Abstract
Background and Aims
Altered sensory processing in interstitial cystitis/painful bladder syndrome (IC/PBS) may result from a deficiency of the central nervous system to adequately filter incoming visceral afferent information. The current study used prepulse inhibition (PPI) as an operational measure of sensorimotor gating to examine early pre-attentive stages of information processing in female IC/PBS and healthy controls (HCs).
Methods
PPI was assessed in female IC/PBS patients (n=14) and HCs (n=17) with 60 ms and 120 ms prepulse-to-startle stimulus intervals. Group differences in PPI and relationships between PPI, neuroticism, and acute stress ratings were evaluated.
Results
Patients showed significantly reduced PPI at both 60 ms and 120 ms prepulse intervals. The PPI deficit was related to acute stress ratings in the IC/PBS patients. However, increased neuroticism appeared to mitigate the PPI deficit in IC/PBS, possibly reflecting greater vigilance.
Conclusions
Compared to HCs, female IC/PBS patients demonstrated a decreased ability to adequately filter incoming information and perform appropriate sensorimotor gating. These results suggest that a possible mechanism for altered interoceptive information processing in IC/PBS may be the presence of a general deficit in filtering mechanisms due to altered pre-attentive processing.
Keywords: Interstitial cystitis, Painful Bladder Syndrome, acoustic startle response, prepulse inhibition of startle
INTRODUCTION
Interstitial cystitis/painful bladder syndrome (IC/PBS) is characterized by chronic pelvic pain, urinary frequency/urgency, and nocturia.1 The specific mechanisms underlying these persistent symptoms remain incompletely understood, but recent evidence suggests a possible role for central pain amplification.2 Given the significant comorbidity of IC/PBS with other persistent pain syndromes and association with anxiety and depression3, 4, it is important to examine both affective and attentional mechanisms that may alter interoceptive awareness and responses. Altered sensory processing including increased pain sensitivity and decreased habituation to non-noxious sensation has been suggested in some studies of IC/PBS patients.5–7 Recently, Rickenbacher et al8 demonstrated that bladder dysfunction produced by partial obstruction in the rat persistently disrupts cortical activity, resulting in decreased low frequency activity and prominent theta oscillations. As theta oscillations have been implicated in sensorimotor integration9, the authors suggested their persistence may reflect constant or disordered processing of bladder signals and noted that this effect on sensorimotor processing could adversely impact focused attention and cortical processing of non-bladder related stimuli.8
The acoustic startle response (ASR) is a rapid defensive response to a sudden intense stimulus, measured in humans via the eyeblink reflex, mediated by brainstem structures, and modulated by higher brain structures.10 The amplitude of the ASR can be reduced by a weak non-startling stimulus (prepulse) presented 30–300 ms before the startle eliciting stimulus. This phenomenon is known as prepulse inhibition (PPI) and is regarded as a neurophysiological measure of the early pre-attentive stages of information processing and an operational measure of sensorimotor gating. Sensorimotor gating is an important protective mechanism related to the ability to regulate transmission of the potentially chaotic flow of sensory information to a motor system. Although PPI has been most commonly studied in relation to schizophrenia and other cognitive disorders, there is evidence that PPI may be reduced (e.g. sensorimotor gating impaired) in patients with anxiety11, children with enuresis12 and animals exposed to early life stressors.13 It is thought that reduced PPI in these disorders reflects an inability to properly inhibit thoughts, sensations and motor responses. Conversely, increases in PPI have been associated with environmental vigilance. In the present study, we examine PPI in female IC/PBS patients and HCs. Specifically, we tested the following hypotheses, suggested by previous reports5–8: 1) compared to HCs, patients show reduced PPI, and 2) based on the hypothesized role of acute and chronic stress on IC/PBS, altered PPI will be associated with increased acute stress ratings and neuroticism (e.g. trait anxiety).
