Abstract
Activation of PKCδ in androgen-dependent LNCaP prostate cancer cells leads to apoptosis via the activation of p38 MAPK and JNK cascades. We have recently shown that treatment of LNCaP cells with phorbol 12-myristate 13-acetate (PMA) leads to a PKCδ-mediated autocrine release of death factors, including the cytokines TNFα and TRAIL, and that conditioned medium (CM) collected from PMA-treated LNCaP cells promotes the activation of the extrinsic apoptotic cascade. Interfering with this autocrine loop either at the level of factor release or death receptor activation/signaling markedly impaired the PMA apoptotic response. In the present study we show that this PKCδ-dependent autocrine mechanism is greatly influenced by androgens. Indeed, upon androgen depletion, which down-regulates PKCδ expression, TNFα and TRAIL mRNA induction and release by PMA are significantly diminished, resulting in a reduced apoptogenic activity of the CM and an impaired ability of the CM to activate p38 MAPK and JNK. These effects can be rescued by addition of the synthetic androgen R1881. Furthermore, RNAi depletion of the androgen-receptor (AR) from LNCaP cells equally impaired PMA responses, suggesting that PKC-mediated induction of death factor secretion and apoptosis in LNCaP prostate cancer cells are highly sensitive to hormonal control.
Keywords: PKC, PMA, apoptosis, androgens, p38 MAPK, JNK
INTRODUCTION
Apoptosis or programmed cell death is a highly regulated process, in which phosphorylation events play key modulatory roles [1–3]. It is well established that protein kinase C (PKC), a family of serine-threonine kinases, modulates both apoptosis and survival in various cell types [4–7]. There are at least 10 PKC family members classified into classical (α, βI, βII, and γ), novel (δ, ε, η, andθ), and atypical (λ and ζ) isozymes, of which only members of the first two classes are subject to regulation by the lipid second messenger diacylglycerol (DAG). Phorbol esters, widely used PKC activators that mimic the actions of DAG, cause profound effects on cell proliferation and differentiation, and they can also induce survival or apoptotic responses in a strict cell type-dependent manner. The marked heterogeneity observed for phorbol ester responses is a direct consequence of the multiplicity of cellular targets for these compounds, and it is largely determined by the differential pattern of PKC isozyme expression in each cell type as well as the characteristic coupling of individual PKCs to downstream effectors, which include the extracellular-signal regulated kinase (ERK), c-Jun N-terminal kinase (JNK), p38 mitogen-activated protein kinase (MAPK), NF-κB, and Stat signaling pathways [4,5,8].
Androgen-dependent prostate cancer cells, such as LNCaP and CWR22-Rv1 cells, undergo apoptosis in response to phorbol 12-myristate 13-acetate (PMA) [9–13] via activation of the novel PKCδ isozyme [12,14]. On the other hand, the novel PKCε isozyme signals for survival in prostate cancer cells [15]. Expression levels of PKC isozymes are deregulated in cancer, including prostate cancer, and disrupting the balance in PKC isozyme expression or function has a considerable impact on cancer progression as well as on the responses to PKC activators [8]. Signaling studies established that PKCδ-dependent apoptosis is mediated by the p38 MAPK and JNK cascades [14,16]. These pathways are well-established mediators of cell death induced by cytokines such as TNFα and tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) [17]. Interestingly, a recent study revealed that PKC activation stimulates the autocrine secretion of death factors from prostate cancer cells, including TNFα and TRAIL. Immunoneutralization of TNFα in conditioned medium (CM) collected from PMA-treated cells significantly impaired its apoptogenic activity. A similar effect is observed upon blockade or RNA interference (RNAi) depletion of TNFα and TRAIL receptors, as well as by interfering with downstream effectors of the extrinsic apoptotic cascade, including the adaptor Fas-associated protein with a death domain (FADD), caspase-8, p38 MAPK and JNK [18], suggesting an essential role for these cytokines in the PKC-mediated autocrine loop.
It has been recently noted that androgens greatly influence the apoptotic responses to phorbol esters in prostate cancer cells. A significant reduction in the ability of PMA to activate apoptotic signaling is observed either upon androgen-depletion of the culture medium or RNAi knock-down of the androgen receptor (AR). A subsequent analysis revealed that androgens modulate the expression of PKCδ in prostate cancer cells at a transcriptional level [19]. We therefore reasoned that PKCδ-dependent induction of the autocrine apoptotic response in prostate cancer cells is modulated by hormonal mechanisms via the AR. To address this issue we decided to analyze whether androgen affects the secretion of death factors from LNCaP cells in response to PKC activation and impact on the activation of proapoptotic signaling events. Our results show that phorbol ester-induced autocrine secretion of death factors and cell death are strictly dependent on androgen, suggesting a major role for hormonal control in the modulation of PKCδ-mediated apoptosis in prostate cancer cells.
MATERIALS AND METHODS
Materials
PMA was purchased from LC Laboratories (Woburn, MA). The synthetic androgen methyl trienolone (R1881) was obtained from Perkin-Elmer (Boston, MA). 4′,6-Diamidino-2-phenylindole (DAPI) was purchased from Sigma (St. Louis, MO). Charcoal/dextran-treated fetal bovine serum (FBS) was from Hyclone (Logan, UT). Other cell culture reagents and media were from ATCC (Rockville, MD).
