Abstract
Objective
To define the role of TNFα in the cascade of gene activation that regulates aortic angiogenesis in response to injury.
Methods and Results
Angiogenesis was studied by culturing rat or mouse aortic rings in collagen gels. Gene expression was evaluated by qRT-PCR, microarray analysis, immunocytochemistry and ELISA. TNFα gene disruption and recombinant TNFα or blocking antibodies against VEGF or TNFRs were used to investigate TNFα-mediated angiogenic mechanisms. Resident aortic macrophages were depleted with liposomal clodronate. Angiogenesis was preceded by overexpression of TNFα and TNFα-inducible genes. Studies with isolated cells showed that macrophages were the main source of TNFα. Angiogenesis, VEGF production, and macrophage outgrowth were impaired by TNFα gene disruption and promoted by exogenous TNFα. Antibody-mediated inhibition of TNFR1 significantly inhibited angiogenesis. The proangiogenic effect of TNFα was suppressed by blocking VEGF or by ablating aortic macrophages. Exogenous TNFα, however, maintained a limited proangiogenic capacity in the absence of macrophages and macrophage-mediated VEGF production.
Conclusions
Overexpression of TNFα is required for optimal VEGF production and angiogenesis in response to injury. This TNFα/VEGF-mediated angiogenic pathway requires macrophages. The residual capacity of TNFα to stimulate angiogenesis in macrophage-depleted aortic cultures implicates the existence of a VEGF-independent alternate pathway of TNFα-induced angiogenesis.
Keywords: neovascularization, wound healing, innate immunity, cytokines, injury
Introduction
Angiogenesis, the formation of new blood vessels, plays an important role in embryonal development, wound healing, and the ovarian cycle, but also contributes to the progression of many diseases including cancer and atherosclerosis. Endothelial cells of preexisting vessels form new vessels in response to angiogenic factors produced by non-endothelial cells.1,2 Among these, vascular endothelial growth factor (VEGF) is recognized as a key regulator of the angiogenic process and important target of anti-angiogenic therapy.3
VEGF is normally expressed in many organs where it regulates capillary permeability and vessel survival under conditions of angiogenic quiescence.4 Increased expression of VEGF above physiological levels leads to the induction of angiogenesis.5 Anti-VEGF therapy blocks angiogenesis, but can also cause side effects such as hypertension, hemorrhage, thrombosis, and proteinuria due to inhibition of the physiologic functions of VEGF.6 Defining the upstream mechanisms responsible for increased VEGF expression is therefore critical to identify additional targets of anti-angiogenic therapy and possibly reduce its side effects. VEGF is characteristically upregulated in ischemic tissues through hypoxia-inducible transcription factors.7 VEGF levels are also elevated in wounded tissues,8,9 but the underlying mechanisms responsible for this VEGF increase are poorly defined. Understanding how the wounded vessel wall generates angiogenic signals may provide new insights into the mechanisms responsible for the formation of new blood vessels in atherosclerosis and other angiogenesis-related disorders associated with vascular injury.10,11
We previously showed that angiogenesis can be replicated ex vivo by culturing aortic rings in three dimensional gels of extracellular matrix.12,13 Angiogenesis in this system is triggered by the injury of the dissection procedure and regulated by paracrine and juxtacrine interactions between endothelial and nonendothelial cells including macrophages, mural cells, and fibroblasts. Injured explants produce VEGF which is released into the culture medium prior to the onset of angiogenesis. Aortic angiogenesis is significantly impaired by blocking VEGF with neutralizing antibodies or VEGF signal transduction inhibitors.14,15 Angiogenic sprouting can also be inhibited by depleting aortic rings of adventitial macrophages which are required for optimal VEGF production.16 Macrophages promote angiogenesis through their ability to orchestrate the inflammatory response in wounded tissues,17 but it remains unclear how the injury process enables macrophages to promote the production of VEGF required for endothelial sprouting.
Among the macrophage products identified in aortic cultures is tumor necrosis factor-α (TNFα), an inflammatory cytokine that has the capacity to modulate the angiogenic process.18,19 TNFα is a homotrimeric transmembrane protein that is released into the extracellular space through proteolytic cleavage by the metalloprotease TNFα converting enzyme (TACE or ADAM17).20 TNFα binds to two cell membrane receptors, TNFα receptor-1 (TNFR1) and TNFR2. Upon TNFα binding TNFRs generate a broad array of downstream signals by variably activating NFκB, MAPK, or caspase dependent cell death pathways depending on different contextual cues.21
TNFα has been shown to stimulate VEGF production by isolated cells,22–24 but there is a gap in our understanding of how this cytokine regulates the angiogenic response to injury in complex multicellular environments. In vitro models have investigated the direct effects of TNFα on isolated endothelial cells,25,26 but there are no studies on how endothelial cells respond to TNFα in the presence of macrophages and other vascular cell types. Using the aortic ring model of angiogenesis we now show that resident macrophage-derived TNFα plays an essential role in the angiogenic response of the vessel wall to injury. Our results demonstrate that TNFα functions as an immediate-response proangiogenic factor in the cascade of gene activation leading to VEGF production and endothelial sprouting following injury of the vessel wall. Our studies also indicate that TNFα plays an important role in the growth and survival of resident aortic macrophages.
Materials and methods
For an expanded Materials and Methods section, see the supplemental data, available online at http://atvb.ahajournals.org.
Aortic ring cultures
Collagen gel cultures of aortic rings from rat and wild type or TNFα-deficient mice were prepared and measured for angiogenic activity as described.27 Rat or mouse aortic rings were cultured with or without TNFα in the absence or presence of anti-VEGF blocking antibody or nonimmune IgG. Co-cultures of aortic rings with aorta-derived macrophages were performed as reported.28 The role of TNFRs in the angiogenic response was studied in cultures of mouse aortic rings treated with anti-TNFR1 or anti-TNFR2 blocking antibodies 29–31 or with nonimmune IgG.
Cell isolation
Aortic macrophages were isolated from colony stimulating factor-1 (CSF-1)-treated aortic cultures, as described.28 Aortic endothelial cells, aortic smooth muscle cells, and bone marrow macrophages were isolated as reported.32
Macrophage ablation
Rat aortic rings were depleted of macrophages with liposomal clodronate16,33 and cultured in collagen gels with or without TNFα (5 ng/ml).
qRT-PCR
The relative expression of TNFα and/or VEGF in aortic cultures at different time points after injury or TNFα treatment was measured by quantitative reverse transcriptase polymerase chain reaction (qRT-PCR).34
Microarray analysis
The transcriptome of injured and TNFα-treated aortic rings was analyzed with Affymetrix Rat Gene 1.0 ST Arrays (Affymetrix, Santa Clara, CA). Microarray data were deposited into the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus (GEO) database (http://www.ncbi.nlm.nih.gov/geo/) accessible through GEO series accession numbers GSE23152 and GSE23153.
Western blot analysis
Western blotting was used to evaluate TNFα protein expression in rat macrophages, endothelial cells, and smooth muscle cells.
Immunocytochemistry
Macrophages were identified in whole mount preparations of aortic ring cultures by immunoperoxidase or immunofluorescence using antibodies against rat CD68, rat CD163, or mouse F4/80.35 Double immunofluorescence staining with anti-TNFα and anti-CD68 antibodies was used to localize TNFα in aortic macrophages.
ELISA
VEGF produced in aortic cultures was measured with the Quantikine rat and mouse ELISA kits (R&D Systems).
Statistical analysis
Data were analyzed by Student’s t test. The statistical significance of differences between experimental groups was set at p<0.05.
