Abstract
We determined the in vitro inhibitory effects of cocoa extracts and procyanidins against pancreatic α-amylase (PA), pancreatic lipase (PL) and secreted phospholipase A2 (PLA2), and characterized the kinetics of such inhibition. Lavado, regular and Dutch-processed cocoa extracts as well as cocoa procyanidins (degree of polymerization (DP) = 2 to 10) were examined. Cocoa extracts and procyanidins dose-dependently inhibited PA, PL and PLA2. Lavado cocoa extract was the most potent inhibitor (IC50 = 8.5 – 47 μg/mL). An inverse correlation between Log IC50 and DP (R2 > 0.93) was observed. Kinetic analysis suggested that regular cocoa extract, the pentamer and decamer inhibited PL activity in a mixed mode. The pentamer and decamer non-competitively inhibited PLA2 activity, whereas regular cocoa extract inhibited PLA2 competitively. Our study demonstrates that cocoa polyphenols can inhibit digestive enzymes in vitro, and may, in conjunction with a low calorie diet, play a role in body weight management.
Keywords: cocoa, Theobroma cacao, procyanidins, phospholipase A2, pancreatic lipase, α-amylase
1. Introduction
Chronic imbalance between energy intake and energy expenditure is the major cause of weight gain and development of obesity (body mass index (BMI) ≥ 30) (1). Today, more than 60 % of Americans are overweight and if the current trajectory continues, the rate will reach 86% by 2030 (2, 3). Elevated BMI is attributable to a global shift in diet towards increased intake of energy-dense foods that are high in fat and carbohydrates but low in vitamins, minerals and other micronutrients as well as increased prevalence of sedentary lifestyle (3). Elevated BMI is also a major risk factor in the development of heart disease, fatty liver disease, cancer, and Type II diabetes (4). One strategy for the prevention of overweight and obesity related disease is the use of agents that interfere with the hydrolysis and absorption of dietary carbohydrates and lipids. Pancreatic α-amylase (PA), pancreatic lipase (PL) and pancreatic phospholipase A2 (PLA2), which are delivered into the intestinal lumen as constituents of pancreatic juices, are the major enzymes involved in the hydrolysis of dietary starch and fat (5). PA is an endoglucosidase that catalyzes the hydrolysis of starch to maltose and maltotriose (6). PL is a key enzyme for absorption of dietary triglycerides and rapidly converts a triglyceride to a 2-monoglycerol and two free fatty acids (5). Orlistat (marketed over-the-counter as Alli in U.S.), a potent competitive inhibitor of PL, is available as an anti-obesity drug. It has been reported that Orlistat promoted both short-term and long-term weight loss and minimized weight regain in overweight or obese subjects (7). PLA2 serves in the initial digestion of phospholipids to free fatty acids and lysolipids. Considerable evidence from cell and animal studies suggest the importance of PLA2 in facilitating the digestion and absorption of lipids (8). Given the key role these three enzymes play in starch and lipid digestion, they represent attractive 57 targets for prevention of excessive body weight gain and of obesity-related diseases including diabetes.
A growing literature has suggested polyphenols from teas, berries and other plants can inhibit some digestive enzymes in vitro and in vivo. For example, Horigome et al. reported that proanthocyanidins from various plants (i.e. black locust, bush clover, wistaria and Japanese knotgrass) have inhibitory effects on lipase, α-amylase, and trypsin (9). Studies have shown green tea catechins can inhibit the intestinal absorption of lipids in vivo (8, 10). This was associated with in vitro inhibitory activities of tea catechins against PLA2. Among the green tea catechins, EGCG is the most potent inhibitor, and it inhibited PLA2 in vitro by 64.9 % at 2 mM. Harach et al. reported rosemary leaf extract, containing 5–10% phenolic compounds, induced a significant reduction of weight and fat mass gain associated with an increase of fecal lipid excretion in high fat –fed mice, and this effect was related to the inhibition of PL activity by the extract (11).
Cocoa (Theobroma cacao) is a rich source of polyphenols with levels reaching 12–18% by dry weight (12). Cocoa polyphenols are primarily composed of monomeric flavanols ((−)-epicatechin and to a lesser degree (+)-catechin) and oligomeric and polymeric C4β-C8 linked B-2 type procyanidins (Fig.1). The monomers account for only about 10 % of the total with the oligomeric and polymeric procyanidins accounting for about 90% of the flavanol content (13). Procyanidins with a degree of polymerization (DP) up to decamer have been identified in cocoa (14). Evidence from the literature indicates that cocoa processing dramatically affects the polyphenol and flavanol content. As cocoa beans are processed on the farm, they are often fermented for two to greater than 6 days, and substantial flavanol loss occurs. Unfermented cocoa that has been immediately water washed and dried is referred to as Lavado cocoa and contains the highest amount of polyphenols. Once fermented and dried, the nib of the cocoa bean is roasted and ground, resulting in the cocoa liquor or separated into cocoa powder and cocoa butter, which are the basis for chocolate manufacture. Dutch-processing (or Alkalization) can also be applied to change the color and develop the flavor of cocoa products. Fermentation and Dutch-processing have been reported to result in the loss of as much as 90% of the cocoa flavanols (15).
