Abstract
Long-term habituation to a novel environment is one of the most elementary forms of nonassociative learning. Here we studied the effect of pre- or posttraining intrahippocampal administration of drugs acting on specific molecular targets on the retention of habituation to a 5-min exposure to an open field measured 24 h later. We also determined whether the exposure to a novel environment resulted in the activation of the same intracellular signaling cascades previously shown to be activated during hippocampal-dependent associative learning. The immediate posttraining bilateral infusion of CNQX (1 μg/side), an AMPA/kainate glutamate receptor antagonist, or of muscimol (0.03 μg/side), a GABAA receptor agonist, into the CA1 region of the dorsal hippocampus impaired long-term memory of habituation. The NMDA receptor antagonist AP5 (5 μg/side) impaired habituation when infused 15 min before, but not when infused immediately after, the 5-min training session. In addition, KN-62 (3.6 ng/side), an inhibitor of calcium calmodulin-dependent protein kinase II (CaMKII), was amnesic when infused 15 min before or immediately and 3 h after training. In contrast, the cAMP-dependent protein kinase (PKA) inhibitor Rp-cAMPS, the mitogen-activated protein kinase kinase (MAPKK) inhibitor PD098059, and the protein synthesis inhibitor anisomycin, at doses that fully block memory formation of inhibitory avoidance learning, did not affect habituation to a novel environment. The detection of spatial novelty is associated with a sequential activation of PKA, ERKs (p44 and p42 MAPKs) and CaMKII and the phosphorylation of c-AMP responsive element-binding protein (CREB) in the hippocampus. These findings suggest that memory formation of spatial habituation depends on the functional integrity of NMDA and AMPA/kainate receptors and CaMKII activity in the CA1 region of the hippocampus and that the detection of spatial novelty is accompanied by the activation of at least three different hippocampal protein kinase signaling cascades.
Long-term memories can be divided into associative and nonassociative depending on the mechanisms required for their formation. Associative memories are based on the acquisition of a predictive link between a specific event and a stimulus. Nonassociative memories are acquired when repeated or continuous exposure to a novel stimulus changes behavioral responses to it. In mammals, some forms of associative and nonassociative memories involve the participation of the hippocampal formation (Izquierdo and Medina 1997; Zhu et al. 1997; Thiel et al. 1998; Eichenbaum 1999; McGaugh 2000).
The molecular events in the hippocampus required for long-term memory formation have been extensively studied in several associative learning tasks, including contextual fear conditioning and one trial inhibitory avoidance training (Bernabeu et al. 1997; Izquierdo and Medina 1997; Atkins et al. 1998; Cammarota et al. 1998; Impey et al. 1998; Taubenfeld et al. 1999; Wong et al. 1999; McGaugh 2000). Much less is known about the molecular substrates in the hippocampus required for memory formation of nonassociative learning tasks (Izquierdo et al. 1992; Acquas et al. 1996; Thiel et al. 1998).
One of the most elementary nonassociative learning tasks is that of behavioral habituation to a novel environment. We have previously shown that hippocampal glutamate NMDA receptors and CaMKII appear to be necessary for the establishment of long-term habituation to a very brief (1–2 min) exposure to an open field (Izquierdo et al. 1992, 1999).
Therefore, to determine whether the molecular events required for long-term memory formation of associative hippocampal-dependent tasks are also involved in habituation to a novel environment, we studied the effect of pre- and posttraining intrahippocampal administration of drugs acting on specific molecular targets on the retention of habituation to a 5-min exposure to an open field measured 24 h later. Also, we determined whether the exposure to a novel environment resulted in the activation of the same intracellular signaling cascades that are activated during memory formation of a one-trial inhibitory avoidance training (Izquierdo and Medina 1997; Taubenfeld et al. 1999; Cammarota et al. 2000).
RESULTS
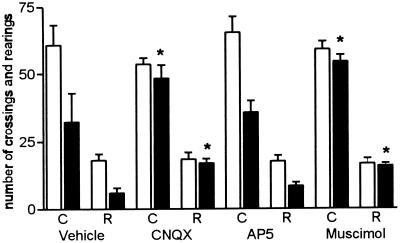
Long-term memory formation of habituation to a 5-min exposure to a novel environment is blocked by the immediate posttraining bilateral infusion of CNQX (1μg/side) or muscimol (0.03 μg/side) (F3,52 = 12,524, P < 0.001), but not of AP5 (5 μg/side), into the CA1 region of the dorsal hippocampus (Fig. 1), indicating that glutamate AMPA/kainate receptors in the hippocampus are required for the consolidation of this nonassociative learning task and that activation of GABAA receptors down-regulates memory processing.
Figure 1.