Results
The angiogenic response of aortic rings to injury is preceded by upregulated expression of TNFα and TNFα-inducible genes
Aortic rings embedded in collagen gels generate a self-limited angiogenic response that is triggered by the injury of the dissection procedure. Aortic explants start sprouting at day 2–3 producing branching neovessels that grow until day 7–8.13 Aortic angiogenesis is associated with overexpression of many pro-angiogenic molecules including TNFα and VEGF which work in concert to stimulate vessel growth.16,36 To investigate the temporal sequence of TNFα and VEGF expression prior to angiogenic sprouting, we performed qRT-PCR time course studies on aortic rings during the first 24 hours of culture. TNFα was upregulated several fold 10 min after cutting the rings and its expression remained elevated at 24 hr. Upregulated expression of TNFα was followed by a gradual increase in VEGF expression over the following 24 hr (Fig. 1). Thus, VEGF expression and angiogenic sprouting were preceded by rapid overexpression of TNFα in response to aortic injury.
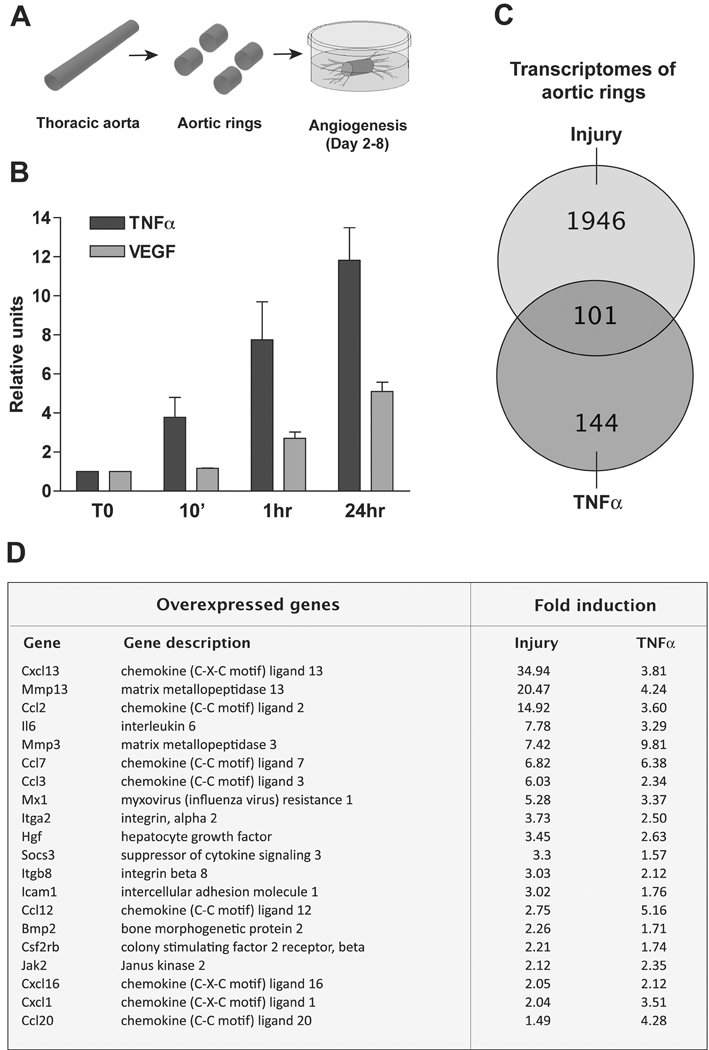
Figure 1. Upregulated expression of TNFα precedes VEGF expression and angiogenic sprouting in aortic ring cultures.
(A) Schematic drawing of aortic ring model of angiogenesis: New vessels sprout from freshly cut aortic explants embedded in collagen gel. (B) qRT-PCR shows rapid upregulation of TNFα gene expression after the aorta has been transected to obtain rings. TNFα mRNA is rapidly overexpressed within minutes after injury whereas highest VEGF mRNA levels are detected at 24 hr. (C) Venn diagram showing the relationship between the transcriptomes of injured and TNFα-treated rings identifies many TNFα-inducible genes in the injury transcriptome. (D) Table shows representative genes upregulated by both injury and TNFα.
To further evaluate the relationship between injury and TNFα gene induction, we performed microarray studies on freshly cut and TNFα-treated aortic rings, and compared the two transcriptomes for the presence of commonly upregulated genes. This study showed that 2,047 genes were upregulated 1.5 fold or higher after injury whereas 245 genes were overexpressed in response to TNFα (Fig. 1). Approximately 40% of TNFα-induced genes were identified in the injury transcriptome. Many of these commonly induced genes were chemokines and angioregulatory molecules. Genes induced by both injury and TNFα comprised chemokines that stimulate the migration of monocytes/macrophages (CCL2, CCL7, CCL12) and macrophage-derived chemokines that attract polymorphonuclear cells to sites of inflammation (CCL3, CCL20, CXCL1). Two of these chemokines (CCL2 and CXCL1) have the additional capacity to stimulate angiogenesis.37,38 TNFα induced many soluble angiogenic regulators including Angiopoietin-1 (Angpt-1), Hepatocyte Growth Factor (HGF), EphrinB1 (Ephn1), interleukin 6 (IL-6)37,39–41, and bone morphogenetic protein 2 (BMP-2).42 HGF, IL-6 and BMP-2 were also upregulated in the injury transcriptome. Taken together these results demonstrate the existence of a TNFα signature of gene expression in the injured aorta prior to angiogenic sprouting.
Resident adventitial macrophages are the main source of TNFα in aortic cultures
Aortic outgrowths are composed of a mixed population of cells including macrophages (Fig. 2). To determine the contribution of different cell types to TNFα production, we analyzed TNFα gene expression in isolated macrophages, endothelial cells, and smooth muscle cells. Sufficient number of macrophages were obtained by treating aortic cultures with CSF-1 which markedly stimulates macrophage outgrowth.28 qRT-PCR showed abundant TNFα expression by isolated aortic macrophages. No significant expression of TNFα was observed in endothelial or smooth muscle cells by qRT-PCR or Western analysis. Aortic macrophages showed higher mRNA levels of TNFα than bone marrow macrophages. TNFα expression in aorta-derived macrophages was confirmed by double staining isolated aortic macrophages for TNFα and CD68 (Fig. 2).
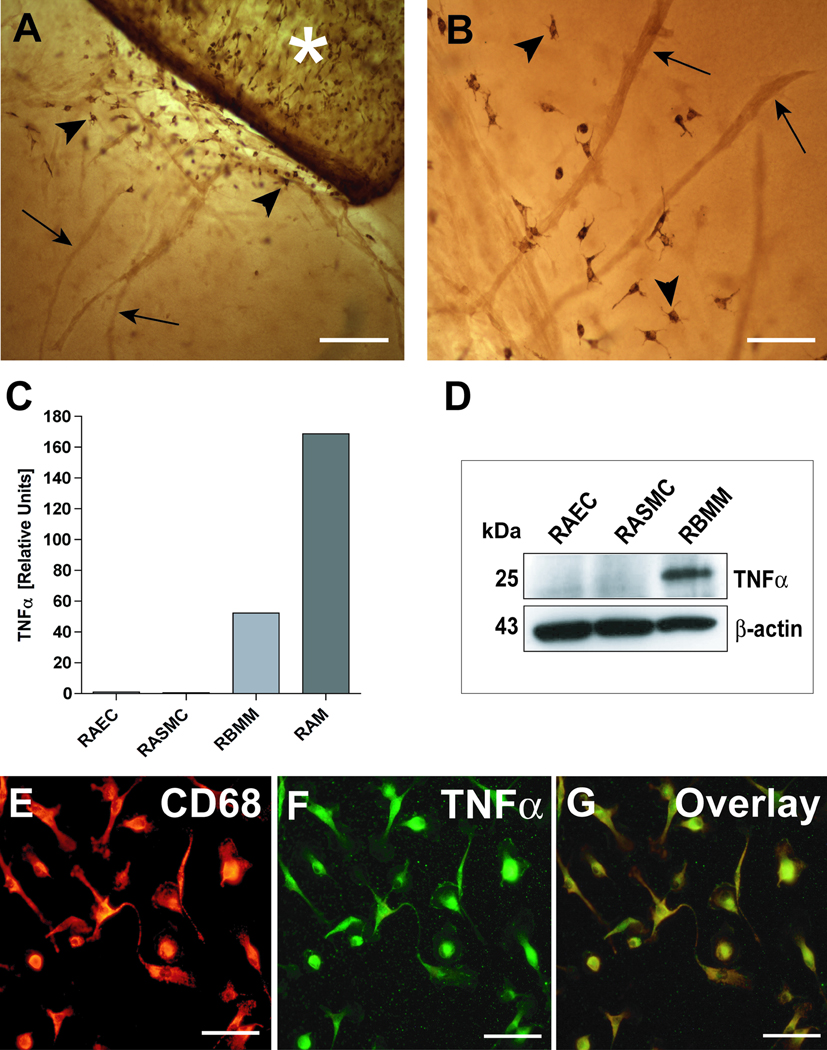
Figure 2. TNFα in aortic cultures is primarily expressed in macrophages.