Fig. 1.
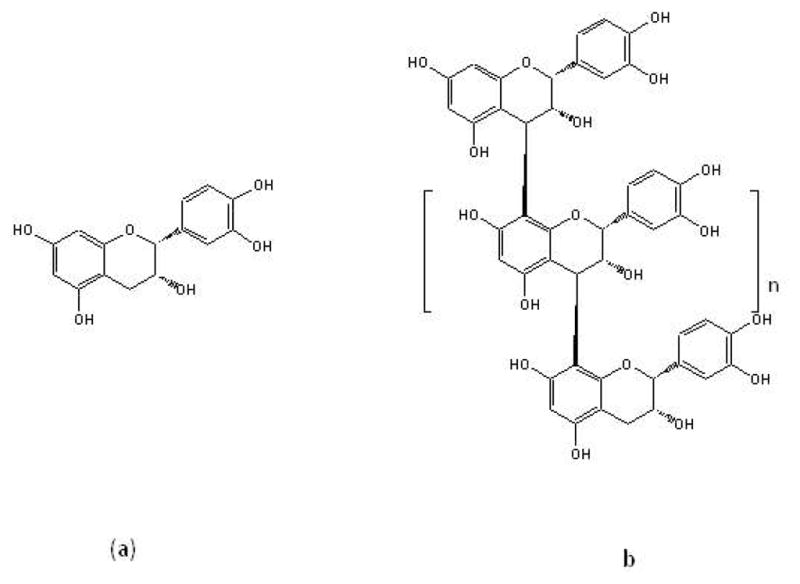
Structure of (a) (−) epicatechin and (b) procyanidin oligomers with C4β-C8 linkage repeating units.
Studies on the health benefits of cocoa have primarily focused on the effects on the risk of cardiovascular disease (16). Recently, a few studies on the anti-obesity and anti-diabetic potential of cocoa have been conducted in animal models. Matsui et al. showed that cocoa supplementation for 3 weeks significantly decreased weight gain in high fat-fed rats comparing to high fat-fed controls (17). Another study by Ruzaidi et al. also showed that dietary cocoa extract (1–3% w/w) dose-dependently reduced body weight gain, serum glucose levels and total triglycerides in diabetic rats compared to control-fed animals (18). Tomaru et al. reported that a diet containing 0.5% or 1.0% cocoa procyanidins decreased the levels of blood glucose and fructosamine in diabetic obese mice compared with the control treatment (19). Jalil et al. found that short-term (acute) supplementation of cocoa extracts significantly reduced the plasma glucose level in obese-diabetic rats at 60 min and 90 min compared with untreated (20). Given recent animal model studies showing that dietary intake of cocoa might be beneficial in preventing the onset of obesity and type II diabetes, as well as cocoa being a rich source of procyanidins, studies of inhibition of key digestion enzymes by cocoa polyphenols were warranted.
The purpose of the present study was to determine the in vitro inhibitory effects of a series of cocoa extracts, ranging from high total flavanols (Lavado) to low flavanols (Dutch-processed) and isolated cocoa procyanidins against PA, PL and PLA2, and to characterize the kinetics of such inhibition.
2. Materials and Methods
2.1 Materials
Cocoa procyanidins (DP = 2 to 10, B type) and three cocoa extracts (from regular, lavado and Dutch-processed cocoa powder) were provided by The Hershey Company (Hershey, PA). The purity of all cocoa procyanidins were greater than 85% by HPLC-MS. The polyphenol levels in the three extracts were assessed using the Folin-Ciocalteu reagent (Sigma Aldrich). (−)- Epicatechin (EC), Orlistat and Lipase from porcine pancreas (Type II) and 4-nitrophenyl butyrate (4-NPB, 98%) were purchased from Sigma-Aldrich (St. Louis, MO). Stock solutions were prepared in dimethyl sulfoxide (EMD Chemicals Inc.) and stored at −80°C. α-Amylase from porcine pancreas and Red-starch were purchased from Megazyme (Wicklow, Ireland). EnzChek® Phospholipase A2 Assay Kit was purchased from Invitrogen (Carlsbad, CA). All the other reagents were of the highest grade commercially available.