Effect of immediate posttraining intrahippocampal infusions of AP5, CNQX, or muscimol on retention of a spatial habituation in rats. Data are expressed as mean ± SEM of crossings (C) and rearings (R) of training (open bars) and test (closed bars) session performance in an open field with a 24-h interval between sessions. n = 10–14 animals per group. Training-test differences were significant in vehicle and AP5 groups, which indicates that in those two groups there was habituation (t = 4.719, P < 0.001 for vehicle and t = 2.177, P = 0.040 for AP5 group in paired t-test). This was not detectable in groups treated with CNQX and muscimol (indicated with an asterisk).
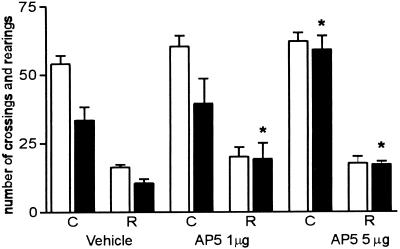
NMDA receptors are essential for the induction of major forms of synaptic plasticity (Bliss and Collingridge 1993) and have been shown to be crucially involved in the acquisition of several associative learning paradigms. To ensure that AP5 has been delivered in time to block NMDA receptor activation we decided to evaluate the effect of AP5 (1 and 5 μg/side) injected 15 min before training into the CA1 region of the dorsal hippocampus (Fig. 2). The time constant for diffusion away from the infusion site for polar molecules is <30 min (Martin 1991). When given before the training session, AP5 impaired long-term memory formation in the two doses tested (F2,24 = 12,524, P = 0.012).
Figure 2.
Effect of 15 min pretraining intrahippocampal infusions of two doses of AP5 on retention of spatial habituation in rats. Data are expressed as mean ± SEM of crossings (C) and rearings (R) of training (open bars) and test (closed bars) session performance in an open field with a 24-h interval between sessions. n = 8–12 animals per group. Training-test differences were significant only in the vehicle group (t = 4.140, P = 0.003 in paired t-test). Habituation was not detectable in groups treated with AP5 in either of the tested doses (indicated with an asterisk).
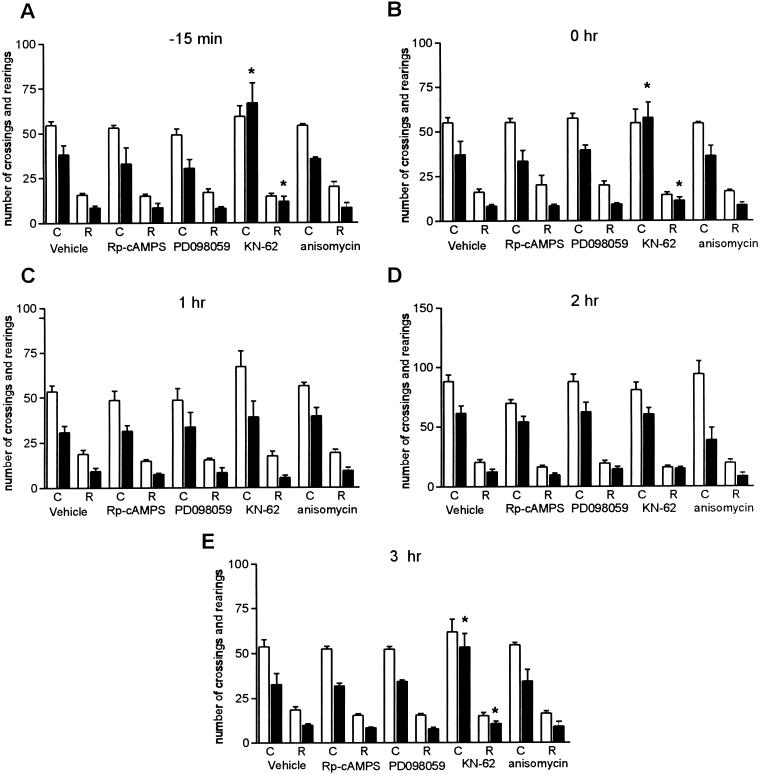
We next determined whether different protein kinase cascades known to be required in memory consolidation of associative learning (for references, see McGaugh 2000) are also involved in memory formation of habituation to a novel environment. As shown in Figure 3A,B,E,3 only the intrahippocampal infusion of KN-62 (3.6 ng/side), an inhibitor of CaMKII, 15 min before (F4,49 = 4,145, P = 0.006) or immediately (F4,65 = 2,325, P = 0.046) and 3 h after training (F4,51 = 2,786, P = 0.036), impaired retention of habituation to the open field. There were no differences among groups in training session performance (Duncan test). All training-test differences in crossings and rearings were significant (Fig. 3) except those from animals treated with KN-62 at the above-mentioned time points. Therefore, inhibitors of PKA (RpcAMPS, 0.5 μg/side) or MAPKK (PD098059, 50 μM) delivered into CA1 region at the time points studied did not affect memory of spatial habituation. Unexpectedly, the same intrahippocampal dose of anisomycin that totally blocked memory formation of inhibitory avoidance training (Quevedo et al. 1999) was ineffective on habituation to a novel environment.