(A) Immuno-peroxidase staining shows CD68+ macrophages (arrowheads) at the root of an angiogenic outgrowth in a collagen gel culture of rat aorta (asterisk). (B) CD68+ macrophages (arrowheads) are closely associated with newly formed microvessels (arrows). (C) qRT-PCR demonstrates much higher expression of TNFα in isolated rat aortic macrophages (RAM) compared to rat aortic endothelial (RAEC) or smooth muscle cells (RASMC); rat aortic macrophages express more TNFα than rat bone marrow derived macrophages (RBMM). (D) Western analysis confirms lack of TNFα expression in endothelial and smooth muscle cells. (E–G) Confocal images of aortic cultures demonstrate co-expression (G, overlay) of CD68 (E, red) and TNFα (F, green) in outgrowing macrophages. Magnification bars = 200 µm (A), 100 µm (B); 50 µm (E,F,G).
Disruption of the TNFα gene causes reduced angiogenesis and macrophage outgrowth
To define the role of endogenous TNFα in the angiogenic response to injury, aortic rings from TNFα knockout mice were compared with normal aortic rings for their capacity to sprout in collagen gel culture. Disruption of the TNFα gene caused a marked reduction in angiogenic sprouting. Impairment of angiogenesis in the absence of TNFα correlated with a significant downregulation in VEGF mRNA expression (data not shown) and reduction in VEGF protein levels (Fig. 3). The angiogenic response of TNFα-deficient aortic rings was restored to normal values by adding exogenous TNFα to the culture medium or by co-culturing the TNFα-deficient rings with TNFα producing normal aortic macrophages (Fig. 3). These observations indicate that TNFα production is required for optimal angiogenesis in response to injury of the aortic wall.
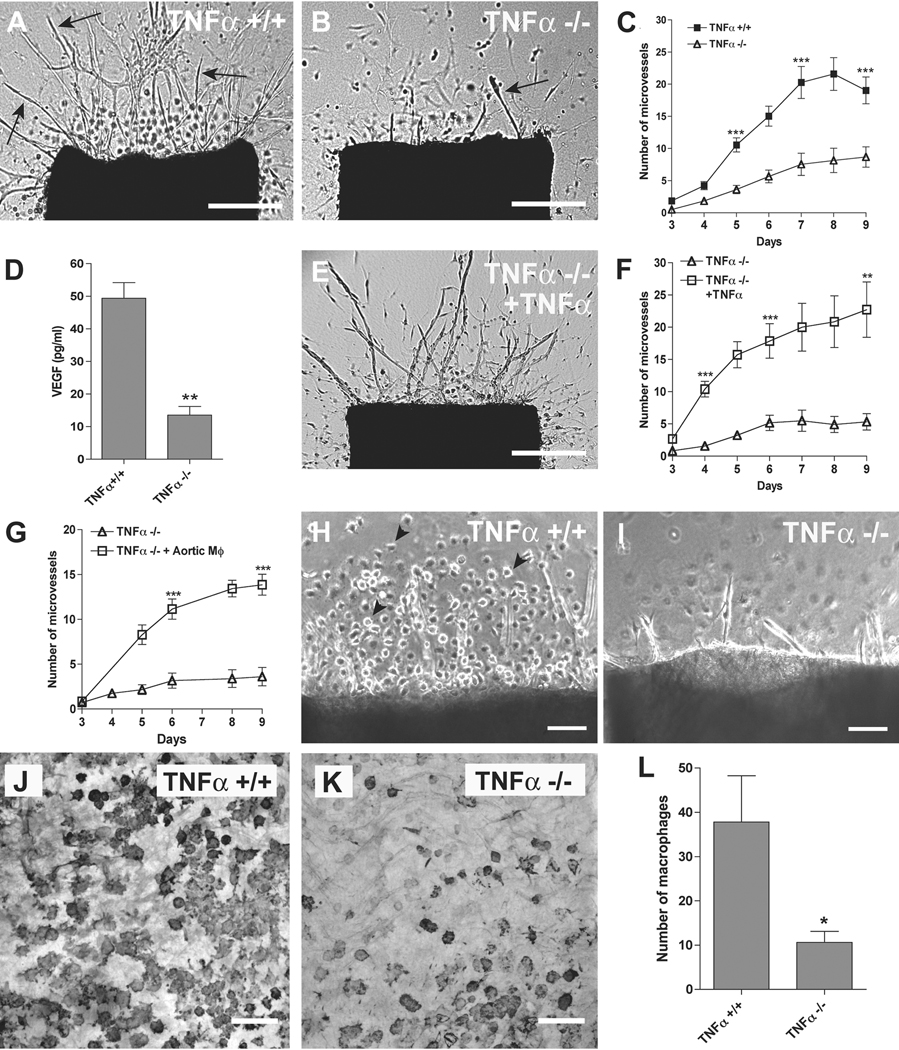
Figure 3. Genetic disruption of TNFα impairs angiogenesis and macrophage outgrowth.
(A) Collagen gel culture of normal aortic ring contains many newly formed microvessels (arrows). (B) Aortic ring from TNFα knockout mouse shows rare microvessels (arrow) due to marked impairment of angiogenic sprouting. (C) Quantitative analysis demonstrates marked reduction of angiogenesis in cultures of TNFα-deficient aortic rings compared to control (N = 52 aortic rings from 7 animals per group). (D) ELISA of conditioned medium shows that VEGF levels in cultures of TNFα-deficient aortic rings are reduced to 20% of control values (N = 3). (E). TNFα-deficient aortic ring treated with exogenous TNFα produces an angiogenic response (arrows) comparable to control. (F, G). Quantitative analysis of aortic angiogenesis shows that exogenous TNFα (F, N=15) or TNFα producing normal aortic macrophages (G, N=8) restore the angiogenic response of TNFα-deficient aortic rings to normal values. (H) Microvessels sprouted from normal mouse aortic rings are surrounded by clusters of single cells with features (rounded morphology, granular cytoplasm) characteristic of macrophages (arrowheads). (I) The angiogenic outgrowth in TNFα-deficient aortic rings shows no evidence of cells with macrophage features. (J) Immunoperoxidase staining demonstrates numerous F4/80+ macrophages in the adventitia of a normal mouse aortic ring in collagen gel culture. (K) The adventitia of TNFα-deficient aortic ring is depleted of F4/80+ macrophages. (L) Quantitative evaluation of F4/80-stained cultures demonstrates marked reduction in macrophages in TNFα-deficient aortic rings compared to normal control (N = 10). Magnification bars = 500 µm (A, B, E), 100 µm (H, I, J, K). * p<0.05; ** p<0.01; *** p<0.001.
