Abstract
Schistosomes (blood flukes) are long lived, intravascular parasites that afflict ~200 million people worldwide. Here we review the potential ability of these parasites to exert control on local vascular physiology. We examine schistosome kallikrein-like proteins that drive vasodilation. We review biogenic amine metabolism in the parasites that involve the vasodilator histamine and its receptors and the vasoconstrictor serotonin and its receptor. Schistosomes can trigger the release of histamine from host cells and can import serotonin. We consider the ability of schistosomes to generate and release the eicosanoid vasodilators PGD2 and PGE2 and the vasoconstrictors LTB4 and LTC4. The literature on nitric oxide metabolism in these blood flukes is assessed. Finally the potential impact of other schistosome metabolic processes (e.g. exogenous adenosine generation and acetylcholine degradation) on vascular function is appraised. An increased understanding of these processes could lead to novel anti-parasitics as well as new therapies to treat vascular dysfunction.
Keywords: schistosome, parasite, vasodilation, vasoconstriction, kinin, histamine, serotonin, ecoisanoid, PGD2, PGE2, LTB4, LTC4, Nitric oxide, adenosine
Schistosomes are intravascular parasitic worms (flukes) belonging to the phylum platyhelminthes. They can cause a chronic, often debilitating disease called schistosomiasis that afflicts more than 200 million people in over 70 countries worldwide1. Mortality is put at over 250,000 deaths annually in sub-Saharan Africa alone 2–3. Three species are especially important for humans; these are Schistosoma mansoni, S. japonicum and S. hematobium4. Humans become infected when larval forms called cercariae emerge from intermediate freshwater snail hosts and penetrate the skin. Here the worms undergo a complex biochemical and morphological transformation into juvenile forms called schistosomula. Schistosomula invade a blood vessel and travel in the vasculature through the heart and lungs to the liver5,6. Here mature males and females mate and migrate to their preferred egg laying sites7,8. For S. mansoni and S. japonicum this is the mesenteric veins and for S. haematobium the vesicle plexus of the urinary bladder. The parasites can live in the blood for years, sometimes decades4.
An ability by intravascular schistosomes to control local vascular tone would be advantageous especially given the large size of the adults (~0.5 mm in diameter and up to 10 mm long) relative to the smaller blood vessels into which they travel to lay eggs. The capacity to e.g. promote vasodilation would permit the worms freer mobility in the vasculature to move temporarily into the smaller blood vessels to deposit eggs and to later migrate freely and safely away from these sites without provoking harmful ischemia and hypoxia that could damage them. The shared evolutionary ancestry of schistosomes and their hosts has led to a degree of shared physiology and biochemistry. Schistosomes are known to be able to generate several metabolites that could impose upon host vascular physiology and these are discussed in this review. The control of vascular tone is complex and can involve a wide variety of biomolecules including peptides, amino acid derivatives, lipids, and gases and schistosomes can produce several of these metabolites. The interaction between schistosomes and these many molecular components that could impinge on host vascular function has not been fully evaluated. Here we review the ability of schistosomes to produce such molecules (kinins, biogenic amines, ecoisanoids, nitric oxide and other metabolites) to potentially exert direct control on specific aspects of local vascular performance.
Schistosomes and kinins
Kinins are small polypeptides that are proteolytically cleaved from globular proteins in blood. The 9-amino acid kinin, bradykinin is generated by the action of the serine protease kallikrein. Bradykinin is a potent vasodilator that increases vascular permeability. An enzyme with kallikrein-like activity was purified from the supernatant of a S. mansoni adult worm homogenate9. The 66kDa enzyme (designated sK1) cleaved bradykinin from purified rat plasma kinin precursor (kininogen) and hydrolysed the kallikrein synthetic substrate d-Pro-Phe-Arg-p-nitroanilide. Activity in adult males was ~ 20 times higher than in females. Intravenous injection of sK1 into an anaesthetized rat induced a drastic reduction in the arterial blood pressure of the animal. This effect lasted for ~ 1 min, and was followed by a progressive recovery of the arterial pressure. Neither bradycardia nor cardiac arrhythmias were noticed, suggesting a peripheral vasodilation effect9. Later work, using a degenerate PCR approach, identified a developmentally regulated S. mansoni putative serine protease (designated SmSP1) with homology to mouse plasma kallikrein10. Since the cloned DNA encodes a ~35kDa protein, this molecule clearly differs from the larger, active sK1 enzyme described above. SmSP1 was detected in schistosomula released products and in male dorsal spines. The sK1 protein was found at the tegumental surface of the parasite9. These localization sites are consistent with a proposed role for the proteins in regulating the parasite’s local vascular environment.
Schistosomes and biogenic amines
Biogenic amines are derivatives of the amino acids tryptophan, tyrosine or histidine that exert control over many functions in the body including vascular performance. Here we consider two such molecules: histamine and serotonin (5-Hydroxytryptamine (5-HT)) which are synthesized from the amino acids histidine and tryptophan, respectively.