Figure 3.
Effect of pretraining (A: −15 min) or posttraining (B: 0 h; C: 1 h; D: 2 h; E: 3 hr) intrahippocampal infusions of Rp-cAMPS, PD098059, KN-62, or anisomycin on retention of spatial habituation in rats. Data are expressed as mean ± SEM of crossings (C) and rearings (R) of training (open bars) and test (closed bars) session performance in an open field with a 24-h interval between sessions. n = 12–16 animals per group. KN-62 causes retrograde amnesia. All the other treatments were ineffective. Training-test differences were significant in all groups (P < 0.05–0.001, paired Student t-test), except those of KN-62 groups in A, B, and E (t = 18.318, P < 0.001; t = 4.063, P = 0.002; and t = 0.913, P = 0.388, respectively; indicated with an asterisk).
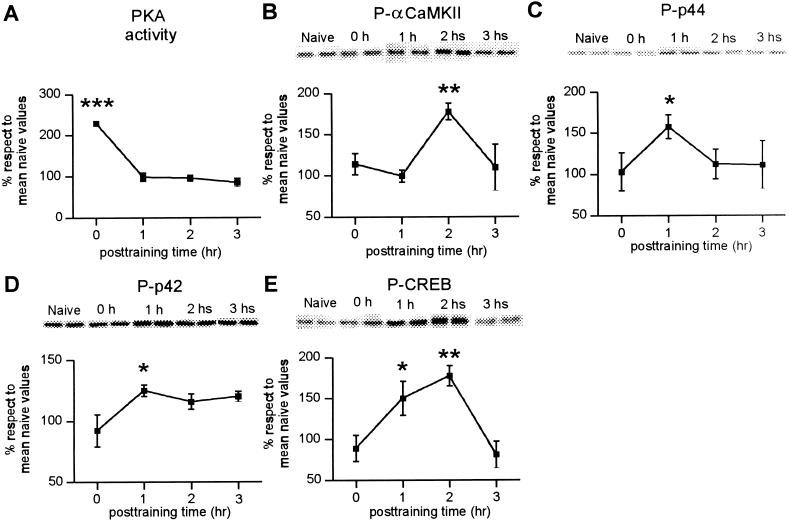
To determine whether exposure to a novel environment resulted in activation of signaling cascades in the hippocampus, we subjected rats to a 5-min session of an open field and sacrificed them at different time points after training. Based on our previous findings demonstrating that PKA activity in the hippocampus increases immediately and 3 h after an inhibitory avoidance training, we decided to assess whether similar changes occur after nonassociative tasks. As shown in Figure 4A, the detection of spatial novelty is associated with a significant increase (+130%, F4,26 = 16,98, P < 0.001) in hippocampal PKA activity immediately after behavioral training. PKA activity did not significantly change at 1, 2, or 3 h after training.
Figure 4.
Exposure to a novel environment is associated with the activation of protein kinases and phosphorylation of CREB in the hippocampus at various time intervals (in hours) after a 5-min exposure to an open field. Data are expressed as mean ± SEM in percentage of naive control values. (A) PKA activity, n = 5–6 independent experiments per time point; *** indicates P < 0.001 in Duncan test after ANOVA. (B) Representative immunoblot of phospho αCaMKII and densitometric analysis of five to eight independent experiments per time point; ** indicates P < 0.01 (Dunnet test ). (C, D) Representative immunoblots of phospho p-44 and phospho p-42 (P-p44 and P-p42, respectively) and densitometric analysis of five to 10 independent experiments per time point; * indicates P < 0.05 (Dunnet test). (E) Representative immunoblot of P-CREB and densitometric analysis of four to eight independent experiments per time point; * indicates P < 0.05, ** indicates P < 0.01 (Dunnet test).
Given that CaMKII activity in the hippocampus increases immediately after inhibitory avoidance learning (Cam-marota et al. 1998), that αCaMKII autophosphorylation increases 2 h after cue and contextual fear conditioning (Atkins et al. 1998), and that an inhibitor of this enzyme blocks spatial habituation (Fig. 3), we next determined the levels of activated αCaMKII in the hippocampus. As shown in Figure 4B, there is an increase in αCaMKII autophosphorylation at 2 h (+77.6%, F4,32 = 8,2, P = 0.01), but not at 0, 1, or 3 h after training.
