Abstract
Malformations of cortical development are frequently identified in surgical resections for intractable epilepsy. Among the more frequently identified are cortical dysplasia, pachygyria and polymicrogyria. The pathogenesis of these common developmental anomalies remains uncertain. Polymicrogyria is particularly vexing because there are multiple described forms (2, 4 and 6 layer) that have been attributed to multiple etiologies (e.g. ischemic, genetic, infectious, and toxic). We reviewed the pathology in 19 cases and performed cortical laminar analysis in 10 of these cases. Our data indicate that a defining feature of polymicrogyria is fusion of the molecular layer and that most often there is a well-defined grey matter-white matter junction. Unexpectedly, the cortical lamina were normally positioned but there were reduced neuronal populations within these lamina, particularly in the subgranular layers. Based on these data, we propose that the categorization of polymicrogyria according to the number of lamina is artificial and should be abandoned and polymicrogyria should be defined according to the presence or absence of coexisting neuropathological features. Furthermore, our data indicate that polymicrogyria is not a cell migration disorder and rather that it should be considered a post-migration malformation of cortical development.
Keywords: Cell migration, Cerebral cortex, Cortical lamina, Malformation of cortical development, Polymicrogyria, Seizures
INTRODUCTION
Polymicrogyria (PMG) was first described by Bielschowsky in 1915 (1). Since then, numerous descriptions have provided a broad and sometimes confusing portrait of this pathological entity (1). The surface of the brain with PMG is frequently described as having a “Moroccan Leather” or fine cobblestone appearance. On cross-sections the involved cerebral cortex appears thickened and may exhibit an undulating but distinct border with the underlying white matter. Microscopic examination is required to distinguish true PMG (which shows cortical lamination defects) from microgyria (also known as ‘polygyria’), in which a normal 6-layer cortex persists and there is no fusion of the molecular layer (1).
The microscopic characteristics of PMG are variable depending on the report: 2, 4 and 6-layer PMG have all been described. Two-layer, also known as “unlayered PMG,” has been defined as having a molecular layer overlying a single disorganized and unlaminated cortex. Four-layer PMG is classically described as having a molecular layer, an upper dense cell layer, an intermediate layer of low cell density containing a few horizontal oriented myelinated fibers in older individuals, and a deep cell layer bordering the white matter (1–3). Finally, 6-layer PMG (which is the least commonly reported) shows the normal neocortical laminar pattern but exhibits fusion of the molecular layer. A relatively abrupt transition between normal cortex and PMG, a relatively well-defined grey matter/white matter junction, and fusion of the molecular layer (layer 1) are common to virtually all reports. While some reports have ascribed specific laminar patterns to specific etiologies (1), these data are largely drawn from anecdotal reports and have not been confirmed in larger series. Furthermore, despite a laminar pattern, whether the remaining layers represent components of one or several of the normal cortical laminae, or whether they are derived by distinct mechanisms, independent of the normal cortical laminae has not been investigated.
To further understand its pathology and pathogenesis, we collected brain specimens with PMG at the Children’s Hospital of Philadelphia (CHOP) between 1990 and 2005. Evaluation of these brains provides several insights into PMG. First, the pathology of PMG is not consistent with a primary cell migration disorder. Second, we commonly identified 2-, 4-, and 6-layer PMG within the same brain, indicating that any stratification of PMG according to cerebral laminae is artificial. Finally, we show that the primary organization of the cerebral laminae is normal in regions of PMG.
MATERIALS AND METHODS
PMG cases were obtained from the collection at the CHOP after approval by the Institution Review Board for research on human subjects. Clinical information was abstracted from the pathology files and from clinical charts when available. Nine cases were excluded due to a lack of adequate material for review. The cases studied included 13 cases from the autopsy and surgical pathology files at the CHOP and 6 brains sent from other institutions for consultation; 17 brains were from CHOP and 2 referrals were from WBD; there were 14 autopsies and 5 surgical specimens (Table).
Table.
