Summary
T helper 17 (Th17) cells are important for host defense against extra-cellular microorganisms. However they are also implicated in autoimmune and chronic inflammatory diseases, and as such need to be tightly regulated. The mechanisms that directly control committed pathogenic Th17 cells in vivo remain unclear. We showed here that IL-17A-producing CD4+ T cells expressed interleukin-10 receptor α (IL-10Rα) in vivo. Importantly, T cell specific blockade of IL-10 signaling led to a selective increase of IL-17A+IFN-γ− (Th17), and IL-17A+IFN-γ+ (Th17+Th1) CD4+ T cells during intestinal inflammation in the small intestine. CD4+ Foxp3− IL-10 producing (Tr1) cells and Foxp3+ regulatory (Treg) were able to control Th17 and Th17+Th1 cells in an IL-10-dependent manner in vivo. Lastly, IL-10 treatment of mice with established colitis decreased Th17 and Th17+Th1 cells frequencies via direct signaling in T cells. Thus IL-10 signaling directly suppresses Th17 and Th17+Th1 cells.
Introduction
T helper 17 (Th17) cells, which are characterized by the production of their signature cytokine IL-17A (Bettelli et al., 2007; Miossec et al., 2009), are a T helper cell lineage distinct from Th1 and Th2 cells. Th17 cells express the transcription factor RORγt, which induces transcription of the Il17A gene (Annunziato et al., 2009; Miossec et al., 2009). Th17 cells have been associated with numerous autoimmune and chronic inflammatory diseases (Miossec et al., 2009). In line with these associations, mice deficient in RORγt exhibit attenuated experimentally induced autoimmune disease and lack tissue-infiltrating Th17 cells (Ivanov et al., 2006). Moreover, adoptive transfer of in vitro differentiated Th17 cells into lymphopenic hosts leads to the development of colitis (Elson et al., 2007; Lee et al., 2009; Wang et al., 2009).
The cytokines responsible for the differentiation of Th17 cells are already well defined; IL-6, TGF-β, and IL-1β are known to drive Th17 differentiation (Bettelli et al., 2006; Mangan et al., 2006; Sutton et al., 2006; Veldhoen et al., 2006). In addition, the cytokine IL-23 maintains the expansion and pathogenicity of Th17 cells (Ahern et al., 2010; McGeachy et al., 2009). Indeed, polymorphisms in the IL-23R gene have been linked with increased susceptibility to inflammatory bowel disease (IBD) in humans (Duerr et al., 2006). Furthermore IL-23 is of major importance for the induction of colitis in mouse models. However it is still unclear whether the role of IL-23 is intrinsically restricted to the development of pathogenic T cell populations. Of note also Th1 cells are unable to induce colitis in the absence of IL-23, which suggests that IL-23 might have other, possibly T cell extrinsic, effects (Ahern et al., 2008). It was recently shown that IL-23 signaling is particularly important for the emergence of IL-17A+IFN-γ+ T cells, which are referred to as ‘Th17+Th1’ cells, in intestinal inflammation. This suggests that these double producing cells play an important pathogenic role in IBD (Ahern et al., 2010). Although Th17 cells can be potentially pathogenic, this subset plays a key role in the defense against external bacteria and fungi (reviewed in (Miossec et al., 2009)). Therefore the mechanism, which fine-tunes Th17 cells, is obviously crucial but as yet is still poorly understood.
IL-10 has a non-redundant role in limiting inflammatory responses in vivo, particularly in the intestine (Kuhn et al., 1993). Mutations in the IL-10R are associated with IBD in humans (Glocker et al., 2009). Mice deficient in IL-10 (Il10−/−) or IL-10 receptor β (Il10rb−/−) are known to develop spontaneous intestinal inflammation (Kuhn et al., 1993; Spencer et al., 1998). However, IL-10 can act on a variety of immune cells: IL-10 acts directly on forkhead box P3 (Foxp3)+CD4+CD25+ regulatory T (Treg) cells to maintain Foxp3 expression and suppressive capacity (Murai et al., 2009), as well as on antigen presenting cells (Ding and Shevach, 1992; Fiorentino et al., 1991). Additionally, several immune cells can produce IL-10. Among these, are the CD4+ regulatory T cells, out of which the two best studied subsets are Foxp3+ Treg cells and T regulatory type 1 (Tr1) cells (Roncarolo and Battaglia, 2007).
Contradictory results have been published about the capacity of Foxp3+ Treg cells to control Th17 cells. It is known that human Foxp3+ Treg cell clones cannot suppress Th17 cell clones (Annunziato et al., 2007). But it has been published recently that the selective deletion of the transcription factor STAT3 in murine Foxp3+ Treg cells leads to spontaneous intestinal inflammation, which is associated with an increase in Th17 cells. These data demonstrated indirectly, that Foxp3+ Treg cells control Th17 response in a STAT3-dependent manner (Chaudhry et al., 2009). However, the mechanism whereby Foxp3+ Treg cells control Th17 cells was not identified in this study. A recent work has showed that a selective reduction of induced Treg (iTreg) cells in the gut associated lymphoid tissue (GALT) unexpectedly did not cause substantial immune-mediate lesion in the intestine, also the frequencies of Th17 cells remained under control (Zheng et al., 2010). This observation strongly suggests, that a compensatory mechanism is present in order to control Th17 cells, at least in the GALT. Interestingly, the authors found that Tr1 cells are strongly expanded in the absence of iTreg cell.
To summarize, it has been shown that IL-10 can control both Th1 and Th17 immune responses (Ding and Shevach, 1992; Fiorentino et al., 1991; Fitzgerald et al., 2007; McGeachy et al., 2007). It is also known that Tr1 and Foxp3+ Treg cells can suppress T cell responses in vivo (Littman and Rudensky, 2010; Roncarolo and Battaglia, 2007). However it is currently unclear, if IL-10 acts directly on the different effector T cells or mediates its inhibitory functions in an indirect manner via antigen presenting cells (APC). Likewise the role of IL-10 signaling in effector T cells themselves for their suppression by Tr1 and Foxp3+ Treg cells is unclear. Moreover whether Tr1 cells can compensate for a possible paucity of Foxp3+ Treg cells and vice versa is currently considered an important unresolved point in this field (Littman and Rudensky, 2010; Zheng et al., 2010). Finally, despite the efficient control of Th17 cell differentiation by IL-27, it is still unclear how mature Th17 cells can be controlled (El-behi et al., 2009).
We found in two models of intestinal inflammation that IL-17A producing CD4+ T cells in contrast to non-IL-17A producing CD4+ T cells expressed high levels of IL-10Rα, and that IL-10 signaling in T cells controlled IL-17A+IFN-γ− and IL-17A+IFN-γ+. Both Foxp3− IL-10 producing Tr1 cells and Foxp3+ Treg cells were independently able to suppress colitis caused by the transfer of in vivo differentiated Th17 cells into lymphopenic hosts in an IL-10 dependent manner.
RESULTS
Differential distribution of pro- and anti-inflammatory T helper cells after anti-CD3 treatment
We previously reported that anti-CD3 treatment led to the accumulation of IL-10 producing T cells in the small intestine (Kamanaka et al., 2006). It was also known that anti-CD3 treatment led to a cytokine storm and systemic increased amounts of TGF-β1 and IL-6 (Chatenoud and Bluestone, 2007). These two cytokines induce the differentiation of Th17 cells (Bettelli et al., 2006). Accordingly we found, that anti-CD3 treatment induced Th17 cells, which accumulated in the small intestine (Figure 1). Based on these data we aimed here to analyze whether IL-10 is important to control Th17 cells in the small intestine after anti-CD3 treatment.
