Abstract
The present study examines the genetic and environmental etiology of the associations among respiratory sinus arrhythmia (RSA), heart rate (HR), skin conductance level (SCL), and non-specific skin conductance responses (NS-SCR)—measures that purportedly index the parasympathetic and sympathetic branches of the autonomic nervous system. The sample was drawn from a cohort of 1,219 preadolescent twins (aged 9–10). Multivariate analyses of the data were conducted using structural equation modeling. Almost all genetic and environmental influences on the measures acted through two latent factors. The first latent factor was largely responsible for the variance in heart rate, SCL and NS-SCR, reflecting sympathetic activity, and its proportions of variance due to genetic and shared environmental influences were 27 and 28% in males, and 31 and 41% in females, respectively. The second latent factor accounted for the variance in RSA and heart rate, reflecting parasympathetic activity; genetic and shared environmental factors explained 27 and 23% of the variance in males, respectively, and 35 and 18% of the variance in females. Measurement-specific genetic effects accounted for 14–27% of the total variance in RSA and SCL, and measurement- specific shared environmental effects accounted for 10–12% in SCL. In general, the validity of separate sympathetic and parasympathetic constructs was supported.
Keywords: RSA, Skin conductance, Heart rate, Heritability, Sympathetic, Parasympathetic
Introduction
Respiratory sinus arrhythmia (RSA), heart rate, and skin conductance are typical psychophysiological measures that index activity of the two independent branches of the autonomic nervous system (ANS): the sympathetic and parasympathetic branches. Because sympathetic and parasympathetic functioning play an important role in regulating physiological arousal and inhibition, it has been suggested that variations in sympathetic and parasympathetic functioning are important biological markers that might discriminate between children with different types of psychopathology (Boyce et al. 2001 691; Garralda et al. 1991 692; Herpertz et al. 2003; van Lang et al. 2007). For example, resting heart rate in children is inversely associated with externalizing psychopathology and positively correlated with anxiety/depression (Lorber 2004; Mezzacappa et al. 1997; Ortiz and Raine 2004; Raine 1996; Rogeness et al. 1990). Several studies have shown a link between RSA and behavioral and emotional regulation (Beauchaine 2001; Porges 1996). Since deficits in behavioral/ emotional regulation characterize much of psychopathology, and are substantially heritable, biological markers of autonomic functioning may be of great importance. They may give clues as to how the genetic predisposition to behavior is ultimately manifested. The purpose of the present study is to examine the genetic and environmental etiology of the variation in RSA, heart rate and skin conductance— measures that purportedly index the sympathetic and parasympathetic branches of the ANS.
RSA is determined largely by vagus-nerve activity that controls fluctuations in heart rate. Given that the vagus nerve inhibits activity of the heart and other organs, RSA is thought to provide a noninvasive index of parasympathetic activity (Berntson et al. 1993; Grossman 1992; Snieder et al. 1997). Skin conductance, on the other hand, is a measure of sympathetic arousal. The basal level of skin conductance (SCL) and frequency of spontaneous or non-specific skin conductance responses (NS-SCR) index eccrine sweat gland activity (Dawson et al. 2007). For example, eccrine sweat glands increase their output during ‘fight-or-flight’ situations (Turner 1989). Although SCL and rate of NS-SCRs are both related to alertness, there is some evidence that they are controlled by separate regions of the cortex. Baseline SCL is highly correlated with neural activity in the orbitofrontal and ventromedial prefrontal cortex (Nagai et al. 2004). On the other hand, transient changes in activity (i.e., SCRs) are dependent on a more distributed network including the diencephalon, limbic system, and lateral regions of the prefrontal cortex. This suggests that the central processes underlying SCL and NS-SCRs may be distinct.
In contrast to RSA and skin conductance, which supposedly provide indices of parasympathetic and sympathetic activation, respectively, the heart receives input from both branches of the ANS. The sinoatrial node, often referred to as the ‘pacemaker’ of the heart, is innervated by both sympathetic and parasympathetic fibers. Whereas the sympathetic fibers speed up cardiovascular activity, the parasympathetic system works to slow it down to conserve energy (Berntson et al. 2007).
Several behavior genetic studies have investigated the relative influence of genetic and environmental factors on individual differences in indices related to parasympathetic and sympathetic arousal. Most studies have focused on RSA and heart rate. In general, non-shared environmental factors (i.e., experiences that make siblings dissimilar) play an essential role in explaining the variance in RSA, with the remaining heritable proportion ranging from 16 to 55% in both adolescent and adult samples (Boomsma et al. 1990; De Geus et al. 2003, 2007; Kupper et al. 2005; Snieder et al. 1997; Snieder et al. 2007). Genetic and non-shared environmental factors have also been found to influence heart rate level (Rice et al. 2002; Snieder et al. 2003), with heritabilities ranging from 36 to 64%. However, there is also some evidence of shared environmental influences (i.e., non-genetic influences that contribute to similarity within pairs of twins) which, in a study of adult twins (aged 18–73), explained 23% of the variance in heart rate (Snieder et al. 2000).
The skin conductance literature is limited to a few studies of adult twins (Lykken et al. 1988; Crider et al. 2004) indicating that genetic, but not shared environmental, factors are important for various indices of skin conductance response. Crider et al. (2004) found that the covariation between rates of non-specific SCRs and habituation of the skin conductance orienting response could be explained by a common latent phenotype that was 53% heritable. Similarly, Lykken et al. (1988) found that genetic factors accounted for 41% of the variance in the number of trials necessary for habituation of the orienting response, with no evidence of shared environmental effects. However, very little is known about the heritability of psychophysiological variables, i.e., electrodermal and cardiovascular measures in children.
No study, to the best of our knowledge, has investigated how genetic and environmental factors contribute to the association among variables reflecting both sympathetic and parasympathetic processes. Since RSA indexes parasympathetic activation and skin conductance largely indexes sympathetic arousal, factor analyses should discriminate between RSA and skin conductance variables. However, heart rate complicates matters somewhat, as it is affected by both branches of the ANS. This suggests that a higher order factor should explain the covariation among heart rate, SCL and NS-SCR. Meanwhile, a second higher order factor should explain the association between RSA and heart rate. By using a genetically informative design it is possible to incorporate these variables simultaneously in a model, and to examine the genetic and environmental etiology of these factors. In sum, we expect that biometrical model-fitting will demonstrate the presence of two latent factors, directly corresponding to the sympathetic and parasympathetic branches.
In the present study, we used data from an ongoing longitudinal twin study of childhood behavior problems, in which psychophysiological recordings were obtained when the twins were 9–10 years old. With these data we investigated the relationships among RSA, heart rate, SCL and NS-SCR in an attempt to understand the genetic and environmental etiology of sympathetic and parasympathetic activity.
Methods
Participants
The sample was drawn from participants in the University of Southern California (USC) Twin Study of Risk Factors for Antisocial Behavior. This is an ongoing prospective longitudinal study of the interplay of genetic, environmental, social, and biological factors on the development of antisocial behavior from childhood to adolescence. The study uses a multi-informant (i.e., self-report, parent-report and teacher-report) and multi-assessment (i.e., interview, observation and mail survey) approach. The twins are evaluated using an extensive protocol, including cognitive, behavioral, psychosocial and psychophysiological measures. The twins and their parents were recruited from the Los Angeles community and the sample is representative of the ethnic and socio-economic diversity of the greater Los Angeles area (Baker et al. 2007). The study includes 605 families (N = 1,219 children). Detailed information regarding the design and recruitment procedures of the project has been provided elsewhere (Baker et al. 2006; Baker et al. 2007). The current study includes data from the first wave of assessment in 2000–2004, when the children were 9–10 years old. The mean age of the full sample was 9.60 years (SD = 0.58). The mean height was 136.7 cm (SD = 7.80) and mean weight was 77.12 lbs (SD = 21.08). There was no sex difference in age, height, or weight.
Zygosity was determined using DNA microsatellite analysis for 87% of twin pairs. For the remaining same-sex twin pairs, zygosity was established by questionnaire items about the twins’ physical similarity and the frequency with which people confuse them. The questionnaire was used only when DNA samples were insufficient for one or both twins. When both questionnaire and DNA results were available, there was a 90% agreement between the two (Baker et al. 2007).
Procedures
The children and their primary caregivers came to the University of Southern California (USC) laboratory for a 6–8 h assessment. The assessment was divided into two sessions, separated by a one-hour lunch break. One twin would be interviewed and do the neurocognitive testing, while the other twin would do psychophysiological assessment. After the lunch break, the twins would switch places. Protocol order was determined randomly prior to the twins’ arrival. The staff was well trained in the psychophysiological lab procedures. While the electrodes and a bellows respiration belt were attached to the child, the interviewer conversed with the child to help the child relax (around 10–20 min). Once the electrodes and the respiration belt were attached to the child, the interviewer left the room and observed the child from a video monitor in an adjacent room.