Clinical and Pathological Data
| Case | Age | Sex | Clinical diagnosis | genotype | Neurologic/imaging manifestations | Extra-neurologic manifestations | Other neuropathology | PMG type |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 mo | M | PMG and PNH | Unbalance translocation resulting in trisomy 6q25, monosomy 22q13.3 | developmental delay seizures | bifid renal collecting system, bilateral cleft lip and palate, bilateral fused ribs (8 and 9), chronic respiratory insufficiency, cardiovascular instability | Multiple, small, bilateral PNH | 4 and 6 layer |
| 2 | 2 mo | M | congenital glomangiomatosis and arteriole obliteration | 46,XY | multifocal ischemic lesions of the brain | thrombocytopenia, coagulopathy, aneurysm and aortic-caval arteriovenous fistula | Old and recent infarcts and vascular changes consistent with glomangiomatosis | 4 layer and disorganized cortex |
| 3 | 7 d | F | multiple cerebral malformations | hydrocephalus, agenesis of the corpus callosum, irregular gyral pattern, small brainstem | pyruvate dehydrogenase deficiency | hypoplasia/aplasia descending cortical spinal tracts, inferior olivary hypoplasia, ACC | 4 layer and increased cellularity in molecular layer | |
| 4 | 11 mo | M | cerebral neuronal migration disorder/PMG (provisional) | 46,XY | congenital diaphragmatic hernia with pulmonary hypertension, RVH and hypoplastic left lung; congenital cataracts | subcortical (small, frontal and parietal lobes) and leptomeningeal heterotopia, partial ACC, inferior olivary dysplasia | 2 layer | |
| 5 | 10 d | F | PMG | carnitine palmitoyl transferase deficiency, type II | 2 and 4 layer | |||
| 6 | 5 mo | M | Cerebral dysplasia with “lissencephaly” | microcephaly | renal vein thrombosis and putative Chlamydia pneumonia, mild congestive heart failure | Hypoplasia descending cortico-spinal tracts; cerebellar dysplasia; single small PNH | Disorganized PMG with loss of grey/white junction | |
| 7* | 2 d | M | encephalocele | 46,XY | encephalocele | twin gestation with polyhydramnios | 4 layer | |
| 8* | 9 mo | M | hemimegalencephaly | intractable seizures | 2 layer with cortical dysplasia including enlarged neurons | |||
| 9 | 5 mo | M | PMG | multiple congenital malformations | 2 layer | |||
| 10* | 2 y | F | cortical dysplasia | seizure disorder | 2 and 4 layer, focal | |||
| 11 | 2 mo | F | PMG | Multiple, small, occipital PNH; hypoplasia descending cortico-spinal track | 4 and 6 layer, Ca++ | |||
| 12 | 1 mo | M | PMG | 46,XY | Microcephaly | micrognathia, micropenis with hypospadias, undescended testes | Olivo-ponto-cerebellar hypoplasia | 2, 4 and 6 layer |
| 13* | 1 mo | F | 2 and 4 layer, with disorganization | |||||
| 14 | 3 d | M | PMG | 46,XY | microcephaly | Potter’s syndrome, congenital heart disease | 2, 4 and 6 layer | |
| 15 | 51 y | F | PMG | seizure disorder, cerebral palsy with left hemiplegia | scoliosis | 4 layer and dysplastic regions | ||
| 16 | 2 mo | F | 2–3 layer, Ca++ | |||||
| 17* | 1 mo | F | 2 and 4 layer | |||||
| 18 | 1 mo | M | 2, 4 and 6 layer | |||||
| 19 | 33 wk ga | Bilateral porencephaly | 4 layer and disorganized cortex |
ACC, agenesis of the corpus callosum; CA++ = calcification; ga, gestational age; PMG, polymicrogyria; PNH, periventricular nodular heterotopia; RVH, right ventricular hypertrophy; M, male; F, female;
surgical cases; all others were autopsy brains.
Hematoxylin and eosin (H&E)-stained sections were reviewed by 2 neuropathologists (A.R.J. and J.A.G.) and consensus of findings was reached on all cases. In an effort to be as inclusive as possible for this study, PMG was defined according to minimum criteria. PMG was defined as small undulating gyri giving a festooned appearance to the cortical surface by microscopy, with fusion of the molecular layer between adjacent sulci. Lamination was not considered in the primary PMG diagnosis. The number of layers was determined using H&E features. All sections from each case were further reviewed to define other pathologic abnormalities.
One to 3 blocks were selected from each case for immunohistochemistry (IHC), which was performed as previously described (4, 5). Primary antibodies included anti-Reelin (MAB5366, Chemicon, Temecula, CA), anti-Calbindin (300, Swant, Bellinzona, Switzerland), anti-cTip2 (ab18465, Abcam, Cambridge, MA), and anti-TBR1 (kindly provided by Dr. Robert Hevner, University of Washington, Seattle, WA). IHC for all antibodies was judged to be successful on 10 of the 19 cases and these served as the basis of the IHC analysis. After reviewing the IHC for adequacy, each slide was converted to a digital image using Aperio Scanscope CS hardware and viewed using Image Scope software. Three regions of interest (ROI) were selected from each case for counting. Each ROI was 100 μm wide and the full thickness of the cortex. All positive cells were counted in the ROI. Labeling index was defined as the mean of the 3 ROI counts. Internal comparisons between PMG and adjacent normal cortex were made in each case. The depths of labeled cells (layers) were extrapolated from the adjacent normal-appearing cortex to the PMG for comparison purposes.