Cell Culture
Human prostate cancer cells LNCaP (passages 2–10), PC3, and DU-145 cells (from ATCC) were cultured in RPMI 1640 medium supplemented with 10% FBS and penicillin (100 U/mL)-streptomycin (100 0 µg/mL) at 37°C in a humidified 5% CO2 atmosphere. For steroid depletion, cells were incubated in Phenol red-free RPMI 1640 supplemented with 2% charcoal/dextrane-treated FBS for 48 h, as previously described [19].
Collection of CM
Cells in 10 cm dishes (~70% confluence) were treated with PMA (100 nM) or vehicle (ethanol) for 1 h and then washed twice with medium to remove the phorbol ester or vehicle. After incubation for 24 h, CM was collected, pass through a 0.45 µM filter, and added to fresh LNCaP cells (~70% confluence).
Western Blot Analysis
Western blot was carried out as described [12]. The following first antibodies were used: anti-PKCδ (Transduction Laboratories, Lexington, KY), anti-AR (Santa Cruz Biotechnology, Santa Cruz, CA), anti-phospho-JNK, anti-total-JNK, anti-phospho-p38 MAPK, and anti-total-p38 MAPK (Cell Signaling Technology, Beverly, MA). All primary antibodies were used at a 1:1000 dilution. Secondary antibodies conjugated to horseradish peroxidase (Bio-Rad, Hercules, CA) were used at a 1:3000 dilution.
Apoptosis Assays
The incidence of apoptosis was determined by assessing morphological changes in chromatin condensation by fluorescence microscopy after DAPI staining, as described before [12]. The incidence of apoptosis in each preparation was analyzed by counting ~300 cells.
RNA Interference (RNAi)
The following sequences were used: AAGCACUGCUACUCUUCAGCA (AR), and AACAUCGCUGUAGCAUCGUCU (control RNAi). RNAi duplexes from Dharmacon (Lafayette, CO) were transfected with the Amaxa Nucleofector system (Amaxa Biosystems, Gaithersburg, MD) following the instructions provided by the manufacturer. Experiments were performed 48 h after transfection.
RNA Isolation and cDNA Synthesis
RNA was extracted using TRIzol (Invitrogen, Carlsbad, CA). Two micrograms of RNA per sample were reverse transcribed using the First-Strand cDNA Synthesis Kit (Amersham Biosciences, Piscataway, NJ).
Real-Time PCR
PCR primers and fluorogenic probes for human TNFα and TRAIL were purchased from Applied Biosystems (Branchburg, NJ). The probes were 5′end-labeled with 6-carboxyfluorescein (FAM). Each PCR amplification was performed in a total volume of 12.5 µL, containing 6.25 µL of 2× TaqMan Universal PCR Master Mix (Applied Biosystems), commercial target primers (300 nM), the fluorescent probe (200 nM), and 1 µL of cDNA, using an ABI PRISM 7700 Detection System. PCR product formation was continuously monitored using the Sequence Detection System software version 1.7 (Applied Biosystems). The FAM signal was normalized to endogenous glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Enzyme-Linked Immunosorbent Assay (ELISA)
TNFα and TRAIL levels were determined by ELISA (Pepro Tech, Inc., Rock Hill, NJ), essentially as described previously [18]. Briefly, 100 µL of CM were added into each well and incubated overnight at 4°C. Subsequently, 100 µL of biotin-labeled anti-TNFα or anti-TRAIL antibodies (0.25 µg/mL) were added for 2 h at room temperature. Bound antibodies were detected by incubation with peroxidase-labeled avidine and SureBlue TMB Microwell Peroxidase Substrate from KPL (Gaithersburg, MD), and absorbance was measured at 405 nm. Non-specific binding was blocked with 1% BSA in PBS.
RESULTS
We have previously determined that the apoptotic activity of phorbol esters in LNCaP cells is associated with their ability to promote the release of death factors, which upon binding to death receptors in LNCaP cells trigger the activation of the extrinsic apoptotic cascade. CM collected from LNCaP cells treated with PMA (CM-PMA), but not from vehicle-treated cells (CM-Veh), has apoptogenic activity when added to a fresh culture of LNCaP cells. This activity is essentially undetectable when cells were pretreated with a PKC inhibitor or subject to PKCδ RNAi before the addition of PMA, thus indicating an essential role for the novel PKCδ isozyme in the release of death factors [18]. PKCδ expression is transcriptionally modulated by androgens and androgen depletion reduces PKCδ levels in LNCaP cells, without changes in PKCε or PKCζ levels, and a slight reduction inPKCα levels [19], we reasoned that PMA induction of death factor secretion should be affected when LNCaP cells are cultured in androgen-depleted medium. To address this issue we collected CM-PMA from LNCaP cells growing either in normal medium or in steroid-depleted medium (charcoal-treated) and determined its ability to trigger an apoptotic response in previously untreated LNCaP cells (Figure 1A). Interestingly, the apoptogenic activity of CM-PMA collected from LNCaP cells growing in steroid-depleted medium was markedly reduced compared to that from cells growing in normal medium (Figure 1B). On the other hand, supplementing the steroid-depleted cultured medium with the synthetic androgen R1881 fully restored the apoptotic response of CM-PMA. We have reported that androgen-independent prostate cancer cells also release death factors capable of promoting LNCaP cell apoptosis [18]. However, as expected, the apoptogenic activity of the CM collected in response to PMA from either DU145 or PC3 androgen-independent cells was not affected when these cells were grown in steroid-depleted medium or by addition of R1881 to the steroid-depleted medium (Figure 1C). As previously reported, PKCδ levels are significantly reduced in LNCaP cell subject to steroid depletion and restored by R1881 supplementation (Figure 1D, upper left panel, see also Ref. [20]), but these effects were not observed in DU145 (lower panel) or PC3 cells (upper right panel). Therefore, it is conceivable that the secretion of apoptotic factors from LNCaP cells is regulated by androgen.