Before administering several psychophysiological tasks, baseline recordings were obtained using both electrodermal and electrocardiographic channels for 3 min during which time the child was instructed to sit quietly and relax (Rest 1). Following completion of a set of tasks (lasting about 1.5 h), a second electrocardiographic baseline was obtained during another 3 min (Rest 2). Data processing yielded second by second values for skin conductance, heart rate, and RSA, which were then averaged across the 3 min period. For the present paper, we examined average RSA, average SCL, and frequency of NS-SCRs collected during Rest 1, and average heart rate for each of the two threeminute rest periods (heart rate at Rest 1; heart rate at Rest 2).
Psychophysiological data attainment
All psychophysiological data were collected with equipment and software from the James Long Company (1999; Caroga Lake, New York). A 31 channel Isolated Bioelectric Amplifier was used and physiological data were recorded online directly into a data acquisition computer. The signal from the amplifier had a sampling rate of 512 Hz. The amplification rates and high-pass filter (HPF) and low-pass filter (LPF) settings were as follows: electrocardiogram (gain = 0.25 × 1000 voltages, HPF = 0.1 Hz, LPF = 1000 Hz), respiration (gain = individually adjusted, HPF = none/DC, LPF = 10 Hz), and skin conductance (gain = 0.1 volts per microsiemen, HPF = none/DC, LPF = 10 Hz).
Recording of respiratory sinus arrhythmia (RSA)
RSA refers to the magnitude of change in heart rate corresponding to the inspiratory and expiratory phases of the respiratory cycle. Respiration was collected with pneumatic bellows, placed around the participant’s chest and fixed with a metal bead chain. An Interbeat Interval (IBI or R-R intervals) analysis software program provided by the James Long Company, which combines respiratory and cardiac data, was used to estimate RSA. R-waves were automatically detected by the computer program. The R-wave file was later manually corrected to identify incorrect R-waves (e.g., movement artifacts that the computer coded as R-waves), or to score R-waves that were missed by the automated detection. The software computes RSA by using respiration and IBI data as suggested by Grossman (Grossman 1983; Grossman and Svebak 1987). The difference between maximum IBI during expiration and the minimum IBI during inspiration was calculated. This difference, which is measured in seconds, is considered to be a measure of RSA, and is measured twice for each respiration cycle (once for each inspiration and once for each expiration). As the software synchronizes respiratory and cardiac data, it is relatively insensitive to arrhythmia due to tonic shifts in heart rate, thermoregulation, and baroreceptor influence. An average RSA was calculated as the mean value during the initial 3 min period during Rest 1.
Recording of electrocardiogram (ECG): heart rate during Rest 1 and Rest 2
The channels of the grounded electrocardiograph (ECG) were recorded through disposable electrodes that were attached to either side of the participant’s lowest ribs. Before attaching the electrodes, target skin areas were cleaned with alcohol wipes. Heart rate data were analyzed using the Interbeat Interval (IBI) analysis software program (James Long Company). The IBI is measured as time elapsed, in milliseconds, between two successive R peaks in the ECG. That is, the time between two consecutive R-waves provides an accurate measure of the frequency with which the heart beats. The IBI is then converted to Beats per Minute (BPM = (60 × 103)/IBI). ECG signal was sampled every 10th of a second. Heart rate was averaged over the course of 3 min for each of the two baseline periods (Rest 1 and Rest 2)1.
Recording of electrodermal activity: skin conductance level (SCL) and non-specific skin conductance responses (NS-SCR)
Electrodermal activity was recorded from bipolar leads on the distal phalanges of the index and middle fingers using a constant voltage system (Lykken and Venables 1971). Silver-silver chloride (Ag–AgCl) electrodes (7 mm in diameter) were placed on the palmar surface of the non-dominant hand. A water soluble lubricant was used, supported by an adhesive electrode collar that maintained full contact with the skin. The electrodes were further fastened in place using waterproof tape.
Skin conductance level (SCL) refers to tonic level of electrical conductivity of the skin. SCL was averaged over 3 min at rest, and is reported here in microsiemens (μS). Skin conductance responses (SCRs) are phasic changes in the level of electrical conductivity, and are here referred to as non-specific skin conductance responses (NS-SCR) because no specific stimuli were presented. Two trained undergraduate students scored NS-SCRs manually based on visual inspection of the continuous wave form, where each non-specific response was counted only if the change in amplitude was greater than 0.05 μS. To minimize the influence of hand movements on the appearance of NS-SCRs, video coding software from the James Long Company was used to record the exact times of finger movements during the recording session. If the time of an NS-SCR coincided with movement, the response was not counted. NS-SCR is reported here as the number of spontaneous responses during 3 min at Rest 1.
Prior to genetic analysis, RSA, SCL and NS-SCR were square-root transformed to reduce the positive skew in their distributions. Heart rate was normally distributed and was not transformed for the genetic analyses.
Of the 1,219 children in the USC Twin Study of Risk Factors for Antisocial Behavior, 1,185 (97%) came to the USC laboratory for assessment. Data were available on at least one of the measures for 1,160 (98%) of these children, and on all five measures for 667 (58%) of the sample. Thirty percent were missing just one measure, and 12% were missing two or more measures. The reasons for missing values varied depending on the measures. In some instances, NS-SCR data were unusable because too much hand or finger movement interfered with the scoring. Data on heart rate were in some cases unusable because there was too much noise to detect the R-waves. In addition, given that some children dropped out during the 1.5 h interval between the two rest periods, fewer subjects completed Rest 2.
Statistical analyses
Descriptive statistics and correlations
Descriptive statistics (including the mean and standard deviation) were compiled for each of the study variables prior to transformation. To account for the dependency between twins, standard deviations were obtained using PROC MIXED (SAS 2005). Next, saturated models, which perfectly capture the observed variances, covariances and means for each twin and zygosity group, were fit to each of the transformed subscales using the structural equation modeling program, Mx (see below). As these summary statistics are obtained for each zygosity group, potential differences in the mean and variance between Twin 1 and Twin 2 as well as across zygosity groups can be formally assessed through the comparison of various sub-models (see details below for description of model comparisons).
To get a first indication of the underlying sources of variance among heart rate, square-root transformed RSA, SCL and NS-SCR, comparisons were made among within-person correlations (i.e., phenotypic correlations among RSA, heart rate, SCL and NS-SCR), twin correlations (i.e., Twin-1 and Twin-2 correlations within each measure) and cross-twin cross-trait correlations (e.g., RSA score in Twin-1 with heart rate in Twin-2). Twin and cross-twin cross-trait correlations were derived from the saturated models in Mx. The magnitude of the twin correlations provides important information about the genetic and environmental etiology of a given trait. For example, a dizygotic (DZ) twin correlation that is approximately half the value of the monozygotic (MZ) twin correlation would indicate the presence of additive genetic effects; a DZ twin correlation that is more than half a MZ twin correlation indicates the presence of shared environmental effects. The cross-twin cross-trait correlations give information about the genetic and environmental effects between traits. Cross-twin cross-trait correlations are interpreted the same way as twin correlations—that is, greater values for MZ compared to DZ pairs suggests genetic influences on a particular trait. However, this is a descriptive approach which does not specifically identify latent factors underlying covariance across measures. Thus, formal genetic modeling is necessary to test the accuracy of the inferences made from these observations.
Genetic model fitting analyses
The classical twin design is a natural experiment that relies on the different levels of genetic relatedness between monozygotic (MZ) and dizygotic (DZ) twins in order to estimate the relative contribution of genetic and environmental factors to individual differences in a phenotype of interest. The total phenotypic variance of a measured trait can be divided into additive genetic factors (A), shared environmental factors (C), and non-shared environmental factors (E). Since MZ twins share all their alleles, additive genetic factors are perfectly correlated in MZ twins. DZ twins correlate 0.5, because DZ twins share on average half of their alleles. Shared environmental factors refer to non-genetic influences that contribute to similarity within pairs of twins. Shared environmental influences contribute equally to the similarity in MZ and DZ twins, and thus shared environmental factors correlate 1.0 in both MZ and DZ twins. Non-shared environmental factors are those experiences that make siblings dissimilar. There is no correlation for the unique environment by definition, and this parameter also includes measurement error. Heritability refers to the proportion of the phenotypic variance that is attributable to genetic influences (Neale and Cardon 1992).