Close examination of TNFα-deficient aortic ring cultures showed a reduced number of single cells with morphologic features of macrophages compared to control. Immunocytochemical staining for the mouse macrophage marker F4/80 confirmed this observation and demonstrated that aortic macrophages in the absence of endogenous TNFα were reduced to 34% of control (Fig. 3).
To identify the TNFα receptor involved in the angiogenic response, aortic cultures were treated with blocking antibodies against TNFR1 or TNFR2. Angiogenic sprouting was markedly impaired in cultures treated with anti-TNFR1 antibody. This inhibitory effect was associated with a marked reduction in the outgrowth of single cells including macrophages (data not shown). The anti-TNFR2 antibody caused a modest but statistically not significant reduction in angiogenesis (supplemental Fig. I).
TNFα stimulates aortic angiogenesis and macrophage outgrowth
To further characterize the angioregulatory function of TNFα, the effect of this cytokine was tested in collagen gel cultures of rat aorta. TNFα potently stimulated angiogenesis in a dose dependent manner causing a marked increase in vessel number and length. The proangiogenic effect of TNFα was most pronounced at 5 ng/ml. TNFα was less stimulatory at higher doses, exhibiting anti-angiogenic activity at 100 ng/ml (Fig. 4). Proangiogenic doses of TNFα markedly stimulated macrophage outgrowth over time. This effect was accompanied by enhanced collagen lysis around the explants. TNFα treatment caused formation of cellular patches composed of CD68+ macrophages on the bottom of the culture dish, beneath the aortic rings. DNA synthesis studies of cultures incubated with a thymidine analogue showed a 285% increase in macrophage proliferation in response to TNFα treatment compared to control (Fig. 4). The effect of TNFα on macrophage outgrowth switched from stimulatory to inhibitory at higher doses. Maximum inhibition of macrophages was obtained with 100 ng/ml (data not shown). Taken together with the TNFα knockout experiments, studies with exogenous TNFα establish a link between the capacity of this cytokine to regulate macrophage outgrowth and its angioregulatory properties.
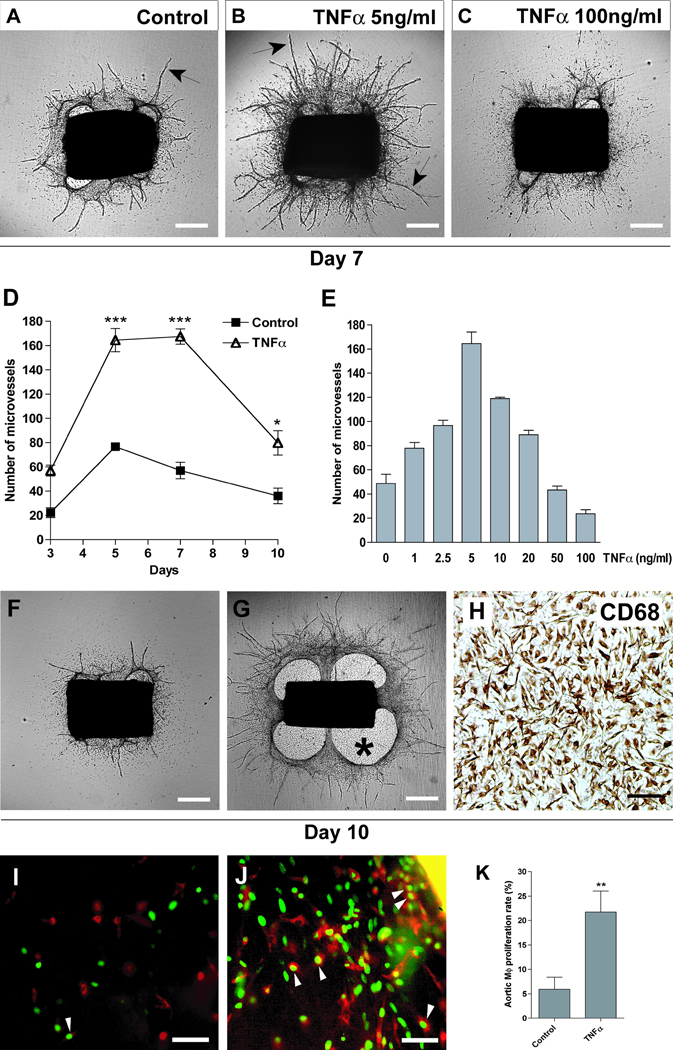
Figure 4. TNFα has dose dependent effects on angiogenesis and macrophage outgrowth.
(A) 7-day-old control aortic ring culture; (B) culture treated with 5 ng/ml TNFα; (C) culture treated with 100 ng/ml TNFα: TNFα has concentration-dependent stimulatory or inhibitory effects on growth of microvessels (arrows). (D) Microvessel counts demonstrate >100% stimulation of angiogenesis over time by 5 ng/ml TNFα (N=4). (E) Treatment of aortic cultures with increasing doses of TNFα identifies 5 ng/ml as the optimal pro-angiogenic dose and demonstrates reduced TNFα efficacy at higher doses, with the highest dose having anti-angiogenic activity (N=4). (F, G) Over time, the proangiogenic dose of TNFα induces the formation of a dense periaortic outgrowth which forms a patch (asterisk) on the bottom of the culture dish (G); this outgrowth is less pronounced in the unstimulated control (F). (H) The TNFα-induced aortic patch is composed of a uniform population of CD68+ macrophages. (I) Studies with the thymidine analogue EDU demonstrate occasional labeled nuclei (green) in CD68+ macrophages (red) of untreated control culture. (J) Macrophages exhibit a higher rate of proliferation in TNFα-treated cultures. (K) Bar graph shows quantitative analysis of macrophage (MΦ) proliferation in CD68 stained control and TNFα-treated aortic cultures (N=10). Magnification bars = 500 µm (A, B, C, F, G), 100 µm (H, I, J). * p<0.05, ** p<0.01, *** p<0.001.
TNFα requires VEGF for optimal stimulation of angiogenesis
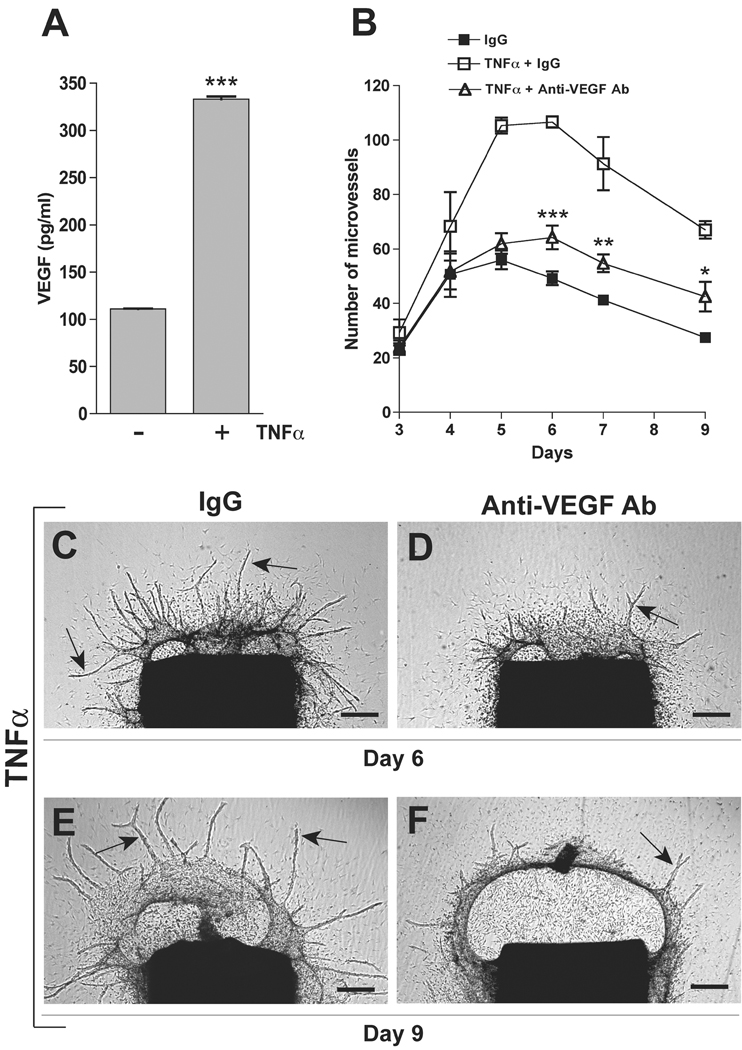
To evaluate the effect of TNFα on the expression of VEGF, conditioned media of aortic cultures were analyzed by VEGF ELISA. VEGF levels at day 3 increased from 100 pg/ml in the untreated control to 320 pg/ml in TNFα-treated cultures (Fig. 5). VEGF concentration remained elevated (220 pg/ml) at day 6 following TNFα treatment while decreasing to 30 pg/ml in control cultures. To define the role of VEGF in TNFα-induced angiogenesis, TNFα-treated aortic cultures were incubated with an anti-VEGF blocking antibody. Inhibition of VEGF almost completely abrogated the stimulatory effect of TNFα on angiogenesis, indicating that VEGF is an important mediator of TNFα-induced angiogenesis. The anti-VEGF antibody had no significant effect on macrophage outgrowth or collagen lysis (Fig. 5).
