Abstract
Introduction
Ceratain classes of antihypertensive drugs have been associated with intraoperative hypotension, and frequently, patients are receiving multiple classes of antihypertensive medications. We sought to determine if one class of antihypertensive medication either alone, or in combination with other classes of antihypertensive medications, increased the probability of intraoperative hypotension, determined by the amount of vasopressor required during carotid endarterectomy (CEA) performed under general anesthesia with specific arterial blood pressure management.
Methods
This is a post hoc analysis of 252 patients scheduled for elective CEA under general anesthesia, all of whom participated in a prospective evaluation of cognitive dysfunction. Patients were characterized by class and number of preoperative antihypertensive medications taken. A predetermined anesthetic regimen was administered to all patients, with a phenylephrine infusion titrated to maintain mean arterial blood pressure at baseline before clamping the carotid artery, and approximately 20% above baseline during clamping. Computerized anesthesia records were used to record hemodynamics and to quantify medication administered intraoperatively.
Results
Patients taking diuretics as part of their antihypertensive regimen required significantly more (1.6 times) total intraoperative phenylephrine than those not taking diuretics, independently of the number of other antihypertensive medications. This difference in the phenylephrine requirement occurs only during the pre-clamp period, i.e., from induction to application of carotid artery clamping for the maintenance of preoperative blood pressure. However, in contrast to this result, there is no difference in pressor requirement comparing classes of antihypertensive medications to increase the mean arterial blood pressure 20 % above baseline during the period when the carotid artery is clamped.
Conclusion
Diuretics are associated with increased vasopressor requirements in patients having a CEA under general anesthesia in the pre-clamp period, which is likely true for any patient having a general anesthetic.
Introduction
Intraoperative hypotension has been associated with adverse perioperative outcomes including stroke1, myocardial ischemia2, cognitive dysfunction3, and increased 1-year mortality4. Therefore, defining the risk factors that predispose patients to intraoperative hypotension, including preoperative chronic antihypertensive therapy,5–8 are of clinical importance.
A number of studies suggest patients receiving chronic angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARBs) experience more hypotension and require more vasopressor than patients receiving other antihypertensive medications during general anesthesia.6,7,9 Some studies suggest formulating guidelines to withhold ACEI and ARBs preoperatively5,8,10–12. However, few studies address the fact that patients with chronic hypertension are often taking multiple antihypertensive medications from different classes, which may interact and confound results,13 and little data are available on the effect of the number and class of antihypertensive drug on intraoperative hypotension.
We hypothesized that the magnitude of the hypotensive effect of a class of antihypertensive drug and the number of antihypertensive drugs could be quantified by the amount of intraoperative vasopressor required in patients undergoing carotid endarterectomy (CEA) under general anesthesia with a specific arterial blood pressure management. Based on these quantitative results, we could determine which classes of antihypertensive drugs, such as ACEIs, ARBs and diuretics, increase the intraoperative vasopressor requirements compared to other classes of antihypertensive medications. We studied quantity of vasopressor as a surrogate for intraoperative hypotension, because we could not allow intraoperative hypotension in the CEA population.
The study is unique because we present data from patients having a single type of procedure, CEA, performed by a small group of surgeons, with a specific and uniform hemodynamic management strategy using arterial blood pressure goals as percentages of the patient's baseline blood pressure. Before clamping the carotid artery, the blood pressure is maintained at the preoperative baseline, during clamping ~20% above baseline and after unclamping, at or slightly below baseline. In addition, it includes an analysis of the amount of vasopressor required as a function of not only drug class, but also the number of different chronic antihypertensive drugs taken by the patient.
Methods
This study, approved by the Columbia University IRB (New York, New York), is a post hoc analysis of 252 patients scheduled for elective CEA under general anesthesia. The patients were prospectively recruited for a study of postoperative cognitive dysfunction after CEA. All patients had vital sign and drug administration data available from computerized intraoperative record systems, eliminating the potential bias of handwritten data collection. Before enrollment, patients provided written consent for study participation.
All patients underwent unilateral CEA under general anesthesia. Preoperative data were collected from clinical documentation entered by anesthesiology residents, attendings and fellows, in addition to data collected by nurses and study coordinators. The baseline arterial blood pressure was obtained by a nurse in the preoperative area on the day of surgery.
The preanesthetic evaluation sheets, completed by an attending anesthesiologist and resident by interview and physical examination on the day of surgery, were used to identify which medications patients were chronically receiving. The data were then categorized into antihypertensive class: ACEI, ARBs, calcium channel blockers (CCBs), beta blockers (BBs) and diuretics. All patients were instructed by the neurosurgical staff to continue any antihypertensive medications they regularly take through and including the morning of surgery. All other medications were withheld. Computerized intraoperative records (CompuRecord; Philips Medical Systems, Andover, MA) were used intraoperatively in every case to record intraoperative hemodynamics, including arterial blood pressure and heart rate, as well as drug dosing.
The anesthetic technique and blood pressure management of patients were determined in advance and standardized for all study patients. Standard monitors which included a blood pressure cuff, pulse oximeter, five lead electrocardiogram, and temperature probe were used for every case. In addition, full montage electroencephalogram (EEG) and transcranial Doppler (TCD) monitoring were performed on every patient. Patients all received their IV lines in the operating room. The IV fluid goal was at least one liter total for each case, regardless of the class of antihypertensive drug. Intraoperative blood pressure was measured using a radial intraarterial catheter that was placed before induction. The anesthetic technique involved a preinduction dose of midazolam (1–5mg) and fentanyl (25–250 micrograms), induction by etomidate (0.15–0.3 mg/kg), propofol (1–2 mg/kg), or thiopental (3–5 mg/kg), and maintenance with 70% nitrous oxide in oxygen and isoflurane (0.3%–0.7%). The anesthesia was performed by 19 anesthesiologists (with one of them performing 86% of the cases), and the surgeries were performed by 8 surgeons (with three performing more than 50 cases each, and the rest performing 1 to 5 cases).