We used Mx (Neale et al. 2003), a structural-equation modeling program, to perform the model-fitting analyses on raw data by the method of maximum likelihood estimation. This method allows the inclusion of subjects who are missing some data and singletons (i.e., where information from only one twin in a pair is available), which ultimately increases power in the analyses. Goodness of fit of models was assessed by a likelihood-ratio χ2-test, which is the difference between −2 log likelihood (−2LL) of the full model from that of the restricted model. This difference is distributed as a χ2. The degrees of freedom (df) for this test are equal to the difference between the number of estimated parameters in the full model and that in the restricted model. The suitability of the models was also determined by comparing the model’s Akaike Information Criterion (AIC). The AIC represents the balance between model fit and the number of parameters (parsimony), with lower values of AIC indicating the most suitable model (Akaike 1987). Finally, a third model-selection statistic was the Bayesian Information Criterion, where increasingly negative values correspond to increasingly better fitting models (Raftery 1995). Both AIC and BIC are based on the log-likelihood statistic and are used to compare the relative goodness of-fit of competing models. Instead of using the log-likelihood itself, each penalize the log-likelihood according to the pre-specified criteria. The AIC penalty is based upon the number of model parameters. The BIC is penalized by not only the number of parameters, but also the sample size. In larger samples, a greater improvement of goodness of fit is needed before a more complex model is selected over a simpler one (Raftery 1995).
First, univariate models were fit to estimate the relative contributions of additive genetic factors (A), shared environmental factors (C), and non-shared environmental factors (E) to RSA, heart rate, SCL and NS-SCR. To test for sex differences in the variance components, we compared a model in which the magnitudes of genetic and environmental effects were allowed to differ between males and females, against a model in which the estimates were constrained to be equal.
Next, in order to investigate the nature of the relationships among RSA, heart rate, SCL and NS-SCR, three multivariate models were tested: (a) Cholesky decomposition, (b) a two-factor common pathway model and (c) and a one-factor common pathway model. These models were compared to a fully saturated model, in which the means and 10 × 10 matrices of covariances among the five measures were freely estimated. The Cholesky model decomposes the variance of each phenotype (RSA, heart rate during Rest 1 and Rest 2, SCL and NS-SCR), as well as the co-variances among the five phenotypes into genetic (A), shared environmental (C) and non-shared (E) environmental factors. Cholesky models have the same number of factors in each of the A, C, and E components as the number of observed variables. The first genetic factor loads on all five (phenotypic) measures, the second genetic factor loads on all measures except the first measure and so on, until the last genetic factor only loads on the final remaining measure; this same procedure repeats for the shared environmental (C) and non-shared (E) environmental components. Because the Cholesky decomposition is fully parameterized in terms of genetic and environmental variance and covariance, it yields the best possible fit to the data. In contrast, the next model, a two-factor common pathway model includes common genetic and environmental effects (AC, CC, and EC) that influence two latent (unobserved) ANS factors that in turn influence all the observed measures in the model. The model also includes specific genetic and environmental factors (AS, CS, and ES) that are unique to each measure. This twofactor model is easily reduced to one that includes a single latent ANS factor, i.e., a one-factor common pathway model (McArdle and Goldsmith 1990). The use of genetic factor models allows us to test specific hypotheses concerning the relationships among our five observed measures.
Results
Descriptive statistics and correlations
Table 1 presents the number of individual twin participants, as well as the means and standard deviations for the raw (untransformed) variables. Complete information from both twins in a pair were available for RSA: 119 MZ male, 76 DZ male, 119 MZ female, 86 DZ female and 127 opposite-sex (DZOS) pairs, HR1: 121 MZ male, 76 DZ male, 119 MZ female, 86 DZ female and 127 DZOS pairs, HR2: 95 MZ male, 59 DZ male, 97 MZ female, 65 DZ female and 105 DZOS pairs, SCL: 125 MZ male, 78 DZ male, 130 MZ female, 91 DZ female and 137 DZOS pairs, NS-SCR: 115 MZ male, 70 DZ male, 126 MZ female, 83 DZ female and 132 DZOS pairs. The vast majority (>94%) of twins used in the genetic analyses were from complete twin pairs.
Table 1.
Means, standard deviations and number of participants (n) for respiratory sinus arrhythmia (RSA), heart rate (HR1 at rest 1, HR2 at rest 2), skin conductance level (SCL) and non-specific skin conductance response (NS-SCR), by sex and zygosity
| Males
|
Females
|
DZ opposite sex
|
||||
|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | Males | Females | |
| Mean (Standard deviation) | ||||||
| RSA | .11 (.07) | .11 (.06) | .11 (.07) | .11 (.07) | .11 (.07) | .12 (.07) |
| N = 1,070 | n = 240 | n = 153 | n = 242 | n = 172 | n = 127 | n = 136 |
| HR1 | 84.58 (11.97) | 85.12 (9.55) | 88.23 (11.90) | 87.69 (11.54) | 84.40 (11.81) | 86.45 (10.94) |
| N = 1,078 | n = 244 | n = 155 | n = 242 | n = 174 | n = 127 | n = 136 |
| HR2 | 82.27 (11.75) | 83.34 (10.62) | 86.48 (11.77) | 86.52 (10.84) | 81.89 (11.86) | 84.67 (10.97) |
| N = 875 | n = 197 | n = 125 | n = 203 | n = 138 | n = 105 | n = 107 |
| SCL | 7.71 (4.51) | 7.84 (4.08) | 7.28 (4.56) | 7.67 (4.22) | 8.31 (4.66) | 7.57 (3.54) |
| N = 1,133 | n = 251 | n = 158 | n = 261 | n = 184 | n = 137 | n = 142 |
| NS-SCR | 7.60 (8.15) | 7.34 (7.33) | 7.15 (8.32) | 7.40 (7.64) | 8.34 (7.80) | 7.97 (6.91) |
| N = 1,069 | n = 233 | n = 143 | n = 254 | n = 171 | n = 132 | n = 136 |
MZ monozygotic, DZ dizygotic, N number of participants
No significant mean or variance differences were found between twin-1 and twin-2 (results available upon request). Moreover, there were no mean or variance differences between MZ and DZ twins of the same sex. For heart rate, mean sex differences were found, with males showing lower mean heart rate than females at both Rest 1 and Rest 2 [HR1: Mmale = 84.69 (SD: 10.74), Mfemale = 87.62 (SD: 10.27), χ2 = 23.78; df = 9; p<.001; HR2: Mmale = 82.49 (SD: 10.61), Mfemale = 86.06 (SD: 10.04), χ2 = 30.89; df = 9; p<.001].
Table 2 shows phenotypic correlations among the measures. Heart rate was strongly correlated across the two rest periods for both boys and girls. Similarly, a significant, albeit somewhat more modest, association between SCL and NS-SCR was found for both sexes. There was a substantial negative association between RSA and heart rate, across Rest 1 and Rest 2 for both sexes, although the relationships between heart rate with SCL and NS-SCR were negligible or modest at best, and were significant only among girls.
Table 2.
Phenotypic correlations for respiratory sinus arrhythmia (RSA), heart rate (HR1 at rest 1, HR2 at rest 2), skin conductance level (SCL) and non-specific skin conductance response (NS-SCR), by sex
| Males/females | RSA | HR1 | HR2 | SCL | NS-SCR |
|---|---|---|---|---|---|
| RSA | 1 | −.58* | −.47* | .02 | .05 |
| HR1 | −.56* | 1 | .85* | .14* | .20* |
| HR2 | −.47* | .82* | 1 | .16* | .16* |
| SCL | .07 | .08 | .06 | 1 | .58* |
| NS-SCR | .11* | .03 | .02 | .61* | 1 |
Females are above the diagonal; RSA, SCL and NS-SCR were square-root transformed,
p<.05
Twin and cross-twin cross-trait correlations are shown in Table 3. The consistently higher MZ as compared to DZ twin correlations suggest genetic influences on all five measures. For example, the twin correlations for RSA were r = .42 for MZ males and r = .31 for MZ females, and the corresponding values for DZ twins were lower: r = .27 and r = .06, respectively. The majority of cross-twin cross-trait correlations were consistently stronger for MZ twins compared to DZ twins, suggesting that genetic effects contribute to the covariation among RSA, HR1 and HR2, and among SCL, NS-SCR, HR1 and HR2. Also of note was the fact that the cross-twin, cross-trait correlations between HR1 and HR2 were nearly as strong as the cross-twin, within-trait correlations for HR1 or HR2, for both MZ and DZ twins, indicating the likely influence of shared measurement error across the two heart rate measures. Etiological patterns suggested by these twin correlations were next tested more formally using structural equation models.
Table 3.
