.8 (90 954) | 25.4 (68 694) | 0.79 | 0.79 | 0.81 | 0.80 | 0.80 | 0.80 |
| Angina | 18.4 (52 580) | 16.9 (45 772) | 0.98 | 0.94 | 0.93 | 0.90 | 0.90 | 0.81 |
| Heart failure | 18.0 (51 306) | 23.3 (63 043) | 1.34 | 1.33 | 1.30 | 1.28 | 1.25 | 1.29 |
| PTCA | 12.8 (36 463) | 8.1 (21 999) | 0.64 | 0.63 | 0.65 | 0.63 | 0.65 | 0.64 |
| CABG | 21.7 (62 044) | 10.5 (28 331) | 0.47 | 0.47 | 0.48 | 0.48 | 0.50 | 0.49 |
| Stroke | 12.1 (34 675) | 13.2 (35 776) | 1.06 | 1.07 | 1.08 | 1.10 | 1.09 | 1.13 |
| Diabetes mellitus | 29.2 (83 511) | 31.2 (84 444) | 1.15 | 1.11 | 1.07 | 1.05 | 1.01 | 0.99 |
| Hypertension | 56.7 (162 002) | 66.9 (181 026) | 1.23 | 1.21 | 1.19 | 1.17 | 1.15 | 1.11 |
| Ever smoked | 14.7 (41 998) | 10.9 (29 388) | 0.72 | 0.76 | 0.73 | 0.74 | 0.70 | 0.79 |
| Clinical characteristics at admission | ||||||||
| Chest pain on presentation | 69.6 (198 972) | 64.6 (174 992) | 0.96 | 0.95 | 0.93 | 0.92 | 0.90 | 0.89 |
| STEMI | 50.4 (143 911) | 51.1 (138 372) | 1.03 | 1.03 | 0.99 | 1.03 | 1.01 | 0.97 |
| Killip class | ||||||||
| 1. No heart failure | 72.9 (208 285) | 67.4 (182 530) | 0.92 | 0.92 | 0.92 | 0.93 | 0.93 | 0.94 |
| 2. Heart failure | 18.5 (52 728) | 22.0 (59 679) | 1.20 | 1.20 | 1.19 | 1.19 | 1.19 | 1.21 |
| 3. Pulmonary edema | 6.9 (19 642) | 8.7 (23 665) | 1.26 | 1.28 | 1.28 | 1.24 | 1.30 | 1.29 |
| 4. Cardiogenic shock | 1.8 (5078) | 1.8 (4840) | 1.05 | 1.11 | 1.00 | 1.00 | 0.94 | 0.88 |
| CK or CK-MB ≥2 times reference range, % | 72.1 (206 120) | 69.0 (186 690) | 0.97 | 0.98 | 0.96 | 0.95 | 0.93 | 0.98 |
| Ejection fraction, % | ||||||||
| ≥40% | 44.2 (126 220) | 44.3 (120 031) | 0.96 | 0.99 | 1.01 | 1.03 | 1.02 | 1.01 |
| <40% | 23.4 (66 805) | 19.4 (52 565) | 0.81 | 0.83 | 0.82 | 0.83 | 0.84 | 0.86 |
| Not assessed | 32.4 (92 710) | 36.2 (98 119) | 1.12 | 1.10 | 1.11 | 1.11 | 1.15 | 1.19 |
| Treatments in first 24 hours | ||||||||
| Aspirin | 84.0 (239 988) | 81.4 (220 256) | 0.96 | 0.97 | 0.97 | 0.97 | 0.97 | 0.97 |
| β-Blockers | 55.8 (159 495) | 54.7 (148 072) | 0.95 | 0.96 | 0.98 | 0.98 | 0.99 | 1.00 |
Abbreviations: CABG, coronary artery bypass graft; CK, creatine kinase; CK-MB, creatine kinase MB; HMO, health maintenance organization; PPO, preferred provider organization; PTCA, percutaneous transluminal coronary angioplasty; STEMI, ST-segment elevation myocardial infarction.
Percentages do not total 100% because of missing values.
The numbers in parentheses do not equal the totals for “Men” and “Women” because a small category, “Other Insurance” was omitted from the Table.
Statistical Analysis
The study period was divided into six 2-year calendar segments from 1994 through 2006. The initial step of the analysis involved the description of patient and hospital characteristics by sex, age, and admission year; to ease the display of descriptive results, age was dichotomized at 65 years, and risk ratios for each characteristic were computed to compare women and men. Next, we plotted hospital mortality rates by sex, age, and time by means of 5 age intervals from younger than 55 years through 85 years or older. The odds ratios (ORs) for hospital death, which compared women to men, were calculated.
