Abstract
Chronic pain is a hallmark of osteoarthritis (OA), yet little is known about its properties and representation in the brain. Here we use fMRI combined with psychophysics to study knee pain in 14 OA patients and 9 healthy controls. Mechanical painful pressure stimuli were applied to the knee in both groups and ratings of evoked pain and related brain activity examined. We observe that psychophysical properties and brain activation patterns of evoked pain are essentially the same between OA patients and healthy subjects, and between worse and better OA knees. In OA patients, stimulus-related brain activity could be distinguished from brain activity associated with spontaneous pain. The former activated brain regions commonly observed for acute painful stimuli in healthy subjects, while the spontaneous pain of OA engaged prefrontal-limbic regions closely corresponding to areas observed for spontaneous pain in other chronic pain conditions, such as chronic back pain and post-herpetic neuralgia. Arthritis-related clinical characteristics of knee OA also mapped to prefrontal-limbic regions. In a subgroup of patients (n = 6) we examined brain activity changes for a 2-week, repeat measure, cyclooxygenase-2 inhibitor (valdecoxib) therapy. Treatment decreased spontaneous pain for the worse knee and clinical characteristics of OA, and increased blood and csf levels of the drug which correlated positively with prefrontal-limbic brain activity. These findings indicate dissociation between mechanically induced and spontaneous OA knee pain, the latter engaging brain regions involved in emotional assessment of the self, and challenge the standard clinical view regarding the nature of OA pain.
Keywords: knee osteoarthritis, prefrontal cortex, limbic, fMRI, COX2 inhibitor, csf, psychophysics
1. Introduction
Osteoarthritis (OA) is the most common type of arthritis, and a leading cause of disability worldwide (Sharma et al., 2006). Pain is the primary complaint associated with OA, serving as a predictor of physical dysfunction and muscular weakness, and is thought to influence subsequent outcomes (O’Reilly et al., 1998; Miller et al., 2001; Kidd et al., 2004; Felson, 2005). Thus characterizing the pain of OA is critical to understanding mechanisms underlying the disease.
OA pain is generally described as a chronic inflammatory response (Felson, 2005), generated in part by up-regulation of Na+ channels (Laird et al., 2001) and cartilage degradation-associated local production of NO (Takahashi et al., 2001). There is poor relationship between joint anatomical changes, as identified by MRI, and OA pain (Conaghan and Felson, 2004). On the other hand, painful OA knees are more likely to demonstrate structural pathology than non-painful knees (Felson et al., 2001; Hill et al., 2007). OA pain is localized and use-related (Gok et al., 2002; Johnson et al., 2007). Most human studies use either pain questionnaires (Bellamy, 2002; Mendoza et al., 2006; Rosemann et al., 2008; van den Akker-Scheek et al., 2008; Victor et al., 2008) or quantitative sensory testing to measure non-painful somatosensory perception and pain thresholds in response to mechanical or thermal stimuli (Hendiani et al., 2003; Ordeberg, 2004; Martinez et al., 2007; Imamura et al., 2008). These studies identify primary and secondary mechanical and heat hyperalgesia, implying peripheral and central sensitization, which seem to resolve following joint replacement (Lindh et al., 1997; Hendiani et al., 2003; Ordeberg, 2004; Neugebauer et al., 2007; Shakoor et al., 2008). OA is also associated with decreased mechanosensation of pressure or vibratory stimuli, yet no study to date has quantitatively examined pressure-induced pain over the diseased joint and its related brain activity. Anti-inflammatory drugs remain the main therapies used to treat OA symptoms, including those preferentially targeting the COX2 enzyme. Although two COX2 selective inhibitors were recently withdrawn from the market, less- and non-selective COX2 inhibitors are still prescribed (Chen et al., 2008). However, the effect of COX2 inhibitor treatment on OA pain-related brain circuitry is not yet known.
Recent studies have begun exploring brain activity in OA in the absence of peripheral stimulation (Kulkarni et al., 2007), for referred pain in hip OA (Gwilym et al., 2009), and for modulation of brain activity in knee OA by lidocaine patch therapy (Baliki et al., 2008). In the present study, we examine knee OA, hypothesizing that painful pressure stimuli applied to the joint will induce greater pain and increase related brain activity in OA patients and, that these responses will be greater for the knee more affected by the disease. Given the inflammatory nature of OA, we expected painful stimulus-evoked brain activity patterns to be similar to those reflecting spontaneous OA pain. We correlated clinical characteristics of OA and the effects of a COX2 inhibitor treatment with brain activity for evoked and spontaneous pain to further elucidate brain regional activity characteristics along these variables.
2. Materials and Methods
2.1. Participants
Fourteen OA patients (10 males; age 56.1 ± 2.09 years, mean±SEM) and nine healthy controls (6 males; age 46.55 ± 2.6 years) participated in this study. Six OA patients also participated in a COX2i drug treatment study. All subjects were right-handed, gave informed consent to procedures approved by the Northwestern University Institutional Review Board, and compensated financially for their time. Healthy subjects had no history of chronic pain, and OA patients were only included if they fulfilled criteria of the American College of Rheumatology for classification of OA (Altman 1986) and had no history of other pain conditions. Patients were also required to have experienced OA pain for a duration longer than 3 months with a pain magnitude of at least 30/100 on a visual analog scale (VAS). During a screening interview patients indicated if one knee is generally more painful than the other, or affects more their daily life; this knee was designated as the ‘worse knee’, and the other designated as the ‘better knee’. OA pain characteristics were determined using the short form of McGill Pain Questionnaire (MPQ) (Melzack, 1987) and the Western Ontario and McMaster Osteoarthritis Index (WOMAC) (Bellamy, 2002). Clinical and demographic data are summarized in Tables S1 and S2. All participants refrained from use of short acting analgesics for 72 hours prior to testing and were not on antidepressants.