2.2 Pancreatic α-Amylase Inhibition Assay in vitro
Inhibition of PA by cocoa extracts and procyanidins was examined using a modification of the chromogenic Red-starch method (Megazyme, IR). PA (0.3 U/ml) in 20 mM phosphate buffer (pH 6.9) containing 6.7 mM sodium chloride and Red-starch (7 mg/ml in 0.5 M potassium chloride) were combined with cocoa procyanidins (0–100 μM) or cocoa extracts (0–200 μg/ml). After incubation at 37°C for 10 min, the reaction was stopped by addition of 95% ethanol. After equilibration to room temperature, the solution was centrifuged at 1000g for 10 min, and the absorbance of the supernatant was measured at 510nm using a BECKMAN DU® 650 spectrophotometer.
2.3 Pancreatic Lipase Inhibition Assay in vitro
Inhibition of PL by cocoa polyphenols was tested by monitoring the cleavage of 4-NPB to release 4-nitrophenol. Cocoa procyanidins (0–20 μM) or cocoa extracts (0–200 μg/ml) were combined with PL (100 μg/ml) in 0.1 M Tris-HCl buffer (pH 8), and 4-NPB (0.2 mM) was added to start the reaction. Following incubation at room temperature for 10 min, absorbance was read at 400 nm. Orlistat was used as a positive control.
2.4 Phospholipase A2 Inhibition Assay in vitro
Inhibition of PLA2 was examined using a commercially available fluorometric method (Invitrogen). Buffered PLA2 solution (1 U/ml, pH 8.9) and cocoa procyanidins (0–100μM) or cocoa extracts (0–200μg/ml) were combined in a 96 well plate. A fluorogenic PLA2 substrate (Red/Green BODIPY® PC-A2, 1.67μM) was dispensed to each well to start the reaction. After incubation at room temperature in the dark for 10 min, fluorescence was determined at λex =485 nm and λem = 538 nm (Fluoroskan Ascent FL, ThermoFisher Scientific Inc.).
2.5 Kinetic Analysis
Cocoa procyanidin pentamer and decamer as well as regular cocoa extract were selected for kinetic analysis of inhibition against PL and PLA2. Reaction conditions were analogous to those above with the following modification. Cocoa procyanidins or cocoa extracts were held at constant concentrations and incubated in the presence of increasing concentrations of substrates (50 – 400 μM, PL substrate; 0.5 – 4 μM, PLA2 substrate) together with enzymes and buffer solutions.
2.6 Data Analysis
The median inhibitory concentration (IC50) of each cocoa procyanidin and extract was determined by interpolation or extrapolation of a dose-response curve using GraphPad Prism software (San Diego, CA). For kinetic analysis, Michaelis-Menten plots were generated using GraphPad Prism, and the maximum velocity (Vmax), Michaelis-Menten constant (Km) and the mode of inhibition were determined from those plots
Data are expressed as mean ± standard deviation (SD) of the mean of at least three independent experiments. Vmax and Km values were compared by one-way ANOVA or Student’s t-test as appropriate. P-values less than 0.05 were considered as statistically significant.
3. Results
3.1 Inhibition of digestive enzymes by cocoa extracts in vitro
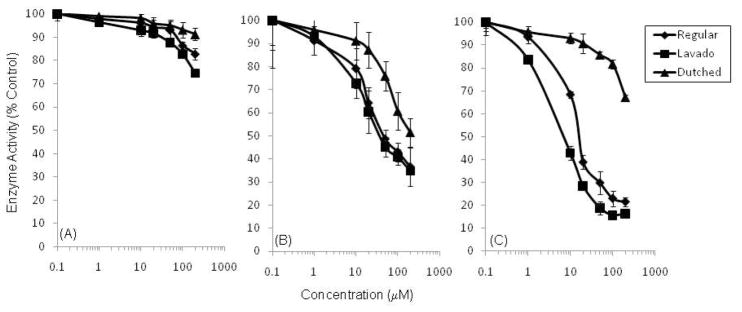
The inhibitory effects of cocoa extracts against PA, PL and PLA2 were dose-dependent (Fig. 2). Lavado, regular, and Dutch-processed cocoa extracts inhibited PA by 25, 20 and 10%, respectively, at 200 μg/ml. PL was more sensitive to all three of the cocoa extracts with IC50 = 47.0, 57.7, and 172 .4 μg/ml for lavado, regular and Dutch-processed cocoa extract, respectively. PLA2 was the most sensitive to cocoa extract with the lavado and regular cocoa extract showing IC50 = 8.5 and 19.7 μg/ml, respectively. The Dutch-processed cocoa extract was not as potent and inhibited PLA2 only by 30% at 200 μg/ml. In order to determine if the inhibition of digestive enzymes correlated with the phenol content of the extracts, we examined the levels of these compounds in the cocoa extracts using the Folin-Ciocalteu reagent (Table 1). We found, as expected, that the lavado extract had the highest levels of phenols, followed by regular cocoa, and lastly Dutch-processed cocoa.