Twin and cross-twin cross-trait correlations between for respiratory sinus arrhythmia (RSA), heart rate (HR1 at rest 1, HR2 at rest 2), skin conductance level (SCL) and non-specific skin conductance response (NS-SCR)
| RSA | HR1 | HR2 | SCL | NS-SCR | RSA | HR1 | HR2 | SCL | NS-SCR | |
|---|---|---|---|---|---|---|---|---|---|---|
| MZ Males | MZ Females | |||||||||
| RSA | 0.42 | 0.31 | ||||||||
| HR1 | −0.22 | 0.42 | −0.19 | 0.48 | ||||||
| HR2 | −0.24 | 0.45 | 0.50 | −0.25 | 0.55 | 0.59 | ||||
| SCL | −0.06 | 0.15 | 0.17 | 0.50 | −0.03 | 0.12 | 0.09 | 0.67 | ||
| NS-SCR | 0.01 | 0.22 | 0.21 | 0.35 | 0.41 | 0.02 | 0.09 | 0.03 | 0.40 | 0.51 |
| DZ Males | DZ Females | |||||||||
| RSA | 0.27 | 0.06 | ||||||||
| HR1 | −0.12 | 0.23 | −0.09 | 0.36 | ||||||
| HR2 | −.014 | 0.33 | 0.37 | −0.05 | 0.37 | 0.36 | ||||
| SCL | 0.08 | −0.18 | −0.08 | 0.32 | −0.03 | 0.07 | 0.09 | 0.45 | ||
| NS-SCR | −0.01 | −0.06 | 0.00 | 0.29 | 0.18 | 0.00 | 0.13 | 0.20 | 0.40 | 0.39 |
| Opposite-sexed twins (male below and female above diagonal) | ||||||||||
| RSA | 0.19 | −0.19 | −0.24 | −0.03 | −0.02 | |||||
| HR1 | −0.31 | 0.41 | 0.47 | −0.04 | −0.03 | |||||
| HR2 | −0.21 | 0.33 | 0.36 | −0.08 | 0.00 | |||||
| SCL | 0.02 | 0.13 | 0.13 | 0.52 | 0.35 | |||||
| NS-SCR | −0.14 | 0.15 | 0.14 | 0.27 | 0.36 | |||||
Twin correlations on the diagonal and cross-twin cross-trait correlations below the diagonal
Univariate model fitting results
Table 4 displays univariate model fitting results for each measure. Variance components were estimated from a full univariate ACE model, which compared favorably to the saturated model in all cases (specific results of model fitting are available from the first author). Genetic and environmental variances could be equated across sex for RSA (Δχ2 = 3.39, df = 3, p = 0.34), HR1 (Δχ2 = 3.66, df = 3, p = 0.30), HR2 (Δχ2 = 2.14, df = 3, p = 0.54), and NS-SCR (Δχ2 = 5.15, df = 3, p = 0.16). When sexes were combined, genetic influences accounted for between 30 and 48% of the variance in each of these measures, shared environmental influences were modest and non-significant, and the non-shared environment accounted for between 41 and 61% of the variance.
Table 4.
Univariate results for respiratory sinus arrhythmia (RSA), heart rate (HR1 at rest 1, HR2 at rest 2) skin conductance level (SCL) and non-specific skin conductance response (NS-SCR)
| Standardized variance components
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Malesa |
Femalesa |
Males = Females
|
|||||||
| A | C | E | A | C | E | A | C | E | |
| RSA | .35 (.00–.60) | .14 (.00–.49) | .52 (.40–.67) | .31 (.00–.46) | .00 (.00–.28) | .69 (.54–.86) | .39 (.19–.49) | .00 (.00–.15) | .61 (.51–.71) |
| HR1 | .28 (.00–.53) | .16 (.00–.46) | .57 (.44–.72) | .35 (.00–.63) | .20 (.00–.49) | .46 (.34–.61) | .30 (.02–.55) | .19 (.00–.39) | .52 (.43–.63) |
| HR2 | .51 (.00–.69) | .08 (.00–.54) | .41 (.30–.58) | .45 (.00–.71) | .16 (.00–.53) | .40 (.28–.56) | .48 (.19–.68) | .11 (.00–.34) | .41 (.32–.52) |
| SCL | .26 (.00–.52) | .27 (.06–.52) | .47 (.36–.60) | .34 (.07–.60) | .31 (.09–.54) | .35 (.27–.45) | – | – | – |
| NS-SCR | .30 (.00–.53) | .11 (.00–.46) | .59 (.46–.75) | .32 (.00–.63) | .22 (.00–.53) | .46 (.35–.59) | .30 (.02–.55) | .17 (.00–.39) | .53 (.44–.63) |
A additive genetic variance, C shared environmental variance, E non-shared environmental variance
Variance estimates for males and females come from univariate ACE models where parameters are allowed to vary across sexes
In contrast, estimates could not be constrained to be equal in males and females for SCL (Δχ2 = 14.35, df = 3, p<0.01). For males, genetic and shared environmental influences each accounted for a quarter of the variance, and the remaining variance was due to non-shared environmental effects. For females, a third of the variance was due to genetic influences, another third was due to shared environmental influences, and the remaining 35% of the variance was due to non-shared environmental influences.
Multivariate model fitting results
To investigate further the nature of the relationships among RSA, HR1 (Rest 1), HR2 (Rest 2), NS-SCR and SCL, we fit a series of multivariate models (Models #2–4 in Table 5). Specifically, a fully saturated model (Model #1 in Table 5) was used as a baseline to which a Cholesky decomposition (Model #2), a two-factor common pathway (Model #3), and a one-factor common pathway (Model #4) were compared. The two-factor common pathway model provided the best fit to the data based on BIC and AIC criteria, and did not significantly differ from the unconstrained fully saturated model (Δχ2 = 294.67; Δdf = 257; p = 0.053). In contrast, the one-factor model fit the data significantly worse than the fully saturated comparison model (Δχ2 = 738.30; Δdf = 271; p<0.001), and also resulted in more positive AIC and BIC values, indicating that one factor was insufficient to account for the genetic and environmental covariance across measures.
Table 5.
Multivariate model fit indices for respiratory sinus arrhythmia (RSA), heart rate (HR1 at rest 1, HR2 at rest 2) skin conductance level (SCL) and non-specific skin conductance response (NS-SCR)
| Model # | Overall fit
|
Compared to model # | Model difference test
|
|||||
|---|---|---|---|---|---|---|---|---|
| −2LL | DF | AIC | BIC | Δχ2 | Δdf | p | ||
| 1 Fully saturated model | −1820.515 | 4900 | −11620.515 | −16537.402 | ||||
| 2 Cholesky | −1577.138 | 5125 | −11827.138 | −17133.286 | 1 | 243.38 | 225 | .191 |
| 3 Two-factor common pathway | −1525.844 | 5157 | −11839.844 | −17209.694 | 1 | 294.67 | 257 | .053 |
| 4 One-factor common pathway | −1082.213 | 5171 | −11424.213 | −17032.528 | 1 | 738.30 | 271 | <.001 |
| 3a Equate parameters in males and females (loadings + common ACE + specific ACE) | −1471.950 | 5186 | −11843.950 | −17275.234 | 3 | 53.89 | 29 | .003 |
| 3b Factor structure: sympathetic and parasympathetic, male and female parameters allowed to differ | −1508.669 | 5163 | −11834.669 | −17220.242 | 3 | 17.18 | 6 | .009 |
| 3c Sympathetic factor structure in both sexes, i.e., drop RSA from factor 1 | −1523.449 | 5159 | −11841.449 | −17214.875 | 3 | 2.40 | 2 | .302 |
| 3d Sympathetic factor in both sexes and parasympathetic factor in males (i.e., drop SCL and NS-SCR from Factor 2) | −1508.814 | 5161 | −11830.814 | −17213.936 | 3 | 17.03 | 4 | <.001 |
| 3e Sympathetic factor in both sexes and parasympathetic factor in females (i.e., drop SCL and NS-SCR from Factor 2) | −1522.439 | 5161 | −11844.439 | −17220.748 | 3 | 3.41 | 4 | .493 |
| 3f Drop common A | −1514.051 | 5165 | −11844.051 | −17229.311 | 3e | 8.39 | 4 | .078 |
| 3g Drop common C | −1512.732 | 5165 | −11842.732 | −17228.652 | 3e | 9.71 | 4 | .046 |
| 3h Drop common A and common C on Factor 1 | −1409.025 | 5165 | −11739.025 | −17176.798 | 3e | 113.42 | 4 | <.001 |
| 3i Drop common A and common C on Factor 2 | −1434.938 | 5165 | −11764.938 | −17189.755 | 3e | 87.50 | 4 | <.001 |
| 3j Drop specific A (HR1, HR2, NS-SCR) and specific C (RSA, HR1, HR2, NS-SCR) | −1513.938 | 5175 | −11863.938 | −17261.147 | 3e | 8.50 | 14 | .862 |
−2LL −2(log-likelihood), AIC Akaike’s Information Criterion, BIC Bayesian Information Criterion, Δχ2 difference in log-likelihoods between nested models, Δdf change in degrees of freedom, A additive genetic influences, C shared environmental influences, E non-shared environmental influences
Given the similarities across sexes in the univariate results, we also explored the extent to which the factor structure and underlying genetic and environmental etiology varied across sexes, using the full two-factor common pathway model (Model #3) as our comparison model. However, we could not equate the factor loadings (i.e., the paths stemming from the two latent ANS factors to the manifest variables—see Fig. 1), common genetic and environmental influences (e.g., the A1, C1, E1 and A2, C2, E2 that explain variance in the two ANS factors), and measurespecific genetic and environmental effects (As, Cs, and Es in Fig. 1) across sex without resulting in a significant deterioration in fit compared to the full two-factor common pathway model (Table 5: Model 3a, Δχ2 = 53.89; df = 29; p = 0.003). This suggests that there are sex differences in the factor structure and underlying genetic and environmental etiology of the five observed variables.
