Abstract
Valproic acid (VPA) is the most highly prescribed epilepsy treatment worldwide and is also used to prevent bipolar disorder and migraine. Surprisingly, very little is known about its mechanisms of cellular uptake. Here, we employ a range of cellular, molecular and genetic approaches to characterize VPA uptake using a simple biomedical model, Dictyostelium discoideum. We show that VPA is taken up against an electrochemical gradient in a dose-dependent manner. Transport is protein-mediated, dependent on pH and the proton gradient and shows strong substrate structure specificity. Using a genetic screen, we identified a protein homologous to a mammalian solute carrier family 4 (SLC4) bicarbonate transporter that we show is involved in VPA uptake. Pharmacological and genetic ablation of this protein reduces the uptake of VPA and partially protects against VPA-dependent developmental effects, and extracellular bicarbonate competes for VPA uptake in Dictyostelium. We further show that this uptake mechanism is likely to be conserved in both zebrafish (Danio rerio) and Xenopus laevis model systems. These results implicate, for the first time, an uptake mechanism for VPA through SLC4-catalysed activity.
Key words: Bicarbonate transporter, Bipolar disorder, Epilepsy, SLC4, Valproic acid
Introduction
Valproic acid (VPA), also called 2-propylpentanoic acid, is a branched short-chain aliphatic fatty acid that was first synthesized in 1882 (Burton, 1882). After being used as an organic solvent for eighty years, its anticonvulsant activity was serendipitously discovered (Meunier et al., 1963), and VPA was first approved as an epilepsy treatment in France in 1967. Since then, it has become the most highly prescribed treatment for generalized and partial epilepsies. VPA is now also used as an effective drug for treatment of bipolar disorder and prevention of migraine attacks (Calabresi et al., 2007; Emrich et al., 1981). Observations that VPA can cause cell growth retardation recently led to investigations of the possibility to use the drug as a treatment for leukaemia and other forms of cancer (Bokelmann and Mahlknecht, 2008). Finally, VPA has also been investigated as a therapy for Alzheimer's disease and latent HIV (Terbach and Williams, 2009).
Therapeutic concentrations of VPA vary considerably between different organs (e.g. brain, kidney) and plasma, suggesting a variable tissue-specific transport mechanism. For example, the concentration required to exert an antiepileptic effect in animals is about 200–500 mg/l in the serum (Chapman et al., 1982) and 150–200 μg/g in the rat brain (Chapman et al., 1982; Loscher et al., 1989). Although highly efficient, VPA efflux transport from the brain at the blood–brain barrier takes place at the same time as less efficient influx (Gibbs et al., 2004); the low permeability of the brain to the drug necessitates a relatively high daily dosage. This aggravates the adverse effects of the drug, including lethargy, weight gain, nausea, alopecia and the most severe side-effects, teratogenicity (Ornoy, 2006) and hepatotoxicity (Bjornsson, 2008). Surprisingly, the mechanism of VPA uptake remains unknown.
Our previous work has employed the simple biomedical model Dictyostelium discoideum to understand cell signalling pathways regulated by VPA. Pathways commonly regulated in the animal brain and Dictyostelium include signalling through Ins(1,4,5)P3 (Berridge et al., 1989; Williams et al., 2002), mitogen-activated protein kinase (MAPK) (Boeckeler et al., 2006) and phospholipase A2 (PLA2) (Chang et al., 2001). Other intracellular targets of VPA include histone deacetylases (Phiel et al., 2001), ion channels (Tian and Alkadhi, 1994), γ-aminobutyric acid (GABA) levels (Mesdjian et al., 1982) and, controversially, glycogen synthase kinase (GSK)-3 (Chen et al., 1999; Ryves et al., 2005). In this paper, we use Dictyostelium to analyse cellular mechanisms of VPA uptake. We show that transport is dependent on pH and the proton gradient and that it occurs not through diffusion but is protein mediated. We identify a potential candidate protein involved in VPA transport, namely the solute carrier 4 bicarbonate transporter, Slc4. We also employ a range of pharmacological and genetic techniques to support a bicarbonate-transporter-dependent uptake mechanism, in addition to showing that bicarbonate competes with VPA for uptake. We further show that bicarbonate transporter inhibitors and bicarbonate transport also reduced the developmental effects of VPA in zebrafish and Xenopus, and VPA uptake in zebrafish, indicating that these results can be translated to higher model systems.
Results
Characterization of VPA uptake
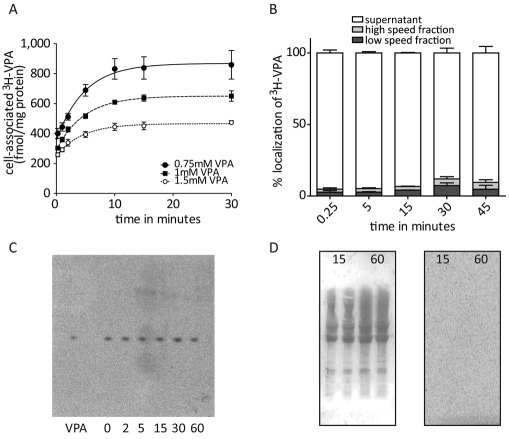
Despite widespread use of VPA as a therapeutic agent, it remains unclear how VPA enters cells, where it localizes within the cells and whether it remains free or is covalently bound to proteins or lipids. To initially investigate these processes, we first examined the dose-dependence of VPA uptake by treating Dictyostelium cells with a constant extracellular concentration of tritiated VPA ([3H]VPA; 6 nM) as a tracer, in the presence of various concentrations of unlabelled VPA (Fig. 1A). VPA uptake showed a very rapid initial diffusion phase, followed by a secondary active phase. Uptake was dose dependent, as increased concentrations of unlabelled VPA gave rise to reduced uptake of the label, and plateaued after 30 minutes. This [3H]VPA tracer-based approach was used in subsequent experiments.
Fig. 1.
Characterization of [3H]VPA uptake in Dictyostelium. (A) Timecourse of VPA uptake (using 6 nM [3H]VPA) in the presence of 0.75 (●), 1 (▪) and 1.5 mM (○) unlabelled VPA (means ± s.e.m.). (B) Subcellular localization of VPA. [3H]VPA content of cellular fractions after 15 seconds, and 5, 15, 30 and 45 minutes (means ± s.e.m.) as determined by differential centrifugation of cell lysates (Williams et al., 1999). (C) Storage phosphoimage of lipids, extracted from [3H]VPA-treated cells following the indicated time (minutes) of treatment, and visualized by lipid extraction and thin-layer chromatography, with [3H]VPA as control (VPA). (D) SDS-PAGE of proteins extracted from cells treated with [3H]VPA for 15 and 60 minutes, showing Ponceau-staining (left-hand side) and a storage phosphoimage (right-hand side).
To calculate the limiting cellular concentration of VPA in Dictyostelium cells, we measured the mean intracellular VPA content in the absence of unlabelled VPA after 30 minutes, and found it to be 502 fmol per mg of protein (±1; s.e.m.). Assuming an average mean cell volume of 565 μm3 per cell and an average cell protein content of 9.3 mg for 108 cells (Soll et al., 1976), this would give an average cell volume of 6.1 μl per mg of protein. This indicates that VPA reaches a steady-state intracellular concentration of ~82 nM.
