Abstract
Background
Alterations in cardiac energy metabolism downstream of neurohormonal stimulation play a crucial role in the pathogenesis of heart failure (HF). The chronic adrenergic stimulation that accompanies HF is a signaling abnormality that leads to the up-regulation of G protein-coupled receptor kinase 2 (GRK2), which is pathological in the myocyte during disease progression in part due to uncoupling of the β-adrenergic receptor (βAR) system. In this study we explored the possibility that enhanced GRK2 expression and activity, as seen during HF, can negatively affect cardiac metabolism as part of its pathogenic profile.
Methods and Results
Positron Emission Tomography (PET) studies revealed that transgenic mice with cardiac-specific overexpression of GRK2 negatively impacted cardiac metabolism by inhibiting glucose uptake and desensitization of insulin signaling, which increases after ischemic injury and precedes HF development. Mechanistically, GRK2 interacts with and directly phosphorylates insulin receptor substrate-1 (IRS1) in cardiomyocytes causing insulin-dependent negative signaling feedback including inhibition of membrane translocation of the glucose transporter, GLUT4. This identifies IRS1 as a novel non-receptor target for GRK2 and represents a new pathological mechanism for this kinase in the failing heart. Importantly, inhibition of GRK2 activity prevents post-ischemic defects in myocardial insulin signaling and improves cardiac metabolism via normalized glucose uptake, which appears to participate in GRK2-targeted prevention of HF.
Conclusions
Our data provide novel insight into how GRK2 is pathological in the injured heart. Moreover, it appears to be a critical mechanistic link within neurohormonal crosstalk governing cardiac contractile signaling/function through βARs and metabolism through the insulin receptor.
Keywords: Cardiac Metabolism, Glucose, Heart Failure, Ischemic Heart Disease, Cardiac PET
Introduction
Despite improvements in the treatment of heart failure (HF) the prognosis of this disease remains poor 1, 2. The reason that HF continues to worsen even in patients receiving optimal therapy is unclear, however, there is increasing evidence that perturbations in cardiac metabolism can contribute to the progression of cardiomyopathy as well as to a loss of pharmacologic effectiveness 3, 4. Therefore, approaches targeting more efficient substrate utilization and preservation of cardiac metabolism are attractive therapeutic strategies 5. In the adult heart the major pathway for ATP production is fatty acid oxidation 6, 7 while the relative contribution of glucose increases during stress or injury, such as during exercise or ischemia 5. During HF there is excessive uptake and myocardial free fatty acid accumulation with reduced glucose utilization 8, 9. In animal models of HF and also in human disease these metabolic alterations reduce myocardial oxygen efficiency and lead to a depletion of intracellular ATP 10, 11.
Insulin receptor signaling is critically involved in increasing glucose uptake in the myocardium and cardiac insulin resistance contributes to the development of left ventricular dysfunction by reducing cardiac efficiency through metabolic shift towards fatty acids utilization12. Indeed, a profound state of insulin resistance has been found in the hearts of ob/ob mice and the ability of these hearts to modulate substrate utilization in response to insulin and changes in fatty acid supply is altered 13. Importantly, normalization of cardiac metabolism by overexpressing a human GLUT4 transgene in mice with insulin resistance recovered the altered cardiac function observed in these animals 14, 15. Therefore, these studies indicate that cardiac insulin resistance reduces the metabolic efficiency of the heart, which can lead to contractile dysfunction. Moreover, insulin resistance is a known and recognized phenomenon leading to HF16, as seen in positron emission tomographic (PET) studies showing that failing human myocardium has reduced glucose uptake and increased free fatty acid uptake 17.
Several hypotheses have been proposed to explain the association between altered cardiac metabolism, insulin resistance, and HF, and among these there is a strong correlation with neurohormonal activation 18, 19, which increases plasma free fatty acid levels and inhibits insulin receptor signaling resulting in a loss of myocyte glucose uptake 20. An important abnormality in the myocyte induced by neurohormonal activation in HF is the up-regulation of G protein-coupled receptor (GPCR) kinase 2 (GRK2), which classically phosphorylates activated GPCRs, such as the β-adrenergic receptors (βARs) in the heart, leading to attenuated signaling 21. In human HF, increased GRK2 is associated with lower cardiac function and prognosis 22, 23.
Recent evidence suggests that GRK2 can have non-GPCR effects including playing an unidentified role in the regulation of insulin signaling in non-cardiac cells 24, 25. Moreover, increases in GRK2 activity following βAR stimulation inhibits insulin-dependent glucose extraction 26. Therefore, we hypothesized that increased levels of GRK2, as seen during HF, directly induces myocardial insulin resistance and, given the importance of cardiac signaling for efficient metabolic substrate usage, we have studied the role of GRK2 in regulating cardiac insulin signaling and metabolism in normal and post-ischemic myocytes and hearts. Our data reveal a role for GRK2 in HF pathogenesis that extends beyond the known negative effects on βAR signaling and cardiac contractility by demonstrating that GRK2 can critically and negatively impact cardiac insulin signaling and metabolism.
Methods
Experimental Procedures
Experimental procedures were performed essentially as describedpreviously 27, 28. An expanded Methods section appears in the online-Data Supplement.
Statistical analyses
All experiments were performed at least in triplicate using cell isolates from different rats and the results were expressed as the mean±s.e.m. We used Prism 4 and SPSS 17 to perform statistical analysis with two-tailed Student t-test for in-vitro studies, and two way and repeated measure Anova with a Bonferroni post hoc test for the in-vivo studies. SPSS was used to evaluate the normal distribution according to the Levene test for normality, and provide different p values for unpaired samples depending on normal distribution. Even when the data is not normally distributed, we still found significant differences between means of different treatments and/or groups. A P< 0.05 was considered statistically significant.