Next, we used generalized linear models for binomial distribution and logit link to estimate time trends in the ORs of mortality for women compared with men in different age groups, before and after adjusting for covariables. In these models, we used the pooled data across the 4 NRMI waves. A series of nested models were fitted for each age group. In the first model, we included sex, time, and the interaction between sex and time. To test for linear trend for time, time and its interaction with sex were tested as an ordinal variable.
In the second model, we adjusted for age, race, insurance status, ever smoking, medical history (previous angina pectoris, MI, diabetes mellitus, stroke, hypertension, coronary bypass surgery, and percutaneous interventions), and severity characteristics on admission (Killip class, STEMI, systolic blood pressure, pulse, myocardial enzyme levels, and left ventricular ejection fraction). In the third model, we added receipt of aspirin and β-blockers in the first 24 hours of admission. Finally, in the fourth model, we adjusted for all these factors plus hospital characteristics (number of beds, medical school affiliation, teaching hospital, urban location, MI volume, hospital ownership, and availability of invasive procedures). This approach allowed us to examine the separate contributions of patients, treatments, and hospital characteristics to the sex differences in hospital mortality over time.
To assess whether the interaction between sex and age on mortality changed over time (ie, whether the excess mortality in younger women vs men decreased or increased with time), we calculated the ratio of the female-to-male ORs of death in the initial period (1994-1995) over the last period (2004-2006) for each age group. This ratio describes the excess mortality in women vs men in 1994-1995 relative to 2004-2006. In addition, we refitted each of the 4 models with all the age groups combined and tested the 3-way interaction among sex, age, and time. Because hospital participation in NRMI changed over time, we conducted a sensitivity analysis restricted to 334 hospitals (of 1964 hospitals) who participated in the NRMI for the full study period, with the inclusion of 348 927 patients. All tests of statistical significance were 2-tailed, and all statistical analyses were performed with SAS statistical software, version 9.1.3, Service Pack 2 (SAS Institute Inc, Cary, North Carolina).
Results
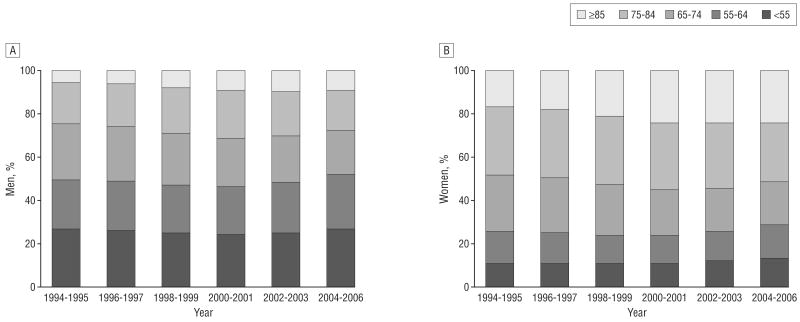
Of the 916 380 patients included in the analysis, approximately 10% of women and 25% of men throughout the study period were younger than 65 years (Figure 1). For both men and women, the overall risk factor profile worsened over time, with proportionally more patients having a history of diabetes, hypertension, hyperlipidemia, and congestive heart failure, whereas the prevalence of STEMI decreased, as previously reported.19 Irrespective of age, women more often had a history of hypertension than men, whereas men more often had a history of MI or previous revascularization. For other characteristics, however, sex differences were more marked among younger patients. Specifically, women younger than 55 years were more likely than men of similar age to have Medicaid insurance, a history of diabetes, heart failure or stroke, and a higher Killip class on admission; sex differences in these factors were less pronounced among older patients (Table 1 and Table 2). Furthermore, for diabetes, hypertension, and Killip class, the sex differences tended to be less pronounced in later years, which suggests that worsening trends for these factors were less marked in women. The sex difference in previous revascularization decreased over time among patients younger than 65 years but not among older patients. In addition, STEMI became less common in women younger than 65 years than in men of similar age in later years, whereas there was no difference in STEMI prevalence among older patients throughout the study period. Time trends in aspirin use in the first 24 hours were similar in women and men, irrespective of age, as they were for β-blockers among older patients. However, in 1994-1995, the use of β-blockers was less among women younger than 65 years than in their male counterparts; this gap decreased progressively in later years.
Figure 1.
Distribution of the study sample according to age and study period. A, Men; B, women.
Women were less likely than men to undergo coronary catheterization and revascularization procedures during admission; sex differences in procedure use were more pronounced among older patients. However, no difference was seen over time in the sex-related risk ratios for these procedures. Over time, there was a slight increase in the representation of hospitals of larger size, with larger MI volume, and with capability for invasive cardiac procedures. These changes followed similar trends over time by sex and age.