We also examined the subcellular localisation of VPA by treating Dictyostelium cells with [3H]VPA for various time periods and measured cellular location, as previously described (Williams et al., 1999) (Fig. 1B). Within 15 seconds, the majority of [3H]VPA was found in the supernatant fraction, assumed to contain the cytosolic content, whereas only 2.7% of the VPA was found in the low-speed fraction, nuclei and cell debris, and 2.1% in the high-speed fraction, assumed to contain membrane and organelles; these ratios showed little change over extended periods (up to 30 minutes). These data thus show a rapid cellular uptake and a predominantly cytoplasmic localization of VPA. To determine whether cellular VPA remains free or is covalently bound to lipids or proteins, as has been previously suggested (Brouwer et al., 1993; Siafaka-Kapadai et al., 1998), we separated lipids (Fig. 1C) and proteins (Fig. 1D) from VPA-labelled cells and examined radiolabel incorporation. No VPA was detected as being covalently bound to either lipids or protein fractions over a 60-minute period, suggesting that VPA remains within the cytosol, without substantial lipid or protein incorporation, although it remains possible that trace quantities (below detection limits) are bound.
VPA uptake is dependent on the pH and proton gradient
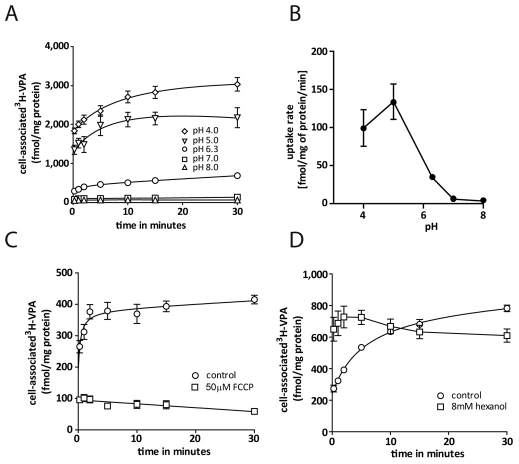
To characterize the basic biochemical parameters of VPA uptake, we employed a range of conditions to assess cellular VPA import. Employing phosphate buffers of pH 4.0–8.0 (Fig. 2A), we showed pH-dependent uptake, given that at an acidic pH of 4.0 the total uptake of VPA was increased by ~sixfold compared with that in control conditions (pH 6.3), whereas a less acidic pH of 5.0 led to a fourfold increase in uptake. By contrast, increasing the buffer pH to 7.0 or 8.0 significantly reduced VPA uptake compared with that in control conditions. It is worth noting here that there is a pH partitioning effect on the initial diffusion phase (the y-axis intersects in Fig. 2A), plus an effect on the secondary active phase. A secondary plot of the rate of VPA uptake in the linear phase, between 15 seconds and 5 minutes, against extracellular pH (Fig. 2B) indicates a nonlinear relationship between pH and uptake rate.
Fig. 2.
Biochemical characterization of VPA uptake. (A) Timecourse of [3H]VPA uptake (means ± s.e.m.) in extracellular buffer with a pH of 4.0 (⋄), 5.0 (▿), 6.3 (○), 7.0 (□) and 8.0 (▵). (B) Secondary plot of uptake rate determined by linear regression of the linear component of uptake against extracellular pH. (C) Uptake in the presence (□) or absence (○) of the proton ionophore FCCP (means ± s.e.m.), added at t=0. (D) Effects of membrane fluidity on VPA uptake. Timecourse of [3H]VPA uptake in the presence (□) or absence (○) of hexanol (means ± s.e.m.).
To analyse further the type of transport involved in VPA uptake, we investigated the effect of the protonophore FCCP on VPA transport (Fig. 2C). This compound permeabilizes the membrane to protons and thus abolishes proton gradients and leads to membrane depolarization. Exposure to 50 μM FCCP resulted in a complete block of VPA uptake, suggesting a proton-gradient-dependent transport mechanism as opposed to simple diffusion. In addition, treatment with 8 mM hexanol has been shown previously to enhance diffusion and block protein-mediated transport in erythrocytes (Deuticke et al., 1982). In Dictyostelium, hexanol enhanced the initial phase of uptake (Fig. 2D) but blocked further movement, supporting the hypothesis that VPA intake is mediated through diffusion in the first 15 seconds, and that uptake is due to protein-mediated transport thereafter. The presence of 10 mM Na+ in the uptake buffer did not change uptake of VPA (data not shown). These combined experiments suggest that VPA uptake occurs through a mechanism that is dependent on the pH and proton gradient, is protein-mediated and is independent of Na+.
Structural specificity of VPA transport
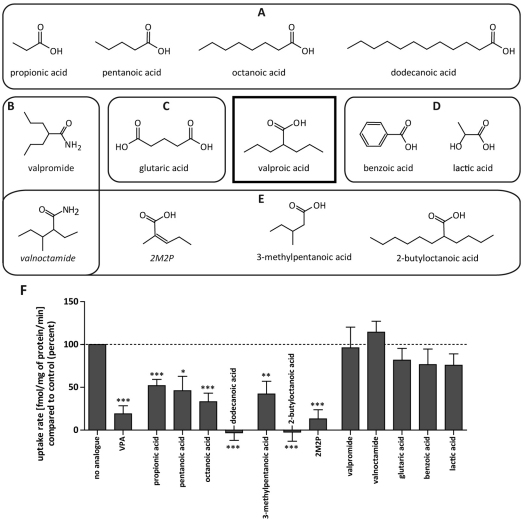
We next investigated the structural specificity of the transport mechanism using competition analysis for VPA uptake with a variety of compounds with structural similarity to VPA (Fig. 3). These experiments employed straight-chain fatty acids of various length (Fig. 3A) and compounds with a different or additional head group (Fig. 3B,C), different branch length and side-chain position (Fig. 3E). In addition, lactic acid and benzoic acid (Fig. 3D) were included owing to previous data indicating competition with VPA uptake through monocarboxylate (MCT) transporters in various cell types (Tamai et al., 1995; Utoguchi and Audus, 2000; Yabuuchi et al., 1998). Uptake of [3H]VPA (6 nM with 0.25 mM unlabelled VPA) was analysed in the presence or absence of 2.75 mM VPA or one of the related compounds. Analysis of the rates of uptake in the linear phase (Fig. 3F), showed that short- to medium-length straight-chain fatty acids strongly competed with uptake (P<0.05), with dodecanoic acid completely abolishing uptake, whereas change of the head group to an amide (valpromide, valnoctamide) or addition of a second carboxy group (glutaric acid) led to a loss of competition with VPA. Compounds with branching in the C-2 position (2-butyloctanoic acid, 2-methyl-2-pentenoic acid) showed enhanced competition with VPA uptake compared with that by the non-branched compounds (octanoic acid, pentanoic acid). Compounds reported to compete with VPA uptake where the proposed mechanism was the MCT transporter (lactic acid, benzoic acid) did not compete with VPA uptake in Dictyostelium.
Fig. 3.
Analogues used in the [3H]VPA competition assay. Straight-chain fatty acids with various lengths (A), alternative head groups (B), different number of functional groups (C) and alternative branching (E) were employed. Two other compounds (D) were included owing to previous competition data. (F) Competition of various analogues with [3H]VPA uptake. Uptake rates of [3H]VPA in the presence of 2.75 mM of analogue compared with that with no analogue (control) are shown (%). ***P<0.001; **P<0.01; *P<0.05 compared with control. 2M2P, 2-methyl-2-pentenoic acid.