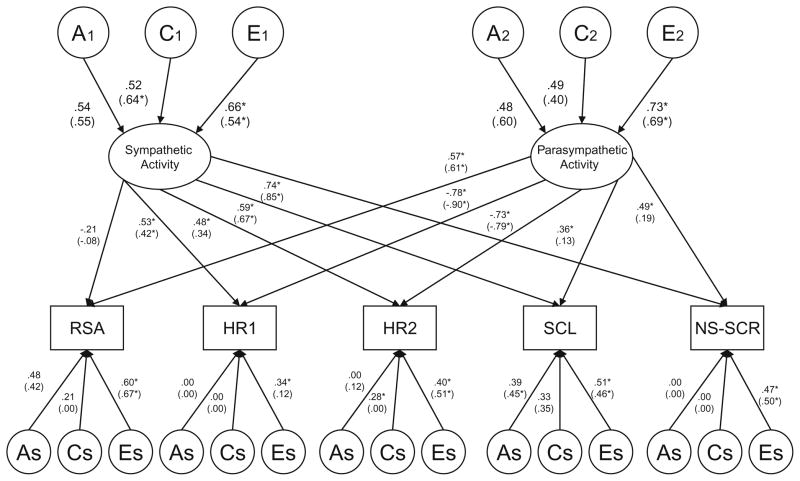
Fig. 1.
Males and (Females). Standardized path estimates from the full two-factor common pathway model for RSA (respiratory sinus arrhythmia), HR1, HR2 (heart rate), SCL (skin conductance level) and NS-SCR (non-specific skin conductance) in 9–10 year old twins. Common additive genetic factors (A), shared environmental factors (C), and non-shared environmental factors (E) are depicted in circles. Ovals denote the two latent factors (i.e., ANS Factor 1 and ANS Factor 2). Measured variables are depicted in rectangles. As: additive genetic residual variance specific to each measure, likewise for shared environment (Cs), and non-shared environment (Es). Female results are presented in parentheses. Significant estimates (p<.05) are marked with *
Based on the magnitude of the factor loadings shown in Fig. 1, for both males and females, the first ANS factor appeared to reflect sympathetic activity, as it was indexed primarily by heart rate, SCL and NS-SCR. Furthermore, the second ANS factor appeared to reflect parasympathetic processes, indexed by strong (and inversely related) factor loadings for RSA and the two heart rate variables. However, loadings for SCL and NS-SCR were also significant for this factor, albeit only in males. Dropping RSA from the first ANS factor as well as SCL and NS-SCR from the second ANS factor for both boys and girls resulted in a significant deterioration in fit compared to the full two-factor common pathway model (Table 5: Model 3b, Δχ2 = 17.18; df = 6; p = 0.009). We consequently proceeded step-by-step, and first tested whether the factor structure could be simplified by dropping RSA from the first ANS factor in both males and females. This could be done without a significant reduction in fit (Table 5: Model 3c, Δχ2 = 2.40; df = 2; p = .302). We next tested whether SCL and NS-SCR could be dropped from the second ANS factor (as would be theoretically predicted) only in males. This resulted in a significant deterioration in fit compared to the full two-factor common pathway model (Table 5: Model 3d, Δχ2 = 17.03; df = 4; p<0.001). However, SCL and NS-SCR could be dropped from the second ANS factor in females without a reduction in fit (Table 5: Model 3e, Δχ2 = 3.41; df = 4; p = 0.493). Thus, the pattern in both sexes indicated that Factor 1 was defined by heart rate, SCL and NS-SCR, reflecting sympathetic activity. Factor 2 was mainly defined by the inverse relationship between heart rate and RSA in females, indicating parasympathetic processes, but the factor structure for Factor 2 could not be further simplified in males. Even though SCL and NS-SCR could not be dropped from the parasympathetic factor in males without a significant reduction in model fit, the factor loadings for these variables were rather small (.17 and .24). This indicates that the structure of the parasympathetic factor was largely similar in males and females.
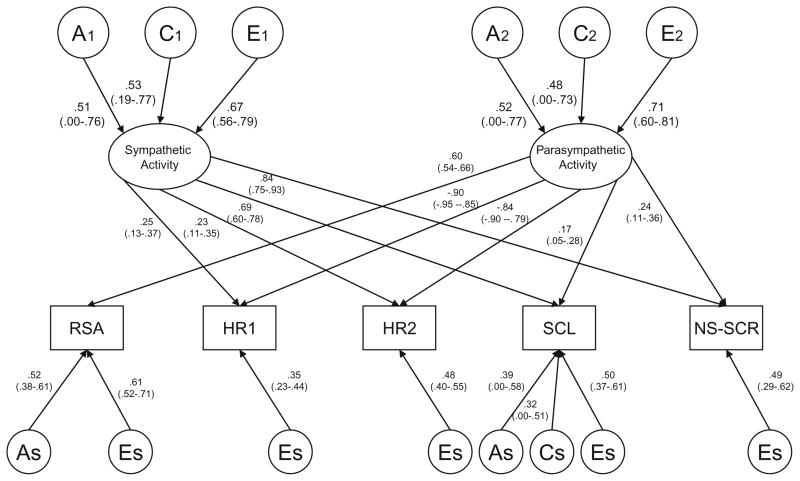
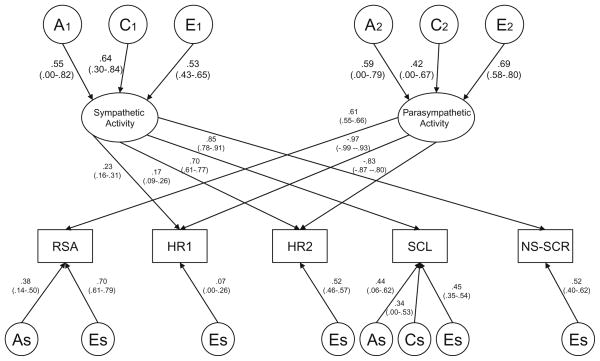
Next, we investigated whether variation in the two latent factors was significantly influenced by both genetic and shared environmental factors by dropping common genetic influences (Model #3f) and common shared environmental influences (Model #3g) from the simplified two-factor common pathway model (Model #3e). While neither model was statistically significant, submodels dropping both common A and common C from Factor 1 (Model #3h) or Factor 2 (Model #3i) did result in a significant decrease in fit. Because the AIC and BIC values were similar for Models #3f and #3g, it was impossible to determine whether genetic or shared environmental influences were more important for variation in the underlying latent factors. Therefore, both common genetic and common shared environmental estimates were kept in the model. Finally, we ran a series of submodels investigating the significance of the genetic and shared environmental factors that were specific to each of the fives measures (results from all submodels available from the first author). The greatest simplification of the model was ultimately achieved by dropping both specific genetic and specific shared environmental influences on HR1, HR2 and NS-SCR, and specific shared environmental influences on RSA (Table 5: Model 3j, Δχ2 = 8.50; df = 14; p = .862). Figures 2 and 3 display standardized parameter estimates from this reduced two-factor common pathway model for males and females, respectively.
Fig. 2.
Final model estimates (males). Standardized path estimates with confidence intervals from the reduced two-factor common pathway model for RSA (respiratory sinus arrhythmia), HR1, HR2 (heart rate), SCL (skin conductance level) and NS-SCR (non-specific skin conductance) in 9–10 year old twins. Common additive genetic factors (A), shared environmental factors (C), and non-shared environmental factors (E) are depicted in circles. Ovals denote the two latent factors (i.e., Factor 1: sympathetic activity and Factor 2: parasympathetic activity). Measured variables are depicted in rectangles. As: additive genetic residual variance specific to each measure, likewise for shared environment (Cs), and non-shared environment (Es)
Fig. 3.