METHODS
Subjects
Female patients with IC/PBS (n=14) were recruited through a urology clinic and by advertisement. Data from this sample of IC/PBS subjects related to fear potentiated startle has been previously reported.14 IC/PBS diagnosis was confirmed using standard symptom criteria15 during a history and clinical exam by a urologist familiar with diagnosing IC/PBS.16 Cystoscopy, urinalysis and urine cytology were conducted to rule out malignancy, calculi, or other bladder pathologies. IC/PBS patients averaged 12.1 years (range 1–31 years) in duration of symptoms. Five IC/PBS patients had co-morbid irritable bowel syndrome using ROME II criteria17 and none had depression as determined by the Hospital Anxiety and Depression Scales.18 IC/PBS patients were discouraged from taking CNS acting mediations on the day of the study but some regularly used medications (summarized in Table 1). Healthy female control subjects (n=17) were selected from ongoing studies of prepulse modulation in functional pain disorders to best match the IC/PBS patients on the basis of age and menstrual cycle information. Control subjects were recruited by advertisement and screened via medical exam for absence of functional pain disorders and other significant health or psychiatric conditions. All study protocols were performed after approval by UCLA and informed consent was obtained from all subjects.
Table 1.
IC/PBS medications and menstrual cycle status
| Subject | %PPI | Menstrual Status | Elmiron® | Antidepressants | Antihistamines | Antimuscarinics | Urinary Analgesics | Narcotic Analgesics |
|---|---|---|---|---|---|---|---|---|
| 1 | 94 | Postmenopausal | Yes | Bupropion | Hydroxyzine | Darifenacin | Hydrocodone, Tramadol | |
| 2 | 91 | Luteal | No | |||||
| 3 | 91 | Luteal | Yes | Cetirizine | ||||
| 4 | 76 | Hysterectomy | No | Amitriptyline | Hydrocodone, Fentanyl | |||
| 5 | 64 | Follicular | Yes | Escitalopram | Solifenacin | Urelle® | Hydrocodone | |
| 6 | 46 | Luteal | Yes | Urised® | ||||
| 7 | 39 | Postmenopausal | No | Fluoxetine | Darifenacin | |||
| 8 | 37 | Follicular | Yes | Amitriptyline | ||||
| 9 | 34 | Luteal | Yes | |||||
| 10 | 30 | OCP | Yes | Citalopram | Hydroxyzine | Oxybutynin | Tramadol | |
| 11 | 22 | Follicular | Yes | Diphenhydramine | Urised® | |||
| 12 | 15 | Postmenopausal | Yes | Fluoxetine | Urelle® | |||
| 13 | 10 | Hysterectomy | No | |||||
| 14 | −22 | Luteal | No |
Note: %PPI was derived from the mean response during Startle Alone and PPI120 trials;
OCP=oral contraceptive pill
Measures
Psychometric instruments
Patients completed the O'Leary-Sant IC Symptom and Problem Indices (ICSI and ICPI, respectively) as a validated measure of IC/PBS symptom severity19 and the Neuroticism scale from the Eysenck Personality Questionnaire (EPQ) as a measure of trait anxiety or anxiety vulnerability.20 All subjects rated their acute stress using the Stress Symptom Rating scale (SSR) before and after completing experimental procedures. The SSR has been validated in studies of acute psychological stress.21
Electrophysiological materials, equipment and data acquisition
The startle stimulus consisted of a 50 ms burst of white noise at 105 dB SPL with a 0 ms rise time presented binaurally through stereophonic earphones. For each trial, startle stimuli were presented alone (Startle Alone) or preceded by a 25 ms 1000 Hz tone at 75 dB SPL with 4 ms rise and fall times initiated 60 or 120 ms before the startle stimulus (PPI60 and PPI120, respectively).
Electromyogram (EMG) activity of the orbicularis oculi was recorded using standard techniques from electrodes placed beneath the right eye.22 EMG activity was full-wave rectified with low and high frequency cut-off values of 30 Hz and 1 kHz, respectively. Experimental timing and data acquisition was performed with custom software programmed in Labview® (National Instruments, Austin, TX, USA).