2.2. Experimental design
Prior to brain scanning subjects learned how to use a finger-spanning device to continuously report changes in pain intensity on a 0 to 100 scale, with 0 = no pain and 100 = worst imaginable pain (Baliki et al., 2006). Pressure was delivered using a custom-made fMRI compatible device designed to apply pressure-pain on deep tissue consisting of a plastic piston 14 mm in diameter with a rounded dull end, propelled by pressurized air and equipped with a feedback sensor to continuously record applied pressure (Fig. S1). Pressure was applied to the most sensitive part of each knee for patients and at the center of the knee joint in controls. During each session, subjects were presented with four 10 minute scan runs of pressure stimulation (two on each knee), while using the finger device to rate ongoing pain. Subjects were instructed to rate the intensity of pain evoked by the stimulus, and ignore touch and/or pressure sensation. Patients were additionally told not to rate their spontaneous OA pain. A measure of spontaneous pain was obtained by interrogating the patients at the start of each scan run as to their level of OA pain on a verbal 0–100 scale in the knee about to be stimulated. In this design we thus obtained, for each scan run, a ten minute ongoing evoked pain rating as well as a single spontaneous pain rating for the specified knee.
Depending on the localization of OA pain, the stimulus was applied to skin just overlying the joint, just over bone, or on soft tissue. Thus absolute applied pressure varied in intensity as a reflection of stimulation site. Therefore we normalized applied pressure, and pain ratings, between subjects by calculating relative stimulus intensities and pain ratings, presented in standardized units (s.u. = number of standard deviations from mean). During each scan run subjects were presented with pressure stimuli distributed in time in a pseudo-random design of variable intensities and durations; the number of stimuli (maximum nine) was determined by monitoring subjects’ responses.
2.3. COX2 Inhibitor treatment study
A subset of OA patients (n = 6) participated in an open-labeled study of the effects of a COX2 selective inhibitor (COX2i, valdecoxib), on OA pain. Our original intention was to carry out this study in all patients. However, halfway through the study the drug was withdrawn from the market in the US, and thus we discontinued the treatment portion. Session 1 was performed prior to the start of drug and after cessation of use of short acting analgesics for 72 hours. OA patients started use of COX2i drug (10 mg twice daily) and continued to refrain from all other analgesic medications during this time. The same procedures were then performed: 24 hours after start of drug (session 2), and 2 weeks after continued use of drug (session 3).
Immediately following each session, samples of cerebrospinal fluid (csf) and heparinized blood plasma were drawn from each participant to to measure drug and PGE2 concentrations. Csf was collected by lumbar puncture, during which patients sat upright, and following betadine prep, sterile draping and infiltration with 1% lidocaine at the L3–L4 interspinous space, an 18g introducer needle was inserted. A 24g pencil-point spinal needle was added using sterile technique and with gentle aspiration a clear 0.2 ml of csf was collected. A solid phase extraction (SPE) procedure was performed on centrifuged csf to obtain 0.10 ml aliquots containing analyte and stable isotope labeled internal standard. Immediately following lumbar puncture, 0.5 ml of blood plasma was drawn from the antecubital fossa using a 21g needle; 0.30 ml aliquots containing analyte and stable isotope labeled internal standard were extracted with SPE procedure. Samples were refrigerated at −20°C and analyzed within established storage stability periods. Extracted csf and blood plasma samples were analyzed by high performance liquid chromatography with tandem mass spectrometry (HPLC/MS/MS). Quantification of samples was performed blinded as to subject identity and time point.
2.4. Psychophysics
Psychophysical properties of knee pressure induced pain collected during fMRI scans were studied in OA patients and healthy subjects by examining average time variability of pain ratings relative to the time course of pressure applied to the knee joint, and by quantifying stimulus-response relationships between applied pressure and resultant pain ratings. To reduce rating noise we subtracted baseline pain ratings 7.5 seconds prior to start of the stimulus from the pain rating of each episode. Stimulation episodes were only included in the analysis if they induced a change in pain rating from baseline greater than 10 out of 100. In order to investigate the time course of the stimulus-response relationship, applied pressure intensity and pain ratings were averaged separately for the start (rise) and end (fall) phases of each stimulation epoch. A 2-way repeated measures analysis of variance was performed for each phase with time a repeat measure factor and grouping (OA vs. healthy, and OA worse vs. better knees) the other independent factor.
2.5. fMRI and anatomical data acquisition
Functional MRI data was acquired with a 3T Siemens Trio whole-body scanner with echo-planar imaging (EPI) capability using a standard 8-channel radio-frequency head coil. Multi-slice T2*-weighted echo-planar images with whole brain coverage were obtained with the following parameters: repetition time TR = 2.5 s, echo time TE = 30 ms, flip angle = 90°, slice thickness = 3 mm, in-plane resolution = 3.475 × 3.475 mm2, and number of slices = 36. T1-weighted anatomical MRI images were also acquired for each subject using the following parameters: TR = 2.1 s, TE = 4.38 ms, flip angle = 8°, FOV = 220 mm, slice thickness = 1 mm, in-plane resolution = 0.86 × 0.86 mm2, and number of sagittal slices = 160.
2.5.1 Preprocessing of fMRI data
Image analysis based on changes in blood oxygen level-dependent (BOLD) signal was performed using FSL Version 4.1 (www.fmrib.ox.ac.uk/fsl) (Jezzard et al., 2001). Preprocessing of each subject’s individual scan run included interleaved slice timing correction, motion correction using MCFLIRT, spatial smoothing using a Gaussian kernel of full-width half-maximum 5 mm, linear high-pass temporal filtering (100 s), and intensity normalization. Scan runs with an absolute magnitude of head motion greater than 2.5 mm were excluded from further analyses (6 scans in controls and 8 in OA patients). Preprocessed data was registered to each subjects’ T1 image before normalization to standard space with the Montreal Neurological Institute (MNI152) template. Following registration, the time course of the BOLD signal for a voxel in the white matter (x = 41, y = 60, z = 46) and another voxel in the ventricle (x = 57, y = 75, z = 46) were extracted for subsequent analyses.
2.6 fMRI statistical analyses
2.6.1 General linear model analysis
The fMRI signal was linearly modeled on a voxel-by-voxel basis using FSL’s improved linear model (FILM) with local autocorrelation correction (Woolrich et al., 2001; Woolrich et al., 2004). For each scan run, pressure-evoked pain ratings were convolved with the hemodynamic response function (gamma function: lag = 6 seconds, standard deviation = 3 seconds). Six head motion vectors (derived from motion correction) and the single-voxel time courses extracted from the white matter and ventricle for each functional run were used at this level as covariates of no interest to further remove residual variance from head motion and scanner and physiological noise. Significance of the model fit to each voxel time-series was calculated to yield statistical parametric maps for each scan in each subject.
