Abstract
Objectives
To examine the association of social and environmental factors with levels of second hand smoke (SHS) exposure, as measured by salivary cotinine, in young inner city children with asthma.
Methods
We used data drawn from a home-based behavioral intervention for young high risk children with persistent asthma post emergency department (ED) treatment (N=198). SHS exposure was measured by salivary cotinine and caregiver report. Caregiver demographic and psychological functioning, household smoking behavior and asthma morbidity were compared with child cotinine concentrations. Chi-square and ANOVA tests and multivariate regression models were used to determine the association between cotinine concentrations with household smoking behavior and asthma morbidity.
Results
Over half (53%) of the children had cotinine levels compatible with SHS exposure and mean cotinine concentrations were high at 2.42 ng/ml (SD 3.2). The caregiver was the predominant smoker in the home (57%) and (63%) reported a total home smoking ban. Preschool age children, and those with caregivers reporting depressive symptoms and high stress had higher cotinine concentrations than their counterparts. Among children living in a home with a total home smoking ban, younger children had significantly higher mean cotinine concentration than older children (Cotinine: 3–5 year olds, 2.24 ng/ml (SD 3.5); 6–10 year olds, 0.63 ng/ml (SD 1.0); p <0.05). In multivariate models, the factors most strongly associated with high child cotinine concentrations were increased number of household smokers (β = 0.24) and younger child age (3–5 years) (β = 0.23; P <0.001, R2 = 0.35).
Conclusion
Over half of young inner-city children with asthma were exposed to second hand smoke and caregivers are the predominant household smoker. Younger children and children with depressed and stressed caregivers are at significant risk of smoke exposures, even when a household smoking ban is reported. Further advocacy for these high-risk children is needed to help caregivers quit and to mitigate smoke exposure.
Keywords: asthma, children, cotinine, second hand smoke
Introduction
Exposure to second hand smoke (SHS) in children has been associated with increased risk for development and severity of asthma, (1–5) difficulty in managing asthma symptoms, (7,8) and chronic airway inflammation. Accordingly, avoidance of SHS exposure is a key component of national and international guideline recommendations for management of childhood asthma. (9–11) Despite parental awareness that SHS exacerbates asthma, 40–67% of inner-city children with asthma reside in a household with at least one smoker, (12–15) with particularly high rates of SHS exposure among children living in poverty. (16)
Several social environmental factors are implicated in high SHS exposure in inner-city children with asthma including caregiver smoking, (17, 18) child age, maternal depression (19, 20) and stress. (19, 21) However, few studies verified SHS exposure using biomarkers. The goal of this study was to examine the association of several social and environmental factors with levels of SHS exposure in young inner city children with asthma using cotinine, a biological marker for SHS exposure. We hypothesized that among children with asthma residing in the inner city, younger children and children with a caregiver who smokes would have more exposure to SHS than their counterparts after controlling for the effects of caregiver depressive symptoms and stress. We also were interested in evaluating the effectiveness of home smoking bans in reducing smoke exposure among these children.
Methods
Study Population
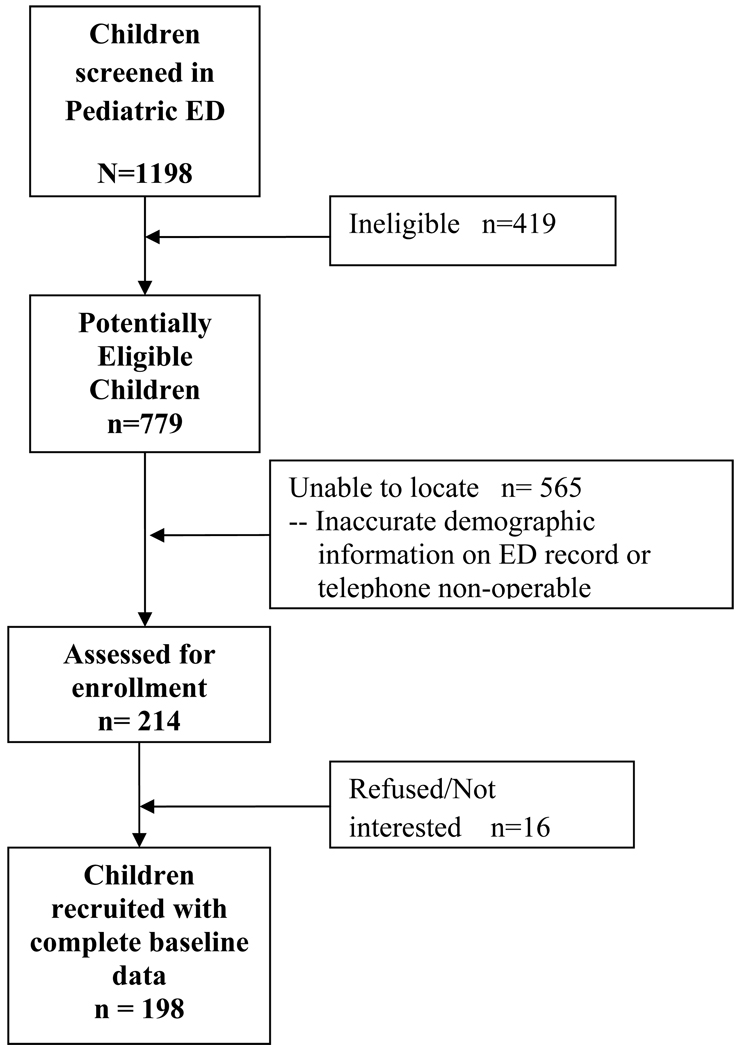
Children with asthma were identified and recruited from daily review of medical records obtained from the Johns Hopkins Hospital and University of Maryland Medical System pediatric emergency departments (EDs). As seen in Figure 1, a total of 779 potentially eligible children were contacted via letters, 214 were assessed for enrollment and 198 (93%) were enrolled n a provider-parent behavioral based randomized controlled trial, Pediatric Asthma Alert Intervention (PAAL). There were no significant differences in age, gender, race/ethnicity, type of health insurance or zipcode noted between the enrolled and a subset of non-enrolled (N=103) subjects. Baseline interviews were completed from December 2008 to June 2010. Eligibility criteria included age 3–10 years, physician-diagnosed asthma with persistent symptoms, and two or more ED visits or one hospitalization for asthma during the past 12 months. Children were excluded if they were diagnosed with other chronic lung diseases or currently enrolled in another asthma study. Parents were informed that the goal of the study was to determine if providing parents and their child’s health care provider with information about the child’s asthma health status. i.e. symptom frequency, medication adherence or cotinine level, would decrease the frequency of the child’s ED visits for asthma. Parents who agreed to participate received a home visit by a trained research assistant to obtain written consent, administer the baseline questionnaire and obtain a saliva sample from the child subject. Up to 5 attempts, at varying times and days, were made to contact all potentially eligible participants by telephone or home visit. Participants received $30.00 for completion of the baseline evaluation. The study was approved by the Institutional Review Boards of the Johns Hopkins University Medical Institutions and the University of Maryland School of Medicine. The trial was registered with clinicaltrials.gov with registration number NCT00860418.