RESULTS
Ten of 18 cases were male; information on gender was not available for 1 of the cases. The mean age was 38.7 months; if the 51-year-old individual (case #15) is excluded, the mean is 5.0 months. The vast majority of brains (16/19 cases) were from patients less than 1 year of age. Cytogenetic analyses were available in only 6 cases; 5 were reported to have a normal karyotype, whereas case #1 showed a complex cytogenetic abnormality involving a translocation that resulted in a partial monosomy 22q13.3 and trisomy 6p12.5 (Table).
Neuropathologic findings in addition to PMG were present in 7 of the 19 cases. Heterotopia, periventricular nodular, or subcortical and deep white matter were the most common of these findings (5 cases). There was evidence of intrauterine injury in 3 of the 7 cases. The intrauterine injury included evidence of perinatal white matter injury (case #1), old cerebral infarcts in a 2-month-old child (case #2), and a 33-week fetus with bilateral porencephaly (termed, “open lip schizencephaly” in the radiologic literature) (case #3). The histology in case #19 included hemosiderin deposition and calcifications, both features associated with intrauterine injury. None of the patients had the linear calcifications found in “band-like calcification with simplified gyration and PMG” (or pseudoTORCH) associated with OCLN mutations (6, 7). There were 2 patients with defined metabolic disorders, 1 with pyruvate dehydrogenase deficiency (case #3) and the other with type II carnitine palmitoyl transferase deficiency (case #5). The available neurologic, neuroradiologic and non-neurologic features of these cases are summarized in the Table.
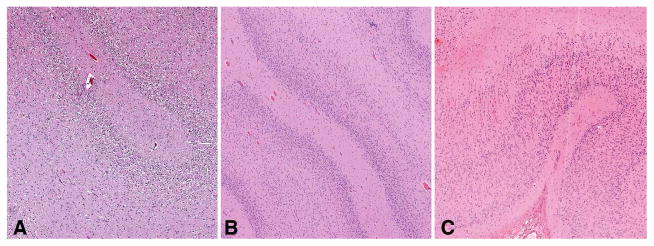
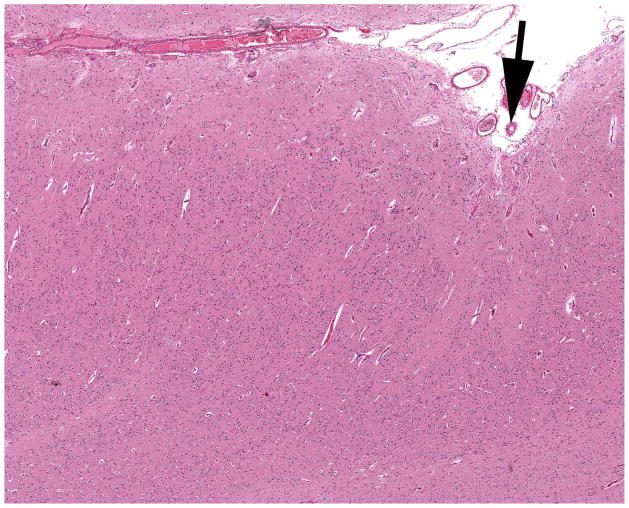
The histopathology of the PMG varied widely within and between cases. The presence of 2-, 4-or 6-layer PMG (Fig. 1) was determined for each brain by consensus of the neuropathologists (Table). The abnormal cortex showed features of 4-layer PMG in 14 of the 19 brains. Of these only 2 had 4-layer PMG alone, 2 had 4-layer PMG along with some disorganized cortex (cases #2 and #19), and the remaining 10 of the 14 brains with 4-layer PMG also had PMG with 2-and/or 6-layer features. PMG with an accompanying disorganized cortex that could not otherwise be definitively classified was present in 2 brains (cases #6 and #13) (Fig. 2). Two other cases each showed a unique pathology. The first exhibited a 4-layer PMG with increased layer 1 cellularity (case #3), and the second exhibited a 4-layer PMG with areas of cerebral cortical dysplasia (case #15). Two cases showed only 2-layer PMG (cases #4 and #9) and an additional case showed 2-layer PMG with dysplastic regions (case #8).