Fig. 2.
Inhibition of (a) PA, (b) PL, and (c) PLA2 activity by cocoa extracts (regular, lavado and Dutch-processed). Values are normalized to vehicle-treated controls and expressed as the mean ± SD of at least three independent experiments.
Table 1.
Phenol content of the lavado, regular, and Dutch-processed cocoa extracts.*
| Cocoa Extract | Phenol Content (mg/g) |
|---|---|
| Lavado | 481.4 ± 9.2a |
| Regular | 271.0 ± 5.8b |
| Dutch-processed | 128.4 ± 4.0c |
Phenol content expressed as gallic acid equivalents. Values not sharing a common superscript are significantly different (p < 0.05)
3.2 Inhibition of digestive enzymes by cocoa procyanidins in vitro
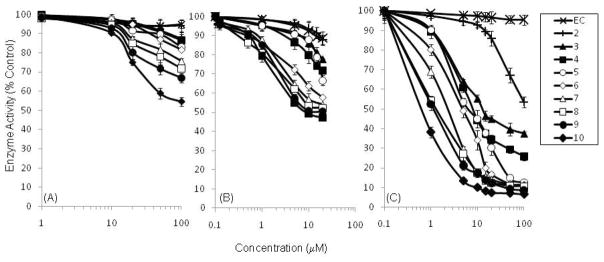
Dose-response curves shown in Fig. 3 summarize the inhibitory activity of cocoa procyanidins (DP=2–10) as well as (−)-epicatechin (EC) against PA, PL and PLA2. Lower molecular weight compounds with DP< 5 showed less than 15% inhibition against PA at a concentration of 100 μM, whereas the higher molecular weight procyanidins (DP=5–10) inhibited PA by 17–45.5% at 100 μM. Cocoa procyanidins generally showed much stronger inhibitory activity against PL. EC and the dimer showed only 15% inhibition at 20 μM, while the trimer and tetramer caused 25% inhibition at 20 μM. Compounds with DP ≥ 5 inhibited PA by 37–53% respectively at 20 μM. Orlistat was used as a positive control and inhibited PL by 72% at 10 μM. Though EC only inhibited PLA2 by 4.5% at a concentration up to 100 μM, the cocoa procyanidins were particularly effective in inhibition of PLA2. Cocoa procyanidins with DP=2–5 inhibited PLA2 by 46–74% at 100 μM. For higher DP procyanidins (DP=6–10), approximately 90% of total enzyme activity was inhibited at 50 μM and the IC50 values for these compounds were less than 5 μM.
Fig. 3.
Inhibition of PA (a), PL (b) and PLA2 (c) activity by EC and cocoa procyanidins (DP=2 – 10). Values are normalized to vehicle-treated controls and expressed as the mean ± SD of at least three independent experiments.
3.3 Correlations between DP of cocoa procyanidins and their IC50 values
Based on the dose-response curves for the pure procyanidins against PL and PLAs, DP appears to be an important factor determining the potency of compound. By regression analysis, we observed a strong inverse relationship between Log IC50 and DP (R2 > 0.93, Fig. 4). Similar analysis comparing Log IC50 and hydrophobicity (Log P) showed no significant correlation (data not shown). Because the procyanidins did not approach the IC50 of PA, similar analysis could not be conducted.
Fig. 4.
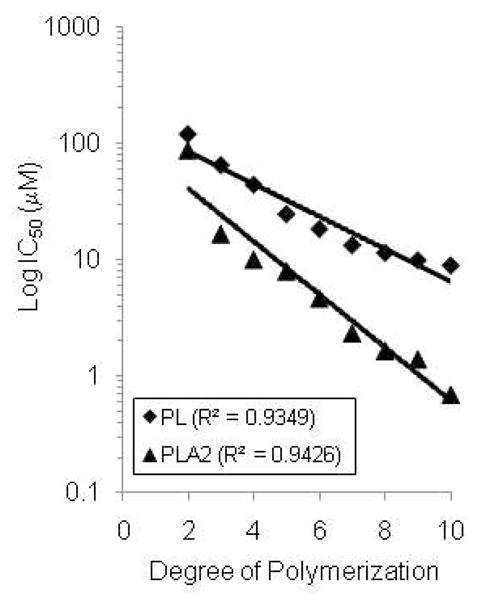
Relationship between degree of polymerization (DP= 2 to 10) of cocoa procyanidins and the IC50 against PL and PLA2. Regression analysis was performed using Graph Pad Prism software (San Diego, CA). R2 values are shown in the figure key.