Figure 5. The proangiogenic effect of TNFα is significantly inhibited by VEGF blockade.
(A) ELISA of aortic culture conditioned medium shows increased production of VEGF in TNFα-treated cultures compared to control (N=3). (B) Antibody-mediated inhibition of VEGF significantly impairs the angiogenic response of aortic rings to TNFα (N=4). (C–F) Micrographs show aortic cultures stimulated with TNFα in the presence of nonimmune IgG (C, E) or anti-VEGF blocking antibody (D, F) and imaged at day 6 (C, D) and 9 (E, F). Note: the anti-VEGF antibody significantly impairs TNFα-stimulated angiogenesis without affecting TNFα-induced macrophage outgrowth and collagen lysis. Representative microvessels in C–F are highlighted by arrows. Magnification bar = 500 µm (C, D, E, F). * p<0.05; ** p<0.01; *** p<0.001.
Macrophages are mediators of TNFα-induced VEGF production and angiogenesis
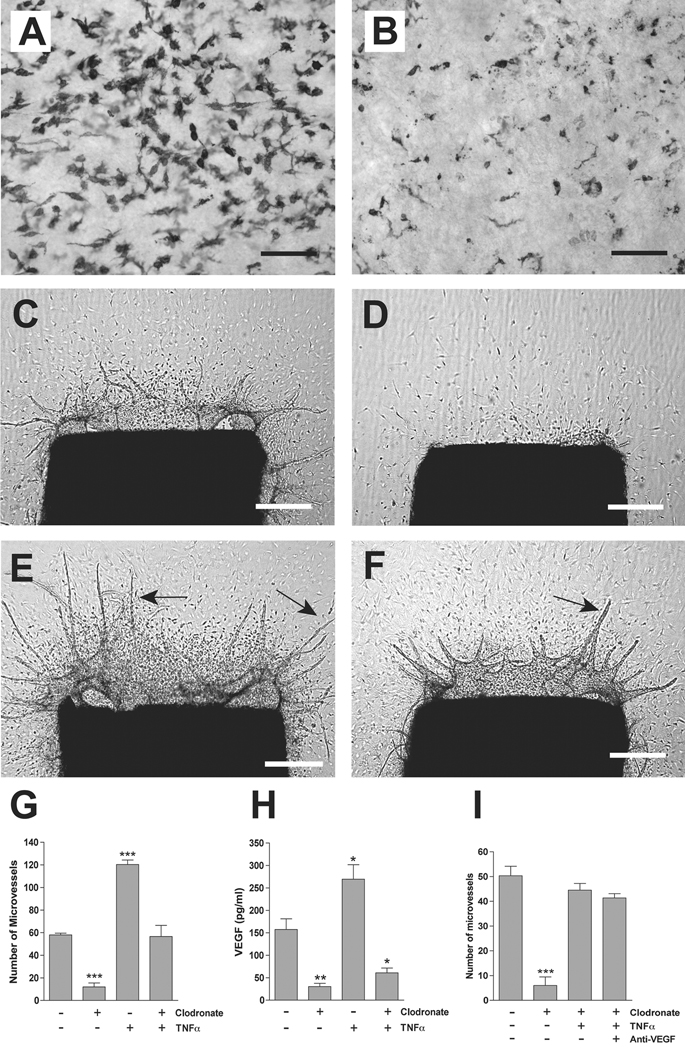
To investigate the role of macrophages in TNFα-induced angiogenesis, aortic rings were depleted of resident adventitial macrophages by overnight treatment with liposomal clodronate prior to collagen embedding. Macrophage ablation markedly impaired the spontaneous angiogenic response of the aortic rings. Addition of exogenous TNFα restored angiogenesis in macrophage-depleted cultures to control values. In the absence of aortic macrophages, however, the TNFα effect was blunted and insufficient to further potentiate the angiogenic response. The reduced efficacy of TNFα in macrophage-depleted cultures correlated with markedly reduced VEGF levels (Fig. 6). This finding suggested that TNFα maintains a limited capacity to stimulate angiogenesis in the absence of macrophages, independently of VEGF production. To evaluate this hypothesis, macrophage-depleted aortic cultures treated with TNFα were supplemented with anti-VEGF blocking antibody or nonimmune IgG control. Inhibition of VEGF had no effect on the residual proangiogenic activity of TNFα in macrophage-depleted cultures. The efficacy of the anti-VEGF antibody was confirmed in VEGF-treated control cultures in which the stimulatory effect of recombinant VEGF was completely abolished by the blocking antibody (data not shown). These results indicate that aortic macrophages, which are the primary source of TNFα, also function as one of the targets and mediators of the TNFα proangiogenic activity during the early stages of the angiogenic response. They also demonstrate the existence of a VEGF-independent pathway of angiogenic stimulation by TNFα which becomes manifest following ablation of resident aortic macrophages.
Figure 6. Macrophage ablation impairs TNFα-induced VEGF production and angiogenesis.
(A, B) Whole mount preparations of rat aorta immunostained for CD163 demonstrate depletion of macrophages in clodronate-treated explants (B) compared to PBS control (A); same results are obtained with other macrophages markers (data not shown). (C–F) Macrophage-depleted aortic rings fail to generate an angiogenic response (D) compared to control rings (C), and produce a reduced number of microvessels when treated with TNFα (F) compared to TNFα-treated rings containing macrophages (E). (G, H) Bar graphs show differences in angiogenesis (G, day 6–7, N=4) and VEGF production (H, N=3) between macrophage-depleted rings and control rings treated with TNFα or left untreated. (I) The residual proangiogenic effect of TNFα in clodronate treated cultures is not affected by antibody-mediated inhibition of VEGF. Magnification bars = 100 µm (A, B), 500 µm (C, D, E, F). * p<0.05; ** p<0.01; *** p<0.001.
Discussion
Angiogenesis in response to tissue injury is a complex process regulated by a cascade of inflammatory stimuli that enable preexisting blood vessels to switch from proliferative quiescence to an angiogenic phenotype.1,43 Although many studies have investigated the role of angiogenic factors, inflammatory cytokines and chemokines in this process, the molecular sequence of events that orchestrates the initial stages of the angiogenic cascade is incompletely understood and not fully characterized.