A. Histamine
Histamine is perhaps best known as an important neuroactive substance in both vertebrates and invertebrates, but it can also act as a powerful vasodilator. In vertebrates, histamine is mostly derived from mast cells and from basophils. In adult schistosomes histamine can be detected widely in the peripheral nervous system including in the subtegumental plexus11. In addition, two G-protein coupled receptors, SmGPCR-1 and SmGPCR-2, have been cloned in schistosomes that respond to histamine11,12. The widespread distribution of histamine and the presence of these two functionally characterized histamine receptors suggest that this transmitter is an important neuroactive substance in these parasites11,12. Whether schistosomes can release histamine into the local environment to exert control on vascular function is unknown.
It has been suggested that the parasites may be able to impinge upon the host’s histamine signaling system. This is because SmGPCR-1, in addition to being expressed in the subtegumental musculature of schistosomula, is particularly highly expressed in the tegument12. In the adult stage too SmGPCR-1 is detected not only in the body wall musculature but mainly in the tegumental tubercles of male worms. The outer tegumental localization of the receptor suggests that it is not part of an endogenous signaling system. Instead, SmGPCR-1 may be activated by exogenous signals and respond to changes in environmental histamine that facilitates migration through the vasculature12. The clustering of SmGPCR-1 in the tubercles, which are enriched in sensory nerve endings13, suggests that the receptor might act through chemosensory circuits that originate at the parasite surface. The neuronal processes that supply the tubercles connect to peripheral elements and ultimately the CNS, so that any signaling through these circuits could have profound effects on worm behavior.
Receptor SmGPCR-2 is expressed in the nervous system and is particularly enriched in the subtegumental neuronal plexus of adults and schistosomula11. Since it is not exposed to the host, SmGPCR-2 seems less likely to be in direct contact with host metabolites. However, both SmGPCR-1 and SmGPCR-2 exhibit a common developmental expression pattern, being up-regulated in the intravascular stages compared with the freshwater, free-living stage and expression levels of both receptor genes peak during the first week of schistosomula development. It has been suggested that the increase in expression at this time may be tied to the need of the young schistosomula to exploit the host’s histamine signaling system to increase vascular permeability, which in turn may facilitate parasite passage through blood vessels during larval migration14.
There is evidence that the parasite may be able to stimulate host histamine release14,15. S. mansoni cercariae, but not schistosomula, are reported to be capable of triggering the release of histamine from rat peritoneal mast cells in vitro14. In addition, a schistosome homolog of the mammalian translationally controlled tumor protein (SmTCTP) has been identified that, in recombinant form, can cause histamine release from rat basophilic leukemia cells15. Histamine release from host cells induced by young, invading parasites could assist the worms in penetrating the tissues. This would help loosen endothelial junctions and facilitate parasite entry into, and migration within, the blood stream. In contrast, other workers have argued that such effects could have the opposite impact; histamine-induced increased vascular permeability could lead to an influx of anti-worm immune effectors which could hinder the parasites16.
B. Serotonin
Serotonin is one of the more ubiquitous neuroactive agents among animal phyla. In the blood of vertebrates, serotonin is taken up and stored by platelets which release it upon damage to blood vessel walls. It acts as a potent vasoconstrictor.
There is ample evidence that serotonin is present in the schistosome nervous system13,17 and is biologically active18. S. mansoni has been shown to possess a functional tryptophan hydroxylase (the enzyme that catalyzes the rate-limiting step in the biosynthesis of serotonin) showing that the parasites have the machinery to synthesize this metabolite19. Despite this, schistosomes have long been known to have the ability to import exogenous serotonin20. A serotonin transporter (SmSERT), existing in two allelic isoforms, has been cloned from S. mansoni21, 22. Following heterologous expression, recombinant SmSERT was found to be selective for serotonin and was inhibited by the same antagonists that also blocked intake of serotonin by intact parasites in culture21. While SmSERT is expressed in free-living infectious schistosomes (cercaria), the level of expression is substantially higher in the intravascular life stages (schistosomula and adults)21.
Serotonin uptake may supplement that synthesized internally. Alternatively, it has been suggested that the intake of host serotonin is part of a signaling mechanism used by the parasite to modulate its motility in the bloodstream21. Some flatworms guide their migration in the host by responding to changes in serotonin concentration23. Perhaps in schistosomes SmSERT plays a role in this process by controlling the intake of serotonin in response to variable concentrations in the host. Whether there is an additional selective advantage for schistosomes to take up serotonin from the blood to thereby decrease the local concentration of this vasoconstrictor is unknown.