Figure 1.
Subject recruitment and retention flow chart.
Measures
Second Hand Smoke (SHS) Exposure
Salivary cotinine measurement and caregiver self-report were used to assess the child’s level of SHS exposure. Saliva samples were collected from each child using two separate sorbettes (an applicator with small sponge the size of a small q-tip) that were placed under the child’s tongue to collect 1 ml of saliva for analysis. The sorbettes were placed in small micro-container and sent to Salimetrics Laboratory, State College, PA for enzyme immunoassay (EIA) analysis and results are reported as nanograms per milliliter. The lower limit of cotinine sensitivity is 0.05 ng/ml and average intra and inter-assay coefficients of variation were less than 5.8% and 7.9%, respectively. Undetectable cotinine levels were recorded as 0 (n= 5). We used 1.0 ng/ml as the cutoff point to define SHS exposure based on prior studies. (22, 23) We achieved a 96% saliva collection rate from all children at baseline (n= 190).
The baseline interview included detailed closed-ended questions regarding household smoking behavior such as number and identification of every household smoker (caregiver, significant other, relatives, sibling, visitor), number of cigarettes smoked per day by each smoker, usual location of smoking (either outdoor, indoor or both locations) and if smoking occurred indoors, the primary location of indoor smoking (living or TV room, kitchen, dining room, parent’s bedroom, child’s bedroom, basement). Because the caregiver was the predominant household smoker (57%), we dichotomized household smokers as caregiver vs. other smoker. Home and car smoking restrictions were ascertained by asking the parent “how is cigarette smoking handled in your home” with one of three close-ended responses of (1) total ban (“no one allowed to smoke in my home without exception”), (2) partial ban (“Only special guests are allowed to smoke in my home or smokers are only allowed to smoke in certain areas of my home”) or (3) no ban (“people are allowed to smoke anywhere in my home”). (24)
Demographic Measures
Child demographic variables included age, gender, race (African American/black, white, or other), ethnicity (Hispanic or non-Hispanic) and insurance status (Medicaid or private insurance/self pay). Caregiver characteristics included age, relationship to child (mother or other), education (less than high school, high school or more), employment (full-time, part-time or not employed) and income (≤$10,000/yr, $11–29,999/yr or ≥ $30,000/yr).
Caregiver daily stress and asthma stress
Routine daily stress and daily asthma stress over the past month were ascertained by using a 10 point visual analog scale (VAS). Moderate to high reliability and validity have been reported with visual analogue scales in assessment of pain (r=0.88–.98), (25) appetite sensations (r=0.32 to 0.53), (26) and mood disorders. (27) We asked caregivers to select the number between 0 and 10 on the VAS that indicated their level of routine daily stress and asthma stress. Daily asthma stress was defined as level of stress related to caring for their child with asthma. Anchors included “ 0” defined as no stress at all and “10” reflecting the highest level of stress based on anchor phrases in prior VAS mood scales. (28)
Caregiver Depression
The Center for Epidemiological Studies-Depression scale (CES-D) (29) is a 20 item self-report instrument, developed by the National Institute for Mental Health Center for Epidemiologic Studies, used to measure caregiver depression. It is designed to measure subject endorsement of depressive symptoms and has strong reliability and discriminant and convergent validity. (29, 30) Participants responded to items such as “I was bothered by things that don’t usually bother me” with responses of “rarely” (0), “ some of the time” (1), “moderate amount of time” (2) or “most of the time” (3). Total CES-D scores were calculated and a clinical cut point score of 16 was used to indicate the presence of depressive symptoms. (31) The cutoff point of “16” has been shown to have high sensitivity (.95) and specificity (.70) in predicting major depressive disorder in low-income minority women. (32)
Asthma Morbidity Measures
Caregiver reports of number of asthma symptom (cough, wheeze, shortness of breath or chest tightness) days and nights over the past 30 days, limitation of activity due to asthma, ED and regular asthma care follow-up visits with the child’s PCP, hospitalizations and controller or rescue medication use in the prior 6 months were collected. Asthma severity was assigned using symptom frequency based on national asthma guidelines categories. (9) Asthma control was based on parent response to the question “was your child’s asthma well controlled in the past four weeks?” (Yes/No). Use of asthma medications were examined using pharmacy refill records. Pharmacy dispensation records of all asthma medications dispensed over the 12 month period prior to the baseline interview were used as an objective measure