Abstract
In a double-blind crossover design, either 30 mg of the noncompetitive NMDA-receptor antagonist memantine or a placebo was administered to 40 healthy male volunteers. Twenty line drawings of objects and 20 photographs of unfamiliar faces were presented on a computer screen. After a retention interval of 80 min, the participants' task was to select the original objects and faces from a set of 80 items. Results were analyzed applying a signal-detection-theory approach. Recognition performance for objects was significantly impaired under memantine as compared to placebo, whereas performance on face recognition was not affected. Findings support the notion of differential effects of NMDA-receptor antagonists on memory functions in humans.
Glutamate is one of the main excitatory neurotransmitters in the mammalian brain. The wide distribution of glutamate within the central nervous system suggests that it mediates normal neural transmission (Collingridge and Singer 1990; Scatton 1993) by activating three major classes of subreceptors: NMDA (N-methyl-D-aspartate), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), and kainate (cf. Danysz and Archer 1994). Abnormal excitatory glutamatergic neurotransmission has been suggested as a pathological mechanism in various disorders such as schizophrenia (Tamminga 1998; Duncan et al. 1999), hypoxic-ischemic brain damage (Choi and Rothman 1990; Scatton 1993), and Alzheimer's dementia (Greenamyre et al. 1988; Foster 1990; Lees 1993). Cognitive symptoms associated with ischemia or Alzheimer's disease are considered a consequence of overactivation of NMDA receptors by endogenous glutamate, which causes neurodegeneration caused by excitotoxicity (Meldrum and Garthwaite 1990; Thomas 1995). Several clinical studies showed that NMDA-receptor antagonists improve cognitive disturbances by inhibiting pathological overactivation of NMDA receptors (Ditzler 1991; Pantev et al. 1993; Müller et al. 1995; Winblad and Poritis 1999).
Under physiological conditions, however, inhibition of NMDA receptors suppresses long-term potentiation (LTP) and thus impairs learning and memory (Izquierdo 1994). Although a large number of animal studies support the notion that NMDA antagonists negatively affect learning and memory processes (for reviews, see Danysz and Archer 1994; Aigner 1995), investigations on memory functions in humans after NMDA-receptor blockade are exceedingly scant. The very few existing studies provide ambiguous results. As can be seen from Table 1, the effects of NMDA antagonists on immediate and delayed verbal and nonverbal memory performance in humans remain unclear, whereas spatial memory was consistently shown to be unaffected by NMDA-antagonistic pharmacological treatment. This pattern of results suggests differential effects of NMDA-receptor antagonists on memory functions in humans.
Table 1.
Effects of Pharmacologically Induced Reduction of NMDA-Receptor Activity
| Study
|
Immediate recall
|
Delayed recall
|
||
|---|---|---|---|---|
| Memory task
|
Outcome
|
Memory task
|
Outcome
|
|
| Krystal et al. 1994 | Verbal | 0 | Verbal | — |
| Krystal et al. 1998 | Verbal | 0 | Verbal | — |
| LaPorte et al. 1996 | Verbal | 0 | Verbal | 0 |
| Nonverbal | 0 | |||
| Newcomer et al. 1999 | Verbal | — | Verbal | — |
| Nonverbal | — | |||
| Spatial | 0 | |||
| Rockstroh et al. 1996 | Verbal | — | Verbal | 0 |
| Nonverbal | — | Nonverbal | — | |
| Spatial | 0 | Spatial | 0 | |
| Schugens et al. 1997 | Verbal | 0 | Verbal | 0 |
| Spatial | 0 | Spatial | 0 | |
(0) not significantly different from placebo; (−) statistically significant impairment compared to placebo.
To date, no human data on the functional relationship between NMDA-receptor activity and other declarative memory functions, such as long-term memory for objects and faces, appear to exist. This is important to note because, most interestingly, recent animal studies suggest that performance on long-term object recognition is positively related to NMDA-receptor activity (Puma et al. 1998; Winnicka and Wisniewski 1999). Therefore, this study was designed to further elucidate the effects of pharmacologically induced NMDA-receptor activity on additional types of declarative memory in humans. For this purpose, the effects of the noncompetitive NMDA-receptor antagonist memantine (1-amino-3,5-dimethyladamantan-hydrochlorid) on long-term memory for objects and faces were investigated in 40 healthy male subjects.