Figure 1.
The apoptotic effect of CM from PMA-treated LNCaP cells is androgen-dependent. Panel A: Schematic representation of the experimental approach. Different prostate cancer cells (LNCaP, DU145, or PC3) growing for 48 h in normal medium (RPMI, 10% FBS), steroid-depleted medium (RPMI, 10% charcoal-treated FBS), or steroid-depleted medium supplemented with R1881 (1 nM), were treated with PMA (100 nM, 1 h) or vehicle. After 24 h CM was collected and added to LNCaP cells growing in normal medium. The percentage of apoptotic cells was determined 24 h later by DAPI staining. Panel B: Effect of CM from androgen-dependent LNCaP cells. Panel C: Effect of CM from androgen-independent DU145 and PC3 cells. Panel D: Expression of PKCδ in prostate cancer cells growing in normal medium, charcoal-treated medium, or charcoal-treated medium supplemented with R1881 (1 nM). Expression levels, relative to normal, have been determined by densitometry and are shown below each corresponding Western blot. For apoptosis assays, results were presented as mean ± SD of an experiment performed in triplicate. Two additional experiments gave similar results. CM-PMA, conditioned medium from PMA-treated cells; CM-Veh, conditioned medium from vehicle-treated cells.
Previous analysis of autocrine factors released from LNCaP cells in response to PMA treatment revealed that TNFα and TRAIL, but not FasL, play a significant role in the pro-apoptotic loop [18]. PMA caused a prominent up-regulation in TNFα mRNA levels in LNCaP cells (Figure 2A). Notably, this effect was significantly lower in LNCaP cells growing in steroid-depleted medium. Addition of R1881 to the steroid-depleted medium, which did not significantly affect TNFα levels in the absence of phorbol ester stimulation, restored the ability of PMA to induce TNFα mRNA (levels were indeed doubled compared to those observed in LNCaP cells growing in normal medium, probably reflecting the presence of additional modulatory factors in serum present in normal medium). While the effect of PMA on TRAIL mRNA induction was modest (~2-fold), it was also abolished in steroid-depleted growing cells, and the addition of R1881 fully restored the PMA response (Figure 2B).
Figure 2.
Androgens regulate TNFα and TRAIL mRNA induction by PMA. LNCaP cells were grown in normal medium, steroid-depleted medium, or steroid-depleted medium supplemented with R1881 (1 nM) for 48 h and then treated with either 100 nM PMA (+PMA) or vehicle (−PMA) for 1 h. Panels A and B: TNFα and TRAIL mRNA levels were determined by real-time PCR 3 h after PMA or vehicle treatment using the TaqMan Gene Expression Assays (Applied Biosystems), and normalized to endogenous GAPDH mRNA levels. Results were expressed as fold-increase relative to mRNA levels in cells growing in normal medium treated with vehicle. Data are presented as mean ± SD of three replicates. Two additional experiments gave similar results. Panels C and D: TNFα and TRAIL levels, as determined by ELISA in CM collected 24 h after PMA or vehicle treatment. Each sample was run by triplicates and results are presented as mean ± SD (n = 3). Two additional experiments gave similar results. CM-PMA, conditioned medium from PMA-treated cells; CM-Veh, conditioned medium from vehicle-treated cells.
PMA promotes a marked release of TNFα from LNCaP cells, as determined by ELISA (Figure 2C and Ref. [18]), an effect that is inhibited by GF109203X (a “pan” PKC inhibitor), rottlerin (a PKCδ inhibitor), or PKCδ RNAi [18]. Interestingly, as shown in Figure 2C, the release of TNFα by PMA was blunted in LNCaP cells growing in steroid-depleted medium. This effect was restored by addition of R1881 to the medium. TRAIL levels in CM-PMA were also higher than in CM-Veh, as determined by ELISA (Figure 2D). Moreover, TRAIL levels were significantly lower in CM-PMA collected from LNCaP cells growing in steroid-depleted medium relative to CM-PMA collected from cells in normal medium. As with TNFα, when the steroid-depleted medium was supplemented with R1881, PMA was able to cause a full release of TRAIL.