of asthma medication use. Pharmacy records were obtained from all pharmacies listed as being used at baseline and were considered complete (97%) if every pharmacy identified by caregivers as used for refills submitted data for the follow-up period and included the dispensing date, product name, strength, dosage form, and quantity dispensed. Pharmacies also reported when child had no fills at their site. Rescue medications were defined as short acting B-agonists (SABA). Controller medications included inhaled corticosteroids (ICS), leukotriene modifiers (LTM), long acting beta agonists (LABA), and mast cell stabilizers (cromolyn). Oral corticosteroid (OCS) prescription fills were analyzed separately. Pharmacy data were analyzed based on frequency and amount of medication dispensed as previously reported. (33, 34)
Statistical Analysis
Child salivary cotinine concentration was the primary outcome measure for this analysis. Because there is no safe level of cigarette smoke exposure for children, (35) cotinine concentrations were considered as a continuous measure in our multivariate analysis even though the lowest cotinine level of detection was low at 0.05 ng/ml. Due to the non-normal distribution of salivary cotinine concentrations, all cotinine values were log transformed for analysis. Student t-test and analysis of variance statistics were used to compare univariate analysis of cotinine levels between age groups of 3–5 years (preschool) versus 6–10 years (school-aged), household smoking behaviors including type of home and car smoking ban, caregiver smoking status and caregiver depression and stress scores. Daily stress scores were analyzed as a continuous variable and then dichotomized into 0–5 vs. 6–10 to compare low versus high daily stress. Unadjusted differences in categorical independent variables by cotinine level were conducted using standard X2 tests and ANOVA for continuous variables. Standardized coefficients were use to examine variables in multivariate models; standardizing all variables with a variance of 1. Multivariate linear regression was used to assess independent effects of child age, type of home and car smoking ban, number of smokers in the home, caregiver depression and stress scores in models predicting level of child cotinine concentrations. Two-sided tests were used and p values ≤ 0.05 were considered significant. All data analyses were performed using SAS V.8.0 (36) and Stata V.8.2.0 (37) software.
Results
Subject Characteristics
Children were primarily male (63%), African American (95%), and enrolled in Medicaid (91%), with a mean age of 5.5 years. (Table 1) Caregivers were predominantly the child’s biological mother (92%), achieved at least a high school education (72%), single (66%), and had a mean age of 32 years. Overall, caregiver daily stress levels were high (mean: 6.2 out of 10, SD 3.0) but somewhat lower for asthma daily stress (mean 5.3 out of 10, SD 4.9). Over one third (34%) of caregivers were categorized with depressive symptoms based on score > 16 on the CESD.
Table 1.
Child and Caregiver Sociodemographic Characteristics at Baseline (N=198)
| Characteristic | Number (%) |
|---|---|
| Child | |
| Gender | |
| Male | 124 (62.6) |
| Mean Age(SD) | 5.5 (2.2) |
| Race/Ethnicity | |
| African American | 188 (95.0) |
| Hispanic | 3 (1.5) |
| White | 2 (1.0) |
| Other | 5 (2.5) |
| Health Insurance type | |
| Medical Assistance | 181 (91.4) |
| Private insurance/Self-pay | 17 (8.6) |
| Caregiver | |
| Birth Mother | 182 (91.9) |
| Birth Father | 7 (3.5) |
| Grandmother/legal guardian/stepfather | 9 (4.6) |
| Education (n=197) | |
| 9–11 years | 56 (28.4) |
| HS grad | 79 (40.1) |
| Some college/technical school | 52 (26.4) |
| College grad | 10 (5.1) |
| Marital status (n=196) | |
| Married | 39 (19.9) |
| Single | 130 (66.3) |
| Divorced/Widowed/other | 27 (13.8) |
| Mean Age (SD) | 32.3 (9.7) |
| Employed (Full or part time) | |
| YES | 87 (45.1) |
| Household Income (n=165) | |
| ≤ $10,000 | 74 (44.8) |
| $11,000–29,000 | 64 (38.8) |
| $30,000 or more | 27 (16.4) |
| Daily Stress Score | |
| Daily Stress scores | |
| Mean (SD) | 6.80 (3.0) |
| 0–5 | 75 (37.9) |
| 6–10 | 123 (62.1) |
| Score of 10 or highest daily stress score = 22% | |
| Asthma Daily Stress Scores (n=197) | |
| Mean (SD) | 4.71 (3.8) |
| 0–5 | 107 (54.3) |
| 6–10 | 90 (45.7) |
| Score of 10 or highest asthma daily stress score = 19% | |
| Caregiver Depression (N=189) | |
| ≥16 score (clinical depressive symptoms) | 65 (34.4) |
Table 2 shows that asthma morbidity was high, as expected by the enrollment criteria, with over half (52%) reporting 5 or more symptom days and 45% reporting 5 or more symptom nights during the past 2 weeks with a mean of 3.1 ED visits over the past 6 months. Almost half (46%) reported daily rescue medication use and limited activity for 4 or more days per last 4 weeks (52%), yet most caregivers (55%) reported feeling that their child’s asthma was controlled. Few children (19%) received asthma specialty care over the past 2 years.
Table 2.