RESULTS
GRK2 levels influence in vivo cardiac glucose uptake
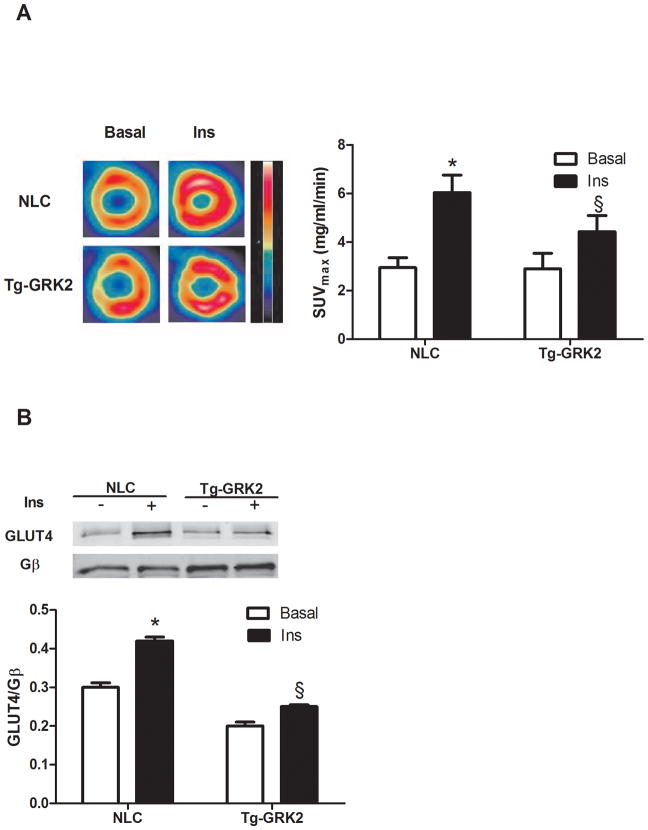
We first examined whether increased GRK2 in cardiomyocytes can alter in vivo glucose uptake in the hearts of mice following insulin treatment. This is important since myocardial GRK2 up-regulation occurs acutely after ischemic injury 29 and may trigger further signaling defects. Therefore, we performed micro-PET using 18 -fluorodeoxyglucose (18-FDG) in transgenic mice with cardiac-specific GRK2 overexpression (Tg-GRK2 30) and their non-transgenic littermate controls (NLCs). An insulin injection produces a robust increase in in vivo 18-FDG accumulation in the hearts of NLC mice, which is significantly lower in Tg-GRK2 mice (Fig. 1A). Mechanistically, GRK2 enhancement appears to cause a loss of insulin-dependent GLUT4 membrane translocation (Fig 1B).
Figure 1. GRK2 levels influence in vivo cardiac glucose uptake.
(A) Tg-GRK2 and NLC mice were studied by PET to evaluate cardiac glucose uptake after intraperitoneal injection of insulin (Ins, 0.075U/Kg, IP). Ins significantly increases glucose uptake however this response was attenuated in BK12 mice (*, P<0.05, Ins vs Basal, §, P<0.05, TG-GRK2 vs NLC, n=8 per group). (B) Plasma membrane GLUT4 protein levels from Tg-GRK2 and NLC hearts. Animals were injected with Ins as above and sacrificed 15 min later. GLUT4 levels on the plasma membrane were increased by Ins in NLC but attenuated in TG-GRK2. GLUT4 level was normalized to the membrane protein Gβ (*, P<0.05, Ins vs Basal, §, P<0.05, fold of increased translocation to the membrane, TG-GRK2 vs NLC, n=3 per group).
GRK2 regulation of insulin signaling in cardiomyocytes
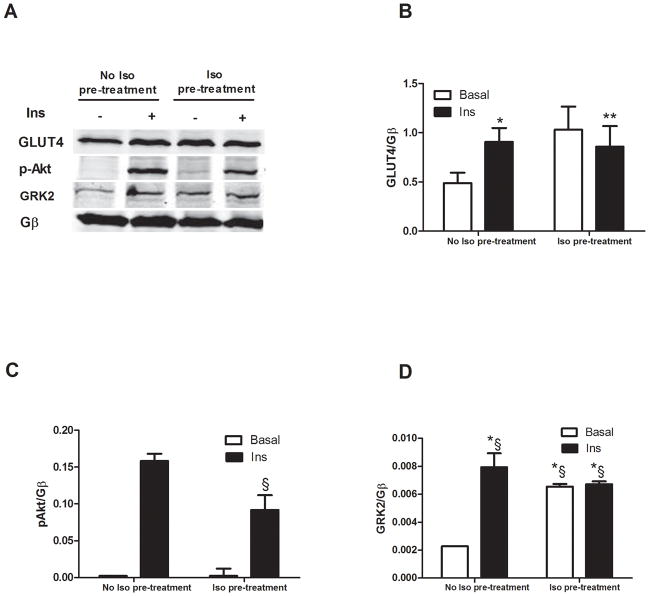
Due to the above provocative in vivo results we explored molecular mechanisms involving GRK2 and insulin signaling in cultured adult rat ventricular cardiomyocytes (ARVMs). As expected, when ARVMs were exposed to insulin, GLUT4 membrane translocation was induced as was the activation of the downstream kinase, Akt (Fig. 2A–C). Unexpectedly, we found that insulin induced the membrane translocation of GRK2 (Fig 2A, D). The cause of GRK2 membrane translocation after insulin administration is unclear but we next evaluated whether increased membrane GRK2 levels could alter downstream insulin receptor signaling. To do this we pre-stimulated ARVMs with the βAR agonist isoproterenol (Iso) to cause Gβγ-dependent GRK2 translocation, which was evident in ARVMs (Fig. 2A, D). Interestingly, following Iso pre-treatment, insulin failed to induce GLUT4 translocation and there was also significantly less insulin-dependent Akt activation (Fig. 2A–C).
Figure 2. Interplay between βAR and insulin receptor signaling.
ARVMs were stimulated with Ins (0.1 μM for 10 min) with or without pre-treatment with the βAR agonist isoproterenol (Iso, 10μM for 5 min). Membrane fractions were prepared and blotted for signaling proteins. (A) Representative western blot showing membrane GLUT4, pAkt and GRK2. Gβ blotting was used as loading control. (B–D) Bar Charts showing GLUT4 translocation (*, P<0.05 Ins vs. Basal, n=3; **, n.s, Iso+Ins vs Iso, n=3per group), Akt was activated in response to Ins but attenuated with Iso pretreatment (§ P<0.05, fold activation, Iso+Ins vs. Ins alone, n=3 per group) and GRK2 translocation induced by Ins with or without Iso pretreatment (*§, P<0.05 Ins, Iso and Iso+Ins vs Basal, n=3 per group).
