Abstract
In bacteria, rapid changes in gene expression can be achieved by affecting the activity of RNA polymerase with small molecule effectors during transcription initiation. An important small molecule effector is the initiating nucleoside triphosphate (iNTP). At some promoters, an increasing iNTP concentration stimulates promoter activity, while a decreasing concentration has the opposite effect. Ribosomal RNA (rRNA) promoters from Gram-positive Bacillus subtilis are regulated by the concentration of their iNTP. Yet, the sequences of these promoters do not emulate the sequence characteristics of [iNTP]-regulated rRNA promoters of Gram-negative Escherichia coli. Here, we identified the 3′-promoter region, corresponding to the transcription bubble, as key for B. subtilis rRNA promoter regulation via the concentration of the iNTP. Within this region, the conserved −5T (3 bp downstream from the −10 hexamer) is required for this regulation. Moreover, we identified a second class of [iNTP]-regulated promoters in B. subtilis where the sequence determinants are not limited to the transcription bubble region. Overall, it seems that various sequence combinations can result in promoter regulation by [iNTP] in B. subtilis. Finally, this study demonstrates how the same type of regulation can be achieved with strikingly different promoter sequences in phylogenetically distant species.
INTRODUCTION
Rapid changes in gene expression can be mediated by changes in the concentration of the transcription initiating nucleoside triphosphate ([iNTP]). The concentration of the iNTP affects the efficiency of transcription initiation. This phenomenon has been documented for a number of bacterial promoters (1–5), for promoters in eukaryotic yeast cells (6,7) and the yeast mitochondrial COX2 promoter (8). The exact molecular mechanism of this regulation may be different for different promoters.
Here, we will focus on regulation of bacterial promoters by [iNTP] in which iNTP facilitates transcription initiation by shifting the equilibrium between initiation intermediates in the forward direction. During transcription initiation in bacteria, RNAP first binds to the promoter DNA and forms the closed complex in which the two DNA strands are still unwound. This step is followed by isomerization through at least one kinetic intermediate. Finally, RNAP melts the DNA and forms the open complex, which is then ready to align the iNTP with the base of the transcription +1 position of the template strand (9–11). Promoters that form relatively unstable open complexes with RNAP can be regulated by the intracellular concentration of the iNTP: an increasing concentration of iNTP stabilizes the open complex by binding of the iNTP and this has a stimulatory effect on promoter activity, whereas a decreasing concentration has the opposite effect (12,13). Such promoters are termed as [iNTP]-sensitive. Promoters that form stable open complexes are not regulated by the concentration of iNTP. The stability of the open complex is not rate limiting for transcription from these promoters. Changes in the intracellular concentration of their iNTPs do not affect them, because even the physiologically occurring low concentration is saturating. Such promoters are termed [iNTP]-insensitive (14).
An extensively studied example of [iNTP]-sensitive promoters are ribosomal RNA (rRNA) promoters from the Gram-negative bacterium Escherichia coli. Transcription from rRNA genes accounts for 70% of all cellular transcription in rapidly dividing cells and represents a major energy investment (10). When nutritional conditions change for the worse, the cell no longer needs massive amounts of new protein and must reduce its translational output to conserve resources/energy. Therefore, the synthesis of new ribosomes must be decreased or even stopped. Conversely, when cells suddenly encounter nutritionally propitious conditions, the synthesis of new ribosomes must be swiftly on. The synthesis of new ribosomes is controlled via the synthesis of rRNA. Regulation of rRNA expression occurs at the transcription initiation level. Regulation by the iNTP concentration is key for E. coli rRNA promoter activity changes during outgrowth from stationary phase and also contributes to the promoter shut-off during entry into stationary phase (15).
At the DNA level, the following sequence characteristics are typical for [iNTP]-sensitive rRNA promoters of E. coli: (i) the spacer between −10 and −35 conserved hexamers is suboptimal—16-bp long instead of the typical length of 17 bp (10); (ii) the −35 hexamer cannot be full consensus (TTGACA), and/or the extended −10 motif (TGX) cannot be present; (iii) the region between −10 and +1 is G/C-rich (the ‘discriminator’); and (iv) a cytosine nucleotide is present at position −7 (2 bp downstream from the −10 hexamer) of the non-template strand and the base makes suboptimal contacts with the 1.2 region of the housekeeping σ70 factor, which decreases the stability of the complex. Changes in these parameters (e.g. extending the spacer length to 17; adding the extended −10 motif; introducing mutations into the discriminator that decrease the G/C content; mutating the −7 C to any other base) result in the loss of regulation by [iNTP] (16–20).
We showed previously that rRNA promoters from the Gram-positive bacterium B. subtilis are regulated by the concentration of their iNTP (21). Unlike in E. coli, where the iNTP of rRNA promoters can be ATP, GTP or CTP (10), B. subtilis rRNA promoters initiate exclusively with GTP (21,22). This identity of the transcription +1 position of B. subtilis rRNA promoters has a physiological role. In some situations, such as the stringent response (starvation for amino acids), ATP and GTP concentrations change in opposite directions in B. subtilis: the ATP level increases while the GTP level decreases (23). Mutating the +1 position of rRNA promoters to an A alters the promoter response to one in the opposite direction. As +1C or +1T would likely not be efficiently utilized as transcription start sites, +1G is the only physiological choice for B. subtilis rRNA promoters. This concept also extends to other stringently regulated promoters, both downregulated (requiring +1G) and upregulated (requiring +1A) (14,24,25).
Another salient difference in the regulation of rRNA promoters between the two model organisms is that while a second small molecule effector, ppGpp, directly affects RNAP from E. coli, B. subtilis RNAP is not directly affected by this molecule. Rather, ppGpp negatively affects the concentration of GTP and thereby indirectly alters the activity of RNAP at rRNA promoters (21). Thus, GTP appears to be the sole direct small molecule effector acting on RNAP at rRNA promoters in B. subtilis.
Finally, the DNA sequence of B. subtilis rRNA promoters dramatically differs from their E. coli counterparts. The B. subtilis rRNA promoters display sequence elements that do not mimic the sequences of iNTP-regulated promoters of E. coli and yet they are [iNTP]-regulated. The characteristics of B. subtilis rRNA promoters are as follows: (i) the spacer region is 17 bp; (ii) the promoters contain the extended −10 motif; (iii) the region between the −10 hexamer and +1 is A/T-rich; and (iv) the −7 position is not a cytosine nucleotide.
As the rRNA promoter sequences of Gram-positive B. subtilis radically differ from their counterparts from Gram-negative E. coli, we decided to identify promoter DNA elements that are required for their regulation by [iNTP] in B. subtilis. In this study, we compare an [iNTP]-sensitive B. subtilis rRNA promoter with an [iNTP]-insensitive promoter and identify the 3′-region of the promoter (the 3′-promoter region is defined here with respect to the non-template strand) as the dominant DNA determinant of its sensitivity to [iNTP] both in vitro and in vivo. We demonstrate that this region affects the open complex stability. Within this region, the base at position −5 is important for this regulation. In addition, we identify a second class of [iNTP]-sensitive, non-rRNA promoters where the sequence determinants of this regulation are not limited to the promoter 3′-region. Finally, by a comparison of selected rRNA promoters from Gram-positive and Gram-negative bacteria we identify the main rRNA promoter sequence types in these species.
MATERIALS AND METHODS
Bacterial strains and plasmids
Strains and plasmids are listed in Table 1. Promoter vector pRLG770 (26) was used to create all promoter constructs used in in vitro multiple-round transcriptions. Promoter fragments created by annealing two complementary oligonucleotides with appropriate overhangs were inserted into the vector using EcoRI and HindIII restriction sites. Restriction enzymes were purchased from Takara. The transcripts that initiate at these promoters terminate at a defined termination site. The length of these transcripts is ∼145 nt. All constructs were verified by DNA sequencing.