Final model estimates (females). See figure caption Fig. 2
Squaring the standardized parameter estimates presented in Fig. 2 (males) and 3 (females) provides the relative contributions to the phenotypic variance. In addition, squaring the loadings for the common A, C, and E influences on the latent factors gives the overall heritability of the underlying latent sympathetic and parasympathetic factors. For the first latent ANS factor (labelled sympathetic activity) in males, 27% of the variance was due to genetic factors, 28% (p<.05) due to shared environmental factors, and 45% (p<.05) due to non-shared environmental factors. Variance in the second latent ANS factor (labelled parasympathetic activity) was decomposed into respective values of 27, 23, and 50% (p<.05). For females, 31% of the variance in sympathetic activity was due to genetic factors, 41% (p<.05) due to shared environmental factors, and 28% (p<.05) due to non-shared environmental factors. The respective proportions of variance for parasympathetic activity were 35, 18, and 47% (p<.05). For both boys and girls, there were significant variable-specific genetic influences on RSA. In addition, there were variable-specific genetic and shared environmental influences on SCL. While neither estimate was statistically significant, our detailed series of analyses investigating the significance of the variable-specific factors indicated that the specific genetic and specific shared environmental influences on SCL could not be dropped simultaneously from the model without a significant reduction in fit (Δχ2 = 19.82; df = 4; p<.001); thus, both estimates were left in the model.
Table 6 shows the variance components for each of the five measures, based on the factor loadings presented in Figs. 2 and 3. The variance components are divided into influences due to genetic (A), shared environmental (C), and non-shared environmental factors (E), and are further differentiated by common variance due to the sympathetic factor (Factor 1), common variance due to the parasympathetic factor (Factor 2), and variable-specific variance. Comparing variance due to Factor 1 versus Factor 2, it can be seen that a larger proportion of variance in RSA, HR1, and HR2 was due to the parasympathetic factor, whereas a greater proportion of the variance in SCL and NS-SCR was due to variation in the sympathetic factor. Despite the fact that we could not drop SCL and NS-SCR from the parasympathetic factor in males, this factor accounted for only a minority of the overall variance in these two variables (i.e., 4–6% of the total phenotypic variance). While genetic variance from the two latent factors accounted for all of the heritabilities of HR1, HR2, and NS-SCR, a large proportion of the heritabilities of RSA and SCL were due to genetic factors that were specific to each variable. Finally, overall, the estimates shown in Table 6 are consistent with the results from the univariate genetic analyses (Table 4). A possible exception is that the non-shared environment accounts for a greater proportion of variance in HR2 in the multivariate analyses (.61 for both males and females) compared to estimates obtained in the univariate analyses (.41 for males and .40 for females). Over half of this nonshared environmental variance comes from non-shared environmental influences on the parasympathetic factor. We noted earlier that the cross-twin, cross-trait correlations for HR1 and HR2 were similar in magnitude to the cross-twin, within-trait correlations for either HR1 or HR2, for both MZ and DZ twins (Table 3). Thus, the higher estimate of non-shared environmental influence on HR2 is likely due to correlated errors of measurement across HR1 and HR2.
Table 6.
Variance Components from Best-Fitting Reduced 2-Factor Model for respiratory sinus arrhythmia (RSA), heart rate (HR1 at rest 1, HR2 at rest 2) skin conductance level (SCL) and non-specific skin conductance response (NS-SCR)
| Ac (F1) | Ac (F2) | As | A (total) | Cc (F1) | Cc (F2) | Cs | C (total) | Ec (F1) | Ec (F2) | Es | E (total) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Males | ||||||||||||
| RSA | – | 0.10 | 0.27 | 0.37 | – | 0.08 | – | 0.08 | – | 0.18 | 0.37 | 0.55 |
| HR1 | 0.02 | 0.22 | – | 0.24 | 0.02 | 0.19 | – | 0.21 | 0.03 | 0.41 | 0.12 | 0.56 |
| HR2 | 0.01 | 0.19 | – | 0.20 | 0.01 | 0.16 | – | 0.17 | 0.02 | 0.36 | 0.23 | 0.61 |
| SCL | 0.12 | 0.01 | 0.15 | 0.28 | 0.13 | 0.01 | 0.10 | 0.24 | 0.21 | 0.02 | 0.25 | 0.48 |
| NS-SCR | 0.18 | 0.02 | – | 0.20 | 0.20 | 0.01 | – | 0.21 | 0.32 | 0.03 | 0.24 | 0.59 |
| Females | ||||||||||||
| RSA | – | 0.13 | 0.14 | 0.27 | – | 0.06 | – | 0.06 | – | 0.18 | 0.49 | 0.67 |
| HR1 | 0.02 | 0.33 | – | 0.35 | 0.02 | 0.17 | – | 0.19 | 0.01 | 0.45 | 0.01 | 0.47 |
| HR2 | 0.01 | 0.24 | – | 0.25 | 0.01 | 0.12 | – | 0.13 | 0.01 | 0.33 | 0.27 | 0.61 |
| SCL | 0.15 | – | 0.19 | 0.34 | 0.20 | – | 0.12 | 0.32 | 0.14 | – | 0.20 | 0.34 |
| NS-SCR | 0.22 | – | – | 0.22 | 0.30 | – | – | 0.30 | 0.20 | – | 0.27 | 0.47 |
F1 Sympathetic Factor, F2 Parasympathetic Factor, Ac common genetic, Cc common shared environment, Ec common non-shared environment, As specific genetic, Cs specific shared environment, Es specific non-shared environment. Parameters that were set to zero in the final model are indicated by dashed lines. The sum of A (total), C (total), and E (total) may sum to slightly more or less than 1.0 due to rounding of decimals
Discussion
To our knowledge, this is the first study to investigate how genetic and environmental factors contribute to the association among RSA, heart rate, SCL and NS-SCR. Based on univariate and multivariate genetic analyses in a sample of 9–10 year old twins, three main conclusions can be drawn. First, from the univariate analyses, RSA, heart rate, SCL and NS-SCR were each moderately influenced by genetic factors (h2 ranged from .26 to .48). Shared environmental influences were only significant for SCL. Nonshared environmental factors accounted for approximately half of the variance in each of these measures. Secondly, when using the measures in a multivariate design, a twofactor common pathway model best fit the data. The first latent ANS factor was largely responsible for positive covariance between heart rate, SCL and NS-SCR, reflecting sympathetic activity. The second latent ANS factor accounted primarily for the inverse relationship between RSA and heart rate, suggesting parasympathetic activity. Thirdly, measurement-specific genetic and shared environmental influences were found only for RSA and SCL. Measure-specific non-shared environmental influences (including uncorrelated measurement error) were observed for all measures.
Both univariate and multivariate analyses indicated that genetic and non-shared environmental influences were primarily responsible for individual differences in RSA. These results are similar to what has been reported in earlier studies on RSA. In keeping with previous research on heart rate (Rice et al. 2002; Snieder et al. 2003), genetic and non-shared environmental effects accounted for most of the variance. Genetic and non-shared environmental influences also accounted for most of the variance in the univariate analyses of NS-SCR, a finding that is well in line with previous research on skin conductance using adult samples (Crider et al. 2004). Further, genetic and environmental influences on SCL could not be constrained to be equal across sex in the univariate analyses. This is probably because familial aggregation of SCL was slightly higher in female-female pairs than in male-male pairs. Also, shared environmental effects on SCL were significant, although these influences were somewhat more modest in the multivariate analyses.
Arguably, the most interesting results from this study are the clear preference for a two-factor model when the five psychophysiological measures were analyzed simultaneously. In addition, the underlying structure of these factors mapped almost perfectly onto sympathetic and parasympathetic processes. Despite the relatively modest phenotypic correlations between HR1 and HR2 with SCL and NS-SCR, for both males and females, the sympathetic factor accounted for positive and significant covariance between the heart rate and skin conductance measures. The sympathetic arousal system is often called the ‘‘fight or flight’’ response. It is interesting that among preadolescent twins, individual differences in skin conductance measures during rest capture this response more strongly than variation in resting heart rate. Parasympathetic processes, in contrast, reflect the body’s ability to return to homeostasis following stress, which in this study was indexed by an inverse relationship between heart rate and RSA. Although the two skin conductance measures could not be dropped from the parasympathetic factor in boys without a significant reduction in model fit, the factor loadings for these variables were quite small (.17 and .24), indicating that the parasympathetic construct was largely similar in boys and girls.
The results of the multivariate analyses showed that both genetic and shared environmental effects contributed to the sympathetic and parasympathetic ANS factors, with genetic factors accounting for 27–35% of the variance, and shared environmental factors accounting for 18–41% of the variance. Estimates were largely similar across males and females. There was some evidence that genetic influences were more important than shared environmental influences for the parasympathetic ANS factor (27% vs. 23% in males; 35% vs. 18% in females), while the reverse was true for the sympathetic ANS factor (27% vs. 28% in males; 31% vs. 41% in females). Nevertheless, these differences were relatively small. Only the shared environmental effects on the sympathetic factor were statistically significant. However, while our model fitting analyses indicated that either common genetic or common shared environmental factors could be dropped from the model, these factors could not be dropped simultaneously. This indicates that familial factors on parasympathetic and sympathetic processes are important.