The length of hospital stay decreased over time more in younger than in older patients but in a similar fashion in women and men within age strata. Between 1994 and 2006, the total hospital time in patients younger than 65 years decreased 37.9% (−2.5 days) in men and 35.1% (−2.6 days) in women. Among patients 65 years or older, it decreased 25.0% (−1.9 days) in men and 25.6% in women (−2.0 days). No significant interaction was seen among sex, age, and year, which suggests that the total days in the hospital decreased in a similar fashion by sex and age.
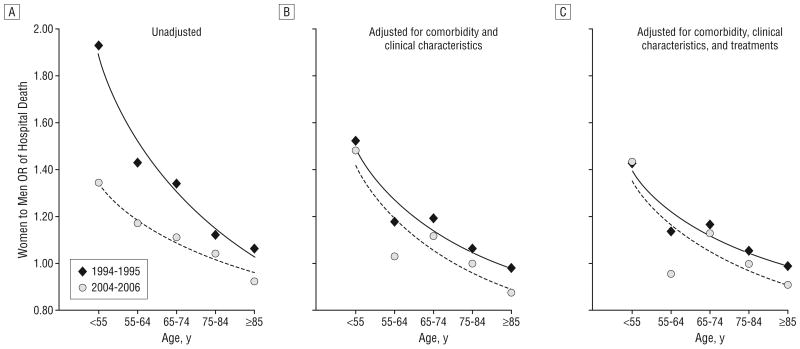
Between 1994 and 2006, hospital mortality rates decreased more in women than in men in virtually all age groups (Table 3). The rate reduction in 2004-2006 relative to the rate in 1994-1995 was particularly pronounced among women younger than 75 years. It was largest in women younger than 55 years (52.9%) and lowest in men younger than 55 years (33.3%). Among patients younger than 55 years, the excess mortality for women was 44.0% larger in 1994-1995 than it was in 2004-2006 (an OR of 1.93 vs an OR of 1.34; Table 4). Even when considered in absolute terms, the decrease in the mortality difference by sex was largest in younger patients, even though older patients had a larger absolute mortality decrease given their higher mortality rates. Among patients younger than 55 years, the absolute rate of decrease was 3 times higher in women (2.7%) than in men (0.9%). Both in absolute (Table 3) and relative (Table 4) terms, the sex difference in mortality decrease became lower in older patients (P=.004 for the interaction among sex, age, and year). As a result, the excess mortality in younger women compared with men decreased progressively over time (Table 4 and Figure 2A).
Table 3. Hospital Mortality Rates in Patients Classified According to Sex, Age, and Admission Year.
| Variable | Hospital Mortality Rate, %a | Total Reduction, % | Absolute Rate Reduction | Ratio (1994-1995 vs 2004-2006)b | |||||
|---|---|---|---|---|---|---|---|---|---|
| 1994-1995 | 1996-1997 | 1998-1999 | 2000-2001 | 2002-2003 | 2004-2006 | ||||
| Men, y | |||||||||
| <55 | 2.7 | 2.6 | 2.5 | 2.4 | 2.2 | 1.8 | 33.3 | 0.9 | 3.00c |
| 55-64 | 5.7 | 5.3 | 4.7 | 4.4 | 4.0 | 3.3 | 42.1 | 2.4 | 1.71d |
| 65-74 | 10.5 | 9.6 | 9.4 | 8.8 | 7.3 | 5.9 | 43.8 | 4.6 | 1.54e |
| 75-84 | 18.1 | 16.1 | 15.3 | 14.3 | 12.6 | 10.9 | 39.8 | 7.2 | 1.17f |
| ≥85 | 24.7 | 23.1 | 21.6 | 19.6 | 18.6 | 16.0 | 35.2 | 8.7 | 1.24g |
| Women, y | |||||||||
| <55 | 5.1 | 4.9 | 3.7 | 3.6 | 3.3 | 2.4 | 52.9 | 2.7 | NA |
| 55-64 | 8.0 | 7.9 | 7.1 | 6.4 | 5.6 | 3.9 | 51.3 | 4.1 | NA |
| 65-74 | 13.6 | 12.3 | 11.1 | 9.8 | 8.5 | 6.5 | 52.2 | 7.1 | NA |
| 75-84 | 19.7 | 17.7 | 16.3 | 14.6 | 13.1 | 11.3 | 42.6 | 8.4 | NA |
| ≥85 | 25.8 | 23.5 | 21.0 | 19.7 | 17.9 | 15.0 | 41.9 | 10.8 | NA |
Abbreviation: NA, not applicable.
P values for trend with time were <.001 for all patients.
This is the ratio of the rate difference (1994-1995 to 2004-2006) in women over the rate difference in men. It indicates the excess mortality in absolute terms in women vs men in 1994-1995 relative to 2004-2006.
P<.001 for interaction between sex and year.
P=.13 for interaction between sex and year.