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Source |
|---|---|---|
| Strain | ||
| Bacillus subtilis | ||
| MH5636 | rpoC-His10 | (29) |
| MO1099 | trpC2 pheA1 amyE::MLS | (27) |
| RLG6943 | MO1099 amyE::Cm rrnO P2 (−77/+50)-lacZ | (21) |
| RLG7553 | MO1099 amyE::Cm rrnB P2 (−38/+1)-lacZ | (21) |
| RLG7554 | MO1099 amyE::Cm rrnB P1 (−39/+1)-lacZ | (21) |
| RLG7555 | MO1099 amyE::Cm Pveg (−38/−1, +1G)-lacZ | (21) |
| LK606 | MO1099 amyE::Cm Pveg-10DBP1-lacZ | This study |
| LK607 | MO1099 amyE::Cm rrnB P1-10Dveg-lacZ | This study |
| Escherichia coli | ||
| LK22 | pCD2 (B. subtilis sigA) | (31) |
| RLG6924 | pDG3661 | (21) |
| Plasmid | ||
| pRLG770 | Promoter vector | (26) |
| pRLG6555 | pRLG770 with Eco-rrnB P1(−66/+9) | (41) |
| pLK1 | pRLG770 with Pveg (−38/−1, +1G), promoter construct #1 | (21) |
| pLK2 | pRLG770 with Pveg+1=8, promoter construct #2 | This study |
| pLK3 | pRLG770 with PvegDiscBP1, promoter construct #3 | This study |
| pLK4 | pRLG770 with Pveg-10DBP1, promoter construct #4 | This study |
| pLK5 | pRLG770 with PveghexDBP1, promoter construct #5 | This study |
| pLK6 | pRLG770 with PvegSp-10DBP1, promoter construct #6 | This study |
| pLK7 | pRLG770 with rrnB P1 (−39/+1), promoter construct #7 | (21) |
| pLK8 | pRLG770 with rrnB P1-39to-2+1G=7, promoter construct #8 | This study |
| pLK9 | pRLG770 with rrnB P1-10Dveg, promoter construct #9 | This study |
| pLK10 | pRLG770 with rrnB P1-5TtoA, promoter construct #10 | This study |
| pLK11 | pRLG770 with Pveg-4AtoT, promoter construct #11 | This study |
| pLK12 | pRLG770 with Pveg-10BP1+1=8G-5AtoT, promoter construct #12 | This study |
| pLK13 | pRLG770 with Pveg-10BP1, promoter construct #13 | This study |
| pLK14 | pRLG770 with rrnA P1 (−39/+1), promoter construct #14 | This study |
| pLK15 | pRLG770 with Pveg-10DAP1, promoter construct #15 | This study |
| pLK16 | pRLG770 with rrnA P1-10Dveg, promoter construct #16 | This study |
| pLK17 | pRLG770 with rrnB P1-10DAP1, promoter construct #17 | This study |
| pLK18 | pRLG770 with rrnJ P1 (-39/+1), promoter construct #18 | This study |
| pLK19 | pRLG770 with Pveg-10DJP1, promoter construct #19 | This study |
| pLK20 | pRLG770 with rrnJ P1-10Dveg, promoter construct #20 | This study |
| pLK21 | pRLG770 with rrnB P1-10DJP1, promoter construct #21 | This study |
| pLK22 | pRLG770 with Pilv (−39/+1, +1G), promoter construct #22 | This study |
| pLK23 | pRLG770 with Pveg-10Dilv, promoter construct #23 | This study |
| pLK24 | pRLG770 with Pilv-10Dveg, promoter construct #24 | This study |
| pLK25 | pRLG770 with rrnB P1-10Dilv, promoter construct #25 | This study |
| pLK26 | pRLG770 with PgcaD (−39/+1), promoter construct #26 | This study |
| pLK27 | pRLG770 with Pveg-10DgcaD, promoter construct #27 | This study |
| pLK28 | pRLG770 with PgcaD-10Dveg, promoter construct #28 | This study |
| pLK29 | pRLG770 with rrnB P1-10DgcaD, promoter construct #29 | This study |
| pLK30 | pRLG770 with PinfC (−39/+1), promoter construct #30 | This study |
| pLK31 | pRLG770 with Pveg-10DinfC, promoter construct #31 | This study |
| pLK32 | pRLG770 with PinfC-10Dveg, promoter construct #32 | This study |
| pLK33 | pRLG770 with rrnB P1-10DinfC, promoter construct #33 | This study |
| pLK541 | pDG3661 with rrnB P1-10Dveg | This study |
| pLK564 | pDG3661 with Pveg-10DBP1 | This study |
The vector pDG3661 (21) was used to create promoter-lacZ fusions for in vivo promoter activity experiments. Promoter fragments were inserted using EcoRI and HindIII restriction sites. The mRNA reporters were constructed with the sequence TCT adjacent to the +1 position to avoid placing an A adjacent to +1, followed by a HindIII site and the lacZ sequence (21). All constructs were verified by DNA sequencing. Promoter-lacZ fusions were integrated at the amyE locus of the B. subtilis chromosome. Recombinants at the amyE locus (double crossing over) were selected for resistance to chloramphenicol (5 µg/ml) and sensitivity to MLS (erythromycin 1 µg/ml and lincomycin 25 µg/ml) (27). Antibiotics were purchased from Sigma.
Media and growth conditions
Cells were grown in LB or MOPS-buffered defined medium: 50 mM MOPS (pH 7.0), 1 mM (NH4)2SO4, 0.5 mM KH2PO4, 2 mM MgCl2, 2 mM CaCl2, 50 µM MnCl2, 5 µM FeCl3, amino acids [50 µg/ml]; all 20 amino acids—referred to as MOPS 20 amino acids and 0.4% glucose. The wt B. subtilis strain (MO1099) used in this work is auxotrophic for Trp and Phe. All experiments with B. subtilis were conducted at 37°C. For outgrowth experiments, the cells were grown through exponential phase in MOPS 20 amino acids, and then shaken for additional 3 h during stationary phase, at which point (time 0) the cells were 10× diluted into the same prewarmed fresh medium. For decoyinine treatment experiments, the cells were grown in MOPS 20 amino acids into early exponential phase (OD600 ∼0.3) and treated with decoyinine at a final concentration of 0.5 mg/ml (28). Decoyinine was purchased from Biomol and the stock solution (100 mg/ml) was dissolved in 1 M KOH. Treatment of the cells with an identical amount of 1 M KOH had no effect on the GTP level (data not shown).
Protein purification
Bacillus subtilis RNAP, histidine tagged on the β′ subunit was purified from strain MH5636 as described by Qi and Hulett (29). The σA subunit of RNAP was overproduced from the pCD2plasmid (30) and purified as described (31). The core RNAP containing the δ subunit was reconstituted with the σA subunit in storage buffer (50 mM Tris–HCl pH 8.0, 0.1 M NaCl, 3 mM 2-mercaptoethanol, 50% glycerol) for 30 min at 30°C. Titration experiments were carried out to ensure saturation of the core RNAP with σA.
Escherichia coli Holo RNAP was purchased from Epicentre Biotechnologies®.
In vitro transcription
The promoters inserted into pRLG770 are listed in Table 2. Agarose gel ethidium bromide electrophoresis was conducted to verify that >90% of the plasmid was in supercoiled form. Multiple-round transcriptions were carried out in 10 µl reactions containing 30 nM RNAP, 1 nM supercoiled plasmid template, 40 mM Tris–HCl pH 8.0, 10 mM MgCl2, 1 mM DTT, 0.1 µg/ml BSA and 150 mM KCl. ATP, CTP and GTP were at 200 µM each. When GTP or ATP was varied, the concentration range was 20–2000 µM. UTP was 10 µM plus 2 µM [α-32P]-UTP. Each sample was preincubated at 30°C for 5 min followed by initiation with RNAP. The reaction was stopped after 15 min at 30°C by 10 µl of formamide loading buffer (95% formamide, 20 mM EDTA pH 8.0) and briefly vortexed. Samples were loaded onto 7 M UREA 7% polyacrylamide gels and separated by electrophoresis. After drying the gels, the gels were scanned with Molecular Imager® FX (BIO-RAD). The amounts of the 145-nt-long transcript (‘Bacterial strains and plasmids’ section) that originated from the tested promoters were quantitated with ImageQuant Software (Molecular Dynamics). Supplementary Figure S1 shows a representative gel. The exponential rise to maximum function of Sigmaplot (Jandel Scientific) was used to fit the data. KNTP values were calculated from the f = a × [1 − exp(−b × x)] equation (f, relative transcription; x, time; a and b, constants). The KNTP values depend on the identity of the salt as well as on the temperature. Therefore, these values are only comparable under identical conditions, when they most faithfully reflect the relative promoter sensitivity to [iNTP].
Table 2.
Alignment of rrn P1 promoters from selected bacteria
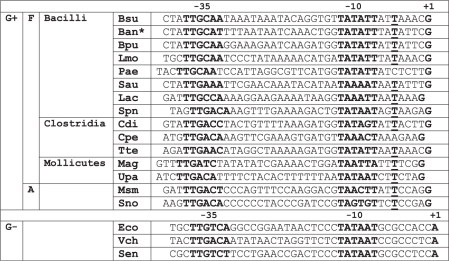 |
F, Firmicutes; A, Actinobacteria; Bsu, Bacillus subtilis; Ban*, Bacillus anthracis, *the same promoter sequence was also found in Bacillus cereus and Bacillus thuringiensis; Bpu, Bacillus pumilus; Lmo, Listeria monocytogenes; Pae, Paenibacillus sp.; Sau, Staphylococcus aureus; Lac, Lactobacillus acidophilus; Spn, Streptomyces pneumoniae; Cdi, Clostridium difficile; Cpe, Clostridium perfringens; Tte, Thermoanaerobacter tencongensis; Mag, Mycoplasma agalactiae; Upa, Ureaplasma parvum; Msm, Mycobacterium smegmatis; Sno, Streptomyces nodosus; Eco, Escherichia coli; Vch, Vibrio cholerae, and Sen, Salmonella enteritica. The −35 and −10 hexamers and +1 positions are shown in bold. −5 T (or a T 3 bp downstream from −10) is in bold and underlined.
Open complex stability
Open complex stability was determined by a transcription assay in the presence of heparin as a competitor as described in (32). Briefly, open complexes between RNAP and promoter DNA on supercoiled plasmids were allowed to form for 15 min at 30°C in transcription buffer (the same as in multiple-round transcriptions) with 30 mM KCl. At time 0, heparin was added at 0.5 µg/ml, and aliquots were withdrawn at time points and added to all four NTPs to initiate transcription. RNAP in the open complex is resistant to heparin and thus this assay measures the fraction of open complexes remaining at selected time points after heparin addition. After 15 min, transcription was stopped with a formamide stop solution (95% formamide, 20 mM EDTA pH 8.0), and the reactions were loaded onto 7 M urea denaturing gel and separated by electrophoresis. The amounts of transcripts were quantitated and plotted as a function of time. The exponential decay function of SigmaPlot (Jandel Scientific) was used to fit the data. Open complex half-lives (t1/2) were calculated from the equation f = a × exp(−b × x) (f, relative transcription; x, time; a and b, constants). Competitor test experiments were also conducted, demonstrating that the concentration of heparin used was sufficient to completely abolish transcription if present in the reaction before the addition of RNAP.
