Highlights
▸ We explore brain mechanisms of cognitive reappraisal in children and adolescents. ▸ Children and adolescents can successfully modulate their emotional responses. ▸ We report age-related effects of activity in prefrontal cortex and amygdala. ▸ Future work should further these findings in pediatric clinical populations.
Keywords: Emotion regulation, Cognitive reappraisal, Development, Functional magnetic resonance imaging
Abstract
The ability to regulate one's emotions is critical to mental health and well-being, and is impaired in a wide range of psychopathologies, some of which initially manifest in childhood or adolescence. Cognitive reappraisal is a particular approach to emotion regulation frequently utilized in behavioral psychotherapies. Despite a wealth of research on cognitive reappraisal in adults, little is known about the developmental trajectory of brain mechanisms subserving this form of emotion regulation in children. In this functional magnetic resonance imaging study, we asked children and adolescents to up- and down-regulate their response to disgusting images, as the experience of disgust has been linked to anxiety disorders. We demonstrate distinct patterns of brain activation during successful up- and down-regulation of emotion, as well as an inverse correlation between activity in ventromedial prefrontal cortex (vmPFC) and limbic structures during down-regulation, suggestive of a potential regulatory role for vmPFC. Further, we show age-related effects on activity in PFC and amygdala. These findings have important clinical implications for the understanding of cognitive-based therapies in anxiety disorders in childhood and adolescence.
1. Introduction
Emotion regulation refers to the set of processes by which we modify the experience and expression of our emotions (Gross, 1998b). This ability is critical to sustained mental health, and impairments in emotion regulation are observed in a range of psychopathologies (Amstadter, 2008, Beauregard et al., 2006, Campbell-Sills et al., 2006, Cisler et al., 2010, Gross and Thompson, 2007, Hermann et al., 2009, Koenigsberg et al., 2002, Mullin and Hinshaw, 2007, Phillips et al., 2003). Furthermore, cognitive-based psychotherapies (Campbell-Sills and Barlow, 2007, Linehan et al., 2007, Taylor and Liberzon, 2007) utilize cognitive reappraisal in their approach to modulating pathological emotional tendencies. The emotion regulatory resources at an individual's disposal change across development commensurate with the refinement of processes including working memory, response inhibition, and self-reflection (Calkins and Hill, 2007, Stegge and Terwagt, 2007, Stuss, 1992, Zelazo and Cunningham, 2007). Many mental illnesses characterized in part by impairments in emotion regulation often manifest initially in childhood or adolescence (Davidson and Slagter, 2000). Thus, from a clinical perspective, a comprehensive exploration of the mechanisms underlying emotion regulatory processes requires a developmental approach.
An extensive literature has focused on the neural mechanisms subserving emotion regulation in adults (e.g., Beauregard et al., 2001, Blair et al., 2007, Kim and Hamann, 2007, Mak et al., 2009, Ochsner et al., 2004, Phan et al., 2005, Urry et al., 2006; see Ochsner and Gross, 2007, Phillips et al., 2008 for review). Most studies rely partially or exclusively on cognitive reappraisal, which involves reinterpreting an emotion-eliciting stimulus in order to modify one's emotional response (Gross, 1998a, Ochsner and Gross, 2005). Regions of prefrontal cortex (PFC) have been consistently implicated in the implementation of regulatory processes that in turn modulate activity in limbic regions such as the amygdala, which exhibits increased or decreased activity in accordance with the direction of regulation (e.g., Eippert et al., 2007, Harenski and Hamann, 2006, Kober et al., 2008, Koenigsberg et al., 2010, McRae et al., 2010, Ochsner et al., 2002, Ohira et al., 2006, Schaefer et al., 2002). The notion that PFC activity modulates the amgydala is supported by anatomical data in monkeys showing direct connections between the amygdala and regions of PFC (Amaral and Price, 1984, Ghashghaei et al., 2007). Medial PFC (mPFC) has also been linked to the down-regulation of amygdala activity (Phelps et al., 2004, Quirk et al., 2003). Additionally, human studies have found inverse correlations between activity in the amygdala and ventromedial PFC (vmPFC) during emotion regulation and have demonstrated that the vmPFC serves as a mediator between lateral PFC (lPFC) and the amygdala (Johnstone et al., 2007, Urry et al., 2006). One study identified a functionally connected network of brain regions associated with amygdala activation during reappraisal, including dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC), anterior cingulate cortex (ACC), and orbitofrontal cortex (OFC; Banks et al., 2007). Finally, the degree of success of reappraisal has been variably correlated with activity in regions of PFC (Eippert et al., 2007, Kober et al., 2008, Levesque et al., 2003, Wager et al., 2008), ACC (Phan et al., 2005), and amygdala (Eippert et al., 2007, Phan et al., 2005), as well as with the degree of correlation between the amgydala and PFC (Banks et al., 2007).
Despite the wealth of neuroimaging studies of cognitive reappraisal in adults, few studies have addressed the neural mechanisms of cognitive reappraisal in children. One study using event-related potentials demonstrated a reduction of the late positive potential, a marker of attention to emotional stimuli which is reduced in adults following reappraisal, after neutral relative to negative interpretations of negatively valenced images, suggesting commonalities in the neural markers of emotion regulation between children and adults (Dennis and Hajcak, 2009). In the only fMRI study to date to examine the neural correlates of cognitive reappraisal in children, Levesque et al. (2004) showed sad film clips to girls aged 8–10 years and instructed them to decrease their emotional response. Consistent with the adult literature, they demonstrated reappraisal-related activity in bilateral lPFC, OFC, and mPFC, right ACC and right ventrolateral PFC (vlPFC). They noted a greater number of prefrontal loci than previously identified in an identical study performed in adults (Levesque et al., 2003), which they interpreted as indicative of immaturity of the prefrontal–limbic circuitry. The authors examined a priori defined regions of interest limited to prefrontal cortex and ACC, and therefore were not able to explore modulation of other brain regions, including the amygdala, during reappraisal. Additionally, with the use of a narrow age range they were unable to examine changes in the neural mechanisms of cognitive reappraisal across development.
The prefrontal cortex is one of the last cortical structures to reach maturity, developing through adolescence and into early adulthood with a more complicated trajectory than phylogenetically older structures such as limbic and visual cortices (Casey et al., 2000, Gogtay et al., 2004, Marsh et al., 2008, Shaw et al., 2008, Toga et al., 2006). Emotion regulation shifts from dependence on caregiver support during infancy (Rothbart et al., 1992) to the development of self-regulatory processes in school age children (Denham, 1998). These self-regulatory abilities, prerequisite for successful cognitive reappraisal, develop in tandem with PFC (Stuss, 1992). Thus, we posit that the neural mechanisms of such regulatory processes should exhibit change across development. The objectives of the present study were to clarify the neural circuitry underling cognitive reappraisal in children, and to explore its developmental trajectory using a cross-sectional design. To this end, we asked children to view disgusting images and to either up- or down-regulate their emotional responses. We chose disgust over other negatively valenced emotions because the experience of disgust has been linked to anxious psychopathologies (Davey et al., 2006, Olatunji and Sawchuk, 2005), including obsessive–compulsive disorder (Berle and Phillips, 2006, Schienle et al., 2005), specific phobias (Davey, 1994, Mulkens et al., 1996, Tolin et al., 1997, Woody and Teachman, 2000), and health anxiety (Davey and Bond, 2005). For example, disgust induction has been shown to lead to a negative interpretation bias similar to that caused by anxiety induction (Davey et al., 2006). Moreover, it has been argued that disgust may play a causal role in the development and maintenance of anxiety disorders via attentional or interpretation biases (Olatunji et al., 2010). Therefore, an investigation of cognitive reappraisal of disgust in children has potential clinical implications for psychotherapeutic approaches to anxiety disorders.
2. Methods
2.1. Participants
We studied a group of 24 typically developing children and adolescents. Prior to group analyses, participants were excluded if, after removing volume acquisitions where movement between two volumes or integrated movement over 4 volumes exceeded 1 mm, more than 25% of the data was removed from the entire experiment or one experimental condition. This excluded 7 participants with a mean age of 11.5 years. Of these participants, 6 had insufficient data in the decrease-gross condition, 6 had insufficient data in the increase-gross condition, 5 had insufficient data in the look-neutral condition, and 3 had insufficient data in the look-gross condition. Two additional participants were excluded; one because professional assessment raised concern for a neuropsychiatric clinical diagnosis, and the other for outlying data in all of the contrasts of interest which raised serious concern that the participant did not perform the task correctly. Specifically, the participant showed significantly greater activation during decrease trials versus both increase and look-negative trials throughout the brain. Thus our final sample consisted of 15 participants (7–17 years, mean 13.03 ± 2.20, 9 male). Written assent was obtained for each participant in addition to informed parental consent according to a protocol approved by the Yale School of Medicine Human Investigations Committee.
2.2. Experimental design
Participants viewed neutral and disgust-inducing images drawn from the International Affective Picture System (Lang et al., 2008) and supplemented from an in-house set of images that were selected to be appropriate for viewing by children, such as images depicting moldy food, people vomiting, and roadkill. Prior to picture presentation, participants were provided an instruction (“Look”, “More gross”, or “Less gross”) as well as a specific strategy for the regulate conditions (i.e., “Pretend it's right in front of you” or “Pretend it's fake”). The instruction and strategy were presented in white text on a black background simultaneously with an audio recording by one of four actors (two male, two female), with a combined duration of 6 s. Images were displayed for 4 s and followed by an affect scale in which individuals were instructed to rate their degree of disgust on a 1-to-5 Likert scale with the prompt “How grossed out are you?”. Responses were selected via an MRI-compatible trackball mouse. The affect scale was presented for 6 s, followed by a “Relax” instruction for 2 s. Thus, each trial lasted 18 s. There were 4 conditions: look-neutral, look-gross, decrease-gross, and increase-gross. Each image was viewed only once by each participant, and the pairing of disgust-inducing images with trial type (look, decrease, increase) was counterbalanced across participants. Nine trials of each condition were presented in an event-related design with a jittered intertrial interval (ITI) of 2, 4 or 6 s. Fixation consisted of a white cross on a black background and was present during each ITI and for 12 s prior to the first trial and 10 s after the last, for a total experiment duration of 13:34 min.
