Abstract
cAMP-dependent protein kinase (PKA) is critical for the expression of some forms of long-term potentiation (LTP) in area CA1 of the mouse hippocampus and for hippocampus-dependent memory. Exposure to spatially enriched environments can modify LTP and improve behavioral memory in rodents, but the molecular bases for the enhanced memory performance seen in enriched animals are undefined. We tested the hypothesis that exposure to a spatially enriched environment may alter the PKA dependence of hippocampal LTP. Hippocampal slices from enriched mice showed enhanced LTP following a single burst of 100-Hz stimulation in the Schaffer collateral pathway of area CA1. In slices from nonenriched mice, this single-burst form of LTP was less robust and was unaffected by Rp-cAMPS, an inhibitor of PKA. In contrast, the enhanced LTP in enriched mice was attenuated by Rp-cAMPS. Enriched slices expressed greater forskolin-induced, cAMP-dependent synaptic facilitation than did slices from nonenriched mice. Enriched mice showed improved memory for contextual fear conditioning, whereas memory for cued fear conditioning was unaffected following enrichment. Our data indicate that exposure of mice to spatial enrichment alters the PKA dependence of LTP and enhances one type of hippocampus-dependent memory. Environmental enrichment can transform the pharmacological profile of hippocampal LTP, possibly by altering the threshold for activity-dependent recruitment of the cAMP-PKA signaling pathway following electrical and chemical stimulation. We suggest that experience-dependent plasticity of the PKA dependence of hippocampal LTP may be important for regulating the efficacy of hippocampus-based memory.
Experience and environmental enrichment can modify the structure (Globus et al. 1973; Greenough and Volkman 1973), growth (Hubel and Wiesel 1977; Stryker and Harris 1986; Kempermann et al. 1997), and physiological efficacy (Wiesel and Hubel 1963; Green and Greenough 1986) of mammalian neurons and their synaptic connections. Environmental enrichment can also enhance learning and memory in rodents (Escorihuela et al. 1995b; Kempermann et al. 1997; van Praag et al. 1999; Rampon et al. 2000; reviewed by Rosenzweig and Bennett 1996). However, the cellular and molecular bases for enrichment-induced modifications of cognitive function remain undefined. Altered intracellular signaling and modified synaptic strength may underlie enrichment-induced improvement of memory (Paylor et al. 1992; Escorihuela et al. 1995a; see also these general reviews on synaptic plasticity: Elgersma and Silva 1999; Micheau and Riedel 1999; Martin et al. 2000).
Hippocampal long-term potentiation (LTP) is an activity-dependent enhancement of synaptic transmission that is believed to be a cellular mechanism for some types of memory in the mammalian brain (for review, see Bliss and Lomo 1973; Micheau and Riedel 1999; Martin et al. 2000). In rodents and humans, area CA1 of the hippocampus is critical for information processing linked to certain forms of memory (Zola-Morgan et al. 1986; Tsien et al. 1996). Exploration of spatially complex, or “enriched,” environments elicits patterns of electrical activity in hippocampal neurons of area CA1 that are similar to patterns of electrical stimulation used to induce LTP in hippocampal slices (O'Keefe 1979; Otto et al. 1991). This suggests that exposure to enriched environments may modify synaptic physiology in hippocampal neurons (Green and Greenough 1986; Foster et al. 1996; van Praag et al. 1999).
One particular signal transduction molecule that is critical for the induction of long-lasting synaptic potentiation in area CA1 is cAMP-dependent protein kinase (PKA). Inhibitors of PKA attenuate maintenance of LTP in an activity-dependent manner: LTP induced by multiple bursts of 100-Hz stimulation in area CA1 of hippocampal slices is long lasting and is critically dependent on PKA (Frey et al. 1993; Huang and Kandel 1994; Blitzer et al. 1995), whereas LTP induced by a single 100-Hz burst of stimulation is relatively less robust and less dependent on PKA activation (Huang and Kandel 1994; Blitzer et al. 1995; Otmakhova et al. 2000). Genetic reduction of hippocampal PKA activity impairs expression of multiburst, but not single-burst, LTP in area CA1 (Abel et al. 1997). These electrophysiological observations are correlated with specific deficits of hippocampus-dependent long-term (but not short-term) memory in PKA mutant mice (Abel et al. 1997).
Although pharmacological and genetic studies have defined a critical requirement for PKA in some forms of LTP and long-term memory, it is not known whether environmental enrichment can modify the PKA dependence of LTP in area CA1. In this study, we have addressed the following question: Can exposure to a spatially enriched environment alter the PKA-dependence of LTP in area CA1?
RESULTS
LTP is Enhanced by a Prolonged Period of Spatial Enrichment
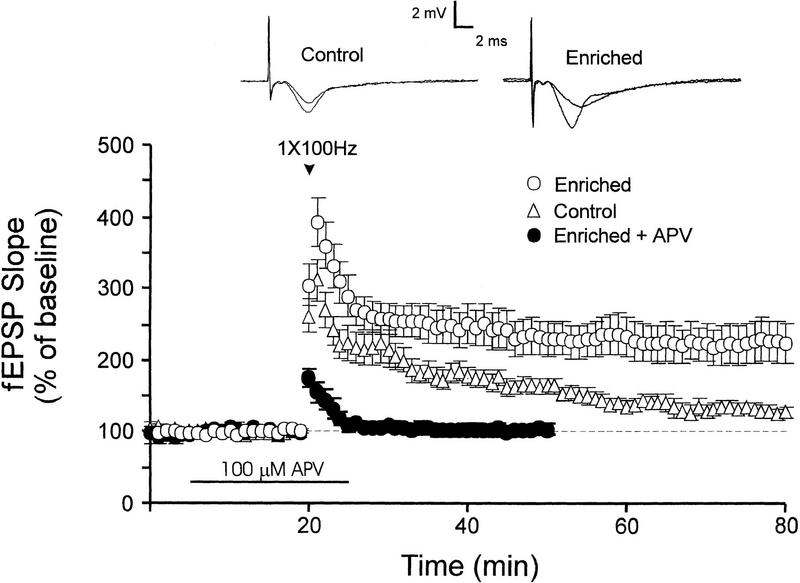
Recent studies have shown that exposure of mice and rats to spatially enriched environments for several weeks can modify neurogenesis, learning, and synaptic strength in the hippocampus (Foster et al. 1996; Kempermann et al. 1997, 1998). We therefore examined the effect of 8 wk of exposure to an enriched environment on hippocampal LTP in area CA1. Hippocampal slices from mice kept in an enriched environment for 8 wk showed robust LTP following just a single 1-sec burst of 100-Hz stimulation; the mean fEPSP slope measured 1 h after induction was 238% ± 36% of baseline (Fig. 1). In contrast, LTP was significantly less robust in slices from age-matched mice reared for 8 wk in standard cages (Fig. 1, open triangles); the mean fEPSP slope was 127% ± 8% of baseline 1 h after LTP induction (P < 0.01 compared to enriched group). In slices from enriched mice, LTP was abolished by the NMDA receptor antagonist, APV (P < 0.001; filled circles, Fig. 1), indicating that LTP in slices from enriched mice was dependent on activation of NMDA receptors. Slices from control nonenriched mice also showed NMDA receptor-dependent LTP following a single burst of 100-Hz stimulation; mean fEPSP slopes in control and APV-treated slices were 175% ± 18% and 105% ± 9% of baseline, respectively, at 20 min post-induction (P < 0.05; Fig. 1).