In twin models, genetic and shared environmental estimates are highly correlated, which suggests that overall, we did not have enough power to detect either influence independently. Further, it is likely that some of the shared environmental effects were due to the fact that twins were tested on the same day (and thus exposed to similar atmospheric conditions related to temperature and season). All of the measures used in the present study were obtained during the same task, which increases the possibility that some of the shared environmental influences found in the study are due to correlated errors of measurement. This would also account for the finding that shared environmental influences accounted for a greater proportion of the variation in the latent factors than they do for variation in individual measures. Future research could use psychophysiological measures obtained from different tasks to determine whether shared environmental influences are attenuated when shared measurement error is reduced. On the other hand, it is also possible that shared environmental influences were a result of the twins living in the same family and therefore exposed to family-wide factors, such as lifestyle habits. For example, healthy dietary habits are associated with increased heart rate variability (Hagstrup Christensen et al. 2001; Williams et al. 2002). Non-shared environmental factors accounted for significant proportions of variance in both sympathetic and parasympathetic factors (28–50%). This may reflect ‘‘true’’ non-shared environmental effects, i.e., environmental experiences not shared by twins in the same family that affect sympathetic and parasympathetic regulation. For instance, one twin in a pair may exercise regularly, but not the other. Physical exercise has been found to be related to resting heart rate level (Gutin et al. 2000; Nagai and Moritani 2004). Non-shared environmental effects are also likely to include correlated errors of measurement that are twin-specific, as measures of RSA, heart rate, SCL, and NS-SCR were obtained during the same procedure. Overall, evidence from this study strongly suggests that both genetic and environmental influences are important in determining individual differences in the parasympathetic and sympathetic ANS factors among pre-adolescents.
Our results show that there are statistically significant sex differences in the genetic and environmental structure. Because many of the standardized estimates appear remarkably similar across sex, this might indicate the presence of subtle sex differences in variance which would affect the equivalence of unstandardized estimates, although we note that we did not find evidence for significant variance differences across boys and girls for any of our measures. Nevertheless, one of the more striking sex differences occurred with respect to the structure of the parasympathetic factor. While the parasympathetic ANS factor in females was indexed solely by RSA and heart rate (as one would expect), the parasympathetic ANS factor in males was also indexed, albeit modestly, by SCL and NS-SCR.
Finally, multivariate results revealed an absence of measure-specific genetic and shared environmental effects for HR1, HR2 and NS-SCR. However, measure-specific genetic effects were found for RSA and SCL. This indicates that there are genetic influences that are important for RSA and SCL which are independent of the underlying familial influences on the sympathetic and parasympathetic ANS factors. These measurement-specific genetic effects accounted for 14–27% of the total variance in RSA and SCL. It is possible that the measurement-specific genetic influence for RSA is partly explained by a genetic overlap between respiration and RSA that is not related to parasympathetic activity. There were also some measurement-specific shared environmental effects in SCL, accounting for 10% of the total variance in males and 12% in females.
Limitations and concluding remarks
This study was limited in that the measures we used were recorded at rest, in the absence of psychological or environmental stimuli. Because we did not include measures from any task conditions that engaged emotional or cognitive processes, it could be argued that our results have limited relevance to psychological phenomena. However, it is important to consider that many behavioral patterns (such as aggression and stimulation seeking) are trait-based, and can be characterized by low resting levels of heart rate and skin conductance (Gatzke-Kopp et al. 2002; Ortiz and Raine 2004). The fact that our results suggest that genetic factors are implicated in individual differences in parasympathetic and sympathetic processing in the absence of physical or psychological stimuli adds further support to the idea that trait-based individual differences in physiological mechanisms may be important for psychopathology. As stated elsewhere, despite the fact that we had data from over 560 complete twin pairs in our analyses, we were clearly underpowered to differentiate between genetic and shared environmental influences. Our hypothesis that some of the observed shared environmental influence may be due to the fact that measures were collected during the same task, and twins were studied on the same day, further reduces our power. However, obtaining such detailed physiological data from larger samples of twin children studied on different days would be virtually impossible. Finally, we note that these data were collected from a sample of preadolescent twins (aged 9–10). Whether the etiology of parasympathetic and sympathetic processes are affected by biological changes associated with puberty is unknown. Because these twins are part of a longitudinal study on risk factors for the development of antisocial behavior, we may be able to examine these issues in future work.
In summary, to our knowledge, this is the first study to use a behavioral genetic design to investigate how genetic and environmental factors contribute to the association among RSA, heart rate, SCL and NS-SCR simultaneously. Almost all genetic and environmental influences on the measures acted through two latent factors representing parasympathetic and sympathetic processes. The findings in this study need to be replicated in other large twin samples and at other ages. In addition, and perhaps more intriguing, a future direction for research would be to examine the extent to which the same genetic and/or environmental factors influencing sympathetic and parasympathetic functioning contribute to psychopathological outcomes. Behavioral genetic studies combining measures reflecting the sympathetic and parasympathetic activities with measures of psychopathology should provide insight into the etiological mechanisms underlying the development of specific forms of psychopathology.
Acknowledgments
This study was funded by NIMH (R01 MH58354). Catherine Tuvblad was supported by post-doctoral stipends from the Swedish Council for Working Life and Social Research (Project 2006-1501) and the Sweden-America Foundation. Adrian Raine was supported by NIMH (Independent Scientist Award K02 MH01114-08). Kristen Jacobson was supported by NIMH (Mentored Scientist Career Development Award K01 MH068484). We thank the Southern California Twin Project staff for their assistance in collecting data, and the twins and their families for their participation.
Footnotes
We had an a priori hypothesis that we would find a parasympathetic factor defined by heart rate (HR) and respiratory sinus arrhythmia (RSA). However, there are problems with model identification for factors defined by only two variables. Thus, we added a second heart rate variable (from Rest 2) in order to help identify the parasympathetic factor. The decision to include a second heart rate variable was arbitrary. Importantly, when models were re-run with two measures of RSA (i.e., from Rest 1 and Rest 2) and only HR from Rest 1, we obtained a similar pattern of results. Thus, although the decision to include both HR1 and HR2 may have influenced our estimates of shared versus non-shared measurement error, it did not effect the main results of the study.
Contributor Information
Catherine Tuvblad, Email: Tuvblad@usc.edu, Department of Psychology (SGM 501), University of Southern, California, 3620 S. McClintock Ave., Los Angeles, CA 90089-1061, USA.
Joshua Isen, Department of Psychology (SGM 501), University of Southern, California, 3620 S. McClintock Ave., Los Angeles, CA 90089-1061, USA.
Laura A. Baker, Department of Psychology (SGM 501), University of Southern, California, 3620 S. McClintock Ave., Los Angeles, CA 90089-1061, USA
Adrian Raine, Departments of Criminology, Psychiatry, and Psychology, University of Pennsylvania, Philadelphia, PA, USA.
Dora-Isabel Lozano, Universidad Autónoma de Ciudad Juárez, Juárez, Mexico.