2.3. Pretask training
All participants received one-on-one task training conducted by trained personnel prior to completing the experiment. First, participants were shown some sample disgust-inducing images and asked to verbally describe their response in order to confirm the experience of disgust. Second, participants were asked to provide the strategies they might use to increase or decrease their emotional response to the pictures. This was done to ensure that participants understood the concept and were not simply echoing instructions. Next, specific strategies for cognitive reappraisal were provided and discussed with the participant. For trials on which participants were instructed to increase their emotional response (“More gross”), we encouraged them to imagine themselves right in front of the contents of the disgusting image, rather than viewing it as a picture (“Pretend it's right in front of you”). For decrease trials, participants were instructed to pretend the contents of the image were fake, for example, props on a television show (“Pretend it's fake”). Participants were instructed in the use of the affect scale and given the opportunity to practice using the trackball mouse. To reduce demand effects, they were instructed explicitly to respond to the prompt “How grossed out are you?” with their actual emotional response to the image, irrespective of whether they were successful in increasing or decreasing their response. Finally, participants completed 3 practice trials with the experimenter and then 9 practice trials on their own.
2.4. Imaging protocol
Images were collected on a Siemens 3T Tim Trio scanner located in the Yale University Magnetic Resonance Research Center. High-resolution T1-weighted anatomical images were acquired using an MPRAGE sequence (TR = 1230 ms; TE = 1.73 ms; FOV = 256 mm; image matrix 2562; 1 mm × 1 mm × 1 mm). Whole-brain functional images were acquired using a single-shot, gradient-recalled echo planar pulse sequence (TR = 2000 ms; TE = 25 ms; flip angle = 60°; FOV = 220 mm; image matrix = 642; voxel size = 3.4 mm × 3.4 mm × 4.0 mm; 34 slices) sensitive to BOLD contrast. Runs consisted of the acquisition of 407 successive brain volumes.
2.5. Data analysis
Data were preprocessed and analyzed using the BrainVoyager QX 2.0 software package (Brain Innovation, Maastricht, The Netherlands). Preprocessing of the functional data included slice time correction (using sinc interpolation), 3-dimensional rigid-body motion correction (using trilinear-sinc interpolation), spatial smoothing with a FWHM 4-mm Gaussian kernel, linear-trend removal, and temporal high-pass filtering (fast-Fourier transform based with a cutoff of 3 cycles/time course). The functional data sets were coregistered to high-resolution, within-session, T1-weighted anatomical images which were in turn normalized to Talairach space (Talairach and Tournoux, 1988), to create 4-dimensional data sets. While it is possible that this normalization process could differentially affect younger participants whose brains may differ more from the Talairach template than the brains of older participants, it was nevertheless important to ensure that participants’ data could be effectively combined and statistically assessed. Additionally, normalizing to Talairach space allows comparison of the present findings to prior adult studies. Kang et al. (2002) provided an empirical validation of normalization for analysis of fMRI data from children. They found very small differences (relative to the resolution of fMRI data) in the spatial correspondence among several brain loci between young children and adults after a standard, nonlinear transformation that warped child and adult fMRI data into a common adult Talairach space. These and other similar findings (Burgund et al., 2002) support the use of a common, adult stereotactic space in this study. An in-house script was used to identify (and exclude) participants for whom, after removing volume acquisitions where movement between two volumes or integrated movement over 4 volumes exceeded 1 mm, more than 25% of the data was removed from the entire experiment or one experimental condition.
To confirm that participants understood and performed the task, behavioral ratings grouped by experimental condition (look-gross, look-neutral, decrease-gross, and increase-gross) were averaged in each participant. These average ratings were then compared in group-wise paired-samples t-tests. The first t-test compared ratings for look-gross to ratings for look-neutral in order to confirm that participants responded to the emotional nature of the stimuli. Additional t-tests compared ratings for look-gross to ratings for decrease-gross and increase-gross, respectively. These two t-tests were performed in order to confirm that participants experienced a change in their emotional reactions to the stimuli when instructed to modulate their reaction to the gross pictures.
To investigate brain regions modulated during the experimental paradigm, a random-effects multi-participant general linear model (GLM)-based analysis was performed. Regressors were defined as boxcar functions peaking during each of the four experimental conditions (predictors of interest), as well as three additional boxcar functions peaking during instruction, affect rating, and “relax” periods (predictors of no interest). These boxcar functions were convolved with a double-gamma hemodynamic response function (HRF) time-locked to the onset of the 4-s image display for the experimental conditions, and to the 6-s instruction period, the 6-s affect rating, and the 2-s “relax” period, respectively. To additionally account for motion during each scan, functions of all of the 3 directions and 3 translations of movement from each participant were included in each single-participant GLM-based analysis as additional predictors of no interest. In all whole-brain analyses, a mask was used to restrict analyses to only voxels located within the brain, determined by the extent of the MNI brain normalized to Talairach space.
To identify brain regions modulated by the emotional nature of the stimuli, brain activation in the contrast of look-gross > look-neutral was assessed at a statistical threshold of p < 0.05, corrected for multiple comparisons with a cluster threshold of 34 contiguous functional voxels (Forman et al., 1995, Xiong et al., 1995). This cluster threshold was calculated by the BrainVoyager cluster-threshold estimator plugin performing 1000 iterations of a Monte-Carlo simulation to correspond to α < 0.05.
To identify brain regions modulated by efforts to emotionally regulate (increase and decrease), a random-effects analysis was performed on the conjunction of both regulation contrasts (decrease-gross > look-gross and increase-gross > look-gross). This conjunction analysis was assessed at a statistical threshold of p < 0.05, corrected to α < 0.05 with a cluster threshold of 34 contiguous functional voxels.
To identify regions modulated by each emotion regulation strategy individually, brain activation in the contrasts of decrease-gross > look-gross and increase-gross > look-gross were assessed separately, each at a statistical threshold of p < 0.05, corrected to α < 0.05 with a cluster threshold of 41 contiguous functional voxels. To explore the effects of each emotion regulation task specifically on regions responsive to gross pictures, a region of interest (ROI) mask was created using regions identified as more active to gross (versus neutral) pictures (identified in this same participant group, in the contrast of look-gross > look-neutral in the multi-participant random-effects GLM analysis at a statistical threshold of p < 0.05, k = 34). Restricting the analyses to only voxels in the ROI mask, the same contrasts of decrease-gross > look-gross and increase-gross > look-gross were assessed at the same statistical threshold as the whole-brain analyses.
More specific ROI analyses were performed in the bilateral insula and amygdala, regions of a priori interest because of their implicated roles in processing negative (and particularly gross) stimuli. Specifically, we chose to investigate the insula given its role in the processing of disgust and our focus on disgust-inducing images (Calder et al., 2000, Ibañez et al., 2010, Lane et al., 1997, Phillips et al., 1997, Schafer et al., 2005, Wicker et al., 2003). We selected the amygdala because prior studies have consistently demonstrated that activity in the amygdala is modified by cognitive reappraisal efforts (e.g., Eippert et al., 2007, Harenski and Hamann, 2006, Kober et al., 2008, Koenigsberg et al., 2010, McRae et al., 2010, Ochsner et al., 2002, Ohira et al., 2006, Schaefer et al., 2002). These ROIs were functionally defined from the multi-participant random-effects GLM analysis in the contrast of look-gross > look-neutral at a more stringent threshold of p < 0.01 which allowed us to discriminate these specific ROIs. Average difference beta values in the contrasts decrease-gross > look-gross and increase-gross > look-gross were calculated for each of the four ROIs and statistically tested in their variance from zero (representing no modulation by emotion regulation) using one-sample t-tests.
In the two regions we found to be significantly decreased by down-regulation (right insula, left amygdala), we used difference values (decrease-gross − look-gross) calculated for each participant as an index of successful down-regulation. We used these values as a covariate in the whole-brain analysis of decrease-gross > look-gross to identify regions where activation significantly correlated with the degree of successful regulation in each participant. Specifically, we looked for regions showing an inverse correlation with the covariate, indicating that increased activation in these regions predicted a larger decrease in insula or amygdala activation during regulation. This covariate analysis was assessed at a statistical threshold of p < 0.05, with a cluster threshold of 10 contiguous functional voxels. We used a more liberal cluster threshold as the low number of active voxels in this analysis precluded the use of BrainVoyager's cluster-threshold estimator plugin.
The age range in the current study's participant sample allowed for the examination of brain regions in which activation correlated with age while viewing gross pictures, as well as during emotion regulation. To this end, whole-brain voxel-wise analyses were performed with chronological age as a covariate in each of the three contrasts of interest (look-gross > look-neutral, decrease-gross > look-gross, increase-gross > look-gross). These covariate analyses were assessed at a statistical threshold of p < 0.05, with a cluster threshold of 34 contiguous functional voxels.