Figure 1.
LTP is enhanced following spatial enrichment for 8 wk. LTP induced by a single 1-sec burst of 100-Hz stimulation (1-sec duration) is less robust in slices from mice housed in standard cages (open triangles, n = 13 mice, 13 slices) than in slices from mice housed in spatially enriched cages (open circles, n = 10 mice, 13 slices). LTP in enriched slices was blocked by an antagonist of NMDA receptors, APV (100 μM; closed circles, n = 7 mice, nine slices). Sample fEPSP traces were recorded from slices 50 min after LTP induction.
Basal synaptic transmission was unaltered by spatial enrichment; the average ratio of fEPSP slope to presynaptic fiber volley amplitude in enriched slices (4.58 ± 0.15 msec−1, n = 7 mice, 20 slices) was not significantly different from the mean ratio measured from nonenriched controls (4.45 ± 0.15 msec−1, n = 7 mice, 19 slices, P > 0.5).
Glutamatergic Synaptic Currents and Spike Frequency Accommodation in Hippocampal Pyramidal Neurons
Activation of NMDA receptors and the resultant Ca2+ influx are necessary for LTP induction in area CA1 (Collingridge et al. 1983; Malenka et al. 1988). Spatial enrichment may facilitate induction of LTP by increasing NMDA receptor currents or by enhancing neuronal membrane excitability. To test this hypothesis, we performed “blind” whole-cell patch clamp recordings on CA1 pyramidal neurons in hippocampal slices from enriched and control mice. We measured evoked postsynaptic glutamatergic currents to determine whether the size of the NMDA receptor-mediated component was altered relative to the non-NMDA receptor-mediated component (Fig. 2a1,a4). We found no significant differences between the relative proportions of NMDA and non-NMDA currents present in cells from control and enriched slices; the mean ratios of NMDA to non-NMDA currents were 0.24 ± 0.03 (n = 32 cells, 13 mice) and 0.27 ± 0.05 (n = 21 cells, six mice), respectively (P > 0.1; Fig. 2a4).
Figure 2.
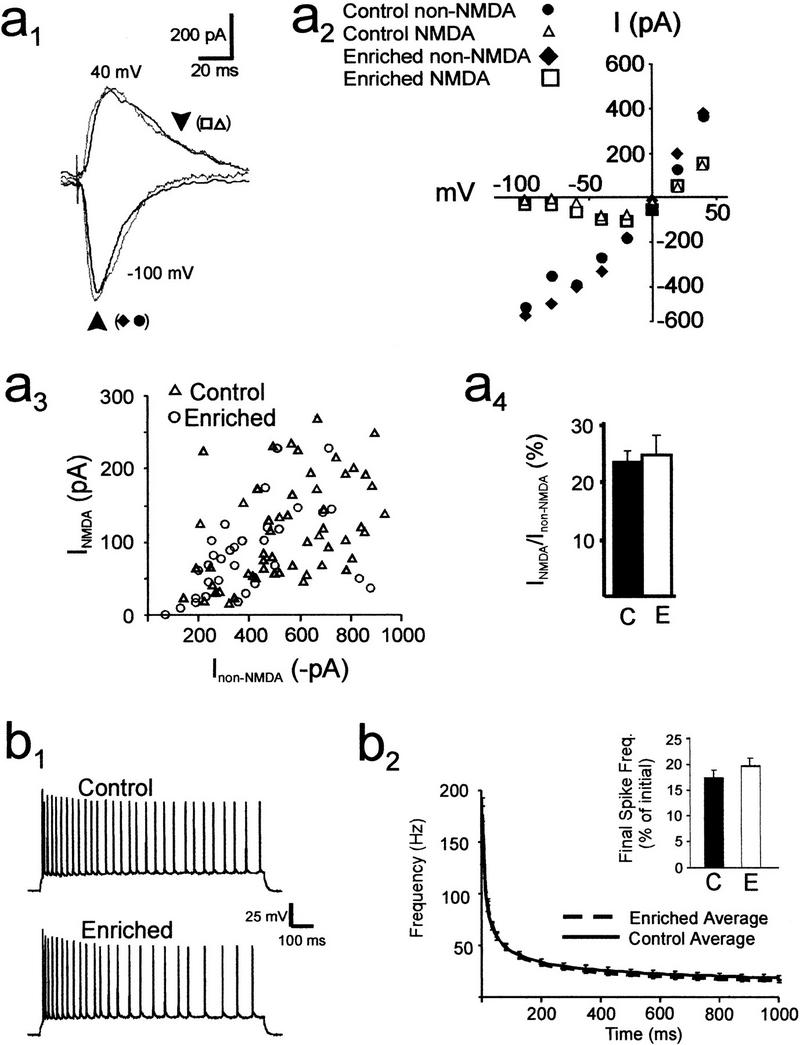
Biophysical and electrophysiological properties of CA1 neurons. (a1) Superimposed sample traces of excitatory postsynaptic currents, evoked by stimulation of Schaffer collateral fibers, in voltage-clamped CA1 pyramidal cells from control (black) and 8-wk enriched mice (gray). Cells were held at −100 mV (lower traces) and +40 mV (upper traces). Arrows demarcate time points for measurements of current amplitudes shown in a2 and a3. The lower arrow indicates peak non-NMDA current, while the upper arrow, placed 35 msec after the peak, is the estimate of NMDA current amplitude. (a2) I-V plot for peak current and the current at 35 msec after the peak in enriched and control cells. (a3) Scatter plot of peak non-NMDA (VM = −100 mV) versus NMDA (VM = +40 mV) currents elicited at various stimulus intensities in CA1 pyramidal neurons from control (open triangles, 32 cells from 13 mice) and enriched mice (open circles, 21 cells from six mice). (a4) The average ratio (±SEM) of NMDA to non-NMDA current amplitudes was derived from the scatter plot in a3 and showed no significant difference between cells from control (C) and enriched (E) mice. (b1) Action potentials in pyramidal CA1 neurons evoked by a 1-sec pulse of intracellular current injection. (b2) Plot of average instantaneous spike frequency (Hz) versus time after the start of a current pulse. No significant differences in these curves were observed for CA1 pyramidal neurons from control (solid line, n = 16 cells from 12 mice) and enriched slices (broken line, n = 19 cells from six mice; P > 0.2). Inset bar graph: histogram showing the mean spike frequency measured during the last 400 msec of a spike train, expressed as a percentage of the average spike frequency measured during the initial 50 msec of the spike train. No significant difference was observed between the two groups (C = control, E = enriched).