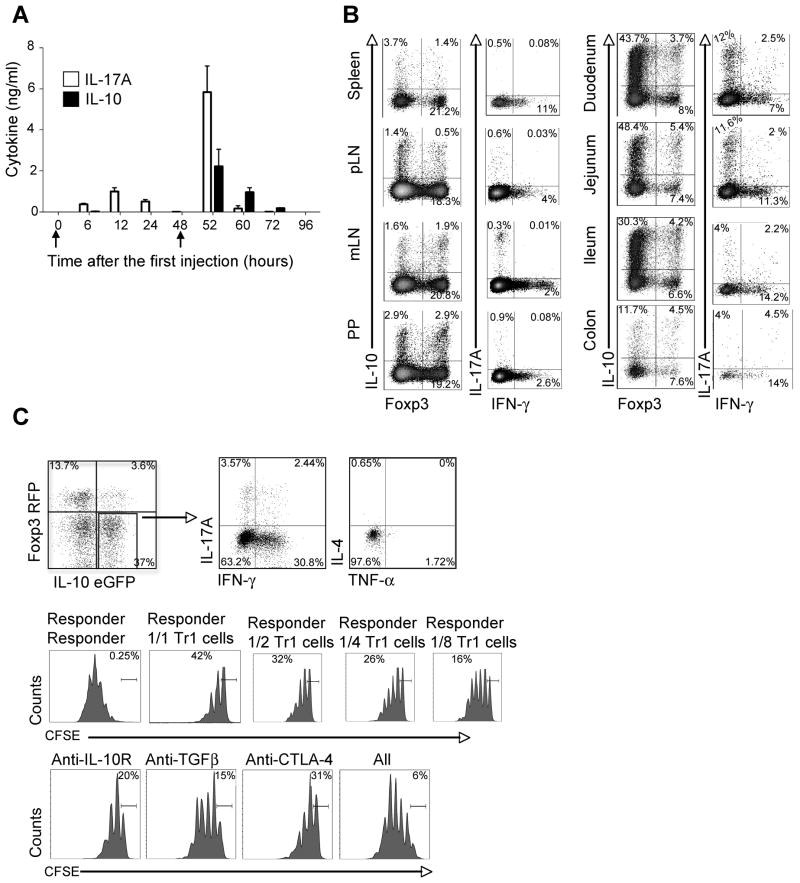
Figure 1. Co-localization of Tr1 and Th17 cells in the small intestine after CD3-specific antibody treatment.
Foxp3 RFP IL-10 eGFP double reporter mice were injected i.p. with anti-CD3 at 0 and 48 hours (see arrows in panel A). (A) Serum concentrations of IL-17A and IL-10 (Mean ± SEM).
(B) Cells were isolated from different organs as indicated. Foxp3 RFP and IL-10 eGFP expression was measured in freshly isolated cells. IL-17A and IFN-γ expression was measured in re-stimulated cells using intracellular cytokine staining (ICS). Cells are gated on CD4+ TCRβ+. Numbers in quadrants indicate percentage of cells. (C) CD4+TCRβ+Foxp3 RFP− IL-10 eGFP+ (CD45.2+) cells were isolated from the small intestine after anti-CD3 treatment. ICS was performed for IL-17A, IFN-γ, IL-4 and TNF-α (Upper panel). The suppressive capacity was measured by CFSE dilution of CD45.1+ responder cells. TGF-β, IL-10Rα and CTLA-4 antibodies were added as indicated (Lower panel). Numbers in quadrants indicate percentage of cells. Results are representative of at least three independent experiments.
We found that the peak of IL-10 and IL-17A in the serum of anti-CD3 treated mice occurred at the same time point, which was at 52 hours after the first injection (Figure 1A). We next analyzed the frequency of CD4+Foxp3−IL-10+ T cells or CD4+Foxp3+ T cells (Foxp3+ Treg cells) using the Foxp3 RFP and IL-10 eGFP double reporter mice (Kamanaka et al., 2006). The highest frequency of CD4+Foxp3−IL-10+ cells was found in the proximal part of the small intestine (duodenum and jejunum), whereas the frequency of Foxp3+ Treg cells was about the same in all parts of the intestine (Figure 1B). We also analyzed the localization of IL-17A+IFN-γ−Foxp3− and IL-17A−IFN-γ+Foxp3− producing CD4+ TCRαβ+ T cells. The highest frequency of IL-17A+IFN-γ−Foxp3− cells was in the small intestine, especially in the duodenum and jejunum, whereas the frequency of IL-17A−IFN-γ+Foxp3− cells was again about the same in the whole intestine (Figure 1B). Thus, IL-17A+ and Foxp3− IL-10+ CD4+ TCRαβ+ T cells are induced in the same time frame and are co-localized in the proximal part of the small intestine after anti-CD3 treatment.
We next characterized the CD4+ Foxp3− IL-10+ T cells localized in the proximal part of the small intestine. We found that about 30% of the IL-10 producing T cells also produced IFN-γ and only 6% produced IL-17A. In contrast, they did not produce IL-4 or TNF-α. Furthermore they were able to suppress the proliferation of T cells in a dose dependent manner in vitro. The suppressive capacity of these cells could also be blocked by a combination of IL-10Rα, TGF-β1, and CTLA-4 antibodies (Figure 1C). In contrast to IL-10 eGFP negative T cells, the IL-10 eGFP positive cells expressed LAG-3 (Figure S1), a marker which has been recently associated with IL-10 producing CD4+CD25−Foxp3− regulatory T cells (Okamura et al., 2009).
Thus, these IL-10 producing CD4+ T cells fulfill all criteria currently defining them as Tr1 cells (Gagliani et al., 2010; Groux et al., 1997; Roncarolo and Battaglia, 2007), and our data show that Th17 and Tr1 cells preferentially accumulated in the proximal part of the small intestine after anti-CD3 treatment, whereas Foxp3+ Treg cells, some of which also produce IL-10, were not exclusively present in the small intestine.
IL-17A producing CD4+ T cells express IL-10Rα
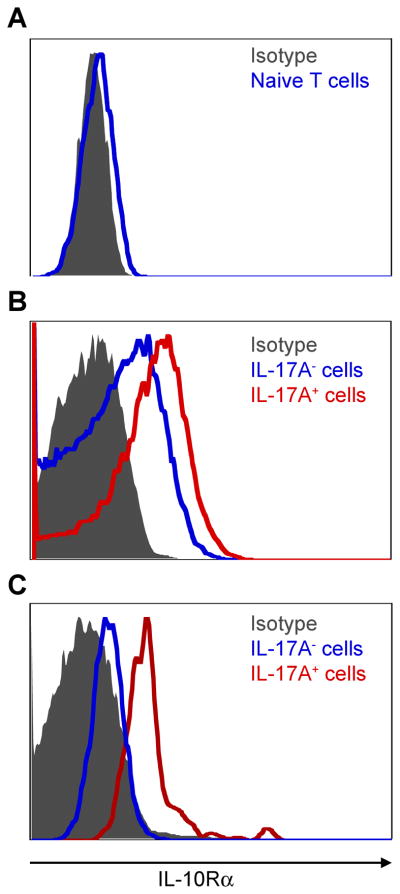
CCR6, IL-23R and IL-7R are known to be key receptors for the differentiation of pathogenic Th17 cells, which are expressed by the Th17 cell itself (Bettelli et al., 2007; Liu et al., 2010; McGeachy et al., 2009). However, it is not known, whether Th17 cells express IL-10Rα. Because Tr1 and Th17 cells were co-localized in the small intestine, we investigated whether Th17 cells express IL-10Rα, and could therefore respond to IL-10. By using IL-17A eGFP reporter mice, we could verify IL-10R expression on freshly isolated IL-17A eGFP+ cells without the need to re-stimulate the cells in vitro. This was important as in vitro stimulation can alter the expression of IL-10Rα. Naïve T cells showed a very low expression of IL-10Rα (Figure 2A). In contrast, in vivo differentiated IL-17A eGFP+ cells, which accumulated in the proximal part of the small intestine after anti-CD3 treatment, expressed IL-10Rα (Figure 2B-red line). In line with this observation, IL-17A eGFP+ cells in the CD45RBhi transfer colitis model also expressed IL-10Rα (Figure 2C-red line). In contrast, IL-17A eGFP− cells expressed lower amounts of IL-10Rα (Figure 2B+C-blue line).
Figure 2. IL-10Rα is highly expressed on IL-17A producing CD4+ T cells.
IL-10Rα expression was measured using flow cytometry. (A) Cells are gated on naive T cells (CD45.2+CD4+TCRβ+Foxp3−CD44−CD62Lhi) isolated from the spleen and lymph nodes of untreated Foxp3 RFP IL-17A eGFP reporter mice. (B) Cells are gated on CD4+TCRβ+CD44hi cells from the small intestine after anti-CD3 treatment. (C) CD4+CD45.2+Foxp3−CD45RBhi cells were isolated from the spleen of Foxp3-RFP IL-17A-eGFP double reporter mice and injected i.p. into CD45.1+Rag1−/− mice. Cells are gated on CD4+CD45.2+CD44hi IL-17A eGFP+ cells isolated from the mLN three weeks after the transfer. Grey area represents the isotype control. Results are representative of at least two independent experiments.