Identification of a VPA uptake mechanism
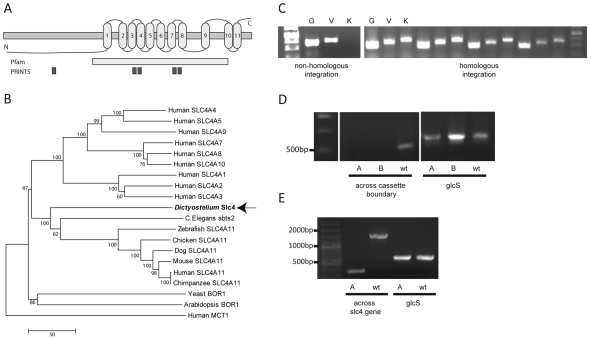
To identify a potential transporter activity responsible for VPA uptake, we screened a Dictyostelium restriction-enzyme-mediated integration library for resistance to VPA, using both growth and development conditions (Williams et al., 1999). VPA is known to block cell growth at a concentration of 2 mM in liquid culture and severely retards development at 1 mM (Boeckeler et al., 2006). Using this approach, 14 mutants in the growth screen and 12 mutants in the developmental screen showed increased resistance to VPA, with one mutant showing partial resistance in both screens. The ablated gene in this mutant, slc4 (DictyBase ID: DDB_G0270422), encodes a protein of 768 amino acids with 11 putative transmembrane segments (Fig. 4A). The protein contains Pfam and PRINTS bicarbonate transporter signatures and shows homology to members of the mammalian bicarbonate transporter family SLC4 (whereby the mammalian protein is represented in uppercase and Slc4 denotes the Dictyostelium protein), ranging from 17 to 30% identity. Phylogenetic analysis shows this protein is related to the mammalian SLC4 family (Fig. 4B) and is not related to the mammalian monocarboxylate transporter MCT1. We then ablated this gene by homologous integration of a deleted gene product, to isolate independent mutants lacking the slc4 gene (Fig. 4C,D,E).
Fig. 4.
Analysis of the Dictyostelium slc4 gene. (A) Schematic showing slc4 topology, Pfam bicarbonate transporter family and PRINTS bicarbonate transporter superfamily signatures. (B) Bootstrap consensus tree showing the evolutionary relationship between the Dictyostelium Slc4 protein and proteins of the SLC4 family in other species. Numbers represent percentage of replicate trees in which the associated taxa clustered together. The scale bar (50) indicates the number of nucleotide substitutions per site. (C) Confirmation of slc4 knockout. Clonal isolates transformed with the slc4-knockout cassette were screened by PCR, indicating non-homologous integrants show amplification of the genomic (G) and vector (V) controls and no amplification of the knockout diagnostic band (K), whereas transformants with homologous integration of the knockout cassette show amplification of all three products. (D) PCR amplification of wild-type (wt) and slc4-knockout derived DNA (A and B labels) using a forward primer inside the ablated region of the gene and a reverse primer inside the genomic DNA (across the cassette boundary) and glycogen synthase (glcS) primers as a control. (E) PCR of wild-type cells and slc4-knockout cells (A label) following removal of the blasticidin resistance cassette using primers on either side of the ablated region of the gene (across the slc4 gene) and glcS primers as a control.
Slc4 participates in VPA uptake
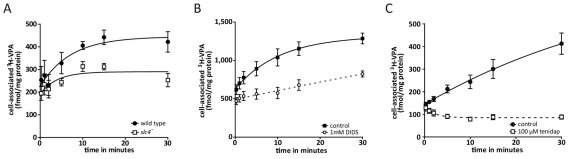
To investigate the role of Slc4 in VPA transport, we first analysed VPA uptake in the slc4-null mutant, and found that the mutant shows strongly reduced uptake compared with that in wild-type cells (P<0.001; Fig. 5A). Furthermore, the anion transporter inhibitors 4,4′-di-isothiocyanostilbene-2,2′-disulfonate (DIDS) (1 mM; Fig. 5B) and tenidap (100 μM; Fig. 5C) (McNiff et al., 1994; Romero et al., 2004), both strongly reduced the VPA uptake rate compared with that in untreated cells (P<0.001), consistent with a role for Slc4 in VPA transport. By comparison, 100 μM probenecid and 50 μM MK571 (inhibitors of multidrug resistance proteins) did not inhibit VPA uptake (data not shown). These results strongly implicate VPA uptake in this system through Slc4 activity.
Fig. 5.
Genetic or pharmacological inhibition of Dictyostelium Slc4 reduces VPA uptake. (A) Timecourse of [3H]VPA in uptake in wild-type (●) and slc4-null mutant (□) cells (±s.e.m.). (B) Uptake in the absence (□) or presence of 1 mM DIDS (○) (means ± s.e.m.). (C) Uptake of [3H]VPA in the absence (●) or presence (□) of 100 μM tenidap (means ± s.e.m.).
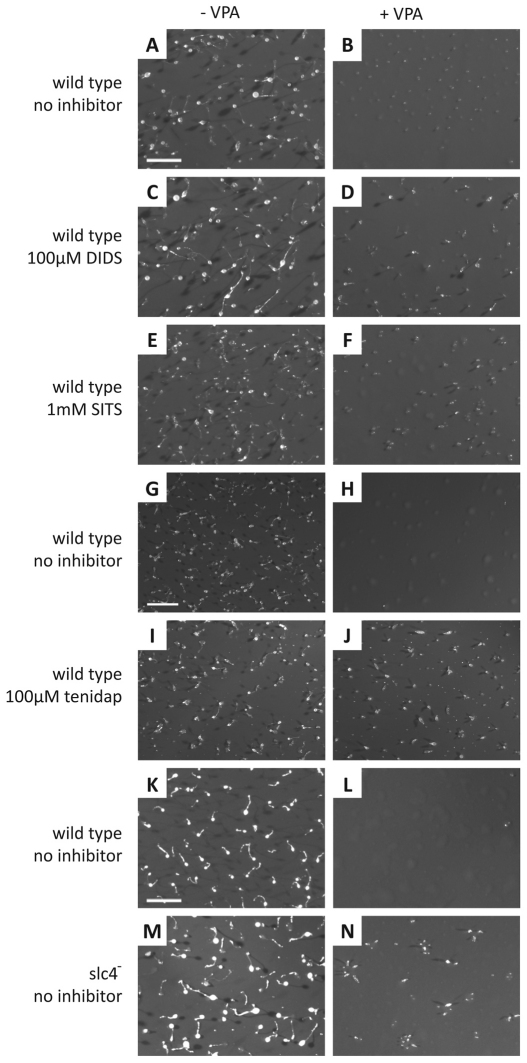
We then examined the chronic effect of slc4 ablation on VPA-sensitive Dictyostelium development. During a 24-hour period, cells aggregate and form a multicellular organism consisting of a stalk and a fruiting body containing spore cells (Fig. 6A,G,K). The presence of VPA during this process leads to the arrest of development before the mound stage (Fig. 6B,H,L) (Williams et al., 2006). Repeating this development assay in the presence of 100 μM DIDS, 1 mM of a second bicarbonate transport inhibitor, 4-acetimido-4′-isothiocyanostilbene-2,2′-disulfonate (SITS), or 100 μM tenidap had no effect on development in untreated cells (Fig. 6C,E and I, respectively) but enabled the formation of small aberrantly formed fruiting bodies in the presence of VPA (Fig. 6D,F,J), suggesting that Slc4 inhibition caused a partial reduction in the effect of VPA on development. The slc4-knockout mutant also showed wild-type fruiting body formation in untreated cells (Fig. 6M), and in the same manner as pharmacological inhibition of bicarbonate transporters, the slc4 mutant showed a similar partial rescue of development in the presence of VPA (Fig. 6N). Other anion transporter inhibitors, including the chloride channel inhibitor 5-nitro-2-(phenylpropylamino)-benzoate (NPPB) (8 μM) did not alter the effect of VPA on Dictyostelium development, and flufenamic acid (FFA) (1 mM), a blocker of Ca2+-activated non-selective cation channels (Gogelein et al., 1990), blocked development in the absence of VPA (data not shown).
