Abstract
β3-Adrenergic receptor agonists are currently under clinical development for the treatment of overactive bladder, a condition that is prevalent in postmenopausal women. These agents purportedly relax bladder smooth muscle through a direct action at the myocyte β3-receptor. The aim of this study was to examine the expression of the individual beta-adrenergic receptors in full thickness sections from ageing human female bladder. We obtained a series of rabbit polyclonal antibodies generated against each of the three β-adrenergic receptors, and validated their receptor specificity in CHOK1 cells expressing each of the individual receptors. Immunostaining for β1, β2, and β3 were each more prominent in the urothelium than in the detrusor, with all receptors expressed in the same cell types, indicating co-expression of all three receptors throughout the urothelium in addition to the detrusor. Staining of all receptors was also observed in suburothelial myofibroblast-like cells, intramural ganglion cells, and in Schwann cells of intramural nerves. The β3-receptor in the human urothelium appears to be functional, as two different selective β3-receptor agonists, TAK677 and BRL37344, stimulate cAMP formation in UROtsa cells. Densitometry analysis indicates a persistent expression of all receptors throughout the bladder with increasing age, with the exception of the β2-receptor in the urothelium of the trigone, which appears to decrease slightly in older women. These data indicate that β3-receptor expression is maintained with age, but may function in concert with other β-receptors. Activation of the myocyte receptor may be influenced by action on non-myocyte structures including the intramural ganglion cells and myofibroblasts.
Keywords: Overactive bladder, β-adrenergic receptors, Immunohistochemistry, Urothelium, Age
Introduction
There is good evidence suggesting that β-adrenoceptors (ARs) are abundant in the detrusor smooth muscle of the urinary bladder of various species and, at least in animals, play an important role in detrusor relaxation during urinary storage (Michel and Vrydag 2006). The β-AR subtype mediating detrusor relaxation appears to be species-dependent: β2-ARs in rabbits (Morita et al. 2000) and mouse (Wuest et al. 2009), both β2- and β3-ARs in rats (Longhurst and Levendusky 1999) and pigs (Yamanishi et al. 2002), and mostly β3-ARs in dogs (Yamazaki et al. 1998) and humans (Fujimura et al. 1999; Takeda et al. 1999; Igawa et al. 1999, 2001; Wuest et al. 2009). β-AR agonists are assumed to relax bladder smooth muscle through a direct effect on the detrusor predominantly through action at the myocyte β3-AR. This assumption is based largely on PCR analysis and/or muscle relaxation experiments performed on isolated detrusor strips from which the underlying mucosa has been removed. However, the β3-ARs are not only expressed on detrusor smooth muscle but have also been localized to other bladder wall structures that seem to contribute to the regulation of bladder function. Indeed, recent studies have shown the expression of β3-ARs in the urothelium of different species including human (Otsuka et al. 2008), rat (Kullmann et al. 2009, 2010) and pig (Masunaga et al. 2010). Previous reports have indicated a functional link between urothelial β-ARs and detrusor smooth muscle, but the molecular biological basis for such a connection has not been clarified. It has been suggested that β-AR agonists, possibly via urothelial β3-ARs (Masunaga et al. 2010), might stimulate the urothelial release of an unidentified factor which inhibits detrusor contractility directly or indirectly (Murakami et al. 2007). In addition, systemic activation of β3-ARs using CL316, 243 inhibits the mechanosensitive Aδ afferent fiber activity and prostangladine E2-induced C-fiber hyperactivity (Aizawa et al. 2009).
In human bladder, it has not been established whether β-ARs are located on other structures in the bladder that may be involved in its functional control, e.g., interstitial cells of Cajal, suburothelial nerves, and vessels. The aim of the present study was to further analyze the distribution of all β-ARs to different structures in the female human bladder wall using well-characterized antibodies to the various subtypes, and to examine whether receptor expression is maintained as a function of age.
Materials and methods
Human bladder sample procurement and qualification
Bladder tissue samples from female donors were acquired by Lifespan Biosciences (Seattle, WA, USA) and Asterand (Royston, Herts, UK). All samples were obtained under IRB approved protocols and patient consent release policies from donor organizations that have approved the tissues for use in contract research. Tissues samples were divided into two cohorts defined as <45-year or >45-year samples, although hormonal status could not be verified retrospectively for the patients corresponding to the tissue samples. Donor pathology reports were provided by the donation site under prior IRB approval and submitted without patient identifying information to preserve patient confidentiality. Only those tissue sections of full thickness bladder regions identified as normal by the pathologist were used for immunohistochemical analysis. Regions of inflammation were avoided.
Transient expression of β-AR for validation of antibodies for IHC in Chinese hamster ovary (CHOK1) cells
CHOK1 cells utilized for immunohistochemical analysis were transfected with hβ1-AR, hβ2-AR, hβ3-AR, or pcDNA3.1+(mock) cDNAs. Briefly, CHOK1 cells were plated at a density of 2–3×106 cells per 100-mm dish the day prior to transfection. Cells were washed three times with 5 ml Optimem and then overlayed with 5 ml Optimem. Then, 5 μg cDNA per 100-mm dish was transfected into cells using Lipofectamine-2000 reagent (Invitrogen) according to manufacturer's instructions. After 5 h, 10 ml of growth medium was added to the transfection and cells were incubated overnight at 37°C, 5% CO2. The following day, cells were split 1:3 and cultured overnight. Cells were washed three times and collected using Cell Stripper (MediaTech). Cells were then washed three times in Dulbecco's-PBS and pelleted. Cells were fixed with 10% buffered formalin (VWR) at room temperature for 15 min, washed three times with 20 ml D-PBS per wash, pelleted, and resuspended in 70% ethanol for storage. For immunohistochemical studies, ethanol was decanted and cells were embedded in 0.5 ml of 3% Agar cooled to 56°C.
Immunostaining and densitometry
Antibody validation in CHOK1 cells over-expressing the individual β-AR receptors was performed at Lifespan Biosciences. Immunostaining of bladder samples was performed by both Lifespan Biosciences and Asterand on paraffin-embedded samples sectioned at 4 μm using the same methods as previously described (Kullmann et al. 2009). Briefly, β1-AR immunostaining was assessed using 2.5 μg/ml polyclonal antisera SC-567 and SC-568 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for both cells and tissue. β2-AR immunostaining was assessed using LS-A2662 (MBL International, Woburn, MA, USA) and SC-9042 (Santa Cruz Biotechnology). Then, 5 μg/ml for LS-A2662 was used for cells and 10 μg/ml was used for tissues, and 5 μg/ml SC-9042 was used for both cells and tissues. β3-AR immunostaining was assessed using 2.5 μg/ml or 10 μg/ml of LS-A4198 (MBL International) for cells and tissues, respectively, or 10 μg/ml KG115 (Trans Genic) for cells. The detection system was a Vector anti-rabbit secondary (BA-100; Vector Laboratories) with a Vector ABC-AP kit (AK-5000) and a Vector Red substrate kit (SK-5100) which produced a fuchsia-colored deposit. The negative control consisted of performing the entire immunohistochemistry procedure on adjacent sections in the absence of primary antibody as described previously (Kullmann et al. 2009, 2010). Immunohistochemistry of bladder tissues from 5 donor women (>75 years) and 3 donor women (<35 years) was performed at Lifespan Biosciences. Immunohistochemistry of bladder tissues from 13 donor women (<45 years) and 10 donor women (>45 years) was performed at Asterand. The bladder samples obtained from LifeSpan Biosciences were from unknown location within the bladder, whereas the bladder samples obtained from Asterand were from the lateral bladder wall and, in some cases, from both lateral wall and trigone (bladder neck) from the same individual. The <45-year (n=13), and >45-year (n=10) bladder samples utilized by Asterand were subjected to quantitative image analysis via densitometry. Three area measurements for each section using a square marquee per region (urothelium or detrusor) were captured and the mean determined. Linear correlation was determined using the Pearson correlation in GraphPad Prism v4.1. Quadratic analysis was performed using GraphPad Prism v4.1.