The anesthetic management of a patient undergoing CEA was predetermined using a protocol anesthetic regimen. CEA surgery was divided into three intervals. The first interval of the case was the pre-clamp interval. This was the period of time starting at the induction of anesthesia and terminating at the clamping of the common carotid artery. During the pre-clamp interval, blood pressure was maintained to within 20% of the patients' mean arterial blood pressure (MAP) baseline (as determined in the holding area) before surgery. During the clamped interval, defined as starting when the clamp is placed on the common carotid artery, and ending when the clamp is removed, blood pressure was titrated up to approximately 20% above the baseline MAP. The third and final interval was the post-clamp interval. During this interval, defined as after the clamp was released from the common carotid artery to the end of surgery, blood pressure was allowed to decrease to within 20% below the patients' baseline MAP. Blood pressure changes were attained using phenylephrine infusions supplemented with boluses. Phenylephrine infusions were administered by Sigma Spectrum infusion pump (Baxter, Deerfield, IL) for all cases, which administers phenylephrine in micrograms/minute. To avoid a “see-saw effect” of multiple boluses, patients were started on a slow rate of phenylephrine soon after intubation, which was then titrated up or down as needed. When the infusion was altered (started or rate changed), the alteration would be entered manually by the anesthesiologist into the computerized record system. The total amount of phenylephrine administered during segments of the anesthetic was determined by the computerized record system based on the manual entry of pump rate into the computerized record system. The computerized record system collects and records data every 15 seconds.
Although a phenylephrine infusion was used from the beginning of the case, after induction of general anesthesia, phenylephrine boluses were also administered as needed at the discretion of the anesthesiologist to maintain the blood pressure at the targeted level. To further characterize the increased intraoperative vasopressor requirement, the phenylephrine requirements were converted into rates of microgram/kilogram/minute, to correct for potential differences in surgical time and patient weight. Ephedrine boluses were also administered if the patient exhibited significant bradycardia in conjuction with hypotension. Total phenylephrine and ephedrine doses were recorded on the intraoperative record system.
To address the interaction of multiple antihypertensive classes, we separated the data by the number of antihypertensive drugs chronically taken. Patients who took multiple classes of antihypertensive medications were characterized by number of classes of drugs taken, as well as which of those classes were taken. To further investigate if there is an effect of antihypertensive class on vasopressor requirement, patients who only took a single class of antihypertensive were isolated to analyze which classes were associated with an increase in vasopressor requirement. For patients receiving only a single class of antihypertensive drug, we determined the amount of phenylephrine required by calculating the rate of phenylephrine administered during pre- and post-clamp intervals of the case.
Statistics
Statistical analyzes were performed using Excel (Microsoft Corp., Redmond, WA) and JMP 7 software (SAS Institute Inc., Cary, NC). All statistical tests were considered significant if the probability of a type I error was inferior to 0.05 (p value).
Univariate analyses were performed to address our hypothesis and determine if there was a difference in the total phenylephrine requirement and the number of antihypertensive classes patients take (Figure 1), and if there was a specific antihypertensive class that resulted in a higher phenylephrine requirement (Figure 2). Furthermore, we analyzed if the inclusion of diuretics, as one of the classes of a multiple antihypertensive regimen, would cause an increase in phenylephrine requirements in groups consisting of the same number of antihypertensive classes (Figure 3).
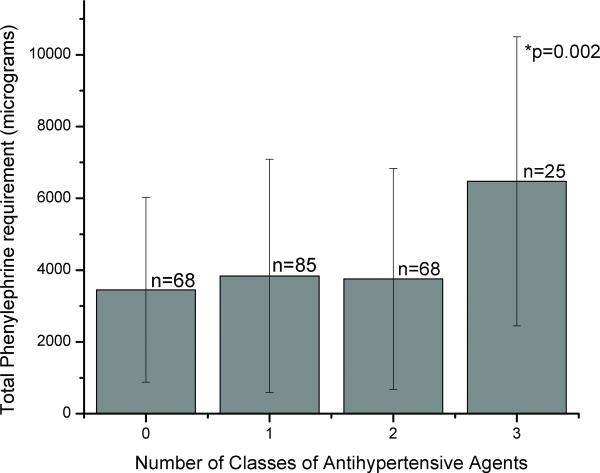
FIGURE 1.
The total intraoperative phenylephrine requirement (in micrograms) during carotid endarterectomy (CEA) under general anesthesia per number of classes of antihypertensive drugs that the patient is chronically taking. Error bars: standard error; p=0.002*: Significant by Kruskal Wallis.
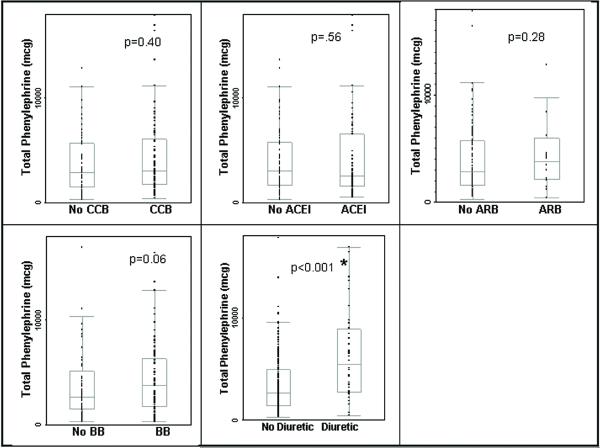
FIGURE 2.