To further elucidate the nature of the age correlations identified in whole brain covariate analyses, we performed similar correlations in anatomically defined regions which overlapped with areas we found to significantly correlate with age and for which we had a priori hypotheses about their importance in the current study, specifically the amygdala and insula. We visualized age correlations with activation in the contrast of decrease-gross versus look-gross in the left amygdala, and increase-gross versus look-gross in the left insula in two separate scatter plots. The left amygdala ROI was defined by the Talairach database (Lancaster et al., 1997, Lancaster et al., 2000), while the left insula ROI was defined by manually drawing insular gray matter on the Montreal Neurological Institute (MNI) 152 standard brain and then converted to Talairach space by normalizing the MNI brain, as previously described (Deen et al., 2010). Difference beta values for increase-gross − look-gross and decrease-gross − look-gross were calculated for the left insula and left amygdala, respectively, and plotted against age to visually inspect the correlation patterns for outliers or binary grouping patterns.
3. Results
3.1. Behavioral data
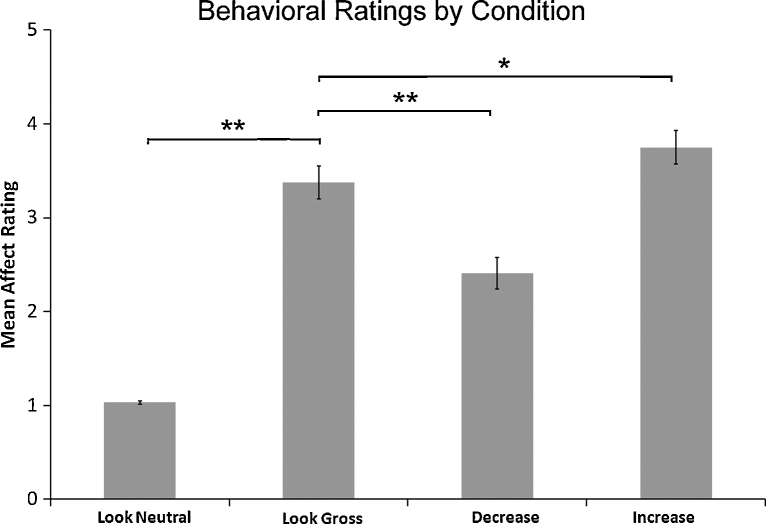
Behavioral results revealed that average disgust ratings of gross pictures were significantly higher than disgust ratings of neutral pictures (t = 12.30, p < 0.001). Further, disgust ratings were significantly lower for gross pictures when participants were asked to decrease (t = −6.06, p < 0.001), and significantly higher for gross pictures when participants were asked to increase (t = 2.82, p = 0.01) versus look (Fig. 1).
Fig. 1.
Mean affect ratings as a function of condition. Error bars represent standard error of the mean (SEM). Single asterisk reflects significance at a threshold of p < 0.01; double asterisk reflects significance at a threshold of p < 0.001.
3.2. Imaging data
3.2.1. Main effect of image valence
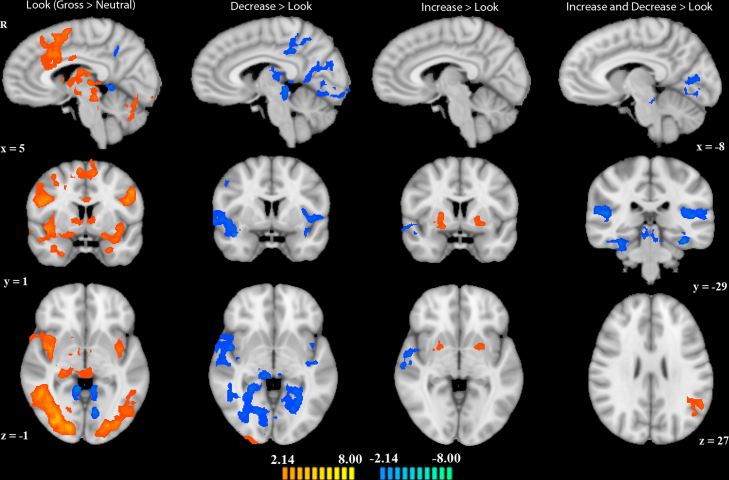
To identify brain regions modulated by looking at disgusting versus neutral pictures, we conducted a whole-brain contrast of look-gross > look-neutral. Regions that showed greater activation while viewing disgusting pictures included bilateral insula, hippocampus, supramarginal gyrus, and occipital cortex. Additionally, regions of the striatum, thalamus, right temporal pole, and right dlPFC and dmPFC were active to disgusting images. Regions that showed greater activation while viewing neutral pictures included right precuneus and bilateral retrosplenial cortex (Table 1, Fig. 2).
Table 1.
Brain regions modulated in each of the three contrasts look-gross > look-neutral, decrease-gross > look-gross, increase-gross > look-gross, as well as the conjunction of the two regulation contrasts. Results for the contrasts look-gross > look-neutral and regulate > look were obtained at a statistical threshold of p < 0.05 with a cluster threshold of 34 contiguous voxels. Results for the contrasts increase-gross > look-gross and decrease-gross > look-gross were obtained at a statistical threshold of p < 0.05 with a cluster threshold of 41 contiguous voxels. All coordinates reported are in standard Talairach space, oriented in radiological convention.
| Brain region | X | Y | Z | Size | t | p |
|---|---|---|---|---|---|---|
| Disgusting > neutral | ||||||
| Right supramarginal gyrus | 27 | −67 | 37 | 20,900 | 7.92 | 0.000002 |
| Left supramarginal gyrus | −24 | −52 | 37 | 9447 | 5.63 | 0.000062 |
| Striatum/thalamus | −12 | −22 | −8 | 11,098 | 5.49 | 0.00008 |
| Left hippocampus | −18 | −4 | −11 | 1965 | 4.86 | 0.000252 |
| Right hippocampus | 21 | −28 | 4 | 3543 | 5.21 | 0.000132 |
| Right occipital cortex | 36 | −43 | −20 | 42,562 | 9.00 | <0.001 |
| Left occipital cortex | −42 | −55 | −8 | 30,436 | 10.36 | <0.001 |
| Right insula/amygdala/TP | 36 | −7 | 7 | 13,419 | 7.35 | 0.000004 |
| Left insula/amygdala | −36 | −7 | 10 | 4692 | 4.68 | 0.000353 |
| Right dlPFC | 45 | 5 | 28 | 11,520 | 5.53 | 0.000074 |
| dmPFC | 12 | 17 | 31 | 15,509 | 7.06 | 0.000006 |
| Left IFG | −33 | 23 | 19 | 1270 | 3.50 | 0.003503 |
| Left dlPFC | −51 | 5 | 28 | 2018 | 5.60 | 0.000065 |
| Neutral > disgusting | ||||||
| Right retrosplenial cortex | 6 | −43 | −2 | 1923 | −4.56 | 0.000445 |
| Right precuneus | 15 | −46 | 28 | 1159 | −4.65 | 0.000377 |
| Left retrosplenial cortex | −9 | −70 | −5 | 2679 | −5.47 | 0.000083 |
| Decrease > look | ||||||
| Right occipital cortex | 18 | −100 | 1 | 1286 | 4.42 | 0.000583 |
| Left SFG | −15 | 11 | 52 | 1459 | 4.43 | 0.000571 |
| Look > decrease | ||||||
| Thalamus | 9 | −25 | −8 | 6341 | −4.93 | 0.000223 |
| Bilateral occipital cortex/hippocampus | 39 | −19 | −14 | 50,616 | −6.27 | 0.000021 |
| Right insula | 45 | −31 | 13 | 18,489 | −8.25 | 0.000001 |
| Left insula | −45 | −28 | 10 | 11,176 | −5.51 | 0.000077 |
| Right dlPFC | 39 | −4 | 43 | 2197 | −5.08 | 0.000168 |
| Right postcentral gyrus | 27 | −61 | 37 | 5988 | −4.67 | 0.000363 |
| Left precentral gyrus | −39 | −10 | 40 | 1438 | −4.07 | 0.001144 |
| Dorsal ACC | −3 | 8 | 40 | 1414 | −4.33 | 0.000692 |
| Increase > look | ||||||
| Right IPL | 67 | −28 | 34 | 2063 | 4.18 | 0.000925 |
| Left IPL | −42 | −64 | 16 | 16,293 | 6.60 | 0.000012 |
| Right LOC | 42 | −73 | 28 | 2450 | 4.32 | 0.000709 |
| Right putamen | 18 | 2 | −5 | 1111 | 4.04 | 0.001215 |
| Left putamen | −21 | −7 | 7 | 1252 | 3.96 | 0.001416 |
| Precuneus | −3 | −64 | 58 | 2103 | 6.81 | 0.000009 |
| Look > increase | ||||||
| Right posterior insula STG | 51 | −31 | 13 | 5483 | −5.28 | 0.000117 |
| Left posterior insula | −39 | −19 | 10 | 3557 | −4.70 | 0.000339 |
| Regulate > look | ||||||
| Left angular gyrus | −54 | −52 | 25 | 3570 | 3.75 | 0.002175 |
| Look > regulate | ||||||
| Right posterior insula/STG | 51 | −31 | 13 | 8886 | −4.85 | 0.00026 |
| Left posterior insula/STG | −42 | −16 | 10 | 7431 | −4.63 | 0.000391 |
| Right MOC | 39 | −52 | 1 | 2302 | −3.97 | 0.001392 |
| Left lingual gyrus | −18 | −64 | 1 | 3336 | −4.02 | 0.001266 |
| Right lingual gyrus | 18 | −67 | 4 | 1263 | −3.67 | 0.002498 |
| Brainstem | 12 | −25 | −17 | 993 | −4.03 | 0.00123 |
| Right PHG | 30 | −28 | −20 | 1825 | −4.43 | 0.000568 |
| Left PHG | −36 | −31 | −17 | 1322 | −7.17 | 0.000005 |
| Left cerebellum | −48 | −52 | −32 | 1252 | −3.37 | 0.00459 |
Abbreviations: ACC, anterior cingulate cortex; dlPFC, dorsolateral prefrontal cortex; dmPFC, dorsomedial prefrontal cortex; IFG, inferior frontal gyrus; IPL, intraparietal lobule; LOC, lateral occipital cortex; MOC, middle occipital cortex; PHG, parahippocampal gyrus; SFG, superior frontal gyrus; STG, superior temporal gyrus; TP, temporal pole.