Binding studies
The antibodies utilized for immunohistochemical analysis were evaluated for specific binding to human β-ARs (hβ1-AR, hβ2-AR, hβ3-AR) transiently expressed in CHOK1 cells using methods described previously (Kullmann et al. 2009). To determine Ki values for unlabeled ligands in competition binding studies, CHOK1 cells were transiently transfected with pcDNA3.1+(mock) or human β-AR cDNAs using Lipofectamine-2000 (5 μg cDNA/100-mm dish; Invitrogen). On the day after transfection, 40,000/well for mock, 30,000/well for hβ3-AR, or 15,000/well for hβ1-AR and hβ2-AR transfectants were plated into 48-well plates and cultured at 37°C, 5% CO2 overnight. Whole cell binding was performed the next day at 4°C for 5 h with 55.9 pM [125I]CYP (GE Healthcare, Piscataway, NJ, USA) and increasing concentrations of isoproterenol in the presence of 1 mM ascorbic acid, 0.5% BSA, 50 mM HEPES, 1 mM CaCl2, and 5 mM MgCl2. Cells were washed three times with ice-cold phosphate-buffered saline. Cell lysates were collected with ice-cold 2% Nonidet P-40/phosphate-buffered saline and counts were measured using a Wizard gamma counter. Parallel wells were collected for cell counting to generate receptor site/cell values. Dose-response curves were analyzed with nonlinear one-site and two-site regression modeling with GraphPad Prism v4.1.
cAMP assay methods
UROtsa cells were maintained in culture media (DMEM, 10% FBS, 1% L-gluatmine, 1% antibiotic/antimycotics, 1% nonessential amino acids). For the cAMP assay, cells (120,000 cells /well) were plated into 24-well plates and cultured overnight at 37°C, 5% CO2. Cells were starved for 30–60 min at 37°C in serum-free DMEM/F12, 0.2% BSA, then pretreated at 37°C (500 μM IBMX in DMEM/F12 (no phenol red) 30 min, 0.2% BSA, 25 mM HEPES), for 30 min, then stimulated with added ligands in the same buffer for 30 min at 37°C. Reactions were stopped and cAMP was measured using the GE Healthcare Biotrack kit (RPN225).
Results
Validation of receptor antibodies
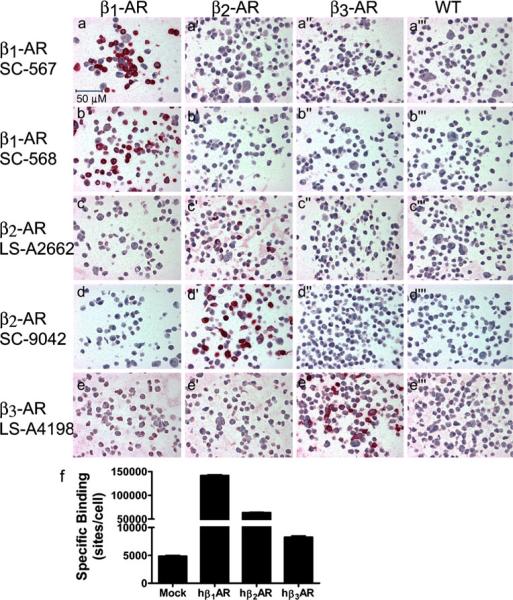
As has been previously discussed, adrenergic receptor antibodies have been known to lack specificity, and as a result more rigorous criteria than incubation in the absence of primary antisera or the appearance of a unique band in a western blot need to be performed (Kullmann et al. 2009; Michel et al. 2009; Pradidarcheep et al. 2009; Hamdani and van der Velden 2009). To ensure that the individual antibodies were specifically recognizing only the receptor protein of interest (as opposed to the antigenic peptide sequence against which the antibodies had been generated), we used two different antibodies for each receptor tested against each of the three individual human β-ARs when transiently over-expressed in CHOK1 cells, as illustrated in Fig. 1. In this type of experiment, one would expect cells to display varying degrees of expression with only a small proportion of cells expressing the receptor to a high degree. Radioligand binding was performed in parallel with receptor antibody validation studies in the transiently transfected CHOK1 cells confirming over-expression of the β-AR receptors (Fig. 1f), as well as demonstrating the presence of a low level of endogenous β–AR receptor binding in the mock transfected CHOK1 cells. As can be seen, both the SC-567 and SC-568 antibodies stain only those cells expressing the β1-AR (Fig. 1a and b, respectively) when compared to the mock and other receptors. Each of the β2-AR specific antibodies stains only those cells expressing the β2-AR (LS-A2662 Fig. 1c′, SC-9042 Fig. 1d′). SC-568 and SC-9042 have been reported to be non-specific to their respective receptors using western analysis (Pradidarcheep et al. 2009; Hamdani and van der Velden 2009). Some IHC antibodies may not be appropriate for western analysis (Skliris et al. 2002). Owing to the inherent differences between methodologies, care was taken to validate these antibodies specifically for immunohistochemical applications. These same antibodies demonstrated a lack of receptor cross-reactivity in this study using immunohistochemical methods in the over-expression cell system. In addition, the tissue staining patterns observed with SC-568 and SC-9042 antibodies were replicated using antibodies (β1-AR: SC-567, and β2-AR: LS-A2662, respectively) generated against distinctly different epitopes (data not shown). The β3-AR specific antibody (LS-A4198) stains only those cells expressing the β3 receptor (Fig. 1e″). The putative β3-AR specific antibody KG115 was also evaluated; however, we were unable to confirm β3 receptor specificity owing to cross-reactivity of that antibody with the β2-AR (supplemental Fig. 1).
Fig. 1.