Health Characteristics at Baseline (N=198)
| Characteristic | Number (%) |
|---|---|
| Number symptom days past 2 weeks | |
| 0–4 | 95 (48.0) |
| 5–13 | 56 (28.3) |
| 14 (everyday) | 47 (23.7) |
| Number of symptom nights past 2 weeks (N=197) | |
| 0–4 | 108 (54.8) |
| 5–13 | 40 (20.3) |
| 14 (every night) | 49 (24.9) |
| Rescue Medication use past 4 weeks (N=185) | |
| Not at all or Once a week | 39 (21.1) |
| Few times per week | 60 (32.4) |
| 1 or 2 times a day | 58 (31.4) |
| 3 or more times a day | 28 (15.1) |
| Number of Steroid courses past 6 months (n=193) | |
| None | 15 (7.8) |
| 1 | 73 (37.8) |
| 2 | 50 (25.9) |
| 3 | 31 (16.1) |
| 4 or more | 24 (12.4) |
| Asthma Control past 4 weeks (Parent Report) | |
| Yes | 108 (54.8) |
| No | 66 (33.5) |
| Unsure | 23 (11.7) |
| Limitation of Activity past 4 weeks (n=197) | |
| Not at all | 44 (22.3) |
| 1–3 days | 50 (25.4) |
| 4–10 days | 46 (23.4) |
| 11 or more days | 57 (28.9) |
| School days missed last 6 months due to asthma (n=190) | |
| None | 37 (19.5) |
| 1–5 | 70 (36.8) |
| 6–10 | 43 (22.6) |
| 11 or more days | 40 (21.1) |
| Health Care Utilization | |
| Regular Asthma Follow-up Visits past 6 months | |
| Mean (SD) | |
| None | 21 (10.6) |
| 1 | 48 (24.2) |
| 2 | 53 (26.8) |
| 3 | 35 (17.7) |
| 4 or more | 41 (20.7) |
| Number of Hospitalizations for Asthma past 6 months (n=194) | |
| None | 132 (68.0) |
| 1 | 48 (24.8) |
| 2 or more | 14 (7.2) |
| Number of ED visits for Asthma past 6 months (n=196) | |
| Mean (SD) | 3.1 (2.7) |
| None | 7 (3.6) |
| 1 | 44 (22.5) |
| 2 | 57 (29.0) |
| 3 or more | 88 (44.9) |
| Seen by Asthma Specialist in past 2 years | |
| Yes | 37 (19.2) |
Second Hand Smoke Exposure and Household Smoking Characteristics
More than half of the children (53%) had evidence of SHS exposure based on a cotinine concentration level >1.0 ng/ml and 63% of the children lived in a home with a smoker by self-report. (Table 3). Mean salivary cotinine level was high at 2.42 ng/ml (SD 3.2) with a range of 0 to 21.3 ng/ml. Mean log cotinine concentration was 0.00g ng/ml (SD 1.5). The child’s caregiver was the predominant smoker in the home (57%) although one in four children lived in a home with more than one smoker. In homes with multiple smokers, the caregiver and her significant other were the predominant smokers (70%) followed by a relative or regular visitors (25%). In homes with only one smoker, the caregiver remained the predominant smoker (43%) followed by her spouse/significant other (25%) and a relative or visitor (32%). Among caregivers who smoked their bedroom was the most common indoor smoking location and 45% reported only smoking outdoors. Total home smoking bans were reported in almost two-thirds (63%) of homes and 76% reported a total car smoking ban.
Table 3.
Child and Caregiver/Household Smoking Characteristics
| Number (%) | |
|---|---|
| Child Characteristics | |
| Positive SHS exposure based on cotinine measurement (cotinine > 1 ng/ml)a | 105 (53%) |
| Positive SHS exposure based on caregiver report of exposure | 124 (63%) |
| Mean (SD) cotinine concentration (range 0–21.3 ng/ml) | 2.39 ng/ml (3.2) |
| Caregiver/Household Smoking Characteristics | |
| Smoker in home based on caregiver report (N=124)b | |
| Caregiver | 70 (56.5) |
| Spouse/significant other | 21 (16.9) |
| Relative | 24 (19.4) |
| Sibling | 3 (2.4) |
| Visitor (regular household visitor) | 6 (4.8) |
| Number of smokers in the homec | |
| 0 | 77 (38.9) |
| 1 | 72 (36.4) |
| 2 | 38 (19.2) |
| 3 or more | 11 (5.5) |
| Caregiver number of cigarettes smoked/day (n=118) | |
| 1–4 cigarettes/day | 41 (34.7) |
| 5 or more cigarettes/day | 77 (65.3) |
| Primary Indoor Smoking location (n=106) | |
| Caregiver bedroom | 40 (37.7) |
| Living room or TV room | 20 (18.9) |
| Kitchen | 21 (19.8) |
| Dining room | 16 (15.1) |
| Child’s bedroom | 9 (4.6 8.5) |
| Caregiver smoking indoor/outdoor | |
| Outdoor only | 56 (45.2) |
| Indoor/Outdoor | 60 (48.4) |
| Indoor only | 8 (6.4) |
| Type of home smoking ban | |
| Total | 124 (62.6) |
| Partial | 50 (25.3) |
| None | 24 (12.1) |
| Type of car smoking ban | |
| Total | 150 (75.8) |
| Partial | 29 (14.6) |
| None | 19 (9.6) |
As shown in Table 4, significantly higher mean cotinine levels were noted in younger children, children residing in homes with a higher number of household smokers, homes with no or a partial home smoking ban, and homes with a caregiver reporting depressive symptoms and greater than moderate levels of daily stress scores. A trend was noted for higher mean cotinine concentration in children when their caregiver was the household smoker (p=0.07). Cotinine concentrations did not differ by any health characteristic. . Cotinine concentrations did not differ by gender, level of asthma control, number of SABA, ICS and OCS fills over the past year, number of symptom days during the past month or number of ED visits over the past 6 months (Table 4).
Table 4.