RNA extraction and reverse transcription
Promoter constructs were fused to lacZ, but activities were assayed by primer extension followed by real-time qPCR rather than by β-galactosidase assay. The half-life of the lacZ mRNA was ∼4 min in the cell (both in exponential phase and outgrowth), allowing to detect rapid changes in promoter activity (21). RNA extractions, primer extensions and real-time qPCRs were carried out as described by Krásný et al. (14). Briefly, a recovery marker RNA (RM RNA, prepared from the B. subtilis strain RLG6943) was added at the time of extraction, controlling for possible differences in degradation during extraction and for variation between samples at later steps; 2 ml of cells was added directly into the mixture of 6 ml of phenol/chloroform (1:1) plus 0.5 ml of lysis buffer (50 mM Tris–HCl pH 8.0, 500 mM LiCl, 50 mM EDTA pH 8.0, 5% SDS). Immediately after a brief vortexing, RM RNA (∼0.5 µg of total RNA in 25 µl) was added, and cells were sonicated for 1 min. After two more phenol/chloroform extractions and two ethanol precipitations, the pellet was typically resuspended in 50 µl of 10 mM Tris–HCl, pH 8.0. RNA was DNased using Turbo DNase from Ambion. Primer extension was performed with M-MLV reverse transcriptase from Promega using the DNased RNA (1–10 µl) and primer βgalR: 5′-CAGTAACTTCCACAGTAGTTCACCAC-3′. The resulting cDNA was quantified by subsequent real-time qPCR.
Real-time qPCR
cDNA was used as a template in qPCR using a Taq polymerase kit purchased from Promega. qPCR was conducted in 8-Tube Strips (BIO-RAD) and an Eppendorf Realplex4 cycler. Each reaction (25 µl) contained 3 µl of cDNA, 1 U Taq DNA polymerase, 0.2 µl SYBR Green (Molecular Probes), 1× buffer, 250 µM dNTPs (each), 3 mM MgCl2 and 0.4 mM primers (each). Two combinations of primers were used with each sample: (i) test RNA-specific primer, #103 5′-TCTAAGCTTCTAGGATCCCC-3′ in combination with the βgalR primer (see ‘RNA extraction and reverse transcription’ section) and (ii) RM RNA-specific primer, #104 5′-GTCGCTTTGAGAGAAGCACA-3′ in combination with the βgalR primer. The 40×-repeated qPCR program [95°C, 15s; 65°C, 20s; 72°C, 30s] was followed by a melting curve analysis to verify the identity of the PCR products. DNased RNA extracts and also reactions without template cDNA were used as controls. The ΔCt method (33) was used to determine the relative quantities of cDNAs. Samples were normalized to the recovery marker and cell density.
Determination of relative in vivo GTP concentration
Cells were grown in MOPS 20 amino acids medium with 0.4% glucose and [32P]-H3PO4 (Phosphorus32, 20 µCi/ml) until early exponential phase (OD600 ∼0.3). At selected time points, aliquots of cells were added to equal volumes of formic acid (13 M), briefly vortexed and stored overnight at −20°C. The samples (4 µl) were then spotted on TLC plates (Polygram® CEL 300 PEI purchased from Macherey-Nagel) followed by running the samples in 0.85 M KH2PO4. After overnight exposure, the spots were quantified by phosphorimaging with Molecular Imager® FX (BIO-RAD). The identity of GTP was verified by comparison with commercial preparations of GTP run in parallel and visualized by UV shadowing (34).
Alignment of rrn P1 promoters
The DNA sequences of the rrn P1 promoters were either retrieved from the literature or identified by visual inspection in DNA sequences upstream of rRNA operons. These DNA sequences were downloaded from the NCBI database (http://www.ncbi.nlm.nih.gov/). Typically, three to five rrn P1 promoters (when available, some organisms have fewer than three rrn P1 promoters) were identified for each species. Subsequently, one typical rrn P1 promoter was selected for the alignment (Table 2). Supplementary Table S1 shows the source (either the NCBI code or reference) from where the promoter sequence was obtained.
In silico modeling of B. subtilis RNAP in complex with DNA
To create the model of B. subtilis RNAP with DNA in the open complex, we used a previously published homology model of B. subtilis RNAP (35). To this model, we added DNA based on a structure of E. coli RNAP in complex with the DNA:DNA duplex in the form of an open transcription bubble (36) (PDB id 3iyd). Structural alignment of the protein structures was performed with the ICM Browser (Molsoft L.L.C.). The alignment enabled the nucleic acids strands to be placed into the correct position in the B. subtilis RNAP model.
RESULTS
Choice of promoters
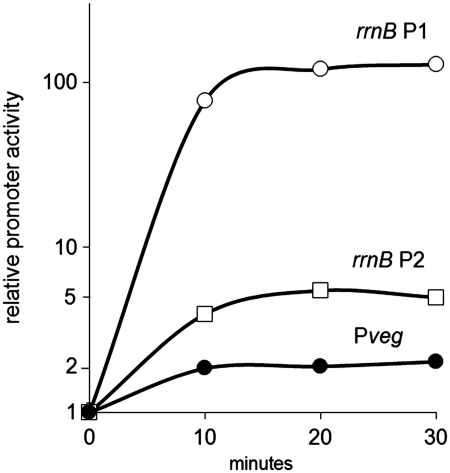
Bacilllus subtilis contains 9–10 rRNA operons, depending on the strain (37). Escherichia coli contains seven rRNA operons (10). In both organisms, each operon is typically transcribed from a pair of promoters, P1 and P2. Both the P1 and P2 promoters in both organisms are sensitive to changes in the concentration of their iNTP. In E. coli, rrn P1 promoters exhibit more pronounced changes with growth rate and growth phase than rrn P2 promoters (10). In B. subtilis, similarly to E. coli, it was documented that an rrn P1 displayed more pronounced changes in activity with changes in growth rate than the corresponding rrn P2 (21). Here we show that this is also valid in B. subtilis for changes in growth phase, using rrnB P1 and rrnB P2 promoters and following their activity during outgrowth from stationary phase. We fused these promoters to a marker gene that gives rise to an in vivo unstable mRNA. We integrated the constructs in a single copy into the B. subtilis chromosome. The intracellular level of the mRNA was a measure of the promoter activity and was quantitated by RT–qPCR. During outgrowth, the rrnB P1 promoter increased its activity ∼100-fold, whereas rrnB P2 increased its activity ∼5-fold (Figure 1). A similar result was obtained with the B. subtilis rrnO P1 and P2 promoters (data not shown). Hence, for further studies, we decided to use an rrn P1 promoter because it displays more dramatic changes in activity than an rrn P2 promoter.
Figure 1.
Changes in activity of selected B. subtilis promoters during outgrowth from stationary phase. Cells were grown in rich MOPS 20 amino acids medium until 3 h into stationary phase (time ‘0’). Subsequently, cells were diluted into fresh medium and RNA was extracted at the indicated time points. RNA transcribed from the tested promoter was quantitated and used as a measure of the promoter’s activity (‘Materials and Methods’ section). To simplify comparison of the promoters in terms of the proportional increase in their activity, the activities of the promoters were normalized to 1 at time 0. The actual relative activities of the promoters normalized to rrnB P2 (set as 1) at time 0 were as follows: rrnB P2 was 1, Pveg was 1.26 and rrnB P1 was 0.02. Thus, the activity of rrnB P1 was most repressed at time 0, allowing for the subsequent large increase in activity. Strains used for the experiment: RLG7554 (rrnB P1, open circles), RLG7553 (rrnB P2, open squares) and RLG7555 (Pveg, black circles). A representative experiment is shown. The experiment was repeated three times with similar results.
Of the B. subtilis rrn P1 promoters, rrnB P1 is typical in terms of its sequence. Its spacer region is 17 bp, which is a length found in five of the seven P1 promoters [rrnO P1 has a length of 16 bp and rrnI P1 18 bp; the seven rrn P1 promoters direct the transcription of all B. subtilis rRNA operons; rrn J-W and rrn I-H-G are transcribed in clusters (38)]. It contains the extended −10 motif (TGX). Its region between the −10 hexamer and +1 is A/T-rich and the transcription +1 position is a G. The +1 position is 8 bp from the −10 hexamer (as opposed to P2 promoters, where this distance is 7 bp). Its position analogous to position −7 of E. coli rrn P1 promoters is not a C.
We selected Pveg as a control promoter. It is not regulated by the concentration of its iNTP and its expression is constitutive. During outgrowth from stationary phase its activity increased relatively slightly in comparison with the rrn promoters (Figure 1). It is a strong promoter that directs the transcription of a single gene transcription unit. The function of the Veg protein is not fully understood (39). As a promoter, however, Pveg is well characterized (21,40). The wt Pveg promoter starts with +1A. Here, we use a +1G version that does not change its properties and simplifies interpretation of the data (21).
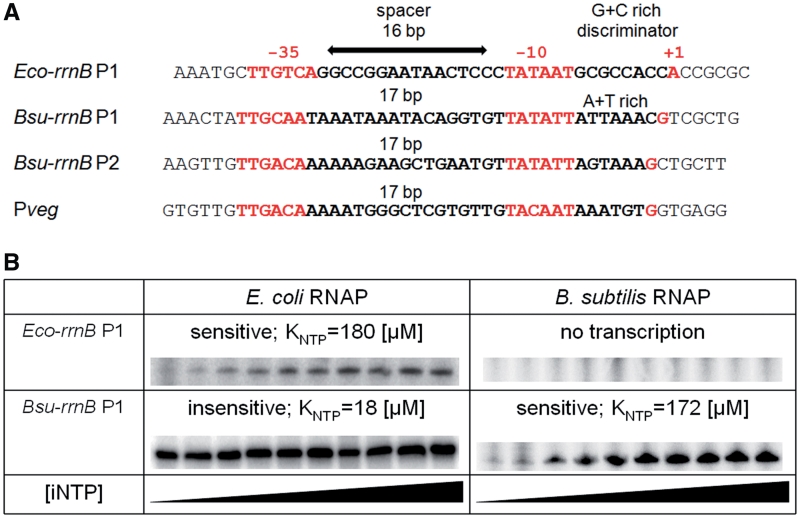
The sequences of B. subtilis rrnB P1, rrnB P2, Pveg and E. coli rrnB P1 are shown in Figure 2A. The same name (rrnB P1) of the B. subtilis and E. coli promoters is purely a coincidence—it does not imply any sequence homology; to distinguish the two promoters, the B. subtilis rrn promoter is designated Bsu-rrnB P1 and the E. coli rrn promoter Eco-rrnB P1. Where only rrnB P1 is mentioned in the text it refers to the B. subtilis rrnB P1 promoter.