2.6.2. Brain activity for stimulus-evoked pain
To standardize comparisons between groups and stimulated knees, stimulus-evoked activity was performed on activity maps after flipping the activation maps for right knee stimulation along the x-axis. This rendered all stimulus runs to reflect a unilateral stimulation of the left knee, thereby enabling group averages and comparison of worse and better knees by controlling for the confound of sidedness. Stimulus pain-related brain activity was identified for each participant by averaging within-subject scan runs in a fixed effects analysis. Group statistical maps were then generated at the next level by averaging OA patients’ and controls’ activation maps with a mixed effects analysis and controlling for potential age effects by regressing out age as a covariate of no interest.
As the mixed effects modeling calculates the true random effects component of the variance and proper degrees of freedom at each voxel, it allows inferences to be made about the wider population from which subjects are drawn. Overlap between activity maps was determined by binarizing and multiplying voxels exceeding intensity and cluster threshold in OA and controls. Contrasts between OA and controls were also performed (unpaired t-test, mixed effects) to identify potential differences in brain activity between the two groups. Similar statistical procedures were employed to investigate brain activity for worse and better knees in OA patients.
2.6.3. Differentiating brain activity for spontaneous versus evoked OA pain
OA patients’ spontaneous pain value was used as a covariate of interest for each scan. The second covariate of interest (evoked pain) was calculated as peak evoked pain averaged across stimulation epochs for each scan. Both covariates were included in a fixed effects multiple regression analysis with individual scan runs (flipped data) as inputs. This approach identifies brain regions involved in one condition while correcting for the influence of the other.
2.6.4. Brain activity co-varying with OA clinical characteristics
To identify brain regions related to clinical characteristics of OA, we performed separate analyses for WOMAC pain, WOMAC function, and MPQ scores. Within-subject averages of scans across both knees were generated using un-flipped data, as stimulus laterality was not of interest. These maps were then input to higher-level fixed effects covariate analyses and corrected for age.
2.6.5. Brain activity modulated by COX2i levels in the blood
Changes in brain activity modulated by treatment were identified by conducting within-subject paired t-tests (fixed effects, un-flipped data) between each of the three scan sessions, yielding 3 maps for each subject (session 2 > session 1, session 3 > session 2, and session 3 > session 1). This identifies brain regions that increase in activity with continued use of the drug. These maps were then input to a higher-level Ordinary Least Squares mixed effects regression model with changes in blood levels of the drug serving as the covariate of interest.
2.6.6. Threshold for identifying brain activations
A gray matter mask was applied to group average maps prior to thresholding. Cluster-based correction of the z-statistic images was thresholded at z > 2.7. For each resulting cluster of spatially connected voxels surviving the z threshold, a cluster probability threshold of P < 0.01 (corresponding to a Family Wise Error Correction of P < 0.0005) was applied to the computed significance of that cluster, properly correcting for multiple comparisons (Friston et al., 1995).
2.6.7. Surface-based mapping
Surface-based mapping was constructed using the Population-Average, Landmark- and Surface-based (PALS) average-fiducial surface from the 23 individual subjects as the atlas target (Van Essen, 2005).
Results
3.1 Psychophysics of knee pressure-evoked pain
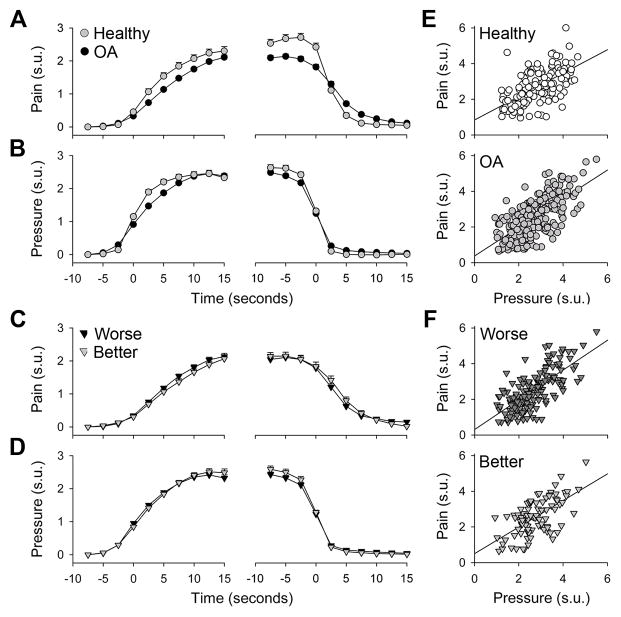
Stimulus-response relationships were examined (per stimulation) regarding perceptual time courses (rise and fall rates) and response magnitudes. Stimulus duration did not differ between controls (mean ± SE 24.9 ± 0.61 seconds) and OA patients (26.5 ± 0.67 seconds) (p = 0.1), or between better and worse knees in OA (p=0.2). Peak pressure intensity for each stimulation was slightly higher in controls (2.8 ± 0.08 s.u.) than OA (mean ± SE: 2.6 ± 0.06 s.u.) (p = 0.02), but did not differ between better and worse knees in OA (p =0.5). Peak pain ratings did not differ between controls (mean ± SE: 2.7 ± 0.09 s.u.) and OA patients (2.5 ± 0.07) (p = 0.07), or between better and worse knees in OA (p =0.7).
The time course for pain ratings during the rise phase of the stimulus was distinct between OA patients and controls (F1, 325 = 9.1, p<0.003), with a significant interaction (time*group, F8, 2600 = 5.5, p<0.00001) (Fig. 1A, first panel). Pressure intensity was similar between the groups (Fig. 1B, first panel), despite a significant interaction effect (time*group, F8, 2600 = 8.8, p<0.00001). At 2.5 seconds prior to stimulus end, OA patients rated stimuli as slightly less painful (F1,325 = 18.9, p<0.00002), with a significant interaction (time*group, 10 time steps, F9, 2925 = 21.4, p<0.00001) (Fig. 1A, second panel). Stimulus-response relationships between pain and pressure were positively correlated in both OA patients (r = 0.67, p<0.0001) and healthy subjects (r = 0.58, p<0.0001); however, these slopes did not differ (Fig. 1E). These results were replicated when using absolute pain and pressure values instead of standardized units (1 s.u. pain = 14/100 absolute pain; 1 s.u. pressure = 1.5 Kg force applied with a 14 mm diameter probe).