Next, to further establish the relevance of these findings, we analyzed the effect of AR depletion on the autocrine secretion of death factors. We have previously determined that AR knock-down using RNAi abrogates the apoptotic effect of PMA in LNCaP cells [19]. Delivery of a specific AR RNAi duplex into LNCaP cells caused a significant reduction in AR levels as well as PKCδ down-regulation (Figure 3A, see also Ref. [19]). No appreciable changes in PKCε or PKCζ levels were observed, and a reduction in PKCα was detected (Supplementary Figure 1), consistent with previous results [19]. Interestingly, CM-PMA collected from AR-depleted LNCaP cells had significantly lower apoptotic activity compared to that from cells transfected with a control RNAi duplex (Figure 3B). The partial inhibition may be a consequence of the incomplete AR and PKCδ depletion achieved in these experiments. The ability of PMA to induce TNFα and TRAIL mRNA was markedly diminished in LNCaP cells subject to AR knockdown (Figure 3C and D). Moreover, in AR-depleted LNCaP cells, the release of TNFα and TRAIL by PMA was impaired (Figure 3E and F).
Figure 3.
AR RNAi inhibits PMA induction of TNFα and TRAIL. LNCaP cells growing in normal medium were transfected with either an RNAi duplex for AR or a control (C) duplex using the Amaxa Nucleofector, and 48 h later treated for 1 h with either 100 nM PMA or vehicle. For mRNA determinations, RNA was extracted 3 h after treatment. For cytokine determinations, CM was collected 24 h after treatment. Panel A: Representative Western blot showing AR depletion and PKCδ down-regulation in AR-depleted cells 48 h after RNAi transfection. Expression levels, relative to control RNAi, have been determined by densitometry and are shown below each corresponding Western blot. Panel B: Apoptotic effect of CM-PMA from LNCaP cells collected from cells subjected to AR or control RNAi. The percentage of apoptotic cells was determined by DAPI staining 24 h after addition of CM-PMA. Panel C: TNFα and TRAIL mRNA levels were determined by real-time PCR 3 h after PMA treatment in LNCaP cells subject to either AR or control RNAi. Results are normalized to endogenous GAPDH mRNA levels and expressed as fold-increase relative to those in control cells treated with vehicle. Panel D: TNFα and TRAIL levels in CM-Veh or CM-PMA collected from LNCaP cells subjected to AR or control RNAi, as determined by ELISA. In all cases, a representative experiment is shown and results are presented as mean ± SD of triplicate samples. Similar results were obtained in two additional experiments. CM-PMA, conditioned medium from PMA-treated cells; CM-Veh, conditioned medium from vehicle-treated cells.
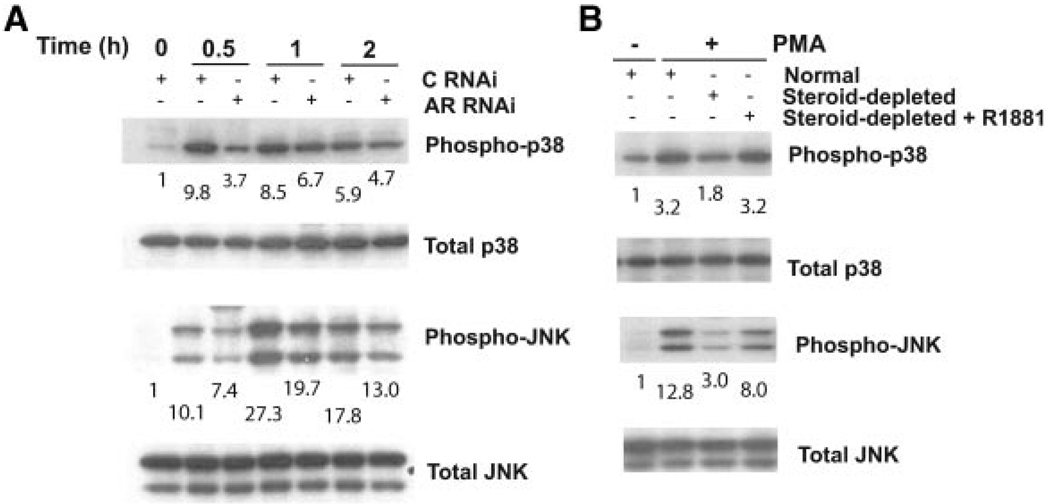
Lastly, since the autocrine-mediated apoptotic effect of phorbol esters in LNCaP cells is mediated by the p38 MAPK and JNK cascades [18], we decided to determine whether androgen depletion impacts on the activation of these signaling pathways. As shown in Figure 4A, addition of CM-PMA to LNCaP cells caused a time-dependent activation of p38 MAPK and JNK. Interestingly, CM-PMA collected from AR-depleted LNCaP cells has a reduced efficacy to activate p38 MAPK and JNK. Likewise, activation of p38 MAPK and JNK by CM-PMA collected from steroid-depleted LNCaP cells was greatly diminished compared to that caused by CM-PMA collected from cells growing in normal medium. This effect was rescued by the addition of R1881 to the medium (Figure 4B). Collectively, our data implicate androgens as key modulators of cytokine release and programmed cell death in response to PKC activation.