Child Cotinine Levels by Sociodemographic and Health Characteristics. N=190d
| Characteristic | Cotinine Mean (SD) |
Statistic for Difference in Cotinine Concentrationsc |
|---|---|---|
| Child Age | ||
| 3–5 years | 2.88 (3.7) | F=8.14, df=1, p=0.005 |
| 6–10 years | 1.75 (2.3)a | |
| Child Gender | ||
| Male | 2.31 (3.3) | F= 0.04, df=1, p=0.84 |
| Female | 2.51 (3.1) | |
| Number of Smokers in Home | ||
| 0 | 1.19 (2.0) | F=12.88, df=4, p < 0.0001 |
| 1 | 2.64 (3.4) | |
| 2 | 3.24 (2.7) | |
| 3 | 4.34 (2.7) | |
| 4 | 11.06 (10.6)b | |
| Smoker in home | ||
| Caregiver | 3.28 (3.0) | F=3.40, df=1, p=0.07 |
| Other household member | 2.93 (4.3) | |
| Type of Home Smoking Ban | ||
| Total | 1.62 (2.6) | F=20.08, df=2, p <0.0001 |
| Partial | 3.45 (4.1) | |
| No restrictions | 4.02 (2.9)b | |
| Type of Car Smoking Ban | ||
| Total | 2.08 (3.0) | F=5.82, df=2, p=0.004 |
| Partial | 2.81 (3.7) | |
| No restrictions | 4.16 (3.8)a | |
| Caregiver Depression (CES-D scores) | ||
| 0–15 | 1.98 (3.1) | F=12.42, df=1, p=0.0005 |
| 16–55 (Depressive symptoms) | 3.20 (3.4)a | |
| Caregiver Daily Stress Score (0–10) | ||
| 0–5 | 1.85 (2.7) | F=3.83, df=1, p=0.05 |
| 6–10 (> moderate daily stress) | 2.71 (3.5) | |
| Caregiver Daily Asthma Stress Score (0–10) | ||
| 0–5 | 2.13 (2.9) | F=1.93, df=1, p=0.17 |
| 6–10 (> moderate daily stress) | 2.70 (3.6) | |
| Health Characteristics | ||
| Asthma Control | ||
| Yes | 2.69 (3.7) | F=0.76, df=1, p=0.39 |
| No | 2.15 (2.8) | |
| SABA fills past 12 months | ||
| 0–2 | 1.74 (2.2) | F=0.58, df=2, p=0.55 |
| 3–5 | 3.38 (3.9) | |
| 6 or more | 2.64 (4.2) | |
| ICS fills past 12 months | ||
| 0–2 | 2.46 (3.3) | F=1.60, df=2, p=0.21 |
| 3–5 | 1.07 (1.1) | |
| 6 or more | 3.49 (4.6) | |
| Oral corticosteroid fills past 12months | ||
| 0–2 | 2.46 (3.3) | F=0.18, df=2, p=0.84 |
| 3–5 | 1.07 (1.1) | |
| 6 or more | 3.49 (4.6) | |
| Symptom days | ||
| 0–3 days | 2.39 (3.2) | F= 0.38, df=2, p=0.69 |
| 4–10 days | 2.85 (3.9) | |
| 11+ days | 2.07 (2.7) | |
| Number ED visits past 6 months | ||
| 0–1 | 2.34 (3.8) | F= 0.33, df=2, p=0.72 |
| 2–3 | 2.61 (3.2) | |
| 4 or more | 2.01 (2.7) | |
p ≤ 0.05
p ≤ 0.01
cotinine was log-transformed for the analysis of differences by variable.
Children with low cotinine levels (less than or equal to 1.0 ng.ml) were significantly more likely to reside in a home with a total home smoking ban than children with >1.0 ng/ml cotinine levels (low cotinine: 85% versus high cotinine: 47%, p<0.001) and were older (mean age, low cotinine: 6.0 versus high cotinine: 5.5 years).
When comparing mean cotinine levels by type of home smoking ban (no restrictions, partial restrictions, or total home smoking ban) and by child age (younger versus older children), mean cotinine concentrations were significantly lower in older children who live in a home with a complete smoking ban, as compared to older children who lived in a home with no or partial restrictions on smoking (mean cotinine: 0.63 vs. 2.58 vs. 4.85 ng/ml, respectively, p <0.05). (Table 5) However this trend was not as striking for younger children. . Younger children who lived in a home with a complete smoking ban had significantly higher cotinine concentrations compared to older children living in a home with a complete smoking ban.(Mean cotinine: 3–5 year olds, 2.24 ng.ml; 6–10 year olds, 0.63 ng/ml).
Table 5.
Mean (SD) Cotinine Levels by Type of Home Smoking Ban for Younger versus Older Children.
| Type of Home Smoking Ban | |||
|---|---|---|---|
| Age Groupa | No Restrictions | Partial Restrictions | Total Ban |
| 3–5 year olds | 3.74 (3.8) ng/ml | 4.28 (4.3) ng/ml | 2.24 (3.5) ng/ml |
| 6–10 year olds | 4.85 (2.4) ng/ml | 2.58 (2.6) ng/ml | 0.63 (1.0) ng/ml |
p < 0.05
Multivariate Analysis of Factors Associated with Baseline Cotinine levels
In a multivariate linear regression model estimating baseline cotinine level, we found that increased number of household smokers (β = 0.24), younger child age (3–5 years) (β = 0.23), having a household smoking ban (β = 0.20), caregiver report of depressive symptoms (β = 0.17) and living with a caregiver who smokes (β =0.16) all were associated with higher cotinine concentrations in the children(P <0.001, R2 =0. 35) (Table 6). When a similar multivariate model was tested using child age and caregiver daily stress scores as continuous variables, child age, having a home smoking ban, and number of smokers in the home were the only variables significantly associated with the child’s cotinine level. Number of symptom days, number of short acting beta agonist and oral corticosteroid fills during past 12 months and interaction terms between child age and type of home smoking ban included in other models were not associated with higher child cotinine concentrations.
Table 6.