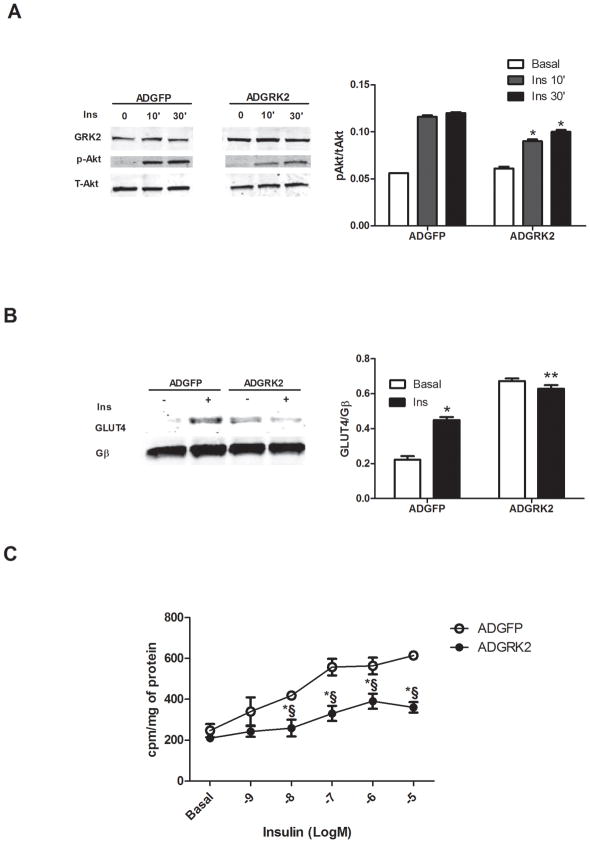
To further explore how GRK2 levels may alter myocyte insulin signaling we overexpressed GRK2 in ARVMs using an adenovirus (ADGRK2). We found that increased GRK2 in ARVMs attenuated insulin-stimulated Akt activation and GLUT4 membrane translocation compared to control myocytes infected with ADGFP (Fig. 3A, B). Reduced GLUT4 translocation is consistent with the above findings in Tg-GRK2 showing that enhanced GRK2 can lead to a loss of insulin-dependent in vivo cardiac glucose uptake. Indeed, we found that insulin-dependent [H3]-deoxyglucose (2DOG) uptake in ARVMs was significantly attenuated by ADGRK2 (Fig. 3C).
Figure 3. GRK2 overexpression in ARVMs inhibits cellular effects of insulin.
Adenoviral-infected ARVMs were treated with or without 0.1uM Ins for either 10 or 30min. (A) Representative Western blot from ARVMs whole lysate. Ins activation of Akt was attenuated by GRK2 overexpression. Total Akt was blotted as loading control (*, P<0.05 fold activation vs ADGFP, n=3 per group). (B) Western blot from plasma membranes of ARVM showing increased GLUT4 translocation in response to 0.1uM Ins for 10min with GFP overexpression but blunted with GRK overexpression (*, P<0.05 vs Basal; **, n.s. vs Basal, n=3), Gβ was used as loading control. (C) ARVMs were stimulated with Ins at different concentrations, ranging from 1 nM to 10 μM for 10 min in the presence of ADGFP or ADGRK2 infection. The rate of glucose uptake was determined by [3H] 2-DOG (*§, P < 0.01 vs. ADGFP, n=3 per group).
GRK2 activity is required for attenuation of cardiomyocyte insulin signaling via direct phosphorylation of IRS-1
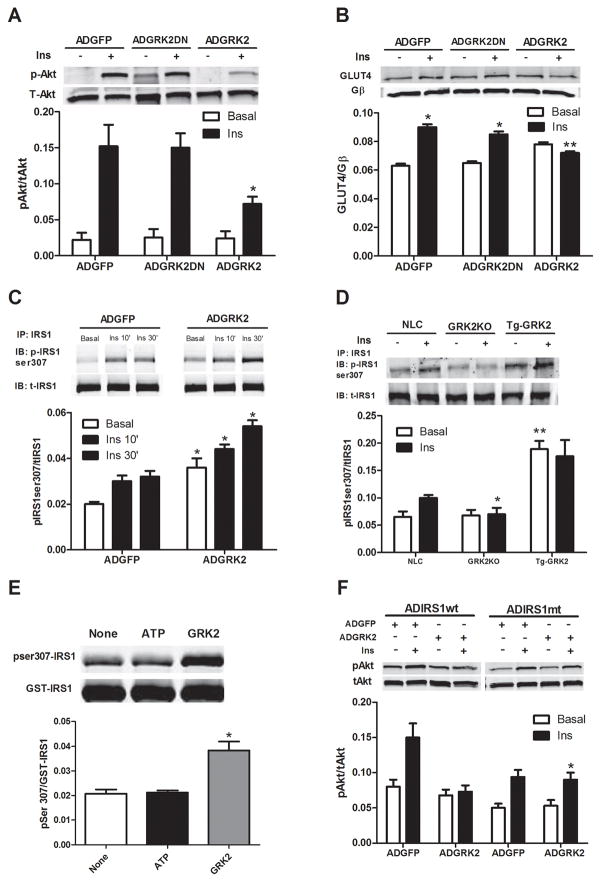
Mechanistically, GRK2 could exert its inhibitory effect on insulin-dependent GLUT4 translocation and cardiomyocyte glucose uptake and metabolism through different pathways. GLUT4 membrane translocation has been shown to be dependent on Gαq/11 signaling 25 and GRK2 has a known RGS domain within its amino-terminus that can interact directly with Gαq and inhibit its activity. Further, GRK2 has recently been shown to interact with and inhibit Akt in endothelial cells 31. We conducted experiments to test these possible mechanisms and found no evidence that GRK2 interacted with either of these proteins in ARVMs, either basally or following stimulation of cells with insulin (data not shown). Therefore, we explored the possibility that GRK2 could regulate insulin signaling in cardiomyocytes in other ways. Of note, kinase activity of GRK2 was necessary for the negative regulation of insulin signaling, because the kinase-dead (K220R) GRK2 dominant negative mutant (GRK2DN) failed to alter Akt activation (Fig. 4A) and GLUT4 membrane translocation in response to insulin (Fig. 4B).
Figure 4. Mechanism of inhibitory effects of GRK2 on insulin signaling in myocytes.