Figure 2.
Comparison of selected B. subtilis and E. coli promoters. (A) Sequence comparison of core promoter regions of E. coli rrnB P1, B. subtilis rrnB P1, rrnB P2 and Pveg. The −35 and −10 hexamers and the transcription start sites (+1) are indicated in red. Spacer and discriminator regions are indicated. (B) Combinatorial comparison of changes in promoter activity as a function of the iNTP concentration with RNAPs from E. coli and B. subtilis and with rrnB P1 promoters from these organisms. Multiple-round transcriptions were conducted with increasing iNTP concentration. Primary data are shown. KiNTPs (NTP concentration required for half maximal transcription) for respective combinations are shown above the primary data. For rrnB P1 from E. coli, the iNTP is ATP. For rrnB P1 from B. subtilis, the iNTP is GTP. The concentrations of iNTP were 20, 40, 100, 200, 400, 600, 1000, 1300, 1600 and 2000 µM.
Escherichia coli RNAP recognizes Bsu-rrnB P1 as an [iNTP]-insensitive promoter
The potential of a promoter to be regulated by [iNTP] can be assessed in vitro by determining the promoter’s KiNTP—the concentration of the iNTP required for half-maximal transcription. Promoters that are [iNTP]-sensitive have relatively high values of KiNTP, whereas [iNTP]-insensitive promoters have relatively low values.
We wished to test whether RNAP from E. coli would indeed display [iNTP]-insensitive behavior at Bsu-rrnB P1, as was predicted based on its sequence. First, we verified that E. coli RNAP required a relatively high concentration of its iNTP (ATP) at Eco-rrnB P1 to reach maximal transcription, displaying typical [iNTP]-sensitive behavior in vitro (Figure 2B). On the contrary and as predicted, E. coli RNAP required a relatively low concentration of the iNTP at Bsu-rrnB P1 (Figure 2B). As a control, we showed that B. subtilis RNAP was sensitive to a wide concentration range of the iNTP at Bsu-rrnB P1 (Figure 2B). Finally, B. subtilis RNAP did not utilize Eco-rrnB P1 as a promoter (Figure 2B) and Pveg was recognized as an [iNTP]-insensitive promoter with RNAPs from both E. coli (data not shown) and B. subtilis (Figure 3B).
Figure 3.
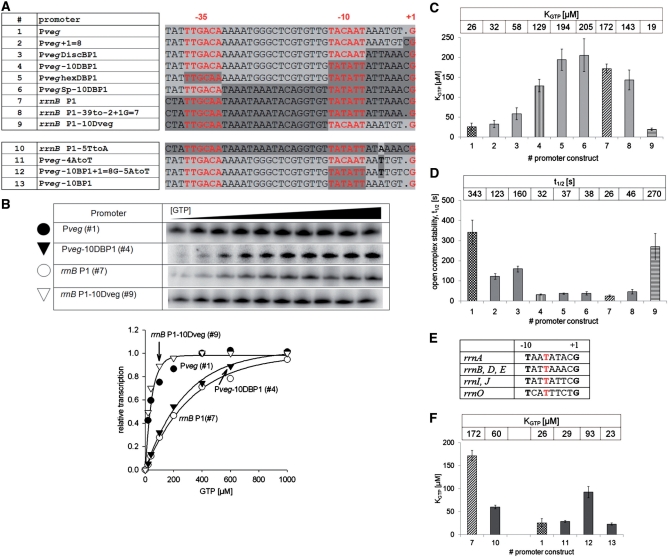
DNA elements of B. subtilis rrnB P1 required for its sensitivity to [iNTP] in vitro. (A) Sequence comparison of Pveg-rrnB P1 chimeric constructs. The sequence is highlighted with two shades of gray, indicating from where this sequence fragment comes: light gray, Pveg; dark gray, rrnB P1. (B) Multiple-round transcriptions as a function of GTP concentration: representative primary data and their graphical comparison for rrnB P1, Pveg and two chimeric constructs (Nos 4 and 9). The graph shows the 0–1000 µM interval. (C) Graphical comparison of KGTP values for construct Nos 1–9. KGTP values are shown above the bars. The values are the averages of three independent experiments. The error bars in this and all subsequent figures represent ±SD of the mean. For construct Nos 1, 4, 7 and 9, distinct bar fill patterns were used to facilitate orientation in this figure. (D) Open complex stability of construct Nos 1–9. Half-lives (t1/2) are indicated above the bars. (E) Sequence alignment of the region between −10 and +1 of B. subtilis rrn P1 promoters. The 100% conserved −5T is indicated in red. (F) Graphical representation of KGTP values for constructs testing the role of −5T. KGTP values are shown above the bars.
We concluded that E. coli RNAP does not recognize Bsu-rrnB P1 as an [iNTP]-sensitive promoter. Bacillus subtilis RNAP, however, does recognize Bsu-rrnB P1 as an [iNTP]-sensitive promoter, whereas the same enzyme recognizes Pveg as an [iNTP]-insensitive promoter.
The 3′-region of Bsu-rrnB P1 is required for its [iNTP]-sensitivity in vitro
Next, we wished to identify the DNA region(s) that differentiate Bsu-rrnB P1 from Pveg in terms of sensitivity to [iNTP] in vitro. We created a set of chimeric promoters, progressively changing Pveg (construct No. 1) into rrnB P1 (construct No. 7) (Figure 3A). Using B. subtilis RNAP, we conducted in vitro multiple-round transcriptions where we varied the concentration of the initiating GTP. Figure 3B shows typical primary data and their quantitation for rrnB P1, Pveg and two other chimeric promoter constructs.
Figure 3C shows the KGTP values of promoter construct Nos 1–9. Shortening the distance between the −10 hexamer and the transcription +1 position in rrnB P1 or lengthening this distance in Pveg had only negligible effects on the respective KGTPs (construct Nos 2 and 8 compared with No. 1 and 7, respectively). Likewise, implanting the region starting downstream of the −10 hexamer and ending at +1 (the discriminator) from rrnB P1 into Pveg had only a moderate effect (construct No. 3). Finally, when the 3′-region from rrnB P1, containing the −10 hexamer and the discriminator was fused to the 5′-region from Pveg (construct No. 4), the KGTP value changed significantly and approached that of rrnB P1 (Figure 3A–C). Adding the −35 hexamer or the spacer (construct Nos 5 and 6) led to further moderate increases in KGTP. In fact, the KGTP values of these two constructs were even slightly higher than that of rrnB P1. In construct No. 5, the increase in KGTP could be due to the suboptimal −35 hexamer. In construct No. 6, however, the −35 hexamer is full-consensus and the spacer even contains the extended −10 motif. Overall, the observed differences are not large and it is apparent that, unlike in E. coli, both the full consensus −35 hexamer and the extended −10 motif can be present and the promoter is still [iNTP]-sensitive with B. subtilis RNAP.
Next, to verify the importance of the identified 3′-region for promoter sensitivity to the relatively high iNTP concentration we prepared the chimeric construct No. 9, which was reciprocal to construct No. 4 (Figure 3A–C). In construct No. 9, the 3′-region [from the −10 hexamer (included) to +1] was from Pveg and the rest was from rrnB P1. Its KGTP value was comparable to the KGTP value of Pveg, corroborating the importance of the 3′-region for promoter sensitivity to [iNTP].
It was previously demonstrated with RNAP from E. coli that promoter sensitivity to [iNTP] inversely correlates with open complex stability (12,41). We determined the stabilities of open complexes of the created promoters with B. subtilis RNAP by measuring their open complex half-lives and observed a similar trend (Figure 3D). Promoters that displayed low KGTP values (e.g. construct Nos 1 and 9) formed more stable open complexes (longer half-lives) than promoters with higher KGTP values (e.g. construct Nos 4 and 7).
We concluded that the 3′-region [from the −10 hexamer (included) to +1] from the B. subtilis rrnB P1 promoter is of key importance for the promoter’s ability to respond to a wide concentration range of its iNTP. This property inversely correlated with the stability of the open complex.
The −5T is required for the [iNTP] sensitivity of Bsu-rrnB P1
Sequence analysis of the region between the −10 hexamer and +1 of all seven rRNA P1 promoters from B. subtilis revealed four sequence variants (Figure 3E). In these four sequence variants, the only 100% conserved base was thymine at the −5 position (3 bp downstream from the −10 hexamer). We created a promoter construct based on rrnB P1 where the −5T was substituted with A (construct No. 10), which is at the analogous position in Pveg. Figure 3F shows that the KGTP of this promoter construct was dramatically decreased relative to wt rrnB P1 (construct No. 7). Subsequently, we prepared a reciprocal promoter construct based on Pveg where −4A (analogous to −5A in rrnB P1: 3 bp downstream from −10) was substituted with T (construct No. 11). No KGTP increase was observed when compared with Pveg (construct No. 1). A more pronounced increase in KGTP was detected when this mutation was placed in the context of the −10 hexamer from rrnB P1 and when the region between the −10 hexamer and +1 was extended from 7 to 8 bp to make this region more rrnB P1-like (construct No. 12). Neither the distance of −10 to +1 (construct No. 2, see previous section) nor the −10 hexamer alone (construct No. 13) had significant effects on the KGTP value.
We concluded that a thymine base at the −5 position is a significant factor in the regulation of rrnB P1 by [iNTP]. However, it does not function as the sole determinant of this regulation and its effect is context dependent.