Fig. 2.
Brain activation in each of the three contrasts look-gross > look-neutral, decrease-gross > look-gross, increase-gross > look-gross, as well as the conjunction of the two regulation contrasts. Orange indicates positive-going activation; blue negative-going activation. All activations are at a threshold of p < 0.05. Images are displayed in radiologic convention. Talairach coordinates displayed to the left apply to the first three columns; those displayed on the right apply to the right-most column.
3.2.2. Regions associated with cognitive reappraisal
In order to identify regions associated with emotion regulation (increase and decrease), we did a whole-brain conjunction analysis of the contrasts decrease-gross > look-gross and increase-gross > look-gross. The one region that showed greater activation in both regulation conditions compared to look was left angular gyrus. Regions that showed greater activation to look conditions versus emotion regulation included bilateral posterior insula extending into superior temporal gyrus, bilateral lingual gyrus, bilateral parahippocampal gyrus, left cerebellum, and right middle occipital cortex (Table 1, Fig. 2).
In a separate analysis of each regulation instruction, brain regions that showed decreased activation when participants were instructed to feel “less gross” (versus look) included bilateral occipital cortex extending into hippocampus, bilateral insula, thalamus, right dlPFC and postcentral gyrus, left precentral gyrus, and dACC. Regions that showed increased activation during decrease versus look trials included right occipital cortex and left superior frontal gyrus (Table 1, Fig. 2).
Regions that showed increased activation when participants were instructed to feel “more gross” (versus look) included bilateral intraparietal lobule and putamen, right lateral occipital cortex, and precuneus. In contrast, regions that decreased activation during increase versus look trials included bilateral posterior insula and right superior temporal gyrus (Table 1, Fig. 2).
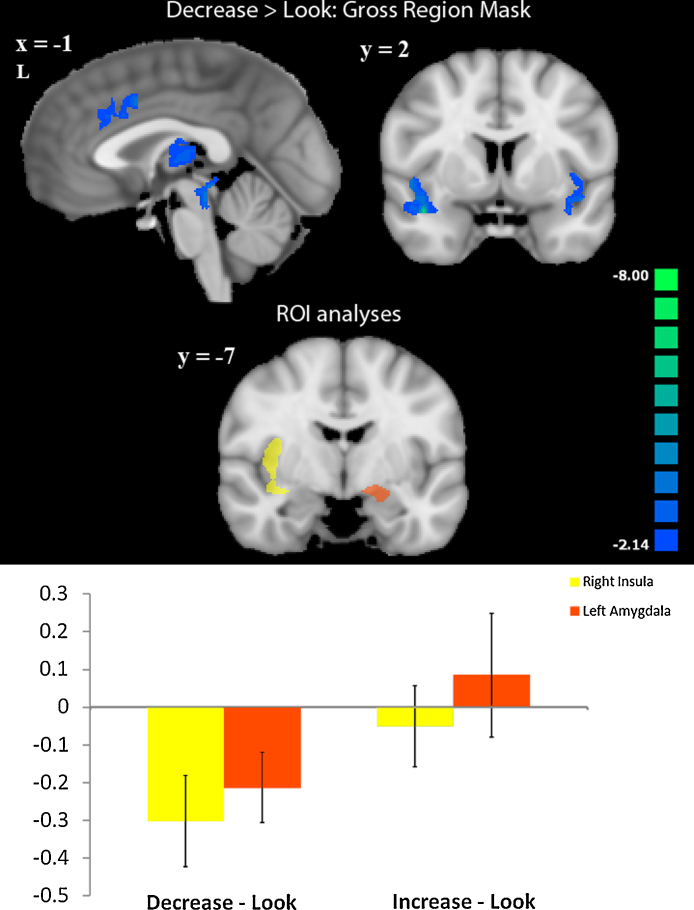
To specifically examine modulation of the brain response to disgusting pictures in each regulation condition, we investigated each of the emotion regulation contrasts (decrease-gross > look-gross, increase-gross > look-gross) in regions that showed activation to disgusting (versus neutral) pictures identified in our first whole-brain analysis. Regions responsive to disgusting pictures that decreased activation when participants were prompted to feel “less gross” included bilateral insula, dACC, right occipital cortex and precentral gyrus, right superior parietal lobule, thalamus, and left parahippocampal gyrus. None of the regions responsive to gross images showed increased activation when participants were instructed to feel “less gross”, and none of these regions were modulated by the “more gross” instruction at the same threshold (Table 2, Fig. 3).
Table 2.
From the areas which showed increased activation in the look-gross > look-neutral contrast, regions that were modulated by the down-regulation (decrease-gross > look-gross). Results were obtained at a statistical threshold of p < 0.05 with a cluster threshold of 41 contiguous voxels. All coordinates reported are in standard Talairach space, oriented in radiological convention.
| Brain region | X | Y | Z | Size | t | p |
|---|---|---|---|---|---|---|
| Look > decrease | ||||||
| Right insula | 39 | 2 | −14 | 3873 | −7.00 | 0.000006 |
| Left insula | −33 | 6 | 13 | 1136 | −4.93 | 0.000223 |
| Right occipital cortex | 39 | −19 | −14 | 17,117 | −6.27 | 0.000021 |
| Right precentral gyrus | 39 | −5 | 43 | 1009 | −5.08 | 0.000168 |
| Right superior parietal lobule | 27 | −61 | 37 | 2123 | −4.67 | 0.000363 |
| Thalamus | 9 | −25 | −8 | 5114 | −4.93 | 0.000223 |
| Dorsal ACC | −3 | 7 | 40 | 1227 | −4.33 | 0.000692 |
| Left PHG | −36 | −31 | −17 | 3540 | −5.96 | 0.000035 |
Abbreviations: ACC, anterior cingulate cortex; PHG, parahippocampal gyrus.
Fig. 3.
Top: Activation modulated by decrease-gross > look-gross in regions that were responsive to disgusting images. Blue indicates negative-going activation. All activations are at a threshold of p < 0.05. Bottom: Functionally defined ROIs in the right insula (yellow) and left amygdala (orange). Images are displayed in radiologic convention. Bar graph: y-axis represents differences in beta weights between the conditions of interest (decrease − look and increase − look).
3.2.3. Region of interest analyses
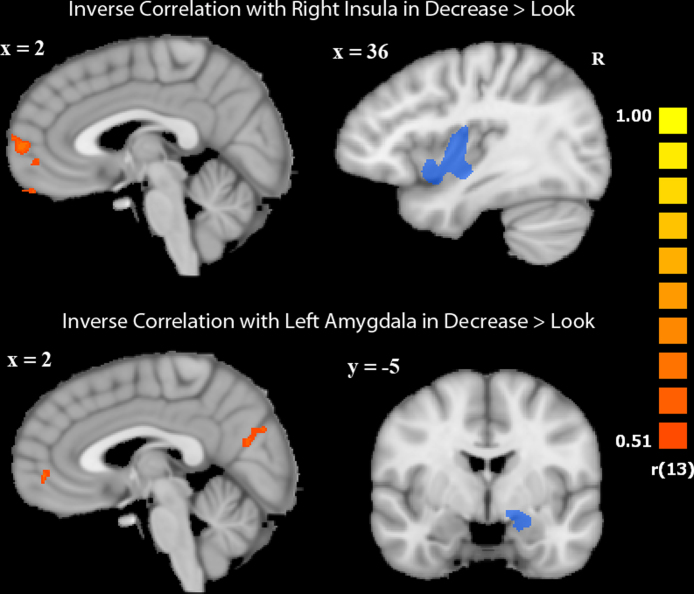
Region of interest analyses in four functionally defined regions responsive to disgusting pictures at p < 0.01 (bilateral insula and amygdala) revealed significant modulation of only two of these regions during the decrease condition (Fig. 2). The right insula and left amygdala showed significant decreases in activation in the “less gross” instruction condition (right insula: t = −2.50, p = 0.03; left amygdala: t = −2.20, p = 0.04). When we examined how individual differences in the amount of right insula and left amygdala down-regulation correlated with whole-brain activation, we identified regions that showed inverse correlations with activation in these two ROIs during the decrease condition. Regions showing a negative correlation with left amygdala activation included right angular gyrus, precuneus, mPFC, left posterior superior temporal sulcus, left posterior middle temporal gyrus, and left inferior occipital gyrus. Regions showing a negative correlation with right insula activation included mPFC, medial OFC, and left fusiform gyrus. A region of mPFC inversely correlated with both left amygdala and right insula activation during the decrease condition (Talairach coordinates: 3, 50, 1; 40 voxels). Modulation of right insula did not correlate with modulation of left amygdala during decrease-gross > look-gross (p > 0.05) (Table 3, Fig. 4).
Table 3.