It has been demonstrated that different associative learn-ings are accompanied by an activation of p44 and p42 MAPKs (Atkins et al. 1998; Berman et al. 1998; Crow et al. 1998). We have recently found that inhibitory avoidance learning is also associated with an activation of p44 and p42 MAPKs 2 h after avoidance training (Cammarota et al. 2000). To test the hypothesis that activation of MAPKs also increases as a consequence of a nonassociative learning task, we used immunoblot techniques to detect dually phosphorylated, activated p44 and p42 MAPKs (Atkins et al. 1998; Cammarota et al. 2000). As shown in Figure 4C,D, exposure to a novel environment results in an increase in phospho-p44 and phospho-p42 MAPKs at 1 h after training (+57.5%, F4.45 = 2,98, P = 0.0435; +25%, F4,32 = 4,358, P = 0.002 for p44 and p42 MAPKs, respectively). These changes are not caused by alterations in total MAPKs levels, as the amount of p44 and p42 MAPKs remained unchanged (p44 MAPK: 94.8% ± 12.6%, p42 MAPK: 89% ± 12%, with respect to naive control values, n = 6).
Given that CREB has an important role in memory formation of associative learning tasks in several species (Bourtchouladze et al. 1994; Yin et al. 1994; Yin and Tully 1996; Guzowski and McGaugh 1997; for references, see Silva et al. 1998), that CREB phosphorylation at ser 133 (P-CREB) is associated with CREB-regulated gene expression (Montminy 1997), and that inhibitory avoidance training results in both a time-dependent increase in P-CREB and in CRE-mediated gene expression (Bernabeu et al. 1997; Impey et al. 1998; Taubenfeld et al. 1999; Cammarota et al. 2000), we next determined the levels of ser 133 P-CREB in rats exposed to the novel environment. As shown in Figure 4E, the detection of spatial novelty is associated with a significant increase in P-CREB levels in the hippocampus (F4,37 = 5.47, P = 0.024) at 1 and 2 h after training (+50% and +77%, respectively). No changes were observed in total CREB levels (CREB 1 h: 95% ± 3.8%, CREB2 h: 98% ± 4.1% with respect to naive control values, n = 6).
DISCUSSION
The crucial role of the hippocampal formation in memory consolidation has been extensively demonstrated in a variety of species and associative learning tasks. In the last decade several intracellular signaling cascades have been shown to be involved in long-term memory formation (Izquierdo and Medina 1997; Atkins et al. 1998; Impey et al. 1998; Silva et al. 1998; Wong et al. 1999; Cammarota et al. 2000; for references, see McGaugh 2000). It also has been shown that de novo protein synthesis is a critical event in associative long-term memory formation (Davis and Squire 1984; Bourtchuladze et al. 1998; for references, see Milner et al. 1998; Schafe et al. 1999) and that blockade of any of these events can cancel the establishment of long-term memory during associative memory consolidation (McGaugh 2000). In this study we investigated the importance of neurotransmitter receptors, intracellular signaling pathways, and protein synthesis in the hippocampus for long-term memory formation of habituation to a novel environment, a nonassociative learning task.
Extending previous findings (Izquierdo et al. 1992), our data indicate that long-term memory for habituation to a novel environment depends on the functionality of AMPA/kainate glutamate receptors in the hippocampus and is regulated by an agent that potentiates intrinsic GABAergic inhibition. The lack of effect of the NMDA receptor antagonist when given immediately after training, shown here, when compared to earlier reports on shorter versions of the task (Izquierdo et al. 1992), could be attributed to the delay introduced by the increased duration of the training session (Izquierdo et al. 1992). It is well known that NMDA receptors are required at the time of induction of plastic events and for a few seconds afterward, but not later (Izquierdo and Medina 1997; Malenka and Nicoll 1999; McGaugh 2000). It might well be the case that posttraining infusion at the end of 5 min of exposure to a novel environment does not reach this initial receptor activation in time to avoid cellular stimulation. This appears to be the case, as the results shown in Figure 2 demonstrate that, when given 15 min before training, AP5 effectively causes amnesia for this task.
The main pharmacological/behavioral finding of the present study is that memory for spatial habituation is not impaired by the blockade of PKA or MAPK cascades or by the inhibition of protein synthesis in the CA1 region of the hippocampus. We and others have previously shown that inhibition of PKA or MAPK cascades hinders memory formation of several hippocampal-dependent associative learning tasks (Bernabeu et al. 1997; Atkins et al. 1998; Bourtchuladze et al. 1998; Vianna et al. 1999; Walz et al. 1999; Wong et al. 1999). The results presented here suggest that PKA or MAPK signaling pathways in the CA1 region are not involved in long-term memory formation of spatial habituation.