The Bsu-rrnB P1 promoter 3′-region is important for [iNTP] sensitivity in vivo
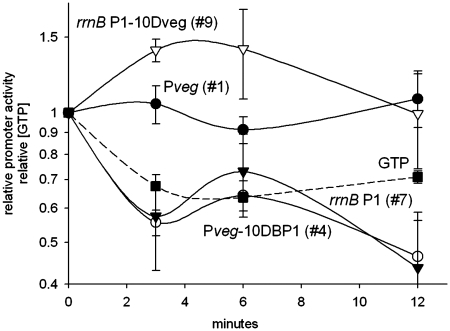
In the next step, we used Pveg, Bsu-rrnB P1, and chimeric promoters No. 4 and 9 to test the importance of the promoter 3′-region for its regulation by [iNTP] in vivo. We created the appropriate constructs and strains. We selected decoyinine treatment as a model situation where the intracellular GTP concentration decreases. Decoyinine inhibits GMP synthase, a protein required for GTP biosynthesis (28). The relative level of GTP was measured by formic acid extraction (‘Materials and Methods’ section).
Upon decoyinine addition, GTP concentration dropped, rrnB P1 activity decreased and Pveg activity remained relatively unchanged (Figure 4) as reported previously. The activity of promoter No. 4 decreased about the same as the activity of rrnB P1. As the half-life of the test mRNA is ∼4 min (‘Materials and Methods’ section), it means that the activities of rrnB P1 and promoter No. 4 almost completely ceased within the first time segment of the experiment (from 0 to the 3 min time point). In contrast, the activity of promoter No. 9 did not decrease. Instead, we observed a moderate increase in its activity. This increase could be, in part, explained by the lower affinity of promoter No. 9 to RNAP in comparison with Pveg (data not shown). As significant amounts of RNAP are presumably liberated from rRNA operons upon the GTP concentration decrease, this increased level of free RNAP can now increase the activity of promoters where the rate of transcription initiation is limited by the concentration of available RNAP.
Figure 4.
Relative changes in promoter activity in B. subtilis after decoyinine treatment. Cells were grown to early log phase (OD600 ∼0.3) and at time 0 treated with decoyinine. RNA was extracted before (time 0) and at indicated time points after the addition of decoyinine. Determination of promoter activity was as in Figure 1. Promoter activities and GTP concentration were set as 1 at time 0. Numbers (#) corresponding to promoter constructs used in in vitro experiments are indicated in the plot together with the name of the construct. Filled squares (dashed line), relative GTP concentration; filled triangles, rrnB P1 (strain RLG7554); closed circles, Pveg (strain RLG7555); open circles, Pveg-10DBP1 (#4) (strain LK606); empty triangles, rrnB P1-10Dveg (#9) (strain LK607). The values are averages of at least four experiments conducted on different days.
We concluded that the 3′-region of rrnB P1 is important for the decrease in promoter activity in response to a drop in [GTP] in vivo: combining this region with the 5′-region of the [iNTP]-insensitive Pveg promoter yields a chimera that is sensitive to changes in the concentration of its iNTP in vivo.
Some non-rRNA promoters differ in [iNTP] sensitivity determinants from rRNA promoters: Class I and Class II promoters
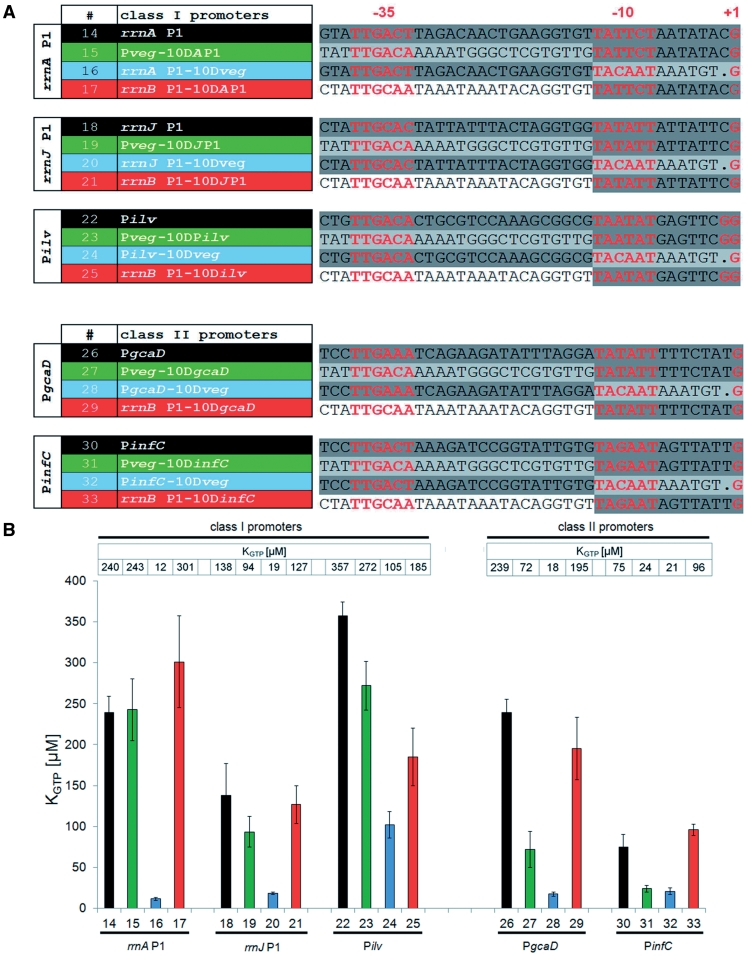
To test whether the effect of the 3′-promoter region on [iNTP] sensitivity is a more general phenomenon in B. subtilis, we extended our studies to other promoters. We selected two rrn P1 promoters (A and J) and three non-rrn promoters (Pilv, PgcaD and PinfC).
Pilv drives the transcription of the genes required for the biosynthesis of isoleucine, leucine and valine (42). Pilv is sensitive to the concentration of its initiating ATP, which plays an important role in the increase in promoter activity during the stringent response (14,25,43). As with Pveg, to facilitate comparison of the results, we used Pilv in which the +1A was replaced with +1G. This change does not alter its sensitivity to [iNTP] (14).
Both PinfC and PgcaD initiate with GTP, and the genes that are transcribed from these promoters are downregulated during the stringent response (43), suggesting that these promoters may be sensitive to [GTP]. PinfC directs transcription of the infC-rpmL-rpkT-ysdA operon coding for the translation initiation factor IF3 and the genes for ribosomal proteins L35 and L20 (44). The ysdA gene encodes a product with an unknown function. It was reported that the expression of the infC-rpmL-rpkT-ysdA gene cluster is autoregulated by a complex transcription attenuation mechanism (45). PgcaD directs transcription of the gcaD gene, which codes for N-acetylglucosamine 1-phosphate uridyltransferase (46).
First, we wanted to verify that it is the core promoter (from −39 to +1) that contains DNA determinants of [iNTP] sensitivity. We selected the two rrn promoters (A and J) as representatives for this experiment and created both core and extended (from −150 to +50) promoter constructs. Both types of constructs yielded approximately the same KGTP value for each promoter (data not shown), suggesting that, similarly to previously reported results (21), it is the core promoter sequence that contains the decisive elements for promoter regulation by [iNTP].
Next, we created four variants of these promoters: (i) core; (ii) chimeras consisting of the 5′-region (from 3 bp upstream of −35 to the −10 hexamer) from Pveg fused to 3′-regions [from the −10 hexamer (included) to +1] of the tested promoters (analogous to construct No. 4 in Figure 3A); (iii) chimeras consisting of 5′-regions from the tested promoters fused to the 3′-region of Pveg (analogous to construct No. 9 in Figure 3A); and (iv) chimeras consisting of the 5′-region from rrnB P1 and 3′-regions from the tested promoter (Figure 5A).
Figure 5.
Class I and Class II promoters. (A) Sequences of B. subtilis rrnA P1, rrnJ P1, Pilv, PgcaD, PinfC promoters and their chimeric variants. −35, −10 and +1 are marked. Transcription from Pilv can initiate at either of the two indicated positions. The +1 position that is 7 bp from −10 is the preferred one. The color coding of the construct names indicates similar types of constructs (e.g. green indicates a chimera with the 5′-region from Pveg and the 3′-region from the tested promoter). The color coding of the sequences indicates the origin of the sequence: dark gray, the tested promoter; light gray, Pveg; white, rrnB P1. (B) Graphical comparison of KGTP values for construct Nos 14–33. The respective KGTP values are shown above the bars and also next to the construct sequence in A. The color coding of the bars corresponds to the color coding of the construct names used in panel A of this figure.
We compared KGTP values of the created promoters (Figure 5B). Both rrnA P1 and rrnJ P1 and to some degree also Pilv exhibited characteristics previously observed for rrnB P1; the 3′-regions of the tested promoters played a dominant role in their sensitivity to [iNTP]. We tentatively classified such promoters as Class I promoters. With PgcaD and PinfC, we did not observe an overriding effect of the 3′-promoter region. Instead, both promoter regions were required for the sensitivity. We termed such promoters Class II promoters. Interestingly, the 5′-promoter region from rrnB P1 was able to complement the 3′-promoter regions of Class II promoters with respect to restoring their sensitivity to [iNTP].
We concluded that at least two classes of promoters exist in B. subtilis in terms of the DNA determinants of promoter sensitivity to [iNTP]: (i) promoters where the dominant determinants of this sensitivity are located within the 3′-region [from the −10 hexamer (included) to +1]. rRNA promoters appear to be typical representatives of this group (Class I promoters) and (ii) promoters where the DNA determinants of [iNTP] sensitivity are not limited to the 3′-region (Class II promoters) (Figure 5A and B).
Bacillus subtilis-like sequences of rRNA promoters are also present in other bacteria
Next, we asked whether the sequence characteristics of B. subtilis rRNA promoters are limited to this species or whether they can be found in other Bacilli and possibly some other Gram-positive bacteria. Table 2 shows an alignment of selected rrn P1 promoters from the two main phyla within Gram-positive bacteria: (i) the larger phylum of Firmicutes (low G+C Gram-positive bacteria) consisting of three groups, Bacilli, Clostridia and Mollicutes (in some phylogenies, Mollicutes are classified as an independent phylum of Tenericutes) (47); and (ii) the smaller phylum of Actinobacteria (high-G+C Gram-positive bacteria) (48). For comparison, three sequences of rrn P1 promoters from three selected Gram-negative species are also shown.