(A–B) ARVMs were infected with ADGFP, ADGRK2, or kinase-dead GRK2 (ADGRK2DN) and stimulated with Ins (0.1 μM for 10 min). (A) Representative immunoblot in whole cell lysates showing reduced pAkt level when GRK2 is overexpressed (*, fold activation, P<0.01 vs ADGFP and ADGRK2DN, n=3 per group). (B) Ins-stimulated GLUT4 membrane levels in ARVMs is attenuated with GRK2 overexpression (*, P<0.05 vs Basal; **, n.s. vs Basal, n=3) (C) IRS1 was immunoprecipitated from whole lysate of ARVMs infected with ADGFP or ADGRK2 and stimulated with Ins as above. Top panel, representative immunoblot for p-ser307 showing increased p-ser307 with ADGRK2 treatment. Lower panel is total IRS-1 (* P<0.05 vs ADGFP, n= 3). (D) Level of p-Ser307 of IRS1 was evaluated by immunoprecipitation of equal amount of IRS1 from isolated myocytes of NLC, Tg-GRK2 and cardiac GRK2 KO mice after stimulation with Ins (10−7 M, 10 min). Representative blots for GRK2, pSer307 IRS1 and total IRS1 are shown. Reduced and increased levels of pSer307IRS1 were respectively observed in GRK2KO and Tg-GRK2 versus NLC (*, P<0.01, fold of activation, GRK2KO vs NLC, n=3; **, P <0.01, Tg-GRK2 vs NLC, n=3 per group). (E) GRK2 phosphorylates IRS1 at Ser307. An in-vitro kinase assay was performed with purified GST-IRS1 alone (none) or in the presence of ATP or ATP plus purified GRK2. Representative blot from 3 independent assays (top panel) and densitometric analysis (lower panel) are shown. (*, P<0.05, fold activation vs ATP and none) (F) NRVMs were co-infected with either ADIRS1wt or ADIRS1 mutant Ser307Ala (mt) in addition to ADGFP or ADGRK2 and stimulated with Ins as above. Representative immunoblot showing p-Akt levels is provided (*, P<0.01, fold activation vs ADGRK2+ADIRS1wt, n=3 per group).
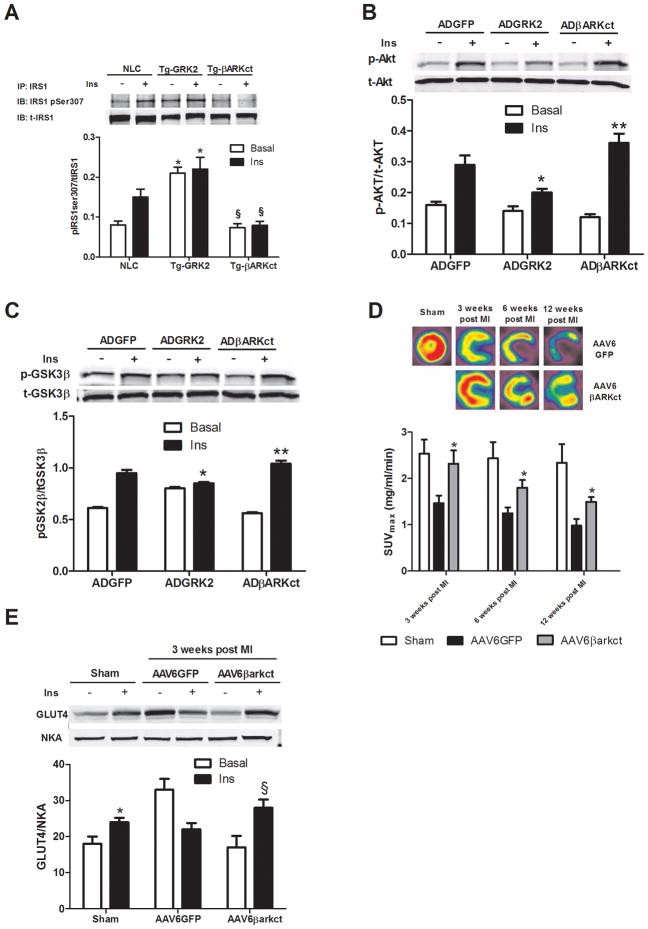
We next focused our attention to a potential regulatory role of GRK2 on insulin receptor substrate-1 (IRS1). This molecule moves to the plasma membrane following insulin stimulation and binds activated insulin receptors leading to Tyr phosphorylation and subsequent activation of downstream signaling 21. IRS1 association with the insulin receptor also initiates events that lead to the attenuation of insulin’s biological effects 32 through phosphorylation of IRS1 at Ser307, which disrupts IRS1 binding to the insulin receptor and promotes its proteasomal degradation 33. Of note, when IRS1 is phosphorylated at Ser307 there is a reduction of Tyr612 phosphorylation and there appears to be a reciprocal relationship between these two sites of post-translational modification 24, 33. A recent study has shown a physical interaction between GRK2 and IRS1 in adipocytes and muscle cells under basal conditions, which is disrupted by insulin treatment 34. However, our experiments in adult cardiac myocytes show that both GRK2 and IRS1 translocate to the plasma membrane. Moreover, they associate together and this interaction increases upon insulin treatment of myocytes (Supplementary Figures 1A, B). Therefore, we pursued the hypothesis that increased GRK2 activity might enhance the feedback loop induced by insulin via IRS1 inhibitory phophorylation. We observed that overexpression of GRK2 itself significantly increased Ser307 phosphorylation of IRS1 under basal conditions and after insulin treatment compared to ADGFP infected cells (Fig 4C).
To address this potential specific role of GRK2 in myocyte insulin signaling, we studied levels of phospho-Ser307 IRS1 in isolated cells from NLC, Tg-GRK2 and cardiac-specific GRK2 knockout (KO) mice 27 after insulin administration. Fig. 4D shows that increased cardiac GRK2 in Tg-GRK2 ARVMs results in increased basal and insulin-stimulated IRS1 pSer307 levels while GRK2KO myocytes have attenuated insulin-stimulated phosphorylation of IRS1 at Ser307 compared to NLC myocytes. Importantly, an in vitro kinase assay shows that purified GRK2 directly phosphorylates purified GST-IRS1 at Ser307 (Fig. 4E). The physiological relevance of GRK2 phosphorylation of Ser307 in insulin signaling regulation was explored by using an IRS1 in which Ser307 was mutated to alanine, which could not be phosphorylated by GRK2 (Supplementary Fig 1C). Overexpression of this mutated IRS1 by adenovirus (ADIRS1mt) in neonatal rat ventricular cardiomyocytes (NRVMs) removes the ADGRK2 inhibitory effects on insulin-mediated activation of Akt (Fig 4F) and GLUT4 translocation to the membrane (Supplementary Fig. 1D).
Consistent with GRK2 activity inducing Ser307 phosphorylation of IRS1, an inhibitory phosphorylation, we found that GRK2, but not the kinase-dead GRK2 mutant (K220R), also reduced the stimulatory-signaling Tyr612 phosphorylation in ARVMs (Supplementary Fig. 1E).