In contrast, CaMKII signaling pathways appear to be critical for both associative (Silva et al. 1992; Wolfman et al. 1994; Mayford et al. 1996; Tan and Liang 1996, 1997; Cammarota et al. 1998) and nonassociative (Izquierdo et al. 1992, 1999; Fig. 2) forms of memories as suggested by the amnesia caused by the CAMKII inhibitor given before, immediately, or 3 h after training, and the rises in p-CAMKII levels observed 2 h after training. The temporal dissociation among the behavioral and biochemical findings is not easily explained and could be attributed to the fact that the p-CAMKII levels were measured in nuclear extracts. Moreover, they could be ascribed to distinct aspects of memory formation as habituation and novel detection itself. As mentioned before, the formation of associative long-term memory has long been known to depend on de novo protein synthesis (Davis and Squire 1984; Milner et al. 1998). Recent reports suggest that long-term memories are sensitive to protein synthesis inhibition in two moments of the consolidation period, one at the time of training and another at a later stage (Freeman et al. 1995; Bourtchuladze et al. 1998). Recently, we have shown that long-term memory of inhibitory avoidance training can be cancelled when anisomycin is delivered into the CA1 region of the dorsal hippocampus around the time of training and at 3 but not 6 h after training (Quevedo et al. 1999). Here, using the same dose of anisomycin that blocked memory of avoidance training, we found no effect of anisomycin on long-term memory formation of spatial habituation. The dose used here is likely to inhibit most hippocampal protein synthesis because it is much higher than doses shown to block protein synthesis in the chick brain (Freeman et al. 1995), in rat hippocampal slices (Frey and Morris 1997, 1998), and in the Hermissenda nervous system (Crow and Forrester 1990). However, we can not rule out the possibility that the hippocampal level of protein synthesis inhibition required to block spatial habituation is higher than that required for impairing avoidance learning. Alternatively, the apparent lack of effect of anisomycin on memory of spatial habituation could be explained by the specific characteristics of our training-test protocol. In this context, it has been previously reported that inhibition of protein synthesis in honeybees and Drosophila does not impair memory formation of classical conditioning when tested 24 or 48 h after training (Wittstock et al. 1993; De Zazzo and Tully 1995), whereas retention over longer periods required protein synthesis (DeZazzo and Tully 1995; Wustenberg et al. 1998). In any case, our results indicate that memory for spatial habituation retrieved 1 d after training is independent of hippocampal de novo protein synthesis.
Recent functional imaging, behavioral, electrophysiological, and neurochemical findings implicate the hippocampus in novelty processing (Knight 1996; Zhu et al. 1997; Honey et al. 1998; Thiel et al. 1998; Manahan-Vaughan and Braunewell 1999; Strange et al. 1999). The detection of novelty depends on the activation of a distributed network involving the hippocampus (Knight and Nakata 1998), and is a memory-dependent process because the novel stimulus has to be compared with stored information to judge its novelty. However, novelty per se might not be the primary factor responsible for activation in the hippocampus. In this regard, it has been recently postulated that the hippocampus is necessary to record new events in any given situation (Eichenbaum et al. 1999; Martin 1999).
It has been previously shown that novelty detection is associated with an increase in the extracellular concentration of acetylcholine in the hippocampus (Acquas et al. 1996; Thiel et al. 1998). In this work we demonstrated for the first time that the detection of a spatial novelty results in the activation of several intracellular signaling pathways in the hippocampus that include a rapid increase in PKA activity followed by an activation of p42 and p44 MAPKs and CaMKII (Fig. 4). Coinciding with the activation of MAPKs and CaMKII there is an increased phosphorylation of CREB. These changes are all transient, as no alterations in PKA activity or phospho-MAPKs, phospho-αCaMKII, or phospho-CREB levels were observed 3 h after novelty. Therefore, the detection of novelty is associated with a rapid and reversible activation of some signaling cascades in the hippocampus exhibiting time courses that differ substantially from those associated with inhibitory avoidance training (Izquierdo and Medina 1997; McGaugh 2000). However, it is interesting to note that the time course of activation of hippocampal MAPKs and CaMKII after spatial novelty is quite similar to those observed after cue and contextual fear conditioning (Atkins et al. 1998).
Taken together, these findings indicate both that long-term memory of spatial habituation, a nonassociative learning task, depends on the functionality of hippocampal NMDA and AMPA/kainate receptors and CaMKII and that spatial novelty activates, in a time-dependent manner, three different protein kinase cascades in the hippocampus. In addition, they suggest that different neural mechanisms are involved in memory formation of hippocampal-dependent associative and nonassociative memories.
MATERIALS AND METHODS
Subjects
Male Wistar rats (age, 3 mo; weight, 200–250 g) were used. They were housed five to a cage with food and water available ad libitum and were maintained on a 12-h light/dark cycle. Behavioral procedures were conducted between 1 and 4 p.m. Rats were bilaterally implanted under deep thionembutal anesthesia with 30-gauge guides 1.0 mm above the CA1 region of the dorsal hippocampus (A −4.3, L +4.0, V 3.4, according to Paxinos and Watson [1986]). This work was approved by the ICBS and the UBA Ethical Committees.
Behavioral Procedures
After recovery from surgery, animals were exposed to a novel environment. This was a 50-cm-high, 50-cm-wide, 39-cm-deep open field with black plywood walls and a brown floor divided into 12 equal squares by black lines. Animals were gently placed on the left rear quadrant. The number of line crossings and rearings (Izquierdo et al. 1999) were measured for a 5-min period. Immediately before or at different time points after exposure to the open field, animals received an infusion into the CA1 region and, 24 h later, were subjected again to a similar open field session (test session).