Thus, in vivo differentiated IL-17A producing CD4+ T cells express IL-10Rα and are therefore in principle able to respond to IL-10.
IL-10 signaling in T cells controls the emergence of IL-17A+IFN-γ− and IL-17A+IFN-γ+ CD4+ T cells
It is known that Il10−/− mice develop more severe tissue damage in the small intestine after anti-CD3 treatment compared to wild-type (WT) mice (Zhou et al., 2004), suggesting an important regulatory role of IL-10 in this model. However, the cellular target of IL-10 was not identified in this study, and the effect of IL-10 deficiency on different T cell subsets was also not addressed.
In order to study the role of IL-10 signaling, specifically in T cells, we previously generated transgenic (Tg) mice, in which IL-10 signaling is specifically blocked in T cells by the over expression of a dominant negative IL-10Rα under the Cd4 promoter (Cd4-DNIL-10R). Of note, these mice had no spontaneous immune-phenotype (Figure S2), and also did not spontaneously develop IBD (Kamanaka et al., 2011).
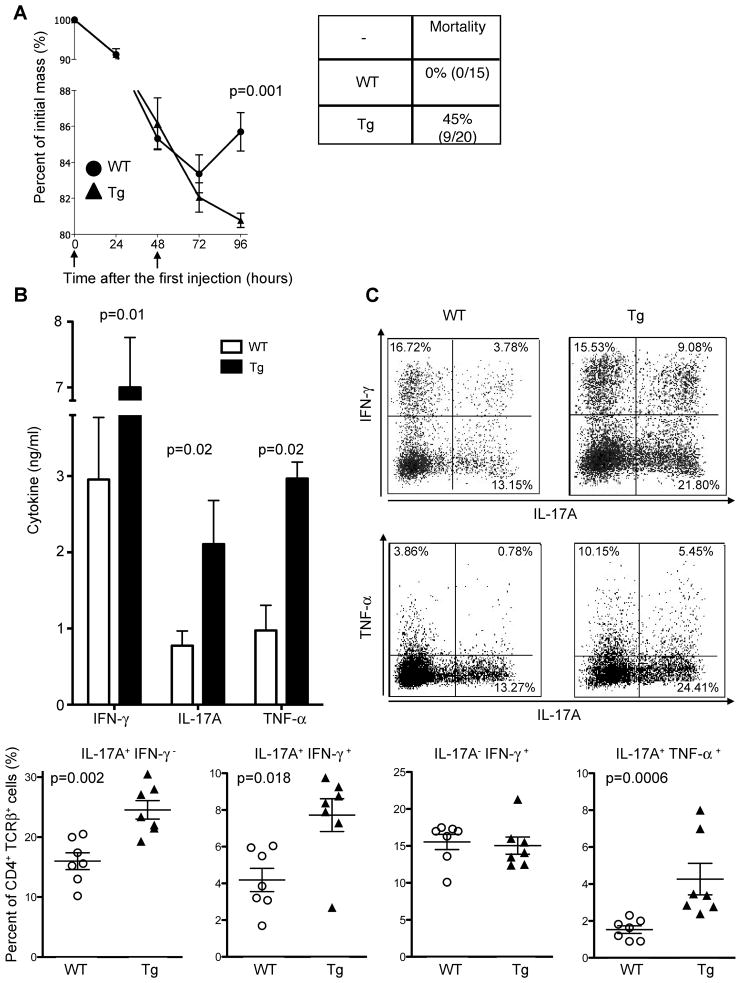
We therefore used the Cd4-DNIL-10R transgenic mice to analyze the role of IL-10 signaling in T cells in this model. Both WT and Cd4-DNIL-10R transgenic mice rapidly lost weight after anti-CD3 treatment. However, 100% of the WT mice recovered after the treatment and started to gain weight 72 hours after the first injection. In contrast, the Cd4-DNIL-10R transgenic mice continued to lose weight and 45% of the transgenic mice died about 72h after the first injection (Figure 3A). In line with the increased mortality, Cd4-DNIL-10R transgenic mice had higher IL-17A, IFN-γ, and TNF-α serum concentrations 52 hours after anti-CD3 treatment compared to WT mice (Figure 3B). Based on intracellular cytokine staining, we compared the frequency of IL-17A, IFN-γ, and TNF-α producing CD4+TCRβ+ T cells in the small intestine 52 hours after CD3-specific antibody treatment in WT and CD4-DNIL-10R transgenic mice. The frequency of IL-17A+IFN-γ− T cells was significantly increased in Cd4-DNIL-10R transgenic mice compared to WT mice, whereas the frequency of IL-17A−IFN-γ+ cells was equal between Cd4-DNIL-10R transgenic and WT mice (Figure 3C and 3D). The increased IFN-γ and TNF-α serum concentrations in Cd4-DNIL-10R transgenic mice correlated with increased frequencies of IL-17A+ IFN-γ+ and also IL-17A+ TNF-α+ double producing cells compared to WT mice (Figure 3C and 3D). We also found increase in TNF-α+IL-17A− T cells in some Cd4-DNIL-10R transgenic compared to WT mice (Figure 3C). This difference was nevertheless not statistically significant (WT: 6.5% +/− 2.2 vs. Tg: 6.7% +/− 1.6; p= 0.6). Of note, the total CD4+TCRβ+ cell numbers in the small intestine were not different at this time point (WT: 262 +/− 18.3 vs. Tg: 313 +/− 28.6 (× 103); p= 0.4). Therefore the total cell numbers of IL-17A+IFN-γ− (WT vs. Tg: 43.7 +/− 5.5 vs. 77.9 +/− 13.2 (× 103); p<0.02) and IL-17A+IFN-γ+ (WT vs. Tg: 12.4 +/− 2 vs. 26.4 +/− 2.8 (× 103); p<0.02), but not IL-17A−IFN-γ+ were different (WT vs. Tg: 39.5 +/− 5.4 vs. 47.4 +/− 5.9 (× 103); p= 0.31). In conclusion, T-cell specific blockade of IL-10 signaling led to increased frequencies and numbers of IL-17A+IFN-γ− and IL-17A+IFN-γ+, but not IL-17A−IFN-γ+ in the small intestine after anti-CD3 treatment.
Figure 3. T-cell specific blockade of IL-10 signaling leads to increased mortality after anti-CD3 treatment.
Cd4-DNIL-10R (Tg) or WT mice were injected with anti-CD3. (A) Mass loss (Mean ± SEM; WT: n=8; Tg: n=10) and mortality (cumulative from three experiments) are shown. (B–D) Mice were analyzed 52 hours after the first injection. (B) IL-17A, IFN-γ and TNF-α serum concentrations (Mean ± SEM; WT: n=7; Tg: n=4). (C) Cells were isolated from the small intestine and IL-17A, IFN-γ and TNF-α expression analyzed using ICS. Numbers in quadrants indicate percentage of cells. (D) Statistical overview of different T cell subsets in the small intestine after anti-CD3 injection as determined by ICS. Each dot represents one mouse. Lines indicate mean. Cells were gated on CD4+TCRβ+ events. Results are representative of at least 3 independent experiments.