Figure 1.
Examples of various forms of polymicrogyria (PMG). (A) 2-layer (unlayered) PMG. (B) 4-layer PMG. (C) 6-layer PMG. H&E stain. Magnification: 100×.
Figure 2.
Polymicrogyria (PMG) (arrow) with adjacent dysplastic cortex (left of arrow) lacking layer organization or molecular layer fusion. H&E stain. Magnification: 100×.
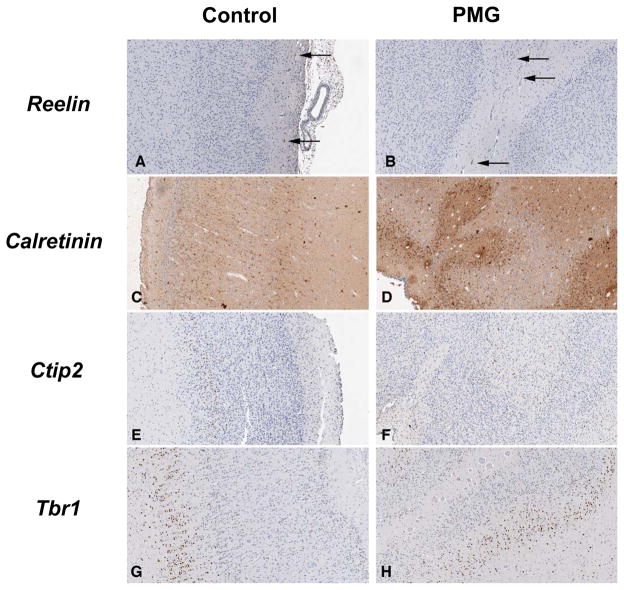
PMG is often considered to be a cell migration disorder. Such disorders exhibit disorganization of the normal neocortical 6-layer arrangement. To determine the internal cortical organization of the neocortex, IHC was performed with a panel of antibodies that delineate a subset of these layers. Reelin was expressed primarily in layer 1, presumably in the Cajal-Retzius neurons (Fig. 3A, B) (8). Calretinin was expressed in all cortical layers, although slightly higher in layers 3 and 5 (Fig. 3C, D). In control brains, Ctip2 was expressed primarily in layer 5 and in slightly fewer cells in layer 6 (Fig. 3E, F) (8). Tbr1 is predominately expressed in layer 6 but showed modest expression in other layers of the postnatal human cortex (Fig. 3G, H) (8).
Figure 3.
Immunohistochemistry for Reelin, Calretinin, Ctip2 and Tbr1 in control and polymicrogyria (PMG) brains. (A, B) Reelin-labeled Cajal-Retzius neurons are present in the molecular layer of both the control and PMG brains (arrows). (C, D) Calretinin-labeled neurons are found in all cortical layers of control and PMG brains with predominance in layers 3 and 5 in controls but not in PMG for layer 5 (see also Fig. 4). (E, F) Most Ctip2+ neurons are located in layer 5 with scattered positive cells in more superficial layers. This pattern is observed in both control and PMG brains, although again there are fewer positive cells in layer 5. (G, H) Tbr1 is predominately expressed in cortical layer 6 in both control and PMG brains. Magnification: 100×.
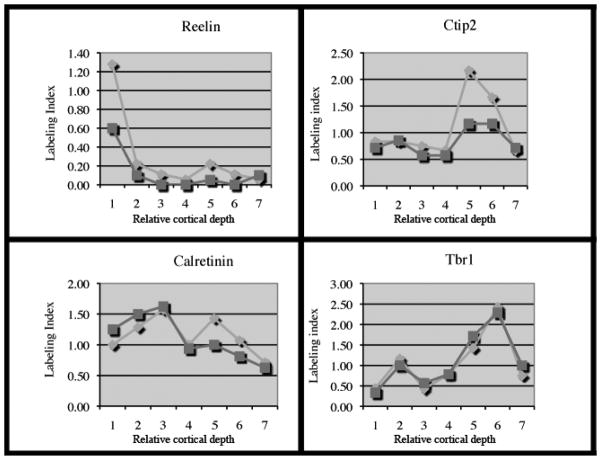
The expression of these 4 neuron markers in the normal neocortex was compared to that in the adjacent PMG in the same histologic section. Although the labeling indices were often reduced in cases of PMG (indicating reduction in the total number of cells), the overall morphology of the labeling index curves was quite similar in the PMG and control regions (Fig. 4). These data suggest that the laminar organization within PMG is similar to that in normal neocortex, despite the histopathologic differences. Interestingly, the Ctip2 and Calretinin data, while paralleling the pattern of cell number in control regions, showed the greatest difference in labeling index of neurons in the deeper layers (i.e. layers 5 and 6). Reduction in deeper layer neurons is commonly described with PMG (1, 3).