The angiogenic process can be accurately replicated ex vivo by culturing rat or mouse vessel explants in collagen gels under serum-free conditions.12,44 Angiogenesis in this system is triggered by injury and mediated by adventitial macrophages which are essential for optimal VEGF production and angiogenic sprouting.16 The angiogenic response of the aortic explants is a self-limited process that begins 2–3 days after injury, lasts 5–6 days, and is followed by vascular regression during the second week of culture. We used this model to define the sequence of molecular events responsible for the initial sprouting of neovessels from the injured aortic wall. Aortic angiogenesis is preceded by an upregulated expression of many inflammatory cytokines and chemokines, which work in concert with VEGF to induce sprouting of new vessels.16,36 Among these is TNFα, a macrophage-derived cytokine, which has been implicated in the regulation of reactive and pathologic angiogenesis.45 Macrophages in aortic cultures act as injury sensors and transducers of the angiogenic response, but the molecular mechanisms by which these cells translate injury signals into VEGF production and angiogenesis are poorly understood. Since TNFα has the capacity to induce VEGF in different vascular cell types,22–24 we hypothesized that macrophages used this cytokine as an early regulator of the angiogenic response of the wounded aortic wall.
Our study demonstrates the following points. (A). Aortic injury induces overexpression of both TNFα and VEGF. (B). TNFα is upregulated within minutes after injury. (C). The increase in TNFα is followed by overexpression of VEGF over the following 24 hr. (D). The transcriptome of injured aortic rings contains a TNFα signature of gene expression. (E). TNFα in aortic cultures is primarily produced by resident macrophages. (F). Disruption of the TNFα gene causes reduced VEGF production, angiogenesis, and macrophage outgrowth. (G) Angiogenesis is markedly impaired by antibody-mediated inhibition of TNFR1. (H). Exogenous TNFα promotes both angiogenesis and macrophage proliferation. (I). The angiogenic response to TNFα is significantly inhibited by neutralizing VEGF with a blocking antibody. (J). Pharmacologic ablation of macrophages markedly inhibits VEGF production and significantly impairs the angiogenic response to exogenous TNFα. (K). Exogenous TNFα retains a limited capacity to stimulate angiogenesis through VEGF independent mechanisms in the absence of macrophages.
Taken together these findings demonstrate that VEGF production in a wounded vasculature is the result of upregulated expression of TNFα which acts as an immediate proangiogenic response gene in macrophages activated by the injury process. We previously reported that the angiogenic response of cultured aortic rings is associated with an increased production of VEGF which peaks during the first 24 hours after injury.14 VEGF plays a central role in the angiogenic response of the aortic wall since endothelial sprouting can be markedly impaired by treating aortic cultures with anti-VEGF neutralizing antibodies or VEGF signal transduction inhibitors.14,15 In the present study we now identify TNFα as an upstream inducer of VEGF expression in the cascade of angiogenic gene activation triggered by the aortic injury. TNFα is upregulated within minutes after wounding of the aortic wall, and disruption of the TNFα gene causes marked reduction in VEGF mRNA expression, VEGF protein levels, and angiogenesis. Our additional finding that aortic angiogenesis can be significantly inhibited by blocking TNFα signaling with an anti-TNFR1 neutralizing antibody supports this conclusion and is in keeping with an earlier study by others in which genetic disruption of TNFR1 was shown to cause impaired VEGF production and neovascularization in a subcutaneous vivo model of inflammatory angiogenesis.46
Our results confirm previous observations that TNFα is rapidly overexpressed in wounded tissues 47–49 and has the capacity to induce VEGF expression in different cell types.22–24 To our knowledge, however, no studies have defined the relationship between the rapid rise in TNFα expression and the induction of VEGF in a model in which the mRNA and protein levels of these molecules can be directly correlated with the angiogenic response. We were able to fill this gap by studying molecular events occurring prior to angiogenic sprouting in the isolated aortic wall. For this particular application, the ex vivo aortic ring model offered considerable advantages over in vitro and in vivo models of angiogenesis. In vitro models of endothelial wound healing are limited by the lack of nonendothelial cell types such as macrophages which are involved in the angiogenic response to injury. Angiogenesis in aortic ring cultures is instead the result of paracrine interactions between endothelial and nonendothelial cells types.13 Compared with the more complex in vivo models, aortic ring cultures can be easily monitored over time and pharmacologically modulated under chemically defined culture conditions.
Microarray analysis revealed a distinct TNFα signature of gene expression in aortic rings 24 hours after injury. Upregulated genes included macrophage stimulatory chemokines such as CCL2, CCL7, and CCL12 and macrophage-derived chemokines that attract polymorphonuclear cells to sites of inflammation (CCL3, CCL20, CXCL1).50 The increase in macrophage outgrowth was associated with enhanced proteolysis resulting in periaortic rarefaction of the collagen gel. Exogenous TNFα induced expression of the interstitial collagenase MMP13 which most likely contributed to the TNFα-stimulated angiogenic sprouting and collagen lysis.51 TNFα also induced MMP3 and MMP9 which together with MMP13 were also upregulated by injury in the absence of exogenous TNFα.
The hypothesis that the stimulatory effect of TNFα on angiogenesis is mediated by leukocytes was originally proposed based on the observation that TNFα inhibits the proliferation of isolated endothelial cell in vitro,52,53 but stimulates inflammatory angiogenesis in vivo.18,54–56 Ablation of macrophages has been shown to reduce angiogenesis during wound healing,57 but there are no in vivo studies on the effect of macrophage depletion on the proangiogenic activity of TNFα. To evaluate the role of macrophages in TNFα-induced angiogenesis, we pretreated aortic rings with liposomal clodronate which selectively kills phagocytic cells.16 Macrophage ablation significantly impaired the proangiogenic effect of TNFα. The reduced proangiogenic activity of TNFα was most likely caused by the marked reduction in VEGF production in these cultures. This result establishes the macrophage as a key mediator of the TNFα proangiogenic activity.
TNFα exhibited a limited capacity to induce vessel sprouting in macrophage-depleted cultures. This residual proangiogenic activity was not due to VEGF because VEGF levels were markedly decreased and antibody-mediated inhibition of VEGF had no effect in the absence of macrophages. This VEGF-independent proangiogenic activity may be due a direct stimulatory effect of TNFα on endothelial cell migration 56,58 and/or the ability of this cytokine to induce expression in mural cells of other angiogenic factors. Our microarray studies showed that TNFα induces expression of HGF, Angpt1, Ephn1, BMP-2 and the angiogenic chemokines CCL2 and CXCL1, which could all function as stimulators of angiogenesis in the absence of VEGF.37,38,40–42,59
A previous study with a subcutaneous in vivo model showed that low doses of TNFα stimulated angiogenesis whereas high doses were inhibitory.19 To evaluate if this bimodal effect of TNFα angiogenesis was related to macrophages, we tested increasing doses of TNFα in the aortic ring model. Our studies confirmed this observation, and showed stimulation of angiogenesis with 5–10 ng/ml of TNFα and marked inhibition with 100 ng/ml. Whereas the proangiogenic low doses of TNFα concurrently stimulated angiogenesis and macrophage outgrowth, the anti-angiogenic high dose were potently inhibitory for both. The inhibitory effect of TNFα was limited to macrophages and microvessels, as mesenchymal cell outgrowths were not significantly affected.