Cardiac glucose uptake defects precede HF development after MI and both are augmented with GRK2 up-regulation
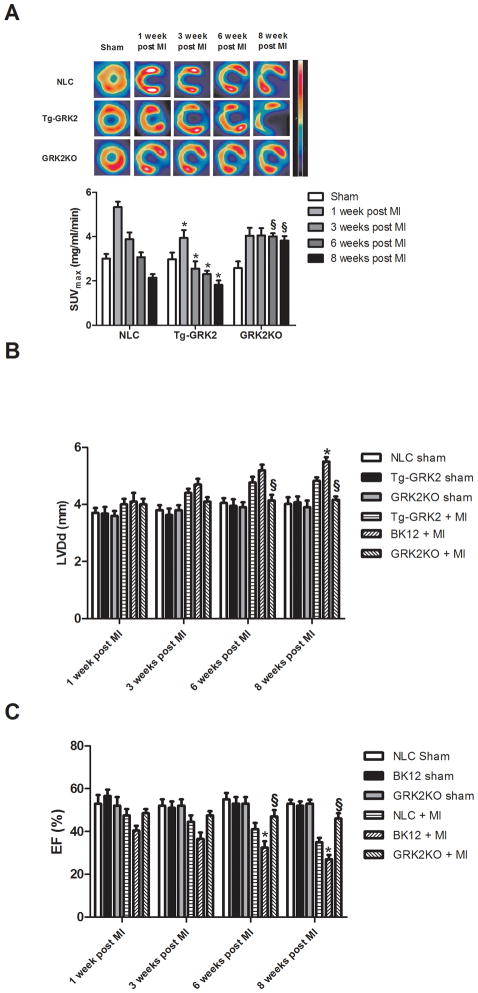
With our findings that increased GRK2 levels in myocytes negatively affects insulin signaling, we next sought to determine if this translates to the pathogenesis of HF. To determine whether GRK2-mediated loss of glucose metabolism occurs in compromised myocardium, we used micro-PET to follow 18-FDG uptake after myocardial infarction (MI). Left coronary artery ligation induced a reproducible left ventricular (LV) infarct in Tg-GRK2, GRK2KO and NLC mice as previously described 27 (data not shown). All groups showed increased 18-FDG uptake 1 week after MI consistent with the heart increasing to the more protective glucose metabolism 5. However, the acute post-MI glucose uptake response was significantly reduced in Tg-GRK2 mice compared to NLC (Fig. 5A). Further, a progressive reduction in glucose uptake after MI is observed in both NLC and Tg-GRK2 mice, which accompanies progressive post-MI cardiac dysfunction and LV remodeling as measured by echocardiography (Fig. 5B). In cardiac GRK2KO mice, there was an initial lower glucose uptake response acutely after MI, however, in vivo glucose uptake remained stable throughout the study period and this resulted in significantly higher uptake compared to the NLC group at 6–8 weeks post MI (Fig. 5A). Interestingly, as in ARVMs (Fig. 3C), attenuated cardiac glucose uptake in vivo over time was significantly attenuated with GRK2 overexpression and this worsened metabolic effect was most evident 1–3 weeks post-MI (Fig. 5A). This data shows that higher levels of myocardial GRK2 cause acute post-MI defects in glucose metabolism that are evident before LV dysfunction appears and accordingly, Tg-GRK2 mice have significantly worse cardiac function and remodeling chronically after MI while cardiac GRK2KO mice showed a preserved glucose utilization, resulting in preserved cardiac volume and function (Fig. 5B,C).
Figure 5. Cardiac glucose uptake in post-MI mice.
(A) 18-FDG cardiac uptake in NLC Tg-GRK2 and cardiac-specific GRK2KO mice under Sham conditions and 1–8 weeks post-MI. Shown at top are representative micro-PET images where infarct is clearly visible and histogram below quantifies 18-FDG uptake in myocardium (*, P<0.05, TG-GRK2 vs NLC; §, P<0.05, GRK2KO vs NLC, n=12 per group). (B–C) LV diameter at diastole and Ejection fraction (EF) in NLC, Tg-GRK2 and GRK2KO mice determined by echocardiography serially after MI. (*, P<0.01, Tg-GRK2 vs NLC; §, P<0.01, GRK2KO vs NLC, n=12, per group).
βARKct improves insulin signaling in adult cardiomyocytes
Our data indicate that excess GRK2 produces serious modifications in cardiac glucose uptake and insulin signaling in vivo and in vitro. Inhibition of GRK2 activity through the carboxyl terminal domain of GRK2 (βARKct) has previously rescued several models of HF21, 29. In light of the above data showing, in particular, the protective role of myocardial GRK2 loss on cardiac metabolism, we hypothesized that the beneficial effect of βARKct in rescuing cardiac function during HF could be due, at least in part, to a favorable effect on cardiac metabolism. Indeed, transgenic mice with cardiac βARKct expression (Tg-βARKct) have significantly lower inhibitory IRS1 Ser307 phosphorylation induced by insulin (Fig. 6A), similar to data in cardiac-specific GRK2 KO mice (Fig 4D). Therefore, βARKct has the potential to enhance insulin signaling and we tested this hypothesis by introducing βARKct in ARVMs via an adenovirus (ADβARKct). We found that βARKct expression restores and enhances Akt phosphorylation as well as a downstream target of Akt, GSK3β, when compared to levels found in ADGRK2 and ADGFP treated myocytes, respectively (Fig 6B,C).
Figure 6. Inhibition of GRK2 in myocytes and myocardium restores normal insulin signaling and prevents defective in vivo glucose uptake post-MI.
(A) Level of Ins-stimulated pSer307 in IRS1 immunoprecipitated from adult mouse ventricular myocytes isolated from NLC, Tg-GRK2, or Tg-βARKct mice (*, P<0.01 vs NLC, n=3; §, P<0.01, Tg-βARKct vs NLC, n=3 per group). (B) ARVMs were infected with either ADGFP, ADGRK2 or ADβARKct. Representative blots for p-Akt and t-Akt are shown (*, P <0.01, fold increase in activation, ADGRK2 vs ADGFP, n=3;**, P <0.01, fold increase in activation, ADβARKct vs ADGRK2 and ADGFP, n=3 per group). (C) Representative western blot from ARVMs whole lysate infected as in (A) and stimulated with Ins showing pGSK3β and total-GSK3β (*, P <0.01, fold increase in activation, ADGRK2 vs ADGFP, n=3; **, P <0.01, fold increase in activation, ADβARKct vs ADGRK2, n=3). (D) Global rat myocardial in vivo glucose uptake evaluated by micro-PET in Sham and 1, 3, 6, and 12 weeks post-MI rats treated with AAV6-GFP or AAV6-βARKct (*, P<0.05, vs AAV6GFP, n=8 per group). (E) Rats 3 weeks post MI and Sham were sacrificed 15 min after an IP injection of insulin (0.075 U/Kg) and hearts removed. Plasma membranes were prepared to evaluate GLUT4 levels as shown in a representative blot (*, P<0.05 vs Basal; §, P< 0.05 vs Basal, n=3 per group).