IL-10 signaling in T cells inhibits the proliferation of IL-17A producing cells
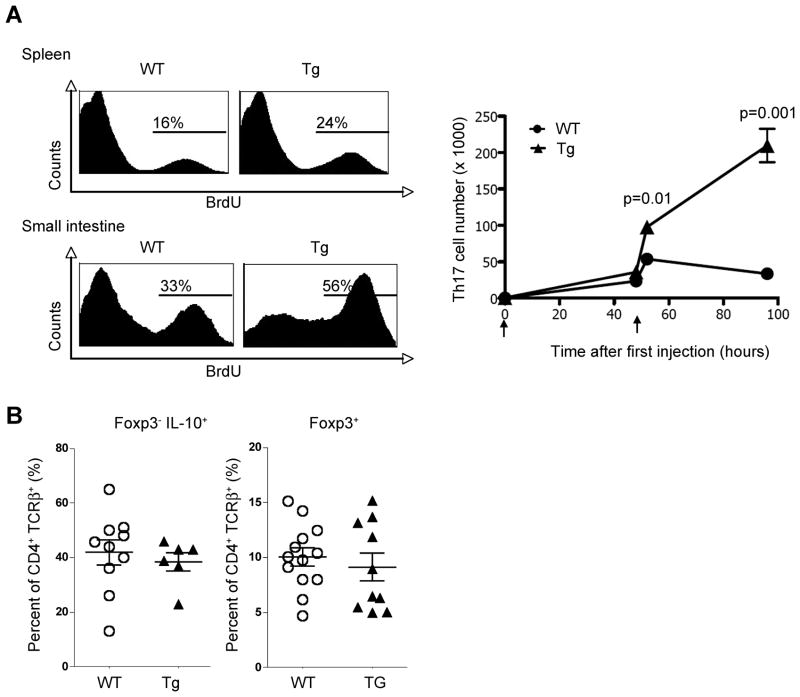
Because we found increased number of IL-17A producing T cells in the small intestine of Cd4-DNIL-10R transgenic mice after anti-CD3 treatment, we next analyzed whether IL-10 controls the proliferation of IL-17A producing T cells. We found that IL-17A eGFP+ transgenic T cells with blocked IL-10 signaling proliferated more in vivo based on BrdU uptake compared to WT (Figure 4A). Consequently, the numbers of IL-17A eGFP+ T cells increased in Cd4-DNIL-10R transgenic mice over time but not in WT mice (Figure 4B). It is important to mention that the frequency of Tr1 and Foxp3+ Treg cells, which are known to be induced or expanded by anti-CD3 treatment, was not different between WT and Cd4-DNIL-10R transgenic mice (Figure 4B). However although the frequency and numbers of Tr1 and Foxp3+ Treg cells were not affected, our data do not exclude a possible effect of IL-10 on the functionality of Tr1 and Foxp3+ Treg cells. As shown in Figure 1C, there were also some IL-17A+IL-10+ double producing T cells in the small intestine. Although the fraction of IL-17A producing cells within the IL-10 producing cell population was relatively small, this fraction represented up to 30% of all IL-17A producing T cells. However we also could not find a significant difference between WT and Cd4-DNIL-10R mice in the emergence of IL-17A+ IL-10+ double producing T cells after anti-CD3 treatment (Figure S3).
Figure 4. IL-10 signaling in T cells inhibits the proliferation of IL-17A producing cells.
(A) BrdU uptake was measured in CD4+TCRβ+IL-17A eGFP+ cells isolated from WT and Cd4-DNIL-10R mice (left panel). BrdU was injected 12 hours before mice were sacrificed. Time course experiment of total numbers of CD4+TCRβ+IL-17A+ cells after CD3-specific antibody treatment (Mean ± SEM; WT: 0h, n=4; 48h, n=2; 52h, n=5; 96h, n=5; Tg: 0h, n=4; 48h, n=2; 52h, n=4; 96h, n=5) (right panel). (B) Foxp3 RFP and IL-10 eGFP expression was measured in freshly isolated cells. Cells are gated on CD4+TCRβ+ events. A (left panel) and B: Mice were analyzed 52 hours after the first anti-CD3 injection. A (right panel) and B: Data are cumulative from three independent experiments. A (left panel): Data are representative of three independent experiments.
Thus, T cell specific blockade of IL-10 signaling led preferentially to the expansion of IL-17A+IFN-γ− and IL-17A+IFN-γ+, but not IL-17A−IFN-γ+ cell numbers. Additionally, the frequency of IL-17A+TNF-α+ T cells was also increased in Cd4-DNIL-10R transgenic mice compared to WT. Moreover, the increased number of these T cells was associated with increased mortality in Cd4-DNIL-10R transgenic mice. By contrast, the frequency of IL-10 producing T cells was not different between WT and Cd4-DNIL-10R mice, indicating that IL-10 signaling in T cells is dispensable for the emergence of these cells, which is in line with a previous study (Maynard et al., 2007).
Tr1 cells inhibit colitis induced by IL-17A+IFN-γ− and IL-17A+IFN-γ+ CD4+ T cells via IL-10
We next hypothesized that IL-10 producing regulatory T cells, such as Tr1 cells, can suppress Th17 cell - mediated disease via IL-10.
We found that after anti-CD3 treatment of WT mice about 30% of the IL-17A+ cells produced IL-10, only few secreted TNF-α, and only 20% produced IFN-γ (Figure 3 + Figure S3). In line with this less pathogenic phenotype, anti-CD3 treatment of WT caused only transient pathology in the small intestine. In contrast 50% of the IL-17A producing T cells in the CD45RBhi T cell transfer colitis model produced IFN-γ. Moreover, most of them also secreted TNF-α (Figure S4). Of note, the IL-17A producing T cells in the transfer colitis model have a more similar phenotype to the Cd4-DNIL-10R transgenic IL-17A producing T cells in the CD3-model (Figure 3, S4).
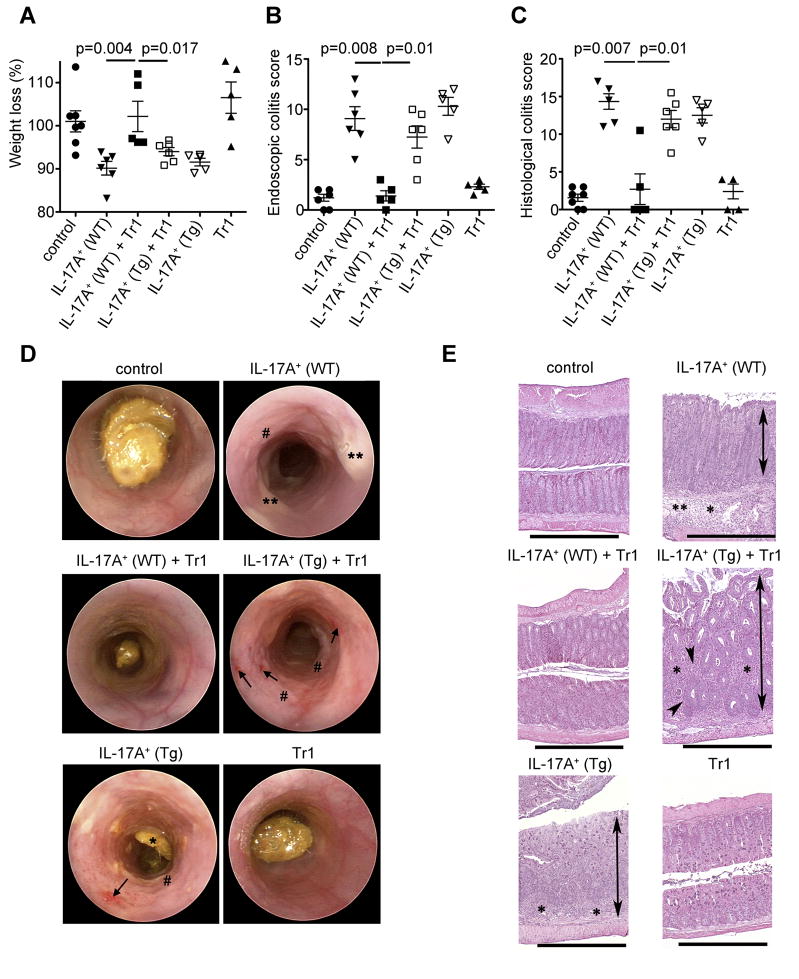
We therefore aimed to test, if Tr1 and Foxp3+ Treg cells could inhibit Th17 cells with a more pathogenic phenotype, which are present in the CD45RBhi T cell transfer colitis model. To that end we transferred CD4+ CD45RBhi T cells isolated from the IL-17A eGFP Foxp3 RFP double reporter mice into Rag1−/− mice and once the recipient mice developed intestinal inflammation, we isolated CD4+ IL-17A+ Foxp3− T cells from the inflamed colon and mesenteric lymph nodes. These CD4+ IL-17A eGFP+ T cells included IL-17A+TNFα+, IL-17A+IFNγ− and IL-17A+IFNγ+ cells (Figure S4). Importantly, all CD4+ IL-17A eGFP+ T cells in the CD45RBhi transfer colitis model expressed high IL-10Rα (Figure 2C). CD4+ IL-17A+ cells were transferred into Rag1−/− mice without any further in vitro expansion or stimulation. Transfer of these in vivo differentiated pathogenic IL-17A producing CD4+ T cells caused disease in the colon of the recipients, based on weight loss, histological and endoscopic findings (Figure 5A–E). In contrast, the development of intestinal disease was blocked by co-transferring Tr1 cells, isolated from the small intestine of anti-CD3 treated mice (Figure 5A–E). The complete suppression of the disease was dependent on IL-10 signaling in the effector T cells, because Tr1 cells were not able to suppress disease mediated by the transfer of in vivo differentiated Cd4-DNIL-10R transgenic effector cells (Figure 5A–E). Tr1 cells alone did not cause disease (Figure 5), although some of these cells produced IFN-γ (Figure 1C).