Fig. 6.
Dictyostelium development in the presence of VPA is partially rescued by slc4 ablation or anion transporter inhibitors. Wild-type cells form fruiting bodies following 24 hours of development (A,G,K). However, the presence of (B) 0.75 mM and (H,L) 1 mM VPA blocks development before the mound stage. Wild-type cells develop normally in the presence of 100 μM of the anion transporter inhibitor DIDS (C), 1 mM of SITS (E) or 100 μM of tenidap (I). In the presence of 0.75 mM VPA, 100 μM DIDS (D) and 1 mM SITS (F) partially rescue Dictyostelium development. (J) 100 μM tenidap partially rescues development in the presence of 1 mM VPA. No developmental phenotype can be observed following ablation of the slc4 gene (M), however, the mutant shows partial rescue in the presence of 1 mM VPA (N). Scale bars: 1 mm.
Bicarbonate transporter inhibition and pH dependence control VPA teratogenicity in zebrafish
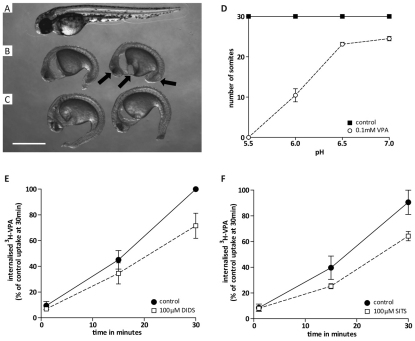
Zebrafish (Danio rerio) are a widely recognized biomedical model and have been used in VPA teratogenicity research (Gurvich et al., 2005). To determine whether VPA uptake in zebrafish is through Slc4-like transport, embryos were cultured in the absence or presence of VPA in various pH conditions or with bicarbonate transport inhibitors (SITS, DIDS) (Fig. 7). VPA and/or inhibitors were added at 5 hours post fertilization (hpf) and embryos were observed the following day at 30 hpf. Under these conditions (at pH 6.0), untreated embryos completed developmental segmentation and had 30 somites (Fig. 7A), whereas VPA retarded development and caused severe deformations, with embryos only reaching the 10-somite stage (Fig. 7B). VPA-induced oedema in the cardiac region was also observed in the latter embryos, together with reduced tail length and eye development (indicated by arrows). Consistent with a role for SLC4 in VPA transport, treatment of developing embryos with 100 μM SITS partially reversed the effects of VPA, with embryos displaying reduced oedema, more advanced yolk sack extension and increased tail development (Fig. 7C) compared with that upon VPA treatment alone. These VPA-dependent changes were also pH dependent; at a higher pH of 7.0, treatment with VPA had virtually no effect on zebrafish development and, at a lower pH of 5.0, VPA showed a stronger effect with no somite formation (Fig. 7D). The bicarbonate transporter inhibitors DIDS and SITS (100 μM) also significantly reduced the uptake of VPA over 30 minutes after addition of the label (P<0.05; Fig. 7E,F). These results are in agreement with an SLC4 transporter playing a role in VPA uptake in both Dictyostelium and zebrafish systems.
Fig. 7.
VPA-catalysed zebrafish teratogenicity and uptake of [3H]VPA are dependent on the bicarbonate transporter and pH. Wild-type zebrafish embryo development at 30 hpf (A) is altered by teratogenic effects of 0.1 mM VPA (B), and this effect is partially reversed by the bicarbonate transporter inhibitor SITS at 0.1 mM (C). Teratogenic effects are also dependent on pH because a VPA-dependent reduction in somite formation correlates with low pH levels when compared with untreated embryos (D). Uptake of [3H]VPA is reduced, compared with that in the control (●), in the presence (□) of 100 μM of DIDS (E) or SITS (F). Scale bar: 0.5 mm.
Bicarbonate reduces VPA uptake in Dictyostelium and zebrafish and teratogenicity in zebrafish and Xenopus
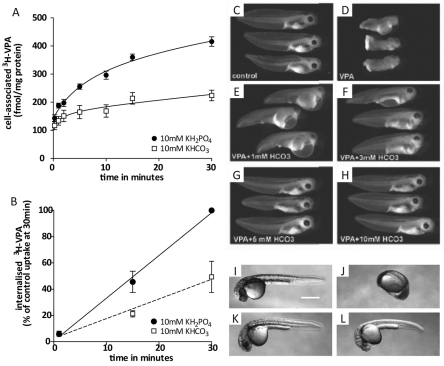
Bicarbonate (HCO3−) is a common substrate for SLC4 proteins (Pushkin and Kurtz, 2006), and our results would therefore suggest a potential competition between VPA and bicarbonate uptake. To examine this, we analysed the effect of KHCO3 on VPA uptake over time (Fig. 8A,B), at a constant pH. Replacing 10 mM KH2PO4 in the uptake medium with an equivalent concentration of KHCO3 significantly reduced uptake of VPA in both Dictyostelium (Fig. 8A; P<0.01) and zebrafish (Fig. 8B; P<0.001). This reduction was dependent on KHCO3 concentration, with lower concentrations leading to a smaller reduction (data not shown).
Fig. 8.
Bicarbonate inhibits VPA uptake in Dictyostelium and zebrafish and reverses VPA-induced teratogenic effects in zebrafish and Xenopus in a dose-dependent manner. Timecourses of Dictyostelium (A) and zebrafish (B) [3H]VPA uptake (means ± s.e.m.) in the presence of 10 mM KHCO3 (□) compared with in the presence of 10 mM KH2PO4 (●). In Xenopus, untreated embryos at stage 41 (C) show wild-type development but following treatment with 2 mM VPA from the 16-cell stage, embryos demonstrate marked developmental delay and anterior–posterior truncation, as well as pericardial oedema (D). The addition of increasing concentrations of NaHCO3 to these VPA-treated embryos at 1 mM (E), 3 mM (F), 5 mM (G) or 10 mM (H) NaHCO3 dose-dependently reverses this VPA-induced teratogenic effect. 5 mM NaHCO3 alone had no effect on development (data not shown). In zebrafish, embryos treated with 50 mM KH2PO4 (I) or 50 mM KHCO3 (K) develop normally. In the presence of 0.1 mM VPA, embryos in KH2PO4 show severe developmental defects (J), whereas development remains unaffected in KHCO3 (L).
We also investigated a bicarbonate-dependent reversal of the teratogenic effects of VPA in zebrafish and Xenopus larvae. As reported previously (Gurvich et al., 2005; Phiel et al., 2001), 2 mM VPA had multiple teratogenic effects in Xenopus compared with control conditions (compare Fig. 8C with 8D), including shortened anterior–posterior axis, developmental delay, and pericardial oedema, similar to zebrafish, and defects in cardiac looping. Addition of bicarbonate provides a dose-dependent reversal of VPA-induced developmental defects in Xenopus, with partial rescue of both developmental delay and growth defects at 1 mM or 3 mM bicarbonate (Fig. 8E,F, respectively) and virtually complete rescue at higher bicarbonate concentrations (compare Fig. 8C and 8G,H). In zebrafish, the addition of 50 mM KHCO3 rescued the development in the presence of 0.1 mM VPA (compare Fig. 8J and 8L). The same effect was observed at a bicarbonate concentration of 10 mM.