Current-clamp recordings revealed no significant changes in membrane excitability. Neither the mean membrane resting membrane potential (Em) nor the average membrane input resistance (Rin) was altered by spatial enrichment (control Em = −63 ± 1 mV, Rin = 124 ± 10 Mohms, n = 24; enriched Em = −63 ± 1 mV, Rin = 124 ± 12 Mohms, n = 15; P > 0.3 for all control vs. enriched comparisons). Also, the average number of action potentials measured in response to a depolarizing current pulse (300 pA for 1 sec) was not significantly different between the two groups (control: 28 ± 3 spikes, n = 16; enriched: 26 ± 2 spikes, n = 19; P > 0.3).
Spike frequency accommodation in hippocampal neurons is a reduction in spike firing rate observed during a sustained stimulus (Schwartzkroin 1977). Experience-induced decreases in spike accommodation may enhance spike firing and synaptic transmission between neurons. We hypothesized that spatial enrichment might alter spike accommodation in CA1 pyramidal neurons. We measured spike frequency accommodation in CA1 neurons as the ratio of the average spike frequency during the final 400 msec of a spike train to the average spike frequency during the first 50 msec of the spike train. In slices from control and enriched mice, the mean ratios were 0.17 ± 0.03 and 0.19 ± 0.04, respectively (P > 0.1; inset histogram, Fig. 2b2). Thus, spatial enrichment under our conditions did not significantly modify spike frequency accommodation in CA1 pyramidal neurons.
Spatial Enrichment Alters the PKA Dependence of LTP in Area CA1
The absence of significant changes in membrane biophysical properties, spike frequency accommodation, and glutamatergic receptor currents in hippocampal neurons of enriched mice prompted further investigation into other possible molecular mechanisms that may underlie the enhanced LTP seen in slices from enriched mice. In particular, we hypothesized that the dependence of LTP expression on particular signaling pathways may be modified by environmental enrichment under these conditions. One specific signaling pathway that has been implicated in the expression of some forms of LTP in area CA1 is the cAMP-PKA pathway.
It is believed that activation of the cAMP-PKA signaling pathway is critical for the expression of some forms of LTP in area CA1 (Huang and Kandel 1994; Blitzer et al. 1995; Abel et al. 1997; Wong et al. 1999). PKA plays a critical role in the expression of LTP induced by strong, multiburst stimulation, whereas LTP produced by a single burst of 100-Hz stimulation is less dependent on hippocampal PKA activity (Huang and Kandel 1994; Blitzer et al. 1995; Abel et al. 1997; Otmakhova et al. 2000). One consequence of exposure to a spatially enriched environment may be altered (increased) PKA dependence of single-burst LTP. To test this hypothesis, we first attempted to block single-burst LTP in enriched slices with a specific inhibitor of PKA. In control slices, a PKA inhibitor, Rp-cAMPS (Dostmann 1995), did not alter LTP evoked by a single 100-Hz burst of stimulation (Fig. 3a). In contrast, the amount of LTP observed in hippocampal slices from enriched mice was reduced by Rp-cAMPS (Fig. 3b; P < 0.05) to levels not significantly different from LTP measured in slices from nonenriched mice (Fig. 3c). Hence, these data show that expression of single-burst LTP in slices from enriched mice is PKA dependent, whereas this same single-burst stimulation protocol induced a form of LTP in nonenriched slices that is less dependent on PKA.
Figure 3.
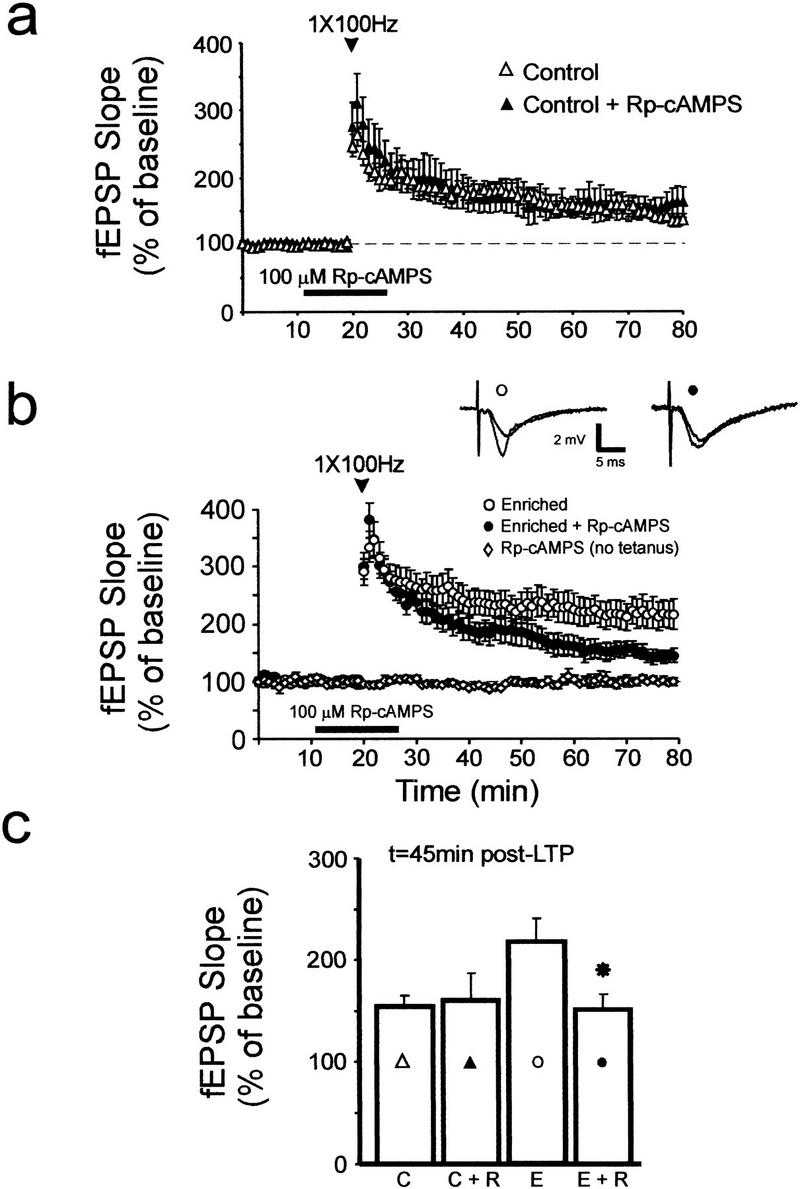
LTP in slices from enriched mice is attenuated by an inhibitor of PKA. (a) A specific inhibitor of PKA, Rp-cAMPS (100 μM), did not alter LTP in slices from control mice (control: open triangles, n = 13 mice, 13 slices; control + Rp-cAMPS: closed triangles, n = 9 mice, nine slices). (b) Rp-cAMPS attenuated LTP in slices from enriched mice (enriched: open circles, n = 11 mice, 13 slices; enriched + Rp-cAMPS: closed circles, n = 8 mice, eight slices). Sample fEPSP traces were recorded from enriched slices in the absence (open circle) and presence (closed circle) of Rp-cAMPS, just before and 1 h after LTP induction. (c) Summary histogram showing mean fEPSP slopes measured 45 min after LTP induction in control (C) and enriched slices (E) in the absence and presence of Rp-cAMPS (R). An asterisk indicates P < 0.05 for comparison with the enriched group (E).