Figure 1.
Pressure-induced pain is similar between OA patients and healthy subjects, and between better and worse knees in OA patients. Time courses for the rise and fall phases of pressure (B) and pain (A) (in standardized units (s.u.), ± SEM) were averaged across all stimulations. Time courses for pressure and pain in better and worse OA knees (C & D) showed no significant differences. Peak pressure and pain for each stimulus positively correlated in all groups (E & F) but did not distinguish between OA and controls, or better and worse OA knees.
Comparison of worse and better knees in OA patients revealed no significant differences in pain or pressure during either the start or end phases of stimulation (Fig. 1C, D). Stimulus-response relationships were again positively correlated for both knees (worse, r = 0.72, p<0.0001; better, r = 0.58, p<0.0001), but the slopes did not differ (Fig. 1F). These results are opposite to our initial hypothesis as they demonstrate no pressure induced pain perception differences between healthy subjects and OA patients, or worse and better knees in OA, either in perceived pain magnitude or in the time course of pain perception.
3.2 Brain activity for pressure-evoked pain is minimally different between OA and healthy subjects and between worse and better knees in OA
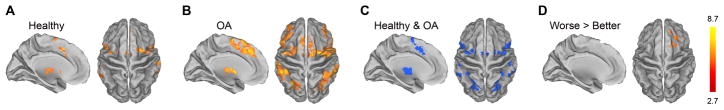
The pressure-induced pain rating task evoked increased brain activity in healthy subjects in bilateral insula, thalamus, basal ganglia (caudate and putamen), amygdala, anterior cingulate (ACc), supplementary motor area (SMA), lateral prefrontal cortex, and posterior parietal cortices, as well as right secondary somatosensory cortex (SII), left premotor cortex, periaqueductal gray and other brainstem regions (Fig. 2A & S2A, Table S3). OA patients exhibited activation in most of the same brain areas, and although it was slightly more widespread in OA (Fig. 2B & S2B, Table S3), the number of significantly activated voxels in controls and OA patients overlapped by 81% (Fig. 2C & S2C). Contrast analyses between the two groups were null in both directions. Thus, there were only small differences in brain activity for pressure-evoked knee pain between OA patients and healthy controls.
Figure 2.
Brain activity for pressure-evoked pain. Group averaged activity for healthy subjects (A) and OA patients (B). Overlapping activations are shown in (C). Contrast analyses between healthy and OA did not show any significant differences. (D) Examination of better and worse OA knees revealed greater activation for the worse knee in the right lateral prefrontal cortex; the opposite contrast (better > worse) showed no difference. In each sub-figure, left panel is left hemisphere midline view, right panel is top view of the brain.
Contrasting brain activity between knees in OA patients identified right lateral prefrontal activity for the worse greater than better knee (Fig. 2D & S2D, Table S3). The opposite contrast (better > worse) was null. Thus, we observe only small differences in brain activity between OA worse and better knees. These results match the psychophysical findings, are opposite to our starting hypothesis, and indicate that between OA and controls, and between worse and better knees, painful pressure stimuli applied to the knee are only minimally different.
3.3 Brain regions associated with pressure-evoked pain versus spontaneous pain in OA
Psychophysically, spontaneous pain ratings were higher for the worse knee (mean ± SE worse knee: 18.9 ± 3.9; better knee: 8.1± 2.8, p=0.04). However, the magnitude of evoked pain did not differ between knees (p=0.9). Moreover, patients’ oral report of spontaneous OA pain at the start of each scan showed no relationship with pressure-evoked pain (r = −0.07, p > 0.6). Given that the evoked pain and spontaneous pain ratings show minimal interaction, they can be used to identify brain regions distinct to each perception.
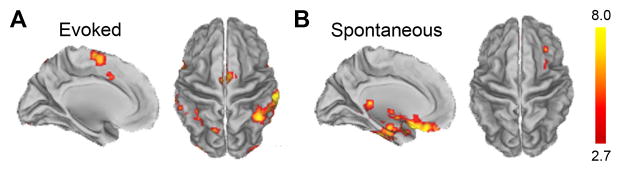
Brain activity related to pressure-evoked pain in OA patients, corrected for the influence of spontaneous pain, localized mainly to bilateral insula, SII, ACc, SMA, inferior and posterior parietal cortices (Fig. 3A & S3A, Table S4). Notably, a number of brain regions present in the group average prior to correcting for spontaneous pain (Fig. 2B & S2B) were no longer significantly activated, specifically thalamus, basal ganglia, amygdala, and lateral prefrontal cortex. Brain activity associated with spontaneous pain, after correcting for pressure-evoked pain, localized to the medial and orbital prefrontal cortex, as well as bilateral accumbens and amygdalae (Fig. 3B & S3B, Table S4). These findings demonstrate that OA spontaneous pain engages brain regions distinct from those activated by pressure-evoked pain, specifically prefrontal-limbic structures.
Figure 3.
Brain activity for evoked versus spontaneous pain in OA patients. (A) Brain activity for pressure-evoked pain, after correcting for spontaneous knee OA pain. (B) Brain activity for spontaneous OA pain, after correcting for pressure-evoked pain. In A and B, left panel is left hemisphere midline view, right panel is top view of the brain.
3.4 Brain activity related to OA clinical characteristics
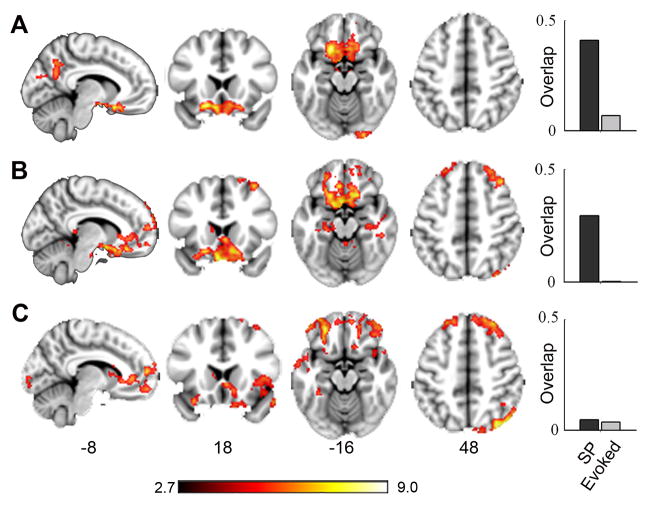
OA patients’ brain activity was determined in relation to MPQ, WOMAC function, and WOMAC pain scores (Fig. 4 & S4, Table S5). All three measures mapped primarily to prefrontal cortical areas: MPQ localized to orbital regions, WOMAC function to orbital, medial, and lateral prefrontal areas, and WOMAC pain to lateral prefrontal cortex, bilateral insula and basal ganglia. Activity maps for MPQ and WOMAC function overlapped with areas involved in spontaneous OA pain. WOMAC pain-related brain activity minimally overlapped with spontaneous and stimulus-evoked brain activity maps.