3.4 Kinetic Analysis of PL and PLA2 inhibition
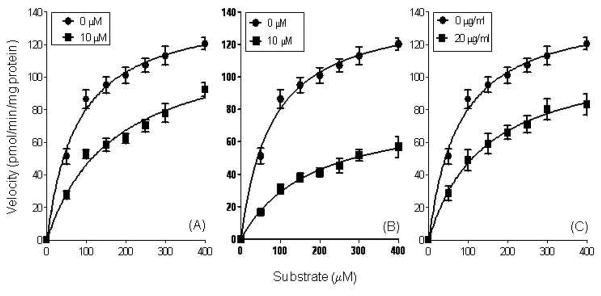
Since PL and PLA2 were more sensitive to inhibition by cocoa extracts and procyanidins, we selected these enzymes for further kinetic analysis to determine the mode of inhibition. The procyanidin pentamer and decamer as well as the regular cocoa extract were selected as test inhibitors. All three test substances reduced the Vmax and increased Km of PL (Fig.5, Table 2). These results suggest a mixed-type inhibition with respect to substrate concentration.
Fig. 5.
Inhibitory kinetics of cocoa procyanidin pentamer (a), decamer (b) and regular cocoa extract (c) on PL were determined using Michaelis-Menten analysis. Values are expressed as the mean ± SD of at least three independent experiments.
Table 2.
Effects of cocoa procyanidin pentamer, decamer and regular cocoa extract on Vmax and Km values of PL.*
| Pentamer (μM) | Decamer (μM) | Regular Cocoa Extract (μg/mL) | ||||
|---|---|---|---|---|---|---|
| 0 | 10 | 0 | 10 | 0 | 20 | |
| Vmax | 142.8a | 123.8b | 142.8a | 81.38b | 142.8a | 113.5b |
| Km | 77.91a | 168.3b | 77.91a | 180.5b | 77.91a | 140.2b |
|
| ||||||
| Inhibition Type | Mixed | Mixed | Mixed | |||
For each inhibitor, values in the same row not sharing a common superscript are significantly different (P<0.05).
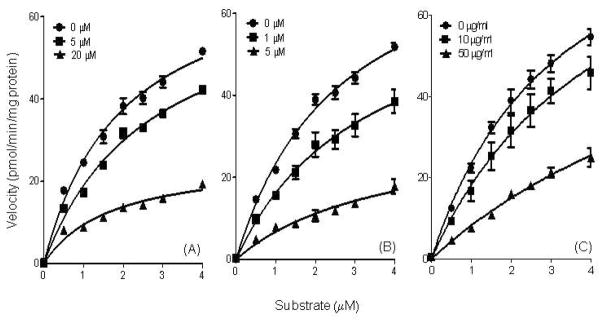
On the other hand, Michealis-Menten plots of PLA2 inhibition by the procyanidin pentamer and decamer decreased the Vmax value but did not affect Km indicating a non-competitive inhibition with respect to substrate concentration (Fig. 6, Table 3). By contrast, the regular cocoa extract increased Km but had no effect on Vmax. These results suggest a competitive mode of inhibition against PLA2 with respect to substrate concentration (Fig. 6, Table 3).
Fig. 6.
Inhibitory kinetics of cocoa procyanidin pentamer (a), decamer (b) and regular cocoa extract (c) on PLA2 were determined using Michaelis-Menten analysis. Values are expressed as the mean ± SD of at least three independent experiments.
Table 3.
Effects of cocoa procyanidin pentamer, decamer and regular cocoa extract 427 on Vmax and Km values of PLA2.*
| Pentamer (μM) | Decamer (μM) | Regular Cocoa Extract (μg/mL) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 5 | 20 | 0 | 1 | 5 | 0 | 10 | 50 | |
| Vmax | 74.66a | 69.97a | 25.53b | 85.14a | 69.53b | 32.37c | 53.14a | 53.36a | 50.91a |
| Km | 1.981a | 2.691a | 1.71a | 2.637a | 3.283a | 3.793a | 3.361a | 4.685a | 11.55b |
|
| |||||||||
| Inhibition Type | Non-competitive | Non-competitive | Competitive | ||||||
For each inhibitor, values in the same row not sharing a common superscript are significantly different (P<0.05).
4. Discussion
In this study, the in vitro inhibitory effects of cocoa extracts and cocoa procyanidins against PA, PL and PLA2 were investigated. Kinetic analysis was performed to determine the mode of inhibition by regular cocoa extracts, the procyanidin pentamer and decamer with respect to substrate concentration. To our knowledge, this is the first detailed study to report the in vitro inhibition of key digestive enzymes by cocoa extracts and cocoa procyanidins. Additionally, this is the first report on the kinetics of inhibition of PL and PLA2 by procyanidins from any source. This study extends previous work by Goncalves et al., showing that these compounds can affect digestive proteases (21, 22).