The total phenylephrine requirement (in micrograms) intraoperatively during carotid endarterectomy (CEA) under general anesthesia of patients taking, versus those not taking each class of antihypertensive including: calcium channel blocker (CCB), angiotensin converting enzyme inhibitor (ACEI), angiotensin receptor blocking agent (ARB), beta blocker (BB), and diuretics. The use of diuretics as an antihypertensive is also the first branch point in the classification and regression tree (CART) diagram (Figure 4). Error bars: standard error; p<0.001*: Significant by Mann Whitney test.
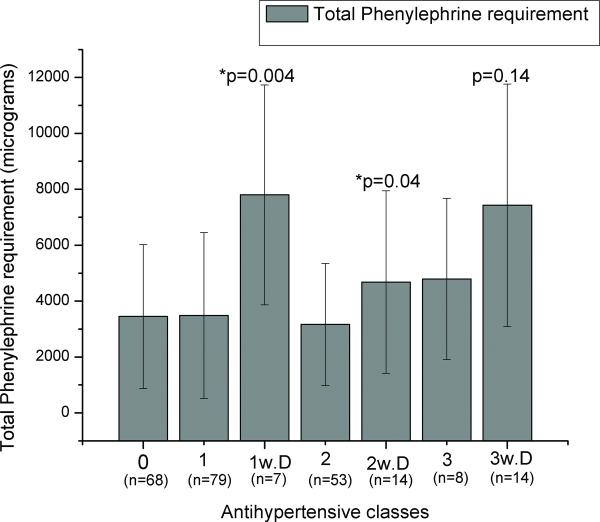
FIGURE 3.
Effect of diuretics on patients taking multi-antihypertensive therapy. Antihypertensive classes: 0: no antihypertensive drug; 1: 1 class of antihypertensive drug; 1 w. D: 1 class of antihypertensive drug, being a diuretic; 2: 2 classes of antihypertensives; 2 w. D.: 2 classes of antihypertensives including a diuretic; 3: 3 classes of antihypertensives; 3 w. D.: 2 classes of antihypertensives including a diuretic. P values significant by Kruskal Wallis test when compared to the previous category (comparing patients taking diuretics as part of regimen to those not). N.S: nonsignificant.
In addition to Univariate analyses, we analyzed our data with a multivariate regression model. Because the main outcome measure, total phenylephrine consumption (PHtotal), followed a log-normal distribution, it was transformed for analyses requiring linearity, and thereby, those results represent median values of phenylephrine use. We used multivariate linear regression to verify the association of antihypertensive medication class with PHtotal while adjusting for potential confounders (duration of surgery, age, body mass index, smoking status, induction drug, ephedrine dose, total fluid administered, coronary artery disease, history of congestive heart failure, and history of diabetes) which were included in the regression model using a forward, backward and stepwise procedure, and the chosen model was identical starting with all available preoperative patient characteristics. (Table 1)
TABLE 1.
Multiple regression model including significant parameters, standard errors and t values. Dependent variable is Log (total phenylephrine dose). The multivariate analysis was repeated with just the data from those taken care of by the anesthesiologist who performed most of these cases with the same results (data not shown).
| Parameter | Estimate | Standard Error | T-value | Pr ( > [t] ) |
|---|---|---|---|---|
| Intercept | 7.92 | 0.07 | 117.309 | <0.001 |
| B.M.I. | 0.18 | 0.06 | 2.807 | 0.005 |
| D.O.S. | 0.22 | 0.06 | 3.441 | <0.001 |
| Ephedrine | 0.15 | 0.06 | 2.451 | 0.015 |
| Diuretic | 0.45 | 0.17 | 2.605 | 0.010 |
B.M.I.: Body mass index, D.O.S.: Duration of surgery, Ephedrine: total dose of ephedrine administered during case, Diuretic: patient chronically takes a diuretic as part of an antihypertensive regimen, Pr ( > [t] ): p-value.
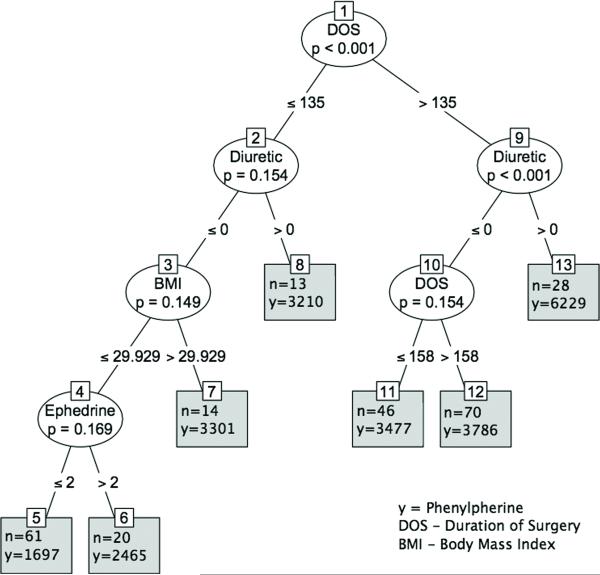
To express the effect size of the independent variables in the regression model, we formulated a classification and regression tree (CART). The duration of surgery was the covariate with the highest association with the outcome. The data were then split into two groups (less than and including 135 minutes or more than 135 minutes). For those above 135 minutes for duration of surgery, the next highly associated covariate was diuretic therapy. The data were again split into two groups: patients taking diuretics (diuretics >0), and patients not taking diuretics (diuretics < 0). For those not taking diuretics, the most associated variable was again duration of surgery. One can similarly interpret other branches of the tree. (Figure 4)
FIGURE 4.