Figure 4.
Brain regions covarying with clinical OA characteristics. Brain activity maps for MPQ total (A), WOMAC Function (B), and WOMAC Pain (C) scores all show activation in prefrontal cortical areas. Right histograms indicate the extent of spatial overlap (0–1 scale) with spontaneous (SP) and pressure evoked pain activity maps.
All clinical measures of OA significantly positively correlated with spontaneous pain, but not with evoked pain ratings (correlations of MPQ total, sensory, and present pain index scores with spontaneous pain were: r=0.35, 0.41, 0.36, with p<0.01 – 0.004; and with evoked pain were: r= 0.18, 0.18, −0.2, p >0.2; similarly correlations of WOMAC total, stiffness, and function with spontaneous pain: r=0.35, 0.27, 0.41, p< 0.06 – 0.004; and with evoked pain: r=0.02, 0.21, −0.02, p>0.2 – 0.9). These relationships along with the brain activity findings indicate that questionnaire outcome measures are not representing stimulus-evoked pain, but instead better reflect characteristics of spontaneous pain in OA.
3.5 COX2 Inhibitor treatment effects on OA pain and related brain activity
With treatment, levels of COX2i increased from undetectable to larger values in blood (F2,10= 21.1, p < 0.0004) and csf (F2,10=19.9, p< 0.0003) (Fig. 5A). Blood and csf levels of COX2i were tightly correlated showing that csf levels are 2% of that in blood (r=0.9, p<0.0001). On the other hand, PGE2 levels did not change with treatment, were not correlated in the blood and csf, and were unrelated to changes in COX2i. Therefore we did not use PGE2 values in further analysis.
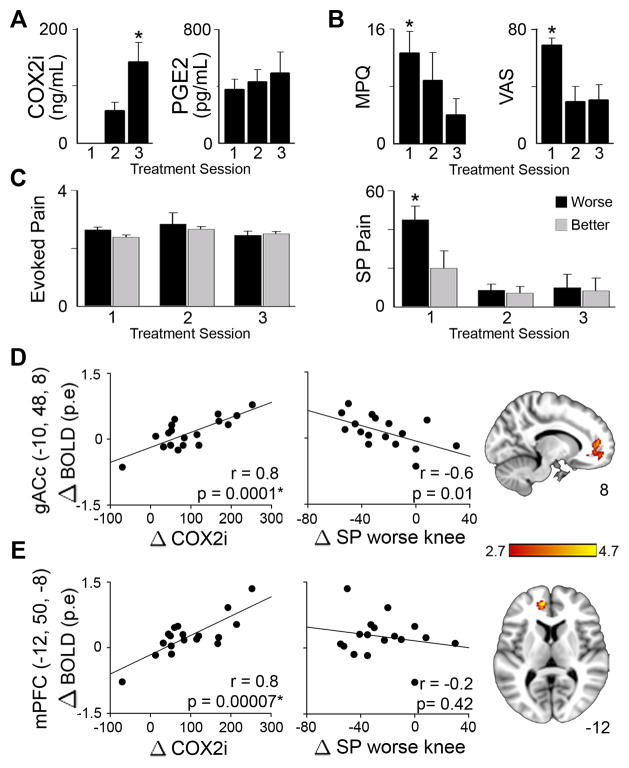
Figure 5.
Effects of COX2i treatment study on OA pain and related brain activity. OA patients were scanned three times (session 1 = prior to drug ingestion, session 2 = 24 hours after start of drug, session 3 = after 2 weeks of drug use). (A) Blood levels of COX2i, but not PGE2, increased with treatment. (B) Treatment significantly lowered MPQ scores and VAS pain ratings. (C) Stimulus-evoked (Ev) pain (standardized units) was unchanged by treatment. However, spontaneous (SP) pain ratings (0–100 scale) for the worse knee significantly decreased. (D & E) Brain activity reflecting changes in COX2i blood concentrations localized to the genu ACc (gACc) and mPFc. Changes in BOLD activity (in parameter estimate units, p.e.) across treatment sessions in both regions positively correlated with changes in COX2i levels. Changes in spontaneous pain for the worse knee only correlated with the gACc, where increased treatment-related activity was associated with decreased pain.
Treatment with COX2i drug over two weeks decreased multiple measures of OA pain. Decreases in clinical indices were observed for VAS ratings (F2, 10 = 5.8, p=0.02), MPQ (F2, 10 = 4.3, p<0.05), WOMAC total (F2, 10 = 8. 7, p=0.007), WOMAC pain (F2, 10 = 6.20, p=0.02), and WOMAC function (F2, 10 = 9.0, p=0.006) scores (correction for multiple measures, division by 5, renders the weaker effects to become borderline significant) (Fig. 5B). Furthermore, there was a significant effect of treatment on OA patients’ spontaneous pain in the worse (F2, 10 = 21.87, p=0.0002) but not the better knee (Fig. 5C), with no effect of COX2i treatment on evoked pain.
As blood levels of COX2i increased with treatment, we identified brain activity reflecting its change across the three scan sessions in OA patients for rating the painful pressure applied to the knee. The two main brain areas identified were located at the genu of the anterior cingulate (gACc) and the medial prefrontal cortex (mPFc) (Fig. 5D & E, Table S6). A region of interest analysis for the two activity foci indicated that changes in BOLD activity were positively correlated with changes in blood levels of COX2i across scan sessions, and gACc activity specifically was also negatively correlated to changes in OA spontaneous pain for the worse knee. Therefore, the gACc activity links treatment related pain perception changes to the blood levels of the COX2i drug.