Representatives of all three main groups of Firmicutes exhibit sequence characteristics typical for the rrn P1 promoters of B. subtilis, including tentative +1 positions as Gs, and in a number of cases the −5T. This suggests that the findings presented in this study may be more general in the context of the large group of Firmicutes. rrn P1 promoters from two selected actinobacterial species contain relatively G/C-rich discriminators (49,50), reminiscent of the discriminators from Gram-negative organisms. Interestingly, the −5 position in these actinobacterial promoters is also a T.
DISCUSSION
rRNA promoter regulation by the iNTP concentration is an important mechanism that links their transcriptional activity to the energy state of the cell. So far, the DNA determinants of this regulation in bacteria have been most thoroughly described for the rRNA promoters from Gram-negative E. coli. In this study, we investigated these determinants in phylogenetically distant Gram-positive B. subtilis, using both rRNA and non-RNA promoters. We identified regions and DNA elements in these promoters that determine their sensitivity to the iNTP concentration. Based on where the determinants are located within the promoter, we divided the [iNTP]-sensitive promoters into two classes. In Class I promoters, the main determinant is the 3′-region [from the −10 hexamer (included) to +1] whereas both the 5′- and 3′-regions are required in Class II promoters.
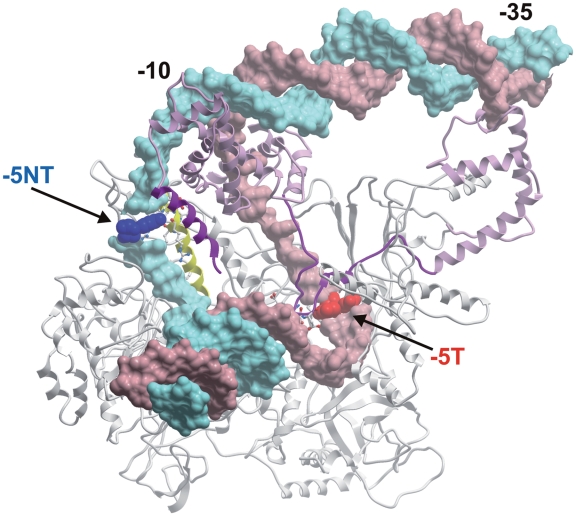
Typical representatives of Class I promoters are rrn P1 promoters. The 3′-promoter region corresponds to the transcription bubble and the promoter sensitivity to [iNTP] inversely correlates with the stability of the open complex. The short time interval available to the incoming iNTP is likely the limiting factor for efficient transcription initiation from these promoters. Within the 3′-promoter region of rrn P1 promoters, the −5T position is an important factor in their sensitivity to [iNTP]. It is reminiscent of the conserved −7C of E. coli rRNA promoters, although it is not exactly homologous to it; it is positioned 1 bp farther from the −10 hexamer than the −7C in E. coli (18,19). In silico modeling with a B. subtilis RNAP homology model suggested that the base of the −5 promoter position of the non-template strand may make contacts with the 428-ERVVRE-433 motif of the β subunit of RNAP (Figure 6). We note that interactions of the transcription bubble non-template strand with β were reported previously (51). Moreover, similarly to the non-template −7 position of E. coli rrn P1 promoters, the B. subtilis promoter non-template −5 position has the potential to interact with σA region 1.2. However, according to the B. subtilis model, interactions involving the base are less likely to be formed in the open complex but can possibly occur during isomerization, when σA region 1.2 may be present in the immediate vicinity of the non-template strand −5 base. The base of the −5 promoter position of the template strand may contact the 272-EEDD-275 motif of σA. Depending on the identity of the base and the DNA strand, optimal or suboptimal interactions may occur and affect the stability of the complex. We stress that these interactions are speculative and their molecular details will have to be addressed by future experiments.
Figure 6.
Model of the promoter −5 position nucleotides and their possible interactions with B. subtilis RNAP. The β′ subunit was removed to view the areas of interest. Light gray, β; light magenta, σA; light pink, DNA template strand; light blue, DNA non-template strand. The regions of β and σA that contain amino acids that may interact with the −5 position bases are in yellow or magenta, respectively. The DNA non-template −5 position is in blue and indicated with ‘−5NT’; the DNA template −5 position is in red and indicated with ‘−5T’. −10 and −35 hexamers are indicated.
We also note that a T at −5 is present in PgcaD and PinfC but not in Pilv. The absence of −5T in Pilv may be compensated for by the relatively high G/C content of the region between −10 and +1.
In Class II promoters, [iNTP] sensitivity determinants are not confined to the 3′-region but a combination of currently unknown elements from both regions is required. We note that the 5′-region (−39 to −14) of rrnB P1 also contains elements that, in combination with the 3′-regions from Class II promoters, yield [iNTP]-sensitive chimeras. The effect of the rrnB P1 5′-promoter region on [iNTP] sensitivity, however, is not dominant, as can be seen with chimera No. 9 (Figure 3C). Overall, it seems that various sequence combinations can result in [iNTP]-sensitive promoters in B. subtilis and a simple general rule cannot be currently established that would apply to all these promoters. Further studies would be required to identify the DNA determinants of the promoter [iNTP] sensitivity of Class II promoters in more detail.
We note that the extended −10 motif (TGX) is found in all B. subtilis rrn P1 promoters (22). It is known to stabilize complexes of RNAP with promoters (52–54) and, therefore, likely to decrease KNTP. However, the presence of this motif in B. subtilis promoters is quite common (55) and its presence in rrn P1 promoters is likely counterbalanced by other elements to achieve their physiologically optimal promoter sensitivity to [iNTP].
A comparison of rrn P1 promoters from various bacterial species revealed two main types of sequences: (i) B. subtilis-like rrn P1 promoters with an A/T-rich region between −10 and +1 and, in many species, with a T at −5. This sequence type appears to be typical for Firmicutes. The −5T, besides in Firmicutes, was also found in Actinobacteria and (ii) E. coli-like rrn P1 promoters with a G/C-rich region between −10 and +1. This sequence type appears to be specific for Gram-negative bacteria and can also be found in Gram-positive Actinobacteria. Thus, the sequences of rrn P1 promoters of Gram-positive and Gram-negative bacteria demonstrate that the same type of regulation can be achieved with strikingly different sequences in phylogenetically distant species.
In general, B. subtilis RNAP forms less stable complexes with promoter DNA than E. coli RNAP (56,57). This is also exemplified by the inability of B. subtilis RNAP to transcribe from the E. coli rrnB P1 promoter (Figure 2B). The differences in the interactions of the two enzymes with promoter DNA stem from the fact that these two enzymes significantly differ. RNAP from B. subtilis is smaller than RNAP from E. coli. It lacks several regions that were shown to contribute to the ability of E. coli RNAP to form stable complexes with promoter DNA. A mutant RNAP from E. coli lacking these regions was shown to form less stable complexes with promoter DNA, reminiscent of the B. subtilis enzyme (58). Hence, relative to E. coli, it is becoming apparent that a higher percentage of B. subtilis promoters are affected/modulated by changes in the intracellular concentration of their iNTPs and that this regulation is an important factor in their response to environmental changes. This is illustrated by Pilv, where [iNTP] sensitivity is one of several regulatory mechanisms acting on this promoter (14,25,59–63).
As evidenced by our study, B. subtilis [iNTP]-sensitive promoters vary in the degree of their sensitivity to changes in [iNTP] in vitro (e.g. compare Pilv with PinfC in Figure 5B). This is most likely relevant in vivo and further studies are required to address this question.
It is possible that additional protein factor(s) may alter B. subtilis RNAP with respect to its sensitivity to [iNTP]. In E. coli, it is DksA, which binds to the secondary channel, and this modifies the RNAP’s sensitivity to [iNTP] (64). Another protein factor, GreB from E. coli, has been shown [apart from its known effects on transcription elongation, (65)] to alter the properties of RNAP in a manner reminiscent of DksA (66,67). In B. subtilis, no obvious homolog of DksA exists and the three closest homologs (yteA, yocK and ylyA) were individually knocked-out without any effect on the activity of [iNTP]-sensitive rrn P1 promoters (21). It is possible that their functions are redundant and multiple knock-outs would be required to observe an effect. Other candidate proteins are GreA and the delta subunit of RNAP (68–70). GreA is the only Gre factor in B. subtilis (71) as opposed to GreA and GreB in E. coli. The delta subunit is specific for Firmicutes (72) and was previously shown to affect the stability of promoter-RNAP complexes (69,73), which may indicate its ability to alter the sensitivity of RNAP to [iNTP]. The effects of B. subtilis GreA and the delta subunit of RNAP on RNAP sensitivity to [iNTP] during transcription initiation are currently being studied in our laboratory.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
Ministry of Education, Youth and Sports of the Czech Republic and from Trios Llc. (2B06065 to L.K. and I.B.; MSM 0021620835 to I.B.); Czech Science Foundation (P302/11/0855 to L.K.); Institutional Research Concept (AV0Z50200510); EC Integrated Project ActinoGEN (LSHM-CT-2004-005224). Funding for open access charge: Ministry of Education, Youth and Sports of the Czech Republic; Czech Science Foundation.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Peter Lewis (University of Newcastle, Australia) for providing us with the homology model of B. subtilis RNAP. We thank Tamas Gaal (University of Wisconsin, USA), Jan Nešvera and Leoš Valášek (Institute of Microbiology, Czech Republic) for critically reading the article. We thank the members of our laboratory for discussions and comments on the article.