Tree diagram from unbiased recursive partitioning under a conditional inference framework20. The plot may be interpreted as follows. To express the effect size of the independent variables in the regression model, we formulated a classification and regression tree (CART). The duration of surgery was the covariate with the highest association with the outcome. The data were then split into two groups (less than and including 135 minutes or more than 135 minutes). For those more than 135 minutes for duration of surgery, the next highly associated covariate was diuretic therapy. The data were again split into two groups: patients taking diuretics (diuretics >0), and patients not taking diuretics (diuretics < 0). For those not taking diuretics, the most associated variable was again duration of surgery. One can similarly interpret other branches of the tree.
Results
Two hundred fifty-two patients were included in this analysis. Of these, 68 patients (27%) were not taking a chronic antihypertensive drug. Of the patients receiving chronic antihypertensive therapy, 85 patients (35%) were taking a single antihypertensive, 68 patients (26%) taking 2, 25 patients (9%) taking 3, and 6 patients (3%) taking 4. (Figure 1) Of the patients taking a single antihypertensive, 27 were taking a BB, 23 taking an ACEI, 22 taking a CCB, 7 taking a diuretic (all receiving a thiazide) and 6 taking an ARB.
The prevalence of preoperative demographic variables of patients taking each class of antihypertensive therapy is shown in Table 2. There was a higher incidence of coronary artery disease in patients taking a BB or a CCB (p<0.01 by Fisher's right-sided exact test). Patients who were taking BBs also had a higher blood urea nitrogen and creatinine (p=0.01 and p=0.03, respectively by Mann-Whitney-Wilcoxon test), and those taking diuretics a higher blood urea nitrogen (p=0.02 by Mann-Whitney-Wilcoxon test) than those not taking this antihypertensive class.
TABLE 2.
Patient demographics including medical conditions, pre-operative labs, baseline parameters and intraoperative parameters.
| Parameter n=total incidence | ACEI (n=70) | CCB (n=80) | ARB (n=28) | BB (n=100) | Diuretic (n=44) |
|---|---|---|---|---|---|
| Medical History | |||||
| Age (years) | 69.6 +/− 10.3 | 71.3 +/− 8.5 | 72.1 +/− 8.1 | 70.6 +/− 8.6 | 71.5 +/− 8.4 |
| BMI (kg/m2) | 27.3 +/− 4.5 | 27.0 +/− 4.1 | 26.5 +/− 2.7 | 27.3 +/− 4.2 | 27.6 +/− 4.0 |
| CAD (n=98) | 30 (43%) | 41 (51%)* (p=0.005) | 8 (29%) | 53 (53%)* (p<0.001) | 23 (52%) |
| CHF (n=9) | 1 (<1%) | 2 (<1%) | 2 (7%) | 4 (4%) | 3 (7%) |
| MI (n=65) | 24 (34%) | 25 (31%) | 4 (14%) | 31 (31%) | 12 (27%) |
| Afib (n=11) | 3 (4%) | 6 (7%) | 1 (4%) | 7 (7%) | 2 (5%) |
| COPD (n=42) | 9 (13%) | 17 (21%) | 2 (8%) | 15 (15%) | 6 (14%) |
| DM (n=50) | 20 (29%) | 16 (20%) | 4 (14%) | 18 (18%) | 10 (23%) |
| Smoker (n=98) | 26 (37%) | 32 (40%) | 10 (36%) | 40 (40%) | 21 (48%) |
| Prev CEA (n=31) | 6 (9%) | 11 (13%) | 2 (7%) | 17 (17%) | 3 (7%) |
| Prev Stroke (n=109) | 30 (43%) | 25 (31%) | 9 (32%) | 38 (38%) | 23 (52%) |
| Pre-operative labs | |||||
| Hematocrit (%) | 40.5 +/− 3.83 | 40.60 +/− 5.2 | 40.4 +/− 3.3 | 40.6 +/− 4.5 | 40.0 +/− 4.8 |
| Creatinine (mg/dL) | 1.15 +/− 0.32 | 1.13 +/− 0.44 | 1.05 +/− 0.32 | 1.18 +/− 0.36* (p=0.01) | 1.15 +/− 0.32 |
| BUN (mg/dL) | 20.2 +/− 7.4 | 20.4 +/− 8.7 | 20.7 +/− 8.1 | 21.8 +/− 8.4* (p=0.03) | 23.6 +/− 10.0* (p=0.02) |
| Baseline parameters | |||||
| SBP (mmHg) | 146.2 +/− 23.9 | 145.7 +/− 22.9 | 148.8 +/− 20.6 | 144.6 +/− 22.7 | 142.3 +/− 20.3 |
| DBP (mmHg) | 76.5 +/− 12.0 | 75.2 +/− 10.0 | 80.2 +/− 12.2 | 77.4 +/− 12.4 | 76.0 +/− 13.1 |
| Intraoperative parameters | |||||
| Surgical duration (mins) | 143 +/− 37 | 140 +/− 39 | 151 +/− 40 | 145 +/− 34 | 150 +/− 34 |
| Cross Clamp duration (mins) | 38 +/− 12 | 36 +/− 14 | 36 +/− 10 | 37 +/− 11 | 40 +/− 11 |
| Fluids (mL) | 1248 +/− 435 | 1268 +/− 484 | 1250 +/− 588 | 1146 +/− 441 | 1175 +/− 362 |
| Total ephedrine (mg) | 6.2 +/− 10.3 | 4.1 +/− 8.2 | 5.8 +/− 14.7 | 5.9 +/− 10.7 | 6.6 +/− 11.7 |
ACEI: angiotensin converting enzyme inhibitor, CCB: calcium channel blocker, ARB: angiotensin receptor blocker, BB: beta blocker, BMI: body mass index, CAD: coronary artery disease, CHF: congestive heart failure, MI: history of previous myocardial infarction, Afib: history of chronic atrial fibrillation, COPD: history of chronic obstructive pulmonary disease, DM: diabetes mellitus, Smoker: currently a smoker, Prev CEA: history of previous carotid endarterectomy, Prev Stroke: history of previous stroke, BUN: blood urea nitrogen, SBP: systolic blood pressure, DBP: diastolic blood pressure. P values compare prevalence of demographic in patients receiving antihypertensive group vs. those not on class.