Figure 4.
Labeling index according to cortical layers for Reelin, Calretinin, Ctip2 and Tbr1. Light grey diamonds correspond to control regions; dark grey squares to correspond to polymicrogyria. Layer 1 = molecular layer; layer 7 = white matter; layers 2 through 6 correspond to relative depth in the cortex.
DISCUSSION
Understanding the pathology of PMG has taken on new interest and importance with the discovery of many PMG syndromes and several associated genes (9). This is combined with the longstanding recognition that PMG may also result from teratogenic, infectious or ischemic insult to the brain (1, 3). While the pathologic features of PMG have been described in many papers, reviews, and chapters, the laminar organization has received little attention. Based on recently defined markers of the laminar organization of the cerebral cortex, our data indicate that, although there is a reduction in the overall thickness of the cortex, neurons are positioned in the appropriate layers in PMG. Our finding that laminar organization is preserved combined with the fact that most cases show few white matter neurons argue against a primary neuronal migration disorder. We also found considerable heterogeneity in the histopathology with overlap in the various types, even within a single case. This also raises doubt regarding the validity of distinguishing PMG by the number of laminae.
A second major implication of our study is that separation of PMG into 2-, 4- or 6-layer types appears artificial (1). We found that different numbers of layers of PMG were frequently present within the same case. Thus, identification of findings accompanying PMG will likely be of greater importance. The presence of porencephaly, extensive leptomeningeal heterotopia, or periventricular nodular heterotopia (all associated pathologies found in our series as well as by others) will likely contribute to understanding the various pathogeneses of PMG. For example, the finding of porencephaly with accompanying abnormalities such as iron deposition more likely indicates a disruption/vascular event underlying the pathogenesis of the PMG (10, 11). In contrast, the presence of extensive bilateral PMG with microcephaly may indicate a specific genetic defect such as a mutation in WDR62 (12).
A number of gene mutations have recently been linked to PMG. In addition to WDR62, mutations in SRPX2 have been associated with perisylvian PMG; the affected patients exhibit language and cognitive defects along with epilepsy (13). Additional genes associated with PMG include PAX6, TBR2, KIAA1279, RAB3GAP1, and COL18A1 (9); these account for a small minority of cases but there is no known pathology for any of these specific genetic disorders. Nonetheless, identifying specific pathologic patterns of PMG in different patients with specific genetic disorders or other known etiologies will significantly aid in guiding genetic evaluations and testing for patients with PMG. A large series of PMG cases highlights its clinical heterogeneity and points to the importance of identifying its genetic and pathobiologic basis (14).
Mutations in Occludin (OCLN), an integral component of tight junctions, were found to be associated with the band-like calcification with simplified gyration and PMG syndrome, also known as pseudo-TORCH syndrome (6). While tight junctions are essential for the structure and function of glial-vascular integrity, it remains uncertain whether OCLN mutations result in vascular disruption as the pathogenesis of the PMG and calcifications, or if there is some other developmental event that is disrupted.
Mutations in GPR56 were first reported to be associated with PMG, specifically frontoparietal PMG and white matter defects (15). Subsequent evaluation of the pathology revealed that the cerebral malformation included extensive leptomeningeal heterotopia and over migration (16). The similarity of the pathology in these cases with that reported in O-glycosylation disorders suggests that mutations in GPR56 are a part of this pathway rather than a PMG gene (16).
Finally, 2 cases in our series had a recognized metabolic defect along with PMG. Although both pyruvate dehydrogenase deficiency and carnitine palmitoyl transferase deficiency have been associated with PMG (17), the pathology of PMG has been confirmed in only rare cases. Our cases provide further confirmation of an association of PMG with these disorders.