Figure 5. Tr1 cells suppress disease mediated by transfer of in vivo differentiated IL-17A+IFN-γ− and IL-17A+IFN-γ+ CD4+ T cells into Rag1−/− mice via IL-10.
IL-17A producing T cells were isolated from the colon and mLN as described in Figure S4. Tr1 cells were isolated from the small intestine of anti-CD3 treated mice and injected i.p. alone or together with IL-17A+ eGFP+ (IL-17A+) cells (WT or Cd4-DNIL-10R (Tg)) into Rag1−/− mice (A) Mass loss, (B) endoscopic and (C) histological colitis score were measured. Each dot represents one mouse. Lines indicate mean. (D) Representative endoscopic findings are shown. Note the bleeding (arrowhead), loss of transluceny, stool inconsistency (*), fibrin (**) and increased mucosal granularity (#) in the mice receiving IL-17A+ (WT), IL-17A+ (Tg) + Tr1 and IL-17A+ (Tg) cells. (E) Representative HE sections of colon are shown. The overall morphology in the colon of mice receiving IL-17A+ (WT)+Tr1 and Tr1 cells was not different from controls. Colons from mice receiving IL-17A+ (WT), IL-17A+ (Tg) + Tr1 and IL-17A+ (Tg) cells all had significant inflammation (*), crypt loss (arrowhead), edema (**), and moderate to marked mucosal hyperplasia (double arrows). HE Scale bars = 1000μm. Results are cumulative from 3 independent experiments.
Of note, the transfer of WT and Cd4-DNIL-10R transgenic IL-17A+ effector T cells caused disease to about the same severity. This indicated that the IL-10 amounts in the Rag1−/− host were not able to inhibit development of disease caused by either WT or CD4-DNIL-10R transgenic IL-17A+ effector T cells.
Thus, through their IL-10 production, Tr1 cells could suppress colitis induced by the transfer of IL-17A+IFN-γ− and IL-17A+IFN-γ+ CD4+ T cells.
Foxp3+ Treg cells suppress colitis induced by IL-17A+IFN-γ− and IL-17A+IFN-γ+ CD4+ T cells via IL-10
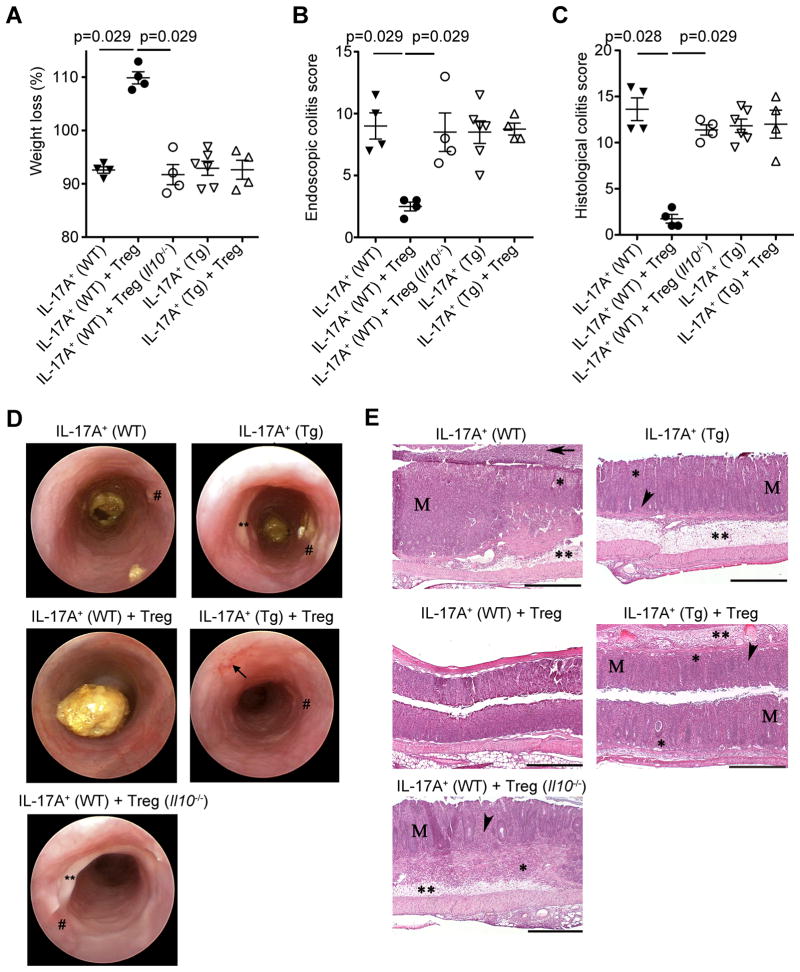
Foxp3+ Treg cells have evolved several mechanisms to suppress effector T cells. But it is still not known whether Foxp3+ Treg cells can directly control Th17 cells in vivo and, if they do, which mechanism they are using. Analogous to the approach we used to analyze the in vivo suppressive capacity of Tr1 cells, we co-transferred in vivo generated IL-17A+IFNγ− and IL-17A+IFNγ+ CD4+ T cells, isolated from the colon and mesenteric lymph nodes of mice with established colitis, with Foxp3+ Treg cell isolated from the spleen of anti-CD3 treated mice, into Rag1−/−mice. The Foxp3+ Treg cells were isolated from the spleen, since it was not possible to isolate sufficient numbers of these cells from the small intestine of anti-CD3 treated mice. Foxp3+ Treg cells were able to suppress disease mediated by the transfer of these effector cells to a similar degree as the Tr1 cells. In contrast, Foxp3+ Treg cells, isolated from Foxp3-cre-YFP-Il10flox/flox mice (Rubtsov et al., 2008), were not able to suppress disease development, suggesting an essential role of Foxp3+ Treg cell derived IL-10 for this suppression (Figure 6A–E). We next co-transferred WT Foxp3+ Treg cells with Cd4-DNIL-10R transgenic IL-17A eGFP+ T cells. Here we did not find a significant suppressive effect of WT Foxp3+ Treg cells on Cd4-DNIL-10R IL-17A eGFP+ T cell mediated disease based on mass loss, endoscopic and histological colitis score (Figure 6A–E).
Figure 6. Treg cells suppress disease mediated by transfer of in vivo differentiated IL-17A+IFN-γ− and IL-17A+IFN-γ+ CD4+ T cells into Rag1−/− mice via IL-10.
IL-17A producing T cells were isolated from the colon and mLN as described in Figure S4. CD4+TCRbeta+Foxp3 YFP+ (Treg cells) were isolated from the spleen of Foxp3-cre-YFP-Il10flox/flox (Treg Il10−/−) or Foxp3-cre-YFP-Il10flox/wt (Treg WT) control mice after anti-CD3 treatment. Treg cells were injected i.p. into Rag1−/− mice alone or together with IL-17A+ eGPF+ (IL-17A+) T cells (WT or Cd4-DNIL-10R (Tg)). (A) Mass loss, (B) endoscopic and (C) histological colitis score were measured. Each dot represents one mouse. Lines indicate mean. (D) Representative endoscopic findings are shown. Note the bleeding (arrowhead), loss of transluceny, stool inconsistency (*), fibrin (**) and increased mucosal granularity (#). (E) Representative HE sections of colon. The overall morphology of the colon from mice receiving IL-17A+ (WT) + Treg (WT) cells was not different from controls (See Figure 5E). Colons from mice receiving IL-17A+ (WT), IL-17A+ (WT)+Treg (Il10−/−), IL-17A+ (Tg), and IL-17A+ (Tg)+Treg cells all had significant inflammation (*), variable crypt loss (arrowheads), edema (**), and moderate to marked mucosal hyperplasia (M). Luminal flooding with neutrophils was observed in some mice with server colitis (arrow). Scale bars = 500μm. Results are representative of three independent experiments using WT Treg and two using Foxp3-cre-YFP-Il10flox/flox Treg cells.