p<0.05: Significant by Fisher exact test (categorical data) or Mann-Whitney-Wilcoxon test (continuous data).
To maintain blood pressure at a steady level within 20% of their baseline pre-clamp and 20% above baseline during carotid clamping, all patients had a phenylephrine infusion started after anesthetic induction. Most of the phenylephrine was administered by continuous infusion (mean of 3888 mcg, standard deviation of 3216 micrograms). The remainder of the phenylephrine was administered through 40 mcg/mL boluses (mean of 132 mcg, standard deviation of 190 micrograms). In addition to the phenylephrine infusion and boluses, one-third of patients (82) received additional ephedrine boluses. The mean total dose of ephedrine was 5.5 milligrams (SD 10.3 mg). There was no association between group of antihypertensive drug and either the number of ephedrine boluses administrated, or the total amount of ephedrine administered during the case. No patient required other drugs such as vasopressin or epinephrine for maintaining blood pressure at the predetermined level.
Patients received a mean of 1250 milliliters (standard deviation of 490 cc) of lactated Ringer solution. There was no difference between the amount of fluids received and antihypertensive class or number of classes of antihypertensive drugs taken.
The total intraoperative amount of phenylephrine required depended on the number of different classes of antihypertensive medications the patients were taking (p=0.002 by Kruskal Wallis test). Patients who were taking 3 classes of medications required an average of 75% more total phenylephrine than those taking zero, one, or two classes. Those patients taking fewer than three classes of medications did not differ in the average amount of phenylephrine administered. (Figure 1)
Univariate analysis of the relationship between intraoperative pressor requirement and class of antihypertensive drug was performed using the Mann Whitney test. Analyses showed that patients taking diuretics required significantly more total phenylephrine than those not taking diuretics (p<0.001). There was no significant difference when comparing patients who were taking other classes of antihypertensives including ACEI, ARBs, BBs, and CCBs to those who were not taking that particular class of drug. (Figure 2) This was true when the total phenylephrine dose was divided into phenylephrine administered by boluses (p=0.0154), and the phenylephrine administered by continuous infusion (p<0.0001). There was no other antihypertensive class in which the total phenylephrine, bolus phenylephrine, or infused phenylephrine amount was different between those taking the antihypertensive and those not taking the antihypertensive.
In addition, when comparing patients only taking one antihypertensive class, only patients taking diuretics required more pressor medication. Furthermore, of those taking two classes of antihypertensive drugs, those who chronically took diuretics required significantly more phenylephrine than those taking two other classes of drugs (p=0.04). (Figure 3) Although patients receiving diuretic therapy as part of a 3-class antihypertensive regimen required more vasopressor than those receiving other drugs, the difference was not significant (p=0.14). (Figure 3) Patients taking diuretics as part of an antihypertensive regimen required 125%, 50%, and 48% more total phenylephrine when taking a total of 1, 2, or 3 classes of antihypertensive drugs respectively. (Figure 3)
When all patients were analyzed by multivariate regression model, including those taking multiple antihypertensive drugs (ACEI, ARBs, BBs, and CCBs), patients who were taking diuretics in their antihypertensive regimen required significantly more total phenylephrine during CEA than those not taking a diuretic. We used a multivariate linear regression to verify the association of antihypertensive medication class with PHtotal while adjusting for potential confounders (duration of surgery, age, body mass index, smoking status, induction drug, ephedrine dose, total fluid administered, coronary artery disease, history of congestive heart failure, and history of diabetes) which were included in the regression model using a forward, backward and stepwise procedure and the chosen model was identical starting with all available preoperative patient characteristics. (Table 1)
Potential confounders that were screened for included duration of surgery, age, body mass index, smoking status, induction drug, ephedrine dose, total fluid administered, coronary artery disease, history of congestive heart failure, and history of diabetes. Of all factors, only duration of surgery (p=0.005), body mass index (p<0.001), ephedrine dose (p=0.015), and chronic diuretic use (p=0.01) remained statistically significant.
To express the effect size of the independent variables in the regression model, we formulated a classification and regression tree (CART). The duration of surgery was the covariate with the highest association with the outcome. The data were then split into two groups (≤135 minutes and >135 minutes). For those >135 minutes for duration of surgery, the next highly associated covariate was diuretic therapy. The data were again split into two groups: patients taking diuretics (diuretics >0), and patients not taking diuretics (diuretics < 0). For those not taking diuretics, the most associated variable was again duration of surgery. One can similarly interpret other branches of the tree. (Figure 4)
Residual analysis showed that there were no large outliers. The residuals are shown to be normally distributed in Figure 5. Moreover, results from robust linear regression were almost identical to those from linear regression. The leverage and Cook's distance was determined for the data (Figure 6) and none of the data points has a Cook's distance more than 0.5. To account for possible co-linearity among the covariates, we standardized the continuous covariates. Also, estimates from ridge regression were almost identical to the one from the linear regression.