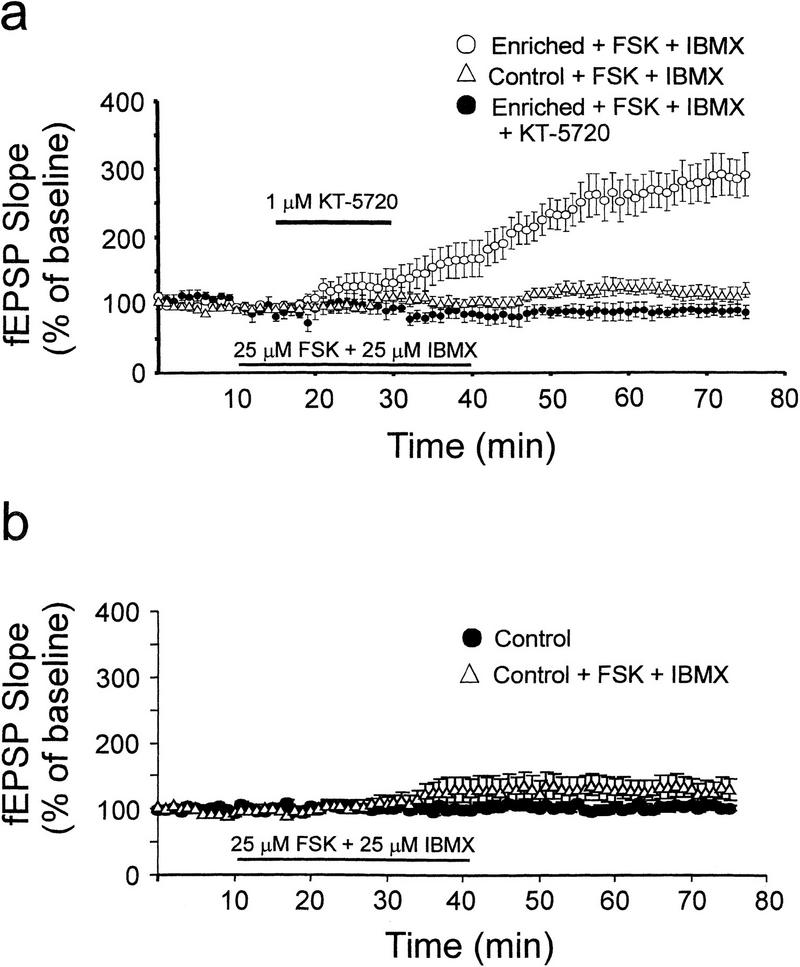
Additional evidence in support of a PKA-dependent enhancement of synaptic potentiation following enrichment was provided by comparing cAMP-induced synaptic facilitation in enriched and control slices. Conjoint application of forskolin and IBMX can facilitate synaptic transmission in hippocampal slices in a cAMP-dependent manner (Chavez-Noriega and Stevens 1992). Forskolin activates adenylate cyclase (Daly et al. 1982), whereas IBMX inhibits phosphodiesterases (Smellie et al. 1979). These drugs activate PKA by raising intracellular cAMP levels. To test the notion that cAMP-dependent synaptic facilitation may be altered following enrichment, we measured fEPSP responses during acute treatment of slices with forskolin and IBMX (25 μM each; Fig. 4). In control, nonenriched slices, treatment with forskolin and IBMX, at 25 μM each, elicited a mild but significant facilitation of synaptic efficacy; the mean fEPSP slope measured from control slices with forskolin/IBMX was 132% ± 12% at 50 min after the start of drug application, whereas the corresponding mean slope measured in control, nonenriched slices was only 100% ± 7% (P < 0.05; Fig. 4b). Also, the mean fEPSP slope measured following forskolin/IBMX treatment was significantly lower in control slices (132% ± 17% of baseline, measured 50 min after the onset of drug application; Fig. 4a) than in enriched slices (256% ± 29% of baseline; P < 0.001). In enriched slices, the synaptic facilitation elicited by forskolin and IBMX was blocked by a specific inhibitor of the catalytic subunit of PKA, KT-5720 (Kase et al. 1987) (P < 0.001; Fig. 4a).
Figure 4.
Differential synaptic facilitation elicited by forskolin and IBMX. (a) Conjoint application of an adenylate cyclase activator, forskolin (FSK, 25 μM), and a phosphodiesterase inhibitor, IBMX (25 μM), increased fEPSP slopes to a greater degree in slices from enriched mice (open circles, n = 8 mice, 11 slices) than in slices from control mice (open triangles, n = 7 mice, 10 slices). A PKA inhibitor, KT-5720, attenuated the synaptic facilitation in enriched slices (closed circles, n = 5 mice, eight slices). (b) Co-application of forskolin (FSK) and IBMX (25 μM each) significantly increased fEPSP slopes in nonenriched slices (open triangles, n = 7 mice, eight slices), whereas no significant change in mean fEPSP slope was observed in control, nonenriched slices in the absence of forskolin and IBMX (closed circles, n = 6 mice, eight slices).
The more robust synaptic facilitation seen in enriched slices following treatment with forskolin and IBMX suggests that spatial enrichment increases the efficacy of cAMP/PKA-dependent forms of synaptic facilitation. It is noteworthy that IBMX blocks adenosine receptors and inhibits phosphodiesterases in the hippocampus (Smellie et al. 1979). Both actions of IBMX can increase cAMP levels, as tonic activation of adenosine receptors in area CA1 (Dunwiddie and Hoffer 1980) is associated with inhibition of adenylate cyclase (Fredholm et al. 1983; Dunwiddie and Fredholm 1989; Chavez-Noriega and Stevens 1992). Our block of forskolin/IBMX-induced synaptic facilitation in enriched slices by a specific inhibitor of PKA confirms that this enhancement of synaptic transmission was mediated through PKA.
These data complement our finding that Rp-cAMPS attenuated stimulation-induced LTP in enriched slices, and the data also provide strong support for the hypothesis that spatial enrichment modifies the PKA dependence of LTP in area CA1 and increases the efficacy of PKA-dependent forms of synaptic facilitation.
Memory for Contextual Fear Conditioning is Improved in Enriched Mice
Memory for contextual fear conditioning is dependent on both the hippocampus and the amygdala, while a related task, cued fear conditioning, requires only the amygdala for its expression (Phillips and LeDoux 1992; Kim et al. 1993; Holland and Bolton 1999). Both tasks require only a single training trial for robust learning to occur, and memory can be tested by measuring freezing behavior 24 h after a training session (e.g., Abel et al. 1997).