REFERENCES
- 1.Sørensen KI, Baker KE, Kelln RA, Neuhard J. Nucleotide pool-sensitive selection of the transcriptional start site in vivo at the Salmonella typhimurium pyrC and pyrD promoters. J. Bacteriol. 1993;175:4137–4144. doi: 10.1128/jb.175.13.4137-4144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker KA, Mallik P, Pratt TS, Osuna R. The Escherichia coli Fis promoter is regulated by changes in the levels of its transcription initiation nucleotide CTP. J. Biol. Chem. 2004;279:50818–50828. doi: 10.1074/jbc.M406285200. [DOI] [PubMed] [Google Scholar]
- 3.Liu C, Heath LS, Turnbough CL. Regulation of pyrBI operon expression in Escherichia coli by UTP-sensitive reiterative RNA synthesis during transcriptional initiation. Genes Dev. 1994;8:2904–2912. doi: 10.1101/gad.8.23.2904. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Turnbough CL. Effects of transcriptional start site sequence and position on nucleotide-sensitive selection of alternative start sites at the pyrC promoter in Escherichia coli. J. Bacteriol. 1994;176:2938–2945. doi: 10.1128/jb.176.10.2938-2945.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwartz M, Neuhard J. Control of expression of the pyr genes in Salmonella typhimurium: effects of variations in uridine and cytidine nucleotide pools. J. Bacteriol. 1975;121:814–822. doi: 10.1128/jb.121.3.814-822.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuehner JN, Brow DA. Regulation of a eukaryotic gene by GTP-dependent start site selection and transcription attenuation. Mol. Cell. 2008;31:201–211. doi: 10.1016/j.molcel.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Thiebaut M, Colin J, Neil H, Jacquier A, Séraphin B, Lacroute F, Libri D. Futile cycle of transcription initiation and termination modulates the response to nucleotide shortage in S. cerevisiae. Mol. Cell. 2008;31:671–682. doi: 10.1016/j.molcel.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Amiott EA, Jaehning JA. Sensitivity of the yeast mitochondrial RNA polymerase to +1 and +2 initiating nucleotides. J. Biol. Chem. 2006;281:34982–34988. doi: 10.1074/jbc.M608638200. [DOI] [PubMed] [Google Scholar]
- 9.Helmann JD, DeHaseth PL. Protein-nucleic acid interactions during open complex formation investigated by systematic alteration of the protein and DNA binding partners. Biochemistry. 1999;38:5959–5967. doi: 10.1021/bi990206g. [DOI] [PubMed] [Google Scholar]
- 10.Paul BJ, Ross W, Gaal T, Gourse RL. rRNA transcription in Escherichia coli. Annu. Rev. Genet. 2004;38:749–770. doi: 10.1146/annurev.genet.38.072902.091347. [DOI] [PubMed] [Google Scholar]
- 11.Haugen SP, Ross W, Gourse RL. Advances in bacterial promoter recognition and its control by factors that do not bind DNA. Nat. Rev. Microbiol. 2008;6:507–519. doi: 10.1038/nrmicro1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaal T, Bartlett MS, Ross W, Turnbough CL, Gourse RL. Transcription regulation by initiating NTP concentration: rRNA synthesis in bacteria. Science. 1997;278:2092–2097. doi: 10.1126/science.278.5346.2092. [DOI] [PubMed] [Google Scholar]
- 13.Revyakin A, Ebright RH, Strick TR. Promoter unwinding and promoter clearance by RNA polymerase: detection by single-molecule DNA nanomanipulation. Proc. Natl Acad. Sci. USA. 2004;101:4776–4780. doi: 10.1073/pnas.0307241101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krásný L, Tišerová H, Jonák J, Rejman D, Šanderová H. The identity of the transcription +1 position is crucial for changes in gene expression in response to amino acid starvation in Bacillus subtilis. Mol. Microbiol. 2008;69:42–54. doi: 10.1111/j.1365-2958.2008.06256.x. [DOI] [PubMed] [Google Scholar]
- 15.Murray HD, Schneider DA, Gourse RL. Control of rRNA expression by small molecules is dynamic and nonredundant. Mol. Cell. 2003;12:125–134. doi: 10.1016/s1097-2765(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 16.Henkin TM, Sonenshein AL. Mutations of the Escherichia coli lacUV5 promoter resulting in increased expression in Bacillus subtilis. Mol. Gen. Genet. 1987;209:467–474. doi: 10.1007/BF00331151. [DOI] [PubMed] [Google Scholar]
- 17.Hirvonen CA, Ross W, Wozniak CE, Marasco E, Anthony JR, Aiyar SE, Newburn VH, Gourse RL. Contributions of UP elements and the transcription factor FIS to expression from the seven rrn P1 promoters in Escherichia coli. J. Bacteriol. 2001;183:6305–6314. doi: 10.1128/JB.183.21.6305-6314.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haugen SP, Berkmen MB, Ross W, Gaal T, Ward C, Gourse RL. rRNA promoter regulation by nonoptimal binding of sigma region 1.2: an additional recognition element for RNA polymerase. Cell. 2006;125:1069–1082. doi: 10.1016/j.cell.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Haugen SP, Ross W, Manrique M, Gourse RL. Fine structure of the promoter—sigma region 1.2 interaction. Proc. Natl Acad. Sci. USA. 2008;2007:2–7. doi: 10.1073/pnas.0709513105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaal T, Barkei J, Dickson RR, DeBoer HA, DeHaseth PL, Alavi H, Gourse RL. Saturation mutagenesis of an Escherichia coli rRNA promoter and initial characterization of promoter variants. J. Bacteriol. 1989;171:4852–4861. doi: 10.1128/jb.171.9.4852-4861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krásný L, Gourse RL. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 2004;23:4473–4483. doi: 10.1038/sj.emboj.7600423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Natori Y, Tagami K, Murakami K, Yoshida S, Tanigawa O, Moh Y, Masuda K, Wada T, Suzuki S, Nanamiya H, et al. Transcription activity of individual rrn operons in Bacillus subtilis mutants deficient in (p)ppGpp synthetase genes, relA, yjbM, and ywaC. J. Bacteriol. 2009;191:4555–4561. doi: 10.1128/JB.00263-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lopez JM, Dromerick A, Freese E. Response of guanosine 5′-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J. Bacteriol. 1981;146:605–613. doi: 10.1128/jb.146.2.605-613.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tojo S, Kumamoto K, Hirooka K, Fujita Y. Heavy involvement of stringent transcription control depending on the adenine or guanine species of the transcription initiation site in glucose and pyruvate metabolism in Bacillus subtilis. J. Bacteriol. 2010;192:1573–1585. doi: 10.1128/JB.01394-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tojo S, Satomura T, Kumamoto K, Hirooka K, Fujita Y. Molecular mechanisms underlying the positive stringent response of the Bacillus subtilis ilv-leu operon, involved in the biosynthesis of branched-chain amino acids. J. Bacteriol. 2008;190:6134–6147. doi: 10.1128/JB.00606-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross W, Thompson JF, Newlands JT, Gourse RL. E.coli Fis protein activates ribosomal RNA transcription in vitro and in vivo. EMBO J. 1990;9:3733–3742. doi: 10.1002/j.1460-2075.1990.tb07586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guérout-Fleury A, Frandsen N, Stragier P. Plasmids for ectopic integration on Bacillus subtilis. Gene. 1996;180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- 28.Mitani T, Heinze JE, Freese E. Induction of sporulation in Bacillus subtilis by decoyinine and hadacidin. Biochem. Biophys. Res. Commun. 1977;77:1118–1125. doi: 10.1016/s0006-291x(77)80094-6. [DOI] [PubMed] [Google Scholar]
- 29.Qi Y, Hulett FM. PhoP-P and RNA polymerase sigmaA holoenzyme are sufficient for transcription of Pho regulon promoters in Bacillus subtilis: PhoP-P activator sites within the coding region stimulate transcription in vitro. Mol. Microbiol. 1998;28:1187–1197. doi: 10.1046/j.1365-2958.1998.00882.x. [DOI] [PubMed] [Google Scholar]
- 30.Chang BY, Doi RH. Overproduction, purification, and characterization of Bacillus subtilis RNA polymerase sigma A factor. J. Bacteriol. 1990;172:3257–3263. doi: 10.1128/jb.172.6.3257-3263.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Juang Y-L, Helmann JD. A promoter melting region in the primary sigma factor of Bacillus subtilis. J. Mol. Biol. 1994;235:1470–1488. doi: 10.1006/jmbi.1994.1102. [DOI] [PubMed] [Google Scholar]
- 32.Barker MM, Gaal T, Josaitis CA, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J. Mol. Biol. 2001;305:673–688. doi: 10.1006/jmbi.2000.4327. [DOI] [PubMed] [Google Scholar]
- 33.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schneider DA, Murray HD, Gourse RL. Measuring control of transcription initiation by changing concentrations of nucleotides and their derivatives. Methods Enzymol. 2003;370:606–617. doi: 10.1016/S0076-6879(03)70051-2. [DOI] [PubMed] [Google Scholar]
- 35.Johnston EB, Lewis PJ, Griffith R. The interaction of Bacillus subtilis sigmaA with RNA polymerase. Protein Sci. 2009;18:2287–2297. doi: 10.1002/pro.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hudson BP, Quispe J, Lara-González S, Kim Y, Berman HM, Arnold E, Ebright RH, Lawson CL. Three-dimensional EM structure of an intact activator-dependent transcription initiation complex. Proc. Natl Acad. Sci. USA. 2009;106:19830–19835. doi: 10.1073/pnas.0908782106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Widom RL, Jarvis ED, LaFauci G, Rudner R. Instability of rRNA operons in Bacillus subtilis. J. Bacteriol. 1988;170:605–610. doi: 10.1128/jb.170.2.605-610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henkin TM. Ribosomes, protein synthesis factors, and tRNA synthetases. In: Sonenshein AL, Hoch JA, Losick R, editors. Bacillus subtilis and Its Closest Relatives: From Genes to Cells. Washington, DC: American Society for Microbiology; 2002. pp. 313–322. [Google Scholar]