FIGURE 5.
The plot shows that the residuals are normally distributed.
FIGURE 6.
The plot shows the leverage and the Cook's distance. Points having Cook's distance more than 1 are considered to have high leverage. The plot shows that none of the data points has a cooks distance more than 0.5.
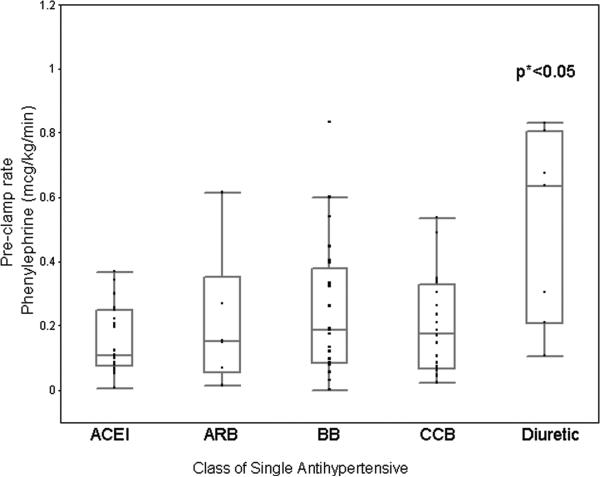
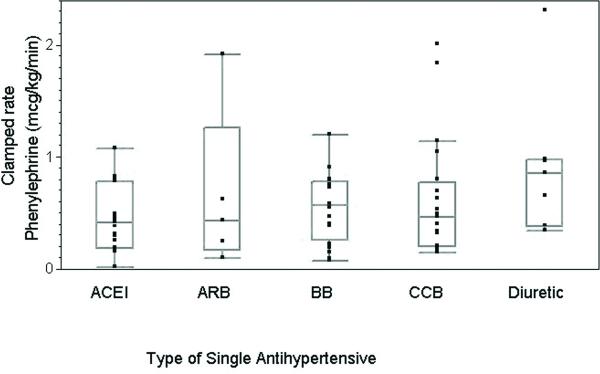
To address the question of when pressor medication is required and to generalize our results, we evaluated the amount or rate of pressor use as a function of the operative interval. Due to the fact that we had fewer patients receiving monotherapy and body mass index and duration of surgery also influenced total phenylephrine requirements, as found by the multivariate analysis above, we expressed the pre and post clamp rates of phenylephrine in terms of mcg/kg/min determined by total mcg of phenylephrine given my infusion in either interval divided by patient weight and interval time. Patients who were taking diuretic therapy required a significantly higher rate of phenylephrine to keep their blood pressure at baseline during the pre-clamp interval than those receiving any other antihypertensive medication (p<0.05). (Figure 7) This result was not found going from the pre-clamp interval to the clamp-interval. During the clamped interval, there was no significant difference in the rate of phenylephrine needed to increase the blood pressure to ~20% above baseline across the different antihypertensive classes, from similar pre-clamp blood pressures, as a fraction of baseline. (Figure 8)
FIGURE 7.
Pre-clamped phenylephrine requirements of carotid endarterectomy patients taking a single class of antihypertensive (micrograms/kilogram/minute). p<0.05 by Kruskal Wallis.
FIGURE 8.
Clamped phenylephrine requirements of carotid endarterectomy patients taking a single class of antihypertensive (micrograms/kilogram/minute).
Discussion
We present a post hoc analysis of vasopressor requirements of patients undergoing CEA under general anesthesia. The results demonstrate that patients taking preoperative diuretic therapy are associated with increased intraoperative vasopressor requirement during CEA when it is used as a single antihypertensive, as well as when it is used as part of a multiple antihypertensive regimen. There is no significant increase of vasopressor requirement for patients taking the other classes of antihypertensives including ACEI, ARBs, CCBs and BBs compared to those not taking antihypertensive medications.
Our results are applicable to patients having general anesthesia for other surgeries. In the pre-clamp interval, before clamping the carotid, blood pressure is kept within 20% of baseline, a similar same situation to patients having other surgery under general anesthesia.
EEG is our accepted standard to determine the adequacy of collateral blood flow to the brain. However, the EEG is insensitive at detecting focal ischemia and requires as much as a 60% decrease in normal cerebral blood flow before subtle EEG changes can be detected. While TCD evaluates the blood flow velocity in one of the four major vessels supplying blood to the brain, to titrate blood pressure to lower values again seems risky by potentially letting focal areas become ischemic. Also, one has to remember that TCD measures cerebral blood flow velocity not cerebral blood flow. Therefore, one does not know where on the normal spectrum of blood flow the patient is when there may be a reduction of cerebral blood flow velocity. EEG has been demonstrated to reflect the adequacy of cortical cerebral blood flow to maintain cerebral activity more directly than TCD. We are aware that some experienced neuroanesthesiologists use the TCD to titrate systemic blood pressure, but we prefer to take a more conservative approach. Therefore, during clamping, our group increases blood pressure, if tolerated cardiovascularly by the patient, to 20 % above their baseline. We then monitor the EEG and TCD monitors and if the perfusion is inadequate, we can use these monitors to view what change an increase in blood pressure can make, often as the surgeon is preparing to place a shunt.