In summary our study identifies TNFα as an immediate response gene in the proangiogenic cascade of gene activation triggered by injury of the aortic wall. Our results indicate that TNFα is required for optimal VEGF production and angiogenesis in aortic cultures. This TNFα/VEGF-mediated pathway of angiogenic induction is critically dependent on macrophages which produce TNFα and mediate its proangiogenic effect. The residual angiogenic activity observed in macrophage-deficient aortic cultures treated with exogenous TNFα implicates the existence of a VEGF-independent alternate pathway of TNFα-induced angiogenesis (supplemental Fig. II). More studies are, however, needed to further characterize the TNFα mediated angiogenic cascade, and to define the mechanisms by which TNFα blocking agents currently used in the clinic affect TNFα-induced angiogenic pathways.60 A greater knowledge of how TNFα regulates macrophage-induced angiogenesis may provide new insights into the molecular pathways responsible for the progression of atherosclerosis, cancer, and other pathologic conditions associated with vascular injury and inflammatory angiogenesis.
Supplementary Material
Acknowledgments
Work presented in the paper was supported by National Heart, Lung, and Blood Institute Grant HL52585 and a Merit Review Grant from US Department of Veterans Affairs, Office of Research and Development, Biomedical Laboratory Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to disclose.
References
- 1.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 2.Fischer C, Schneider M, Carmeliet P. Principles and therapeutic implications of angiogenesis, vasculogenesis and arteriogenesis. Handb Exp Pharmacol. 2006:157–212. doi: 10.1007/3-540-36028-x_6. [DOI] [PubMed] [Google Scholar]
- 3.Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789–791. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 4.Senger DR. Vascular endothelial growth factor: much more than an angiogenesis factor. Mol Biol Cell. 2010;21:377–379. doi: 10.1091/mbc.E09-07-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw. 2009;20:158–163. doi: 10.1684/ecn.2009.0170. [DOI] [PubMed] [Google Scholar]
- 6.Kamba T, McDonald DM. Mechanisms of adverse effects of anti-VEGF therapy for cancer. Br J Cancer. 2007;96:1788–1795. doi: 10.1038/sj.bjc.6603813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fong GH. Regulation of angiogenesis by oxygen sensing mechanisms. J Mol Med. 2009;87:549–560. doi: 10.1007/s00109-009-0458-z. [DOI] [PubMed] [Google Scholar]
- 8.Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van de WL. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med. 1992;176:1375–1379. doi: 10.1084/jem.176.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153:347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross JS, Stagliano NE, Donovan MJ, Breitbart RE, Ginsburg GS. Atherosclerosis and cancer: common molecular pathways of disease development and progression. Ann N Y Acad Sci. 2001;947:271–292. [PubMed] [Google Scholar]
- 11.Albert DM, Neekhra A, Wang S, Darjatmoko SR, Sorenson CM, Dubielzig RR, Sheibani N. Development of choroidal neovascularization in rats with advanced intense cyclic light-induced retinal degeneration. Arch Ophthalmol. 2010;128:212–222. doi: 10.1001/archophthalmol.2009.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nicosia RF, Ottinetti A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Lab Invest. 1990;63:115–122. [PubMed] [Google Scholar]
- 13.Nicosia RF. The aortic ring model of angiogenesis: a quarter century of search and discovery. J Cell Mol Med. 2009;13:4113–4136. doi: 10.1111/j.1582-4934.2009.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicosia RF, Lin YJ, Hazelton D, Qian X. Endogenous regulation of angiogenesis in the rat aorta model. Role of vascular endothelial growth factor. Am J Pathol. 1997;151:1379–1386. [PMC free article] [PubMed] [Google Scholar]
- 15.Ruggeri B, Singh J, Gingrich D, Angeles T, Albom M, Yang S, Chang H, Robinson C, Hunter K, Dobrzanski P, Jones-Bolin S, Pritchard S, Aimone L, Klein-Szanto A, Herbert JM, Bono F, Schaeffer P, Casellas P, Bourie B, Pili R, Isaacs J, Ator M, Hudkins R, Vaught J, Mallamo J, Dionne C. CEP-7055: a novel, orally active pan inhibitor of vascular endothelial growth factor receptor tyrosine kinases with potent antiangiogenic activity and antitumor efficacy in preclinical models. Cancer Res. 2003;63:5978–5991. [PubMed] [Google Scholar]
- 16.Gelati M, Aplin AC, Fogel E, Smith KD, Nicosia RF. The angiogenic response of the aorta to injury and inflammatory cytokines requires macrophages. J Immunol. 2008;181:5711–5719. doi: 10.4049/jimmunol.181.8.5711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DiPietro LA. Wound healing: the role of the macrophage and other immune cells. Shock. 1995;4:233–240. [PubMed] [Google Scholar]
- 18.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 19.Fajardo LF, Kwan HH, Kowalski J, Prionas SD, Allison AC. Dual role of tumor necrosis factor-alpha in angiogenesis. Am J Pathol. 1992;140:539–544. [PMC free article] [PubMed] [Google Scholar]
- 20.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–733. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 21.MacEwan DJ. TNF ligands and receptors--a matter of life and death. Br J Pharmacol. 2002;135:855–875. doi: 10.1038/sj.bjp.0704549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez-Ruiz M, Ros J, Morales-Ruiz M, Navasa M, Colmenero J, Ruiz-del-Arbol L, Cejudo P, Claria J, Rivera F, Arroyo V, Rodes J, Jimenez W. Vascular endothelial growth factor production in peritoneal macrophages of cirrhotic patients: regulation by cytokines and bacterial lipopolysaccharide. Hepatology. 1999;29:1057–1063. doi: 10.1002/hep.510290416. [DOI] [PubMed] [Google Scholar]
- 23.Chu SC, Tsai CH, Yang SF, Huang FM, Su YF, Hsieh YS, Chang YC. Induction of vascular endothelial growth factor gene expression by proinflammatory cytokines in human pulp and gingival fibroblasts. J Endod. 2004;30:704–707. doi: 10.1097/01.don.0000129962.65752.c6. [DOI] [PubMed] [Google Scholar]
- 24.Alagappan VK, McKay S, Widyastuti A, Garrelds IM, Bogers AJ, Hoogsteden HC, Hirst SJ, Sharma HS. Proinflammatory cytokines upregulate mRNA expression and secretion of vascular endothelial growth factor in cultured human airway smooth muscle cells. Cell Biochem Biophys. 2005;43:119–129. doi: 10.1385/CBB:43:1:119. [DOI] [PubMed] [Google Scholar]
- 25.Koolwijk P, van Erck MG, de Vree WJ, Vermeer MA, Weich HA, Hanemaaijer R, van Hinsbergh VW. Cooperative effect of TNFalpha, bFGF, and VEGF on the formation of tubular structures of human microvascular endothelial cells in a fibrin matrix. Role of urokinase activity. J Cell Biol. 1996;132:1177–1188. doi: 10.1083/jcb.132.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aplin AC, Fogel E, Zorzi P, Nicosia RF. The aortic ring model of angiogenesis. Methods Enzymol. 2008;443:119–136. doi: 10.1016/S0076-6879(08)02007-7. [DOI] [PubMed] [Google Scholar]