Drugs and Infusion Procedures
Fifteen minutes before or 0, 1, 2, or 3 h after the exposure to the novel environment, a 27-gauge infusion cannula attached to a microsyringe was fitted into the guide cannula and infusions were carried out over 30 sec, first on one side and then on the other. In each case the infusion cannula was left in place for 15 sec after the infusion had been completed; therefore, the entire bilateral infusion procedure took slightly over 90 sec (Wolfman et al. 1994; Bernabeu et al. 1997; Izquierdo et al. 1992, 1999; Vianna et al. 1999).
The treatments were saline, vehicle (2% dimethysulfoxide in saline), the NMDA receptor antagonist AP5 (1.0 or 5.0 μg/side), the AMPA/kainate receptor antagonist CNQX (1 μg/side), the GABAA receptor agonist muscimol (0.03 μg/side), the CaMKII inhibitor KN-62 (3.6 ng/side), the PKA inhibitor Rp-cAMPS (0.5 μg/side), the MAPKK inhibitor PD098059 (50 μM solution), or the protein synthesis inhibitor anisomycin (80 μg/side). Infusion volumes were 0.5 μL in all cases except for anisomycin (0.8 μL). Anisomycin was dissolved in a minimal volume of 3N HCl and the solution was adjusted to pH 7.2, when it was brought to a concentration of 100 μg/μL by addition of 3 N NaOH (Tiunova et al. 1996).
The specific doses of the different drugs used in this study were chosen precisely because they had all been previously shown to have clear effects on memory formation of a one-trial inhibitory avoidance task (Izquierdo et al. 1992; Wolfman et al. 1994; Izquierdo and Medina 1997; Quevedo et al. 1999; Vianna et al. 1999; Walz et al. 1999).
Histology
Twenty-four hours after the end of the behavioral procedures, 0.5 μL of a solution of 4% methylene blue in saline was infused as indicated above into each implanted site. Animals were killed by decapitation 1 h later and the brains were stored in formalin for histological localization of the infusion sites as explained elsewhere (Bernabeu et al. 1997; Izquierdo et al. 1992, 1999; Quevedo et al. 1999). Infusions spread with a radius of <1.0 mm3, as described before (Martin 1991; Izquierdo et al. 1992, 1999; Bernabeu et al. 1997; Cammarota et al. 2000), and were found to be correct (i.e., within 1.5 mm3 of the intended site) in 93% of the animals. Only the behavioral data from animals with the cannula located in the intended site were included in the final analysis.
Biochemical Measurements
Nonimplanted animals were exposed to the open field for 5 min and, at different time points after training, they were killed by decapitation. After sacrifice, the brains were immediately removed and the hippocampi were dissected out, pooled, and homogenized in ice-chilled buffer (20 mM Tris-HCl at pH 7.4, 0.32 M sucrose, 1 mM EDTA, 1 mM EGTA, 1 mM PMSF, 10 μg/mL aprotinin, 15 μg/mL leupeptin, 50 mM NaF and 1mM sodim orthovanadate). The homogenate was centrifugated 10 min at 900g and the obtained nuclear pellet was resuspended in buffer (20 mM Tris-HCl at pH 7.4, 1 mM PMSF, 50 mM NaF, and 1 mM sodium orthovanadate). The procedure was carried out at 4°C. The samples were stored at −70°C until used.
Samples of nuclear extracts (12–25 μg of protein, determined by the method of Bradford) were subjected to SDS-PAGE (10% gels) (Cammarota et al. 1998, 2000). The proteins were electrophoretically transferred (1 h 100 V) to PVDF membranes. After preincubation in blocking buffer (25 mM Tris HCl at pH 7.4, 3% BSA or 5% dried milk, 150 mM NaCl, 0.05% v/v Tween 20), the blots were incubated with the following antibodies: anti-CREB (1 : 1000; New England BioLabs, directed against the CREB residues 123–137), anti-pCREB (1 : 1000; New England BioLabs, corresponding to Ser 133 phosphorylated form of CREB and directed against residues 129–137), anti-p42 and -p44 MAPKs (1 : 2000; New England Biolabs), antiactivated p42 and p44 MAPKs (1 : 2000; New England Biolabs), and antiphospho αCaMKII (1 : 8,000; Promega). Antibody–antigen complexes were detected with a goat antirabbit IgG conjugated to horseradish peroxidase (BioRad) and visualized by the ECL method as described by the manufacturer (Amersham). Densitometric analysis of the films was performed by using a MCID Image Analysis System (5.02 v, Image Research). Western blots were developed to be linear in the range used for densitometry. PKA activity was determined in cellular extracts as previously described (Bernabeu et al. 1997), using kemptide as specific substrate of PKA.