Diuretics are unique in altering the circadian rhythm of blood pressure. Hypertensive patients can be grouped into two categories based on their nocturnal (or sleeping) blood pressure: “dippers” are patients whose nocturnal blood pressure decreases by 10%, whereas “nondippers” are patients who do not experience this decrease. These different patterns are of clinical significance due to the results of a large prospective trial that showed that nondippers at an increased risk of cardiovascular morbidity than dippers, whereas “extreme dippers” (those with an exaggerated 20% or more nocturnal blood pressure decrease) are at an increased risk of stroke14,15. The period of “dipping” is characterized by a response to the decrease of sympathetic activation16. Dipping is restored to patients taking hydrochlorothiazide diuretics probably due to increased sodium excretion during the daytime.17
In the perioperative hypertensive patient, a thiazide diuretic such as hydrochlorothiazide may enhance a decrease of blood pressure or dipping with the induction of anesthesia, similar to the dipping enhanced in patients taking thiazide diuretics during sleep. Both periods are characterized by a decrease in sympathetic stimulation. Therefore, the benefit to cardiovascular morbidity offered to the patient by maintaining circadian blood pressure, may increase blood pressure decrease in the operating room upon a decrease in sympathetic stimulation.
We can expect that many patients presenting for anesthesia will be taking chronic thiazide diuretics as a first-line antihypertensive drug since thiazide diuretics have been confirmed to be equal or superior to other antihypertensive drugs as a first-line therapy by The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT)18.
There are a number of case reports of refractory hypotension of patients taking chronic ACEI and ARBs undergoing general anesthesia. Currently there is debate whether to withhold these medications preoperatively. To address this debate, a number of small studies have been conducted to evaluate if patients taking chronic ACEI or ARBs experience hypotension and require more vasopressors than patients taking other antihypertensive medications during general anesthesia6,7,9. The major limitation of these studies is their small sample size and a large number of other antihypertensive drugs taken.
Kheterpal13 et al. performed a large retrospective study of 65,043 patients having noncardiac surgery in which they analyzed the number of vasopressor boluses (of equivalent doses of ephedrine and phenylephrine) in patients who were receiving different combinations of antihypertensives. They concluded that patients receiving chronic diuretic therapy along with ACEI/ARB therapy had greater vasopressor requirements compared to patients receiving an ACEI/ARB combined with a CCB. However, patients who were taking a CCB along with a diuretic required the same number of vasopressor boluses as the group that was taking an ACE/ARB plus diuretic. This observation is in concordance with our data, which show that when a diuretic is added to antihypertensive management, intraoperative pressor requirements increase. Similar to our findings, it suggests that ACEI and ARBs, are not responsible for increased intraoperative vasopressor requirements alone, and that diuretics play a key role in these requirements. The major limitations of the study by Kheterpal et al. were that although the study was large, the type and length of surgery varied widely and there was no set anesthetic protocol. The number of equivalent vasopressor boluses were calculated, but this information was not combined with vasopressor infusion data to yield the total amount or rate of vasopressor used. In addition, BBs were excluded from their analysis, so it is not possible to analyze exactly how many antihypertensive medications each patient was receiving.
Our study is unique due to the fact that the anesthetic technique was standardized. In contrast to previous studies, this study involved a single type of surgery performed by one of three surgeons at a single institution. The entire intraoperative interval was addressed, and the data were divided into three intervals corrected for time, thus correcting for potential differences in surgical length. Because the patients were all undergoing the same type of surgery, they had similar preoperative factors. During the intraoperative course, the hemodynamic variables were measured precisely and the vasopressor used was standardized. In addition, all data were recorded by a computerized medical record keeper, which made drug infusion dose calculations, as well as blood pressure measurement recordings more accurate19.
This study has several limitations, including its post hoc design and small sample size. In addition, it was not clear how long the patients were taking their current medication regimen and the exact amount of time before the surgery that their last dose was administered. Patient noncompliance may also interfere with the results of the study, especially due to the fact that patients taking multiple medications are less likely to be taking all the medications appropriately. However, we do show the response of the patient in the “real world” clinical scenario, in which we can assume a certain degree of noncompliance. In addition, the rate of phenylephrine set on the pump was added to a computerized record system separately, and frequent changes in phenylephrine rate may not have all been recorded. However, a typical case involved 5–8 changes to the rate of phenylephrine.
Although the results of the study are subject to some limitations, they are in agreement with previous studies. Our study design addresses vasopressor requirements in patients managed with a standardized protocol-controlled anesthetic technique and blood pressure management strategy. The analysis of the clinical response of the hypertensive patient to different antihypertensive therapy is of clinical significance to the anesthesiologist in the prediction and prevention of intraoperative hypotension during general anesthesia. The fact that this difference occurs in the pre-clamp period makes this study relevant to other surgical procedures performed under general anesthesia.
The results address the fact that diuretics are a large confounding factor, if not an independent factor of attributing the cause of intraoperative hypotension under general anesthesia. In addition, patients taking three different classes of antihypertensive drugs require significantly more phenylephrine than those taking a fewer number of classes. The group of patients that are described in this study are of a specific category: patients with carotid disease and hypertension. Further laboratory work and prospective clinical studies must be performed to elucidate the mechanism of this interaction, as well as the other possible patient populations to which the findings may be applicable.
Acknowledgements
We would like to acknowledge Drs. Alastair and Margret Wood for their help and inspiration in preparing the manuscript, as well as SaeJin Kim for her help in preparing the manuscript.
Funding: E.J.H. was supported in part by a grant from the NIA (R01 AG17604).