It is increasingly recognized that polyphenols can regulate carbohydrate and lipid metabolism by affecting the activity of digestive enzyme. Inhibition of α-amylase in vitro by cocoa phenolic extracts has been noted earlier in Quesada et al. (20). In our study, all of three cocoa extracts demonstrated inhibitory activities in vitro. Among three cocoa extracts, lavado (meaning “washed” in Spanish) cocoa undergoes the least processing (without fermentation or Dutch-processing), and this extract exerted the highest inhibitory activity against all of three digestive enzymes. By contrast, the Dutch-processed or alkali treated cocoa, which is the most highly processed, showed the least inhibitory effect against the enzymes tested. Since it is expected that the lavado cocoa extract is the highest in polyphenols and flavanols, followed by the regular cocoa extract and the least would be found in the Dutch-processed cocoa extract, these results suggest that the inhibitory effects of cocoa extracts are related to their polyphenol content. In this study, the lavado cocoa extract was a potent enzyme inhibitor, and our results are comparable or superior to some recent studies with other polyphenol-rich extracts. Moreno et al. found that grape seeds extract at a concentration of 1 mg/ml resulted in 80% inhibition against PL, and they also suggested that the inhibitory effect may be caused by a synergistic action of several phenolic compounds including procyanidins within the extracts (23). Polyphenol-rich berry fruits such as strawberry and raspberry have been shown to inhibit PA, but the effects were relatively weak (e.g. 25 mg/ml strawberry extract inhibited PA by 14.7 % in vitro) (24, 25).
In general, the cocoa procyanidins (DP=2–10) showed greater inhibitory activity against PLA2 than PL and PA. These results mirror the results of our study on cocoa extracts and suggest that procyanidins are the components in cocoa responsible for inhibition of these digestive enzymes. The inhibitory potency of the cocoa procyanidins is increased as a function of DP. These results are in agreement with some recent studies. Sugiyama et al. reported that the oligomeric procyanidins in apples significantly decreased the plasma triglyceride levels in both mice and humans, and inhibited PL activity in vitro. They also suggested that DP was an important factor in determining the inhibitory potency and a strong inverse correlation was observed (24). Another study found that procyandins from persimmon peel showed strong inhibitory activity against α-amylase in vitro (IC50 < 100 μg/ml), and the inhibition of α-amylase activity was dependent on the DP (26).
The results of kinetic analysis suggested that regular cocoa extracts, the procyanidin pentamer and decamer inhibited PL activity in a mixed mode. By contrast, the procyanidin pentamer and decamer noncompetitively inhibited PLA2 activity, whereas the regular cocoa extracts inhibited PLA2 in a competitive fashion. These results demonstrate the diversity of potential interactions between the procyanidins, the enzyme surface and/or the substrate, and such interactions need further study by in silico or crystallographic methods. These results suggest that other compounds in cocoa beyond the procyanidins might also contribute to the inhibitory potency of the extract. In addition to the flavanols, cocoa is also rich in methylxanthines (caffeine, theobromine and theophylline), which have been shown to have thermogenic, diuretic and appetite-suppressing properties that may aid in obesity and diabetes prevention (27). However, scientific data in relation to the in vitro inhibition of digestive enzymes by methylxanthines are still limited.
Biological properties of cocoa polyphenols are modulated by their bioavailability. One proposed limitation of cocoa procyanidins is their low systemic bioavailability. Studies have shown that monomers and dimers in cocoa can be absorbed, and they began to appear in plasma within 30 min −60 min post consumption (28, 29). Despite their presence in cocoa in high amounts, procyanidin oligomers larger than dimers have not been detected in human plasma following the consumption of cocoa products (30). However, because our studies are focused on the small intestine lumen as the site of action, we believe the bioavailability is not a limiting factor. Previous studies have shown that these compounds are stable in the stomach and small intestinal milieu and are expected to be present in the small intestinal lumen at relatively high concentrations following consumption of cocoa products, particularly those with high polyphenol content (e.g. dark chocolate) (31, 32). We believe that the effective concentrations in our enzyme inhibition assays are physiologically achievable in this situation, although further studies are needed to confirm the in vivo activity and small intestinal bioavailability of these compounds.
In summary, the present study provides the first evidence that cocoa extracts and cocoa procyanidins are potent inhibitors of key enzymes in digestion of carbohydrates and lipids in vitro, and these inhibitory activities are related to polyphenol content in cocoa extracts and the degree of polymerization of cocoa procyanidins. Further in vivo studies are needed to examine whether cocoa extracts and/or cocoa procyanidins can inhibit digestive enzymes in vivo and related down-stream pathways such as aberrant eicosanoid metabolism at dose levels achievable in the diets.