Procedure
Subjects were fitted with headphones and watched a muted movie while receiving startle stimuli. The first trial consisted of a startle stimulus presented alone and was not included in analyses. Following this initial trial, subjects received 6 Startle Alone, 6 PPI60, and 6 PPI120 trials. In addition, subjects received 6 trials in which a long continuous tone preceded the startle stimulus to provide a measure of prepulse facilitation. The long prepulse led to only very small facilitation and no group differences so these trials were not included in the analyses reported below. All subjects received the same order of startle trials based on a Latin square design.
The general procedures for stimulus presentation and recording follow those of previous studies of prepulse modulation of startle in our laboratory, e.g.22. Following completion of the experiment, subjects completed a hearing test. None of the subjects displayed a hearing impairment.
Data Analysis
Startle blink responses
Response amplitude, expressed in microvolts (μV), was calculated as the difference between mean EMG in the 200 ms prior to the startle stimulus onset and the response peak. 5% of all trials were rejected for artifact and 3% of Startle Alone and 19% of PPI60/PPI120 trials showed zero response. Trials with 0 startle response were included in the analyses.
Statistical Analyses
The main analyses used the Proc Mixed procedure in SAS to perform a mixed linear model analysis [SAS Institute Incorporated, Carey, NC] on natural log-transformed data due to positive skew (index=1.87; standard error=0.11). The primary approach was individual growth curve modeling for repeated measures data. Differences in startle response amplitude and habituation for the Startle Alone trials were evaluated using a Diagnosis (IC/PBS; Control) × Trial Number (1:6) mixed model ANOVA. To evaluate prepulse effects, a Diagnosis (IC/PBS; Control) × Trial Type (Startle Alone; PPI60; PPI120) design was used. Age of the subject was entered into the models as a covariate to control for age effects. Prepulse inhibition at 60 ms and 120 ms was estimated as the difference between log-transformed Startle Alone trials and PPI60/PPI120 trials, respectively. Following the convention in the field, more positive values of PPI indicate greater inhibition. Diagnosis effects on PPI were tested using contrast statements of a priori planned comparisons. Since the difference between log-transformed values is the logarithm of the ratio of the responses, PPI estimates were equivalent to proportional changes in amplitude.
Exploratory analyses were performed to examine the relationship between acute stress, trait neuroticism and PPI in the IC/PBS subjects. For these analyses, neuroticism and stress measures were entered as continuous covariates in the prepulse analysis model described above. Due to the small sample and low variability in neuroticism and stress measures in HCs, parallel analyses were not performed in this group. Group differences in age were evaluated by independent samples t-test and SSR Stress ratings were analyzed by repeated measures ANOVAs in PASW Statistics 17.0 [SPSS Incorporated, Chicago, IL]. Post hoc comparisons were Sidak adjusted.
RESULTS
Subject data
IC/PBS patients and HCs did not significantly differ in age (p>.05; see Table 2). Patient ratings on the O'Leary-Sant Symptom and Problem Indices and EPQ Neuroticism scale are also presented in Table 2.
Table 2.
Mean, standard errors, and range for age, stress ratings before and after the experimental procedures, neuroticism, and symptom ratings
| Age | Stress Before | Stress After | Neuroticism | ICSI | ICPI | |
|---|---|---|---|---|---|---|
| IC/PBS | 45.9(3.0; 23.1–62.7) | 2.8(0.5; 0.3–7.5) | 4.5(0.7; 0.5–9.0)* | 10.0(1.7; 1–22) | 13.1(1.0; 7–20) | 11.2(0.9; 5–16) |
| HCs | 39.5(2.9; 22.1–56.3) | 2.2(0.4; 0–6.0) | 1.8(0.4; 0–5.3) | |||
Significant effect for diagnosis (IC/PBS vs HCs) and time (Stress Before vs Stress After)
SSR Stress
The repeated measure ANOVA of SSR ratings of stress revealed significant interactions between diagnosis and time (F(1,29)=13.652, p=.001). The ratings were similar across the two groups at the beginning of the study (p>.05; see Table 2). However, at the end of the study, IC/PBS patients had significantly higher ratings of stress compared to HCs (p=.001; see Table 2). The increase in stress was significant for IC/PBS patients (p=.001).