These data indicate that Foxp3+ Treg cell derived IL-10 is essential for the suppression of disease mediated by the transfer of IL-17A+IFNγ− and IL-17A+IFNγ+ CD4+ T cells, and that the IL-10 produced by the Rag1−/− host is not able to compensate for this T cell derived IL-10. Furthermore, the suppression is dependent on IL-10 signaling in the transferred effector IL-17A+ T cells.
IL-10 suppresses IL-17A+IFN-γ− and IL-17A+IFN-γ+ CD4+ T cells in mice with established colitis
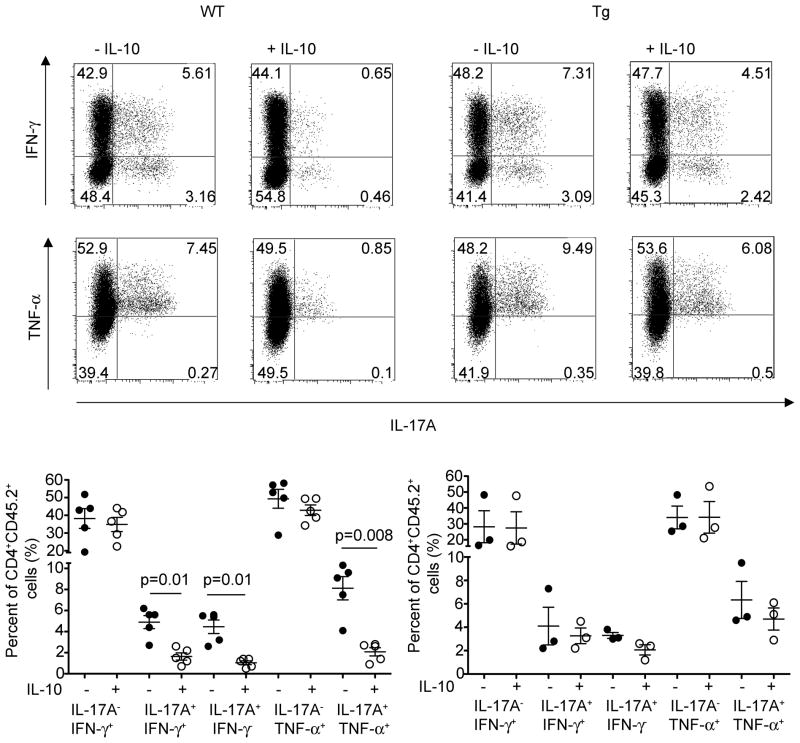
Through production of IL-10, both Tr1 cells and Foxp3+ Treg cells were able to suppress disease mediated by the transfer of in vivo differentiated IL-17A+IFNγ− and IL-17A+IFNγ+ CD4+ T cells. Unfortunately, in the previous transfer model we could not address which T cell subset (IL-17A+IFN-γ− or IL-17A+IFN-γ+) was affected by the co-transferred regulatory T cells. The reason was the limited number of cells that could be used for these transfer-experiments and consequently recovered from the protected mice. To overcome this problem, we took advantage of the CD45RBhi T cell transfer colitis model, the same model that we used to isolate IL-17A eGFP+ T cells for the previous described transfer experiments. CD4+Foxp3−CD45RBhi T cells from Foxp3 RFP reporter mice were transferred into Rag1−/− mice. As already mentioned before, IL-17A eGFP+ cells expressed high IL-10Rα (Figure 2C). As shown in Figure S4, IFN-γ+IL-17A−, IL-17A+IFN-γ−, and IL-17A+IFN-γ+ T cells were present in the Rag1−/− host four weeks after the transfer of CD4+Foxp3−CD45RBhi cells. In order to analyze the effect of IL-10 on these different T cell subsets, we treated the Rag1−/− mice at this time point with recombinant IL-10. IL-10 treated mice demonstrated a reduced frequency of IL-17A+IFN-γ− and IL-17A+IFN-γ+ compared to untreated animals in the mesenteric lymph nodes and colon (Figure 7, data not shown). In this colitis model almost all IL-17A+ T cells were also TNF-α+. Accordingly, the frequency of IL-17A+TNF-α+ T cells was also reduced in IL-10 treated mice compared to untreated controls (Figure 7). In contrast, the frequency of IL-17A− IFN-γ+ and TNF-α+IL-17A− cells was not different between IL-10-treated and untreated animals. In order to test whether IL-10 signaling in T cells was involved in the reduced frequency of IL-17A+IFN-γ− and IL-17A+IFN-γ+, we repeated the experiment mentioned above using Cd4-DNIL-10R transgenic CD4+Foxp3−CD45RBhi. If CD4+Foxp3−CD45RBhi with impaired IL-10 signaling were transferred, we could not find any significant change in the frequency of any of these T cells subsets, indicating that the reduction of IL-17A+IFN-γ− and IL-17A+IFN-γ+ T cell frequencies were dependent on IL-10 signaling in T cells (Figure 7).
Figure 7. IL-10 inhibits IL-17A+IFN-γ− and IL-17A+IFN-γ+ CD4+ T cells in the T-cell transfer colitis model.
CD4+CD45.2+Foxp3−CD45RBhi cells (WT and Cd4-DNIL-10R transgenic) were injected i.p. into CD45.1+Rag1−/− mice. Three to four weeks later Rag1−/− mice were injected three times on every other day with IL-10 (75ng/mouse) or PBS i.v.. Cells were isolated from the mLN and ICS for IL-17A, IFN-γ, and TNF-α was performed (top). Cells were gated on CD4+CD45.2+ events. Numbers in quadrants indicate percentage of cells. (Bottom) Each dot represents one mouse. Lines indicate mean. Results are cumulative from three independent experiments.
In conclusion, these data show that IL-10 signaling in T cells preferentially controls the emergence of both IL-17A+IFN-γ− and IL-17A+IFN-γ+. This is in line with IL-10Rα expression, which is high in IL-17A producing cells, but low in non-IL-17A producing cells (Figure 2). However although the frequency of IFN-γ+IL-17A− T cells was not affected, our data do not exclude a possible effect of IL-10 on the functionality of Th1 cells.
Discussion
Mechanisms that can control directly previously committed pathogenic Th17 cells are still not well understood. In this study we tested whether Tr1 and Foxp3+ Treg cells are able to control Th17, and if they do, which mechanism they use. We found that all IL-17A producing T cells, which include IL-17A+IFNγ− and IL-17A+IFNγ+ cells, expressed IL-10Rα. In line with this observation, IL-10 produced by Tr1 or Foxp3+ Treg cells controlled mature Th17, and Th17+Th1, but not Th1 cells, in a direct manner.
Prior studies have shown that IL-10 can inhibit Th17 cell immune responses in vivo. However these studies are based on the use of neutralizing IL-10 mAb or IL-10 deficient T cells (Fitzgerald et al., 2007; McGeachy et al., 2007). It is therefore not clear, whether IL-10 acts directly on Th17 cells or exerts its suppressive function indirectly, for example via APCs. Also if IL-10 signaling in Th17 cells themselves is important for their suppression by Foxp3+ Treg and Tr1 cells is unknown. We recently found that IL-10 signaling in T cells is particularly important to control the CD4+Foxp3−CD45RBlo T cell pool upon transfer into a lymphopenic host (Kamanaka et al., 2011). However, from this study, it is still not clear, whether IL-10 can directly control Th17 or Th1 cells. Of note two in vitro studies aiming to analyze the role of IL-10 on Th1 and Th17 cells (Gu. Y et al. EJI 2008; Naundorf S. et al EJI 2009) apparently contradict each other. The in vitro study by Naundorf et al. (Naundorf et al., 2009), who used human PBMCs, suggested that IL-10 might directly inhibit TCR induced IFN-γ, but not IL-17A production. In contrast, Gu et al. found that IL-10 inhibits IL-17A production in T cells in vitro in a total mouse splenocyte culture, and that APCs contribute to the negative regulation of Th17 cell differentiation by IL-10. Th17 cells are defined as IL-17A producing CD4+TCRαβ+ T cells. But further complexity may derive from the existence of IL-17A+IFN-γ+ double producing cells, which are referred as ‘Th17+Th1’ cells (Annunziato et al., 2009). Th17+Th1 cells have been described in patients with Crohn’s disease (Annunziato et al., 2007) and it has been suggested that they play a major role in the development of intestinal inflammation in murine colitis models (Ahern et al., 2010). We found that IL-10 signaling in T cells controls Th17 and Th17+Th1 cells, but not Th1 cells in vivo and the main source of IL-10 in our experiments appears to be Tr1 and Foxp3+ Treg cells. However, our data do not exclude a possible further contribution of APC-derived IL-10 for the suppression of Th17 cells in vivo.