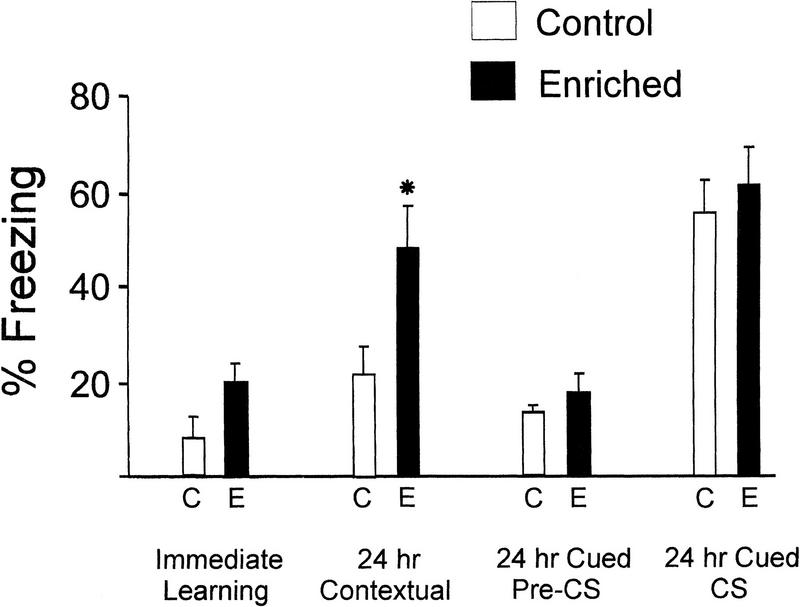
Does spatial enrichment under the present conditions improve memory for contextual fear conditioning? To address this question, we compared fear conditioning between mice that had experienced spatial enrichment for 8 wk and mice that were housed for the same period of time in nonenriched cages. We found that immediate learning following presentation of the unconditioned stimulus (US; footshock) was slightly, but not significantly, increased in enriched mice (Fig. 5). Levels of freezing, measured during a 30-sec time period immediately after the US, were 20% ± 4% for enriched mice and 8% ± 4% for control mice (P > 0.1, n = 7 for controls, n = 10 for enriched group). However, memory for contextual fear conditioning (measured 24 h posttraining) was significantly enhanced in enriched mice (Fig. 5). Levels of freezing were 48% ±9% for enriched and 22% ± 6% for control mice (n = 10 for each group; P < 0.01).
Figure 5.
Contextual and cued fear conditioning. Mice reared in an enriched environment for 8 wk (dark bars) displayed significantly enhanced memory for contextual fear conditioning (24 h contextual) as compared to control mice kept in standard nonenriched cages (open bars; * P < 0.01). Ordinate shows freezing behavior expressed as a percentage of total time spent in the test chamber. Immediate learning, measured after presentation of the unconditioned stimulus, was slightly, but not significantly, increased by enrichment. Memory for cued fear conditioning, measured before and during the CS (24 h pre-CS and 24 h CS, respectively), was not significantly different between enriched and control mice. CS = conditioned stimulus (tone).
Conditioning was also compared between enriched and control mice during testing for memory for cued fear conditioning. At 24 h after training, the levels of freezing observed before presentation of the conditioned stimulus (CS; tone) were not significantly different between enriched and control mice (“pre-CS” bars, Fig. 5). Levels of freezing were 18% ± 5% for enriched and 13% ± 3% for control mice (n = 7 for controls, n = 10 for enriched group, P > 0.2). During presentation of the CS, the levels of freezing behavior also did not differ significantly between these two groups (CS bars, Fig. 5); the measured values were 62% ± 8% for enriched and 56% ± 7% for control mice (n = 10 for each group, P > 0.1).
In summary, enriched mice showed enhanced memory for contextual fear conditioning when compared to control mice. Memory for cued fear conditioning, which requires the amygdala, was not significantly altered by spatial enrichment under these conditions. Hence, these data show that enrichment improves hippocampus-dependent contextual fear conditioning in mice (see also Rampon et al. 2000).
DISCUSSION
Our data indicate that significant enhancement of LTP can occur in hippocampal area CA1 following 8 wk of spatial enrichment. Enhanced LTP in slices from enriched mice was attenuated by an inhibitor of PKA, and greater forskolin/IBMX-induced (and PKA-dependent) synaptic facilitation was observed in slices from enriched mice. These experience-induced changes in synaptic efficacy were not associated with altered biophysical or electrophysiological characteristics of postsynaptic CA1 pyramidal neurons.
Previous studies have shown that synaptic potentiation induced by a single burst of 100-Hz stimulation in area CA1 is less sensitive to inhibitors of PKA than LTP induced by multiple 100-Hz bursts (Huang and Kandel 1994; Blitzer et al. 1995; Otmakhova et al. 2000). Our observation that slices from enriched mice showed larger single-burst LTP than slices from control nonenriched mice suggests that the threshold for induction of LTP may have decreased after 8 wk of spatial enrichment. In addition, the enhancement of LTP seen in enriched slices was attenuated by an inhibitor of PKA. These findings indicate that exposure to spatial enrichment under the present conditions can elicit an experience-dependent transformation of the pharmacological profile of single-burst LTP in area CA1, such that this modified form of single-burst LTP resembles multiburst LTP in its requirement for PKA.
Our finding that the sizes of synaptically evoked glutamatergic currents were not significantly altered after 8 wk of enrichment suggests that substantial, long-lasting increases in the amplitudes of NMDA- or AMPA-receptor currents are not critically involved in causing the enhanced LTP seen in enriched slices. This is consistent with a previous study that showed no changes in the expression levels of GluR1 and GluR2/3 proteins in hippocampal membranes of spatially enriched animals (Gagne et al. 1998). Furthermore, we observed no significant changes in membrane excitability, mean resting potential, mean input resistance, or spike frequency accommodation in postsynaptic CA1 pyramidal neurons. However, subtle alterations in the regulation of glutamatergic receptor function cannot be ruled out.
Previous studies have shown that running per se enhances neurogenesis and LTP in the dentate gyrus, but not in area CA1, of mouse hippocampal slices (van Praag et al. 1999). These studies did not examine the effects of spatial complexity on CA1 LTP in the absence of running activity (i.e., an enriched environment without a running wheel). One reason for the difference between the CA1 LTP data of van Praag et al. (1999) and our results is that we have used a single burst of 100-Hz stimulation to induce LTP, whereas van Praag et al. used a stronger tetra-burst protocol. Tetra-burst stimulation induces persistent LTP in area CA1, even in slices from aged, senescent mice (Bach et al. 1999). Hence, this stimulation protocol may saturate or strongly activate synaptoplastic processes in area CA1, and such saturation may mask more subtle modifications in the expression, or threshold for induction, of LTP. A single-burst, 100-Hz stimulation protocol may be better suited for examining putative experience-induced changes in the expression or induction threshold of LTP (see Barad et al. 1998).