Startle response amplitude and habituation
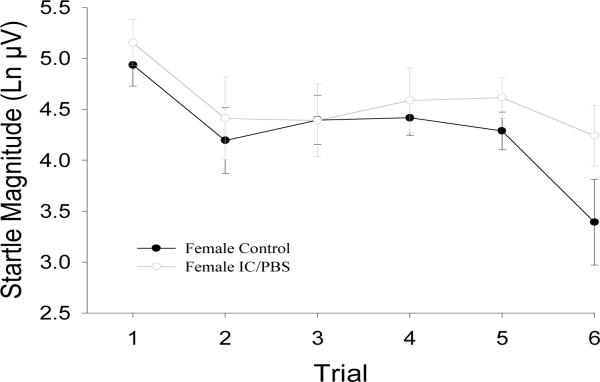
No significant group differences in response during Startle Alone trials (F(1,29)=0.09, p=.76) or in habituation of this response were found (F(1,26)=0.37, p=.548; see Figure 1).
Figure 1.
Unmodulated acoustic startle response magnitude. Values are means (with standard error bars) for each startle alone trial throughout the experimental session. Ln μV = natural log transformed microvolts
Prepulse inhibition
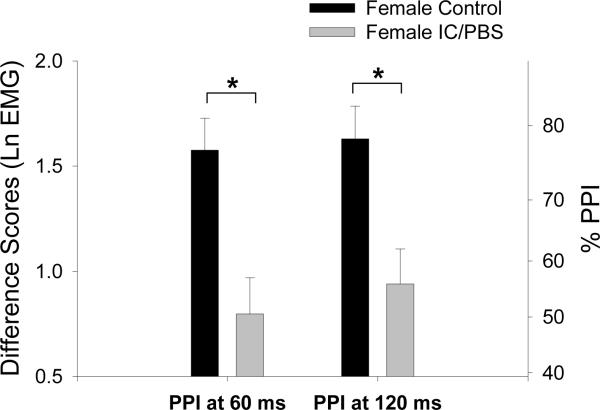
The mixed model ANOVA revealed a significant main effect for Trial Type (F(2,491)=59.42, p<.0001) reflecting larger startle responses for all subjects during Startle Alone trials compared to prepulse trials. In addition, there was a significant Diagnosis × Trial Type interaction (F(2,491)=4.91, p=.0077). Linear contrasts were performed to interpret the significant interaction in terms of PPI. IC/PBS patients had significantly reduced PPI at 60 ms and 120 ms compared to HCs (F(1,491)=8.27, p=.0042; F(1,491)=6.25, p=.0127, respectively; see Figure 2).
Figure 2.
Prepulse inhibition of the acoustic startle response. Values are estimated means (with standard error bars). Greater prepulse inhibition is indicated by a more positive difference score. The right axes shows percent change derived from the log-transformed difference scores. Significance is indicated for group differences in percent PPI. Ln μV = natural log transformed microvolts.
PPI covariates
Exploratory analyses of the relationship between PPI and neuroticism and acute stress ratings for the patients were examined using mixed model ANCOVAs.
Acute Stress
A significant interaction between stress ratings taken after the PPI task and Trial Type interaction was found (F(2,219)=5.33, p=.0055). Stress ratings were not related to responses during Startle Alone trials (p's>.05) but increased stress was significantly related to decreased PPI at 120 ms (t(219)=3.26, p=.0013; slope=−0.164±0.05 (standard error)).
Neuroticism
A significant Neuroticism × Trial Type interaction was revealed (F(2,219)=4.66, p=.01). Neuroticism was not related to responses during Startle Alone trials (p's>.05) but increased neuroticism was significantly related to increased PPI at 120 ms (t(219)=2.62, p=.0095; slope=0.053±0.02 (standard error)).
There were no significant correlations between neuroticism and acute stress ratings in this small sample (p's>.05).