RESULTS
Means (±SEM) of all dependent variables for the memantine and the placebo condition are given in Table 2. Because of the fact that 50% of the subjects received memantine first and the other 50% received the placebo first, in an initial analysis the potential effects of order were examined by comparing recognition performance of both these groups under placebo. There were no effects of order for both recognition tasks; mean performance on face recognition as indicated by the sensitivity measure d′ was 1.68 (±0.24) and 1.85 (±0.24; [t(38) = 0.62, nonsignificant], and mean performance on object recognition was 4.09 (±0.28) and 4.08 (±0.24; [t(38) = 0.99, nonsignificant] for subjects who received placebo first and second, respectively. Furthermore, because the same set of stimuli was used in each experimental session, two-way analyses of variance with drugs (placebo and memantine) as two levels of a repeated-measurement factor and order of drug administration (placebo first and memantine first) as a grouping variable were computed to test for a significant interaction between drug status and order of drug administration on recognition memory. Neither for performance on face recognition (F(1,38) = 0.91, nonsignificant) nor for performance on object recognition (F(1,38) = 0.06, nonsignificant) could a statistically significant interaction be shown. This finding suggests that the factorial combinations of order of pharmacological treatment and drug status applied in this study did not exert any systematic effect on recognition memory, although the same set of stimuli was presented in each experimental session.
Table 2.
Means and Standard Errors of the Mean of Indicators of Performance on Object and Face Recognition
|
|
Placebo
|
Memantine
|
||
|---|---|---|---|---|
| Mean
|
SEM
|
Mean
|
SEM
|
|
| Object recognition: | ||||
| hits (%) | 89.75 | 1.56 | 84.88 | 1.97 |
| false alarm (%) | 3.12 | 0.85 | 5.62 | 1.23 |
| d‘ | 4.09 | 0.18 | 3.43 | 0.23 |
| c | 0.44 | 0.09 | 0.44 | 0.07 |
| Face recognition: | ||||
| hits (%) | 70.25 | 3.35 | 68.00 | 3.04 |
| false alarm (%) | 20.25 | 2.54 | 17.50 | 2.31 |
| d‘ | 1.77 | 0.17 | 1.78 | 0.15 |
| c | 0.20 | 0.10 | 0.32 | 0.09 |
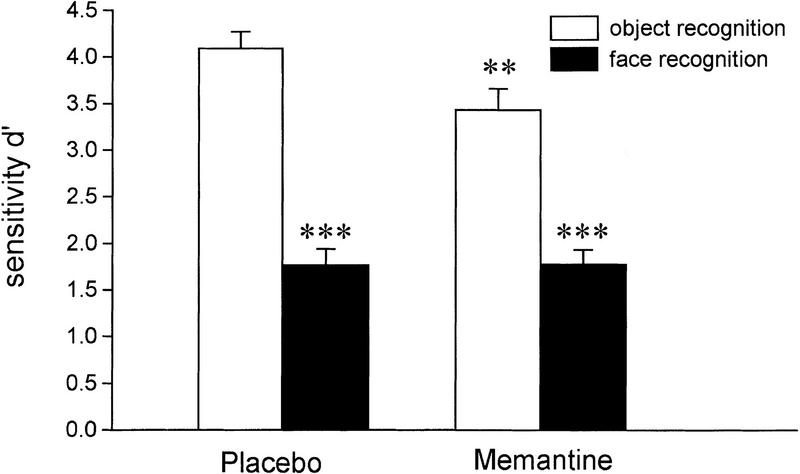
Two-way analysis of variance for repeated measurements with Drug and Memory (objects and faces) as two grouping factors revealed that recognition performance (d′) was markedly impaired by memantine (F(1,39) = 5.35, P < 0.05). There also was a significant main effect of Memory (F(1,39) = 119.97, P < 0.001), indicating a much higher sensitivity for objects than for faces. Furthermore, a statistically significant interaction between Drug and Memory could also be shown (F(1,39) = 6.97, P < 0.05). Recognition performance for objects was significantly impaired under memantine as compared to placebo (P < 001), whereas performance on face recognition was not affected by pharmacologically induced changes in glutamatergic neurotransmission (see Fig. 1).