Schistosomes and eicosanoids
Eicosanoids are important signaling molecules derived from essential fatty acids. There are four families of eicosanoids—the prostaglandins, prostacyclins, the thromboxanes and the leukotrienes. Eicosanoids exert complex control over many functions in the body including vascular performance. They have specific effects on target cells close to their site of formation. They are rapidly degraded, so they are not transported to distal sites within the body. A number of studies have revealed that schistosomes can synthesize and secrete eicosanoids24–27. These studies have largely focused on the potential ability of released schistosome eicosanoids to impede host immune function24,25. However a number of the metabolites, such as prostaglandin D2 (PGD2) and prostaglandin E2 (PGE2) that can be released by larval and adult worms, have powerful vasodilator capabilities28,29. Additional eicosanoids synthesized by schistosomes include leukotriene B4 (LTB4) and leukotriene C4 (LTC4) and these can exert vasoconstrictive effects30,31. Schistosomes have been shown to release the vasoconstrictors LTB4 and LTC4 in culture at substantially lower levels when compared with the vasodilators PGD2 and PGE224. These data suggest that intravascular schistosomes could use their endogenous eicosanoid metabolism to impinge upon host vascular dynamics. What controls parasite release of its vasoactive eicosanoids and whether they actually impact the vasculature is not yet known.
Schistosomes and nitric oxide (NO)
Nitric oxide (NO) is a widespread gaseous messenger molecule that plays a role in multiple physiological processes including neuronal communication and cell survival. NO is synthesized from oxygen and the amino acid L-arginine by various nitric oxide synthase (NOS) enzymes, such as neuronal NOS and inducible NOS. NO is highly reactive, diffuses freely across membranes and has a half-life of just a few seconds. NO, generated and released by several types of immune effector cells, is toxic to many pathogens. In terms of its vasoactivity, NO, released from endothelial cells, relaxes vascular smooth muscle and this results in vasodilation. Some of the vasodilators previously discussed, e.g. bradykinin and histamine exert their effects, at least in part, by stimulating the formation of NO.
Using heterologous anti-NOS antiserum, NOS immunoreactivity has been detected in S. mansoni adult parasites32,33. Neuronal NOS-like immunoreactivity is found in the main nerve cords, the peripheral nervous system and putative sensory neurons32. Presumably in these locations, any NO generated is involved in signaling cascades that are part of normal, internal parasite physiology. However, anti-inducible NOS antibodies label a variety of predominantly non-neuronal tissues, in parts of the gastrointestinal tract and with intense labeling at or near the surface of the worm32. It has been suggested that NO-related pathways derived from these sources may play a role in parasite–host interactions, perhaps by permitting the parasites to respond not only to endogenous NO but also to host-derived NO. Indeed it has been demonstrated that exposure of adult schistosomes to an NO donor in vitro does induce rapid changes in gene expression, though these changes are not large-scale34. Some NO upregulated genes, such as cyclic nucleotide phosphodiesterase and calcineurin, and some down regulated genes, such as Rac GTPase, share similarity with genes that have previously been reported to be involved in NO signaling in other systems34. The roles of other NO upregulated genes, (e.g. encoding a metal ion transport associated protein and a subunit of the coatomer complex), and some down regulated genes, (e.g. encoding a subunit of vacuolar ATP synthase, a putative coated vesicle membrane protein, and a putative ligand gated ion channel) are entirely unknown. Several other genes, both up and down regulated, encode hypothetical proteins with no homologs outside of the schistosomes34.
Using a fluorescent indicator to directly detect NO in living adult S. mansoni males revealed patchy staining in “epithelial-like cells” at least some of which are likely to be sensory35. Whether NO is ever released by the parasites to act e.g. as a vasodilator is unknown. A complication is that worms secrete the heme-containing metabolite hemozoin as a waste product of hemoglobin digestion36 and it is known that heme products can inactivate NO37–39. It is noteworthy that infection with the vascular nematode parasite Dirofilaria immitis can lead to an alteration in the relaxation behavior of endothelial cells of the pulmonary artery, and NO has been implicated in this effect40.
It seems likely that schistosomes would act as obstacles in blood vessels and this should lead to severe disturbances of blood flow including increased blood velocity past the endothelium. This is a known stimulus for NO release by endothelial cells41. Acting as a vasodilator, such NO release would help restore blood flow levels and make it less imperative for the parasites to interfere directly in the process. In this view, schistosomes, rather than directly impinging on host vascular biochemistry in the many ways outlined in this review, act as simple parasites and rely completely on the host to maintain vascular homeostasis.
Vascular control by ions and other metabolic products
Many different ions and metabolic products can dilate or constrict local blood vessels. For instance, an increase in calcium ion concentration can cause vasoconstriction41. In contrast, increases in potassium ion, magnesium ion or hydrogen ion concentrations can cause vasodilation, largely by inhibiting smooth muscle contraction41. An increase in carbon dioxide concentration can also cause vasodilation41. Other metabolites that are vasodilatory include lactate42, adenosine43, and acetylcholine44. General schistosome metabolism, by generating and releasing molecules of this nature, may therefore act to impose control on local vascular tone. For instance, intravascular schistosomes import and consume copious quantities of glucose45,46 which they catabolize via glycolysis to generate considerable amounts of lactic acid47–49 and lactate is transported out of the parasites49,50. The release of large amounts of lactate and hydrogen ions into the environment around the worms could by itself have a profound vasodilatory effect. Perhaps this is one reason the intravascular worms are homolactate fermentors - generating and releasing lactate helps promote a vasodilatory environment?