Multivariate Regression Model Predicting log Cotinine Levels
| Predicting log Cotinine Level (N=190) | ||
|---|---|---|
| Standardized β |
P value | |
| Child Age | ||
| 3–5 years | 0.23 | <0.001 |
| 6–10 years | a | |
| Number of Smokers in Home | 0.24 | 0.002 |
| Type of Home Smoking Ban | ||
| Total | a | |
| Partial | 0.20 | 0.003 |
| None | 0.20 | 0.004 |
| Caregiver CES-D score | ||
| 0–15 | a | |
| ≥16 | 0.17 | 0.02 |
| Caregiver Daily Stress Score | ||
| 0–5 | a | |
| 6–10 | −0.03 | 0.69 |
| Caregiver is Smoker | ||
| Yes | 0.16 | 0.03 |
| No | a | |
Referent group
Discussion
Young inner city children with asthma continue to be exposed to second hand smoke, despite its known association with increased chronic airway inflammation, risk for severity of asthma, (1–6) and difficulty in managing asthma symptoms. (7,8) Alarmingly, in our community-based, inner-city sample of high-risk young children with asthma, over half were exposed to second hand smoke and their caregiver was most commonly the household smoker. This rate of SHS exposure exceeds the 2007–2008 U.S. rate (18.2%) of children aged 3–11years living with someone who smoked inside the home (38) and is consistent with previously reported ranges of low income children with asthma who reside with ≥ 1 smoker (47% to 69%). (12,22,39,40)
Young child age and increased number of household smokers had the strongest associations with the child’s SHS exposure. Young children are particularly at risk for high SHS exposure because they may spend up to 90% of their time in the home. (41) This is a particular concern in light of our data indicating that many children lived in a home with two or more household smokers. The predominant household smoker was the child’s caregiver who generally was the biological mother. Our rate of caregiver smoking is comparable to prior reports of low income children with asthma. (24) For young, preschool aged children, caregiver smoking likely results in close proximity and prolonged exposure between the smoker and the child. Moreover, most caregivers reported smoking in their own bedroom, which is likely in close proximity to the child.
Importantly, younger children had significantly higher cotinine levels than older children even when a total smoking ban was reported in the home. This suggests that total home smoking bans may be less effective for younger children. One explanation for this is that the young child may need to accompany the caregiver outside the home for the caregiver to smoke, thus the child remains exposed to SHS. Alternatively, caregivers may be less likely to truly implement a total home ban due to the difficulty leaving a young child alone in the home in order to smoke elsewhere. Moreover, the child also may experience significant exposure to smoke at an outside location, such as at a childcare site or relative’s home.
Caregiver depression and stress were high in this group of families with over one third of the caregivers endorsing symptoms compatible with depression and almost two-thirds endorsing moderate to high daily stress. Interestingly, daily asthma stress was somewhat less prevalent, suggesting that the management of a child’s asthma may have been less stressful than the daily stress of food and housing insecurity, neighborhood violence and ongoing family illnesses. We observed an association between caregiver report of depressive symptoms and higher child cotinine levels as noted in prior studies. (22) This suggests that counseling for caregivers who smoke needs to incorporate both psychosocial screening and support.
Of note is the discordance between caregiver’s perception of the child’s well controlled asthma and moderately high symptom frequency and increased limitation of activity. Over half of caregivers rated their child’s asthma as controlled yet 52% reported limitation of activity of the child at least 4 or more days per month. This discordance may suggest caregiver tolerance of suboptimal respiratory status of the child, inappropriate expectation of child exercise capability or poor symptom recognition by the caregiver or child and highlights the need for caregiver education of accurate symptom recognition.
Pediatric providers can play an important role in reducing child SHS exposure by providing counseling for smoking cessation. (42) Nationally 70% of smokers report a desire to quit each year, yet only 34% attempt and only 10% succeed in quitting and remain tobacco-free for a year. (35) Unfortunately, quit rates are lowest in less educated adults (43) represented by one out of four caregivers in our study population. Opportunities for primary care providers to address caregiver smoking cessation are numerous if families adhere to the schedule of well child and asthma care visits.(42) For caregivers who are not ready to quit, providers can promote implementation of home smoking bans to protect their child from SHS exposures. However, it is important to note that we found that cotinine concentrations were elevated even among children whose caregivers reported a total home smoking ban, particularly for those less than 6 years of age. It may be that more specific counseling for parents of young children is need to help them implement a truly effective home smoking ban, in that our data may reflect parental inability to enforce a total home smoking ban. Further, periodic child cotinine testing may provide valuable feedback to families about the effectiveness of their efforts.
Our data also support the need for stronger policies for restricting SHS exposure in locations outside the home where children spend a large proportion of time. A child may be exposed to smoke in various locations, including the homes of extended family, neighbors and child care settings. (44,45) Recent data also suggest that children living in apartments might be exposed to SHS from smokers in nearby apartment units. (46) Promoting a larger ecological change in culture that includes community-wide adjustment in restricting smoking in private residences may help to reduce SHS exposure for children. (44,47)
Our study has limitations to be noted. First, we were unable to account for all SHS exposures outside of the home and household smoking behavior was based only on caregiver report. Salivary cotinine concentrations reflect SHS exposure at one point in time and may not reflect long term exposure. However, caregiver report of SHS exposure among children with asthma remains relatively stable over time. (12) We purposely enrolled high risk children with significant asthma morbidity into our study, and this may have limited our ability to detect differences in asthma morbidity by levels of smoke exposure. Lastly, our findings can only be generalized to similar populations.
Conclusion
Over half of young high-risk children with asthma were exposed to second hand smoke and their caregiver was the predominant household smoker in this sample of inner city children. Younger children and children with depressed and stressed caregivers were at significant risk of smoke exposure, even when a household smoking ban was reported. Further advocacy for these children needs to include stronger efforts to reduce SHS exposure in private spaces, especially for young children, with the goal to eliminate SHS exposure.