P<.001 for interaction between sex and year.
P=.03 for interaction between sex and year.
P=.02 for interaction between sex and year.
Table 4. Unadjusted and Adjusted Female-to-Male Odds Ratios (95% Confidence Intervals) for Hospital Mortality According to Age and Admission Year.
| Age Group, y | 1994-1995 | 1996-1997 | 1998-1999 | 2000-2001 | 2002-2003 | 2004-2006 | Ratio (1994-1995 vs 2004-2006)a | P Value for Interaction | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Sex and Yearb | Sex, Age, and Yearc | |||||||||
| Model 1: Unadjusted | ||||||||||
| <55 | 1.93 (1.67-2.24) | 1.89 (1.68-2.13) | 1.53 (1.33-1.75) | 1.54 (1.34-1.79) | 1.50 (1.26-1.77) | 1.34 (1.07-1.67) | 1.44 | <.001 |
|
.004 |
| 55-64 | 1.43 (1.28-1.59) | 1.55 (1.42-1.69) | 1.53 (1.39-1.68) | 1.46 (1.32-1.63) | 1.43 (1.26-1.62) | 1.17 (0.99-1.38) | 1.22 | .08 | ||
| 65-74 | 1.34 (1.25-1.43) | 1.33 (1.25-1.41) | 1.21 (1.14-1.29) | 1.12 (1.05-1.21) | 1.18 (1.08-1.29) | 1.11 (0.99-1.26) | 1.21 | <.001 | ||
| 75-84 | 1.12 (1.05-1.18) | 1.12 (1.06-1.17) | 1.07 (1.02-1.13) | 1.03 (0.97-1.08) | 1.05 (0.98-1.12) | 1.04 (0.96-1.14) | 1.08 | .02 | ||
| ≥85 | 1.06 (0.98-1.16) | 1.02 (0.96-1.09) | 0.97 (0.91-1.03) | 1.01 (0.95-1.07) | 0.96 (0.89-1.03) | 0.92 (0.84-1.01) | 1.15 | .02 | ||
|
| ||||||||||
| Model 2: Adjusted for Comorbidity and Clinical Characteristics at Admissiond | ||||||||||
| <55 | 1.52 (1.26-1.83) | 1.41 (1.21-1.64) | 1.35 (1.13-1.60) | 1.27 (1.06-1.54) | 1.12 (0.90-1.39) | 1.48 (1.12-1.95) | 1.03 | .12 |
|
.34 |
| 55-64 | 1.18 (1.03-1.34) | 1.26 (1.13-1.40) | 1.34 (1.19-1.51) | 1.23 (1.08-1.40) | 1.19 (1.02-1.39) | 1.03 (0.84-1.27) | 1.15 | .64 | ||
| 65-74 | 1.19 (1.10-1.28) | 1.21 (1.13-1.29) | 1.15 (1.07-1.24) | 1.01 (0.93-1.10) | 1.09 (0.98-1.21) | 1.12 (0.97-1.30) | 1.06 | .003 | ||
| 75-84 | 1.06 (0.99-1.13) | 1.06 (1.00-1.12) | 1.04 (0.99-1.10) | 0.99 (0.93-1.05) | 1.00 (0.93-1.08) | 1.00 (0.90-1.11) | 1.06 | .04 | ||
| ≥85 | 0.98 (0.89-1.08) | 1.00 (0.93-1.08) | 0.95 (0.89-1.03) | 0.92 (0.86-1.00) | 0.91 (0.84-0.99) | 0.88 (0.78-0.99) | 1.11 | .07 | ||
|
| ||||||||||
| Model 3: Also Adjusted for Admission Treatments in First 24 Hourse | ||||||||||
| <55 | 1.42 (1.17-1.71) | 1.33 (1.14-1.55) | 1.25 (1.04-1.49) | 1.22 (1.01-1.48) | 1.04 (0.83-1.31) | 1.43 (1.07-1.90) | 0.99 | .30 |
|
.47 |
| 55-64 | 1.13 (0.99-1.29) | 1.23 (1.11-1.37) | 1.30 (1.16-1.47) | 1.20 (1.05-1.36) | 1.18 (1.01-1.38) | 0.95 (0.77-1.17) | 1.19 | .79 | ||
| 65-74 | 1.16 (1.07-1.26) | 1.20 (1.12-1.28) | 1.14 (1.05-1.22) | 1.00 (0.92-1.09) | 1.06 (0.95-1.19) | 1.13 (0.97-1.31) | 1.03 | .007 | ||
| 75-84 | 1.05 (0.98-1.12) | 1.05 (1.00-1.11) | 1.04 (0.99-1.11) | 0.98 (0.92-1.05) | 1.00 (0.92-1.08) | 0.99 (0.89-1.10) | 1.06 | .04 | ||
| ≥85 | 0.98 (0.89-1.08) | 0.99 (0.92-1.07) | 0.96 (0.89-1.03) | 0.93 (0.86-1.00) | 0.92 (0.84-1.00) | 0.90 (0.80-1.01) | 1.09 | .14 | ||
|
| ||||||||||
| Model 4: Also Adjusted for Hospital Characteristicsf | ||||||||||
| <55 | 1.42 (1.18-1.72) | 1.35 (1.15-1.58) | 1.23 (1.03-1.48) | 1.23 (1.01-1.50) | 1.07 (0.85-1.34) | 1.42 (1.06-1.90) | 1.00 | .27 |
|
.50 |
| 55-64 | 1.13 (0.99-1.30) | 1.24 (1.11-1.38) | 1.32 (1.17-1.49) | 1.19 (1.04-1.36) | 1.19 (1.02-1.40) | 0.95 (0.76-1.17) | 1.19 | .78 | ||
| 65-74 | 1.16 (1.07-1.26) | 1.19 (1.11-1.28) | 1.13 (1.04-1.21) | 1.02 (0.93-1.11) | 1.06 (0.95-1.19) | 1.13 (0.96-1.32) | 1.03 | .01 | ||
| 75-84 | 1.05 (0.99-1.13) | 1.05 (0.99-1.11) | 1.04 (0.98-1.10) | 0.98 (0.92-1.05) | 1.00 (0.92-1.08) | 1.00 (0.90-1.11) | 1.05 | .05 | ||
| ≥85 | 0.98 (0.89-1.09) | 0.99 (0.92-1.07) | 0.95 (0.88-1.03) | 0.93 (0.86-1.00) | 0.92 (0.84-1.01) | 0.90 (0.79-1.01) | 1.09 | .13 | ||