- 39.Fukushima T, Ishikawa S, Yamamoto H, Ogasawara N, Sekiguchi J. Transcriptional, functional and cytochemical analyses of the veg gene in Bacillus subtilis. J. Biochem. 2003;133:475–483. doi: 10.1093/jb/mvg062. [DOI] [PubMed] [Google Scholar]
- 40.Le Grice SF, Shin CC, Whipple F, Sonenshein AL. Separation and analysis of the RNA polymerase binding sites of a complex Bacillus subtilis promoter. Mol. Gen. Genet. 1986;204:229–236. doi: 10.1007/BF00425503. [DOI] [PubMed] [Google Scholar]
- 41.Barker MM, Gourse RL. Regulation of rRNA transcription correlates with nucleoside triphosphate sensing. J. Bacteriol. 2001;183:6315–6323. doi: 10.1128/JB.183.21.6315-6323.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward JB, Zahler SA. Genetic studies of leucine biosynthesis in Bacillus subtilis. J. Bacteriol. 1973;116:719–726. doi: 10.1128/jb.116.2.719-726.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eymann C, Homuth G, Scharf C, Hecker M. Bacillus subtilis functional genomics : global characterization of the stringent response by proteome and transcriptome analysis. J. Bacteriol. 2002;184:2500–2520. doi: 10.1128/JB.184.9.2500-2520.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wipat A, Carter N, Brignell SC, Guy BJ, Piper K, Sanders J, Emmerson PT, Harwood CR. The dnaB-pheA (256°-240°) region of the Bacillus subtilis chromosome containing genes responsible for stress responses , the utilization of plant cell walls and primary metabolism. Microbiology. 1996;142:3067–3078. doi: 10.1099/13500872-142-11-3067. [DOI] [PubMed] [Google Scholar]
- 45.Choonee N, Even S, Zig L, Putzer H. Ribosomal protein L20 controls expression of the Bacillus subtilis infC operon via a transcription attenuation mechanism. Nucleic Acids Res. 2007;35:1578–1588. doi: 10.1093/nar/gkm011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hove-Jensen B. Identification of tms-26 as an allele of the gcaD gene, which encodes N-acetylglucosamine 1-phosphate uridyltransferase in Bacillus subtilis. J. Bacteriol. 1992;174:6852–6856. doi: 10.1128/jb.174.21.6852-6856.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolf M. Phylogeny of Firmicutes with special reference to Mycoplasma (Mollicutes) as inferred from phosphoglycerate kinase amino acid sequence data. Int. J. Syst. Evol. Microbiol. 2004;54:871–875. doi: 10.1099/ijs.0.02868-0. [DOI] [PubMed] [Google Scholar]
- 48.Ventura M, Canchaya C, Tauch A, Chandra G, Fitzgerald GF, Chater KF, van Sinderen D. Genomics of Actinobacteria: tracing the evolutionary history of an ancient phylum. Microbiol. Mol. Biol. Rev. 2007;71:495–548. doi: 10.1128/MMBR.00005-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yap WH, Wang Y. Molecular cloning and comparative sequence analyses of rRNA operons in Streptomyces nodosus ATCC 14899. Gene. 1999;232:77–85. doi: 10.1016/s0378-1119(99)00112-2. [DOI] [PubMed] [Google Scholar]
- 50.Gonzalez-y-Merchand JA, Colstonl MJ, Cox RA, Hill M, Nw L. The rRNA operons of Mycobacterium smegmatis and Mycobacterium tuberculosis: comparison of promoter elements and of neighbouring upstream genes. Microbiology. 1996;142:667–674. doi: 10.1099/13500872-142-3-667. [DOI] [PubMed] [Google Scholar]
- 51.Naryshkin N, Revyakin A, Kim Y, Mekler V, Ebright RH. Structural organization of the RNA polymerase-promoter open complex. Cell. 2000;101:601–611. doi: 10.1016/s0092-8674(00)80872-7. [DOI] [PubMed] [Google Scholar]
- 52.Camacho A, Salas M. Effect of mutations in the “extended -10” motif of three Bacillus subtilis sigmaA-RNA polymerase-dependent promoters. J. Mol. Biol. 1999;286:683–693. doi: 10.1006/jmbi.1998.2526. [DOI] [PubMed] [Google Scholar]
- 53.Voskuil MI, Chambliss GH. The -16 region of Bacillus subtilis and other gram-positive bacterial promoters. Nucleic Acids Res. 1998;26:3584–3590. doi: 10.1093/nar/26.15.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Voskuil MI, Chambliss GH. The TRTGn motif stabilizes the transcription initiation open complex. J. Mol. Biol. 2002;322:521–532. doi: 10.1016/s0022-2836(02)00802-1. [DOI] [PubMed] [Google Scholar]
- 55.Helmann JD. Compilation and analysis of Bacillus subtilis sigma A-dependent promoter sequences: evidence for extended contact between RNA polymerase and upstream promoter DNA. Nucleic Acids Res. 1995;23:2351–2360. doi: 10.1093/nar/23.13.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whipple FW, Sonenshein AL. Mechanism of initiation of transcription by Bacillus subtilis RNA polymerase at several promoters. J. Mol. Biol. 1992;223:399–414. doi: 10.1016/0022-2836(92)90660-c. [DOI] [PubMed] [Google Scholar]
- 57.Ishikawa S, Oshima T, Kurokawa K, Kusuya Y, Ogasawara N. RNA polymerase trafficking in Bacillus subtilis cells. J. Bacteriol. 2010;192:5778–5787. doi: 10.1128/JB.00489-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Artsimovitch I, Svetlov V, Murakami KS, Landick R. Co-overexpression of Escherichia coli RNA polymerase subunits allows isolation and analysis of mutant enzymes lacking lineage-specific sequence insertions. J. Biol. Chem. 2003;278:12344–12355. doi: 10.1074/jbc.M211214200. [DOI] [PubMed] [Google Scholar]
- 59.Grandoni JA, Zahler SA, Calvo JM. Transcriptional regulation of the ilv-leu operon of Bacillus subtilis. J. Bacteriol. 1992;174:3212–3219. doi: 10.1128/jb.174.10.3212-3219.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mader U, Hennig S, Hecker M, Homuth G. Transcriptional organization and posttranscriptional regulation of the Bacillus subtilis branched-chain amino acid biosynthesis genes. J. Bacteriol. 2004;186:2240–2252. doi: 10.1128/JB.186.8.2240-2252.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shivers RP, Sonenshein AL. Bacillus subtilis ilvB operon: an intersection of global regulons. Mol. Microbiol. 2005;56:1549–1559. doi: 10.1111/j.1365-2958.2005.04634.x. [DOI] [PubMed] [Google Scholar]
- 62.Tojo S, Satomura T, Morisaki K, Yoshida K, Hirooka K, Fujita Y. Negative Transcriptional regulation of the ilv-leu operon for biosynthesis of branched-chain amino acids through the Bacillus subtilis global regulator TnrA. J. Bacteriol. 2004;186:7971–7979. doi: 10.1128/JB.186.23.7971-7979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tojo S, Satomura T, Morisaki K, Deutscher J, Hirooka K, Fujita Y. Elaborate transcription regulation of the Bacillus subtilis ilv-leu operon involved in the biosynthesis of branched-chain amino acids through global regulators of CcpA, CodY and TnrA. Mol. Microbiol. 2005;56:1560–1573. doi: 10.1111/j.1365-2958.2005.04635.x. [DOI] [PubMed] [Google Scholar]
- 64.Rutherford ST, Lemke JJ, Vrentas CE, Gaal T, Ross W, Gourse RL. Effects of DksA, GreA, and GreB on transcription initiation: insights into the mechanism of factors that bind in the secondary channel of RNA polymerase. J. Mol. Biol. 2007;366:1243–1257. doi: 10.1016/j.jmb.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roberts JW, Shankar S, Filter JJ. RNA polymerase elongation factors. Annu. Rev. Microbiol. 2008;62:211–233. doi: 10.1146/annurev.micro.61.080706.093422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rutherford ST, Villers CL, Lee J-H, Ross W, Gourse RL. Allosteric control of Escherichia coli rRNA promoter complexes by DksA. Genes Dev. 2009:236–248. doi: 10.1101/gad.1745409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Potrykus K, Vinella D, Murphy H, Szalewska-Palasz A, D’Ari R, Cashel M. Antagonistic regulation of Escherichia coli ribosomal RNA rrnB P1 promoter activity by GreA and DksA. J. Biol. Chem. 2006;281:15238–15248. doi: 10.1074/jbc.M601531200. [DOI] [PubMed] [Google Scholar]
- 68.Motáčková V, Šanderová H, Žídek L, Nováček J, Padrta P, Švenková A, Korelusová J, Jonák J, Krásný L, Sklenář V. Solution structure of the N-terminal domain of Bacillus subtilis delta subunit of RNA polymerase and its classification based on structural homologs. Proteins. 2010;78:1807–1810. doi: 10.1002/prot.22708. [DOI] [PubMed] [Google Scholar]
- 69.López de Saro FJ, Yoshikawa N, Helmann JD. Expression, abundance, and RNA polymerase binding properties of the delta factor of Bacillus subtilis. J. Biol. Chem. 1999;274:15953–15958. doi: 10.1074/jbc.274.22.15953. [DOI] [PubMed] [Google Scholar]
- 70.Doherty G, Fogg M, Wilkinson A, Lewis P. Small subunits of RNA polymerase: localisation, levels and implications for core enzyme composition. Microbiology. 2010;156:3532–3543. doi: 10.1099/mic.0.041566-0. [DOI] [PubMed] [Google Scholar]
- 71.Doherty GP, Meredith DH, Lewis PJ. Subcellular partitioning of transcription factors in Bacillus subtilis. J. Bacteriol. 2006;188:4101–4110. doi: 10.1128/JB.01934-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xue X, Tomasch J, Sztajer H, Wagner-Döbler I. The delta subunit of RNA polymerase, RpoE, is a global modulator of Streptococcus mutans environmental adaptation. J. Bacteriol. 2010;192:5081–5092. doi: 10.1128/JB.00653-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Achberger EC, Whiteley HR. The role of the delta peptide of the Bacillus subtilis RNA polymerase in promoter selection. J. Biol. Chem. 1981;256:7424–7432. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.