Figure 1.
Performance on object and face recognition as indicated by the sensitivity measure d′ under placebo and memantine. Note that better recognition performance is indicated by higher d′ values. (**) significantly different from performance on object recognition under placebo (P < 0.01). (***) significantly different from respective performance on object recognition (P < 0.001).
Although there was neither a significant main effect of Drug (F(1,39) = 0.70, nonsignificant) nor a significant interaction between Drug and Memory (F(1,39) = 0.64, nonsignificant) with regard to response bias (c), a statistically significant main effect of Memory (F(1,39) = 4.37, P < 0.05] could be established. The tendency to respond “new” was much more pronounced for the object than for the face recognition task, as indicated by c values of 0.44 and 0.26, respectively, but was unaffected by pharmacological treatment.
DISCUSSION
The major finding of this study was that NMDA-antagonistic pharmacological treatment produced a substantial performance decrement in delayed object recognition. This result indicates that the functional relationship between NMDA-receptor activity and performance on long-term object recognition, as observed in animal studies (Puma et al. 1998; Winnicka and Wisniewski 1999), also holds for human subjects. Unlike delayed recognition of objects, long-term memory for faces was not affected by memantine. In addition, recognition performance, as indicated by the sensitivity measure d′, was reliably more than twice as high for object than for face recognition regardless of the pharmacological treatment applied. This finding is consistent with previous studies (Sergent et al. 1992; Farah et al. 1998; Rammsayer et al. 2000) also reporting better recognition performance with objects than with faces. As a possible explanation to account for this finding, Farah et al. (1998) put forward the notion that faces are recognized more holistically, that is, using less part decomposition than other types of objects.
It should be noted, however, that there were some differences between both memory tasks that could be responsible for the differential effects of memantine. First, the objects were line drawings and thus may be easier to discriminate between and recognize than pictures of faces. This could imply that the differential effects of memantine were caused by perceptual differences rather than different brain areas involved in long-term memory for objects and faces. There is some evidence, however, that perceptual differences are unlikely to account for the results of this study. In several studies, NMDA-receptor antagonists were shown to produce only small effects (Krystal et al. 1994, 1998; Schulz et al. 1996) or no changes at all (LaPorte et al. 1996; Rockstroh et al. 1996; Newcomer et al. 1999) on various attentional, perceptual, or vigilance tasks. Similarly, in this study, there were no effects of memantine on a series of perceptual and psychomotor tasks (temporal discrimination, reaction time, critical flicker fusion frequency, and signal detection) completed by the subjects during the retention interval. Taken together, the available data do not support the notion that attentional factors or perceptual differences between processing of faces and objects were responsible for the differential effects of memantine.
Second, one may argue that the objects were all recognizable, nameable objects, whereas the faces were unfamiliar, unnameable faces. Furthermore, during the initial presentation phase subjects were instructed to name each object but only tell whether it was a male or female face (see Materials and Methods). Thus, the object recognition task could have become more of a verbal word-learning task, whereas verbal encoding was not possible for the faces. From this perspective, the significant memantine-induced performance decrement in delayed object recognition corresponds to the reports of substantial decreases in delayed verbal memory produced by NMDA-receptor antagonists (Krystal et al. 1994, 1998; Newcomer et al. 1999). However, the lack of an effect of memantine on delayed face recognition observed in this study, in combination with the consistent finding that spatial memory is not affected by NMDA-receptor antagonists (Rockstroh et al. 1996; Schugens et al. 1997; Newcomer et al. 1999), points to the conclusion that some declarative memory functions, such as spatial memory and memory for faces, may be less susceptible to pharmacologically induced changes in NMDA-receptor activity than long-term verbal memory or object recognition.