4. Discussion
The main result of this study is the demonstration of the existence of two distinct pain states in knee OA patients. Knee pressure-evoked pain activated brain regions commonly observed for acute pain (Apkarian et al., 2005; Baliki et al., 2009), while ongoing spontaneous OA pain engaged medial prefrontal-limbic cortical areas. We were surprised that applying deep pressure to the knee joint psychophysically showed a minor increase in perceived pain in healthy subjects, and yet the related brain activity was slightly greater in OA patients. Following correction for contribution of spontaneous OA pain, we observe that the stimulus-evoked pain related map becomes more circumscribed and dominated by somatosensory nociceptive processing regions, shows minimal overlap with brain regions modulated by clinical characteristics of OA (MPQ, WOMAC function, and WOMAC pain), and is unaffected by treatment with a COX2 inhibitor. We observe almost an opposite pattern for spontaneous OA pain: 1) Brain regions involved in spontaneous pain did not overlap with those for stimulus-evoked activity, but instead engaged medial prefrontal-limbic areas, indicating that it is more of an emotional state (Dolan, 2002; Gallagher and Frith, 2003; Phelps et al., 2004). 2) They showed close correspondence with brain regions modulated by clinical characteristics of OA, and many clinical characteristics decreased with COX2i drug treatment. 3) The main brain area (gACc) reflecting COX2i drug concentration in the blood also reflected decreases in OA spontaneous pain. Thus, prefrontal-limbic activity seems more relevant to OA pain as different parts of the region reflect emotional assessment, clinical characteristics, and modulation of spontaneous pain by drug treatment.
Recent clinical studies emphasize the critical role pain plays in OA (O’Reilly et al., 1998; Miller et al., 2001; Kidd et al., 2004; Felson, 2005). To our knowledge this is the first quantitative psychophysical examination of pain perception for the knee joint in OA. Our initial hypothesis was that mechanically stimulating at and around the OA joint should result in allodynia and/or hyperalgesia. Instead we observe only a small decrease in pain sensitivity. Given that the stimulus is applied externally across the skin, we cannot unequivocally state that the pain ratings reflected only perturbations of deep tissue structures, such as bone, joint, muscle. On the other hand, given the geometry of the stimulating probe, its placement, and OA patients’ confirmation that the applied pressure was provoking the OA pain, it is also highly unlikely that the ratings of pain were purely due to skin indentation. Electrophysiological studies of the inflamed knee in animal models provide ample evidence for afferent sensitization as well as activation of silent high-threshold nociceptors (Neugebauer et al., 2007; Schaible et al., 2009). However, these animal studies are done shortly after peripheral inflammation. Therefore, we suspect that the lack of pressure-induced pain sensitization of the OA knee is a reflection of chronicity of OA in the human clinical population, suggesting a mismatch between animal models and clinical OA. Our stimulus paradigm only examined pressure-pain, and other stimulus modalities may still exhibit evidence for sensitized joint afferents. It should be emphasized that systematic quantitative studies of OA pain remain urgently needed, and the current study is only a first attempt in this direction.
Here we observe that the stimulus-evoked pain has a distinct representation from spontaneous OA pain. Components of these activations have been observed in two earlier studies of OA related brain activity (Kulkarni et al., 2007; Gwilym et al., 2009). Our initial hypothesis was that spontaneous pain and evoked pain would map to the same brain areas, as inflammation is expected to enhance sensitivity and spontaneous firing of peripheral nociceptors. Based on this idea in an earlier study we attributed pressure stimulus-evoked activity to the inflammatory component of OA (Baliki et al., 2008). The current results clarify this relationship by dissociating stimulus-evoked brain activity from spontaneous pain-related brain activity. Notably, there is a close correspondence between brain regions seen here for spontaneous pain in OA and those observed for spontaneous pain in other chronic pain conditions, including chronic back pain (Baliki et al., 2006) and post-herpetic neuralgia (Geha et al., 2007). In the present study we identified brain activity for spontaneous pain using a covariate analysis for subjects’ ratings of stimulus-evoked pain. In two earlier studies spontaneous pain-related activity was determined using continuous ratings of fluctuations of ongoing pain (Baliki et al., 2006; Geha et al., 2007). Despite methodological differences, in all three chronic pain patient groups we observe similar brain regions identified for spontaneous pain, primarily medial prefrontal cortex-limbic structures including the amygdala and nucleus accumbens. As the latter structures are implicated in emotional assessment of the environment relative to the self (Dolan, 2002; Gallagher and Frith, 2003; Phelps et al., 2004), we can state that spontaneous chronic pain is generally an emotional state, and this applies specifically to knee OA pain too, a conclusion also reached by Kulkarni et al. (2007). Moreover, the preoccupation of these regions with spontaneous pain may distort assessment and prediction of outcomes based on emotional cues (Dembo et al., 2005; Apkarian, 2008; Seymour and Dolan, 2008; van Roozendaal and Krass, 2009), and may underlie the decision-making deficits observed in different chronic pain conditions (Apkarian et al., 2004), suggesting that similar deficits should also be observable in OA patients. Even though spontaneous pain generally engages mPFc-limbic circuitry, there is also a differential gradient of activity across the three clinical conditions implying unique emotional properties for spontaneous pain in different clinical conditions: mPFc is most prominently active in chronic back pain (Baliki et al., 2006) and amygdala and accumbens activity best reflect clinical characteristics of post herpetic neuralgia (Geha et al., 2007), whereas in OA more orbitofrontal cortical regions seem engaged in the condition, with orbital, medial and lateral PFC activity reflecting its primary clinical characteristics. To our knowledge this is the first study mapping clinical characteristics of OA pain to brain activity, and the tight correlation between clinical parameters and magnitude of spontaneous OA pain provide further evidence as to the physiological source of these clinical measures in OA, and indicate that these medial prefrontal-limbic regions encode complex properties, expectations and consequences of OA pain.