In summary, our analysis of 19 cases of PMG, the largest series of PMG reported in the pathology literature, has identified several important pathologic characteristics of PMG. First, the histopathologic distinction of PMG into 2-, 4-, and 6-layer PMG is likely artificial and does not have biological significance. Second, neurons in the cases of PMG we studied appear to be positioned in the appropriate laminae, suggesting that PMG is not a migration disorder. It remains possible that other cases of PMG, when similarly studied, will identify laminar disruptions; these will be of extreme interest and may point to specific genetic defects. Finally, we propose that the minimal criteria for PMG should be fusion of the molecular layer with festooning of the cortical surface independent of other cortical changes.
Acknowledgments
This work was supported by NIH grant RO1-NS45034.
References
- 1.Friede R. Developmental Neuropathology. 2. Berlin: Springer-Verlag; 1989. [Google Scholar]
- 2.Golden J, Harding BN. Developmental Neuropathology. Basel: ISN Neuropath Press; 2004. [Google Scholar]
- 3.Norman M, McGillivray B, Kalousek D, et al. Congenital malformations of the brain: pathological, embryological, clinical, radiolological and genetic aspects. New York: Oxford University Press; 1995. [Google Scholar]
- 4.Pancoast MM, Dobyns WB, Golden JA. Interneuron deficits in patients with the Miller-Dieker syndrome. Acta Neuropathol (Berl) 2005;109:400–4. doi: 10.1007/s00401-004-0979-z. [DOI] [PubMed] [Google Scholar]
- 5.Santi MR, Golden JA. Periventricular heterotopia may result from radial glial fiber disruption. J Neuropathol Exp Neurol. 2001;60:856–62. doi: 10.1093/jnen/60.9.856. [DOI] [PubMed] [Google Scholar]
- 6.O’Driscoll MC, Daly SB, Urquhart JE, et al. Recessive mutations in the gene encoding the tight junction protein occludin cause band-like calcification with simplified gyration and polymicrogyria. Am J Hum Genet. 2010;87:354–64. doi: 10.1016/j.ajhg.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briggs TA, Wolf NI, D’Arrigo S, et al. Band-like intracranial calcification with simplified gyration and polymicrogyria: a distinct “pseudo-TORCH” phenotype. Am J Med Genet A. 2008;146A:3173–80. doi: 10.1002/ajmg.a.32614. [DOI] [PubMed] [Google Scholar]
- 8.Hevner RF. Layer-specific markers as probes for neuron type identity in human neocortex and malformations of cortical development. J Neuropathol Exp Neurol. 2007;66:101–9. doi: 10.1097/nen.0b013e3180301c06. [DOI] [PubMed] [Google Scholar]
- 9.Guerrini R, Dobyns WB, Barkovich AJ. Abnormal development of the human cerebral cortex: genetics, functional consequences and treatment options. Trends Neurosci. 2008;31:154–62. doi: 10.1016/j.tins.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Ferrer I, Catala I. Unlayered polymicrogyria: structural and developmental aspects. Anat Embryol (Berl) 1991;184:517–28. doi: 10.1007/BF01236058. [DOI] [PubMed] [Google Scholar]
- 11.Barth PG. Prenatal clastic encephalopathies. Clin Neurol Neurosurg. 1984;86:65–75. doi: 10.1016/0303-8467(84)90068-4. [DOI] [PubMed] [Google Scholar]
- 12.Bilguvar K, Ozturk AK, Louvi A, et al. Whole-exome sequencing identifies recessive WDR62 mutations in severe brain malformations. Nature. 2010;467:207–10. doi: 10.1038/nature09327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roll P, Rudolf G, Pereira S, et al. SRPX2 mutations in disorders of language cortex and cognition. Hum Mol Genet. 2006;15:1195–207. doi: 10.1093/hmg/ddl035. [DOI] [PubMed] [Google Scholar]
- 14.Leventer RJ, Jansen A, Pilz DT, et al. Clinical and imaging heterogeneity of polymicrogyria: a study of 328 patients. Brain. 2010;133:1415–27. doi: 10.1093/brain/awq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piao X, Hill RS, Bodell A, Chang BS, et al. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033–6. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- 16.Bahi-Buisson N, Poirier K, Boddaert N, et al. GPR56-related bilateral frontoparietal polymicrogyria: further evidence for an overlap with the cobblestone complex. Brain. 2010;133:3194–209. doi: 10.1093/brain/awq259. [DOI] [PubMed] [Google Scholar]
- 17.van Straaten HL, van Tintelen JP, Trijbels JM, et al. Neonatal lactic acidosis, complex I/IV deficiency, and fetal cerebral disruption. Neuropediatrics. 2005;36:193–9. doi: 10.1055/s-2005-865713. [DOI] [PubMed] [Google Scholar]