Acknowledgments
The present study was supported by a grant from The Hershey Company and by a grant from the National Center for Complementary and Alternative Medicine (AT004678).
Abbreviations
- BMI
body mass index
- DP
degree of polymerization
- EC
(−)-epicatechin
- IC50
median inhibitory concentration
- Km
Michaelis-Menten constant
- PA
pancreatic α-amylase
- PL
pancreatic lipase
- PLA2
secreted phospholipase A2
- Vmax
maximum velocity
Footnotes
Safety
There are no issues related to safety in the present manuscript.
Literature Cited
- 1.Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;104:531–43. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- 2.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. Jama. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 2008;16:2323–30. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 4.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest. 2004;114:1752–61. doi: 10.1172/JCI21625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe ME. Pancreatic triglyceride lipase and colipase: insights into dietary fat digestion. Gastroenterology. 1994;107:1524–36. doi: 10.1016/0016-5085(94)90559-2. [DOI] [PubMed] [Google Scholar]
- 6.Damager I, Numao S, Chen H, Brayer GD, Withers SG. Synthesis and characterisation of novel chromogenic substrates for human pancreatic alpha-amylase. Carbohydr Res. 2004;339:1727–37. doi: 10.1016/j.carres.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 7.Rossner S, Sjostrom L, Noack R, Meinders AE, Noseda G. Weight loss, weight maintenance, and improved cardiovascular risk factors after 2 years treatment with orlistat for obesity. European Orlistat Obesity Study Group. Obes Res. 2000;8:49–61. doi: 10.1038/oby.2000.8. [DOI] [PubMed] [Google Scholar]
- 8.Wang S, Noh SK, Koo SI. Green tea catechins inhibit pancreatic phospholipase A(2) and intestinal absorption of lipids in ovariectomized rats. J Nutr Biochem. 2006;17:492–8. doi: 10.1016/j.jnutbio.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Horigome T, Kumar R, Okamoto K. Effects of condensed tannins prepared from leaves of fodder plants on digestive enzymes in vitro and in the intestine of rats. Br J Nutr. 1988;60:275–85. doi: 10.1079/bjn19880099. [DOI] [PubMed] [Google Scholar]
- 10.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. J Nutr Biochem. 2007;18:179–83. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harach T, Aprikian O, Monnard I, Moulin J, Membrez M, Beolor JC, Raab T, Mace K, Darimont C. Rosemary (Rosmarinus officinalis L.) leaf extract limits weight gain and liver steatosis in mice fed a high-fat diet. Planta Med. 76:566–71. doi: 10.1055/s-0029-1240612. [DOI] [PubMed] [Google Scholar]
- 12.Kim H, Keeney PG. (−)-Epicatechin content in fermented cocoa beans. J Food Sci. 1984;49:1090–1092. [Google Scholar]
- 13.Wollgast J, Pallaroni L, Agazzi ME, Anklam E. Analysis of procyanidins in chocolate by reversed-phase high-performance liquid chromatography with electrospray ionisation mass spectrometric and tandem mass spectrometric detection. J Chromatogr A. 2001;926:211–20. doi: 10.1016/s0021-9673(01)00994-3. [DOI] [PubMed] [Google Scholar]
- 14.Hammerstone JF, Lazarus SA, Mitchell AE, Rucker R, Schmitz HH. Identification of procyanidins in cocoa (Theobroma cacao) and chocolate using high-performance liquid chromatography/mass spectrometry. J Agric Food Chem. 1999;47:490–6. doi: 10.1021/jf980760h. [DOI] [PubMed] [Google Scholar]
- 15.Miller KB, Hurst WJ, Payne MJ, Stuart DA, Apgar J, Sweigart DS, Ou B. Impact of alkalization on the antioxidant and flavanol content of commercial cocoa powders. J Agric Food Chem. 2008;56:8527–33. doi: 10.1021/jf801670p. [DOI] [PubMed] [Google Scholar]
- 16.Cooper KA, Donovan JL, Waterhouse AL, Williamson G. Cocoa and health: a decade of research. Br J Nutr. 2008;99:1–11. doi: 10.1017/S0007114507795296. [DOI] [PubMed] [Google Scholar]