AAV6-βARKct gene therapy prevents GRK2-mediated defects in cardiac glucose metabolism prior to the rescue of subsequent HF
Next, we asked whether inhibiting GRK2 could lift the negative effects of this kinase on in vivo myocardial insulin signaling and measured myocardial glucose uptake and insulin responses in post-MI rat hearts that expressed βARKct. To do this, we injected AAV6-GFP or AAV6-βARKct into the myocardium of rats during surgery to induce a MI as we have done previously 28. Then, we serially measured in vivo glucose uptake in the heart via micro-PET along with LV dimension and function via echocardiography and LV catheterization. As expected, our method of cardiac gene transfer led to chronic and robust βARKct expression in the rat heart (Supplementary Fig. 2A). AAV6-βARKct delivery compared to AAV6-GFP did not produce any significant differences in cardiac function and volume in sham-treated rats (data not shown), therefore in our data presentation we only show one sham control group.
MI induces progressive dilatation and LV dysfunction (Supplementary Fig 2C,D-Table 1) and consistent with human and animal models of HF, we found a progressive increase in myocardial GRK2 levels after MI (Supplementary Fig 2B). Rats treated with AAV6-βARKct had significantly improved post-MI cardiac function and significantly less remodeling throughout the 12 weeks of study (Supplementary Fig 2C,D and Supplementary Table 1) with respect to the AAV6-GFP group. When we assessed in vivo glucose uptake post-MI via micro-PET, we found that MI caused an acute defect in 18-FDG uptake in control AAV6-GFP treated rats and this metabolic abnormality was evident before any cardiac dysfunction or remodeling was found (Fig. 6D), a finding that was similar to the above studies in mice (Fig. 5). Interestingly, AAV6-βARKct treated rats actually had normal myocardial glucose uptake 3 weeks post-MI and this was significantly improved over post-MI GFP treated rats throughout the study (Fig 6D). To evaluate the potential in vivo mechanism of the above PET results, we took a cohort of 3 week post-MI rats and challenged them with insulin to measure GLUT4 membrane localization and found this significantly blunted in GFP-treated rats, however, AAV6-βARKct treated animals had significantly improved insulin responsiveness (Fig. 6E).
Discussion
Our data adds significantly to the dynamic role of GRK2 in the pathogenesis of HF as it uncovers a new mechanism upon which up-regulated GRK2 in the injured myocardium promotes ventricular dysfunction. Importantly, enhanced GRK2 activity not only negatively impacts cardiac contractile function after myocardial injury but it is also causes abnormal cardiac metabolism. Further, GRK2 may represent a molecular link between the excessive neurohormonal activation that follows cardiac stress and initiation of defects in myocyte energy substrate utilization. Of note, our data reveals that GRK2 inhibition via βARKct expression or its gene deletion in myocytes corrects metabolic alterations observed during the early stages of post-ischemic HF. This facilitates myocyte usage of glucose which is a protective metabolic substrate, and delays the development of HF.
As observed in our micro-PET studies the modification in cardiac glucose uptake is an early event after induction of MI. In particular, the initial increase is followed by a progressive reduction, which is then accompanied by a progressive cardiac dilation and reduced function. This behavior observed in NLC control mice is significantly modified in mice with altered GRK2 levels in myocytes. Tg-GRK2 mice with enhanced GRK2 cardiac expression significantly reduces protective myocyte glucose uptake while cardiac-targeted GRK2KO mice have preserved glucose uptake throughout the 8 weeks post-MI. Given the higher efficiency of glucose in ATP production and the lower impact in oxidative stress with respect to other substrates, we show that GRK2-mediated pathogenesis in HF is mediated at least in part through negative alterations in cardiac metabolism.
Our findings once again highlight the deleterious effect of increased GRK2 levels that accompany myocardial ischemia, and most importantly, confirm the importance of identifying strategies aimed specifically to reduce its activity in the heart as potential HF therapy. Moreover, GRK2 inhibition with the βARKct, which has been demonstrated to rescue or prevent animals models of HF and also improves contractile function of failing human ventricular myocytes 28, 35, has beneficial effects that extend beyond the functional recovery of βARs and contractility. Indeed, previous studies showed that GLUT4 overespression improves cardiac function in mice with cardiac insulin resistance 14, 15 and here we demonstrate that lowering GRK2 during HF improves glucose uptake and delays the development of the disease. We attribute at least part of the improvement to restored insulin-stimulated GLUT4 membrane translocation. GRK2 also represents the first identification of a molecule that represents a nodal link between excessive neurohormonal stimulation and metabolic abnormalities, supporting the hypothesis that strategies aimed to preserve myocardial metabolism could be beneficial in the treatment or prevention of HF.
Insulin signaling is known to be protective in the heart by inhibiting apoptosis and oxidative stress 36 and is also an important regulator of cardiac mass 37. These effects are primarily mediated by activation of PI3K-Akt and inhibition of GSK3β 38. Based on the results of this study, injury-induced increases in GRK2 within the cardiomyocyte may increase oxidative stress, which expands our mechanistic understanding of why Tg-GRK2 mice progress more rapidly to HF. Therefore, GRK2 clearly has effects in the myocyte that extend beyond GPCR-mediated functions improving our mechanistic understanding of the pathological nature of GRK2 in the heart.
Of interest, recent studies have described a correlation between insulin resistance and increased levels of GRK2 in pathological conditions. In particular, Garcia-Guerra et al. have recently found that overexpression of either GRK2 or the kinase-dead mutant reduce insulin sensitivity in myoblasts and adipocytes, 34 and that finding was consistent with other studies25, 31 that suggested a kinase independent mechanism was involved, possibly by sequestering molecules involved in insulin signaling, such as Gαq/11, Akt, and IRS1. However, our data demonstrates for the first time that the catalytic activity of GRK2 is fundamental and a requirement in post-ischemic cardiac metabolic alterations - in particular, the dampening insulin signaling.