This is the ratio of the relative risks and is a measure of the interaction between sex and year. It indicates the excess mortality in women vs men in 1994-1995 relative to 2004-2006.
This P value indicates whether there is a significant difference in the trend over time for mortality in comparison between women and men.
This P value indicates whether there is a significant difference in the trend over time for mortality in comparison between women and men according to different age groups.
Model 2 adjusted forage, race, insurance status, ever smoked, medical history (previous angina pectoris, myocardial infarction, diabetes mellitus, stroke, hypertension, coronary bypass surgery, and percutaneous interventions) and severity characteristics on admission (Killip class, ST-segment elevation myocardial infarction, systolic blood pressure, pulse, myocardial enzyme levels, and left ventricular ejection fraction).
Model 3, in addition to the variables listed for model 2, adjusted for aspirin and β-blocker use in the first 24 hours.
Model 4, in addition to the variables listed for models 2 and 3, adjusted for hospital characteristics (number of beds, medical school affiliation, teaching hospital, urban location, myocardial infarction volume, hospital ownership, and availability of invasive procedures).
Figure 2.
Unadjusted and adjusted odds ratios (ORs) for death during hospitalization for myocardial infarction in women compared with men, according to age and study period. The unadjusted ORs (A) were derived from the model that included sex; age (5 groups); study admission year (6 groups); the interaction among sex, age, and year; and all lower-level interactions. The ORs adjusted for comorbidity and clinical characteristics (B) were derived from the model that also included age, race, insurance status, ever smoked, medical history, and severity characteristics on admission. The ORs adjusted for comorbidity, clinical characteristics, and treatments (C) were derived from the model that, in addition to all these factors, included aspirin and β-blocker use in the first 24 hours.
Adjustment for comorbidity and clinical characteristics on admission accounted for a large part of the excess mortality of younger women compared with younger men in the entire period by lowering the female-to-male ORs in the younger age groups in each study year (Table 4). It also substantially explained the differential mortality trends over time by bringing the OR for 1994-1995 closer to the OR for 2004-2006 (Figure 2B) and making the interaction among sex, age, and year no longer significant (P=.34). Overall, time changes in comorbidity and clinical features explained 93% of the changes in mortality in younger women relative to men, as shown by the decrease in the ORs (1994-1995 vs 2004-2006) from 1.44 to 1.03 (Table 4). In contrast, treatment did not explain further the differential mortality trends over time, although it somewhat lowered the mortality difference between women and men in all years (Figure 2C and Table 4). Further adjustment for hospital characteristics had no impact on the women-to-men estimates (Table 4). When the analysis was restricted to only hospitals that participated in the NRMI for the entire study period, results were similar, which suggests that variations in hospital participation in the NRMI had no substantial impact on our results. Finally, when analyses were restricted to patients without a previous history of MI (n=680 995; 74%), the results remained substantially unchanged, which suggests that our results are not driven by better recognition or treatment of previous MI events. In this subgroup, time changes in comorbidity and clinical features explained 91% of the changes in mortality in younger women relative to men.