Validation of the β-AR antibodies β2-AR, SC-9042 of Fig. 1 was previously published as supplemental data (Fig. 1) with permission of the Journal of Pharmacology and Experimental Therapeutics. a–a‴ Validation of specific staining for the human β1-AR with the SC-567 antibody. The antibody was generated against AA 456–475 from the COOH terminus. The antibody was used at a concentration of 2.5 μg/ml, and specific staining (red) was only observed in CHOK1 cells transiently over-expressing the human β1-AR (a), with no staining observed in cells transiently over-expressing human β2-(a′), or β3-(a‴ ARs, or in cells transfected with empty vector (a‴). b–b‴ Validation of specific staining for the human β1-AR with the SC-568 antibody. The antibody was generated against AA 420–470 from the COOH terminus. The antibody was used at a concentration of 2.5 μg/ml, and specific staining (red) was only observed in CHOK1 cells transiently over-expressing the human β1-AR (b), with no staining observed in cells transiently over-expressing human β2-(b′), or β3-(b″) ARs, or in cells transfected with empty vector (b‴). c–c‴ Validation of specific staining for the human β2-AR with the LS-A2662 antibody. The antibody was generated against AA 13–28 from the human β2-AR NH2 terminus. The antibody was used at a concentration of 2.5 μg/ml, and specific staining (red) was only observed in CHOK1 cells transiently over-expressing the human β2-AR (c′), with no staining observed in cells transiently over-expressing human β1-(c), or β3-(c″) ARs, or in cells transfected with empty vector (c‴). d–d‴ Validation of specific staining for the human β2-AR with the SC-9042 antibody. The antibody was generated against AA 338–413 from the human β2-AR extreme COOH-terminus. The antibody was used at a concentration of 5 μg/ml, and specific staining (red) was only observed in CHOK1 cells transiently over-expressing the human β2-AR (d′), with no staining observed in cells transiently over-expressing human β1-(d), or β3-(d″) ARs, or in cells transfected with empty vector (d‴). e–e‴ Validation of specific staining for the human β3-AR with the LS-A4198 antibody. The antibody was generated against AA 1–20 from the human β3-AR NH2 terminus. The antibody was used at a concentration of 2.5 μg/ml, and high levels of specific staining (red) were only observed in CHOK1 cells transiently over-expressing the human β3-AR (e″), with low levels of staining observed in cells transfected with empty vector (e‴), and those transiently over-expressing human β1-(e), or β2-(e′) ARs; indicating potential low level expression of β3 in the CHOK1 cell line. f Whole cell binding was performed in parallel samples of CHOK1 cells transiently transfected with pcDNA3.1 (Mock), hβ1-AR, hβ2-AR, or hβ3-AR cDNA to illustrate overexpression of hβ-ARs in CHOK1 cells that were generated for IHC studies. The binding study was performed on a sampling of the cells utilized for IHC- antibody validation studies. 55.9 pM [125I] I-CYP tracer was used for the experiment shown. Isoproterenol was used as the competitor. Error bars SEM of three determinations
The low level of staining that is observed with the LS-A4198 antibody in cells expressing the β1- and β2-ARs (Fig. 1e, e′, respectively) is also observed in cells transfected with the empty vector (Fig. 1e‴), indicating that the CHOK1 cells potentially express a low level of the β3-AR. Although a low level of non-receptor-related binding of the LS-A4198 antibody to CHOK1 cells cannot be completely discounted, the presence of low levels of specific β-AR binding sites suggests the presence of β-AR receptors in the mock CHOK1 cells (Fig. 1f). To determine whether or not these endogenous β-AR binding sites represented β3 receptors, a competition analysis was performed with β3-AR selective ligands (BRL37344, TAK677) in mock CHOK1 cells (supplemental Fig. 2). These data demonstrate that the mock CHOK1 cells bind the β-AR ligands with a similar selectivity profile to that of β3-AR transfected cells (Kullmann et al. 2009). Taken together with the low level of staining observed with the LS-A4198 antibody, the data suggest the presence of a low level expression of endogenous β3 receptors in mock CHOK1 cells.
Antibodies can also be validated by obtaining similar staining patterns with distinct antibodies generated against different epitopes, as illustrated for the β2-AR antibodies (Fig. 1c and d, and see also Fig. 4b, e). This was also observed when two different β2-AR antibodies or two different β1-AR antibodies were used on full thickness bladder samples, although differences in staining intensity were seen with each of the two different β2-AR or β1-AR antibodies (data not shown). Although LS-A4198 was the only validated β3-AR antibody that worked in human tissues, the cellular staining pattern observed with this antibody in rat bladder is similar to that observed in rat with a distinct antibody generated against the rat receptor, providing additional evidence for the specificity of this antibody for the β3-AR (Kullmann et al. 2009, 2010).
Fig. 4.
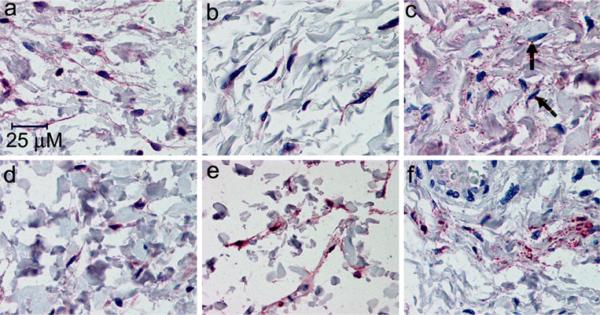
All three β-ARs are expressed in the suburothelial myofibroblast-like cells. The expression of β1-(a,d), β2-(b,e), and β3-(c,f) ARs was detected with the SC-567, (SC-9042, LS-A2662), and LS-A4198 antibodies, respectively, as described in “Materials and methods”. a,d Positive immunoreactivity for the β1-AR (red) is seen in the isolated fibroblast-like cells and the associated cell processes in the lamina propria taken from the bladder of an 80-year-old female (a) and 16-year-old female (d). b,e Positive immunoreactivity for the β2-AR (red) is seen in the isolated fibroblast-like cells and the associated cell processes in the lamina propria taken from the bladder of an 80-year-old female (b) and 16-year-old female (e). c,f Some of the blush positive immunoreactivity (light red) for the β3-AR in the lamina propria is associated with individual cells that most likely represent myofibroblasts, highlighted by the arrows (c). This is illustrated in samples taken from the bladder of an 80-year-old female (c) and 28-year-old female (f). Images provided at high magnification to visualize myofibroblasts
All three β-ARs are expressed in different cell types of the human female bladder
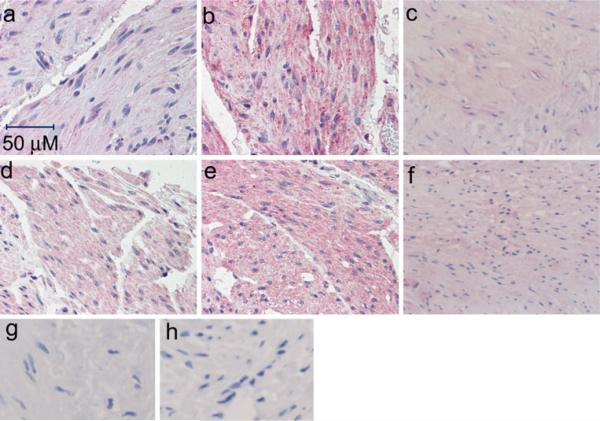
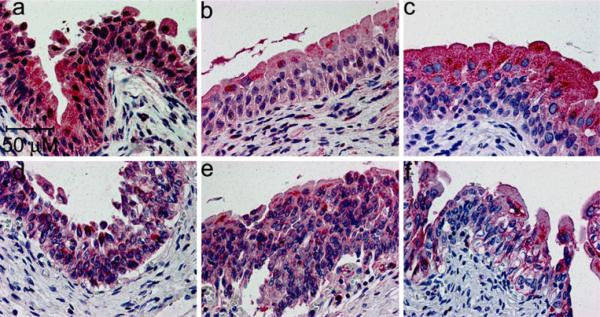
Figures 2 and 3 illustrate that each of the three β-ARs are expressed in the detrusor (Fig. 2) and urothelium (Fig. 3)of the female bladder. No conclusions can be drawn about the relative abundance of the different receptor subtypes based on the immunohistochemistry data because no data are available about the relative affinity of the different antibodies for the individual receptors. It is noteworthy that expression of the β3-AR appears to be stronger in the umbrella cells in comparison to the innermost urothelial cell layers, as illustrated by the more intense uniform staining of the umbrella cells relative to the basal and intermediate cell layers (Fig. 3c, f). This is in contrast to what is observed in the rat bladder, where stronger expression is seen in the basal and intermediate urothelial cell layers (Kullmann et al. 2010). This non-homogeneous urothelial cell staining, which was observed in ~70% (20/28) of the samples examined, was not observed for the β1-(Fig. 3a, d)and β2-(Fig. 3b, e) ARs. Occasional expression of each receptor can also be seen in the suburothelial myofibroblast-like cells located within the mucosa below the urothelium. These myofibroblast-like cells are visible as elongated cells in suburothelial space illustrated at higher magnification in Fig. 4. It is more difficult to discern suburothelial myofibroblast-like expression of the β3-AR within the mucosa owing to the prevalence of adipocytes in this area, and the expected expression of the β3-AR on adipocytes (Ursino et al. 2009). Nevertheless, some of the myofibroblast-like cells within the adipose tissue do appear to express the β3-AR (arrow in Fig. 4c).