- 17.Matsui N, Ito R, Nishimura E, Yoshikawa M, Kato M, Kamei M, Shibata H, Matsumoto I, Abe K, Hashizume S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition. 2005;21:594–601. doi: 10.1016/j.nut.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Ruzaidi A, Amin I, Nawalyah AG, Hamid M, Faizul HA. The effect of Malaysian cocoa extract on glucose levels and lipid profiles in diabetic rats. J Ethnopharmacol. 2005;98:55–60. doi: 10.1016/j.jep.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 19.Tomaru M, Takano H, Osakabe N, Yasuda A, Inoue K, Yanagisawa R, Ohwatari T, Uematsu H. Dietary supplementation with cacao liquor proanthocyanidins prevents elevation of blood glucose levels in diabetic obese mice. Nutrition. 2007;23:351–5. doi: 10.1016/j.nut.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Jalil AM, Ismail A, Pei CP, Hamid M, Kamaruddin SH. Effects of cocoa extract on glucometabolism, oxidative stress, and antioxidant enzymes in obese-diabetic (Ob-db) rats. J Agric Food Chem. 2008;56:7877–84. doi: 10.1021/jf8015915. [DOI] [PubMed] [Google Scholar]
- 21.Goncalves R, Mateus N, de Freitas V. Study of the interaction of pancreatic lipase with procyanidins by optical and enzymatic methods. J Agric Food Chem. 2010;58:11901–6. doi: 10.1021/jf103026x. [DOI] [PubMed] [Google Scholar]
- 22.Goncalves R, Soares S, Mateus N, de Freitas V. Inhibition of trypsin by condensed tannins and wine. J Agric Food Chem. 2007;55:7596–601. doi: 10.1021/jf071490i. [DOI] [PubMed] [Google Scholar]
- 23.Moreno DA, Ilic N, Poulev A, Brasaemle DL, Fried SK, Raskin I. Inhibitory effects of grape seed extract on lipases. Nutrition. 2003;19:876–9. doi: 10.1016/s0899-9007(03)00167-9. [DOI] [PubMed] [Google Scholar]
- 24.McDougall GJ, Shpiro F, Dobson P, Smith P, Blake A, Stewart D. Different polyphenolic components of soft fruits inhibit alpha-amylase and alpha-glucosidase. J Agric Food Chem. 2005;53:2760–6. doi: 10.1021/jf0489926. [DOI] [PubMed] [Google Scholar]
- 25.da Silva Pinto M, Kwon YI, Apostolidis E, Lajolo FM, Genovese MI, Shetty K. Functionality of bioactive compounds in Brazilian strawberry (Fragaria x ananassa Duch.) cultivars: evaluation of hyperglycemia and hypertension potential using in vitro models. J Agric Food Chem. 2008;56:4386–92. doi: 10.1021/jf0732758. [DOI] [PubMed] [Google Scholar]
- 26.Lee YA, Cho EJ, Tanaka T, Yokozawa T. Inhibitory activities of proanthocyanidins from persimmon against oxidative stress and digestive enzymes related to diabetes. J Nutr Sci Vitaminol (Tokyo) 2007;53:287–92. doi: 10.3177/jnsv.53.287. [DOI] [PubMed] [Google Scholar]
- 27.Dulloo AG, Seydoux J, Girardier L, Chantre P, Vandermander J. Green tea and thermogenesis: interactions between catechin-polyphenols, caffeine and sympathetic activity. Int J Obes Relat Metab Disord. 2000;24:252–8. doi: 10.1038/sj.ijo.0801101. [DOI] [PubMed] [Google Scholar]
- 28.Richelle M, Tavazzi I, Enslen M, Offord EA. Plasma kinetics in man of epicatechin from black chocolate. Eur J Clin Nutr. 1999;53:22–6. doi: 10.1038/sj.ejcn.1600673. [DOI] [PubMed] [Google Scholar]
- 29.Schroeter H, Heiss C, Balzer J, Kleinbongard P, Keen CL, Hollenberg NK, Sies H, Kwik-Uribe C, Schmitz HH, Kelm M. (−)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc Natl Acad Sci U S A. 2006;103:1024–9. doi: 10.1073/pnas.0510168103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwik-Uribe C, Bektash RM. Cocoa flavanols - measurement, bioavailability and bioactivity. Asia Pac J Clin Nutr. 2008;17(Suppl 1):280–3. [PubMed] [Google Scholar]
- 31.Zhu QY, Holt RR, Lazarus SA, Ensunsa JL, Hammerstone JF, Schmitz HH, Keen CL. Stability of the flavan-3-ols epicatechin and catechin and related dimeric procyanidins derived from cocoa. J Agric Food Chem. 2002;50:1700–5. doi: 10.1021/jf011228o. [DOI] [PubMed] [Google Scholar]
- 32.Rios LY, Bennett RN, Lazarus SA, Remesy C, Scalbert A, Williamson G. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr. 2002;76:1106–10. doi: 10.1093/ajcn/76.5.1106. [DOI] [PubMed] [Google Scholar]