Regions that inversely correlated with left amygdala and right insula activation in the contrast decrease-gross > look-gross. Results were obtained at a statistical threshold of p < 0.05 with a cluster threshold of 10 contiguous voxels. All coordinates reported are in standard Talairach space, oriented in radiological convention.
| Brain region | X | Y | Z | Size | r | p |
|---|---|---|---|---|---|---|
| Left amygdala correlation | ||||||
| Right angular gyrus | 45 | −55 | 28 | 374 | −0.70 | 0.003791 |
| Precuneus | −6 | −76 | 19 | 1381 | −0.72 | 0.002432 |
| mPFC | −9 | 50 | 1 | 366 | −0.70 | 0.003919 |
| Left pSTS | −63 | −55 | 10 | 626 | −0.77 | 0.000744 |
| Left posterior MTG | −60 | −55 | −2 | 461 | −0.82 | 0.000162 |
| Left IOG | −39 | −82 | −17 | 3199 | −0.85 | 0.00005 |
| Right insula correlation | ||||||
| mPFC | 3 | 50 | 1 | 994 | −0.66 | 0.007426 |
| mOFC | −13 | 47 | −8 | 562 | −0.42 | 0.115795 |
| Left FFG | −30 | −88 | −20 | 524 | −0.75 | 0.001326 |
Abbreviations: FFG, fusiform gyrus; IOG, inferior occipital gyrus; mOFC, medial orbitofrontal cortex; mPFC, medial prefrontal cortex; MTG, middle temporal gyrus; pSTS, posterior superior temporal sulcus.
Fig. 4.
Activation inversely correlated with left amygdala and right insula activation in the contrast decrease-gross > look-gross. Orange indicates regions of inverse correlation with seed regions, depicted in blue. All activations are at a threshold of p < 0.05. Images are displayed in radiologic convention.
3.2.4. Effects of age on brain mechanisms for reappraisal
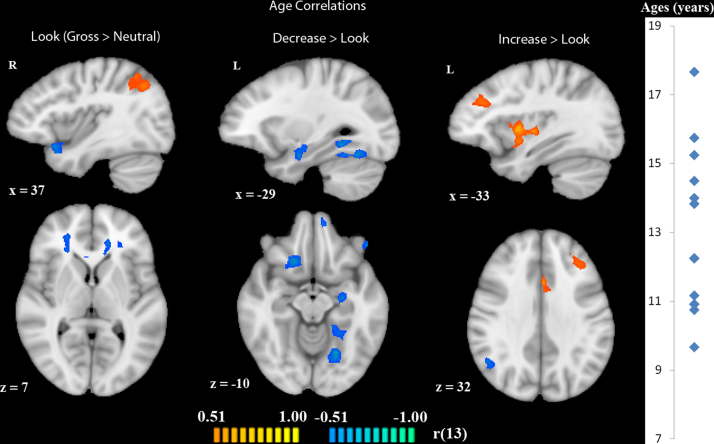
In our exploration of developmental changes in brain activation in our three contrasts of interest, we identified regions that significantly correlated with chronological age. While viewing disgusting (versus neutral) pictures, activation in bilateral inferior parietal lobule positively correlated with age. Regions that negatively correlated with age in this contrast included bilateral ACC and right anterior insula extending into superior temporal gyrus. In the decrease-gross > look-gross contrast, no regions positively correlated with age. Regions that negatively correlated with age in this contrast included right medial OFC, mPFC, left parahippocampal gyrus, left inferior frontal gyrus, and left amygdala. Finally, in the increase-gross > look-gross contrast, regions that positively correlated with age included dACC, left insula extending into putamen, medial frontal gyrus, right posterior cingulate gyrus, and left middle frontal gyrus. Only right inferior parietal lobule negatively correlated with age in this contrast (Table 4, Fig. 5).
Table 4.
Regions that correlated with age in each of the three contrasts look-gross > look-neutral, decrease-gross > look-gross, and increase-gross > look-gross. Results were obtained at a statistical threshold of p < 0.05 with a cluster threshold of 34 contiguous voxels. All coordinates reported are in standard Talairach space, oriented in radiological convention.
| Brain region | X | Y | Z | Size | r | p |
|---|---|---|---|---|---|---|
| Look-gross > look-neutral | ||||||
| Right IPL | 42 | −55 | 46 | 4951 | 0.76 | 0.001125 |
| Left IPL | −51 | −52 | 37 | 2589 | 0.72 | 0.002457 |
| Right anterior insula/STG | 54 | 12 | −17 | 1926 | −0.82 | 0.000184 |
| Right ACC | 15 | 26 | 16 | 3353 | −0.83 | 0.000117 |
| Left ACC | −12 | 23 | 16 | 2608 | −0.74 | 0.001687 |
| Decrease > look | ||||||
| Right mOFC | 18 | 20 | −14 | 1205 | −0.90 | 0.000005 |
| mPFC | −9 | 53 | −8 | 1296 | −0.84 | 0.000101 |
| Left PHG | −21 | −61 | −14 | 4089 | −0.83 | 0.000123 |
| Left amygdala | −27 | −7 | −17 | 1151 | −0.85 | 0.000066 |
| Left IFG | −52 | 29 | −2 | 1233 | −0.80 | 0.000308 |
| Increase > look | ||||||
| Right posterior cingulate gyrus | 6 | −28 | 46 | 1275 | 0.90 | 0.000005 |
| Dorsal ACC | −3 | 14 | 28 | 1090 | 0.86 | 0.000034 |
| Medial frontal gyrus | −3 | −7 | 58 | 1628 | 0.71 | 0.003096 |
| Left insula/putamen | −33 | 2 | 4 | 2910 | 0.84 | 0.000077 |
| Left MFG | −36 | 32 | 28 | 989 | 0.77 | 0.00071 |
| Right IPL | 45 | −61 | 34 | 1166 | −0.78 | 0.000651 |
Abbreviations: ACC, anterior cingulate cortex; IFG, inferior frontal gyrus; IPL, intraparietal lobule; mOFC, medial orbitofrontal cortex; mPFC, medial prefrontal cortex; MFG, middle frontal gyrus; PHG, parahippocampal gyrus; STG, superior temporal gyrus.
Fig. 5.
Activation correlated with age in each of the three contrasts look-gross > look-neutral, decrease-gross > look-gross, and increase-gross > look-gross. Orange indicates regions that positively correlate with age; blue indicates regions that negatively correlate with age. All activations are at a threshold of p < 0.05. Images are displayed in radiologic convention. The graph to the right demonstrates the age distribution of the participants.
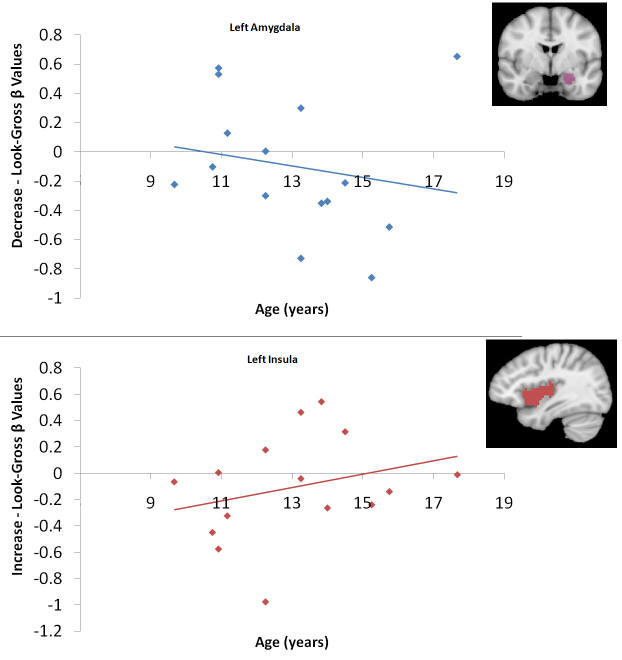
Using anatomically defined ROIs to visualize the age correlations in left amygdala and left insula yielded non-significant results, however, this analysis allowed for the visual inspection of meaningful correlation findings in our whole brain analyses to ensure they were not driven by outliers or binary groupings of older versus younger participants. In the structurally defined left insula, activation to increase-gross (versus look-gross) increased with age (r = 0.28, p = 0.155, one-tailed). In the structurally defined left amygdala, activation to decrease-gross (versus look-gross) decreased with age (r = −0.19, p = 0.25, one-tailed). These correlations suggest that outliers or binary grouping were not driving our whole brain correlation effects (Supplemental Fig. 1).
4. Discussion
4.1. Behavioral effects of cognitive reappraisal
Comparison of self-report ratings confirms that children and adolescents experienced more disgust in response to disgusting versus neutral images. Furthermore, participants were successful in regulating their self-reported emotional responses in accordance with instructions as evidenced by significantly lower ratings on decrease-gross trials and higher ratings on increase-gross trials relative to look-gross trials. None of these behavioral effects were significantly modulated by age.
4.2. Brain effects of cognitive reappraisal
4.2.1. Brain mechanisms for processing disgust
We identified brain regions involved in the processing of disgust-inducing images, including regions previously implicated in the processing of disgust such as insula, amygdala, mPFC, thalamus and striatum (Calder et al., 2000, Lane et al., 1997, Phillips et al., 1997, Schafer et al., 2005, Wicker et al., 2003). These regions show significant overlap with those identified in a prior study of emotion regulation of disgust-inducing film clips in adults (Goldin et al., 2008), suggesting similarities in the neural circuitry underlying disgust processing between children and adults. However, secondary analyses revealed that activation to disgusting pictures in bilateral intraparietal lobule increased with age, and activation in ACC and right anterior insula decreased with age, demonstrating the presence of age-related differences in the neural network underlying the processing of disgust.