Fig. 2.
All three β-ARs are expressed in human detrusor. These samples of normal bladder were obtained from older (80-year-old (a,b), 66-year-old (c)) females, versus young (34-year-old (d,e), 38-year-old (f)) females and stained for expression of the β1-(a,d), β2 -(b,e), and β3-(c,f) ARs with the SC-567 (a,d) LS-A2662 (b,e), and LS-A4198 (c,f) antibodies, respectively. Panels g,h demonstrate an absence of staining obtained in the presence of non-immune rabbit IgG antibody for the corresponding tissues (66- and 38-year-old female samples for the β3 antibody, respectively). Similar negative results were obtained in the absence of primary antibody on the samples for the β1- and β2-AR antibodies (data not shown). Blush to faint staining was observed in the muscularis propria for the β1 receptor, and was similar in the younger and older samples. The muscularis propria was faint to moderately positive for β2 -ARs in both the younger and older samples. A similar faint expression of the β3-AR was observed in the muscularis propria in all samples regardless of age. The antibodies were used at 2.5, 10, and 10 μg/ml, respectively
Fig. 3.
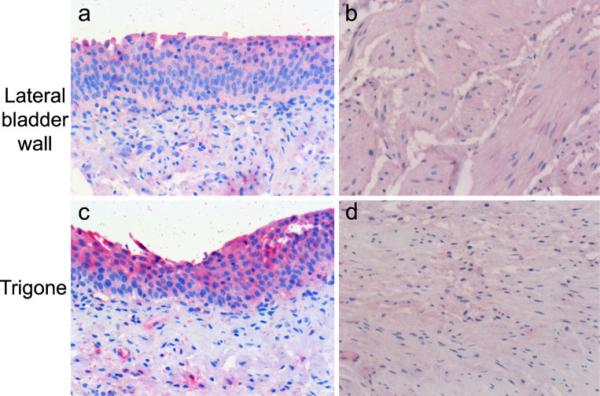
All three β-ARs are strongly expressed in human urothelium. These samples of normal bladder were obtained from an 85-year old (a–c) or 28 year old (d–f) female, and stained for expression of the β1-(a,d), β2 (b,e), and β3-(c,f) ARs with the SC-567 (a,d) LS-A2662 (b,e), and LS-A4198 (c,f) antibodies, respectively. Note the non-homogeneous distribution of β3-ARs in the urothelium (c,f), with more concentrated staining in the umbrella cells relative to the other cell layers, whereas more uniform staining is seen in the urothelium of the β1-(a,d) and β2-(b,e) AR-stained bladders. Similar results were seen in 71% of the samples examined (20/28 slides). The antibodies were used at 2.5, 10, and 10 μg/ml, respectively
An additional level of complexity is a potential regional variation in receptor expression within the bladder. We obtained samples from trigone and lateral bladder wall from the same donors in six cases and examined three pairs of tissue sets per cohort. The general trend was that, where present, expression of all three receptors was approximately equal, or tended to be higher in the trigone, particularly in the urothelium. This observation was more pronounced in the younger cohort (<45 years old), in which all three donors where matched pairs were available, exhibited trigone > lateral bladder wall immunoreactivity for at least the β3- and/or β2-AR. This is illustrated for the β3-AR in Fig. 5.
Fig. 5.
Expression of β3-AR in both urothelium and bladder smooth muscle in different bladder regions. The illustrated samples from trigone and bladder wall were obtained from a 38-year-old female and stained with 10 μ/ml of LSA-4198 as specified in “Materials and methods”. Specific staining for the β3-AR (red) is observed in urothelium (a,c), vascular smooth muscle, and in the detrusor (b,d). Immunoreactivity appeared to be stronger in the urothelium versus the detrusor in all samples examined. In 2/3 bladders from the <45-year-old cohort in which matched samples were available from the same donor from different bladder regions, staining tended to be more pronounced in the urothelium in the trigone (c) versus bladder wall (a). In contrast, there did not appear to be a difference in staining intensity in the detrusor obtained from trigone (b) versus bladder wall (d) samples across all samples evaluated
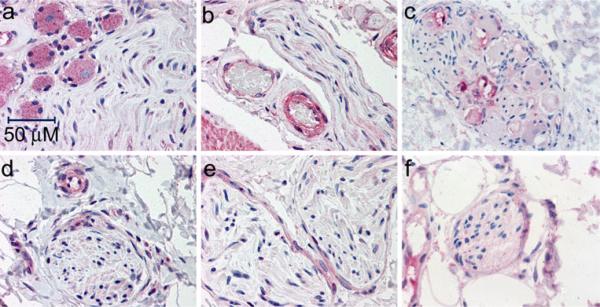
The different types of bladder peripheral nerve cell staining are illustrated in Fig. 6, where faint cytoplasmic expression of the β1-, (Fig. 6a, d), β2- (Fig. 6b, e) and β3-(Fig. 6c, f) ARs is seen in peripheral nerves. Stronger staining of the endothelium and vascular smooth muscle of the small vessels juxtaposing the nerves is also apparent for the β1- (Fig. 6d) and β2- (Fig. 6b) ARs. Expression of the β1- (Fig. 6a) and β3- (Fig. 6c) ARs is also apparent in some ganglion cells. Faint expression of both β1 (Fig. 6d) and β3 (Fig. 6f) is evident in the cytoplasm of Schwann cells. It should be clarified that, while many positive peripheral nerves were identified that were immunoreactive for β3-ARs throughout the fatty components of the bladder (i.e. the mucosa) in the various samples, many negative peripheral nerve fibers were also identified (data not shown), indicating that the expression of the β3-AR is not present in all peripheral nerves of the bladder.