Differences between our data and those of previous studies on the synaptoplastic effects of enrichment (e.g., Green and Greenough 1986; Foster et al. 1996) may reflect variations in experimental protocols. In this study, area CA1 was examined, whereas synaptic physiology was studied in the dentate gyrus of hippocampal slices in previous work (Green and Greenough 1986; Foster et al. 1996). Some forms of physiological plasticity in the dentate gyrus, such as LTP, may be less susceptible to robust modifications or are less readily detectable following environmental manipulations or slice stimulation (see Nguyen and Kandel 1996). Also, differences in the duration of exposure to enrichment, species, gender, and age of animals used in all of these studies may influence the amount of synaptoplasticity detected after enrichment. For example, we have used young female mice in this study, whereas male rats were used in previous work on enrichment-induced synaptic modifications (Foster et al. 1996). Male mice were not examined in the present study because aggressive behavior (fighting) among male mice may confound the interpretation of our spatial enrichment experiments. Also, differences between the genetic backgrounds of animals exposed to enrichment might have an important impact on the amount of synaptic plasticity seen following enrichment (Haemisch et al. 1994; van de Weerd et al. 1994; Nguyen et al. 2000a,b). Hence, the interactions of multiple factors (such as stress, hormones, handling, genetic background, development, and exercise) may importantly influence the outcomes of experiments using spatial enrichment to explore mechanisms of neural and cognitive plasticity.
What specific aspects of an enriched environment caused the changes in synaptic plasticity reported here? It may be impossible to clearly dissociate the contributions (to experience-induced plasticity) of motor activity per se from increased sensory stimulation that occurs during exploration of inanimate objects in an enriched environment. Exploration of a spatially enriched environment almost invariably involves some physical activity, and such activity will stimulate muscle sensory receptors and might increase the synthesis and release of neurotrophic chemicals (Neeper et al. 1995; Torasdotter et al. 1998). These neurotrophic factors may then modulate the activity and plasticity of neurons (Kang and Schuman 1996; Boulanger and Poo 1999).
In this study, we used similar-sized cages with identical animal group sizes and spatial enrichment conditions. The food provided to enriched and control mice in this study was identical; hence, nutritional enrichment cannot explain our results. Finally, the enhancement of LTP seen here was evident after 8 wk of enrichment, but it was absent after only 2 wk of enrichment (S. Duffy, unpubl.). This suggests that the effects of acute handling stress may not be important in causing the synaptic changes seen here. Indeed, stress is associated with a decrease in LTP magnitude (Foy et al. 1987; Pavlides et al. 1993) and stress impairs hippocampus-dependent spatial learning (Luine et al. 1994). In contrast, we observed enhanced LTP magnitude and improved hippocampus-dependent memory for contextual fear conditioning after enrichment. In summary, these considerations, and our data, provide strong support for an important role of spatial enrichment in eliciting the enhancement of LTP seen here.
Numerous studies have shown that exposure of rodents to enriched environments can improve learning and memory (for review, see Rosenzweig and Bennett 1996; see also Kempermann et al. 1997; van Praag et al. 1999; Rampon et al. 2000). Our behavioral experiments revealed a significant improvement in memory for contextual fear conditioning in enriched mice. In contrast, memory for cued fear conditioning was unaffected in enriched mice. The neural bases for both types of fear conditioning have been identified: Contextual fear conditioning depends on the hippocampus and the amygdala, while cued fear conditioning relies on the amygdala alone (Phillips and LeDoux 1992; Kim et al. 1993; Holland and Bolton 1999). This suggests that spatial enrichment might have modified hippocampus-dependent processes that contribute to these behavioral changes. Such processes may include, but might not be restricted to, synaptic LTP, which was enhanced in slices from enriched mice.
Our behavioral data also indicate that experience-dependent enhancement of LTP in area CA1 may contribute to enrichment-induced improvement of hippocampus-dependent memory for contextual fear conditioning. Transgenic mice with genetically reduced levels of hippocampal PKA activity show defective maintenance of LTP and impaired hippocampus-dependent long-term memory for contextual fear conditioning (Abel et al. 1997). In this study, enhancement of LTP in slices from enriched mice was attenuated by Rp-cAMPS, whereas LTP in nonenriched slices was unaffected by this same inhibitor of PKA. It is noteworthy that the persistence of single-burst LTP in mouse hippocampal slices can also be facilitated by pharmacological enhancement of levels of hippocampal cAMP (Barad et al. 1998). Also, memory formation during contextual fear conditioning of mice is blocked by intracranial injection of Rp-cAMPS (Bourtchouladze et al. 1998). Further work is needed to determine whether the improved hippocampus-dependent memory seen here following enrichment is affected by PKA inhibitors. Experience-dependent plasticity of the PKA dependence of hippocampal LTP may be important for regulating the efficacy of hippocampus-based memory.
MATERIALS AND METHODS
Spatial Enrichment
C57BL/6J mice (female, age 4 wk, Jackson Labs) were housed in groups of seven in 47 × 37 × 21-cm cages, each containing one 40-cm hollow plastic J-shaped tunnel; three linked 5-cm-diameter, metallic swinging rings (Tarzan rings); one 13-cm-diameter metallic running wheel; short, hollow plastic tubes for burrowing; and one two-story colored, hollow plastic house (10 × 12 × 14 cm) with multiple entrances/exits accessible to mice. Age-matched mice were housed in groups of seven in similar-sized cages without these added spatial components. Mice were housed for 8 consecutive wk before electrophysiological or behavioral experimentation. A shorter duration of enrichment has been used to produce experience-related changes in neurogenesis and synaptic efficacy (Kempermann et al. 1997). Multiple hippocampal slices from a given animal were used for the different types of experiments described in the Results section (i.e., different treatments were interleaved between separate slices from the same animal). Both enriched and nonenriched groups of mice received identical types of rodent chow and water ad libitum, and they were kept on a 12-h light/dark cycle. All mice were maintained in the animal care facility at the University of Alberta, consistent with CCAC guidelines.
Electrophysiology
Electrophysiological experiments were performed over a period of one to four consecutive days after 8 wk of environmental enrichment. Hippocampal slices (400 μm thickness) were prepared and maintained at 28°C in an interface chamber, as described in Nguyen and Kandel (1997). Slices were superfused with artificial cerebrospinal fluid (ACSF, 1 mL/min flow rate) containing (in mM) 125 NaCl, 4.4 KCl, 1.5 MgSO4, 1 NaH2PO4, 26 NaHCO3, 10 glucose, 2.5 CaCl2. Extracellular recordings of excitatory postsynaptic field potentials (fEPSPs) were obtained from stratum radiatum of area CA1 with 2–4 MΩ glass microelectrodes filled with ACSF. The Schaffer collateral pathway was stimulated with a bipolar nickel-chromium electrode positioned in stratum radiatum of area CA1. fEPSPs were elicited at a stimulus intensity (0.08-msec pulse width) sufficient to produce fEPSP amplitudes that were 40% of maximal sizes. Pre-LTP baseline was measured by delivering single pulses of stimulation once per minute. A single 100-Hz burst (1-sec duration) of stimulation was used to induce LTP.