4.2.2. Brain mechanisms for cognitive reappraisal
Next, we demonstrated significant modulation of brain activity by engaging in cognitive reappraisal in general as well as up-regulation and down-regulation of emotion individually. Taken together with our behavioral findings, this suggests that our participants were responsive to the task in general, and differentially responsive when increasing and decreasing. The one region with increased activation to regulating in general (conjunction of increase and decrease) was the left angular gyrus. The cognitive reappraisal strategies we provided instructed participants to “pretend it's fake” or “pretend it's right in front of you”. Angular gyrus has been implicated in out of body experiences, potentially relating to failures to integrate somatosensory information (Blanke et al., 2002, Blanke et al., 2004). Similar processes of dissociating oneself from sensory inputs might underlie regulatory strategies induced by our prompts. In addition, adjacent temporoparietal regions have been implicated in the use and observation of pretense (German et al., 2004, Lewis and Carmody, 2008). Thus, the left angular gyrus activity observed in our participants during cognitive reappraisal may reflect the use of pretense in the task. Alternatively, adjacent temporoparietal junction (TPJ) has been implicated in mentalizing, or reasoning about others’ mental states (Aichhorn et al., 2006, Gallagher et al., 2000, Krach et al., 2008, Saxe and Kanwisher, 2003, Saxe et al., 2006, Saxe and Powell, 2006, Saxe and Wexler, 2005). Saxe et al. (2006) specifically demonstrated the role of TPJ in reflecting on others’ mental states, but not during self-reflection. It is conceivable that, in manipulating one's own mental states during cognitive reappraisal, participants experienced a degree of detachment which led to the recruitment of structures necessary for reflecting on another person's thoughts. While intriguing, this possibility is speculative and would require further investigation to confirm.
By performing the contrast of each regulation type separately over a whole-brain mask of the gross > neutral contrast, we were able to identify task-related modulation of regions involved in the processing of disgusting pictures. When participants engaged in decreasing their emotional responses, we observed down-regulation of activity in regions including bilateral insula, thalamus, and dACC. The thalamus has been implicated in the processing of emotion in general, regardless of type (Lane et al., 1997). Moreover, of the regions implicated in the processing of disgust, the insula has most classically been identified as involved not merely in the observation of disgust, but in the experience of disgust as well (Calder et al., 2000, Wicker et al., 2003). Therefore down-regulation of these regions in response to decreasing one's emotional response to disgusting images may reflect a reduction in emotional experience. This interpretation is further supported by our behavioral data which show decreased disgust ratings after down-regulation of emotion. Surprisingly, despite behavioral reports of increased emotional responses on the increase trials, we did not see modulation of disgust-specific regions during up-regulation of emotion. It is possible that these regions were already maximally activated to disgusting pictures, such that they could not be measurably up-regulated, despite the participants’ efforts to increase their emotional responses. It is also possible that differences in the difficulty of up- and down-regulation underlie this null finding.
4.2.3. Modulation of insula and amygdala activity
Region of interest analyses of bilateral insula and amygdala revealed down-regulation of right insula and left amygdala during decrease trials, consistent with prior reports in adults of decreased activity in the insula (Goldin et al., 2008, Harenski and Hamann, 2006) and amygdala (Blair et al., 2007, Eippert et al., 2007, Goldin et al., 2008, Kim and Hamann, 2007, Kober et al., 2008, Koenigsberg et al., 2010, McRae et al., 2008, McRae et al., 2010, Ochsner et al., 2002, Ochsner et al., 2004, Ohira et al., 2006, Phan et al., 2005, van Reekum et al., 2007, Walter et al., 2009, Winecoff et al., 2010) during emotion down-regulation. Furthermore, the degree of decreased activation in both of these regions correlated with increasing activation in vmPFC. Prior studies of emotion regulation in adults have similarly shown an inverse correlation between vmPFC and amygdala, and have further demonstrated that the vmPFC mediates a top-down influence of lateral PFC on the amgydala (Johnstone et al., 2007, Urry et al., 2006). These findings are in line with evidence of anatomical connections between vmPFC and amygdala (Amaral and Price, 1984, Ghashghaei et al., 2007) as well as connections between vmPFC and dorsal and lateral PFC (Barbas, 1995, Ongur and Price, 2000). Conversely, our finding of an inverse correlation between vmPFC and insula has not been previously reported in the emotion regulation literature. However, Hermann et al. (2009) demonstrated that in spider phobic individuals, there was increased insula and decreased vmPFC activity when viewing pictures of spiders relative to other aversive images. They interpreted this as evidence for a deficit of automatic emotion regulatory processes in specific phobia. Our findings suggest the possibility that the vmPFC exhibits top-down inhibitory modulation of insula activity similar to its effect on the amygdala, a phenomenon evident in this study likely due to our use of exclusively disgust-inducing images. Alternatively, prior research has suggested that the insula both receives input from and sends output to the central-executive network and the default mode network (including vmPFC), serving as a hub enabling switching between these networks (Menon and Uddin, 2010, Sridharan et al., 2008). Thus, it is also conceivable that the inverse correlation between vmPFC and insula demonstrated here is reflective of a regulatory role for the insula on vmPFC. Ultimately, in the absence of statistical assessments of causality we cannot draw conclusions regarding the directionality of the inverse relationships between vmPFC and the amygdala and insula. Future research will benefit from investigating the causality of such correlations.
4.2.4. Developmental changes
Secondary analyses exploring the developmental trajectory of brain mechanisms for each emotion regulation condition revealed that the magnitude of activation in prefrontal cortex regions during down-regulation decreases with age. In a prior fMRI study of cognitive reappraisal in children, Levesque et al. (2004) compared their results with a separate but identical study performed in adults (Levesque et al., 2003) and suggested a decrease in the number of prefrontal loci of activation between childhood and adulthood. The authors hypothesized that this age difference reflects a developmental maturation of the prefrontal regulatory system, implying greater regulation efficiency. However, while prior research has pointed to greater volumes of prefrontal activity in younger children and equated this with immaturity of prefrontal circuitry (i.e., Casey et al., 1995, Tamm et al., 2002), other studies have shown that the strength of prefrontal activity increases with age, a process known as frontalization (Lewis et al., 2006, Rubia et al., 2000, Yurgelun-Todd and Killgore, 2006). It is possible that decreased PFC activity with age in combination with our concurrent finding that left amygdala activation decreased with age reflects a reduction in the effort required to downregulate emotion and greater success in down-regulation of the amygdala.
This same decrease in prefrontal regulatory activation was not seen in the emotional up-regulation condition. Group analyses suggested that as a whole, participants’ psychological up-regulation of emotion was not coupled with enhanced activation in regions responsive to gross pictures. Thus, the lack of evidence for increasing prefrontal efficiency in this condition is not surprising. Instead, insula and dACC activation increases with age. Both of these regions were responsive to viewing disgusting pictures in the absence of the regulatory task, suggesting that successful enhancement of brain responses to gross pictures may develop later in adolescence. Further, the increasing activation in more lateral frontal regions with age may account for this increasing up-regulation success.
Given that the age range of our sample encompasses the typical onset of puberty and that puberty brings with it associated changes with respect to brain and behavior (Dahl, 2004), it is worth noting that in addition to age, pubertal stage could play a role in the development of emotion regulatory processes observed in our participants. As we did not collect data on the pubertal development of our participants, we are unable to investigate the differential contributions of age and puberty.
4.3. Departure from prior studies
The present study differs from past studies of cognitive reappraisal, particularly in terms of increases in prefrontal activity during emotion regulation as observed in the prior literature (e.g., Beauregard et al., 2001, Goldin et al., 2008, Kim and Hamann, 2007, Ochsner et al., 2004, Urry et al., 2006). In an effort to make the cognitive reappraisal task more accessible to children, we instructed participants to engage in pretend. Studies utilizing variations on emotion regulation strategies, such as distraction (McRae et al., 2010) and distancing (Koenigsberg et al., 2010), have yielded differing results from more classical approaches to cognitive reappraisal. For example, the distancing regulation strategy recruited loci of activity in parietal and temporal cortex as well as posterior cingulate cortex, but no prefrontal cortical activation was observed in the main effect of instruction type (Koenigsberg et al., 2010). As mentioned previously, the use of pretense as a reappraisal strategy recruited a temporoparietal region in our participants. The recruitment of variable networks for differing reappraisal strategies is a likely explanation for the discrepancy between our findings and some of the existing literature. Furthermore, secondary analyses revealed prefrontal effects with age, suggesting that differences in prefrontal activation between our study and others may also be secondary to age differences in the participant samples.
Most prior studies have not exclusively focused on the cognitive reappraisal of disgust (but see Goldin et al., 2008). While the study of disgust may have important implications for understanding anxiety disorders, it is not clear to what extent these findings reflect the brain mechanisms of emotion regulation of negatively valenced stimuli in general. A study comparing cognitive reappraisal of disgust to another negative emotion, such as fear, could clarify this point.
5. Conclusions
This is the first study to our knowledge to examine developmental trends in the neural mechanisms of cognitive reappraisal in children and adolescents. We have demonstrated that children and adolescents are able to successfully regulate their emotional responses to disgusting images utilizing pretense as a cognitive reappraisal strategy. Our results suggest a regulatory effect between vmPFC and the amygdala and insula during down-regulation of emotion. Further, we show age-related effects on activity in PFC and amygdala suggestive of decreased regulatory effort and increased success across development. These findings have important clinical implications for the understanding of processes relevant to cognitive therapies in anxiety disorders in childhood and adolescence, in particular, the extent to which changes in emotion regulation relevant circuitry may predict and track clinical outcomes. Future studies may further our understanding by exploring the neural and behavioral effects of cognitive reappraisal in pediatric clinical populations.
Acknowledgments
This research was supported by grants from the National Institute of Mental Health, the John Merck Scholars Fund, and the Simons Foundation. This work was further supported by a grant from the Doris Duke Charitable Foundation to Yale University to fund Clinical Research Fellow Naomi Pitskel. Kevin Pelphrey was supported by a Career Development Award from the National Institutes of Health (NIMH Grant MH071284).