Figure 4.
Androgen depletion and AR RNAi impair the ability of CM-PMA to activate p38 MAPK and JNK. Panel A: LNCaP cells growing in normal medium were transfected with RNAi duplexes for either AR or a control (C) duplex using the Amaxa Nucleofector, and 48 h later treated for different times with 100 nM PMA. Panel B: LNCaP cells were grown in normal medium, steroid-depleted medium, or steroid-depleted medium supplemented with R1881 (1 nM) for 48 h, and then treated with either 100 nM PMA (+PMA) or vehicle (−PMA) for 1 h. For Panels A and B, CM was collected 24 h after treatment and added to LNCaP cells. Cell extracts were prepared and subjected to Western blot analysis using the antibodies indicated in the figures. Phospho-38 and phospho-JNK levels, normalized to the corresponding total levels, were determined by densitometry. Values were expressed as fold-increase relative to t = 0 (Panel A) or to cells growing in normal medium and untreated with PMA (Panel B). Values are shown under each corresponding Western blot. Similar results were observed in three independent experiments.
DISCUSSION
Apoptosis in response to phorbol esters occurs in a number of cellular models, such as prostate cancer cells, keratinocytes, and hemopoietic cells, and it is mediated in most cases by the novel PKCδ isozyme. Our studies have underscored the existence of a PKC-activated autocrine loop responsible for the apoptosis induced by phorbol esters in prostate cancer cells which involves the death factors TNFα and TRAIL. Constant removal of the factors released to the medium abrogates apoptosis induced by PMA in LNCaP cells, arguing that the autocrine mechanism is necessary for the apoptotic effect [18]. These studies also revealed the involvement of the extrinsic apoptotic cascade, which could be inferred from the induction of caspase-8 cleavage and activation of p38 MAPK, JNK and NF-κB, well-established death receptor effectors. Interfering with the extrinsic apoptotic cascade by various means, including RNAi depletion of caspase-8 or the adaptor FADD, or pharmacological inhibition of p38 MAPK and JNK, reduces the apoptotic effect of PMA [18]. PKCδ has a dual role, both in the secretion of death factors as well as an effector downstream of death receptors [18]. While other studies have established a potential contribution of autocrine factor release to mitogenic and transforming events induced by PKC activation [20–22], our studies established a paradigm of PKC-mediated autocrine apoptotic signaling in prostate cancer cells.
In this manuscript we report that the PKC-triggered pro-apoptotic autocrine loop is sensitive to androgen control. Steroid depletion from LNCaP cell culture medium greatly influenced TNFα and TRAIL mRNA induction and their accumulation in the CM in response to PMA treatment. This is consistent with the reduced apoptogenic activity of PMA in LNCaP cells growing in steroid-depleted (charcoal-treated) serum [18], as well as with the limited ability of CM-PMA from steroid-deprived cells to promote apoptosis. All these effects can be rescued by supplementing the culture medium with R1881, thereby implicating androgens as modulators of the autocrine response. This conclusion is supported by studies using CM-PMA from androgen-independent DU145 and PC-3 cell lines, which were insensitive to androgen depletion. Moreover, the fact that AR RNAi mimics the responses observed upon androgen-depletion strongly argues for the androgen control of the autocrine release of death factors in response to PKC activation. Only androgens but not other steroids such as glucocorticoids, progesterone, or T3, were able to rescue PMA-induced apoptosis in LNCaP cells growing in steroid-depleted medium, and the androgen rescue was not observed when cells were treated with the AR specific antagonist Casodex [19]. The reduced apoptotic index is independent of the reduced growth rate of steroid-deprived LNCaP cells, as growth factors that reinstate growth of these cells do not restore the apoptotic effect of PMA [19]. Androgen depletion causes a marked reduction in PKCδ expression levels, and since PKCδ mediates the autocrine release of death factors from LNCaP cells it is conceivable that PKCδ down-regulation restricts the release of autocrine factors by PMA. A strict correlation exists between PKCδ levels and the ability of PMA to trigger LNCaP cell death [18,19]. The human PKCδ gene possesses several putative androgen responsive elements (AREs), and at least one located − 4.7 kb from the transcription start site is functionally relevant in vivo in LNCaP cells, as revealed by ChIP analysis and luciferase reporter studies. The transcriptional regulation of PKCδ by androgens in prostate cancer cells has been recently confirmed by others [23]. Androgen-dependent PKCδ up-regulation has been also found in coronary smooth muscle [24]. Interestingly, changes in PKCδ expression have been reported in other models in response to other hormones such as estrogens, vitamins, or mechanical forces [25–28], which like androgens are probably mediated through genomic mechanisms. The ability of androgen to regulate multiple apoptotic proteins [29–31] makes it likely that the impaired apoptotic effect of phorbol esters in androgen-deprived LNCaP cells involves additional mechanisms not necessarily related to PKCδ depletion.