Comment
Our study documents remarkable reductions in hospital mortality after MI for both men and women during the past decade. However, women experienced larger improvements in mortality than men, with the largest relative improvement among younger women relative to men of similar age. As a result, the higher MI mortality rates for younger women compared with men, although still present, have substantially narrowed during this period.
Whether there are true sex-related differences in mortality after MI or whether they are owing to older age or higher prevalence of coexisting diseases in women has been debated for more than 20 years. Only recently has it become apparent that sex-related mortality differences are confined to younger patients, typically those younger than 60 years. This is an age group in which an MI occurs rarely in women. If women in this age bracket develop an MI, they show higher mortality and complication rates than men, whereas no mortality differences, or even better outcomes for women, are found when older women are compared with older men.2-10 The reasons for this age-dependent disparity in mortality are not clear. Although patient characteristics and treatments do not entirely account for it,3,4 a disproportionate burden of coronary risk factors and comorbidities is a clear feature of younger women with MI, with the inclusion of conditions such as diabetes, heart failure, and previous stroke. These women also tend to be treated less aggressively.3 On the other hand, indicators of infarct size or severity, such as myocardial enzyme levels, left ventricular ejection fraction, and ST-segment elevation or Q waves on the initial electrocardiogram, tend to be similar or more favorable in younger women than men.3,4 Thus, differences in symptom presentation, the accuracy of diagnostic tests for coronary heart disease, or other factors that lead to differential triage, evaluation, or early treatment of younger women with acute ischemia compared with men could contribute to their worse outcome.20,21
We hypothesized that better awareness of heart disease in younger women in recent years or improved diagnosis or treatment13,22 could have reduced their risk profile compared with men and contributed to lowering the sex difference in mortality. We found that certain clinical factors on admission, particularly history of diabetes, increased less in younger women than men of similar age, whereas STEMI and Killip class decreased more, which led to a narrowing of the sex differences in presentation characteristics over time among younger patients. A reduction of the sex difference in previous revascularization was also noted in younger patients. A large part (93%) of this sharper decrease in mortality of younger women compared with men in recent years was because the risk status of women on admission improved compared with that of men. Such improvement may be due to better recognition and management of coronary heart disease and its risk factors in women before the acute MI event, as suggested by the narrowing in the sex difference in previous revascularization. Given the changing pattern over time, our data also suggest that the excessive burden of risk factors consistently reported in younger women with MI relative to men is in part a result of delay in recognition or treatment rather than being exclusively owing to differential pathophysiologic findings.
Other studies23-26 have described decreased mortality after MI in recent years, and most attributed such a decrease to improved treatment. Although in our study use of therapies on the first day of admission helped explain some of the overall sex differences in mortality, they did not account for the differential mortality trends by sex and age. This finding is not surprising because treatment use over time followed similar trends in men and women, both young and old, which confirms our previous observations of no substantial narrowing of differential management of MI by demographic groups in recent years.26,27
A number of possible limitations of our study should be mentioned. We only had data on in-hospital mortality; therefore, our results may not be extrapolated to long-term outcomes. In addition, because information on prehospital deaths was not available, we were unable to determine whether sex differences in hospital mortality trends were owing to changing patterns of mortality before hospitalization. A decreasing length of hospital stay could also influence mortality trends by inflation of the decrease in in-hospital mortality if deaths occurred more often after discharge. However, this possibility is unlikely to affect our estimates of sex differences because hospital length of stay decreased in a similar fashion in women and men. Detailed patient-level data, such as socioeconomic factors, functional status, and psychosocial factors, which may have an impact on sex differences in mortality, were not available for our analysis. On the other hand, the simplicity of the protocol and the brief case ascertainment form enable the NRMI to be the largest and most contemporary MI registry in the United States. Another limitation is that information with regard to the use of statins was not available early in the registry. Therefore, we were unable to examine whether sex differences in prescription of statins over time could explain sex differences in mortality. The NRMI hospitals lacked independent verification of data collection; however, the data collection process has been validated through a comparison with the Cooperative Cardiovascular Project.16 Participation in NRMI is voluntary, and participating hospitals tend to differ from those that choose not to participate by being larger, more procedure-oriented centers with an interest in the improvement of quality metrics and processes.15 Hospital characteristics also slightly changed over time. However, men and women did not differ in the characteristics of their admitting hospital during the entire study period. Therefore, a self-selection of hospitals into the registry should not have introduced bias in our estimate of sex differences in mortality. In fact, addition of hospital data to the statistical models had virtually no impact on the study results.