Acknowledgments
Funding: National Institute of Nursing Research, NIH. NR008544
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Morgan WJ. Group Health Medical Associates. Asthma and wheezing in the first six years of life. NEJM. 1995;332(3):133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Henderson FW, Henry MM, Ivins SS, Morris R, Neebe EC, Leu SY, Stewart PW. The Physicians of Raleigh Pediatric Associates. Correlates of recurrent wheezing in school-age children. Am J Respir Crit Care Med. 1995;151(6):1786–1793. doi: 10.1164/ajrccm.151.6.7767521. [DOI] [PubMed] [Google Scholar]
- 3.DiFranza JR, Lew RA. Morbidity and mortality in children associated with the use of tobacco products by other people. Pediatrics. 1996;97(4):560–568. [PubMed] [Google Scholar]
- 4.Martinez FD, Antognoni G, Macri F. Parental smoking enhances bronchial responsiveness in nine-year-old children. Am Review of Respir Dis. 1988;138(3):518–523. doi: 10.1164/ajrccm/138.3.518. [DOI] [PubMed] [Google Scholar]
- 5.Morkjaroenpong V, Rand CS, Butz AM, Eggleston PA. Environmental tobacco smoke exposure and nocturnal symptoms among inner-city children with asthma. J Allergy and Clin Immun. 2002;110(1):147–153. doi: 10.1067/mai.2002.125832. [DOI] [PubMed] [Google Scholar]
- 6.Mannino DM, Homa DM, Redd SC. Involuntary smoking and asthma severity in children. Chest. 2002;122(2):409–415. doi: 10.1378/chest.122.2.409. [DOI] [PubMed] [Google Scholar]
- 7.Halterman JS, Szilagyi PG, Yoos HL, Conn KM, Kaczorowski JM, Holzhauer RJ, Lauver SC, Neely TL, Callahan PM, McConnochie KM. Benefits of a school-based asthma treatment program in the absence of secondhand smoke exposure. Arch Pediatric Adoles Med. 2004;158(5):460–467. doi: 10.1001/archpedi.158.5.460. [DOI] [PubMed] [Google Scholar]
- 8.Ehrlich R, Jordan E, Du TD, Potter P, Volmink J, Zwarenstein M, Weinberg E. Household smoking and bronchial hyper responsiveness in children with asthma. J Asthma. 2001;38(3):239–251. doi: 10.1081/jas-100000111. [DOI] [PubMed] [Google Scholar]
- 9.U. S. Department of Health and Human Services. Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Institutes of Health; National Heart, Lung, and Blood Institute, National Asthma Education and Prevention Program. Full Report 2007. NIH Publication No. 07-4051. [Google Scholar]
- 10.Global Initiative for Asthma. 2006. GINA Workshop Report: Global Strategy for Asthma Management and Prevention. [Google Scholar]
- 11.U.S. Department of Health and Human Services. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. [Google Scholar]
- 12.Halterman JS, Borrelli B, Tremblay P, Conn KM, Fagnano M, Montes G, Hernandez T. Screening for environmental tobacco smoke exposure among inner-city children with asthma. Pediatrics. 2008;122(6):1277–1283. doi: 10.1542/peds.2008-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kattan M, Mitchell H, Eggleston PA, Gergen P, Crain E, Redline S, Weiss K, Evans R, 3rd, Kaslow R, Kercsman C, Leickly F, Malveaux F, Wedner HJ. Characteristics of inner-city children with asthma: the National Cooperative Inner-City Asthma Study. Pediatric Pul. 1997;24(4):253–262. doi: 10.1002/(sici)1099-0496(199710)24:4<253::aid-ppul4>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 14.Eggleston PA, Buckley TJ, Wils-Karp M, Kleeberger SR, Jaakkola JJ. The environment and asthma in U.S. inner cities. Environ Health Perspect. 1999;107(supple 3):439–450. doi: 10.1289/ehp.99107s3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swartz LJ, Callahan KA, Butz AM, Rand CS, Kanchanaraksa S, Diette GB, Krishnan JA, Breysse PN, Buckley TJ, Mosley AM, Eggleston PA. Methods and issues in conducting a community based environmental randomized trial. Environ Res. 2004;95(2):156–165. doi: 10.1016/j.envres.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Delva J, Tellez M, Finlayson TL. Cigarette smoking among low-income African Americans: a serious public health problem. Am J Prev Med. 2005;29(3):218–220. doi: 10.1016/j.amepre.2005.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oddoze C, Dubus JC, Badier M, Thirion X, Pauli AM, Pastor J, Bruguerolle B. Urinary cotinine and exposure to parental smoking in a population of children with asthma. Clini Chemistry. 1999;45(4):505–509. [PubMed] [Google Scholar]
- 18.Oliveti JF, Kercsmar CM, Redline S. Pre- and perinatal risk factors for asthma in inner city African-American children. Am J Epidemiol. 1996;143(6):570–577. doi: 10.1093/oxfordjournals.aje.a008787. [DOI] [PubMed] [Google Scholar]
- 19.Kub J, Jennings JM, Donithan M, Walker JM, Land CL, Butz AM. Life events, chronic stressors, and depressive symptoms in low-income urban mothers with asthmatic children. Public Health Nurs. 2009;26(4):297–306. doi: 10.1111/j.1525-1446.2009.00784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Otsuki M, Eakin MN, Arceneaux LL, Rand CS, Butz AM, Riekert KA. Prospective relationship between maternal depressive symptoms and asthma morbidity among inner-city African American children. J Pediatr Psychol. 2010 Aug;35(7):758–767. doi: 10.1093/jpepsy/jsp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen E, Chim LS, Strunk RC, Miller GE. The role of the social environment in children and adolescents with asthma. Am J Respir Crit Care Med. 2007;176(7):644–649. doi: 10.1164/rccm.200610-1473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar R, Curtis LM, Khiani S, Moy J, Shalowitz MU, Sharp L, Durazo-Arvizu RA, Shannon JJ, Weiss KB. A community-based study of tobacco smoke exposure among inner-city children with asthma in Chicago. J Allergy Clin Immunol. 2008;122(4):754–759. doi: 10.1016/j.jaci.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 23.Scherer G, Meger-Kosslen I, Riedel K, Renner T, Meger M. Assessment of the exposure of children to environmental tobacco smoke (ETS) by different methods. Hum Exp Toxicol. 1999;18(4):297–301. doi: 10.1191/096032799678840075. [DOI] [PubMed] [Google Scholar]