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.dcn.2011.03.004.
Appendix A. Supplementary data
Supplemental Fig. 1.
Top: Scatterplot of age versus difference β values for the contrast of decrease-gross > look-gross in the anatomically defined left amygdala. Bottom: Scatterplot of age versus difference β values for the contrast of increase-gross > look-gross in the anatomically defined left insula. The anatomical regions of interest are displayed in the upper right corner of each graph.
References
- Aichhorn M., Perner J., Kronbichler M., Staffen W., Ladurner G. Do visual perspective tasks need theory of mind? Neuroimage. 2006;30:1059–1068. doi: 10.1016/j.neuroimage.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Amaral D.G., Price J.L. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J. Comp. Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amstadter A. Emotion regulation and anxiety disorders. J. Anxiety Disord. 2008;22:211–221. doi: 10.1016/j.janxdis.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Anatomic basis of cognitive–emotional interactions in the primate prefrontal cortex. Neurosci. Biobehav. Rev. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Beauregard M., Levesque J., Bourgouin P. Neural correlates of conscious self-regulation of emotion. J. Neurosci. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M., Paquette V., Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Berle D., Phillips E.S. Disgust and obsessive–compulsive disorder: an update. Psychiatry. 2006;69:228–238. doi: 10.1521/psyc.2006.69.3.228. [DOI] [PubMed] [Google Scholar]
- Blair K.S., Smith B.W., Mitchell D.G., Morton J., Vythilingam M., Pessoa L., Fridberg D., Zametkin A., Sturman D., Nelson E.E., Drevets W.C., Pine D.S., Martin A., Blair R.J. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35:430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanke O., Landis T., Spinelli L., Seeck M. Out-of-body experience and autoscopy of neurological origin. Brain. 2004;127:243–258. doi: 10.1093/brain/awh040. [DOI] [PubMed] [Google Scholar]
- Blanke O., Ortigue S., Landis T., Seeck M. Stimulating illusory own-body perceptions. Nature. 2002;419:269–270. doi: 10.1038/419269a. [DOI] [PubMed] [Google Scholar]
- Burgund E.D., Kang H.C., Kelly J.E., Buckner R.L., Snyder A.Z., Petersen S.E., Schlaggar B.L. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17:184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Calder A.J., Keane J., Manes F., Antoun N., Young A.W. Impaired recognition and experience of disgust following brain injury. Nat. Neurosci. 2000;3:1077–1078. doi: 10.1038/80586. [DOI] [PubMed] [Google Scholar]
- Calkins S.D., Hill A. Caregiver influences on emerging regulation: biological and environmental transactions in early development. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford; New York: 2007. pp. 229–248. [Google Scholar]
- Campbell-Sills L., Barlow D.H. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford; New York: 2007. pp. 542–559. [Google Scholar]
- Campbell-Sills L., Barlow D.H., Brown T.A., Hofmann S.G. Effects of suppression and acceptance on emotional responses of individuals with anxiety and mood disorders. Behav. Res. Ther. 2006;44:1251–1263. doi: 10.1016/j.brat.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Cohen J.D., Jezzard P., Turner R., Noll D.C., Trainor R.J., Giedd J., Kaysen D., Hertz-Pannier L., Rapoport J.L. Activation of prefrontal cortex in children during a nonspatial working memory task with functional MRI. Neuroimage. 1995;2:221–229. doi: 10.1006/nimg.1995.1029. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Giedd J.N., Thomas K.M. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cisler J.M., Olatunji B.O., Feldner M.T., Forsyth J.P. Emotion regulation and the anxiety disorders: an integrative review. J. Psychopathol. Behav. Assess. 2010;32:68–82. doi: 10.1007/s10862-009-9161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl R.E. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Ann. N. Y. Acad. Sci. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Davey G.C.L. Self-reported fears to common indigenous animals in a UK population: the role of disgust sensitivity. Br. J. Psychol. 1994;85:541–554. doi: 10.1111/j.2044-8295.1994.tb02540.x. [DOI] [PubMed] [Google Scholar]
- Davey G.C.L., Bickerstaffe S., MacDonald B.A. Experienced disgust causes a negative interpretation bias: a causal role for disgust in anxious psychopathology. Behav. Res. Ther. 2006;44:1375–1384. doi: 10.1016/j.brat.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Davey G.C.L., Bond N. Using controlled comparisons in disgust psychopathology research: the case of disgust, hypochondriasis and health anxiety. J. Behav. Ther. Exp. Psychiatry. 2005;37:4–15. doi: 10.1016/j.jbtep.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Davidson R.J., Slagter H.A. Probing emotion in the developing brain: functional neuroimaging in the assessment of the neural substrates of emotion in normal and disordered children and adolescents. Ment. Retard. Dev. Disabil. Res. Rev. 2000;6:166–170. doi: 10.1002/1098-2779(2000)6:3<166::AID-MRDD3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Deen B., Pitskel N.B., Pelphrey K.A. Three Systems of Insular Functional Connectivity Identified with Cluster Analysis. Cereb Cortex. 2010 doi: 10.1093/cercor/bhq186. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denham S.A. Guilford; New York: 1998. Emotional Development in Young Children. [Google Scholar]
- Dennis T.A., Hajcak G. The late positive potential: a neurophysiological marker for emotion regulation in children. J. Child Psychol. Psychiatry. 2009;50:1373–1383. doi: 10.1111/j.1469-7610.2009.02168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F., Veit R., Weiskopf N., Erb M., Birbaumer N., Anders S. Regulation of emotional responses elicited by threat-related stimuli. Hum. Brain Mapp. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn. Reson. Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Gallagher H.L., Happé F., Brunswick N., Fletcher P.C., Frith U., Frith C.D. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38:11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- German T.P., Niehaus J.L., Roarty M.P., Giesbrecht B., Miller M.B. Neural correlates of detecting pretense: automatic engagement of the intentional stance under covert conditions. J. Cogn. Neurosci. 2004;16:1805–1817. doi: 10.1162/0898929042947892. [DOI] [PubMed] [Google Scholar]
- Ghashghaei H.T., Hilgetag C.C., Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C., Nugent T.F., 3rd, Herman D.H., Clasen L.S., Toga A.W., Rapoport J.L., Thompson P.M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin P.R., McRae K., Ramel W., Gross J.J. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol. Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. Antecedent- and response-focused emotion regulation: divergent consequences for experience expression, and physiology. J. Pers. Soc. Psychol. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross J.J. The emerging field of emotion regulation: an integrative review. Rev. Gen. Psychol. 1998;2:271–299. [Google Scholar]
- Gross J.J., Thompson R.A. Emotion regulation: conceptual foundations. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford; New York: 2007. pp. 3–24. [Google Scholar]
- Harenski C.L., Hamann S. Neural correlates of regulating negative emotions related to moral violations. Neuroimage. 2006;30:313–324. doi: 10.1016/j.neuroimage.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Hermann A., Schafer A., Walter B., Stark R., Vaitl D., Schienle A. Emotion regulation in spider phobia: role of the medial prefrontal cortex. Soc. Cogn. Affect. Neurosci. 2009;4:257–267. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibañez A., Gleichgerrcht E., Manes F. Clinical effects of insular damage in humans. Brain Struct. Funct. 2010;214:397–410. doi: 10.1007/s00429-010-0256-y. [DOI] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. Failure to regulate: counterproductive recruitment of top-down prefrontal–subcortical circuitry in major depression. J. Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H.C., Burgund E.D., Lugar H.M., Petersen S.E., Schlaggar B.L. Comparison of functional activation foci in children and adults using a common stereotactic space. Neuroimage. 2002;19:16–28. doi: 10.1016/s1053-8119(03)00038-7. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Hamann S. Neural correlates of positive and negative emotion regulation. J. Cogn. Neurosci. 2007;19:776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Kober H., Barrett L.F., Joseph J., Bliss-Moreau E., Lindquist K., Wager T.D. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Fan J., Ochsner K.N., Liu X., Guise K., Pizzarello S., Dorantes C., Tecuta L., Guerreri S., Goodman M., New A., Flory J., Siever L.J. Neural correlates of using distancing to regulate emotional responses to social situations. Neuropsychologia. 2010;48:1813–1822. doi: 10.1016/j.neuropsychologia.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigsberg H.W., Harvey P.D., Mitropoulou V., Schmeidler J., New A.S., Goodman M., Silverman J.M., Serby M., Schopick F., Siever L.J. Characterizing affective instability in borderline personality disorder. Am. J. Psychiatry. 2002;159:784–788. doi: 10.1176/appi.ajp.159.5.784. [DOI] [PubMed] [Google Scholar]
- Krach S., Hegel F., Wrede B., Sagerer G., Binkofsji F., Kircher T. Can machines think? Interaction and perspective taking with robots investigated via fMRI. PLoS ONE. 2008;3:1–11. doi: 10.1371/journal.pone.0002597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Rainey L.H., Summerlin J.L., Freitas C.S., Fox P.T., Evans A.C., Toga A.W., Mazziotta J.C. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum. Brain Mapp. 1997;5:238–242. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster J.L., Woldorff M.G., Parsons L.M., Liotti M., Freitas C.S., Rainey L., Kotchunov P.V., Nickerson D., Mikiten S.A., Fox P.T. Automated Talairach Atlas labels for functional brain mapping. Hum. Brain Mapp. 2000;10:120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane R.D., Reiman E.M., Ahern G.L., Schwartz G.E., Davidson R.J. Neuroanatomical correlates of happiness, sadness, and disgust. Am. J. Psychiatry. 1997;154:926–933. doi: 10.1176/ajp.154.7.926. [DOI] [PubMed] [Google Scholar]
- Lang, P.J., Bradley, M.M., Cuthbert, B.N. (2008) International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. Technical Report A-8, University of Florida, Gainesville, FL.