Footnotes
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Limburg M, Wijdicks EF, Li H. Ischemic stroke after surgical procedures: clinical features, neuroimaging, and risk factors. Neurology. 1998;50:895–901. doi: 10.1212/wnl.50.4.895. [DOI] [PubMed] [Google Scholar]
- 2.Barone JEM, FACS, FCCM, Bull Marcia B., MD, FACP, FACCP, FCCC, FACC, Cussatti Edward H., MD, Miller Kevin D., MD, Tucker James B., RN, MBA Perioperative Myocardial Infarction in Low-Risk Patients Undergoing Noncardiac Surgery Is Associated With Intraoperative Hypotension. Journal of Intensive Care Medicine. 2002;17:250–255. [Google Scholar]
- 3.Yocum GT, Gaudet JG, Teverbaugh LA, Quest DO, McCormick PC, Connolly ES, Jr., Heyer EJ. Neurocognitive performance in hypertensive patients after spine surgery. Anesthesiology. 2009;110:254–61. doi: 10.1097/ALN.0b013e3181942c7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monk TG, Saini V, Weldon BC, Sigl JC. Anesthetic management and one-year mortality after noncardiac surgery. Anesth Analg. 2005;100:4–10. doi: 10.1213/01.ANE.0000147519.82841.5E. [DOI] [PubMed] [Google Scholar]
- 5.Bertrand M, Godet G, Meersschaert K, Brun L, Salcedo E, Coriat P. Should the angiotensin II antagonists be discontinued before surgery? Anesth Analg. 2001;92:26–30. doi: 10.1097/00000539-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Brabant SM, Bertrand M, Eyraud D, Darmon PL, Coriat P. The hemodynamic effects of anesthetic induction in vascular surgical patients chronically treated with angiotensin II receptor antagonists. Anesth Analg. 1999;89:1388–92. doi: 10.1097/00000539-199912000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Brabant SM, Eyraud D, Bertrand M, Coriat P. Refractory hypotension after induction of anesthesia in a patient chronically treated with angiotensin receptor antagonists. Anesth Analg. 1999;89:887–8. doi: 10.1097/00000539-199910000-00012. [DOI] [PubMed] [Google Scholar]
- 8.Comfere T, Sprung J, Kumar MM, Draper M, Wilson DP, Williams BA, Danielson DR, Liedl L, Warner DO. Angiotensin system inhibitors in a general surgical population. Anesth Analg. 2005;100:636–44. doi: 10.1213/01.ANE.0000146521.68059.A1. [DOI] [PubMed] [Google Scholar]
- 9.Rosenman DJ, McDonald FS, Ebbert JO, Erwin PJ, LaBella M, Montori VM. Clinical consequences of withholding versus administering renin-angiotensin-aldosterone system antagonists in the preoperative period. J Hosp Med. 2008;3:319–25. doi: 10.1002/jhm.323. [DOI] [PubMed] [Google Scholar]
- 10.Coriat P, Richer C, Douraki T, Gomez C, Hendricks K, Giudicelli JF, Viars P. Influence of chronic angiotensin-converting enzyme inhibition on anesthetic induction. Anesthesiology. 1994;81:299–307. doi: 10.1097/00000542-199408000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Pigott DW, Nagle C, Allman K, Westaby S, Evans RD. Effect of omitting regular ACE inhibitor medication before cardiac surgery on haemodynamic variables and vasoactive drug requirements. Br J Anaesth. 1999;83:715–20. doi: 10.1093/bja/83.5.715. [DOI] [PubMed] [Google Scholar]
- 12.Raja SG, Fida N. Should angiotensin converting enzyme inhibitors/angiotensin II receptor antagonists be omitted before cardiac surgery to avoid postoperative vasodilation? Interact Cardiovasc Thorac Surg. 2008;7:470–5. doi: 10.1510/icvts.2007.174698. [DOI] [PubMed] [Google Scholar]
- 13.Kheterpal S, Khodaparast O, Shanks A, O'Reilly M, Tremper KK. Chronic angiotensin-converting enzyme inhibitor or angiotensin receptor blocker therapy combined with diuretic therapy is associated with increased episodes of hypotension in noncardiac surgery. J Cardiothorac Vasc Anesth. 2008;22:180–6. doi: 10.1053/j.jvca.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 14.Verdecchia P, Porcellati C, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Guerrieri M, Gatteschi C, Zampi I, Santucci A, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 15.Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension. 1996;27:130–5. doi: 10.1161/01.hyp.27.1.130. [DOI] [PubMed] [Google Scholar]
- 16.Kario K, Schwartz JE, Pickering TG. Changes of nocturnal blood pressure dipping status in hypertensives by nighttime dosing of alpha-adrenergic blocker, doxazosin : results from the HALT study. Hypertension. 2000;35:787–94. doi: 10.1161/01.hyp.35.3.787. [DOI] [PubMed] [Google Scholar]
- 17.Uzu T, Kimura G. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation. 1999;100:1635–8. doi: 10.1161/01.cir.100.15.1635. [DOI] [PubMed] [Google Scholar]
- 18.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 19.Reich DL, Wood RK, Jr., Mattar R, Krol M, Adams DC, Hossain S, Bodian CA. Arterial blood pressure and heart rate discrepancies between handwritten and computerized anesthesia records. Anesth Analg. 2000;91:612–6. doi: 10.1097/00000539-200009000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Hothorn THK, Zeileis A. Unbiased Recursive Partitioning. Journal of Computational and Graphical Statistics. 2006;15:651–674. [Google Scholar]