Hippocampal CA1 neurons were patch-clamped in the “blind” whole-cell mode (Blanton et al. 1989) using an Axopatch-1D amplifier and PClamp-7 software (Axon Instruments). All cells were in the CA1 pyramidal cell layer, as judged by visual placement of the patch-clamp electrode. Spike frequency accommodation was used as the physiological criterion for identifying these neurons as pyramidal cells (Kandel and Spencer 1961; Schwartzkroin 1977; Schwartzkroin and Mathers 1978). Cells with resting potentials more depolarized than −60 mV or spike amplitudes less than 80–90 mV were rejected. The patch electrode's internal solution for current-clamp recording contained (in mM) 130 potassium gluconate, 10 HEPES, 10 NaCl, 5 MgCl2, 0.05 CaCl2, 2 NaATP, 0.3 NaGTP (pH = 7.3). For voltage-clamping, the electrode's internal solution was slightly modified from that of Hestrin et al. (1990), and it contained (in mM) 130 CsF, 10 HEPES, 10 NaCl, 10 EGTA, 5 lidocaine N-ethyl bromide (QX-314; pH = 7.3). Fluoride was used because it enhanced the duration of these recordings. Similar recordings were obtained with potassium phosphate in the electrode solution, and no substantial differences in our results were observed for these two types of intracellular solutions (see also Hestrin et al. 1990). For voltage-clamp measurements of glutamatergic currents, GABAA currents were blocked with bath-applied 10 μM picrotoxin (RBI).
Rp-adenosine 3′,5′-cyclic monophosphothioate triethylamine (Rp-cAMPS), forskolin, 3-isobutyl-1-methylxanthine (IBMX), and D(-)-2-amino-5-phosphonopentanoic acid (APV) were purchased from RBI. KT-5720 was purchased from Biomol. Drugs were bath applied at 1 mL/min. All drugs, except for Rp-cAMPS and APV, were prepared as stock solutions in DMSO and were diluted in ACSF to the desired concentrations. The final DMSO concentration was 0.1% and did not affect synaptic responses in our experiments. Rp-cAMPS and APV were dissolved in ACSF to the desired concentrations. For some experiments with Rp-cAMPS, fEPSPs were recorded during stimulation (once per minute at test intensity) of an adjacent, untetanized pathway in stratum radiatum, as described in Nguyen et al. (1994).
Student's t-test was used for statistical comparisons of mean fEPSP slopes or cellular biophysical characteristics between slices from enriched and nonenriched mice or between drug-treated and drug-free slices.
Contextual and Cued Fear Conditioning
Fear conditioning experiments were performed as described in Abel et al. (1997). For training, a mouse was placed in a conditioning chamber (Med Associates) for 2 min before the onset of a conditioned stimulus (CS; an 85-dB tone), which lasted for 30 sec. The last 2 sec of the CS was paired with an unconditioned stimulus (US), consisting of footshock (0.7 mA). Each mouse then remained in the chamber for an additional 30 sec with no further presentation of the CS. This training protocol was identical for all behavioral experiments. Following this training regimen, each mouse was returned to its home cage. Testing for memory for contextual fear conditioning was performed 24 h after training by measuring freezing behavior during a 5-min extinction test in the conditioning chamber. Freezing was defined as a complete lack of movement in 5-sec intervals. For tests of memory for cued fear conditioning, the mice were placed, 24 h after training, in a novel context for 2 min, after which they were exposed to the CS for 3 min. Freezing behavior was scored throughout the testing session. Student's t-test was used to compare the amounts of behavioral freezing (expressed as a percentage of total time spent in the conditioning chamber) displayed by enriched and control mice during testing of memory for contextual or cued fear conditioning. All statistical values cited are means ± standard error of the mean (SEM).
Acknowledgments
We thank Newton Woo for help with preparing the manuscript. This research was supported by grants and funds from the National Institutes of Health (T.A.), the Alberta Heritage Foundation for Medical Research (AHFMR), the Canadian Institutes of Health Research (CIHR), and the University of Alberta Faculty of Medicine (the latter three to P.V.N.). S.N.D. holds a Fellowship from the AHFMR, and K.J.C. was supported by a Summer Studentship from the AHFMR. T.A. is a John Merck Scholar. P.V.N. is a Scholar of the AHFMR and a New Investigator of the CIHR.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL Peter.Nguyen@ualberta.ca; FAX (780) 492-8915.
Article and publication are at www.learnmem.org/cgi/doi/10.1101/lm.36301.
REFERENCES
- Abel T, Nguyen PV, Barad M, Deuel TAS, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by grugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Meth. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate of the anesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Boulanger L, Poo MM. Gating of BDNF-induced synaptic potentiation by cAMP. Science. 1999;284:1982–1984. doi: 10.1126/science.284.5422.1982. [DOI] [PubMed] [Google Scholar]
- Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- Chavez-Noriega LE, Stevens CF. Modulation of synaptic efficacy in field CA1 of the rat hippocampus by forskolin. Brain Res. 1992;574:85–92. doi: 10.1016/0006-8993(92)90803-h. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. The antagonism of amino acid–induced excitations of rat hippocampal neurones in vitro. J Physiol. 1983;334:19–31. doi: 10.1113/jphysiol.1983.sp014477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly JW, Padgett W, Seamon KB. Activation of cAMP-generating systems in brain membranes and slices by the diterpene forskolin: Augmentation of receptor-mediated responses. J Neurochem. 1982;38:532–544. doi: 10.1111/j.1471-4159.1982.tb08660.x. [DOI] [PubMed] [Google Scholar]
- Dostmann WRG. Rp-cAMPS inhibits the cAMP-dependent protein kinase by blocking the cAMP-induced conformational transition. FEBS Lett. 1995;375:231–234. doi: 10.1016/0014-5793(95)01201-o. [DOI] [PubMed] [Google Scholar]
- Dunwiddie TV, Fredholm BB. Adenosine A1 receptors inhibit adenylate cyclase activity and neurotransmitter release and hyperpolarize pyramidal neurons of rat hippocampus. J Pharmacol Exp Ther. 1989;249:31–37. [PubMed] [Google Scholar]
- Dunwiddie TV, Hoffer BJ. Adenine nucleotides and synaptic transmission in the in vitro rat hippocampus. Brit J Pharmacol. 1980;69:59–68. doi: 10.1111/j.1476-5381.1980.tb10883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Silva AJ. Molecular mechanisms of synaptic plasticity and memory. Curr Opin Neurobiol. 1999;9:209–213. doi: 10.1016/s0959-4388(99)80029-4. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Fernandez-Tereul A, Tobena A, Vivas NM, Marmol F, Badia A, Dierssen M. Early environmental stimulation produces long-lasting changes on β-adrenoceptor transduction systems. Neurobiol Learn Mem. 1995a;64:49–57. doi: 10.1006/nlme.1995.1043. [DOI] [PubMed] [Google Scholar]
- Escorihuela RM, Tobena A, Fernandez-Tereul A. Environmental enrichment and postnatal handling prevent spatial learning deficits in aged hypoemotional (Roman high-avoidance) and hyperemotional (Roman low-avoidance) rats. Learn Mem. 1995b;2:40–48. doi: 10.1101/lm.2.1.40. [DOI] [PubMed] [Google Scholar]
- Foster TC, Gagne J, Massicotte G. Mechanism of altered synaptic strength due to experience: Relation to long-term potentiation. Brain Res. 1996;736:243–250. doi: 10.1016/0006-8993(96)00707-x. [DOI] [PubMed] [Google Scholar]