DISCUSSION
This is the first study to evaluate sensorimotor gating in patients with a diagnosis of IC/PBS. The results demonstrate that IC/PBS subjects as a group have a significant deficit in PPI compared to HCs, suggesting a decreased ability to adequately filter incoming information and perform appropriate sensorimotor gating. This deficit was seen at both the 60ms and 120 ms prepulse lead intervals. The PPI deficit was related to acute stress ratings during the experiment in the IC/PBS patients, with the greatest PPI decline seen in patients with increased stress reported during the study procedures. Although the prepulse paradigm is generally considered as not stress-inducing, patients reported significantly greater stress following the procedure than HCs. In contrast, increased neuroticism, in IC/PBS patients was associated with greater PPI.
Prior studies suggest that symptoms in IC/PBS may result in part from altered processing of interoceptive information, including augmented pain sensitivity and increased response to non-noxious sensations.5–7 IC/PBS patients demonstrate a deficit in the ability to habituate to non-noxious electrical and thermal stimulation to the T12 and S3 dermatomes.5, 6 In addition, IC/PBS patients demonstrate generalized hypersensitivity to deep tissue stimulation7 and pain during intravesical instillation of ice water.23 The current results indicate that a possible mechanism contributing to altered interoceptive information processing in IC/PBS may be the presence of a general deficit in filtering mechanisms for intero- and exteroceptive stimuli due to altered pre-attentive processing. While this alteration is evident in the IC/PBS subjects as a group and is greater in those patients with increased subjective ratings of the experimental situation as stressful, it appears that neuroticism had a significant moderating effect on the lowered PPI. Swerdlow et al24 reported increased PPI to be associated with higher scores on a psychological scale of illness fears and concerns (the Hysteria scale of the MMPI) in healthy subjects. Also, previous studies have shown that increased PPI may be associated with increased vigilance. PPI is greater when task demands require attention to the prepulse and less when instructions are to ignore the prepulse10 and is enhanced by threat conditions, presumably due to increased vigilance.25 IC/PBS patients score higher on the Kohn Reactivity Scale suggesting increased vigilance to a variety of stimuli.7 In the current data, IC/PBS subjects with greater neuroticism, a measure of trait anxiety associated with vigilance26, showed greater PPI. Therefore, the data support a model in which the overall deficit in PPI associated with IC/PBS is less pronounced in subjects who are high in neuroticism and likely high in vigilance to relatively innocuous environmental cues like the prepulse.
While the brain structures mediating sensorimotor gating are in the pons, areas of the frontal cortex, in particular the anterior cingulate and lateral prefrontal cortex (PFC) provide `top down' influence via the thalamus.10 Lateral PFC also responds to experimental changes in bladder sensation and may play a crucial role in controlling the desire to void.27 Lateral PFC is involved in attentional processes and corticolimbic inhibition and has been implicated in endogenous pain inhibition. Alterations in corticolimbic-pontine interactions may therefore play a role in altered intero- and exteroceptive information processing, including visceral hyperalgesia in female IC/PBS patients. Additionally, medial PFC activity has been negatively associated with both neuroticism28 and attention modulated prepulse inhibition29 and may be involved in increased vigilance to the prepulse. We have previously shown in this same sample of IC/PBS patients an increase in startle during anticipation of an abdominal threat suggesting an increased sensitivity of the extended amygdala to some forms of stress.14 Further research that specifically examines processing of both exteroceptive and interoceptive stimuli is needed to clarify what may be overlapping brain mechanisms involved in increased pain sensitivity, increased trait anxiety and alterations in attention and information processing that are involved in vulnerability to develop and maintain IC/PBS symptoms.
Procedural differences that affect the signal to noise ratio of the prepulse, such as the presence or absence of a constant background sound can influence both the amount of PPI and group differences in PPI30. Although the current methods led to robust levels of PPI, it will be important to test the consistency of these results using other PPI parameters and signal to noise ratios.