Discussion
Despite the widespread use of VPA for epilepsy, bipolar disorder and migraine treatment, the mechanism of its cellular import remains poorly understood. Here, we employed a variety of cellular, molecular and genetic approaches to characterize VPA uptake in the simple biomedical model Dictyostelium.
Biochemical properties of VPA uptake
We showed that VPA crosses the cell membrane and predominantly localizes to the cytosol. Although some researchers have proposed therapeutic activity of VPA on the cell membrane (Kessel et al., 2001), our results are consistent with intracellular activity, which is required for the widely reported effects of VPA in a range of pathways, such as inositol signalling (Eickholt et al., 2005; Williams et al., 2002) and MAPK signalling (Boeckeler et al., 2006; Chen et al., 1999). VPA uptake is dose dependent and slows considerably after 30 minutes. This plateau could be due to saturation of the cell with VPA or to the presence of efflux transport, but given that exposure of cells to 6 nM extracellular [3H]VPA stabilized the intracellular concentration at 82 nM, our data suggest that VPA uptake occurs against a concentration gradient. This is consistent with uptake plateaus of 26 nM in intestinal epithelial (Caco-2) and 47 nM in brain endothelial (RBE4) cells (Fischer et al., 2008) and suggests the presence of an active transport mechanism in both Dictyostelium and mammalian cells. The energy source for this transport could be ATP, as is the case for multidrug resistance transporters. These proteins are responsible for efflux transport of many drugs, although VPA has been shown to not be a substrate of these transporters (Baltes et al., 2007) and we show that inhibitors of multidrug resistance transporters do not alter VPA uptake in Dictyostelium. Alternatively, energy for active uptake could be released by simultaneous transport of a different molecule down its concentration gradient by a coupled transporter, as seen in the bicarbonate transporters of the SLC4 family that are investigated here.
We also demonstrated a pH dependence of VPA uptake in both Dictyostelium and zebrafish. Uptake increases significantly at lower pH, whereas it decreases at higher pH. Because VPA is present mainly in its ionized form at physiological pH (it has a pKa of 4.8), variation of uptake with extracellular pH is consistent with simple diffusion of the non-ionized form. For example, the percentage of non-ionized VPA molecules is highest at low pH (86% of VPA is in its non-ionized form at pH 4.0 and 39% at pH 5.0), compared with that at physiological pH 6.3 (3.1% non-ionized), and converges towards zero at higher pH (0.6% at pH 7.0 and 0.1% at pH 8.0); this mirrors changes in cellular uptake due to changing the pH environment. However, the majority of VPA at physiological pH is in its ionized form, thus would not enter cells through diffusion. The pH dependence of VPA uptake is also inferred in zebrafish experiments, whereby VPA effects on development are eliminated in high pH conditions (pH 7.0). This pH dependence has also been shown in human placental brush border cells (Nakamura et al., 2002), supporting a similar uptake behaviour in animal systems.
The pH-dependent uptake of VPA could also point to the presence of a pH-dependent active transport mechanism. The intracellular pH (pHi) of Dictyostelium has been measured with a variety of different methods and has been reported to be between 6.7 and 7.5 (Liu et al., 2002; Ratner, 1986). At an extracellular pH (pHo) below this pHi, an inward-directed proton gradient is present that could act as the driving force for active transport. If transport were dependent on the proton gradient, a pHo higher than the pHi would inhibit uptake. This is consistent with our observations reported here. In order to determine whether transport is dependent on extracellular pH or the proton gradient, we employed the proton ionophore FCCP. This compound abolished transport, indicating an uptake mechanism dependent upon proton gradient or membrane potential for VPA. SLC4 proteins are important regulators of intracellular pH (Pushkin and Kurtz, 2006) and have previously been shown to be themselves regulated by pH (Alper et al., 2002), thus, SLC4 proteins are a feasible transport mechanism for VPA.
Structural specificity of the VPA transporter
A total of 12 compounds were tested for competition with VPA uptake. Medium-length fatty acids (dodecanoic acid, 2-butyl octanoic acid) showed the greatest competition for uptake, and short straight-chain compounds (propionic and pentanoic acid) showed less competition, suggesting a chain-length dependence of inhibition. These results are consistent with previously published data whereby medium-chain fatty acids, including pentanoic acid and octanoic acid, competed with VPA uptake in placenta, intestine and blood–brain barrier model cell lines (Fischer et al., 2008; Utoguchi and Audus, 2000). However, this was not shown in rat microvessels or rat choroid plexus (Adkison and Shen, 1996), suggesting the presence of different uptake mechanisms in different organisms or cell types.
Comparison of the uptake competition for VPA-related compounds shows considerable structural specificity. Compounds possessing an amide group instead of the carboxy head group of VPA, such as valpromide or valnoctamide, or those with an additional carboxy group (glutaric acid) did not compete with VPA uptake, indicating that the uptake mechanism examined here is dependent on the presence of a single carboxy head group; these results are consistent with the previously reported placental cell line data (Adkison and Shen, 1996; Utoguchi and Audus, 2000). All three of the tested branched compounds significantly reduced the initial rate of VPA uptake. Comparison of these branched compounds and their related straight-chain counterparts suggests that the affinity for transport is significantly increased by branching in the C-2 position. Benzoic acid and lactic acid did not significantly inhibit the initial rate of VPA uptake and these have been shown to be substrates of MCT (Tamai et al., 1995; Utoguchi and Audus, 2000), although benzoic acid is also transported by SLC4 activity (Yabuuchi et al., 1998).
Slc4 is involved in transport of VPA across the cell membrane
In screens of a Dictyostelium mutant library for increased resistance to VPA in growth and development, a potential transporter, encoded by the slc4 gene, was identified. This gene was shown to encode a homologue to members of the mammalian SLC4 family of bicarbonate transporters, proteins that have also been linked to fatty acid transport in various human colon adenocarcinoma cell lines and HEK-293 cells (Lecona et al., 2008; Yabuuchi et al., 1998) and to flippase activity in erythrocytes (Ortwein et al., 1994), consistent with our findings. Sequence identity between the identified Dictyostelium Slc4 and members of the mammalian bicarbonate transporter family is between 17 and 30%. Although it is difficult to specify the subtype of SLC4 protein, the Dictyostelium homologue groups within the same clade as SLC4A11. No other gene in the Dictyostelium genome shows homology to the SLC4 family, so it is likely to represent the only member of its class in the model organism. The identification of this mutant showing resistance to VPA in both growth and development suggests that Slc4 provides a mechanism for VPA uptake.