Finally, the outcome of this study provides pharmacological evidence for the notion of different brain mechanisms underlying object and face recognition in humans. The brain mechanisms involved in facial processing were shown to be less susceptible to pharmacologically induced changes in NMDA-receptor activity than the ones associated with cognitive processing of objects. Whereas performance on face recognition was virtually unaffected by NMDA-receptor antagonistic treatment, performance on object recognition was markedly impaired.
Although recognition of objects and faces represents genuine declarative-memory functions (Zola-Morgan and Squire 1990; Squire et al. 1993), several lines of research suggest different brain systems underlying object and face recognition. Within the conceptual framework of declarative memory, the notion that face recognition involves different brain systems than do other types of object recognition is supported by neuropsychological studies demonstrating that face recognition can be selectively impaired (Levine 1989; Sergent and Signoret 1992; McNeil and Warrington 1993; Farah 1996; Henke et al. 1998). Similarly, EEG and PET studies also indicate a substantial degree of anatomic and functional specificity in face recognition (Seeck and Grüsser 1992; Sergent et al. 1992; Haxby et al. 1993; Allison et al. 1999), and electrophysiological studies in primates revealed neuronal assemblies that respond selectively to faces (Desimone 1991; Mesulam 1998; O'Scalaidhe et al. 1999). Neurophysiological studies in humans suggest that discrete regions of inferior extrastriate, midtemporal, and temporopolar cortex are specifically involved in facial recognition (Allison et al. 1999; Andreasen et al. 1996; Mesulam 1998). However, object recognition appears to depend on the perirhinal and postrhinal cortices (Meunier et al. 1993; Ennaceur et al. 1996; Ennaceur and Aggleton 1997; Buckley and Gaffan 1998a,b; Murray and Mishkin 1998; Parker and Gaffan 1998; Bussey et al. 1999). Thus, the observed detrimental effect of NMDA-antagonistic pharmacological treatment on long-term object recognition may be located in those brain systems specifically involved in object recognition.
An alternative explanation, however, is based on recent neuropsychological studies in nonhuman primates (Buckley and Gaffan 1997, 1998a,c; Murray et al. 2000). These studies suggest that perceptual difficulty of an identification task can change the reliance of the task on perirhinal cortex. Therefore, one may assume that in this experiment the differential pharmacological effects of memantine could be caused by different ways of encoding visual stimuli of differing perceptual difficulty rather than different brain areas recognizing faces and objects. This interpretation may fit the observation that in all cases face recognition was poorer than object recognition. Although highly speculative at this point, one may also consider the hypothesis that such perceptual difficulty may effectively modulate the extent to which a given task is dependent on NMDA-receptor activity.
Because NMDA receptors exist throughout the entire cortical mantle, it remains an open question why different regions encoding objects and faces appeared to respond in different ways to NMDA-receptor antagonistic treatment. Differences in compensatory mechanisms, such as transneuronal feedback, may represent a possible explanation for the differential susceptibility of different brain regions (cf. Rammsayer 1989). A low level of transneuronal feedback may cause those functions mediated by a specific region to be particularly susceptible to any pharmacologically induced changes in neuronal activity. For example, dopaminergic mechanisms may modulate glutamatergic activity in the cortex by means of interaction at the level of the basal forebrain (Pralong and Jones 1993; Freed 1994; Svensson et al. 1994). Therefore, further studies applying a single behavior–multiple brain systems strategy (Solomon 1986) appear to be highly desirable to discover how different neurotransmitter systems in the brain contribute to specific memory functions.
MATERIALS AND METHODS
In a double-blind crossover design, either 30 mg of the noncompetitive NMDA-receptor antagonist memantine (1-amino-3,5-dimethyladamantan-hydrochlorid) or a placebo were applied in a single oral dose in balanced order to 40 healthy male volunteers ranging in age from 20 to 35 yr (mean = 26.0 yr). The subjects were tested at 1-wk intervals, 5 h after drug intake. The study was approved by the research committee of the German Psychological Association.