Several publications during the last years have suggested that a balance between effector T cells, such as Th1 and Th17 cells, and different types of regulatory T cells, such as Tr1 or Foxp3+ Treg cells is essential in order to maintain immune homeostasis, especially in the intestine; reviewed in (Littman and Rudensky, 2010). It was however not clear, whether Tr1 or Foxp3+ Treg cells can directly inhibit Th17 cells in vivo. An additional point is that most studies of Th17 and also Tr1 cells were based on the use of in vitro differentiated Th17 (Elson et al., 2007; Lee et al., 2009; Wang et al., 2009) or Tr1 (Groux et al., 1997) cells respectively. Here we used reporter mice to purify in vivo differentiated Tr1, Foxp3+ Treg and Th17 cells to address these questions. IL-17A+ producing CD4+ T cells, comprising Th17 and Th17+Th1 cells, isolated from the CD45RBhi T cell transfer colitis model caused disease in the colon upon transfer into another lymphopenic host. Both Tr1 and Foxp3+ Treg cells were independently capable of inhibiting this disease, and this suppression was dependent on IL-10 signaling in the effector T cells.
By using this model, it was however not possible to analyze, whether IL-10 inhibits IL-17A+IFNγ− and IL-17A+IFNγ+ individually or both. Another possibility would be that IL-10 acted on the effector T cells after they converted into IL-17A−IFN-γ+ cells. To address this question, we used the CD4+CD45RBhi T cell transfer colitis model, in which IL-17A+IFNγ−, IL-17A+IFNγ+, and IL-17A−IFNγ+ cells are present (Ahern et al., 2010). We found that treatment with IL-10 in this T-cell transfer colitis model led to a selective reduction of IL-17A+IFN-γ− and IL-17A+IFN-γ+, but not IL-17A−IFNγ+. Furthermore, the effect was dependent on IL-10 signaling in T cells. This result was in line with our data obtained using the model of anti-CD3 treatment, in which also the frequency of IL-17A+IFN-γ− and IL-17A+IFN-γ+, but not IL-17A−IFNγ+ was significantly changed by the blockade of IL-10 signaling. Thus, our results demonstrated that IL-10 acts preferentially on IL-17A+IFN-γ− (Th17) and IL-17A+IFN-γ+ (Th17+Th1), rather than on IL-17A−IFNγ+ (Th1) cells. These data seem to be in contrast with previous publications, which described an inhibitory role of IL-10 on Th1 cells (Berg et al., 1996; Fiorentino et al., 1991). However, the reduction of the Th1 cell response by IL-10 was shown to be indirect, and instead mediated by inhibition of APC (Darrah et al., 2010; Ding and Shevach, 1992; Fiorentino et al., 1991). Additionally, the role of IL-10 on IL-17A+IFN-γ+ (Th17+Th1) double producing CD4+ T cells was not analyzed in these studies. We found that IL-10 signaling in T cells inhibited the emergence of these double producing T cells, but not IFN-γ single producing T cells in a direct manner.
IL-27 can inhibit the differentiation of Th17 cells, whereas it has little direct influence on already committed Th17 cells (El-behi et al., 2009; Fitzgerald et al., 2007; Stumhofer et al., 2006; Stumhofer et al., 2007). It was therefore still an open question which cytokine directly controls committed pathogenic Th17 cells. Of note, our study provides evidence that IL-10 controls Th17 and Th17+Th1 in a direct manner in vivo. Taken together, IL-27 can inhibit the differentiation of Th17 cells, whereas IL-10, which can also be induced by IL-27 (Fitzgerald et al., 2007), controls committed Th17 cells in a direct manner.
It is currently still unclear which factors drive the pathogenicity of Th17 cells. Others and we have shown that IL-22 and IL-17A, both cytokines produced by Th17 cells, have a protective function in colitis (O’Connor et al., 2009; Ogawa et al., 2004; Zenewicz et al., 2008). The CD45RBhi T cell transfer colitis is typically characterized by ulceration of the mucosa. Interestingly, transfer of IL-17A deficient T cells is associated with increased ulceration of the mucosa in the colon (O’Connor et al., 2009). We found here that transfer of in vivo differentiated IL-17A+ producing CD4+ T cells (including IL-17A+IFN-γ− and IL-17A+IFN-γ+ cells) into Rag1−/− mice caused colitis, which was characterized by inflammation, edema, crypt loss, and hyperplasia. However, ulceration was not characteristically seen in our studies, with only 40% of Rag1−/− mice that were injected with in vivo differentiated Th17 and Th17+Th1 cells demonstrating even mild ulceration of the mucosa (7 out of 18 mice). Taken together, the data presented here are consistent with the notion that although Th17 cells are pro-inflammatory, selected Th17-associated molecules, including IL-17A, IL-22 and possibly others, might also have tissue-protective properties (O’Connor et al., 2009; Zenewicz et al., 2008).
It should be mentioned, that CD3-specific antibody is already used as a therapy in human disease studies currently, most successfully for type 1 diabetes. Although the mechanisms of CD3-specific antibody induced tolerance is only still partially understood (Chatenoud and Bluestone, 2007). We found that this treatment, at least in a mouse model also induced Th17 cells. However we also found, in line with previous in vitro findings (McGeachy et al., 2007), that these Th17 cells can produce IL-10 in vivo. We extended this finding and showed that Th17 cells also expressed IL-10Rα and could therefore in principle be regulated by autocrine IL-10 production. However, it seems that pathogenic Th17 cells, which can be found for example in EAE or IBD do not produce IL-10, and therefore need to be controlled by regulatory T cells.
In conclusion, our data show that both Tr1 and Foxp3+ Treg cells are able to inhibit mature and pathogenic Th17 and Th17+Th1 cells independently via IL-10, suggesting that Tr1 cells can compensate for a possible paucity of Foxp3+ Treg cell and vice versa. Moreover, IL-10 can act directly on the T cells to inhibit Th17 and Th17+Th1 cells. The overlapping protective function of Foxp3+ Treg and Tr1 cells is likely to be of selective benefit to the host when confronted with an inflammatory insult at its mucosa.
Experimental Procedures
Mice
C57BL/6 mice (B6), C57BL/6 Rag1−/− mice, C57BL/6 CD45.1+ were purchased from The Jackson Laboratories. Foxp3 reporter mice (Wan and Flavell, 2005), IL-17A eGFP reporter mice and IL-10 eGFP mice (Kamanaka et al., 2006) were crossed with dominant negative IL-10Ralpha mice (Cd4-DNIL-10R, (Kamanaka et al., 2011)). Foxp3-cre-YFP-Il10flox/flox mice were provided by Y.A. Rudensky (Rubtsov et al., 2008). Age and sex matched littermates between 6–12 weeks of age were used. All animal procedures were approved by the Institutional Animal Care and Use Committee of Yale University.
Flow cytometry
Anti-CD4, anti-CD62L, anti-CD44, anti-CD45.1 and anti-CD45.2, anti-TCR-β, anti-IFN-γ, anti-IL-17A, anti-TNF-α, anti-IL-4, anti-BrdU were purchased from (BD). For intracellular cytokine staining (ICS), cells were re-stimulated with PMA (Sigma, 50 ng/mL) and ionomycin (Sigma, 1mM) for 4 hr. Golgistop (BD Bioscience) was added during the last 3 hours of re-stimulation. After staining for the extra cellular markers, cells were fixated, permeabilized and stained intracellular for specific cytokines and anti-GFP-FITC antibody (Invitrogen) to detect IL-10 and IL-17A eGFP. Cells were acquired on a LSRII flow cytometer (BD) and data analyzed using FlowJo software (Treestar) or FCS express.
For purification of T cell substes CD4+ T cells were first enriched by magnetic-activated cell sorting beads (MACS; Miltenyi Biotec) and then further purified using a FACSVantage (BD). Purity of sorted cells was higher than 97%.