- Foy MR, Stanton ME, Levine S, Thompson RF. Behavioral stress impairs LTP in rodent hippocampus. Behav Neural Biol. 1987;48:138–149. doi: 10.1016/s0163-1047(87)90664-9. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Jonzon B, Lindstrom K. Adenosine receptor–mediated increases and decreases in cAMP in hippocampal slices treated with forskolin. Acta Physiol Scand. 1983;117:461–463. doi: 10.1111/j.1748-1716.1983.tb00022.x. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Gagne J, Gelinas S, Baudry M, Massicotte G, Martinoli MG, Foster TC, Ohayon M, Thompson RF, et al. AMPA receptor properties in adult rat hippocampus following environmental enrichment. Brain Res. 1998;799:16–25. doi: 10.1016/s0006-8993(98)00451-x. [DOI] [PubMed] [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973;82:157–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Green EJ, Greenough WT. Altered synaptic transmission in dentate gyrus of rats reared in complex environments: Evidence from hippocampal slices maintained in vitro. J Neurophysiol. 1986;55:739–750. doi: 10.1152/jn.1986.55.4.739. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Volkman FR. Pattern of dendritic branching in occipital cortex of rats reared in complex environments. Exp Neurol. 1973;40:491–504. doi: 10.1016/0014-4886(73)90090-3. [DOI] [PubMed] [Google Scholar]
- Haemisch A, Voss T, Gartner K. Effects of environmental enrichment on aggressive behavior, dominance hierarchies, and endocrine states in male DBA/2J mice. Physiol Behav. 1994;56:1041–1048. doi: 10.1016/0031-9384(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. J Physiol (Lond) 1990;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland PC, Bolton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol. 1999;9:195–202. doi: 10.1016/s0959-4388(99)80027-0. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and PKA-dependent LTP in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Functional architecture of macaque monkey visual cortex. Proc R Soc Lond B. 1977;198:1–59. doi: 10.1098/rspb.1977.0085. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Spencer WA. Electrophysiology of hippocampal neurons. II. After-potentials and repetitive firing. J Neurophysiol. 1961;24:243–259. doi: 10.1152/jn.1961.24.3.243. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. A requirement for local protein synthesis in neurotrophin-induced hippocampal synaptic plasticity. Science. 1996;273:1402–1406. doi: 10.1126/science.273.5280.1402. [DOI] [PubMed] [Google Scholar]
- Kase H, Iwahashi K, Nakanishi S, Matsuda Y, Yamada K, Takahashi M, Murakata C, Sato A, Kaneko M. K-252 compounds, novel and potent inhibitors of PKC and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987;142:436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- ————— Experience-induced neurogenesis in the senescent dentate gyrus. J Neurosci. 1998;18:3206–3212. doi: 10.1523/JNEUROSCI.18-09-03206.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Rison RA, Fanselow MS. Effects of amygdala, hippocampus, and periaqueductal gray lesions on short- and long-term contextual fear. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Kauer JA, Zucker R, Nicoll RA. Postsynaptic calcium is sufficient for potentiation of hippocampal synaptic transmission. Science. 1988;242:81–84. doi: 10.1126/science.2845577. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RGM. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Micheau J, Riedel G. Protein kinases: Which one is the memory molecule? Cell Mol Life Sci. 1999;55:534–548. doi: 10.1007/s000180050312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper SA, Gomez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of LTP requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ————— Brief θ-burst stimulation induces a transcription-dependent late phase of LTP requiring cAMP in area CA1 of the mouse hippocampus. Learn Mem. 1997;4:230–243. doi: 10.1101/lm.4.2.230. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000a;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Duffy SN, Young JZ. Differential maintenance and frequency-dependent tuning of LTP at hippocampal synapses of specific strains of inbred mice. J Neurophysiol. 2000b;84:2484–2493. doi: 10.1152/jn.2000.84.5.2484. [DOI] [PubMed] [Google Scholar]
- O'Keefe J. A review of hippocampal place cells. Prog Neurobiol. 1979;13:419–439. doi: 10.1016/0301-0082(79)90005-4. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Otmakhov N, Mortenson LH, Lisman JE. Inhibition of the cAMP pathway decreases early LTP at CA1 hippocampal synapses. J Neurosci. 2000;20:4446–4451. doi: 10.1523/JNEUROSCI.20-12-04446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto T, Eichenbaum H, Wiener SI, Wible CG. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal LTP. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Watanabe Y, McEwen B. Effects of glucocorticoids on hippocampal LTP. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- Paylor R, Morrison SK, Rudy JW, Waltrip LT, Wehner JM. Brief exposure to an enriched environment improves performance on Morris water task and increases hippocampal cytosolic PKC activity. Behav Brain Res. 1992;52:49–59. doi: 10.1016/s0166-4328(05)80324-9. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Rampon C, Tang Y-P, Goodhouse J, Shimizu E, Kyin M. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nature Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behav Brain Res. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA. Further characteristics of hippocampal CA1 cells in vitro. Brain Res. 1977;128:53–68. doi: 10.1016/0006-8993(77)90235-9. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA, Mathers LH. Physiological and morphological identification of a nonpyramidal hippocampal cell type. Brain Res. 1978;157:1–10. doi: 10.1016/0006-8993(78)90991-5. [DOI] [PubMed] [Google Scholar]
- Smellie FW, Davis CW, Daly JW, Wells JN. Alkylxanthines: Inhibition of adenosine-elicited accumulation of cAMP in brain slices and of brain phosphodiesterase activity. Life Sci. 1979;24:2475–2482. doi: 10.1016/0024-3205(79)90458-2. [DOI] [PubMed] [Google Scholar]
- Stryker MP, Harris WA. Binocular impulse blockade prevents the formation of ocular dominance columns in cat visual cortex. J Neurosci. 1986;6:2117–2133. doi: 10.1523/JNEUROSCI.06-08-02117.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torasdotter M, Metsis M, Henriksson BG, Winblad B, Mohammed AH. Environmental enrichment results in higher levels of NGF mRNA in rat visual cortex and hippocampus. Behav Brain Res. 1998;93:83–90. doi: 10.1016/s0166-4328(97)00142-3. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Huerta PT, Tonegawa S. The essential role of hippocampal CA1 NMDA receptor-dependent synaptic plasticity in spatial memory. Cell. 1996;87:1327–1338. doi: 10.1016/s0092-8674(00)81827-9. [DOI] [PubMed] [Google Scholar]
- van de Weerd HA, Baumans V, Koolhaas JM, van Zutphen LF. Strain specific behavioral response to environmental enrichment in the mouse. J Exp Anim Sci. 1994;36:117–127. [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and LTP in mice. Proc Natl Acad Sci. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Wong ST, Athos J, Figueroa XA, Pineda VV, Schaefer ML, Chavkin CC, Muglia LJ, Storm DR. Calcium-stimulated adenylyl cyclase activity is critical for hippocampus-dependent long-term memory and late phase of LTP. Neuron. 1999;23:787–793. doi: 10.1016/s0896-6273(01)80036-2. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG. Human amnesia and the medial temporal region: Enduring memory impairment following a bilateral lesion limited to field CA1 of hippocampus. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]