Genetic and pharmacological ablation of Slc4 in Dictyostelium significantly reduced both acute VPA transport and chronic VPA-mediated developmental effects, consistent with a role for Slc4 in VPA uptake. Pharmacological ablation employed two structurally independent transport inhibitors, DIDS, a bicarbonate transporter inhibitor, and tenidap, a non-steroidal anti-inflammatory drug (McNiff et al., 1994; Romero et al., 2004). Note that these bicarbonate inhibitors are not specific to SLC4 transporters and potentially block a range of anion channels and exchangers (Izumi et al., 2003; Utoguchi and Audus, 2000); however, their functional overlap allows identification of the most likely target reducing the uptake of VPA. DIDS, SITS and tenidap are known to inhibit chloride channels, as well as SLC4 (Koszela-Piotrowska et al., 2007; McNiff et al., 1994). However, chloride channels and monocarboxylate transporters of the MCT family (Goncalves et al., 2009) are also blocked by NPPB, but this inhibitor did not block the effects of VPA on development. Two inhibitors for multidrug resistance proteins, MK571 and probenecid, did not reduce uptake of VPA, suggesting that these proteins are also not involved in VPA transport in Dictyostelium. Although neither genetic ablation nor pharmacological inhibition completely blocked the effects of VPA, the presence of non-ionised VPA (~3% in pH 6.3 buffered medium), transported through diffusion, might explain low-level uptake or developmental effects. The reduction in VPA uptake following genetic and pharmaceutical ablation of Slc4 is in agreement with a role for this transporter in VPA uptake.
The anion transporter SITS also reduced teratogenic effects of VPA on zebrafish development, and both DIDS and SITS reduced VPA uptake in this model, showing that the results from Dictyostelium are likely to be applicable to other systems. Final confirmation of a potential role of SLC4 bicarbonate transporters in VPA uptake is shown in bicarbonate competition assays, whereby reduced VPA uptake in Dictyostelium occurs through competition with increasing levels of bicarbonate. Furthermore, this effect is present in more complex animal systems, as the uptake of VPA is also reduced by bicarbonate in zebrafish, and the teratogenic effect of VPA in Xenopus and zebrafish larvae is rescued in the presence of increasing levels of bicarbonate. Although it remains a possibility that SLC4 inhibition has an indirect effect on VPA uptake by, for example, altering intracellular ion levels, resulting in altered uptake or excretion of VPA, our results support our proposition that SLC4 proteins are involved in VPA transport in multiple model systems.
Identification of a novel uptake mechanism for VPA
We have characterized the uptake of the most highly prescribed conventional antiepileptic treatment VPA in the simple biomedical model Dictyostelium. We show protein-mediated uptake is dependent on VPA conentration, pH, proton gradient and structure and occurs against a concentration gradient, suggesting an active mechanism of uptake. We have identified a gene in Dictyostelium that encodes a protein homologous to mammalian bicarbonate transporters of the SLC4 family showing increased specificity for medium-chain fatty acids branched at the C-2 position. This is the first identification of an active transport mechanism for VPA. We further show that this transport mechanism is consistent with transport in two more complex animal models, zebrafish and Xenopus. This discovery, suggesting Slc4 as a putative VPA transporter in animal systems, might enable a better regulation of cellular VPA levels during therapy. For example, regulation of neuronal uptake might provide better epilepsy, bipolar disorder and/or migraine protection, and reduced uptake in the liver or embryo might lead to reduced hepatotoxicity (Bjornsson, 2008) or teratogenicity (Ornoy, 2006). Furthermore, the design of second-generation VPA-related drugs for VPA-treatable disorders might benefit from improving SLC4-mediated uptake in target tissues.
Materials and Methods
Materials
Valproic acid (VPA), carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP), 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS), 4-acetamido-4′-isothiocyanatostilbene-2,2′-disulfonic acid (SITS), 5-nitro-2-(phenylpropylamino)-benzoate (NPPB), flufenamic acid (FFA), hexanol, probenecid and potassium bicarbonate (KHCO3) were purchased from Sigma-Aldrich. MK571 was purchased from Enzo Life Sciences. Tenidap was obtained from Tocris Bioscience. [4,5-3H]VPA was obtained from Hartmann Analytic, Germany. Dictyostelium gene and protein information was obtained from DictyBase (http://www.dictybase.org).
Dictyostelium cell culture and gene isolation
Dictyostelium (Ax2) were grown in association with Raoultella planticola or in shaking culture (HL5 medium) at 21°C. Cells were maintained and harvested in mid-exponential phase (1×106–4×106 cells per ml). Mutagenesis was carried out as described previously (Williams et al., 1999). The knockout of slc4 was recapitulated by electroporation of the pLPBLP vector (Faix et al., 2004) containing N- and C-terminal fragments of slc4 into wild-type (Ax2) cells, confirmed by PCR amplification. Dictyostelium developmental assays were performed as described previously (Boeckeler et al., 2006; Williams et al., 2002).
Phylogenetic analysis of Slc4
Evolutionary history was inferred using the neighbour-joining method. The bootstrap test was performed with 500 replicates. Phylogenetic analyses were conducted in MEGA.
VPA uptake assays in Dictyostelium
For uptake assays, 1×108 cells per condition were washed twice and pulsed with 30 nM cAMP at 2×107 cells per ml for 5 hours (Boeckeler et al., 2006). Cells were then washed and resuspended in 5 ml phosphate buffer (KK2: 16.5 mM KH2PO4, 3.8 mM K2HPO4) and aerated on a platform shaker at 21°C (120 r.p.m.). For pH-dependent uptake, buffers were adjusted to the appropriate pH using H3PO4 or KOH. In each experiment, solutions comprising 1 ml KK2 with 6 nM of [3H]VPA and an appropriate amount of unlabelled VPA (0.25 mM unless stated otherwise) were prepared and added to the shaking cells at the start of the assay. At specific intervals, 300-μl samples were taken and placed on ice to stop uptake. Samples were washed with cold KK2 once, resuspended in 250 μl of KK2 and analysed by liquid scintillation. The experiments shown normally comprise triplicate independent experiments with duplicate samples for each experiment. The total intracellular concentration of [3H]VPA after 30 minutes was determined by performing the above assay without the addition of unlabelled VPA. Protein levels were measured using the Pierce BCA protein assay kit (Thermo Scientific). For subcellular localization of [3H]VPA, cells were prepared as described above and 1 ml cell samples was taken at indicated time points, washed once with KK2, lysed and fractionated by differential centrifugation as described previously (Williams et al., 1999). To measure VPA incorporation into lipids, these were extracted with acidified methanol and separated by thin-layer chromatography, as described previously (Pawolleck and Williams, 2009). For VPA protein incorporation, cells were prepared and lysed, as described previously (Williams et al., 1999), and proteins were separated by SDS-PAGE and transferred onto a Hybond N+ membrane. A tritium-sensitive phosphoimager screen was used to record incorporation, and analysed using a Typhoon phosphoimager.
Zebrafish protocols
Fish were maintained and bred at 26.5°C and embryos were raised at 28.5°C and maintained as previously described (Westerfield, 2000). Both AB and TL wild-type strains were used for these studies. For the analysis of VPA effects on zebrafish development, fertilized eggs were separated from unfertilized eggs at 4–6 hours after spawning and 10 eggs per condition were distributed into each well of a six-well tissue culture plate with 4 ml of either embryo medium alone or supplemented with the appropriate drugs. The phenotype was assessed after 30, 54 and 78 hours post fertilization (hpf). For [3H]VPA uptake assays, 10 larvae (102 hpf) per time point per condition were exposed to [3H]VPA and 0.1 mM of unlabelled VPA. After 1, 15 and 30 minutes, larvae were washed five times and the [3H]VPA content was measured using liquid scintillation. All animal experiments were performed according to the relevant regulatory standards.