In conclusion, between 1994 and 2006, women have experienced larger improvements in mortality when hospitalized for MI than men. The mortality reduction relative to men was most pronounced among younger women and is mostly the result of improved risk profile on presentation. As a result, the higher mortality rates of younger women with MI compared with men, as described in earlier years, has narrowed considerably.
Acknowledgments
Funding/Support: The NRMI is funded by Genentech Inc. Dr Vaccarino is supported by grant K24HL077506 from the National Institutes of Health.
Footnotes
Author Contributions: Study concept and design: Vaccarino and Canto. Analysis and interpretation of data: Vaccarino, Parsons, Peterson, Kiefe, and Canto. Drafting of the manuscript: Vaccarino and Canto. Critical revision of the manuscript for important intellectual content: Vaccarino, Parsons, Peterson, Rogers, Kiefe, and Canto. Statistical analysis: Parsons. Study supervision: Vaccarino.
Financial Disclosure: Ms Parsons is a paid consultant for Genentech Inc.
Additional Information: Neither this manuscript nor one with substantially similar content under our authorship has been published or is being considered for publication elsewhere, except as an abstract.
Publisher's Disclaimer: Disclaimer: Funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
References
- 1.Lloyd Jones D, Adams R, Carnethon M, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Vaccarino V, Horwitz RI, Meehan TP, Petrillo MK, Radford MJ, Krumholz HM. Sex differences in mortality after myocardial infarction: evidence for a sex-age interaction. Arch Intern Med. 1998;158(18):2054–2062. doi: 10.1001/archinte.158.18.2054. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex-based differences in early mortality after myocardial infarction: National Registry of Myocardial Infarction 2 participants. N Engl J Med. 1999;341(4):217–225. doi: 10.1056/NEJM199907223410401. [DOI] [PubMed] [Google Scholar]
- 4.Vaccarino V, Krumholz HM, Yarzebski J, Gore JM, Goldberg RJ. Sex differences in 2-year mortality after hospital discharge for myocardial infarction. Ann Intern Med. 2001;134(3):173–181. doi: 10.7326/0003-4819-134-3-200102060-00007. [DOI] [PubMed] [Google Scholar]
- 5.Rosengren A, Spetz CL, Koster M, Hammar N, Alfredsson L, Rosen M. Sex differences in survival after myocardial infarction in Sweden: data from the Swedish National Acute Myocardial Infarction Register. Eur Heart J. 2001;22(4):314–322. doi: 10.1053/euhj.2000.2368. [DOI] [PubMed] [Google Scholar]
- 6.Andrikopoulos GK, Tzeis SE, Pipilis AG, et al. Investigators of the Hellenic Study of AMI. Younger age potentiates post myocardial infarction survival disadvantage of women. Int J Cardiol. 2006;108(3):320–325. doi: 10.1016/j.ijcard.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 7.Mukamal KJ, Muller JE, Maclure M, Sherwood JB, Mittleman MA. Evaluation of sex-related differences in survival after hospitalization for acute myocardial infarction. Am J Cardiol. 2001;88(7):768–771. doi: 10.1016/s0002-9149(01)01848-3. [DOI] [PubMed] [Google Scholar]
- 8.MacIntyre K, Stewart S, Capewell S, et al. Gender and survival: a population-based study of 201,114 men and women following a first acute myocardial infarction. J Am Coll Cardiol. 2001;38(3):729–735. doi: 10.1016/s0735-1097(01)01465-6. [DOI] [PubMed] [Google Scholar]
- 9.Koek HL, de Bruin A, Gast F, et al. Short- and long-term prognosis after acute myocardial infarction in men versus women. Am J Cardiol. 2006;98(8):993–999. doi: 10.1016/j.amjcard.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Simon T, Mary krause M, Cambou JP, et al. USIC Investigators. Impact of age and gender on in-hospital and late mortality after acute myocardial infarction: increased early risk in younger women: results from the French nationwide USIC registries. Eur Heart J. 2006;27(11):1282–1288. doi: 10.1093/eurheartj/ehi719. [DOI] [PubMed] [Google Scholar]
- 11.Wexler LF. Studies of acute coronary syndromes in women—lessons for everyone. N Engl J Med. 1999;341(4):275–276. doi: 10.1056/NEJM199907223410409. [DOI] [PubMed] [Google Scholar]
- 12.Ayanian JZ. Increased mortality among middle-aged women after myocardial infarction: searching for mechanisms and solutions. Ann Intern Med. 2001;134(3):239–241. doi: 10.7326/0003-4819-134-3-200102060-00015. [DOI] [PubMed] [Google Scholar]