Object stimuli were 40 unambiguous two-dimensional black-and-white line drawings of simple common objects. Face stimuli were black-and-white photographs of 40 unfamiliar faces of 20 males and 20 females ranging from 20 to 65 yr of age. All faces were portrayed in three-quarter view and presented at a size of ∼12 × 8.5 cm. All stimuli were presented at a size of ∼12 × 8.5 cm on a computer monitor. Half of the objects and half of the faces were randomly selected and displayed as target stimuli, and the remainder were used as distractors. Objects and faces were randomly presented, one at a time, on the monitor screen for 4 sec each, with an interstimulus interval (ISI) of 1 sec.
Subjects were instructed that each target object would be shown only once for a few seconds and that they should try to remember as many of the items as possible for a later recognition test. Furthermore, to control for attentional and/or perceptual difficulties, subjects were required to name each object and, in the case of faces, to tell whether it is a male or female face that is presented on the screen.
After a retention interval of 80 min during which the subjects completed several tasks (temporal discrimination, reaction time, CFF, and a signal-detection task) not related to the object and face recognition, instructions for the recognition phase were given. The subjects' task was to select the original 20 target objects and target faces from a set of 40 objects and 40 faces by indicating those items that had been previously shown by the verbal response “old” and those items that had not been previously shown (i.e., distractor items) by the verbal response “new.” The target and distractor items were presented in a random sequence for a duration of 4 sec each with an ISI of 1 sec. Subjects' verbal responses were recorded by the experimenter.
As an indicator of recognition performance, frequency of correct recognitions and frequency of false alarms, that is, responding “old” to a (new) distractor item, were computed separately for objects and faces. Subsequently, these data were analyzed applying a signal-detection-theory (SDT) approach (Swets 1964; Green and Birdsall 1978). The advantage of this approach is that SDT supplies a pure index of recognition performance (“sensitivity”) that is independent of whatever criterion is adopted by the individual for making a particular decision (“response bias”). As a measure of recognition performance, the sensitivity measure d′ and, as a measure of response bias, the criterion c were computed according to the procedure described by Macmillan and Creelman (1991). With these measures, better recognition performance is indicated by increasing d′ values, whereas a positive response bias represents a tendency to say “new.”
Acknowledgments
This work was supported in part by grants from the Deutsche Forschungsgemeinschaft.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL trammsa@uni-goettingen.de; FAX 49-551-393662.
Article and publication are at www.learnmem.org/cgi/doi/10.1101/lm.33701.
REFERENCES
- Aigner TG. Pharmacology of memory: Cholinergic-glutamatergic interactions. Curr Opin Neurobiol. 1995;5:155–160. doi: 10.1016/0959-4388(95)80021-2. [DOI] [PubMed] [Google Scholar]
- Allison T, Puce A, Spencer DD, McCarthy G. Electrophysiological studies of human face perception. I. Potentials generated in occipitotemporal cortex by face and non-face stimuli. Cereb Cortex. 1999;9:415–430. doi: 10.1093/cercor/9.5.415. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O'Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Boles Ponto L, Hichwa RD. Neural substrates of facial recognition. J Neuropsychiat. 1996;8:139–146. doi: 10.1176/jnp.8.2.139. [DOI] [PubMed] [Google Scholar]