Fig. 6.
All three β-ARs are detectable in peripheral neurons of the human bladder. The expression of β1-(a,d), β2-(b,e), and β3-(c,f) ARs was detected with the SC-567, LS-A2662, and LS-A4198 antibodies, respectively, as described in “Materials and methods”. a,d Positive immunoreactivity (red) for the β1-AR in the cytoplasm of ganglion cells and peripheral nerve, taken from the bladder of an 80-year-old female (a). Similar expression is observed in a peripheral nerve and capillary taken from the bladder of a 16-year-old female (d). b,e Positive immunoreactivity (red) for the β2-AR is observed in the nerve sheath and in the cytoplasm of a peripheral nerve, as well as in the adjacent capillaries, taken from the bladder of an 82-year-old female (b), with similar expression observed in peripheral nerves taken from the bladder of a 16-year-old female (e). c Positive immunoreactivity (pink) of the β3-AR is observed in ganglia of a peripheral nerve fiber taken from the trigone of the bladder of a 40-year-old female. f Positive immunoreactivity (pink) for the β3 receptor in the cytoplasm of a peripheral nerve taken from the bladder mucosa of a 37-year-old female
Human urothelial cells express functional β3-ARs
The immortalized UROtsa cell line has been utilized as a potential cell culture model of human urothelium (Rossi et al. 2001), and non-selective ligands have been used to characterize β-AR responses in this cell line (Harmon et al. 2005). In order to demonstrate that the strong immunoreactivity observed in the urothelium (Figs. 3 and 5) represented functionally relevant β3-ARs, we determined the relative potency series of the β3-AR selective agonists, TAK677 and BRL37344, to elicit a cAMP response in the UROtsa cells, as shown in Fig. 7 and Table 1. This same relative potency series has also been observed at the isolated β3-AR when expressed in a heterologous cell expression system (Kullmann et al. 2009). Unlike in the heterologous cell systems over-expressing the β3-AR, both TAK677 and BRL37344 elicit a relatively small increase of cAMP in the UROtsa cell system as compared to isoproterenol, a broad-spectrum β-AR agonist, indicating a low efficacy in comparison to that of the nonselective β-AR agonist isoproterenol (Table 1). This observation indicates that β3-ARs compose a small proportion of the total β-AR receptor population in this urothelial cell line.
Fig. 7.
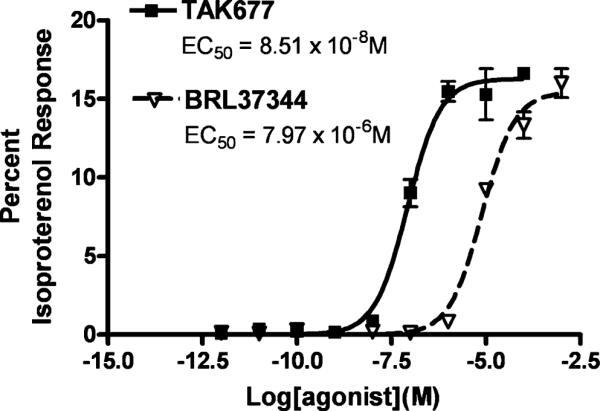
cAMP Assay of UROTSA cells stimulated with TAK677 and BRL37344. The data are illustrated for TAK677 and BRL37344 only. The data points were normalized to the maximal isoproterenol response (Emax) in the same assay. Therefore the fitted curve efficacies (Emax) represent a normalized efficacy relative to isoproterenol. The Emax values (relative to the maximal isoproterenol response) are 16.28 and 15.4% for TAK677 and BRL37344, respectively for the illustrated experiment. The fitted potency values for the individual agonists are: TAK677 EC50=8.51×10−8 M; BRL37344 EC50=7.97×10−6 M; Isoproterenol EC50=1.01×10−7 M). Error bars SEM of three determinations. Similar results were obtained in two additional experiments (see Table 1)
Table 1.
cAMP response of β3-AR agonists in UROtsa cells
| Log EC50 ± SEM | EC50 (M) | n | % Isoproterenol Response | |
|---|---|---|---|---|
| Isoproterenol | −7.39 ± 0.24 | 4.12×10−8 | 5 | — |
| BRL 37344 | −5.65 ± 0.39 | 2.24×10−6 | 4 | 17.29 ± 2.73 |
| TAK677 | −6.44 ± 0.55 | 6.44×10−7 | 3 | 19.53 ± 2.39 |
Quantative analysis of bladder β-AR expression with age
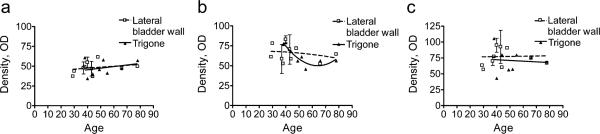
It has previously been demonstrated that the β-AR density decreases in the ageing human bladder (Li et al. 2003). However, those studies utilized [3H]dihydroalprenolol ([3H] DHA) as the radioligand, and [3H]DHA is known to be a very poor ligand for the β3-AR (Niclauss et al. 2006). In order to determine whether the expression of the individual β-ARs changed with age, we performed a quantitative immunohistochemical analysis of the expression of the individual receptors in each bladder sample in the Asterand sample group. The data are plotted as a function of age for the urothelial receptor distribution. Figure 8 illustrates only the urothelial data, as no variation in expression with age was observed for any receptor in the detrusor samples (Table 2). Correlation analysis was performed on all of the data sets, but the data for the β2-AR in the urothelium of the trigone appeared to best fit a quadratic equation, rather than a linear equation (Fig. 8b, solid triangles). The fit of these data to the quadratic polynomial equation yielded an r2=0.8170, and the F test for the quadratic polynomial fit yielded a p value of 0.014, indicating a non-linear decreasing density in the β-ARs with age in the urothelium of the trigone. In contrast, the β2-AR in the lateral bladder wall exhibited a linear correlation with a p value of 0.5537 (Table 2), indicating a lack of significant change in receptor density with age. Similarly, there was no significant change in receptor density with age for either the β1- or β3-AR in the urothelium of the trigone or of the lateral bladder wall (Fig. 8, Table 2).
Fig. 8.
Quantitative image analysis of the urothelial β-AR expression as a function of age. The density of expression of the β1-, β2-, and β3-ARs was quantified after staining with the SC-567, SC-9042, and LS-A4198 antibodies as described in “Materials and methods”. Each data point represents the average of three density measurements for the area comprising all urothelial cell layers for a single individual. No clear correlation is observed between expression of either the β1-(a SC-567) or β3- (c LS-A4198) ARs with age. However, the data indicate a non-linear decreasing density in β2-AR expression with age only in the urothelium from the trigone region (b SC-9042 antibody). Similar results were obtained when the data were quantitated after staining for the β2-AR with the LS-A2662 antibody (data not shown)
Table 2.