In Vivo T Cell Stimulation and Intestinal Lymphocyte Isolation
Mice were injected with anti-CD3 (15 μg, 145-2C11), isotype antibody or PBS i.p. two-times every other day. After removal of the Peyer’s Patches intraepithelial lymphocytes (IEL) and lamina propria lymphocytes (LPL) were isolated using incubation with 5 mM EDTA at 37°C for 30 min (for IEL), followed by further digestion with collagenase IV and DNase at 37°C for 1 hr (for LPL). Cells were then further separated using a Percoll gradient. If not indicated differently cells were isolated from the upper part of the small intestine (duodenum + jejunum) of anti-CD3 treated mice.
Endoscopic procedure
Colonoscopy was performed in a blinded fashion for colitis scoring using the Coloview system (Karl Storz, Germany) (Becker et al., 2006). Briefly: Colitis scoring was based on granularity of mucosal surface, stool consistence, vascular pattern, translucency of the colon and fibrin visible (0–3 points for each).
Histopathology Procedure
Colons and small intestines were fixed in Bouin’s fixative solution and embedded in paraffin. Colons were assigned scores by investigators blinded to experimental manipulation. Each section was evaluated by a semi quantitative criterion-based method (score 0–5) as described before (O’Connor et al., 2009).
Cytokine assays
Cytokines were quantified in the plasma using Cytometric Bead Array (BD Bioscience) following manufacturer’s instructions.
Suppression Assays
CD45.1+ CD4+CD25− T cells (responder cells) were labeled with CFSE (1μM; Invitrogen) and cultured in a 96-well round bottom plates (50 × 103 cells/well) with or without FACS sorted CD45.2+CD4+IL-10 eGFP+Foxp3 RFP− (Tr1 cells) isolated from the proximal part of the small intestine (duodenum + jejunum) of anti-CD3 antibody treated mice. Irradiated APCs (spleenocytes MACS depleted for CD4+and CD8+ T cells) were used as feeder cells (400 × 103 cells/well). Cells were stimulated with 2 μg/ml of anti-CD3 antibody (2C11) in the presence or absence of anti-TGF-β (1D11), anti-CTLA-4 (9H10) and anti-IL10Rα (1B1). After 4 days CFSE dilution in CD45.1+CD4+ (responder cells) was analyzed by flow cytometry.
Statistical analysis
The non-parametric Mann-Whitney U test was used to calculate statistical significance using Prism. A p-value of less than 0.05 was considered significant.
Supplementary Material
Acknowledgments
The authors would like to thank F. Manzo for expert administrative assistance, E. Eynon and J. Alderman for managing the mouse program. We also thank T. Taylor for expert help with the FACS sorting. R.A.F. is an Investigator of the Howard Hughes Medical Institute. S.H. was supported by the DFG (HU 1714/1-1) and by a James Hudson Brown – Alexander B. Coxe Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahern PP, Izcue A, Maloy KJ, Powrie F. The interleukin-23 axis in intestinal inflammation. Immunol Rev. 2008;226:147–159. doi: 10.1111/j.1600-065X.2008.00705.x. [DOI] [PubMed] [Google Scholar]
- Ahern PP, Schiering C, Buonocore S, McGeachy MJ, Cua DJ, Maloy KJ, Powrie F. Interleukin-23 drives intestinal inflammation through direct activity on T cells. Immunity. 2010;33:279–288. doi: 10.1016/j.immuni.2010.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Liotta F, Maggi E, Romagnani S. Type 17 T helper cells-origins, features and possible roles in rheumatic disease. Nat Rev Rheumatol. 2009;5:325–331. doi: 10.1038/nrrheum.2009.80. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nat Protoc. 2006;1:2900–2904. doi: 10.1038/nprot.2006.446. [DOI] [PubMed] [Google Scholar]
- Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- Chatenoud L, Bluestone JA. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat Rev Immunol. 2007;7:622–632. doi: 10.1038/nri2134. [DOI] [PubMed] [Google Scholar]
- Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah PA, Hegde ST, Patel DT, Lindsay RW, Chen L, Roederer M, Seder RA. IL-10 production differentially influences the magnitude, quality, and protective capacity of Th1 responses depending on the vaccine platform. J Exp Med. 2010;207:1421–1433. doi: 10.1084/jem.20092532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Shevach EM. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–3139. [PubMed] [Google Scholar]
- Duerr RH, Taylor KD, Brant SR, Rioux JD, Silverberg MS, Daly MJ, Steinhart AH, Abraham C, Regueiro M, Griffiths A, et al. A genome-wide association study identifies IL23R as an inflammatory bowel disease gene. Science. 2006;314:1461–1463. doi: 10.1126/science.1135245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-behi M, Ciric B, Yu S, Zhang GX, Fitzgerald DC, Rostami A. Differential effect of IL-27 on developing versus committed Th17 cells. J Immunol. 2009;183:4957–4967. doi: 10.4049/jimmunol.0900735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–2370. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- Gagliani N, Jofra T, Stabilini A, Valle A, Atkinson M, Roncarolo MG, Battaglia M. Antigen-specific dependence of Tr1-cell therapy in preclinical models of islet transplant. Diabetes. 2010;59:433–439. doi: 10.2337/db09-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glocker EO, Kotlarz D, Boztug K, Gertz EM, Schaffer AA, Noyan F, Perro M, Diestelhorst J, Allroth A, Murugan D, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med. 2009;361:2033–2045. doi: 10.1056/NEJMoa0907206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kamanaka M, Huber S, Zenewicz LA, Gagliani N, Rathinam C, O’Connor W, Wan YY, Nakae S, Iwakura Y, Hao L, Flavell RA. Memory/effector (CD45RBlo) CD4 T cells are controlled directly by IL-10 and cause IL-22 dependent colitis. 2011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamanaka M, Kim ST, Wan YY, Sutterwala FS, Lara-Tejero M, Galan JE, Harhaj E, Flavell RA. Expression of interleukin-10 in intestinal lymphocytes detected by an interleukin-10 reporter knockin tiger mouse. Immunity. 2006;25:941–952. doi: 10.1016/j.immuni.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. Interleukin-10-deficient mice develop chronic enterocolitis. Cell. 1993;75:263–274. doi: 10.1016/0092-8674(93)80068-p. [DOI] [PubMed] [Google Scholar]
- Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Liu X, Leung S, Wang C, Tan Z, Wang J, Guo TB, Fang L, Zhao Y, Wan B, Qin X, et al. Crucial role of interleukin-7 in T helper type 17 survival and expansion in autoimmune disease. Nat Med. 2010;16:191–197. doi: 10.1038/nm.2077. [DOI] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Maynard CL, Harrington LE, Janowski KM, Oliver JR, Zindl CL, Rudensky AY, Weaver CT. Regulatory T cells expressing interleukin 10 develop from Foxp3(+) and Foxp3(−) precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Bak-Jensen KS, Chen Y, Tato CM, Blumenschein W, McClanahan T, Cua DJ. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat Immunol. 2007;8:1390–1397. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- McGeachy MJ, Chen Y, Tato CM, Laurence A, Joyce-Shaikh B, Blumenschein WM, McClanahan TK, O’Shea JJ, Cua DJ. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- Murai M, Turovskaya O, Kim G, Madan R, Karp CL, Cheroutre H, Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naundorf S, Schroder M, Hoflich C, Suman N, Volk HD, Grutz G. IL-10 interferes directly with TCR-induced IFN-gamma but not IL-17 production in memory T cells. Eur J Immunol. 2009;39:1066–1077. doi: 10.1002/eji.200838773. [DOI] [PubMed] [Google Scholar]
- O’Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Okamura T, Fujio K, Shibuya M, Sumitomo S, Shoda H, Sakaguchi S, Yamamoto K. CD4+CD25-LAG3+ regulatory T cells controlled by the transcription factor Egr-2. Proc Natl Acad Sci U S A. 2009;106:13974–13979. doi: 10.1073/pnas.0906872106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarolo MG, Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat Rev Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2–4 is an essential subunit of the interleukin 10 receptor. J Exp Med. 1998;187:571–578. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal Immunol. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, Rudensky AY. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Streutker C, Borojevic R, Wang Y, Croitoru K. IL-10 modulates intestinal damage and epithelial cell apoptosis in T cell-mediated enteropathy. Am J Physiol Gastrointest Liver Physiol. 2004;287:G599–604. doi: 10.1152/ajpgi.00063.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.