Dissecting the mechanisms that modulate the release and function of cytokines from prostate cancer cells is highly relevant, as therapeutic strategies based on targeting death receptors or their ligands are under development [32–35]. While LNCaP cells have a limited apoptotic response to TNFα and TRAIL when used as single agents, chemoterapeutic drugs or irradiation sensitize cells to these cytokines [36–38]. Anti-androgen therapy represents a standard approach for prostate cancer and it is therefore likely that down-regulation of PKCδ occurs in androgen-responsive tissues upon androgen withdrawal. Not surprisingly, a recent study found reduced PKCδ levels in prostate cancer biopsies from patients undergoing androgen ablation therapy as compared to those from untreated patients [23]. Since PKCδ is not only required for phorbol ester-mediated cytokine release but also acts as an effector downstream of death receptors [18,39], a speculation is that PKCδ down-regulation in androgen-depleted LNCaP cells may also contribute to the resistance to prostate cancer cell killing by cytokines.
In summary, our results revealed that androgens sensitize LNCaP prostate cancer cells to the release of cytokines by phorbol esters. In the absence of androgen, PMA fails to release TNFα and TRAIL, and consequently the overall apoptotic effect of the phorbol ester is impaired. As phorbol ester effects depend on the balance between the activation of pro-apoptotic and pro-survival PKC isozymes (e.g., PKCδ vs. PKCε), it would be important to determine whether androgen affects the expression of other PKCs. By fine-tuning the expression of PKC isozymes, androgens may greatly alter the effectiveness of chemotherapeutic agents that depend on PKC activation. Of interest, studies have shown that the cell killing effects of etoposide and radiation are dependent on PKCδ [4,40,41]. Given the emerging interest in PKCδ as a therapeutic target [5,8,41–43], and considering that PKC activators are in clinical trials for various types of cancers and greatly enhance the antitumor effects of other agents and radiation in prostate tumors [15,44–46], our studies suggest that modifiers of PKCδ expression such as hormonal control may have significant impact on the responsiveness to therapeutic agents.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grants RO1-CA89202 (NIH) and PC061328 (Department of Defense) to M.G.K., and a post-doctoral training award from Department of Defense (PC060387) to L.X.
Abbreviation used
- PKC
protein kinase C
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- PMA
phorbol 12-myristate 13-acetate
- TNF
tumor necrosis factor
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- CM
conditioned medium
- RNAi
RNA interference
- AR
androgen receptor
- FBS
fetal bovine serum
- ELISA
enzyme-linked immunosorbent assay
REFERENCES
- 1.Cross TG, Scheel-Toellner D, Henriquez NV, et al. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- 2.Okada H, Mak TW. Pathways of apoptotic and non-apoptotic death in tumour cells. Nat Rev Cancer. 2004;4:592–603. doi: 10.1038/nrc1412. [DOI] [PubMed] [Google Scholar]
- 3.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;6:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 4.Brodie C, Blumberg PM. Regulation of cell apoptosis by protein kinase c delta. Apoptosis. 2003;8:19–27. doi: 10.1023/a:1021640817208. [DOI] [PubMed] [Google Scholar]
- 5.Gavrielides MV, Frijhoff AF, Conti CJ, Kazanietz MG. Protein kinase C and prostate carcinogenesis: Targeting the cell cycle and apoptotic mechanisms. Curr Drug Targets. 2004;5:431–443. doi: 10.2174/1389450043345380. [DOI] [PubMed] [Google Scholar]
- 6.Reyland ME. Protein kinase C delta and apoptosis. Biochem Soc Trans. 2007;35:1001–1004. doi: 10.1042/BST0351001. [DOI] [PubMed] [Google Scholar]
- 7.Basu A, Sivaprasad U. Protein kinase Cepsilon makes the life and death decision. Cell Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 9.Powell CT, Brittis NJ, Stec D, Hug H, Heston WD, Fair WR. Persistent membrane translocation of protein kinase C alpha during 12-0-tetradecanoylphorbol-13-acetate-induced apoptosis of LNCaP human prostate cancer cells. Cell Growth Differ. 1996;7:419–428. [PubMed] [Google Scholar]
- 10.Zhao X, Gschwend JE, Powell CT, Foster RG, Day KC, Day ML. Retinoblastoma protein-dependent growth signal conflict and caspase activity are required for protein kinase C-signaled apoptosis of prostate epithelial cells. J Biol Chem. 1997;272:22751–22757. doi: 10.1074/jbc.272.36.22751. [DOI] [PubMed] [Google Scholar]