Statistical Analysis
Statistical analysis was performed by one-way analysis of variance (ANOVA) using the Duncan test (for behavioral data) and Dunnet test (for biochemical assays) for comparison among groups and Student t-test for training-test performance comparisons within each group.
Acknowledgments
This work was supported by Pronex (Brazil) and CONICET and UBA (Argentina). The authors thank Dr. S.P.R. Rose for his helpful discussion.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL mrmvianna@yahoo.com; FAX 55-51-316-55-40.
Article and publication are at www.learnmem.org/cgi/doi/10.1101/lm.34600.
REFERENCES
- Acquas E, Wilson C, Fibiger HC. Conditioned and unconditioned stimuli increase frontal cortical and hippocampal release: Effects of novelty, habituation and fear. J Neurosci. 1996;16:3089–3096. doi: 10.1523/JNEUROSCI.16-09-03089.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nature Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- Berman DE, Hazvi S, Rosenblum K, Seger R, Dudai Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J Neurosci. 1998;18:10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Schultz G, Silva A. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Cammarota M, Bernabeu R, Levi de Stein M, Izquierdo I, Medina JH. Learning-specific, time-dependent increases in hippocampal Ca2+/calmodulin-dependent protein kinase II activity and AMPA GluR1 subunit immunoreactivity. Eur J Neurosci. 1998;10:2669–2676. doi: 10.1046/j.1460-9568.1998.00254.x. [DOI] [PubMed] [Google Scholar]
- Cammarota M, Bevilaqua LRM, Ardenghi P, Paratcha G, Levi de Stein M, Izquierdo I, Medina JH. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: Abolition by NMDA receptor blockade. Mol Brain Res. 2000;76:36–46. doi: 10.1016/s0169-328x(99)00329-0. [DOI] [PubMed] [Google Scholar]
- Crow T, Forrester J. Inhibition of protein synthesis blocks long-term enhancement of generator potentials produced by one-trial in vivo conditioning in Hermissenda. Proc Natl Acad Sci. 1990;87:4490–4494. doi: 10.1073/pnas.87.12.4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow T, Xue-bian JJ, Siddiqi V, Kang Y, Neary JT. Phosphorylation of mitogen-activated protein kinase by one-trial and multi-trial classical conditioning. J Neurosci. 1998;18:3480–3487. doi: 10.1523/JNEUROSCI.18-09-03480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis HP, Squire LR. Protein synthesis and memory: A review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- DeZazzo J, Tully T. Dissection of memory formation: From behavioral pharmacology to molecular genetics. Trends Neurosci. 1995;18:212–218. doi: 10.1016/0166-2236(95)93905-d. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. The hippocampus and mechanisms of declarative memory. Behav Brain Res. 1999;103:123–133. doi: 10.1016/s0166-4328(99)00044-3. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- Freeman F, Rose SPR, Scholey A. Two time windows of anisomycin-induced amnesia for passive avoidance training in the day-old chick. Neurobiol Learn Mem. 1995;63:291–295. doi: 10.1006/nlme.1995.1034. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RGM. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- ————— Synaptic tagging: Implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey RC, Watt A, Good M. Hippocampal lesions disrupt an associative mismatch process. J Neurosci. 1998;15:2226–2230. doi: 10.1523/JNEUROSCI.18-06-02226.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nature Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Medina JH. Memory formation: The sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, da Cunha C, Rosat R, Ferreira MBC, Jerusalinsky D, Medina JH. Neurotransmitter receptors involved in memory processing by the amygdala, medial septum and hippocampus of rats. Behav Neural Biol. 1992;58:16–25. doi: 10.1016/0163-1047(92)90847-w. [DOI] [PubMed] [Google Scholar]
- Izquierdo I, Schroeder N, Netto CA, Medina JH. Novelty causes time-dependent retrograde amnesia for one-trial avoidance in rats through NMDA receptor- and CAMKII-dependent mechanisms in hippocampus. Eur J Neurosci. 1999;11:3323–3328. doi: 10.1046/j.1460-9568.1999.00742.x. [DOI] [PubMed] [Google Scholar]