Xenopus laevis protocols
Xenopus embryos were cultured by standard methods (Sive et al., 2000). For the analysis of VPA effects on Xenopus development, embryos were collected by in vitro fertilization and staged as previously described (Nieuwkoop and Faber, 1994). These embryos were simultaneously administered 2 mM VPA and indicated doses of bicarbonate at the 16-cell stage (~3 hours post fertilization), cultured in 0.1× MMR (10 mM NaCl, 0.2 mM KCl, 0.2 mM CaCl2, 0.1 mM MgSO4 and 0.5 mM HEPES pH 7.8) at 21°C until stage 40 or 41 (3 days post fertilization) and fixed in MEMFA (0.1 M MOPS pH 7.4, 2 mM EGTA, 1 mM MgSO4 and 3.7% formaldehyde). All experimental conditions were confirmed at constant pH (7.8).
Acknowledgments
This work was supported by Wellcome Trust Project Grant 082640 and NC3Rs grant G0900775 to R.S.B.W., a Royal Holloway and St George's University of London postgraduate Scholarship and University of London CRF Equipment Grant to N.T. and NIH grants MH58324 and GM076621 to P.S.K. R.S. was supported by a Cancer Pharmacology Training grant from the NCI (5R25CA101871-07). Deposited in PMC for release after 6 months.
References
- Adkison K. D., Shen D. D. (1996). Uptake of valproic acid into rat brain is mediated by a medium-chain fatty acid transporter. J. Pharmacol. Exp. Ther. 276, 1189-1200 [PubMed] [Google Scholar]
- Alper S. L., Chernova M. N., Stewart A. K. (2002). How pH regulates a pH regulator: a regulatory hot spot in the N-terminal cytoplasmic domain of the AE2 anion exchanger. Cell Biochem. Biophys. 36, 123-136 [DOI] [PubMed] [Google Scholar]
- Baltes S., Fedrowitz M., Tortos C. L., Potschka H., Loscher W. (2007). Valproic acid is not a substrate for P-glycoprotein or multidrug resistance proteins 1 and 2 in a number of in vitro and in vivo transport assays. J. Pharmacol. Exp. Ther. 320, 331-343 [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. (1989). Neural and developmental actions of lithium: a unifying hypothesis. Cell 59, 411-419 [DOI] [PubMed] [Google Scholar]
- Bjornsson E. (2008). Hepatotoxicity associated with antiepileptic drugs. Acta Neurol. Scand. 118, 281-290 [DOI] [PubMed] [Google Scholar]
- Boeckeler K., Adley K., Xu X., Jenkins A., Jin T., Williams R. S. (2006). The neuroprotective agent, valproic acid, regulates the mitogen-activated protein kinase pathway through modulation of protein kinase A signalling in Dictyostelium discoideum. Eur. J. Cell Biol. 85, 1047-1057 [DOI] [PubMed] [Google Scholar]
- Bokelmann I., Mahlknecht U. (2008). Valproic acid sensitizes chronic lymphocytic leukemia cells to apoptosis and restores the balance between pro- and antiapoptotic proteins. Mol. Med. 14, 20-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer K. L., Hall E. S., Pollack G. M. (1993). Protein binding and hepatobiliary distribution of valproic acid and valproate glucuronide in rats. Biochem. Pharmacol. 45, 735-742 [DOI] [PubMed] [Google Scholar]
- Burton B. S. (1882). On the propyl derivatives and decomposition products of ethylacetoacetate. Am. Chem. J. 3, 385-395 [Google Scholar]
- Calabresi P., Galletti F., Rossi C., Sarchielli P., Cupini L. M. (2007). Antiepileptic drugs in migraine: from clinical aspects to cellular mechanisms. Trends Pharmacol. Sci. 28, 188-195 [DOI] [PubMed] [Google Scholar]
- Chang M. C., Contreras M. A., Rosenberger T. A., Rintala J. J., Bell J. M., Rapoport S. I. (2001). Chronic valproate treatment decreases the in vivo turnover of arachidonic acid in brain phospholipids: a possible common effect of mood stabilizers. J. Neurochem. 77, 796-803 [DOI] [PubMed] [Google Scholar]
- Chapman A., Keane P. E., Meldrum B. S., Simiand J., Vernieres J. C. (1982). Mechanism of anticonvulsant action of valproate. Prog. Neurobiol. 19, 315-359 [DOI] [PubMed] [Google Scholar]
- Chen G., Huang L. D., Jiang Y. M., Manji H. K. (1999). The mood-stabilizing agent valproate inhibits the activity of glycogen synthase kinase-3. J. Neurochem. 72, 1327-1330 [DOI] [PubMed] [Google Scholar]
- Deuticke B., Beyer E., Forst B. (1982). Discrimination of three parallel pathways of lactate transport in the human erythrocyte membrane by inhibitors and kinetic properties. Biochim. Biophys. Acta 684, 96-110 [DOI] [PubMed] [Google Scholar]
- Eickholt B. J., Towers G. J., Ryves W. J., Eikel D., Adley K., Ylinen L. M., Chadborn N. H., Harwood A. J., Nau H., Williams R. S. (2005). Effects of valproic acid derivatives on inositol trisphosphate depletion, teratogenicity, glycogen synthase kinase-3beta inhibition, and viral replication: a screening approach for new bipolar disorder drugs derived from the valproic acid core structure. Mol. Pharmacol. 67, 1426-1433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrich H. M., von Zerssen D., Kissling W., Moller H. J. (1981). Therapeutic effect of valproate in mania. Am. J. Psychiatry 138, 256 [DOI] [PubMed] [Google Scholar]
- Faix J., Kreppel L., Shaulsky G., Schleicher M., Kimmel A. R. (2004). A rapid and efficient method to generate multiple gene disruptions in Dictyostelium discoideum using a single selectable marker and the Cre-loxP system. Nucleic Acids Res. 32, e143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W., Praetor K., Metzner L., Neubert R. H., Brandsch M. (2008). Transport of valproate at intestinal epithelial (Caco-2) and brain endothelial (RBE4) cells: mechanism and substrate specificity. Eur. J. Pharm. Biopharm. 70, 486-492 [DOI] [PubMed] [Google Scholar]
- Gibbs J. P., Adeyeye M. C., Yang Z., Shen D. D. (2004). Valproic acid uptake by bovine brain microvessel endothelial cells: role of active efflux transport. Epilepsy Res. 58, 53-66 [DOI] [PubMed] [Google Scholar]
- Gogelein H., Dahlem D., Englert H. C., Lang H. J. (1990). Flufenamic acid, mefenamic acid and niflumic acid inhibit single nonselective cation channels in the rat exocrine pancreas. FEBS Lett. 268, 79-82 [DOI] [PubMed] [Google Scholar]
- Goncalves P., Araujo J. R., Pinho M. J., Martel F. (2009). Modulation of butyrate transport in Caco-2 cells. Naunyn Schmiedebergs Arch. Pharmacol. 379, 325-336 [DOI] [PubMed] [Google Scholar]
- Gurvich N., Berman M. G., Wittner B. S., Gentleman R. C., Klein P. S., Green J. B. (2005). Association of valproate-induced teratogenesis with histone deacetylase inhibition in vivo. FASEB J. 19, 1166-1168 [DOI] [PubMed] [Google Scholar]
- Izumi H., Torigoe T., Ishiguchi H., Uramoto H., Yoshida Y., Tanabe M., Ise T., Murakami T., Yoshida T., Nomoto M., et al. (2003). Cellular pH regulators: potentially promising molecular targets for cancer chemotherapy. Cancer Treat. Rev. 29, 541-549 [DOI] [PubMed] [Google Scholar]