The finding that Ser307 of IRS1 is a target for GRK2 phosphorylation in cardiomyocytes significantly increases the overall knowledge about regulation of insulin signaling in physiology and pathology. Several serine residues within IRS1 are involved in the physiological mechanism of IRS1 inhibition; however Ser307 has specific properties and characteristics that make it particularly interesting as a target of GRK2. This site is found proximal to the IRS1 phospho-Tyr binding domain and Ser307 phosphorylation rapidly reduces its affinity for binding to the insulin receptor 33, a process that resembles βAR desensitization. Thus, GRK2 appears to directly modulate signaling through this non-GPCR and participates in the physiological regulation of insulin signaling. Accordingly, GRK2 inhibition reduces insulin-mediated Ser307 phosphorylation and improves signaling and as a result can improve glucose uptake and insulin resistance in the injured cardiomyocyte. Indeed, elevated levels of phospho-Ser307 of IRS1 have been observed in animal models of insulin resistance and the equivalent Ser 312 site in human IRS1 has been demonstrated to be hyperphosphorylated in insulin-resistant subjects 39, 40. Therefore, our findings can hypothetically be extended beyond the heart and suggests that βARKct gene therapy could be employed for the possible treatment of reduced skeletal muscle and liver glucose uptake as is observed in type II Diabetes.
Overall, our data show that GRK2 inhibition clearly delays the reduction in glucose uptake and protects insulin signaling in the heart, preserving cardiac dimension and function. These data support the novel hypothesis that part of the therapeutic effect of GRK2 inhibition in HF includes correction of abnormal cardiac metabolism.
Supplementary Material
Clinical Perspective
A large number of studies have demonstrated that the increased level of G protein-coupled receptor kinase 2 (GRK2) seen in injured myocardium has deleterious effects on the progression of heart failure (HF). Importantly, GRK2 lowering is associated with improved cardiomyocyte signaling and function in failing human hearts mechanically unloaded with assist devices. However, the mechanisms through which GRK2 promotes HF or why its inhibition is therapeutic have not been fully elucidated. Interestingly, therapies targeting the neurohormonal axis, such as β-blockers, can attenuate cardiac remodeling and improve prognosis in patients with HF and these drugs can significantly decrease GRK2 in the heart. On the other hand, agents aimed to directly increase cardiac contractility have failed. Thus, it appears that GRK2 lowering and inhibition, which does improve contractility, must have additional effects on pathological components of HF development to account for the overwhelming positive effects seen in various animal models. The current study provides the first evidence that there is a close relationship between GRK2 activity and unfavorable modifications of cardiac metabolism, including insulin resistance and a loss of glucose uptake that can occur after ischemic injury and through the progression to HF. Indeed, we have identified IRS1 as a novel substrate of GRK2 kinase activity that can attenuate insulin signaling in the myocyte. These data also include the demonstration that insulin resistance and defective glucose uptake appear in the early stages of HF, suggesting the use of therapies aimed at restoring correct metabolic substrate utilization in the myocyte as soon as possible after ischemic injury. In this scenario, lowering GRK2, which is known to be up-regulated acutely after myocardial injury, appears to be a feasible and exciting approach to maintain metabolic homeostasis in the myocyte, which may contribute significantly in preventing the development of subsequent ischemic HF.
Acknowledgments
We thank Dr. M. Thakur and K. Devadalas of the Jefferson PET core facility for conducting the PET imaging and analysis of Micro-PET data. We also thank Filomena Severino and Dr Daniela Femminella for their technical support and encouragement.
Funding Resources
This work was supported in part by US National Institutes of Health grants R01 HL085505, R37 HL61690 and P01 HL075443 (Project 2) (to W.J.K.) and a fellowship from the Great Rivers Affiliate of the American Heart Association (to M.C.).
Footnotes
Subject codes: Chronic Ischemic Heart Disease; Animal Models of Human disease; Biochemistry and Metabolism.
Disclosures.
None.
References
- 1.Braunwald E. The Denolin lecture. Congestive heart failure: a half century perspective. Eur Heart J. 2001;22:825–836. doi: 10.1053/euhj.2001.2614. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 4.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Cir Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 5.Ashrafian H, Frenneaux MP. Metabolic modulation in heart failure: the coming of age. Cardiovasc Drugs Ther. 2007;21:5–7. doi: 10.1007/s10557-007-6000-z. [DOI] [PubMed] [Google Scholar]
- 6.Bing RJ, Siegel A, Ungar I, Gilbert M. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am J Med. 1954;16:504–515. doi: 10.1016/0002-9343(54)90365-4. [DOI] [PubMed] [Google Scholar]
- 7.Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C-labeled substrates in humans. J Clin Invest. 1987;79:359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lommi J, Kupari M, Yki-Jarvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Am J Cardiol. 1998;81:45–50. doi: 10.1016/s0002-9149(97)00804-7. [DOI] [PubMed] [Google Scholar]
- 9.Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, Stone CK. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F]fluoro-6-thia-heptadecanoic acid and [18F]FDG in Patients with Congestive Heart Failure. J Nucl Med. 2001;42:55–62. [PubMed] [Google Scholar]
- 10.Horn M, Remkes H, Stromer H, Dienesch C, Neubauer S. Chronic phosphocreatine depletion by the creatine analogue beta-guanidinopropionate is associated with increased mortality and loss of ATP in rats after myocardial infarction. Circulation. 2001;104:1844–1849. doi: 10.1161/hc3901.095933. [DOI] [PubMed] [Google Scholar]
- 11.Neubauer S, Krahe T, Schindler R, Horn M, Hillenbrand H, Entzeroth C, Mader H, Kromer EP, Riegger GA, Lackner K. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease. Altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86:1810–1818. doi: 10.1161/01.cir.86.6.1810. [DOI] [PubMed] [Google Scholar]