- Buckley MJ, Gaffan D. Impairment of visual object-discrimination learning after perirhinal cortex ablation. Behav Neurosci. 1997;111:467–475. doi: 10.1037//0735-7044.111.3.467. [DOI] [PubMed] [Google Scholar]
- ————— Perirhinal cortex ablation impairs visual object identification. J Neurosci. 1998a;18:2268–2275. doi: 10.1523/JNEUROSCI.18-06-02268.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Perirhinal cortex ablation impairs configural learning and paired-associate learning equally. Neuropsychologia. 1998b;36:535–546. doi: 10.1016/s0028-3932(97)00120-6. [DOI] [PubMed] [Google Scholar]
- ————— Learning and transfer of object-reward associations and the role of the perirhinal cortex. Behav Neurosci. 1998c;112:15–23. doi: 10.1037//0735-7044.112.1.15. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Muir JL, Aggleton JP. Functionally dissociating aspects of event memory: The effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J Neurosci. 1999;19:495–502. doi: 10.1523/JNEUROSCI.19-01-00495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci. 1990;13:171–182. doi: 10.1146/annurev.ne.13.030190.001131. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Singer W. Excitatory amino acid receptors and synaptic plasticity. Trends Pharmacol Sci. 1990;11:290–296. doi: 10.1016/0165-6147(90)90011-v. [DOI] [PubMed] [Google Scholar]
- Danysz W, Archer T. Glutamate, learning and dementia—Selection of evidence. Amino Acids. 1994;7:147–163. doi: 10.1007/BF00814157. [DOI] [PubMed] [Google Scholar]
- Desimone R. Face-selective cells in the temporal cortex of monkeys. J Cognit Neurosci. 1991;3:1–8. doi: 10.1162/jocn.1991.3.1.1. [DOI] [PubMed] [Google Scholar]
- Ditzler K. Efficacy and tolerability of memantine in patients with dementia syndrome. Drug Res. 1991;8:773–780. [PubMed] [Google Scholar]
- Duncan GE, Sheitman BB, Lieberman JA. An integrated view of pathophysiological models of schizophrenia. Brain Res Rev. 1999;29:250–264. doi: 10.1016/s0165-0173(99)00002-8. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Aggleton JP. The effects of neurotoxic lesions of the perirhinal cortex combined to fornix transection on object recognition memory in the rat. Behav Brain Res. 1997;88:181–193. doi: 10.1016/s0166-4328(97)02297-3. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Neave N, Aggleton JP. Neurotoxic lesions of the perirhinal cortex do not mimic the behavioural effects of fornix transection in the rat. Behav Brain Res. 1996;80:9–25. doi: 10.1016/0166-4328(96)00006-x. [DOI] [PubMed] [Google Scholar]
- Farah MJ. Is face recognition “special”? Evidence from neuropsychology. Behav. Brain Res. 1996;76:181–189. doi: 10.1016/0166-4328(95)00198-0. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Wilson KD, Drain M, Tanaka JN. What is “special” about face perception? Psychol Rev. 1998;105:482–498. doi: 10.1037/0033-295x.105.3.482. [DOI] [PubMed] [Google Scholar]
- Foster AC. Physiology and pathophysiology of excitatory amino acid neurotransmitter systems in relation to Alzheimer”s disease. Adv Neurol. 1990;51:97–102. [PubMed] [Google Scholar]
- Freed WJ. Glutamatergic mechanisms mediating stimulant and antipsychotic drug effects. Neurosci Biobehav Rev. 1994;18:111–120. doi: 10.1016/0149-7634(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Green DM, Birdsall TG. Detection and recognition. Psychol Rev. 1978;85:192–206. [Google Scholar]
- Greenamyre JT, Maragos EF, Albin RL, Penney JB, Young AB. Glutamate transmission and toxicity in Alzheimer's disease. Prog Neuro-Psych Biol Psych. 1988;12:421–430. doi: 10.1016/0278-5846(88)90102-9. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Grady CL, Horwitz B, Salerno J, Ungerleider LG, Mishkin M, Schapiro MB. Dissociation of object and spatial visual processing pathways in human extrastriate cortex. In: Gulyas B, Ottoson D, Roland PE, editors. Functional organisation of the human visual cortex. Oxford: Pergamon Press; 1993. pp. 329–340. [Google Scholar]