- Kessel A., Musafia B., Ben-Tal N. (2001). Continuum solvent model studies of the interactions of an anticonvulsant drug with a lipid bilayer. Biophys. J. 80, 2536-2545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszela-Piotrowska I., Choma K., Bednarczyk P., Dolowy K., Szewczyk A., Kunz W. S., Malekova L., Kominkova V., Ondrias K. (2007). Stilbene derivatives inhibit the activity of the inner mitochondrial membrane chloride channels. Cell. Mol. Biol. Lett. 12, 493-508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecona E., Olmo N., Turnay J., Santiago-Gomez A., Lopez de Silanes I., Gorospe M., Lizarbe M. A. (2008). Kinetic analysis of butyrate transport in human colon adenocarcinoma cells reveals two different carrier-mediated mechanisms. Biochem. J. 409, 311-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T., Mirschberger C., Chooback L., Arana Q., Dal S. Z., MacWilliams H., Clarke M. (2002). Altered expression of the 100 kDa subunit of the Dictyostelium vacuolar proton pump impairs enzyme assembly, endocytic function and cytosolic pH regulation. J. Cell Sci. 115, 1907-1918 [DOI] [PubMed] [Google Scholar]
- Loscher W., Fisher J. E., Nau H., Honack D. (1989). Valproic acid in amygdala-kindled rats: alterations in anticonvulsant efficacy, adverse effects and drug and metabolite levels in various brain regions during chronic treatment. J. Pharmacol. Exp. Ther. 250, 1067-1078 [PubMed] [Google Scholar]
- McNiff P., Svensson L., Pazoles C. J., Gabel C. A. (1994). Tenidap modulates cytoplasmic pH and inhibits anion transport in vitro. I. Mechanism and evidence of functional significance. J. Immunol. 153, 2180-2193 [PubMed] [Google Scholar]
- Mesdjian E., Ciesielski L., Valli M., Bruguerolle B., Jadot G., Bouyard P., Mandel P. (1982). Sodium valproate: kinetic profile and effects on GABA levels in various brain areas of the rat. Prog. Neuropsychopharmacol. Biol. Psychiatry 6, 223-233 [DOI] [PubMed] [Google Scholar]
- Meunier H., Carraz G., Meunier Y., Eymard P., Aimard M. (1963). Pharmacodynamic properties of N-dipropylacetic acid. Therapie 18, 435-438 [PubMed] [Google Scholar]
- Nakamura H., Ushigome F., Koyabu N., Satoh S., Tsukimori K., Nakano H., Ohtani H., Sawada Y. (2002). Proton gradient-dependent transport of valproic acid in human placental brush-border membrane vesicles. Pharm. Res. 19, 154-161 [DOI] [PubMed] [Google Scholar]
- Nieuwkoop P. D., Faber J. (1994). Normal Table of Xenopus laevis (Daudin). A Systematical and Chronological Survey of the Development of the Fertilized Egg till the end of Metamorphosis. New York: Garland Publishing; [Google Scholar]
- Ornoy A. (2006). Neuroteratogens in man: an overview with special emphasis on the teratogenicity of antiepileptic drugs in pregnancy. Reprod. Toxicol. 22, 214-226 [DOI] [PubMed] [Google Scholar]
- Ortwein R., Oslender-Kohnen A., Deuticke B. (1994). Band 3, the anion exchanger of the erythrocyte membrane, is also a flippase. Biochim. Biophys. Acta 1191, 317-323 [DOI] [PubMed] [Google Scholar]
- Pawolleck N., Williams R. S. (2009). Quantifying in vivo phosphoinositide turnover in chemotactically competent dictyostelium cells. Methods Mol. Biol. 571, 283-290 [DOI] [PubMed] [Google Scholar]
- Phiel C. J., Zhang F., Huang E. Y., Guenther M. G., Lazar M. A., Klein P. S. (2001). Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J. Biol. Chem. 276, 36734-36741 [DOI] [PubMed] [Google Scholar]
- Pushkin A., Kurtz I. (2006). SLC4 base (HCO3−, CO32−) transporters: classification, function, structure, genetic diseases, and knockout models. Am. J. Physiol. Renal Physiol. 290, F580-F599 [DOI] [PubMed] [Google Scholar]
- Ratner D. I. (1986). Equivalence of intracellular pH of differentiating Dictyostelium cell types. Nature 321, 180-182 [Google Scholar]
- Romero M. F., Fulton C. M., Boron W. F. (2004). The SLC4 family of HCO 3-transporters. Pflugers Arch. 447, 495-509 [DOI] [PubMed] [Google Scholar]
- Ryves W. J., Dalton E. C., Harwood A. J., Williams R. S. (2005). GSK-3 activity in neocortical cells is inhibited by lithium but not carbamazepine or valproic acid. Bipolar Disord. 7, 260-265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siafaka-Kapadai A., Patiris M., Bowden C., Javors M. (1998). Incorporation of [3H]valproic acid into lipids in GT1-7 neurons. Biochem. Pharmacol. 56, 207-212 [DOI] [PubMed] [Google Scholar]
- Sive H. L., Grainger R. M., Harland R. M. (2000). Early Development of Xenopus laevis: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; [Google Scholar]
- Soll D. R., Yarger J., Mirick M. (1976). Stationary phase and the cell cycle of Dictyostelium discoideum in liquid nutrient medium. J. Cell Sci. 20, 513-523 [DOI] [PubMed] [Google Scholar]
- Tamai I., Takanaga H., Maeda H., Sai Y., Ogihara T., Higashida H., Tsuji A. (1995). Participation of a proton-cotransporter, MCT1, in the intestinal transport of monocarboxylic acids. Biochem. Biophys. Res. Commun. 214, 482-489 [DOI] [PubMed] [Google Scholar]
- Terbach N., Williams R. S. (2009). Structure-function studies for the panacea, valproic acid. Biochem. Soc. Trans. 37, 1126-1132 [DOI] [PubMed] [Google Scholar]
- Tian L. M., Alkadhi K. A. (1994). Valproic acid inhibits the depolarizing rectification in neurons of rat amygdala. Neuropharmacology 33, 1131-1138 [DOI] [PubMed] [Google Scholar]
- Utoguchi N., Audus K. L. (2000). Carrier-mediated transport of valproic acid in BeWo cells, a human trophoblast cell line. Int. J. Pharm. 195, 115-124 [DOI] [PubMed] [Google Scholar]
- Westerfield M. (2000). The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio). Eugene: University of Oregon Press; [Google Scholar]
- Williams R. S., Eames M., Ryves W. J., Viggars J., Harwood A. J. (1999). Loss of a prolyl oligopeptidase confers resistance to lithium by elevation of inositol (1,4,5) trisphosphate. EMBO J. 18, 2734-2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. S., Cheng L., Mudge A. W., Harwood A. J. (2002). A common mechanism of action for three mood-stabilizing drugs. Nature 417, 292-295 [DOI] [PubMed] [Google Scholar]
- Williams R. S., Boeckeler K., Graf R., Muller-Taubenberger A., Li Z., Isberg R. R., Wessels D., Soll D. R., Alexander H., Alexander S. (2006). Towards a molecular understanding of human diseases using Dictyostelium discoideum. Trends Mol. Med. 12, 415-424 [DOI] [PubMed] [Google Scholar]
- Yabuuchi H., Tamai I., Sai Y., Tsuji A. (1998). Possible role of anion exchanger AE2 as the intestinal monocarboxylic acid/anion antiporter. Pharm. Res. 15, 411-416 [DOI] [PubMed] [Google Scholar]