- Knight RT. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Knight RT, Nakata T. Cortico-limbic circuits and novelty: A review of EEG and blood flow data. Rev Neurosci. 1998;9:57–70. doi: 10.1515/revneuro.1998.9.1.57. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Manahan-Vaughan D, Braunewell KH. Novelty acquisition is associated with induction of hippocampal long-term depression. Proc Natl Acad Sci. 1999;96:8739–8744. doi: 10.1073/pnas.96.15.8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. Automatic activation of the medial temporal lobe during encoding: Lateralized influences of meaning and novelty. Hippocampus. 1999;9:71–82. doi: 10.1002/(SICI)1098-1063(1999)9:1<62::AID-HIPO7>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Martin JH. Autoradiographic estimation of the extent of reversible inactivation produced by microinjection of lidocaine and muscimol in the rat. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- Mayford M, Bach ME, Huang Y, Wang L, Hawkins RD, Kandel ER. Control of memory formation through regulated expression of a CAMK II transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory—A century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. San Diego: Academic Press; 1986. [Google Scholar]
- Quevedo J, Vianna MRM, Roesler R, de Paris F, Izquierdo I, Rose SPR. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: Protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn Mem. 1999;6:600–607. doi: 10.1101/lm.6.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Nadel NV, Sullivan GM, Harris A, LeDoux JE. Memory consolidation for contextual and auditory fear conditioning is dependent upon protein synthesis, PKA and MAP kinase. Learn Mem. 1999;6:97–110. [PMC free article] [PubMed] [Google Scholar]
- Silva AC, Paylor R, Wehner J, Tonegawa S. Impaired spatial learning in α-calcium-calmodulin-kinase II mutant mice. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- Silva AJ, Kogan JH, Frankland PW, Kida S. CREB and memory. Annu Rev Neurosci. 1998;21:127–148. doi: 10.1146/annurev.neuro.21.1.127. [DOI] [PubMed] [Google Scholar]
- Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S, Liang K-C. Spatial learning alters calcium/calmodulin-dependent protein kinase II activity in rats. Brain Res. 1996;711:234–240. doi: 10.1016/0006-8993(95)01411-x. [DOI] [PubMed] [Google Scholar]
- ————— Inhibitory avoidance learning alters the amygdala calcium/calmodulin-dependent protein kinase II activity in rats. Brain Res. 1997;748:227–233. doi: 10.1016/s0006-8993(96)01298-x. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Bear MF, Alberini CM. A molecular correlate of memory and amnesia in the hippocampus. Nature Neurosci. 1999;2:309–310. doi: 10.1038/7217. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Huston JP, Schwarting RJK. Hippocampal acetylcholine and habituation learning. Neuroscience. 1998;85:1253–1262. doi: 10.1016/s0306-4522(98)00030-x. [DOI] [PubMed] [Google Scholar]
- Tiunova A, Anokhin K, Rose SPR, Mileusnic R. Involvement of glutamate receptors, protein kinases, and protein synthesis in memory for visual discrimination in the young chick. Neurobiol Learn Mem. 1996;65:233–243. doi: 10.1006/nlme.1996.0028. [DOI] [PubMed] [Google Scholar]
- Vianna MRM, Izquierdo LA, Barros D, Medina JH, Izquierdo I. Intrahippocampal infusion of an inhibitor of protein kinase A separates short- from long-term memory. Behav Pharmacol. 1999;10:223–227. doi: 10.1097/00008877-199903000-00011. [DOI] [PubMed] [Google Scholar]
- Walz R, Roesler R, Barros DM, de Souza MM, Rodrigues C, Sant'Anna MK, Quevedo J, Choi H, Neto WP, DeDavid e Silva TL, et al. Effects of post-training infusions of a mitogen-activated protein kinase kinase inhibitor into the hippocampus or entorhinal cortex on short- and long-term retention of inhibitory avoidance. Behav Pharmacol. 1999;10:723–730. doi: 10.1097/00008877-199912000-00003. [DOI] [PubMed] [Google Scholar]
- Wittstock S, Kaatz HH, Menzel R. Inhibition of protein synthesis by cycloheximide does not affect formation of long-term memory in honeybees after olfactory conditioning. J Neurosci. 1993;13:1379–1386. doi: 10.1523/JNEUROSCI.13-04-01379.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman C, Fin C, Dias M, Bianchin M, Da Silva R, Schmitz P, Medina JH, Izquierdo I. Intrahippocampal or intraamygdala infusion of KN62, a specific inhibitor of calcium/calmodulin-dependent protein kinase II, causes retrograde amnesia in the rat. Behav Neural Biol. 1994;61:203–205. doi: 10.1016/s0163-1047(05)80001-9. [DOI] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late-phase LTP. Neuron. 1999;23:787–798. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Wustenberg D, Gerber B, Menzel R. Long—but not medium—term retention of olfactory memories in honeybees is impaired by actinomycin D and anisomycin. Eur J Neurosci. 1998;10:2742–2745. doi: 10.1046/j.1460-9568.1998.00319.x. [DOI] [PubMed] [Google Scholar]
- Yin JCP, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:204–208. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- Yin JCP, Wallach JS, Del Vecchio M, Wilder EL, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Zhu XO, McCabe BJ, Aggleton JP, Brown MW. Differential activation of the rat hippocampus and perirhinal cortex by novel visual stimuli and a novel environment. Neurosci Lett. 1997;229:141–143. doi: 10.1016/s0304-3940(97)00437-0. [DOI] [PubMed] [Google Scholar]