- 24.Wakefield M, Banham D, Martin J, Ruffin R, McCaul K, Badcock N. Restrictions on smoking at home and urinary cotinine levels among children with asthma. Am J Prev Med. 2000;19(3):188–192. doi: 10.1016/s0749-3797(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 25.Price DD, Bush FM, Long S, Harkins SW. A comparison of pain measurement characteristics of mechanical visual analogue and simple numeric rating scales. Pain. 1994;56:217–226. doi: 10.1016/0304-3959(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 26.Flint A, Raben A, Blundell JE, Aastrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single tests meals studies. International J Obesity and Related Metabolic Dis. 2000;24(1):38–48. doi: 10.1038/sj.ijo.0801083. [DOI] [PubMed] [Google Scholar]
- 27.Ahearn EP. The use of visual analog scales in mood disorders: a critical review. J Psychiat Res. 1997;31(5):569–579. doi: 10.1016/s0022-3956(97)00029-0. [DOI] [PubMed] [Google Scholar]
- 28.Folstein MF, Luria R. Reliability, validity and clinical application of the visual analogue mood scale. Psychol Med. 1973;3(4):479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- 29.Andersen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale.) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 31.Goethe JW, Maljanian R, Wolf S, Hernandez P, Cabrera Y. The impact of depressive symptoms on the functional status of inner-city patients with asthma. Ann Allergy Asthma Immunol. 2001;87:205–210. doi: 10.1016/S1081-1206(10)62227-2. [DOI] [PubMed] [Google Scholar]
- 32.Thomas JL, Jones GN, Scarinci IC, Mehan DJ, Brantley PJ. The utility of the CES-D as a depression screening measure among low-income women attending primary care clinics. The Center for Epidemiologic Studies-Depression. Int J Psychiatry Med. 2001;31:25–40. doi: 10.2190/FUFR-PK9F-6U10-JXRK. [DOI] [PubMed] [Google Scholar]
- 33.Broder MS, Gutierrez B, Chang E, Meddis D, Schatz M. Ratio of controller to total asthma medications: Determinants of the Measure. Am J Manag Care. 2010;16(3):170–178. [PubMed] [Google Scholar]
- 34.Schatz M, Zeiger RS, Vollmer WM, Mosen D, Mendoza G, Apter AJ, Stibolt TB, Leong A, Johnson MS, Cook EF. The controller-to-total asthma medication ratio is associated with patient-centered as well as utilization outcomes. Chest. 2006;130(1):43–50. doi: 10.1378/chest.130.1.43. [DOI] [PubMed] [Google Scholar]
- 35.Institute of Medicine (IOM) Clearing the smoke: Assessing the science base for tobacco harm reduction. Washington, DC: National Academy of Sciences; 2001. ISBN 978-0-309-07282-3. [Google Scholar]
- 36.SAS. Version 8.0. Cary, NC: SAS Institute; 1999. [Google Scholar]
- 37.Stata, Version 8.2. College Station, Tex: StatCorp; 2007. [Google Scholar]
- 38.Centers for Disease Control and Prevention (CDC) MMWR. 35. Vol. 59. 2010. Morbidity and Mortality Weekly Report (MMWR). Vital Signs: Nonsmokers’ exposure to secondhand smoke. United States, 1999–2008; pp. 1141–1146. [PubMed] [Google Scholar]
- 39.Gerald LB, Gerald JK, Gibson L, Patel K, Zhang S, McClure LA. Changes in environmental tobacco smoke exposure and asthma morbidity among urban school children. Chest. 2009;135(4):911–916. doi: 10.1378/chest.08-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eggleston PA, Buckley TJ, Breysse PN, Wills-Karp M, Kleeberger SR, Jaakkola JJ. The environment and asthma in U.S. inner cities. Environ Health Perspect. 1999;107(suppl 3):439–450. doi: 10.1289/ehp.99107s3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klepeis NE, Nelson WC, Ott WR, Robinson JP, Tsang AM, Switzer P, Behar JV, Hern SC, Engelmann WH. The National Human Activity Pattern Survey (NHAPS): a resource for assessing exposure to environmental pollutants. J Exposure Anal Environ Epi. 2001;11(3):231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 42.Winickoff JP, Park ER, Hipple BJ, Berkowitz A, Vieira C, Friebely J, Healy EA, Rigotti NA. Clinical effort against secondhand smoke exposure: Development of framework and intervention. Pediatrics. 2008;122(2):e363–e375. doi: 10.1542/peds.2008-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention. Morbidity and Mortality Weekly Report (MMWR) 44. Vol. 54. 2009. Cigarette smoking among adults and trends in smoking cessation—United States, 2008; pp. 1227–1232. [PubMed] [Google Scholar]
- 44.Matt GE, Bernert JT, Hovell MF. Measuring secondhand smoke exposure in children: an ecological measurement approach. J Pediatric Psychology. 2008;33(2):156–175. doi: 10.1093/jpepsy/jsm123. [DOI] [PubMed] [Google Scholar]
- 45.Moon RY, Biliter WM, Croskell SE. Examination of state regulations regarding infants and sleep in licensed child care centers and family child care settings. Pediatrics. 2001;107(5):1029–1036. doi: 10.1542/peds.107.5.1029. [DOI] [PubMed] [Google Scholar]
- 46.Wilson KM, Klein JD, Blumkin AK, Gottlieb M, Winickoff JP. Tobacco-smoke exposure in children who live in multiunit housing. Pediatrics. 2010 December 13; doi: 10.1542/peds.2010-2046. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Winickoff JP, Buckley VJ, Palfrey JS, Perrin JM, Rigotti NA. Intervention with parental smoking in an outpatient pediatric clinic using counseling and nicotine replacement. Pediatrics. 2003;112(5):1127–1133. doi: 10.1542/peds.112.5.1127. [DOI] [PubMed] [Google Scholar]