- Levesque J., Eugene F., Joanette Y., Paquette V., Mensour B., Beaudoin G., Leroux J.M., Bourgouin P., Beauregard M. Neural circuitry underlying voluntary suppression of sadness. Biol. Psychiatry. 2003;53:502–510. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Levesque J., Joanette Y., Mensour B., Beaudoin G., Leroux J.M., Bourgouin P., Beauregard M. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–369. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Lewis M., Carmody D.P. Self-representation and brain development. Dev. Psychol. 2008;44:1329–1334. doi: 10.1037/a0012681. [DOI] [PubMed] [Google Scholar]
- Lewis M.D., Lamm C., Segalowitz S.J., Stieben J., Zelazo P.D. Neurophysiological correlates of emotion regulation in children and adolescents. J. Cogn. Neurosci. 2006;18:430–443. doi: 10.1162/089892906775990633. [DOI] [PubMed] [Google Scholar]
- Linehan M.M., Bohus M., Lynch T.R. Dialectical behavior therapy for pervasive emotion dysregulation: theoretical and practical underpinnings. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford; New York: 2007. pp. 581–605. [Google Scholar]
- Mak A.K., Hu Z.G., Zhang J.X., Xiao Z., Lee T.M. Sex-related differences in neural activity during emotion regulation. Neuropsychologia. 2009;47:2900–2908. doi: 10.1016/j.neuropsychologia.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Marsh R., Gerber A.J., Peterson B.S. Neuroimaging studies of normal brain development and their relevance for understanding childhood neuropsychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2008;47:1233–1251. doi: 10.1097/CHI.0b013e318185e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Hughes B., Chopra S., Gabrieli J.D., Gross J.J., Ochsner K.N. The neural bases of distraction and reappraisal. J. Cogn. Neurosci. 2010;22:248–262. doi: 10.1162/jocn.2009.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Ochsner K.N., Mauss I.B., Gabrieli J.D.E., Gross J.J. Gender differences in emotion regulation: an fMRI study of cognitive reappraisal. Group Process. Intergroup Relat. 2008;11:145–162. doi: 10.1177/1368430207088035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214:655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkens S.A.N., de Jong P.J., Merckelbach H. Disgust and spider phobia. J. Abnorm. Psychol. 1996;105:464–468. doi: 10.1037//0021-843x.105.3.464. [DOI] [PubMed] [Google Scholar]
- Mullin B.C., Hinshaw S.P. Emotion regulation and externalizing disorders in children and adolescents. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford; New York: 2007. pp. 523–541. [Google Scholar]
- Ochsner K.N., Bunge S.A., Gross J.J., Gabrieli J.D. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J. Cogn. Neurosci. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The neural architecture of emotion regulation. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford; New York: 2007. pp. 87–109. [Google Scholar]
- Ochsner K.N., Ray R.D., Cooper J.C., Robertson E.R., Chopra S., Gabrieli J.D., Gross J.J. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohira H., Nomura M., Ichikawa N., Isowa T., Iidaka T., Sato A., Fukuyama S., Nakajima T., Yamada J. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–733. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Olatunji B.O., Sawchuk C.N. Disgust: characteristic features social manifestations, and clinical implications. J. Soc. Clin. Psychol. 2005;24:932–962. [Google Scholar]
- Olatunji B.O., Cisler J., McKay D., Phillips M.L. Is disgust associated with psychopathology? Emerging research in the anxiety disorders. Psychiatry Res. 2011;175:1–10. doi: 10.1016/j.psychres.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Ongur D., Price J.L. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb. Cortex. 2000;10:206–219. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- Phan K.L., Fitzgerald D.A., Nathan P.J., Moore G.J., Uhde T.W., Tancer M.E. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol. Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps E.A., Delgado M.R., Nearing K.I., LeDoux J.E. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception. II. Implications for major psychiatric disorders. Biol. Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13(829):833–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Young A.W., Senior C., Brammer M., Andrew C., Calder A.J., Bullmore E.T., Perrett D.I., Rowland D., Williams S.C., Gray J.A., David A.S. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Likhtik E., Pelletier J.G., Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart M., Ziaie H., O’Boyle C. Self-regulation and emotion in infancy. In: Eisenberg N., Fabes R., editors. Emotion and Its Regulation in Early Development. Jossey–Bass/Pfeiffer; San Francisco: 1992. pp. 7–23. [Google Scholar]
- Rubia K., Overmeyer S., Taylor E., Brammer M., Williams S.C.R., Simmons A., Andrew C., Bullmore E.T. Functional frontalisation with age: mapping neurodevelopmental trajectories with fMRI. Neurosci. Behav. Rev. 2000;24:13–19. doi: 10.1016/s0149-7634(99)00055-x. [DOI] [PubMed] [Google Scholar]
- Saxe R., Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in ‘theory of mind’. Neuroimage. 2003;19:1835–1842. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Saxe R., Moran J.M., Scholz J., Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Soc. Cogn. Affect. Neurosci. 2006;1:229–234. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe R., Powell L.J. It's the thought that counts: specific brain regions for one component of theory of mind. Psychol. Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Saxe R., Wexler A. Making sense of another mind: the role of the right temporo-parietal junction. Neuropsychologia. 2005;43:1391–1399. doi: 10.1016/j.neuropsychologia.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Schaefer S.M., Jackson D.C., Davidson R.J., Aguirre G.K., Kimberg D.Y., Thompson-Schill S.L. Modulation of amygdalar activity by the conscious regulation of negative emotion. J. Cogn. Neurosci. 2002;14:913–921. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Schafer A., Schienle A., Vaitl D. Stimulus type and design influence hemodynamic responses towards visual disgust and fear elicitors. Int. J. Psychophysiol. 2005;57:53–59. doi: 10.1016/j.ijpsycho.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Schienle A., Schafer A., Stark R., Walter B., Vaitl D. Relationship between disgust sensitivity, trait anxiety and brain activity during disgust induction. Neuropsychobiology. 2005;51:86–92. doi: 10.1159/000084165. [DOI] [PubMed] [Google Scholar]
- Shaw P., Kabani N.J., Lerch J.P., Eckstrand K., Lenroot R., Gogtay N., Greenstein D., Clasen L., Evans A., Rapoport J.L., Giedd J.N., Wise S.P. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D., Levitin D.J., Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegge H., Terwagt M.M. Awareness and regulation of emotion in typical and atypical development. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford; New York: 2007. pp. 269–286. [Google Scholar]
- Stuss D.T. Biological and psychological development of executive functions. Brain Cogn. 1992;20:8–23. doi: 10.1016/0278-2626(92)90059-u. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical; New York: 1988. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging. [Google Scholar]
- Taylor S.F., Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn. Sci. 2007;11:413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Tamm L., Menon V., Reiss A. Maturation of brain function associated with response inhibition. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:1231–1238. doi: 10.1097/00004583-200210000-00013. [DOI] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends Neurosci. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolin D.F., Lohr J.M., Sawchuk C.N., Lee T.C. Disgust and disgust sensitivity in blood-injection-injury and spider phobia. Behav. Res. Ther. 1997;35:949–953. doi: 10.1016/s0005-7967(97)00048-x. [DOI] [PubMed] [Google Scholar]
- Urry H.L., van Reekum C.M., Johnstone T., Kalin N.H., Thurow M.E., Schaefer H.S., Jackson C.A., Frye C.J., Greischar L.L., Alexander A.L., Davidson R.J. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J. Neurosci. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Reekum C.M., Johnstone T., Urry H.L., Thurow M.E., Schaefer H.S., Alexander A.L., Davidson R.J. Gaze fixations predict brain activation during the voluntary regulation of picture-induced negative affect. Neuroimage. 2007;36:1041–1055. doi: 10.1016/j.neuroimage.2007.03.052. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Davidson M.L., Hughes B.L., Lindquist M.A., Ochsner K.N. Prefrontal–subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter H., von Kalckreuth A., Schardt D., Stephan A., Goschke T., Erk S. The temporal dynamics of voluntary emotion regulation. PLoS ONE. 2009;4:e6726. doi: 10.1371/journal.pone.0006726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B., Keysers C., Plailly J., Royet J.P., Gallese V., Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Winecoff A., Labar K.S., Madden D.J., Cabeza R., Huettel S.A. Cognitive and neural contributors to emotion regulation in aging. Soc. Cogn. Affect. Neurosci. 2010 doi: 10.1093/scan/nsq030. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody S.R., Teachman B.A. Intersection of disgust and fear: normative and pathological views. Clin. Psychol. Sci. Pract. 2000;7:291–311. [Google Scholar]
- Xiong J., Gao J.H., Lancaster J.L., Fox P.T. Clustered pixels analysis for functional MRI activation studies of the human brain. Hum. Brain Mapp. 1995;3:287–301. [Google Scholar]
- Yurgelun-Todd D.A., Killgore W.D.S. Fear-related activity in the prefrontal cortex increases with age during adolescence: a preliminary fMRI study. Neurosci. Lett. 2006;406:194–199. doi: 10.1016/j.neulet.2006.07.046. [DOI] [PubMed] [Google Scholar]
- Zelazo P.D., Cunningham W.A. Executive function: mechanisms underlying emotion regulation. In: Gross J.J., editor. Handbook of Emotion Regulation. Guilford; New York: 2007. pp. 135–158. [Google Scholar]