The action of a tegumental sodium-potassium ATPase (sodium pump), by pumping potassium ions out of the worms (and sodium ions in), might additionally have a local vasodilation effect51. Likewise, the presence of host-interactive tegumental phosphatases would tend to promote adenosine formation in the local environment of the worms and promote vasodilation52. The presence of an active acetylcholinesterase at the parasite surface would likely impinge on local acetylcholine concentrations53, again with possible implications for vascular tonality.
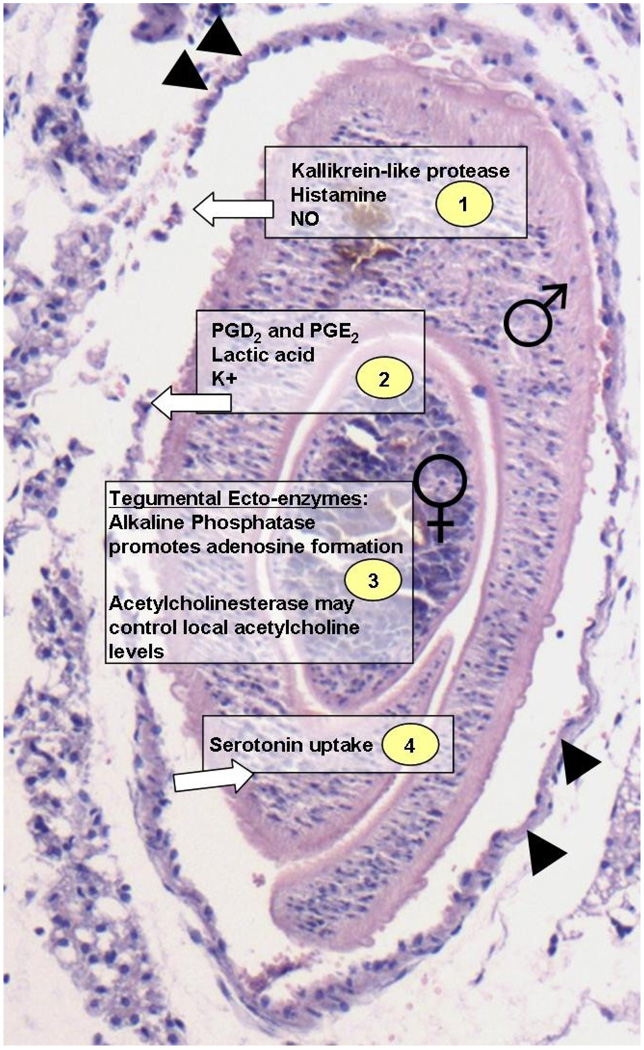
Figure 1 summarizes some of the data reviewed here. The figure shows a cross section through a blood vessel that contains a male/female schistosome couple. The female can be seen contained within the gynaecophoric canal of the male. Note that the parasites occupy a large portion of the vessel. The vascular endothelium is indicated (arrowheads, figure 1). In the figure, aspects of schistosome biochemistry that may pertain to vascular physiology are highlighted in 4 boxes. Box 1 lists metabolites that could impinge powerfully on host vasculature. These include the schistosome kallikrein-like protease (SK1 and/or SmSP1), histamine and NO. There is ample evidence that schistosomes do possess and utilize these metabolites but whether any of them is released by the parasites to impose upon vascular function is not known. The second group of metabolites, listed in box 2, include molecules that the worms are known to produce and release, at lease in vitro. These include the eicosanoids PGD2 and PGE2, lactate and potassium ions. Box 3 highlights the fact that the parasites may not necessarily release materials to exert an effect. The host-exposed, surface enzyme alkaline phosphatase (SmAP) is capable of generating the vasodilator, adenosine from the exogenous substrate AMP and the surface acetylcholinesterase may lessen local acetylcholine concentrations. The final box (number 4, figure 1) notes the ability of the parasites to take up the vasoconstrictor serotonin. Lessening local serotonin concentration around the worms could promote a more vasorelaxed environment.
Fig. 1.
Haemotoxylin and eosin stained section of the vasculature of a mouse infected 6 weeks previously with Schistosoma mansoni. A cross section of an adult male and female worm is shown. The female is contained within the male’s gynaecophoric canal. The vascular endothelium bounding the couple is indicated (arrowheads). Aspects of schistosome biochemistry that may pertain to vascular physiology are highlighted in 4 boxes. Box 1 (top) lists metabolites that could impinge powerfully on host vasculature. These include the schistosome kallikrein-like protease (SK1 and/or SmSP1), histamine and NO. The second group of metabolites, listed in box 2, include molecules that the worms are known to produce and release, at lease in vitro. These include the eicosanoids PGD2 and PGE2, lactate and potassium ions. Box 3 highlights the fact that some tegumental ecto-enzymes (e.g. alkaline phosphatase and acetylcholinesterase) may to exert an effect on vascular function. Box 4 (bottom) highlights the ability of the parasites to take up the vasoconstrictor serotonin. The while arrows indicate the potential movement of the selected metabolites into, or out of, the parasites.