- 13.Mosca L, Mochari H, Christian A, et al. National study of women's awareness, preventive action, and barriers to cardiovascular health. Circulation. 2006;113(4):525–534. doi: 10.1161/CIRCULATIONAHA.105.588103. [DOI] [PubMed] [Google Scholar]
- 14.Ford ES, Capewell S. Coronary heart disease mortality among young adults in the U.S. from 1980 through 2002. J Am Coll Cardiol. 2007;50(22):2128–2132. doi: 10.1016/j.jacc.2007.05.056. [DOI] [PubMed] [Google Scholar]
- 15.Rogers WJ, Bowlby LJ, Chandra NC, et al. Observations from the National Registry of Myocardial Infarction. Treatment of myocardial infarction in the United States (1990 to 1993): observations from the National Registry of Myocardial Infarction. Circulation. 1994;90(4):2103–2114. doi: 10.1161/01.cir.90.4.2103. [DOI] [PubMed] [Google Scholar]
- 16.Every NR, Frederick PD, Robinson M, Sugarman J, Bowlby L, Barron HV. A comparison of the National Registry of Myocardial Infarction 2 with the Cooperative Cardiovascular Project. J Am Coll Cardiol. 1999;33(7):1886–1894. doi: 10.1016/s0735-1097(99)00113-8. [DOI] [PubMed] [Google Scholar]
- 17.Rogers WJ, Canto JC, Lambrew CT, et al. Temporal trends in the treatment of over 1.5 million patients with myocardial infarction in the U.S. from 1990 through 1999. J Am Coll Cardiol. 2000;36(7):2056–2063. doi: 10.1016/s0735-1097(00)00996-7. [DOI] [PubMed] [Google Scholar]
- 18.Killip T, III, Kimball JT. Treatment of myocardial infarction in a coronary care unit: a two-year experience with 250 patients. Am J Cardiol. 1967;20(4):457–464. doi: 10.1016/0002-9149(67)90023-9. [DOI] [PubMed] [Google Scholar]
- 19.Rogers WJ, Frederick PD, Stoehr E, et al. Trends in presenting characteristics and hospital mortality among patients with ST elevation and non-ST elevation myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156(6):1026–1034. doi: 10.1016/j.ahj.2008.07.030. [DOI] [PubMed] [Google Scholar]
- 20.Pope JH, Aufderheide TP, Ruthazer R, et al. Missed diagnoses of acute cardiac ischemia in the emergency department. N Engl J Med. 2000;342(16):1163–1170. doi: 10.1056/NEJM200004203421603. [DOI] [PubMed] [Google Scholar]
- 21.Wenger NK, Shaw LJ, Vaccarino V. Coronary heart disease in women: update 2008. Clin Pharmacol Ther. 2008;83(1):37–51. doi: 10.1038/sj.clpt.6100447. [DOI] [PubMed] [Google Scholar]
- 22.Mosca L, Linfante AH, Benjamin EJ, et al. National study of physician awareness and adherence to cardiovascular disease prevention guidelines. Circulation. 2005;111(4):499–510. doi: 10.1161/01.CIR.0000154568.43333.82. [DOI] [PubMed] [Google Scholar]
- 23.Watkins S, Thiemann D, Coresh J, Powe N, Folsom AR, Rosamond W. Fourteen-year (1987 to 2000) trends in the attack rates of, therapy for, and mortality from non–ST-elevation acute coronary syndromes in four United States communities. Am J Cardiol. 2005;96(10):1349–1355. doi: 10.1016/j.amjcard.2005.07.037. [DOI] [PubMed] [Google Scholar]
- 24.Masoudi FA, Foody JM, Havranek EP, et al. Trends in acute myocardial infarction in 4 US states between 1992 and 2001: clinical characteristics, quality of care, and outcomes. Circulation. 2006;114(25):2806–2814. doi: 10.1161/CIRCULATIONAHA.106.611707. [DOI] [PubMed] [Google Scholar]
- 25.Setoguchi S, Glynn RJ, Avorn J, Mittleman MA, Levin R, Winkelmayer WC. Improvements in long-term mortality after myocardial infarction and increased use of cardiovascular drugs after discharge: a 10-year trend analysis. J Am Coll Cardiol. 2008;51(13):1247–1254. doi: 10.1016/j.jacc.2007.10.063. [DOI] [PubMed] [Google Scholar]
- 26.Peterson ED, Shah BR, Parsons L, et al. Trends in quality of care for patients with acute myocardial infarction in the National Registry of Myocardial Infarction from 1990 to 2006. Am Heart J. 2008;156(6):1045–1055. doi: 10.1016/j.ahj.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 27.Vaccarino V, Rathore SS, Wenger NK, et al. National Registry of Myocardial Infarction Investigators. Sex and racial differences in the management of acute myocardial infarction, 1994 through 2002. N Engl J Med. 2005;353(7):671–682. doi: 10.1056/NEJMsa032214. [DOI] [PMC free article] [PubMed] [Google Scholar]