- 12.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, Davila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–1404. doi: 10.1016/j.jacc.2003.10.062. [DOI] [PubMed] [Google Scholar]
- 13.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, Boudina S, Abel ED. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53:2366–2374. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 14.Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 15.Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283:H976–982. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- 16.Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, Abel ED. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119:1272–1283. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witteles RM, Tang WH, Jamali AH, Chu JW, Reaven GM, Fowler MB. Insulin resistance in idiopathic dilated cardiomyopathy: a possible etiologic link. J Am Coll Cardiol. 2004;44:78–81. doi: 10.1016/j.jacc.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 18.Kostis JB, Sanders M. The association of heart failure with insulin resistance and the development of type 2 diabetes. Am J Hypertens. 2005;18:731–737. doi: 10.1016/j.amjhyper.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 19.Zucker IH. Novel mechanisms of sympathetic regulation in chronic heart failure. Hypertension. 2006;48:1005–1011. doi: 10.1161/01.HYP.0000246614.47231.25. [DOI] [PubMed] [Google Scholar]
- 20.Opie LH, Thandroyen FT, Muller C, Bricknell OL. Adrenaline-induced “oxygen-wastage” and enzyme release from working rat heart. Effects of calcium antagonism, beta-blockade, nicotinic acid and coronary artery ligation. J Mol Cell Cardiol. 1979;11:1073–1094. doi: 10.1016/0022-2828(79)90395-x. [DOI] [PubMed] [Google Scholar]
- 21.Iaccarino G, Koch WJ. Transgenic mice targeting the heart unveil G protein-coupled receptor kinases as therapeutic targets. Assay Drug Dev Technol. 2003;1:347–355. doi: 10.1089/154065803321204484. [DOI] [PubMed] [Google Scholar]
- 22.Iaccarino G, Barbato E, Cipolletta E, De Amicis V, Margulies KB, Leosco D, Trimarco B, Koch WJ. Elevated myocardial and lymphocyte GRK2 expression and activity in human heart failure. Eur Heart J. 2005;26:1752–1758. doi: 10.1093/eurheartj/ehi429. [DOI] [PubMed] [Google Scholar]
- 23.Bonita RE, Raake PW, Otis NJ, Chuprun JK, Spivack T, Dasgupta A, Whellan DJ, Mather PJ, Koch WJ. Dynamic changes in lymphocyte GRK2 levels in cardiac transplant patients: a biomarker for left ventricular function. Clin Transl Sci. 2008;3:14–18. doi: 10.1111/j.1752-8062.2010.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahid G, Hussain T. GRK2 negatively regulates glycogen synthesis in mouse liver FL83B cells. J Biol Chem. 2007;282:20612–20620. doi: 10.1074/jbc.M700744200. [DOI] [PubMed] [Google Scholar]
- 25.Usui I, Imamura T, Babendure JL, Satoh H, Lu JC, Hupfeld CJ, Olefsky JM. G protein-coupled receptor kinase 2 mediates endothelin-1-induced insulin resistance via the inhibition of both Galphaq/11 and insulin receptor substrate-1 pathways in 3T3-L1 adipocytes. Mol Endocrinol. 2005;19:2760–2768. doi: 10.1210/me.2004-0429. [DOI] [PubMed] [Google Scholar]
- 26.Cipolletta E, Campanile A, Santulli G, Sanzari E, Leosco D, Campiglia P, Trimarco B, Iaccarino G. The G protein coupled receptor kinase 2 plays an essential role in beta-adrenergic receptor-induced insulin resistance. Cardiovasc Res. 2009;84:407–415. doi: 10.1093/cvr/cvp252. [DOI] [PubMed] [Google Scholar]
- 27.Raake PW, Vinge LE, Gao E, Boucher M, Rengo G, Chen X, DeGeorge BR, Jr, Matkovich S, Houser SR, Most P, Eckhart AD, Dorn GW, 2nd, Koch WJ. G protein-coupled receptor kinase 2 ablation in cardiac myocytes before or after myocardial infarction prevents heart failure. Circ Res. 2008;103:413–422. doi: 10.1161/CIRCRESAHA.107.168336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaARKct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature. 2002;415:206–212. doi: 10.1038/415206a. [DOI] [PubMed] [Google Scholar]
- 30.Koch WJ, Rockman HA, Samama P, Hamilton RA, Bond RA, Milano CA, Lefkowitz RJ. Cardiac function in mice overexpressing the beta-adrenergic receptor kinase or a beta ARK inhibitor. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, Premont RT, Kontos CD, Zhu S, Rockey DC. A crucial role for GRK2 in regulation of endothelial cell nitric oxide synthase function in portal hypertension. Nat Med. 2005;11:952–958. doi: 10.1038/nm1289. [DOI] [PubMed] [Google Scholar]
- 32.Sun XJ, Goldberg JL, Qiao LY, Mitchell JJ. Insulin-induced insulin receptor substrate-1 degradation is mediated by the proteasome degradation pathway. Diabetes. 1999;48:1359–1364. doi: 10.2337/diabetes.48.7.1359. [DOI] [PubMed] [Google Scholar]
- 33.Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Guerra L, Nieto-Vazquez I, Vila-Bedmar R, Jurado-Pueyo M, Zalba G, Diez J, Murga C, Fernandez-Veledo S, Mayor F, Jr, Lorenzo M. G Protein-Coupled Receptor Kinase 2 (Grk2) Plays a Relevant Role in Insulin Resistance and Obesity. Diabetes. 2010;59:2407–2417. doi: 10.2337/db10-0771. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Williams ML, Hata JA, Schroder J, Rampersaud E, Petrofski J, Jakoi A, Milano CA, Koch WJ. Targeted beta-adrenergic receptor kinase (betaARK1) inhibition by gene transfer in failing human hearts. Circulation. 2004;109:1590–1593. doi: 10.1161/01.CIR.0000125521.40985.28. [DOI] [PubMed] [Google Scholar]
- 36.Aikawa R, Nawano M, Gu Y, Katagiri H, Asano T, Zhu W, Nagai R, Komuro I. Insulin prevents cardiomyocytes from oxidative stress-induced apoptosis through activation of PI3 kinase/Akt. Circulation. 2000;102:2873–2879. doi: 10.1161/01.cir.102.23.2873. [DOI] [PubMed] [Google Scholar]
- 37.Decker RS, Cook MG, Behnke-Barclay M, Decker ML. Some growth factors stimulate cultured adult rabbit ventricular myocyte hypertrophy in the absence of mechanical loading. Circ Res. 1995;77:544–555. doi: 10.1161/01.res.77.3.544. [DOI] [PubMed] [Google Scholar]
- 38.Markou T, Cullingford TE, Giraldo A, Weiss SC, Alsafi A, Fuller SJ, Clerk A, Sugden PH. Glycogen synthase kinases 3alpha and 3beta in cardiac myocytes: regulation and consequences of their inhibition. Cell Signal. 2008;20:206–218. doi: 10.1016/j.cellsig.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Werner ED, Lee J, Hansen L, Yuan M, Shoelson SE. Insulin resistance due to phosphorylation of insulin receptor substrate-1 at serine 302. J Biol Chem. 2004;279:35298–35305. doi: 10.1074/jbc.M405203200. [DOI] [PubMed] [Google Scholar]
- 40.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.