- 11.Garzotto M, White-Jones M, Jiang Y, et al. 12-O-tetradecanoylphorbol- 13-acetate-induced apoptosis in LNCaP cells is mediated through ceramide synthase. Cancer Res. 1998;58:2260–2264. [PubMed] [Google Scholar]
- 12.Fujii T, García-Bermejo ML, Bernabó JL, et al. Involvement of protein kinase C delta (PKCdelta) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCdelta. J Biol Chem. 2000;275:7574–7582. doi: 10.1074/jbc.275.11.7574. [DOI] [PubMed] [Google Scholar]
- 13.Truman JP, Gueven N, Lavin M, et al. Down-regulation of ATM protein sensitizes human prostate cancer cells to radiation-induced apoptosis. J Biol Chem. 2005;280:23262–23272. doi: 10.1074/jbc.M503701200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem. 2003;278:33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- 15.McJilton MA, Van Sikes C, Wescott GG, et al. Protein kinase Cepsilon interacts with Bax and promotes survival of human prostate cancer cells. Oncogene. 2003;22:7958–7968. doi: 10.1038/sj.onc.1206795. [DOI] [PubMed] [Google Scholar]
- 16.Ikezoe T, Yang Y, Taguchi H, Koeffler HP. JNK interacting protein 1 (JIP-1) protects LNCaP prostate cancer cells from growth arrest and apoptosis mediated by 12-0-tetradecanoylphorbol-13-acetate (TPA) Br J Cancer. 2004;90:2017–2024. doi: 10.1038/sj.bjc.6601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 18.Gonzalez-Guerrico AM, Kazanietz MG. Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade: A key role for protein kinase C delta. J Biol Chem. 2005;280:38982–38991. doi: 10.1074/jbc.M506767200. [DOI] [PubMed] [Google Scholar]
- 19.Gavrielides MV, Gonzalez-Guerrico AM, Riobo NA, Kazanietz MG. Androgens regulate protein kinase Cdelta transcription and modulate its apoptotic function in prostate cancer cells. Cancer Res. 2006;66:11792–11801. doi: 10.1158/0008-5472.CAN-06-1139. [DOI] [PubMed] [Google Scholar]
- 20.Cacace AM, Ueffing M, Han EK, Marmè D, Weinstein IB. Overexpression of PKCepsilon in R6 fibroblasts causes increased production of active TGFbeta. J Cell Physiol. 1998;175:314–322. doi: 10.1002/(SICI)1097-4652(199806)175:3<314::AID-JCP9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Chattopadhyay N, Tfelt-Hansen J, Brown EM. PKC, p42/44 MAPK and p38 MAPK regulate hepatocyte growth factor secretion from human astrocytoma cells. Brain Res Mol Brain Res. 2002;102:73–82. doi: 10.1016/s0169-328x(02)00215-2. [DOI] [PubMed] [Google Scholar]
- 22.Wheeler DL, Ness KJ, Oberley TD, Verma AK. Protein kinase Cepsilon is linked to 12-O-tetradecanoylphorbol-13-acetate-induced tumor necrosis factor-alpha ectodomain shedding and the development of metastatic squamous cell carcinoma in protein kinase C epsilon transgenic mice. Cancer Res. 2003;63:6547–6555. [PubMed] [Google Scholar]
- 23.Jariwala U, Prescott J, Jia L, et al. Identification of novel androgen receptor target genes in prostate cancer. Mol Cancer. 2007;6:39. doi: 10.1186/1476-4598-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowles DK, Maddali KK, Dhulipala VC, Korzick DH. PKCdelta mediates anti-proliferative, pro-apoptic effects of testosterone on coronary smooth muscle. Am J Physiol Cell Physiol. 2007;293:805–813. doi: 10.1152/ajpcell.00127.2007. [DOI] [PubMed] [Google Scholar]
- 25.Peters CA, Cutler RE, Maizels ET, et al. Regulation of PKC delta expression by estrogen and rat placental lactogen-1 in luteinized rat ovarian granulosa cells. Mol Cell Endocrinol. 2000;162:181–191. doi: 10.1016/s0303-7207(00)00193-3. [DOI] [PubMed] [Google Scholar]
- 26.Berry DM, Antochi R, Bhatia M, Meckling-Gill KA. 1,25-DihydroxyvitaminD3stimulates expression and translocation of protein kinase Calpha and Cdelta via a nongenomic mechanism and rapidly induces phosphorylation of a 33-kDa protein in acute promyelocytic NB4 cells. J Biol Chem. 1996;271:16090–16096. doi: 10.1074/jbc.271.27.16090. [DOI] [PubMed] [Google Scholar]
- 27.Geng WD, Boskovic G, Fultz ME, et al. Regulation of expression and activity of four PKC isozymes in confluent and mechanically stimulated UMR-108 osteoblastic cells. J Cell Physiol. 2001;189:216–228. doi: 10.1002/jcp.10019. [DOI] [PubMed] [Google Scholar]
- 28.Shanmugam M, Krett NL, Maizels ET, et al. Regulation of protein kinase Cdelta by estrogen in the MCF-7 human breast cancer cell line. Mol Cell Endocrinol. 1999;148:109–118. doi: 10.1016/s0303-7207(98)00229-9. [DOI] [PubMed] [Google Scholar]