Significance of correlation for receptor density with age
| Target | Tissue | Slope | p | n | Deviation from zero |
|---|---|---|---|---|---|
| β1-AR | Lateral bladder wall | 0.1236±0.1681 | 0.4775 | 13 | Not significant |
| β2-AR | Lateral bladder wall | −0.1730±0.2831 | 0.5537 | 13 | Not significant |
| β3-AR | Lateral bladder wall | 0.03297±0.3621 | 0.9291 | 13 | Not significant |
| β1-AR | Trigone | 0.2008±0.2767 | 0.4954 | 8 | Not significant |
| β2-AR | Trigone | N/A | 0.014 | 8 | Significant in polynomial fit |
| β3-AR | Trigone | −0.1088±0.5730 | 0.8557 | 8 | Not significant |
| β1AR | Detrusor | 0.0267±0.0737 | 0.7242 | 13 | Not significant |
| β2-AR | Detrusor | 0.07042±0.0558 | 0.2337 | 13 | Not significant |
| β3-AR | Detrusor | 0.05686±0.1342 | 0.6799 | 13 | Not significant |
Discussion
We have demonstrated that each of the three β-ARs is expressed in a variety of cell types in addition to the detrusor smooth muscle in the human female bladder using a series of antibodies validated for receptor specificity. However, the data do not speak to the relative abundance in expression between different receptor subtypes because staining intensity is influenced by both individual tissue sample preparation and relative antibody affinity, all of which remain unknown variables. Nevertheless, valid comparisons can be made for a given receptor subtype across different cell types.
A number of studies point to the existence of multiple β-AR subtypes in bladder tissue from a variety of species including human (reviewed in Michel and Vrydag 2006). β1-AR and β2-AR binding sites have been demonstrated in human bladder homogenates (Goepel et al. 1997). However, the lack of a suitable radioligand for the β3-AR has prevented quantitation of the relative amounts of each receptor subtype (Niclauss et al. 2006; Vrydag and Michel 2007), and our immunohistochemical results appear to indicate prominent expression of all three receptor subtypes throughout the human female bladder. These results may at first seem to contradict more recent studies that have indicated a predominant expression of the β3-AR subtype in the human bladder. It should be noted that those studies were based solely on the expression of receptor mRNA (Nomiya and Yamaguchi 2003; Wuest et al. 2009; Igawa et al. 1999), and expression of mRNA is not necessarily equivalent to, nor predictive of, protein expression. Studies in isolated human muscle strips demonstrate that the β3-AR subtype is the predominant functional receptor in the detrusor, but those studies removed the mucosa prior to the measurement of ligand-stimulated relaxation (Nomiya and Yamaguchi 2003; Wuest et al. 2009; Igawa et al. 1999). Separate studies have demonstrated the ability of selective β2-AR agonists to relax human detrusor muscle (Badawi et al. 2007), and it has also been observed that the nonsubtype selective β-AR agonist isoproterenol produces a greater maximal relaxation in human and rat bladder strips than that obtained with selective β3-AR agonists (Igawa et al. 2001; Kullmann et al. 2009, 2010), implying a potential role for additional β-ARs in adrenergic-mediated bladder relaxation. Indeed, a role for β2-AR-mediated relaxation is consistent with the known ability of both terbutaline and clenbuterol to improve incontinence symptoms in females suffering from urge incontinence (Lindholm and Lose 1986; Ushiroyama et al. 2000).
The clinical effectiveness of the β2-AR selective agonists is at odds with the seemingly predominant effect of β3-AR agonists on detrusor relaxation, unless one considers the expression of these receptors on cell types distinct from the detrusor myocyte. Indeed, it has recently been demonstrated that removal of the urothelium alters the ability of isoproterenol to relax human detrusor, implying that adrenergic receptor stimulation within the urothelium leads to the release of an inhibitory factor that influences muscle relaxation (Otsuka et al. 2008). The same study also demonstrated the presence of all three β-ARs in human urothelium by immunohistochemistry, but we were unable to confirm their results with the β3-AR antibody used in that study owing to strong cross-reactivity of that antibody with the β2-AR (supplemental Fig. 1). Despite the inability to validate the staining with the TransGenic β3-receptor antibody used in that study, the results demonstrated with the validated antibodies used herein show specific staining of all three β-ARs in both the urothelium and in the detrusor, as well as in myofibroblast-like cells and certain neural tissue. Using the UROtsa cells as a model of human urothelium (Rossi et al. 2001), we demonstrate for the first time that two different selective β3-AR agonists (Kullmann et al. 2009) are capable of stimulating the cAMP response, albeit with much lower efficacy than with that observed with the non-subtype selective agonist isoproterenol (Fig. 7, Table 1). In CHOK1 cells transfected with human β1, β2, β3-AR, we have shown previously that BRL37344 was only a partial agonist relative to isoproterenol at the β3 receptor, whereas TAK677 maintained full agonist efficacy. Although both agonists were inactive at the rat β2-AR, they exhibited at least 50-fold greater selectivity for the human β3-AR versus the human β2-AR, where they exhibited comparable efficacy to isoproterenol. TAK677 was somewhat less selective for the human β3 versus the human β1 than for the human β2 receptor in the transient expression system, but it only behaved as a partial agonist at the human β1 receptor relative to isoproterenol (Kullmann et al. 2009). In light of the immunohistochemistry data indicating the presence of all three β-ARs in the urothelium (Fig. 3) and previous data in the UROtsa cells (Harmon et al. 2005), the data suggest that the urothelial β3-AR, while clearly functional, may not be the sole mediator of urothelial responsiveness to the nonselective sympathomimetic neurotransmitters in the human female bladder.
The role of the suburothelial myofibroblast-like and peripheral nerve β-AR expression in the relaxation response to β3-AR agonists is currently unknown. The sensation of bladder fullness is thought to be relayed by the afferent nerves in the mucosa, which are activated by mediators originating in the urothelium in response to stretch upon bladder filling (de Groat 2004; Fowler et al. 2008), with the suburothelial myofibroblasts possibly acting as intermediaries in the sensory process (Fry et al. 2004). Recently, it has been shown that systemic injection of β3-AR agonists in the rat decreased Aδ fiber activity in response to bladder filling (Aizawa et al. 2009). β-AR stimulation of urothelial cells in the rat bladder releases nitric oxide, providing a potential mechanism to influence afferent function (Birder et al. 2002).
β-AR stimulation has been demonstrated to facilitate ganglionic transmission in a subset of post-ganglionic nerve filaments on the surface of the cat bladder (de Groat and Saum 1972; Keast et al. 1990), and expression of the β3-AR in ganglion in the myenteric plexus of the human colon is associated with inhibition of cholinergically-mediated muscle contraction and somatostatin release (Cellek et al. 2007). Together these data suggest that ganglionic β-ARs in the bladder may play a role in the cross-talk between the adrenergic and muscarinic cholinergic receptors in the bladder (Klausner et al. 2009).