It is possible that schistosomes exert an effect on several other key factors involved in the control of circulation such as the vasoconstrictive hormones norepinephrine and epinephrine, or other potent vasoconstrictive substances such as angiotensin II, vasopressin and endothelin. However, nothing has been reported on these issues.
Schistosome impact on vascular function
Complicating the potential involvement of the various vasoactive molecules and pathways that schistosomes may engage to control their environment is the fact that the worms are pathogenic and can directly damage vascular tissue. Schistosome eggs released by adult females, lodge in the vascular tissue and provoke a strong immune response. There are clear and well described changes in the vascular function of individuals infected with schistosomes that are generally attributed to the granulomatous host response to these eggs54,55. Portal hypertension, accompanied by anatomical changes of the portal vasculature, can develop as a longer term consequence of the granulomatous response to eggs. However, it is becoming increasingly clear that the intravascular parasites themselves (and not just worm eggs) impinge on vascular biochemistry. To disentangle the impact of the worms from the impact of their eggs on the vasculature, experimental animals can be infected with male-only cercariae. This leads to a single sex infection where, in the absence of female worms, egg laying is not possible. Starting at about 5 weeks after these animals are infected with 300 male cercariae, periportal spaces are observed to contain inflammatory cells56. Endothelial hyperplasia is reported and with time the inflammatory infiltrate is progressively substituted by connective tissue cells leading to fibrosis (increased collagen deposition)57. Other workers have confirmed the histopathological changes in the vasculature of male-only infected mice58. Isolated portal vein segments from such mice exhibit an increased response to the vasoconstrictor serotonin or to acetylcholine versus that seen in the portal vein of control, uninfected mice. This is manifest as an increase in maximal contraction and sensitivity to these reagents. No differences in responses to KCl were noted58,59. Blocking NOS function had no significant impact on the responses of veins from infected individuals to these vasoconstrictors. In contrast, blocking NOS function lessened vasoconstrictor action in control vein tissue58,59. This led to the suggestion that NO production is impaired in the infected tissue58,59. Perhaps this occurs due to the fact, as mentioned earlier, that secreted hemozoin may act to inactivate NO37–39.
It is worth recalling that schistosome released products (including waste products of digestion that contains hemozoin and constitutes schistosome vomitus), the host response to such products, as well as the physical presence of adult worm tubercles with tegumental spines in direct contact with the endothelium (see figure 1), all sustain host responses that ultimately can damage endothelial cells and impair function. It is possible that schistosome vasoactive metabolites must exert their effects on damaged host tissue and must be capable of compensating for any parasite-induced damage.
Finally, whether any of the schistosome derived molecules reviewed here actually do impact host vascular function has not been demonstrated. Many could be simply part of an entirely internal parasite metabolism. In addition, it is worth reiterating that several of the metabolites discussed here can have multiple functions. For instance, nitric oxide contributes to vessel homeostasis not only by inhibiting vascular smooth muscle contraction but by restraining platelet aggregation and leukocyte adhesion to the endothelium32. Adenosine is not only a vasodilator but is also a potent immunosuppressant. In addition to acting as vasoactive agents, several prostaglandins exert immunomodulatory roles. This makes it difficult to disentangle any proposed vasoactive role for the listed schistosome metabolites from an impact on host immune, or other, function. Nonetheless, it is clear that an increased understanding of these processes could lead to novel anti-parasitic therapies as well as new therapeutics to treat other vascular dysfunction.
Acknowledgment
This work was supported by grant AI-056273 from the NIH-NIAID.
Role of the funding source
The funders had no role in study design, data collection, analysis and interpretation, or in the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that no conflict of interest exists.
Research Agenda
The precise roles and relative importance of the various parasite-derived metabolites (including kinins, biogenic amines, eicosanoids, nitric oxide) in modulating host vascular physiology.