- 29.Rothermund CA, Gopalakrishnan VK, Eudy JD, Vishwanatha JK. Casodex treatment induces hypoxia-related gene expression in the LNCaP prostate cancer progression model. BMC Urol. 2005;5:5. doi: 10.1186/1471-2490-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bozdogan O, Atasoy P, Bozdogan N, et al. BAG-1 expression in hyperplastic and neoplastic prostate tissue: Is there any relationship with BCL-related proteins and androgen receptor status? Tumori. 2005;91:539–545. doi: 10.1177/030089160509100615. [DOI] [PubMed] [Google Scholar]
- 31.Jia Y, Hikim AP, Lue YH, et al. Signaling pathways for germ cell death in adult cynomolgus monkeys (Macaca fascicularis) induced by mild testicular hyperthermia and exogenous testosterone treatment. Biol Reprod. 2007;77:83–92. doi: 10.1095/biolreprod.106.058594. [DOI] [PubMed] [Google Scholar]
- 32.Jin Z, El-Deiry WS. Overview of cell death signaling pathways. Cancer Biol Ther. 2005;4:139–163. doi: 10.4161/cbt.4.2.1508. [DOI] [PubMed] [Google Scholar]
- 33.Guseva NV, Taghiyev AF, Rokhlin OW, Cohen MB. Death receptor-induced cell death in prostate cancer. J Cell Biochem. 2004;91:70–99. doi: 10.1002/jcb.10707. [DOI] [PubMed] [Google Scholar]
- 34.Shankar S, Chen X, Srivastava RK. Effects of sequential treatments with chemotherapeutic drugs followed by TRAIL on prostate cancer in vitro and in vivo. Prostate. 2005;62:165–186. doi: 10.1002/pros.20126. [DOI] [PubMed] [Google Scholar]
- 35.O’Kane HF, Watson CJ, Johnston SR, Petak I, Watson RW, Williamson KE. Targeting death receptors in bladder, prostate and renal cancer. J Urol. 2006;175:432–438. doi: 10.1016/S0022-5347(05)00160-6. [DOI] [PubMed] [Google Scholar]
- 36.Shankar S, Singh TR, Srivastava RK. Ionizing radiation enhances the therapeutic potential of TRAIL in prostate cancer in vitro and in vivo: Intracellular mechanisms. Prostate. 2004;61:35–49. doi: 10.1002/pros.20069. [DOI] [PubMed] [Google Scholar]
- 37.Hu H, Jiang C, Schuster T, Li GX, Daniel PT, Lü J. Inorganic selenium sensitizes prostate cancer cells to TRAIL-induced apoptosis through superoxide/p53/Bax-mediated activation of mitochondrial pathway. Mol Cancer Ther. 2006;5:1873–1882. doi: 10.1158/1535-7163.MCT-06-0063. [DOI] [PubMed] [Google Scholar]
- 38.An J, Sun YP, Adams J, Fisher M, Belldegrun A, Rettig MB. Drug interactions between the proteasome inhibitor bortezomib and cytotoxic chemotherapy, tumor necrosis factor (TNF) alpha, and TNF-related apoptosis-inducing ligand in prostate cancer. Clin Cancer Res. 2003;9:4537–4545. [PubMed] [Google Scholar]
- 39.Kilpatrick LE, Sun S, Mackie D, Baik F, Li H, Korchak HM. Regulation of TNF mediated antiapoptotic signaling in human neutrophils: Role of delta-PKC and ERK1/2. J Leukoc Biol. 2006;80:1512–1521. doi: 10.1189/jlb.0406284. [DOI] [PubMed] [Google Scholar]
- 40.Sumitomo M, Ohba M, Asakuma J, et al. Protein kinase Cdelta amplifies ceramide formation via mitochondrial signaling in prostate cancer cells. J Clin Invest. 2002;109:827–836. doi: 10.1172/JCI14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitsutake N, Namba H, Shklyaev SS, et al. PKC delta mediates ionizing radiation-induced activation of c-Jun NH(2)-terminal kinase through MKK7 in human thyroid cells. Oncogene. 2001;20:989–996. doi: 10.1038/sj.onc.1204179. [DOI] [PubMed] [Google Scholar]
- 42.Michie AM, Nakagawa R. Elucidating the role of protein kinase C in chronic lymphocytic leukaemia. Hematol Oncol. 2006;24:134–138. doi: 10.1002/hon.789. [DOI] [PubMed] [Google Scholar]
- 43.Mackay HJ, Twelves CJ. Targeting the protein kinase C family: Are we there yet? Nat Rev Cancer. 2007;7:554–562. doi: 10.1038/nrc2168. [DOI] [PubMed] [Google Scholar]
- 44.Fang B, Song Y, Han Z, et al. Synergistic interactions between 12-0-tetradecanoylphorbol-13-acetate (TPA) and imatinib in patients with chronic myeloid leukemia in blastic phase that is resistant to standard-dose imatinib. Leuk Res. 2007;31:1441–1444. doi: 10.1016/j.leukres.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Zheng X, Chang RL, Cui XX, et al. Effects of 12-O-tetradecanoylphorbol-13-acetate (TPA) in combination with paclitaxel (Taxol) on prostate Cancer LNCaP cells cultured in vitro or grown as xenograft tumors in immunodeficient mice. Clin Cancer Res. 2006;12:3444–3451. doi: 10.1158/1078-0432.CCR-05-2823. [DOI] [PubMed] [Google Scholar]
- 46.Garzotto M, Haimovitz-Friedman A, Liao WC, et al. Reversal of radiation resistance in LNCaP cells by targeting apoptosis through ceramide synthase. Cancer Res. 1999;59:5194–5201. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.