A number of studies have addressed the issue of an age-related decline in β-AR responsiveness in rat and human bladder tissue, and decreases in receptor number and alterations in receptor-G-protein coupling have been implicated (Li et al. 2003; Frazier et al. 2006; Wheeler et al. 2005; Derweesh et al. 2000; Nishimoto et al. 1995). It has previously been observed that the number of total β-ARs decreases with age in the human bladder, when receptor density is measured with [3H]DHA (Li et al. 2003). This ligand preferentially labels β2-ARs and is not suitable for labeling β3-ARs (Niclauss et al. 2006). This loss in receptor density with age is consistent with what we observed with the age-related loss in immunostaining of the trigone urothelial β2-AR in the human female bladder (Fig. 8). In the absence of a reference stain, the small decrease (~30%) in total urothelial β2-AR staining that is observed here could be attributed to other factors, such as thinning of the urothelium with age, rather than a loss of receptor density per unit area per se. If this were the case, however, then one would also have expected to see a loss in urothelial β3 receptor staining, and the small loss in receptor staining appeared to be observed only for the β2 receptors, consistent with what has been observed with receptor binding methods with radioligands that do not adequately label the β3 receptor population (discussed above). In tissues where both β2- and β3-ARs are co-expressed, unique signals can be mediated through the β2/β3-AR heterodimer (Breit et al. 2004). The possibility exists that selective loss of the β2-AR in the ageing female urothelium would shift the signaling potential from the heterodimeric species to the β3-AR homodimeric species, allowing for further selectivity in ligand-mediated signaling as preferences for homodimeric versus heterodimeric receptor complexes are discriminated. The finding that the expression of the β2-AR decreases slightly while expression of the β3-AR remains unchanged with age will need to be verified with a larger sample and a more varied age range than that utilized here. Nevertheless, the data suggest that the efficacy of the β3-AR agonists currently under development for the treatment of overactive bladder in postmenopausal women will be maintained in the ageing population.
Acknowledgements
We thank the staff of Asterand Ltd for excellent technical assistance with the expanded human IHC samples, and Dr. Michael P. Meredith (The Procter & Gamble Company) for help with statistical analysis during the preparation of this manuscript.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00441-010-1053-x) contains supplementary material, which is available to authorized users.
References
- Aizawa N, Igawa Y, Nishizawa O, Wyndaele JJ. Effects of CL316, 243 a β3-adrenoceptor agonist, and intravesical prostaglandin E2 on the primary bladder afferent activity of the rat. Neurourol Urodynam. 2009;29:771–776. doi: 10.1002/nau.20826. [DOI] [PubMed] [Google Scholar]
- Badawi JK, Seja T, Uecelehan H, Honeck P, Kwon ST, Bross S, Langbein S. Relaxation of human detrusor muscle by selective beta-2 and beta-3 agonists and endogenous catecholamines. Urology. 2007;69:785–790. doi: 10.1016/j.urology.2007.01.059. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. beta -adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002;22:8063–8070. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit A, Lagace M, Bouvier M. Hetero-oligomerization between {beta}2- and {beta}3-adrenergic receptors generates a {beta}-adrenergic signaling unit with distinct functional properties. J Biol Chem. 2004;279:28756–28765. doi: 10.1074/jbc.M313310200. [DOI] [PubMed] [Google Scholar]
- Cellek S, Thangiah R, Bassil AK, Campbell CA, Gray KM, Stretton JL, Lalude O, Vivekanandan S, Wheeldon A, Winchester WJ, Sanger GJ, Schemann M, Lee K. Demonstration of functional neuronal beta3-adrenoceptors within the enteric nervous system. Gastroenterology. 2007;133:175–183. doi: 10.1053/j.gastro.2007.05.009. [DOI] [PubMed] [Google Scholar]
- de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004;64:7–11. doi: 10.1016/j.urology.2004.08.063. [DOI] [PubMed] [Google Scholar]
- de Groat WC, Saum WR. Sympathetic inhibition of the urinary bladder and of pelvic ganglionic transmission in the cat. J Physiol. 1972;220:297–314. doi: 10.1113/jphysiol.1972.sp009708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derweesh IH, Wheeler MA, Weiss RM. Alterations in G-proteins and beta -adrenergic responsive adenylyl cyclase in rat urinary bladder during aging. J Pharmacol Exp Ther. 2000;294:969–974. [PubMed] [Google Scholar]
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9:453–466. doi: 10.1038/nrn2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier EP, Schneider T, Michel MC. Effects of gender, age and hypertension on beta-adrenergic receptor function in rat urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 2006;373:300–309. doi: 10.1007/s00210-006-0077-y. [DOI] [PubMed] [Google Scholar]
- Fry CH, Ikeda Y, Harvey R, Wu C, Sui GP. Control of bladder function by peripheral nerves: avenues for novel drug targets. Urology. 2004;63:24–31. doi: 10.1016/j.urology.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Fujimura T, Tamura K, Tsutsumi T, Yamamoto T, Nakamura K, Koibuchi Y, Kobayashi M, Yamaguchi O. Expression and possible functional role of the β3-adrenoceptor in human and rat detrusor muscle. J Urol. 1999;161:680–685. [PubMed] [Google Scholar]
- Goepel M, Wittman A, Rubben H, Michel MC. Comparison of adrenoceptor subtype expression in porcine and human bladder and prostate. Urol Res. 1997;25:199–206. doi: 10.1007/BF00941983. [DOI] [PubMed] [Google Scholar]
- Hamdani N, van der Velden J. Lack of specificity of antibodies directed against human beta-adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:403–407. doi: 10.1007/s00210-009-0392-1. [DOI] [PubMed] [Google Scholar]
- Harmon E, Porter J, Porter J. beta-adrenergic receptor activation in immortalized human urothelial cells stimulates inflammatory responses by PKA-independent mechanisms. Cell Communication and Signaling. 2005;3:10. doi: 10.1186/1478-811X-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa Y, Yamazaki Y, Takeda H, Hayakawa K, Akahane M, Ajisawa Y, Yoneyama T, Nishizawa O, Andersson KE. Functional and molecular biological evidence for a possible β3-adrenoceptor in the human detrusor muscle. Br J Pharmacol. 1999;126:819–825. doi: 10.1038/sj.bjp.0702358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igawa Y, Yamazaki Y, Takeda H, Kaidoh K, Akahane M, Ajisawa Y, Yoneyama T, Nishizawa O, Andersson KE. Relaxant effects of isoproterenol and selective β3-adrenoceptor agonists on normal, low compliant and hyperreflexic human bladders. J Urol. 2001;165:240–244. doi: 10.1097/00005392-200101000-00071. [DOI] [PubMed] [Google Scholar]