- 28.Zorzi P, Aplin AC, Smith KD, Nicosia RF. Technical Advance: The rat aorta contains resident mononuclear phagocytes with proliferative capacity and proangiogenic properties. J Leukoc Biol. 2010 doi: 10.1189/jlb.0310178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sheehan KC, Pinckard JK, Arthur CD, Dehner LP, Goeddel DV, Schreiber RD. Monoclonal antibodies specific for murine p55 and p75 tumor necrosis factor receptors: identification of a novel in vivo role for p75. J Exp Med. 1995;181:607–617. doi: 10.1084/jem.181.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruuls SR, Hoek RM, Ngo VN, McNeil T, Lucian LA, Janatpour MJ, Korner H, Scheerens H, Hessel EM, Cyster JG, McEvoy LM, Sedgwick JD. Membrane-bound TNF supports secondary lymphoid organ structure but is subservient to secreted TNF in driving autoimmune inflammation. Immunity. 2001;15:533–543. doi: 10.1016/s1074-7613(01)00215-1. [DOI] [PubMed] [Google Scholar]
- 31.Bruggeman LA, Drawz PE, Kahoud N, Lin K, Barisoni L, Nelson PJ. TNFR2 interposes the proliferative and NF-kappaB-mediated inflammatory response by podocytes to TNF-alpha. Lab Invest. 2011 doi: 10.1038/labinvest.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicosia RF, Villaschi S, Smith M. Isolation and characterization of vasoformative endothelial cells from the rat aorta. In Vitro Cell Dev Biol Anim. 1994;30A:394–399. doi: 10.1007/BF02634360. [DOI] [PubMed] [Google Scholar]
- 33.van Rooijen N, Kors N, ter Hart H, Claassen E. In vitro and in vivo elimination of macrophage tumor cells using liposome-encapsulated dichloromethylene diphosphonate. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54:241–245. doi: 10.1007/BF02899217. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Zhu WH, Nicosia RF. The thin prep rat aortic ring assay: a modified method for the characterization of angiogenesis in whole mounts. Angiogenesis. 2002;5:81–86. doi: 10.1023/a:1021509004829. [DOI] [PubMed] [Google Scholar]
- 36.Aplin AC, Gelati M, Fogel E, Carnevale E, Nicosia RF. Angiopoietin-1 and vascular endothelial growth factor induce expression of inflammatory cytokines before angiogenesis. Physiol Genomics. 2006;27:20–28. doi: 10.1152/physiolgenomics.00048.2006. [DOI] [PubMed] [Google Scholar]
- 37.Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, Oppenheim JJ, Murphy WJ. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- 38.Bechara C, Chai H, Lin PH, Yao Q, Chen C. Growth related oncogene-alpha (GRO-alpha): roles in atherosclerosis, angiogenesis and other inflammatory conditions. Med Sci Monit. 2007;13:RA87–RA90. [PubMed] [Google Scholar]
- 39.Huang SP, Wu MS, Shun CT, Wang HP, Lin MT, Kuo ML, Lin JT. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11:517–527. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- 40.Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Angiopoietin-1 induces sprouting angiogenesis in vitro. Curr Biol. 1998;8:529–532. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- 41.Huynh-Do U, Vindis C, Liu H, Cerretti DP, McGrew JT, Enriquez M, Chen J, Daniel TO. Ephrin-B1 transduces signals to activate integrin-mediated migration, attachment and angiogenesis. J Cell Sci. 2002;115:3073–3081. doi: 10.1242/jcs.115.15.3073. [DOI] [PubMed] [Google Scholar]
- 42.Langenfeld EM, Langenfeld J. Bone morphogenetic protein-2 stimulates angiogenesis in developing tumors. Mol Cancer Res. 2004;2:141–149. [PubMed] [Google Scholar]
- 43.Augustin HG. Tubes, branches, and pillars: the many ways of forming a new vasculature. Circ Res. 2001;89:645–647. [PubMed] [Google Scholar]
- 44.Zhu WH, Iurlaro M, MacIntyre A, Fogel E, Nicosia RF. The mouse aorta model: influence of genetic background and aging on bFGF- and VEGF-induced angiogenic sprouting. Angiogenesis. 2003;6:193–199. doi: 10.1023/B:AGEN.0000021397.18713.9c. [DOI] [PubMed] [Google Scholar]
- 45.Sethi G, Sung B, Aggarwal BB. TNF: a master switch for inflammation to cancer. Front Biosci. 2008;13:5094–5107. doi: 10.2741/3066. [DOI] [PubMed] [Google Scholar]
- 46.Barcelos LS, Talvani A, Teixeira AS, Vieira LQ, Cassali GD, Andrade SP, Teixeira MM. Impaired inflammatory angiogenesis, but not leukocyte influx, in mice lacking TNFR1. J Leukoc Biol. 2005;78:352–358. doi: 10.1189/jlb.1104682. [DOI] [PubMed] [Google Scholar]
- 47.Welham NV, Lim X, Tateya I, Bless DM. Inflammatory factor profiles one hour following vocal fold injury. Ann Otol Rhinol Laryngol. 2008;117:145–152. doi: 10.1177/000348940811700213. [DOI] [PubMed] [Google Scholar]
- 48.Grellner W. Time-dependent immunohistochemical detection of proinflammatory cytokines (IL-1beta, IL-6, TNF-alpha) in human skin wounds. Forensic Sci Int. 2002;130:90–96. doi: 10.1016/s0379-0738(02)00342-0. [DOI] [PubMed] [Google Scholar]
- 49.Herskowitz A, Choi S, Ansari AA, Wesselingh S. Cytokine mRNA expression in postischemic/reperfused myocardium. Am J Pathol. 1995;146:419–428. [PMC free article] [PubMed] [Google Scholar]
- 50.Keeley EC, Mehrad B, Strieter RM. Chemokines as mediators of neovascularization. Arterioscler Thromb Vasc Biol. 2008;28:1928–1936. doi: 10.1161/ATVBAHA.108.162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zijlstra A, Aimes RT, Zhu D, Regazzoni K, Kupriyanova T, Seandel M, Deryugina EI, Quigley JP. Collagenolysis-dependent angiogenesis mediated by matrix metalloproteinase-13 (collagenase-3) J Biol Chem. 2004;279:27633–27645. doi: 10.1074/jbc.M313617200. [DOI] [PubMed] [Google Scholar]
- 52.Schweigerer L, Malerstein B, Gospodarowicz D. Tumor necrosis factor inhibits the proliferation of cultured capillary endothelial cells. Biochem Biophys Res Commun. 1987;143:997–1004. doi: 10.1016/0006-291x(87)90350-0. [DOI] [PubMed] [Google Scholar]
- 53.Frater-Schroder M, Risau W, Hallmann R, Gautschi P, Bohlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987;84:5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vanderslice P, Munsch CL, Rachal E, Erichsen D, Sughrue KM, Truong AN, Wygant JN, McIntyre BW, Eskin SG, Tilton RG, Polverini PJ. Angiogenesis induced by tumor necrosis factor-agr; is mediated by alpha4 integrins. Angiogenesis. 1998;2:265–275. doi: 10.1023/a:1009296700991. [DOI] [PubMed] [Google Scholar]
- 55.Roesel JF, Nanney LB. Assessment of differential cytokine effects on angiogenesis using an in vivo model of cutaneous wound repair. J Surg Res. 1995;58:449–459. doi: 10.1006/jsre.1995.1071. [DOI] [PubMed] [Google Scholar]
- 56.Montrucchio G, Lupia E, de Martino A, Battaglia E, Arese M, Tizzani A, Bussolino F, Camussi G. Nitric oxide mediates angiogenesis induced in vivo by platelet-activating factor and tumor necrosis factor-alpha. Am J Pathol. 1997;151:557–563. [PMC free article] [PubMed] [Google Scholar]
- 57.Leibovich SJ, Ross R. The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol. 1975;78:71–100. [PMC free article] [PubMed] [Google Scholar]
- 58.Gao B, Saba TM, Tsan MF. Role of alpha(v)beta(3)-integrin in TNF-alpha-induced endothelial cell migration. Am J Physiol Cell Physiol. 2002;283:C1196–C1205. doi: 10.1152/ajpcell.00064.2002. [DOI] [PubMed] [Google Scholar]
- 59.Rosen EM, Grant DS, Kleinman HK, Goldberg ID, Bhargava MM, Nickoloff BJ, Kinsella JL, Polverini P. Scatter factor (hepatocyte growth factor) is a potent angiogenesis factor in vivo. Symp Soc Exp Biol. 1993;47:227–234. [PubMed] [Google Scholar]
- 60.Taylor PC. Pharmacology of TNF blockade in rheumatoid arthritis and other chronic inflammatory diseases. Curr Opin Pharmacol. 2010;10:308–315. doi: 10.1016/j.coph.2010.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.