References
- 1.Koukounari A, Gabrielli AF, Toure S, Bosque-Oliva E, Zhang Y, Sellin B, et al. Schistosoma haematobium infection and morbidity before and after large-scale administration of praziquantel in Burkina Faso. J Infect Dis. 2007;196:659–669. doi: 10.1086/520515. [DOI] [PubMed] [Google Scholar]
- 2.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–1569. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- 3.King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4:65–79. doi: 10.1177/1742395307084407. [DOI] [PubMed] [Google Scholar]
- 4.Skelly P. Fighting killer worms. Sci Am. 2008;298:94–99. doi: 10.1038/scientificamerican0508-94. [DOI] [PubMed] [Google Scholar]
- 5.Wilson RA, Draskau T, Miller P, Lawson JR. Schistosoma mansoni: the activity and development of the schistosomulum during migration from the skin to the hepatic portal system. Parasitology. 1978;77:57–73. doi: 10.1017/s0031182000048721. [DOI] [PubMed] [Google Scholar]
- 6.Crabtree JE, Wilson RA. Schistosoma mansoni: an ultrastructural examination of pulmonary migration. Parasitology. 1986;92(Pt 2):343–354. doi: 10.1017/s0031182000064118. [DOI] [PubMed] [Google Scholar]
- 7.Despommier DD, Gwadz RW, Hotez PJ. Parasitic Diseases. 3ed. New York: Springer-Verlag; 1994. [Google Scholar]
- 8.Faust EC, Jones CA, Hoffman WA. Studies on schistosomiasis in Puerto Rico. III Biological Studies. 2. The mammalian phase of the life cycle. Puerto Rican Journal of Public Health and Tropical Medicine. 1934;10:133–196. [Google Scholar]
- 9.Carvalho WS, Lopes CT, Juliano L, Coelho PM, Cunha-Melo JR, Beraldo WT, et al. Purification and partial characterization of kininogenase activity from Schistosoma mansoni adult worms. Parasitology. 1998;117(Pt 4):311–319. doi: 10.1017/s0031182098003175. [DOI] [PubMed] [Google Scholar]
- 10.Cocude C, Pierrot C, Cetre C, Fontaine J, Godin C, Capron A, et al. Identification of a developmentally regulated Schistosoma mansoni serine protease homologous to mouse plasma kallikrein and human factor I. Parasitology. 1999;118(Pt 4):389–396. doi: 10.1017/s0031182098003874. [DOI] [PubMed] [Google Scholar]
- 11.El-Shehabi F, Ribeiro P. Histamine signalling in Schistosoma mansoni: Immunolocalisation and characterisation of a new histamine-responsive receptor (SmGPR-2) Int J Parasitol. 2010;40(12):1395–1406. doi: 10.1016/j.ijpara.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 12.El-Shehabi F, Vermeire JJ, Yoshino TP, Ribeiro P. Developmental expression analysis and immunolocalization of a biogenic amine receptor in Schistosoma mansoni. Exp Parasitol. 2009;122:17–27. doi: 10.1016/j.exppara.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gustafsson MK. Immunocytochemical demonstration of neuropeptides and serotonin in the nervous systems of adult Schistosoma mansoni. Parasitol Res. 1987;74:168–174. doi: 10.1007/BF00536029. [DOI] [PubMed] [Google Scholar]
- 14.Catto BA, Lewis FA, Ottesen EA. Cercaria-induced histamine release: a factor in the pathogenesis of schistosome dermatitis? Am J Trop Med Hyg. 1980;29:886–889. doi: 10.4269/ajtmh.1980.29.886. [DOI] [PubMed] [Google Scholar]
- 15.Rao KV, Chen L, Gnanasekar M, Ramaswamy K. Cloning and characterization of a calcium-binding, histamine-releasing protein from Schistosoma mansoni. J Biol Chem. 2002;277:31207–31213. doi: 10.1074/jbc.M204114200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerken SE, Mota-Santos TA, Vaz NM, Correa-Oliveira R, Dias-da-Silva W, Gazzinelli G. Recovery of schistosomula of Schistosoma mansoni from mouse skin: involvement of mast cells and vasoactive amines. Braz J Med Biol Res. 1984;17:301–307. [PubMed] [Google Scholar]
- 17.Bennett J, Bueding E, Timms AR, Engstrom RG. Occurrence and levels of 5-hydroxytryptamine in Schistosoma mansoni. Mol Pharmacol. 1969;5:542–545. [PubMed] [Google Scholar]
- 18.Day TA, Bennett JL, Pax RA. Serotonin and its requirement for maintenance of contractility in muscle fibres isolated from Schistosoma mansoni. Parasitology. 1994;108(Pt 4):425–432. doi: 10.1017/s0031182000075983. [DOI] [PubMed] [Google Scholar]
- 19.Hamdan FF, Ribeiro P. Characterization of a stable form of tryptophan hydroxylase from the human parasite Schistosoma mansoni. J Biol Chem. 1999;274:21746–21754. doi: 10.1074/jbc.274.31.21746. [DOI] [PubMed] [Google Scholar]
- 20.Bennett JL, Bueding E. Uptake of 5-hydroxytryptamine by Schistosoma mansoni. Mol Pharmacol. 1973;9:311–319. [PubMed] [Google Scholar]