- Keast JR, Kawatani M, de Groat WC. Sympathetic modulation of cholinergic transmission in cat vesical ganglia is mediated by alpha 1- and alpha 2-adrenoceptors. Am J Physiol. 1990;258:R44–R50. doi: 10.1152/ajpregu.1990.258.1.R44. [DOI] [PubMed] [Google Scholar]
- Klausner AP, Rourke KF, Miner AS, Ratz PH. Potentiation of carbachol-induced detrusor smooth muscle contractions by beta-adrenoceptor activation. Eur J Pharmacol. 2009;606:191–198. doi: 10.1016/j.ejphar.2009.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann FA, Limberg BJ, Artim DE, Shah M, Downs TR, Contract D, Wos J, Rosenbaum JS, de Groat WC. Effects of β3-adrenergic receptor activation on rat urinary bladder hyperactivity induced by ovariectomy. J Pharmacol Exp Ther. 2009;330:704–717. doi: 10.1124/jpet.109.155010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann FA, Downs TR, Artim D, Limberg BJ, Shah M, Contract D, de Groat WC, Rosenbaum JS. Urothelial beta3 adrenergic receptors in the rat bladder. Neurourol Urodynam. 2010 doi: 10.1002/nau.20965. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Li K, Li Z, Wang P. Age-dependent changes in beta-adrenoceptor function in human detrusors and possible mechanisms. Chin Med J (Engl ) 2003;116:1511–1514. [PubMed] [Google Scholar]
- Lindholm P, Lose G. Terbutaline (Bricanyl) in the treatment of female urge incontinence. Urol Int. 1986;41:158–160. doi: 10.1159/000281188. [DOI] [PubMed] [Google Scholar]
- Longhurst PA, Levendusky M. Pharmacological characterization of [beta]-adrenoceptors mediating relaxation of the rat urinary bladder in vitro. Br J Pharmacol. 1999;127:1744–1750. doi: 10.1038/sj.bjp.0702709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masunaga K, Chappl CR, McKay NG, Yoshida M, Sellers DJ. The β3-adrenoceptor mediates the inhibitory effects of β-adrenoceptor agonists via the urothelium in pig bladder dome. Neurourol Urodynam. 2010;29:1320–1325. doi: 10.1002/nau.20838. [DOI] [PubMed] [Google Scholar]
- Michel MC, Vrydag W. alpha(1)-, alpha(2)- and beta-adrenoceptors in the urinary bladder, urethra and prostate. Br J Pharmacol. 2006;147(Suppl 2):S88–S119. doi: 10.1038/sj.bjp.0706619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Wieland T, Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol. 2009;379:385–388. doi: 10.1007/s00210-009-0395-y. [DOI] [PubMed] [Google Scholar]
- Morita T, Iizuka H, Iwata T, Kondo S. Function and distribution of β3-adrenoceptors in rat, rabbit and human urinary bladder and external urethral sphincter. J Smooth Muscle Res. 2000;36:21–32. doi: 10.1540/jsmr.36.21. [DOI] [PubMed] [Google Scholar]
- Murakami S, Chapple CR, Akino H, Sellers DJ, Chess-Williams R. The role of the urothelium in mediating bladder responses to isoprenaline. BJU Int. 2007;99:669–673. doi: 10.1111/j.1464-410X.2006.06679.x. [DOI] [PubMed] [Google Scholar]
- Niclauss N, Michel-Reher MB, Alewijnse AE, Michel MC. Comparison of three radioligands for the labelling of human beta-adrenoceptor subtypes. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:99–105. doi: 10.1007/s00210-006-0104-z. [DOI] [PubMed] [Google Scholar]
- Nishimoto T, Latifpour J, Wheeler MA, Yoshida M, Weiss RM. Age-dependent alterations in beta-adrenergic responsiveness of rat detrusor smooth muscle. J Urol. 1995;153:1701–1705. [PubMed] [Google Scholar]
- Nomiya M, Yamaguchi O. A quantitative analysis of mRNA expression of alpha 1 and beta-adrenoceptor subtypes and their functional roles in human normal and obstructed bladders. J Urol. 2003;170:649–653. doi: 10.1097/01.ju.0000067621.62736.7c. [DOI] [PubMed] [Google Scholar]
- Otsuka A, Shinbo H, Matsumoto R, Kurita Y, Ozono S. Expression and functional role of beta-adrenoceptors in the human urinary bladder urothelium. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:473–481. doi: 10.1007/s00210-008-0274-y. [DOI] [PubMed] [Google Scholar]
- Pradidarcheep W, Stallen J, Labruyere WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:397–402. doi: 10.1007/s00210-009-0393-0. [DOI] [PubMed] [Google Scholar]
- Rossi MR, Masters JR, Park S, Todd JH, Garrett SH, Sens MA, Somji S, Nath J, Sens DA. The immortalized UROtsa cell line as a potential cell culture model of human urothelium. Environ Health Perspect. 2001;109:801–808. doi: 10.1289/ehp.01109801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skliris GP, Parkes AT, Limer JL, Burdall SE, Carder PJ, Speirs V. Evaluation of seven oestrogen receptor β antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol. 2002;197:155–162. doi: 10.1002/path.1077. [DOI] [PubMed] [Google Scholar]
- Takeda M, Obara K, Mizusawa T, Tomita Y, Arai K, Tsutsui T, Hatano A, Takahashi K, Nomura S. Evidence for beta 3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther. 1999;288:1367–1373. [PubMed] [Google Scholar]
- Ursino MG, Vasina V, Raschi E, Crema F, De PF. The β3-adrenoceptor as a therapeutic target: current perspectives. Pharmacol Res. 2009;59:221–234. doi: 10.1016/j.phrs.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Ushiroyama T, Ikeda A, Ueki M. Clinical efficacy of clenbuterol and propiverine in menopausal women with urinary incontinence: improvement in quality of life. J Med. 2000;31:311–319. [PubMed] [Google Scholar]
- Vrydag W, Michel MC. Tools to study β3-adrenoceptors. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:385–398. doi: 10.1007/s00210-006-0127-5. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Ayyagari RR, Wheeler GL, Weiss RM. Regulation of cyclic nucleotides in the urinary tract. J Smooth Muscle Res. 2005;41:1–21. doi: 10.1540/jsmr.41.1. [DOI] [PubMed] [Google Scholar]
- Wuest M, Eichhorn B, Grimm MO, Wirth MP, Ravens U, Kaumann AJ. Catecholamines relax detrusor through beta 2-adrenoceptors in mouse and beta 3-adrenoceptors in man. J Pharmacol Exp Ther. 2009;328:213–222. doi: 10.1124/jpet.108.142562. [DOI] [PubMed] [Google Scholar]
- Yamanishi T, Chapple CR, Yasuda K, Yoshida K, Chess-Williams R. The role of β3-adrenoceptors in mediating relaxation of porcine detrusor muscle. Br J Pharmacol. 2002;135:129–134. doi: 10.1038/sj.bjp.0704470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y, Takeda H, Akahane M, Igawa Y, Nishizawa O, Ajisawa Y. Species differences in the distribution of [beta]-adrenoceptor subtypes in bladder smooth muscle. Br J Pharmacol. 1998;124:593–599. doi: 10.1038/sj.bjp.0701870. [DOI] [PMC free article] [PubMed] [Google Scholar]