- Henke K, Schweinberger SR, Grigo A, Klos T, Sommer W. Specificity of face recognition: Recognition of exemplars of non-face objects in propagnosia. Cortex. 1998;34:289–296. doi: 10.1016/s0010-9452(08)70756-1. [DOI] [PubMed] [Google Scholar]
- Izquierdo I. Pharmacological evidence for the role of long-term potentiation in memory. FASEB J. 1994;8:1139–1145. [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB, Jr, Charney DS. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psych. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Karper LP, Bennett A, D'Souza DC, Abi-Dargham A, Morrisey K, Abi-Saab D, Bremner JD, Bowers MB, Jr, Suckow RF, et al. Interactive effects of subanesthetic ketamine and subhypnotic lorazepam in humans. Psychopharmacology. 1998;135:213–229. doi: 10.1007/s002130050503. [DOI] [PubMed] [Google Scholar]
- LaPorte DJ, Lahti AC, Koffel B, Tamminga CA. Absence of ketamine effects on memory and other cognitive functions in schizophrenic patients. J Psychiat Res. 1996;30:321–330. doi: 10.1016/0022-3956(96)00018-0. [DOI] [PubMed] [Google Scholar]
- Lees GJ. Contributory mechanisms in the causation of neurodegenerative disorders. Neuroscience. 1993;54:287–322. doi: 10.1016/0306-4522(93)90254-d. [DOI] [PubMed] [Google Scholar]
- Levine SC. The question of faces: Special is in the brain of the beholder. In: Young AW, Ellis HD, editors. Handbook of research on face processing. Amsterdam: Elsevier; 1989. pp. 37–48. [Google Scholar]
- Macmillan NA, Creelman CD. Cambridge: Cambridge University Press; 1991. [Google Scholar]
- McNeil JE, Warrington EK. Prosopagnosia: A face specific disorder. J Exp Psychol. 1993;46A:1–10. doi: 10.1080/14640749308401064. [DOI] [PubMed] [Google Scholar]
- Meldrum B, Garthwaite J. Excitatory amino acid neurotoxicity and neurodegenerative disease. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- Mesulam M-M. From sensation to cognition. Brain. 1998;121:1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller WE, Mutschler E, Riederer P. Noncompetitive NMDA receptor antagonists with fast open-channel blocking kinetics and strong voltage-dependency as potential therapeutic agents for Alzheimer's dementia. Pharmacopsychiatry. 1995;28:113–124. doi: 10.1055/s-2007-979603. [DOI] [PubMed] [Google Scholar]
- Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ, Hampton RR, Saksida LM. The parahippocampal region and object identification. Ann NY Acad Sci. 2000;911:166–174. doi: 10.1111/j.1749-6632.2000.tb06725.x. [DOI] [PubMed] [Google Scholar]
- Newcomer JW, Farber NB, Jevtovic-Todorovic V, Selke G, Melson AK, Hershey T, Craft S, Olney JW. Ketamine-induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118. doi: 10.1016/S0893-133X(98)00067-0. [DOI] [PubMed] [Google Scholar]
- O'Scalaidhe SP, Wilson FAW, Goldman-Rakic PS. Face-selective neurons during passive viewing and working memory performance of rhesus monkeys: Evidence for intrinsic specialization of neuronal coding. Cereb Cortex. 1999;9:459–475. doi: 10.1093/cercor/9.5.459. [DOI] [PubMed] [Google Scholar]
- Pantev MR, Ritter R, Görtelmeyer R. Clinical and behavioral evaluation in long-term care patients with mild to moderate dementia under memantine treatment. Z Gerontol Psychiat. 1993;6:103–117. [Google Scholar]
- Parker A, Gaffan D. Interaction of frontal and perirhinal cortices in visual object recognition memory in monkeys. Eur J Neurosci. 1998;10:3044–3057. doi: 10.1046/j.1460-9568.1998.00306.x. [DOI] [PubMed] [Google Scholar]
- Pralong E, Jones RSG. Interactions of dopamine with glutamate- and GABA-mediated synaptic transmission in the rat entorhinal cortex in vitro. Eur J Neurosci. 1993;5:760–767. doi: 10.1111/j.1460-9568.1993.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Puma C, Baudoin C, Bizot JC. Effects of intraseptal infusions of N-methyl-D-aspartate receptor ligands on memory in an object recognition task in rats. Neurosci Lett. 1998;244:97–100. doi: 10.1016/s0304-3940(98)00137-2. [DOI] [PubMed] [Google Scholar]