- 21.Patocka N, Ribeiro P. Characterization of a serotonin transporter in the parasitic flatworm, Schistosoma mansoni: cloning, expression and functional analysis. Mol Biochem Parasitol. 2007;154:125–133. doi: 10.1016/j.molbiopara.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Fontana AC, Sonders MS, Pereira-Junior OS, Knight M, Javitch JA, Rodrigues V, et al. Two allelic isoforms of the serotonin transporter from Schistosoma mansoni display electrogenic transport and high selectivity for serotonin. Eur J Pharmacol. 2009;616:48–57. doi: 10.1016/j.ejphar.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho CH, Mettrick DF. Circadian variation in the distribution of Hymenolepis diminuta (Cestoda) and 5-hydroxytryptamine levels in the gastro-intestinal tract of the laboratory rat. Parasitology. 1982;84:431–441. doi: 10.1017/s0031182000052732. [DOI] [PubMed] [Google Scholar]
- 24.Angeli V, Faveeuw C, Roye O, Fontaine J, Teissier E, Capron A, et al. Role of the parasite-derived prostaglandin D2 in the inhibition of epidermal Langerhans cell migration during schistosomiasis infection. J Exp Med. 2001;193:1135–1147. doi: 10.1084/jem.193.10.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salafsky B, Fusco AC. Schistosoma mansoni: a comparison of secreted vs nonsecreted eicosanoids in developing schistosomulae and adults. Exp Parasitol. 1987;64:361–367. doi: 10.1016/0014-4894(87)90048-8. [DOI] [PubMed] [Google Scholar]
- 26.Abdel Baset H, O'Neill GP, Ford-Hutchinson AW. Characterization of arachidonic-acid-metabolizing enzymes in adult Schistisoma mansoni. Mol Biochem Parasitol. 1995;73:31–41. doi: 10.1016/0166-6851(95)00085-f. [DOI] [PubMed] [Google Scholar]
- 27.Nevhutalu PA, Salafsky B, Haas W, Conway T. Schistosoma mansoni and Trichobilharzia ocellata: comparison of secreted cercarial eicosanoids. J Parasitol. 1993;79:130–133. [PubMed] [Google Scholar]
- 28.Giles H, Leff P. The biology and pharmacology of PGD2. Prostaglandins. 1988;35:277–300. doi: 10.1016/0090-6980(88)90093-7. [DOI] [PubMed] [Google Scholar]
- 29.Hristovska AM, Rasmussen LE, Hansen PB, Nielsen SS, Nusing RM, Narumiya S, et al. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension. 2007;50:525–530. doi: 10.1161/HYPERTENSIONAHA.107.088948. [DOI] [PubMed] [Google Scholar]
- 30.Back M. Leukotriene receptors: crucial components in vascular inflammation. ScientificWorld Journal. 2007;7:1422–1439. doi: 10.1100/tsw.2007.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Back M. Functional characteristics of cysteinyl-leukotriene receptor subtypes. Life Sci. 2002;71:611–622. doi: 10.1016/s0024-3205(02)01733-2. [DOI] [PubMed] [Google Scholar]
- 32.Kohn AB, Moroz LL, Lea JM, Greenberg RM. Distribution of nitric oxide synthase immunoreactivity in the nervous system and peripheral tissues of Schistosoma mansoni. Parasitology. 2001;122(Pt 1):87–92. doi: 10.1017/s0031182000007022. [DOI] [PubMed] [Google Scholar]
- 33.Long XC, Bahgat M, Chlichlia K, Ruppel A, Li YL. Detection of inducible nitric oxide synthase in Schistosoma japonicum and S. mansoni. J Helminthol. 2004;78:47–50. doi: 10.1079/joh2003202. [DOI] [PubMed] [Google Scholar]
- 34.Messerli SM, Morgan W, Birkeland SR, Bernier J, Cipriano MJ, McArthur AG, et al. Nitric oxide-dependent changes in Schistosoma mansoni gene expression. Mol Biochem Parasitol. 2006;150:367–370. doi: 10.1016/j.molbiopara.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kohn AB, Lea JM, Moroz LL, Greenberg RM. Schistosoma mansoni: use of a fluorescent indicator to detect nitric oxide and related species in living parasites. Exp Parasitol. 2006;113:130–133. doi: 10.1016/j.exppara.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Oliveira MF, d'Avila JC, Torres CR, Oliveira PL, Tempone AJ, Rumjanek FD, et al. Haemozoin in Schistosoma mansoni. Mol Biochem Parasitol. 2000;111:217–221. doi: 10.1016/s0166-6851(00)00299-1. [DOI] [PubMed] [Google Scholar]
- 37.Martin W, Villani GM, Jothianandan D, Furchgott RF. Selective blockade of endothelium-dependent and glyceryl trinitrate-induced relaxation by hemoglobin and by methylene blue in the rabbit aorta. J Pharmacol Exp Ther. 1985;232:708–716. [PubMed] [Google Scholar]
- 38.Martin W, Smith JA, White DG. The mechanisms by which haemoglobin inhibits the relaxation of rabbit aorta induced by nitrovasodilators, nitric oxide, or bovine retractor penis inhibitory factor. Br J Pharmacol. 1986;89:563–571. doi: 10.1111/j.1476-5381.1986.tb11157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang XD, Xu R, Reynolds MF, Garcia ML, Heinemann SH, Hoshi T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 2003;425:531–535. doi: 10.1038/nature02003. [DOI] [PubMed] [Google Scholar]