This study is also the first to examine brain activity in OA in relation to an anti-inflammatory drug treatment, and investigate COX2i and PGE2 blood and csf levels during the course of treatment. The csf levels of COX2i and PGE2 are similar to the only two other reports documenting these values in humans (Hsu et al., 2001; Dembo et al., 2005). Although this was an open labeled study done in a small group of OA patients, we observe specific changes in clinical outcome measures, spontaneous OA pain, and robust brain activity. We expected a negative correlation between PGE2 levels and COX2i (Samad et al., 2001), but this was not observed. The lack of relationship between central COX2i and PGE2 suggests that the anti-inflammatory effects of COX2i may not be centrally mediated (Samad et al., 2001; Vardeh et al., 2009), although this needs to be tested in a larger study. Multiple clinical outcome measures decreased with continued use of COX2i as well as the magnitude of spontaneous pain for the worse OA knee, but did not affect the magnitude of stimulus-evoked pain. These results raise questions as to time, dose, and site of action for this COX2i drug, and for anti-inflammatory drug action in OA in general, which require larger and placebo controlled future studies. The brain regions related to COX2i are also informative as to possible mechanisms of OA pain and its modulation by anti-inflammatory drugs: The gACc activity related to changing COX2i blood levels also reflected changes in spontaneous OA pain for the worse knee. We do not know whether this activity is driven by changes in pain or in COX2i. Moreover, the general area of the gACc and mPFc are in close proximity to the regions encoding clinical characteristics of OA and thus may be influenced by changes in those parameters as well. This region is also shown to be involved in placebo effects (Wager et al., 2007; Eippert et al., 2009) and engaged in top-down modulation (Fields, 2006). Therefore, it is unlikely that the brain activity reflecting blood levels of COX2i are due to local inhibition of COX2 enzyme. Instead, it likely reflects effects of pain modulation by efficacy of the drug at the joint itself, coupled with a top-down modulation based on expectation as well as peripheral analgesia, which in turn would diminish the central gain for the afferent spontaneous nociceptive barrage emanating from the OA joint, through spino-cephalad-spinal interactions.
Overall, this study provides multiple lines of evidence indicating that pressure-evoked knee pain and spontaneous OA pain have distinct mechanisms. The pressure-evoked pain and related brain activity is minimally different between OA and healthy subjects. In contrast, spontaneous knee OA pain has a brain representation similar to that seen for spontaneous pain in other clinical chronic pain conditions, and these brain regions relate to clinical characteristics and are modulated by COX2i therapy. These results challenge the clinical ideas regarding the nature of chronic OA pain and point to the intriguing possibility that the prefrontal-limbic brain regions identified for spontaneous pain, and molecular mechanisms underlying chronic pain in these areas (Millecamps et al., 2006; Centeno et al., 2009), may provide novel therapeutic opportunities for OA.
Acknowledgments
We thank all participants in the study. We also thank Rami Jabakhanji for aid in building the pressure stimulator. Funding was provided by NIH NINDS NS 35115 and partially by Pfizer Pharmaceuticals. Pfizer Pharmaceuticals provided financial aid, drug, and assisted in drug quantification for csf measurements, but had no involvement in other aspects of the project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apkarian AV, Sosa Y, Krauss BR, Thomas PS, Fredrickson BE, Levy RE, Harden RN, Chialvo DR. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18:464–468. doi: 10.1016/j.conb.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Chialvo DR, Geha PY, Levy RM, Harden RN, Parrish TB, Apkarian AV. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Jabakhanji R, Harden N, Schnitzer TJ, Apkarian AV. A preliminary fMRI study of analgesic treatment in chronic back pain and knee osteoarthritis. Mol Pain. 2008;4:47. doi: 10.1186/1744-8069-4-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki MN, Geha PY, Apkarian AV. Parsing pain perception between nociceptive representation and magnitude estimation. J Neurophysiol. 2009;101:875–887. doi: 10.1152/jn.91100.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy N. WOMAC: a 20-year experiential review of a patient-centered self-reported health status questionnaire. J Rheumatol. 2002;29:2473–2476. [PubMed] [Google Scholar]
- Centeno MV, Mutso A, Millecamps M, Apkarian AV. Prefrontal cortex and spinal cord mediated anti-neuropathy and analgesia induced by sarcosine, a glycine-T1 transpoter inhibitor. Pain. 2009;145:176–183. doi: 10.1016/j.pain.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YF, Jobanputra P, Barton P, Bryan S, Fry-Smith A, Harris G, Taylor RS. Cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs (etodolac, meloxicam, celecoxib, rofecoxib, etoricoxib, valdecoxib and lumiracoxib) for osteoarthritis and rheumatoid arthritis: a systematic review and economic evaluation. Health Technol Assess. 2008;12:1–278. iii. doi: 10.3310/hta12110. [DOI] [PubMed] [Google Scholar]
- Conaghan PG, Felson DT. Structural associations of osteoarthritis pain: lessons from magnetic resonance imaging. Novartis Found Symp. 2004;260:191–201. discussion 201–195, 277–199. [PubMed] [Google Scholar]
- Dembo G, Park SB, Kharasch ED. Central nervous system concentrations of cyclooxygenase-2 inhibitors in humans. Anesthesiology. 2005;102:409–415. doi: 10.1097/00000542-200502000-00026. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell ED, Yacubian J, Klinger R, Lorenz J, Buchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, Kazis L, Gale DR. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- Felson DT. The sources of pain in knee osteoarthritis. Curr Opin Rheumatol. 2005;17:624–628. doi: 10.1097/01.bor.0000172800.49120.97. [DOI] [PubMed] [Google Scholar]
- Fields HL. A motivation-decision model of pain: the role of opioids. Proceedings of the 11th world congress on pain; Seattle: IASP press; 2006. pp. 449–459. [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistic parametric maps in functional imaging: A general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends Cogn Sci. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Geha PY, Baliki MN, Chialvo DR, Harden RN, Paice JA, Apkarian AV. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128:88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gok H, Ergin S, Yavuzer G. Kinetic and kinematic characteristics of gait in patients with medial knee arthrosis. Acta Orthop Scand. 2002;73:647–652. doi: 10.1080/000164702321039606. [DOI] [PubMed] [Google Scholar]