- Rammsayer T. Is there a common dopaminergic basis of time perception and reaction time? Neuropsychobiology. 1989;21:37–42. doi: 10.1159/000118549. [DOI] [PubMed] [Google Scholar]
- Rammsayer T H, Rodewald S, Groh D. Dopamine-antagonistic, anticholinergic, and GABAergic effects on declarative and procedural memory functions. Cognit Brain Res. 2000;9:61–71. doi: 10.1016/s0926-6410(99)00045-2. [DOI] [PubMed] [Google Scholar]
- Rockstroh S, Emre M, Tarral A, Pokorny R. Effects of the novel NMDA-receptor antagonist SDZ EAA 494 on memory and attention in humans. Psychopharmacology. 1996;124:261–266. doi: 10.1007/BF02246666. [DOI] [PubMed] [Google Scholar]
- Scatton B. The NMDA receptor complex. Fundam Clin Pharmacol. 1993;7:389–400. doi: 10.1111/j.1472-8206.1993.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Schugens MM, Egerter R, Daum I, Schepelmann K, Klockgether T, Loschmann PA. The NMDA antagonist memantine impairs classical eyeblink conditioning in humans. Neurosci Lett. 1997;224:57–60. doi: 10.1016/s0304-3940(97)13452-8. [DOI] [PubMed] [Google Scholar]
- Schulz H, Jobert M, Coppola R, Herrmann WM, Pantev M. The use of diurnal vigilance changes in the EEG to verify vigilance-enhancing effects of memantine in a clinical pharmacological study. Neuropsychobiology. 1996;33:32–40. doi: 10.1159/000119246. [DOI] [PubMed] [Google Scholar]
- Seeck M, Grüsser O-J. Category-related components in visual evoked potentials: Photographs of faces, persons, flowers and tools as stimuli. Exp Brain Res. 1992;92:338–349. doi: 10.1007/BF00227976. [DOI] [PubMed] [Google Scholar]
- Sergent J, Signoret JL. Varieties of functional deficits in prosopagnosia. Cereb Cortex. 1992;2:375–388. doi: 10.1093/cercor/2.5.375. [DOI] [PubMed] [Google Scholar]
- Sergent J, Ohta S, Macdonald B. Functional neuroanatomy of face and object processing. Brain. 1992;115:15–36. doi: 10.1093/brain/115.1.15. [DOI] [PubMed] [Google Scholar]
- Solomon PR. Strategies for studying brain-behavior relationships. Behav Brain Sci. 1986;9:344–345. [Google Scholar]
- Squire LR, Knowlton B, Musen G. The structure and organization of memory. Annu Rev Psychol. 1993;44:453–495. doi: 10.1146/annurev.ps.44.020193.002321. [DOI] [PubMed] [Google Scholar]
- Svensson L, Zhang J, Johannessen K, Engel JA. Effect of local infusion of glutamate analogues into the nucleus accumbens of rats: An electrochemical and behavioural study. Brain Res. 1994;643:155–161. doi: 10.1016/0006-8993(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Swets JA. Signal detection and recognition by human observers. New York: Wiley; 1964. [Google Scholar]
- Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- Thomas RJ. Excitatory amino acids in health and disease. J Am Geriatr Soc. 1995;43:1279–1289. doi: 10.1111/j.1532-5415.1995.tb07407.x. [DOI] [PubMed] [Google Scholar]
- Winblad B, Poritis N. Memantine in severe dementia: Results of the sup-9M-Best study (benefit and efficacy in severely demented patients during treatment with memantine) J Geriatr Psych. 1999;14:135–146. doi: 10.1002/(sici)1099-1166(199902)14:2<135::aid-gps906>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Winnicka MM, Wisniewski K. Disruption of temporo-entorhinal connections abolishes recognition memory-enhancing effect of angiotensins in rats. Gen Pharmacol. 1999;33:91–97. doi: 10.1016/s0306-3623(98)00246-8. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR. Neuropsychological investigations of memory and amnesia: Findings from humans and nonhuman primates. In: Diamond A, editor. The development and neural bases of higher cognitive functions. New York: New York Academy of Sciences; 1990. pp. 434–456. [DOI] [PubMed] [Google Scholar]