Kristen C. Jacobson, Department of Psychiatry and Behavioral Neuroscience, University of Chicago, Chicago, IL, USA
References
- Akaike AC. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- Baker LA, Barton M, Lozano DI, Raine A, Fowler JH. The Southern California twin register at the University of Southern California: II. Twin Res Hum Genet. 2006;9:933–940. doi: 10.1375/183242706779462912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Jacobson KC, Raine A, Lozano DI, Bezdjian S. Genetic and environmental bases of childhood antisocial behavior: a multi-informant twin study. J Abnorm Psychol. 2007;116(2):219–235. doi: 10.1037/0021-843X.116.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Dev Psychopathol. 2001;13(2):183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS. Respitory sinus arrhythmia: autonomic origins, physiological mechanisms, and psychophysiological implications. Psychophysiology. 1993;30:183–196. doi: 10.1111/j.1469-8986.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Quigley KS, Lozano DI. Cardiovascular psychophysiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. Cambridge University Press; Cambridge: 2007. pp. 182–210. [Google Scholar]
- Boomsma DI, van Baal GC, Orlebeke JF. Genetic influences on respiratory sinus arrhythmia across different task conditions. Acta Genet Med Gemellol (Roma) 1990;39(2):181–191. doi: 10.1017/s0001566000005419. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Quas J, Alkon A, Smider NA, Essex MJ, Kupfer DJ Development MABWGotMFRNoPa. Autonomic reactivity and psychopathology in middle childhood. Br J Psychiatry. 2001;179:144–150. doi: 10.1192/bjp.179.2.144. [DOI] [PubMed] [Google Scholar]
- Crider A, Kremen WS, Xian H, Jacobson KC, Waterman B, Eisen SA, Tsuang MT, Lyons MJ. Stability, consistency, and heritability of electrodermal response lability in middle-aged male twins. Psychophysiology. 2004;41(4):501–509. doi: 10.1111/j.1469-8986.2004.00189.x. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. Cambridge University Press; Cambridge: 2007. pp. 159–181. [Google Scholar]
- De Geus EJ, Boomsma DI, Snieder H. Genetic correlation of exercise with heart rate and respiratory sinus arrhythmia. Med Sci Sports Exerc. 2003;35(8):1287–1295. doi: 10.1249/01.MSS.0000079073.20399.11. [DOI] [PubMed] [Google Scholar]
- De Geus EJ, Kupper N, Boomsma DI, Snieder H. Bivariate genetic modeling of cardiovascular stress reactivity: does stress uncover genetic variance? Psychosom Med. 2007;69(4):356–364. doi: 10.1097/PSY.0b013e318049cc2d. [DOI] [PubMed] [Google Scholar]
- Garralda ME, Connell J, Taylor DC. Psychophysiological anomalies in children with emotional and conduct disorders. Psychol Med. 1991;21:947–957. doi: 10.1017/s0033291700029937. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp L, Raine A, Loeber R, Stouthamer-Loeber M, Steinhauer S. Serious delinquent behavior, sensation seeking, and electrodermal arousal. J Abnorm Child Psychol. 2002;30:477–486. doi: 10.1023/a:1019816930615. [DOI] [PubMed] [Google Scholar]
- Grossman P. Respiration, stress and cardiovascular function. Psychophysiology. 1983;23:284–299. doi: 10.1111/j.1469-8986.1983.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Grossman P. Respiratory and cardiac rhythms as windows to central and autonomic biobehavioral regulation: selection of window frames, keeping the panes clean, and viewing the neural topography. Biol Psychol. 1992;34:131–161. doi: 10.1016/0301-0511(92)90013-k. [DOI] [PubMed] [Google Scholar]
- Grossman P, Svebak S. Respiratory sinus arrhythmia as an index of parasympathetic cardiac control during active coping. Psychophysiology. 1987;24:228–235. doi: 10.1111/j.1469-8986.1987.tb00284.x. [DOI] [PubMed] [Google Scholar]
- Gutin B, Barbeau P, Litaker MS, Ferguson M, Owens S. Heart rate variability in obese children: relations to total body and visceral adiposity, and changes with physical training and detraining. Obes Res. 2000;8(1):12–19. doi: 10.1038/oby.2000.3. [DOI] [PubMed] [Google Scholar]
- Hagstrup Christensen J, Aarup Skou H, Fog L, Ellegaard Hansen V, Vesterlund T, Dyerberg J, Toft E, Berg Schmidt E. Marine n-3 fatty acids, wine intake, and heart rate variability in patients referred for coronary angiography. Circulation. 2001;103:651–657. doi: 10.1161/01.cir.103.5.651. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Mueller B, Wenning B, Qunaibi M, Lichterfeld C, Herpertz-Dahlmann B. Autonomic responses in boys with externalizing disorders. J Neural Transm. 2003;110(10):1181–1195. doi: 10.1007/s00702-003-0026-6. [DOI] [PubMed] [Google Scholar]
- Kupper N, Willemsen G, Posthuma D, De Boer D, Boomsma DI, De Geus EJC. A genetic analysis of ambulatory cardiorespitory coupling. Psychophysiology. 2005;42:201–212. doi: 10.1111/j.1469-8986.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychol Bull. 2004;130(4):531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Venables PH. Direct measurement of skin conductance: a proposal for standardization. Psychophysiology. 1971;8:656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- Lykken DT, Iacono WG, Haroian K, McGue M, Bouchard TJJ. Habituation of the skin conductance response to strong stimuli: a twin study. Psychophysiology. 1988;25(1):4–15. doi: 10.1111/j.1469-8986.1988.tb00949.x. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Goldsmith HH. Alternative common factor models for multivariate biometric analyses. Behav Genet. 1990;20:569–608. doi: 10.1007/BF01065873. [DOI] [PubMed] [Google Scholar]
- Mezzacappa E, Tremblay RE, Kindlon D, Saul JP, Arseneault L, Seguin J, Pihl RO, Earls F. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. J Child Psychol Psychiatry. 1997;38(4):457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Nagai N, Moritani T. Effect of physical activity on autonomic nervous system function in lean and obese children. Int J Obes. 2004;2004(28):1. doi: 10.1038/sj.ijo.0802470. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a ‘default mode’ of brain function. NeuroImage. 2004;22(1):243–251. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Kluwer Academic Publications; Dordrecht: 1992. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes H. Mx: statistical modeling. Department of Psychiatry, Medical College of Virginia; Richmond: 2003. [Google Scholar]
- Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2004;43(2):154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Porges SW. Physiological regulation in high-risk infants: a model for assessment and potential intervention. Dev Psychopathol. 1996;8:43–58. [Google Scholar]
- Raftery AE. Bayesian model selection in social research. Sociol Methodol. 1995;25:111–163. [Google Scholar]
- Raine A. Autonomic nervous system factors underlying disinhibited, antisocial, and violent behavior. Biosocial perspectives and treatment implications. Ann N Y Acad Sci. 1996;20(794):45–59. doi: 10.1111/j.1749-6632.1996.tb32508.x. [DOI] [PubMed] [Google Scholar]
- Rice T, An P, Gagnon J, Leon AS, Skinner JS, Wilmore JH, Bouchard C, Rao DC. Heritability of HR and BP response to exercise training in the HERITAGE Family Study. Med Sci Sports Exerc. 2002;34(6):972–979. doi: 10.1097/00005768-200206000-00011. [DOI] [PubMed] [Google Scholar]
- Rogeness GA, Cepeda C, Macedo CA, Fischer C, Harris WR. Differences in heart rate and blood pressure in children with conduct disorder, major depression, and separation anxiety. Psychiatry Res. 1990;33(2):199–206. doi: 10.1016/0165-1781(90)90074-f. [DOI] [PubMed] [Google Scholar]
- SAS. SAS/STAT software: changes and enhancements through release 9.2. SAS Institute Inc; Cary: 2005. [Google Scholar]
- Snieder H, Boomsma DI, Van Doornen LJ, De Geus EJ. Heritability of respiratory sinus arrhythmia: dependency on task and respiration rate. Psychophysiology. 1997;34(3):317–328. doi: 10.1111/j.1469-8986.1997.tb02402.x. [DOI] [PubMed] [Google Scholar]
- Snieder H, Hayward CS, Perks U, Kelly RP, Kelly PJ, Spector TD. Heritability of central systolic pressure augmentation: a twin study. Hypertension. 2000;35(2):574–579. doi: 10.1161/01.hyp.35.2.574. [DOI] [PubMed] [Google Scholar]
- Snieder H, Harshfield GA, Treiber FA. Heritability of blood pressure and hemodynamics in African- and European-American youth. Hypertension. 2003;41(6):1196–1201. doi: 10.1161/01.HYP.0000072269.19820.0D. [DOI] [PubMed] [Google Scholar]
- Snieder H, van Doornen LJ, Boomsma DI, Thayer JF. Sex differences and heritability of two indices of heart rate dynamics: a twin study. Twin Res Hum Genet. 2007;10(2):364–372. doi: 10.1375/twin.10.2.364. [DOI] [PubMed] [Google Scholar]
- Turner JR. Individual differences in heart rate response during behavioral challenge. Psychophysiology. 1989;26:497–505. doi: 10.1111/j.1469-8986.1989.tb00701.x. [DOI] [PubMed] [Google Scholar]
- van Lang NDJ, Tulen JHM, Kallen VL, Rosbergen B, Dieleman G, Ferdinand RF. Autonomic reactivity in clinically referred children attention-deficit/hyperactivity disorder versus anxiety disorder. Eur Child Adolesc Psychiatry. 2007;16(2):71–78. doi: 10.1007/s00787-006-0575-y. [DOI] [PubMed] [Google Scholar]
- Williams CL, Hayman LL, Daniels SR, Robinson TN, Steinberger J, Paridon S, Bazzarre T. Cardiovascular Health in Child-hood, A Statement for Health Professionals From the Committee on Atherosclerosis, Hypertension, and Obesity in the Young (AHOY) of the Council on Cardiovascular Disease in the Young. Am Heart Assoc Circ. 2002;106:143–160. doi: 10.1161/01.cir.0000019555.61092.9e. [DOI] [PubMed] [Google Scholar]