- Gwilym SE, Keltner JR, Warnaby CE, Carr AJ, Chizh B, Chessell I, Tracey I. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–1234. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- Hendiani JA, Westlund KN, Lawand N, Goel N, Lisse J, McNearney T. Mechanical sensation and pain thresholds in patients with chronic arthropathies. J Pain. 2003;4:203–211. doi: 10.1016/s1526-5900(03)00557-1. [DOI] [PubMed] [Google Scholar]
- Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, Gale D, Grainger A, Conaghan P, Felson DT. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu MM, Chou YY, Chang YC, Chou TC, Wong CS. An analysis of excitatory amino acids, nitric oxide, and prostaglandin E2 in the cerebrospinal fluid of pregnant women: the effect on labor pain. Anesth Analg. 2001;93:1293–1296. doi: 10.1097/00000539-200111000-00053. [DOI] [PubMed] [Google Scholar]
- Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, Cutait MM, Fregni F, Camanho GL. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum. 2008;59:1424–1431. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Mathews P, Smith SM. Functional MRI: An introduction to methods. Oxford: Oxford University Press; [Google Scholar]
- Johnson SR, Archibald A, Davis AM, Badley E, Wright JG, Hawker GA. Is self-reported improvement in osteoarthritis pain and disability reflected in objective measures? J Rheumatol. 2007;34:159–164. [PubMed] [Google Scholar]
- Kidd BL, Photiou A, Inglis JJ. The role of inflammatory mediators on nociception and pain in arthritis. Novartis Found Symp. 2004;260:122–133. discussion 133–128, 277–129. [PubMed] [Google Scholar]
- Kulkarni B, Bentley DE, Elliott R, Julyan PJ, Boger E, Watson A, Boyle Y, El Deredy W, Jones AK. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007;56:1345–1354. doi: 10.1002/art.22460. [DOI] [PubMed] [Google Scholar]
- Laird JM, Carter AJ, Grauert M, Cervero F. Analgesic activity of a novel use-dependent sodium channel blocker, crobenetine, in mono-arthritic rats. Br J Pharmacol. 2001;134:1742–1748. doi: 10.1038/sj.bjp.0704428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindh C, Liu Z, Lyrenas S, Ordeberg G, Nyberg F. Elevated cerebrospinal fluid substance P-like immunoreactivity in patients with painful osteoarthritis, but not in patients with rhizopatic pain from a herniated lumbar disc. Scand J Rheumatol. 1997;26:468–472. doi: 10.3109/03009749709065721. [DOI] [PubMed] [Google Scholar]
- Martinez V, Fletcher D, Bouhassira D, Sessler DI, Chauvin M. The evolution of primary hyperalgesia in orthopedic surgery: quantitative sensory testing and clinical evaluation before and after total knee arthroplasty. Anesth Analg. 2007;105:815–821. doi: 10.1213/01.ane.0000278091.29062.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- Mendoza T, Mayne T, Rublee D, Cleeland C. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10:353–361. doi: 10.1016/j.ejpain.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Millecamps M, Centeno MV, Berra HH, Rudick CN, Lavarello S, Tkatch T, Apkarian AV. D-cycloserine reduces neuropathic pain behavior through limbic NMDA-mediated circuitry. Pain. 2006 doi: 10.1016/j.pain.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ME, Rejeski WJ, Messier SP, Loeser RF. Modifiers of change in physical functioning in older adults with knee pain: the Observational Arthritis Study in Seniors (OASIS) Arthritis Rheum. 2001;45:331–339. doi: 10.1002/1529-0131(200108)45:4<331::AID-ART345>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Han JS, Adwanikar H, Fu Y, Ji G. Techniques for assessing knee joint pain in arthritis. Mol Pain. 2007;3:8. doi: 10.1186/1744-8069-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57:588–594. doi: 10.1136/ard.57.10.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordeberg G. Characterization of joint pain in human OA. Novartis Found Symp. 2004;260:105–115. discussion 115–121, 277–109. [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Rosemann T, Laux G, Szecsenyi J, Wensing M, Grol R. Pain and osteoarthritis in primary care: factors associated with pain perception in a sample of 1,021 patients. Pain Med. 2008;9:903–910. doi: 10.1111/j.1526-4637.2008.00498.x. [DOI] [PubMed] [Google Scholar]
- Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. Interleukin-1beta-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature. 2001;410:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Richter F, Ebersberger A, Boettger MK, Vanegas H, Natura G, Vazquez E, Segond von Banchet G. Joint pain. Exp Brain Res. 2009;196:153–162. doi: 10.1007/s00221-009-1782-9. [DOI] [PubMed] [Google Scholar]
- Seymour B, Dolan R. Emotion, decision making, and the amygdala. Neuron. 2008;58:662–671. doi: 10.1016/j.neuron.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Shakoor N, Agrawal A, Block JA. Reduced lower extremity vibratory perception in osteoarthritis of the knee. Arthritis Rheum. 2008;59:117–121. doi: 10.1002/art.23241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma L, Kapoor D, Issa S. Epidemiology of osteoarthritis: an update. Curr Opin Rheumatol. 2006;18:147–156. doi: 10.1097/01.bor.0000209426.84775.f8. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Hashimoto S, Kubo T, Hirasawa Y, Lotz M, Amiel D. Hyaluronan suppressed nitric oxide production in the meniscus and synovium of rabbit osteoarthritis model. J Orthop Res. 2001;19:500–503. doi: 10.1016/S0736-0266(00)90024-X. [DOI] [PubMed] [Google Scholar]
- van den Akker-Scheek I, Zijlstra W, Groothoff JW, Bulstra SK, Stevens M. Physical functioning before and after total hip arthroplasty: perception and performance. Phys Ther. 2008;88:712–719. doi: 10.2522/ptj.20060301. [DOI] [PubMed] [Google Scholar]
- Van Essen DC. A Population-Average, Landmark- and Surface-based (PALS) atlas of human cerebral cortex. Neuroimage. 2005;28:635–662. doi: 10.1016/j.neuroimage.2005.06.058. [DOI] [PubMed] [Google Scholar]
- van Roozendaal BW, Krass I. Development of an evidence-based checklist for the detection of drug related problems in type 2 diabetes. Pharm World Sci. 2009;31:580–595. doi: 10.1007/s11096-009-9312-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardeh D, Wang D, Costigan M, Lazarus M, Saper CB, Woolf CJ, Fitzgerald GA, Samad TA. COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J Clin Invest. 2009;119:287–294. doi: 10.1172/JCI37098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TW, Jensen MP, Gammaitoni AR, Gould EM, White RE, Galer BS. The dimensions of pain quality: factor analysis of the Pain Quality Assessment Scale. Clin J Pain. 2008;24:550–555. doi: 10.1097/AJP.0b013e31816b1058. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proc Natl Acad Sci U S A. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage. 2001;14:1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]