- 40.Mupanomunda M, Williams JF, Mackenzie CD, Kaiser L. Dirofilaria immitis: heartworm infection alters pulmonary artery endothelial cell behavior. J Appl Physiol. 1997;82:389–398. doi: 10.1152/jappl.1997.82.2.389. [DOI] [PubMed] [Google Scholar]
- 41.Guyton AC, Hall JE. Textbook of Medical Physiology. 11 ed. Philadelphia: Elsevier; 2006. [Google Scholar]
- 42.Hein TW, Xu W, Kuo L. Dilation of retinal arterioles in response to lactate: role of nitric oxide, guanylyl cyclase, and ATP-sensitive potassium channels. Invest Ophthalmol Vis Sci. 2006;47:693–699. doi: 10.1167/iovs.05-1224. [DOI] [PubMed] [Google Scholar]
- 43.Gebremedhin D, Weinberger B, Lourim D, Harder DR. Adenosine can mediate its actions through generation of reactive oxygen species. J Cereb Blood Flow Metab. 2010;30(10):1777–1790. doi: 10.1038/jcbfm.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treiber G, Csepregi A, Malfertheiner P. The pathophysiology of portal hypertension. Dig Dis. 2005;23:6–10. doi: 10.1159/000084720. [DOI] [PubMed] [Google Scholar]
- 45.Bueding E. Carbohydrate metabolism of Schistosoma mansoni. J Gen Physiol. 1950;33(5):475–495. doi: 10.1085/jgp.33.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Skelly PJ, Tielens AGM, Shoemaker CB. Glucose transport and metabolism in mammalian stage schistosomes. Parasitol Today. 1998;14:402–406. doi: 10.1016/s0169-4758(98)01319-2. [DOI] [PubMed] [Google Scholar]
- 47.Shapiro TA, Talalay P. Schistosoma mansoni: mechanisms in regulation of glycolysis. Exp Parasitol. 1982;54:379–390. doi: 10.1016/0014-4894(82)90047-9. [DOI] [PubMed] [Google Scholar]
- 48.Thompson DP, Morrison DD, Pax RA, Bennett JL. Changes in glucose metabolism and cyanide sensitivity in Schistosoma mansoni during development. Mol Biochem Parasitol. 1984;13:39–51. doi: 10.1016/0166-6851(84)90100-2. [DOI] [PubMed] [Google Scholar]
- 49.Githui EK, Damian RT, Aman RA. In vitro efflux of lactic acid by schistosomes cultured in varying concentrations of glucose: potential toxicity of accumulated lactic acid. J Trop Microbiol Biotechnol. 2006;2:31–36. [Google Scholar]
- 50.Faghiri Z, Camargo SM, Huggel K, Forster IC, Ndegwa D, Verrey F, et al. The tegument of the human parasitic worm schistosoma mansoni as an excretory organ: the surface aquaporin SmAQP is a lactate transporter. PLoS One. 2010;5:e10451. doi: 10.1371/journal.pone.0010451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skelly P, Wilson R. Making Sense of the Schistosome Surface. Advances in Parasitology. 2006;63:185–284. doi: 10.1016/S0065-308X(06)63003-0. [DOI] [PubMed] [Google Scholar]
- 52.Bhardwaj R, Skelly PJ. Purinergic signaling and immune modulation at the schistosome surface? Trends Parasitol. 2009;25:256–260. doi: 10.1016/j.pt.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Camacho M, Alsford S, Agnew A. Molecular forms of tegumental and muscle acetylcholinesterases of Schistosoma. Parasitology. 1996;112(Pt 2):199–204. doi: 10.1017/s0031182000084766. [DOI] [PubMed] [Google Scholar]
- 54.Nagy BA, File SK, Smith JH. Changes in the enteric vasculature of mice infected with Schistosoma mansoni. Am J Trop Med Hyg. 1981;30:999–1009. doi: 10.4269/ajtmh.1981.30.999. [DOI] [PubMed] [Google Scholar]
- 55.de Cleva R, Pugliese V, Zilberstein B, Saad WA, Pinotti HW, Laudanna AA. Systemic hemodynamic changes in mansonic schistosomiasis with portal hypertension treated by azygoportal disconnection and splenectomy. Am J Gastroenterol. 1999;94:1632–1637. doi: 10.1111/j.1572-0241.1999.01086.x. [DOI] [PubMed] [Google Scholar]
- 56.Baki CA, Grimaud JA. Unisexual murine schistosomiasis: portal hepatitis in subacute infections. Experientia. 1985;41:1423–1426. doi: 10.1007/BF01950016. [DOI] [PubMed] [Google Scholar]
- 57.Baki CA, Guerret S, Grimaud JA, Chevallier M. Liver fibrosis in unisexual murine Schistosomiasis: quantitative study and morphological changes in mice with chronic infection. Cell Mol Biol. 1998;44:627–633. [PubMed] [Google Scholar]
- 58.Silva CL, Morel N, Lenzi HL, Noel F. Increased reactivity to 5-hydroxytryptamine of portal veins from mice infected with Schistosoma mansoni. Comp Biochem Physiol A Mol Integr Physiol. 1998;120:417–423. doi: 10.1016/s1095-6433(98)10041-7. [DOI] [PubMed] [Google Scholar]
- 59.Silva CL, Lenzi HL, Silva VF, Paulo FO, Noel F. Cellular mechanisms involved in the increased contraction of portal veins from Schistosoma mansoni-infected mice. Parasitol Res. 2003;89:16–22. doi: 10.1007/s00436-002-0711-7. [DOI] [PubMed] [Google Scholar]