Conclusions
Although preliminary, this study suggests that IC/PBS is associated with deficits in early stage information processing and, specifically, inefficient gating of incoming sensory information. While altered sensorimotor gating may reflect a longstanding or even genetically based neurocognitive vulnerability factor for development of IC/PBS, it could also be a result of chronically altered interoceptive signals from the bladder. In any case, improper inhibition of responses to low level interoceptive stimulation may be a significant factor in long term symptom maintenance. Given previous studies that have shown vigilance can increase PPI, IC/PBS subjects with greater neuroticism/trait anxiety may have increased vigilance to environmental events, apparently mitigating a deficit in PPI. Further study is needed to examine the dynamics of the relationship between symptoms and altered information processing in IC/PBS patients.
Acknowledgements
Supported in part by NIH grants NR007768, P50 DK64539, R24 AT002681, VA Medical Research, NIH GI Training Grant T32-DK07180-34 and a gift from the Virginia Friedhofer Charitable Trust.
Footnotes
Conflicts of Interest Statement: No conflicts of interest exist for this study. Subjects were informed of any potential conflicts of interest.
REFERENCES
- 1.Dell JR, Mokrzycki ML, Jayne CJ. Differentiating interstitial cystitis from similar conditions commonly seen in gynecologic practice. Eur J Obstet Gynecol Reprod Biol. 2009;144:105. doi: 10.1016/j.ejogrb.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 2.Clemens JQ. Male and female pelvic pain disorders--is it all in their heads? J Urol. 2008;179:813. doi: 10.1016/j.juro.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez MA, Afari N, Buchwald DS. Evidence for overlap between urological and nonurological unexplained clinical conditions. J Urol. 2009;182:2123. doi: 10.1016/j.juro.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Naliboff BD, Rhudy J. Anxiety in Functional Pain Disorders. In: Mayer EA BM, editor. Fucntional Pain Syndromes: Presentation and Pathophysiology. International Association for the Study of Pain; Seattle, WA: 2009. pp. 185–214. [Google Scholar]
- 5.Lowenstein L, Kenton K, Mueller ER, et al. Patients with painful bladder syndrome have altered response to thermal stimuli and catastrophic reaction to painful experiences. Neurourol Urodyn. 2009;28:400. doi: 10.1002/nau.20676. [DOI] [PubMed] [Google Scholar]
- 6.Fitzgerald MP, Koch D, Senka J. Visceral and cutaneous sensory testing in patients with painful bladder syndrome. Neurourol Urodyn. 2005;24:627. doi: 10.1002/nau.20178. [DOI] [PubMed] [Google Scholar]
- 7.Ness TJ, Powell-Boone T, Cannon R, et al. Psychophysical evidence of hypersensitivity in subjects with interstitial cystitis. J Urol. 2005;173:1983. doi: 10.1097/01.ju.0000158452.15915.e2. [DOI] [PubMed] [Google Scholar]
- 8.Rickenbacher E, Baez MA, Hale L, et al. Impact of overactive bladder on the brain: central sequelae of a visceral pathology. Proc Natl Acad Sci U S A. 2008;105:10589. doi: 10.1073/pnas.0800969105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorimotor integration. Behav Brain Res. 2001;127:119. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Du Y, Li N, et al. Top-down modulation of prepulse inhibition of the startle reflex in humans and rats. Neurosci Biobehav Rev. 2009;33:1157. doi: 10.1016/j.neubiorev.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Grillon C, Morgan CA, Southwick SM, et al. Baseline startle amplitude and prepulse inhibition in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1996;64:169. doi: 10.1016/s0165-1781(96)02942-3. [DOI] [PubMed] [Google Scholar]
- 12.Ornitz EM, Russell AT, Hanna GL, et al. Prepulse inhibition of startle and the neurobiology of primary nocturnal enuresis. Biol Psychiatry. 1999;45:1455. doi: 10.1016/s0006-3223(98)00205-4. [DOI] [PubMed] [Google Scholar]
- 13.Ellenbroek BA, Cools AR. Early maternal deprivation and prepulse inhibition: the role of the postdeprivation environment. Pharmacol Biochem Behav. 2002;73:177. doi: 10.1016/s0091-3057(02)00794-3. [DOI] [PubMed] [Google Scholar]
- 14.Twiss C, Kilpatrick L, Craske M, et al. Increased startle responses in interstitial cystitis: evidence for central hyperresponsiveness to visceral related threat. J Urol. 2009;181:2127. doi: 10.1016/j.juro.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrams P, Cardozo L, Fall M, et al. The standardisation of terminology in lower urinary tract function: report from the standardisation subcommittee of the International Continence Society. Urology. 2003;61:37. doi: 10.1016/s0090-4295(02)02243-4. [DOI] [PubMed] [Google Scholar]
- 16.Bogart LM, Berry SH, Clemens JQ. Symptoms of interstitial cystitis, painful bladder syndrome and similar diseases in women: a systematic review. J Urol. 2007;177:450. doi: 10.1016/j.juro.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 17.Thompson WG, Longstreth GF, Drossman DA, et al. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(Suppl 2):II43. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zigmond As SRP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 19.O'Leary MP, Sant GR, Fowler FJ, Jr., et al. The interstitial cystitis symptom index and problem index. Urology. 1997;49:58. doi: 10.1016/s0090-4295(99)80333-1. [DOI] [PubMed] [Google Scholar]
- 20.Eysenck HJ, Eysenck SBG. Manual of the Eysenck Personality Questionnaire: (EPQ-R Adult) comprising the EPQ-Revised (EPQ-R) and EPQ-R short scale. Educational and Industrial Testing Service; San Diego: 1994. [Google Scholar]
- 21.Dickhaus B, Mayer EA, Firooz N, et al. Irritable bowel syndrome patients show enhanced modulation of visceral perception by auditory stress. Am J Gastroenterol. 2003;98:135. doi: 10.1111/j.1572-0241.2003.07156.x. [DOI] [PubMed] [Google Scholar]
- 22.Ornitz EM, Guthrie D, Kaplan AR, et al. Maturation of startle modulation. Psychophysiology. 1986;23:624. doi: 10.1111/j.1469-8986.1986.tb00681.x. [DOI] [PubMed] [Google Scholar]
- 23.Mukerji G, Waters J, Chessell IP, et al. Pain during ice water test distinguishes clinical bladder hypersensitivity from overactivity disorders. BMC Urol. 2006;6:31. doi: 10.1186/1471-2490-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swerdlow NR, Filion D, Geyer MA, et al. “Normal” personality correlates of sensorimotor, cognitive, and visuospatial gating. Biol Psychiatry. 1995;37:286. doi: 10.1016/0006-3223(94)00138-S. [DOI] [PubMed] [Google Scholar]
- 25.Grillon C, Davis M. Effects of stress and shock anticipation on prepulse inhibition of the startle reflex. Psychophysiology. 1997;34:511. doi: 10.1111/j.1469-8986.1997.tb01737.x. [DOI] [PubMed] [Google Scholar]
- 26.Burton L, Pfaff D, Bolt N, et al. Effects of gender and personality on the Conners Continuous Performance Test. J Clin Exp Neuropsychol. 2009:1. doi: 10.1080/13803390902806568. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto S, Ishikawa A, Kume H, et al. Near infrared spectroscopy study of the central nervous activity during artificial changes in bladder sensation in men. Int J Urol. 2009;16:760. doi: 10.1111/j.1442-2042.2009.02358.x. [DOI] [PubMed] [Google Scholar]
- 28.Kim SH, Hwang JH, Park HS, et al. Resting brain metabolic correlates of neuroticism and extraversion in young men. Neuroreport. 2008;19:883. doi: 10.1097/WNR.0b013e328300080f. [DOI] [PubMed] [Google Scholar]
- 29.Hazlett EA, Buchsbaum MS, Tang CY, et al. Thalamic activation during an attention-to-prepulse startle modification paradigm: a functional MRI study. Biol Psychiatry. 2001;50:281. doi: 10.1016/s0006-3223(01)01094-0. [DOI] [PubMed] [Google Scholar]
- 30.Franklin JC, Bowker KB, Blumenthal TD. Anxiety and prepulse inhibition of acoustic startle in a normative sample: The importance of signal-to-noise ratio. Personality and Individual Differences. 2009;46:369. [Google Scholar]