Abstract
Deficits in tongue function in conjunction with airway compromise can contribute to dysphagia associated with Parkinson disease (PD). However, it is unknown if these deficits are related to the primary disease pathology in PD, nigrostriatal dopamine depletion. To directly study the impact of striatal dopamine depletion on tongue function, we used unilateral infusion of the neurotoxin 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle and measured tongue force and timing parameters during a complex tongue protrusion task for a water reward. Maximal and average forces were significantly diminished and average press time was significantly longer after neurotoxin administration, reflecting aspects of bradykinesia and hypokinesia associated with PD. Our findings suggest that even unilateral deficits to the nigrostriatal dopamine system may be contributing to some of the lingual sensorimotor deficits seen in PD. Because previous research in rat models of PD has shown that targeted training of the limb can rescue behavioral deficits and spare striatal dopamine neurons, early intervention for cranial sensorimotor deficits may also be indicated.
Keywords: Parkinson disease, 6-hydroxydopamine, tongue deficits, dopamine, dysphagia, rat
1. Introduction
Oropharyngeal dysphagia occurs in up to 95% of people with Parkinson disease (PD) and contributes to mortality and decreased quality of life.[20,27,30,33,34] Deficits in tongue function in conjunction with airway compromise can significantly contribute to dysphagia.[1,24,26,31,36] Previous research has found that force and timing measures, such as average tongue force and average tongue press rate in rats were sensitive to disrupting dopaminergic synaptic transmission, even with low doses of the dopamine antagonist haloperidol (0.05mg/kg). [8] Similar dose-dependent findings have been reported by others in rats.[13,17,19] However, haloperidol has widespread antidopaminergic effects that are not limited to the nigrostriatal pathway, which is the main dopaminergic pathway compromised in PD.[5,6,16]
To directly study the impact of nigrostriatal dopamine depletion on craniomotor function, we have employed a unilateral infusion of the neurotoxin 6-hydroxydopamine (6-OHDA) into the medial forebrain bundle. Unlike haloperidol, 6-OHDA acts directly on the nigrostriatal dopamine pathway and thus provides a model of the primary disease pathology in early PD.[3,21,25] In these previous studies using this model, cranial sensorimotor deficits were shown to be similar to those observed in humans with PD. Specifically, ultrasonic vocalizations in rats werere analogous to human vocalization behaviors [7,23] and after unilateral 6-OHDA-induced infusion, rat ultrasonic vocalizations were less complex in type, reduced in frequency range, and reduced in loudness.[7,9] Previous studies in other laboratories have examined effects of these lesions on tongue function and found reductions in lick rhythm and average peak force according to severity of lesion.[40] As such, 6-OHDA lesions present an interesting and useful model for study of cranial sensorimotor impairments and can be applied to the study of lingual behaviors.[10]
The unilateral lesions used in our studies more closely approximate an earlier stage of PD prior to the bilateral progression that characterizes later stages [5,6,16] The importance of defining and characterizing early deficits may be substantial given that early therapeutic intervention has been associated with striatal dopaminergic sparing and behavioral recovery of deficits in animal studies of limb actions.[2,11,42] In the current study, we hypothesized that a severe unilateral 6-OHDA-induced lesion to the medial forebrain bundle swouldlead to decreased average tongue forces and altered timing characteristics in rats.
2. Materials and Methods
2.1 Animals
Fifteen 9-month-old male Fisher 344/Brown Norway rats were used in this experiment. Animals were housed in pairs in standard polycarbonate cages on a 12:12 hour light-dark reversed light cycle. Rats were obtained from Charles River (Raleigh, NC) 8 weeks prior to the start of the experiment to allow acclimation to the animal care facility, reversal of light cycle, water restriction, and familiarization to the tongue force operandum. Food was given ad libitum. Water was restricted to 3 hours per day to encourage the animals to press a disk for a water reward. All tongue press training was performed during the dark period of the light cycle. Experimental methods for tongue press measurements in rats have been detailed previously [8,12] but are discussed briefly below. All experiments were approved by the University of Wisconsin Institutional Animal Care and Use Committee (IACUC).
2.2 Experimental Overview
The experiment involved a 2-week introductory period to allow water restriction and familiarization with the tongue force operandum. After familiarization to the task, we completed baseline behavioral assessments for limb use and tongue force measurements. After baseline testing, rats were infused with the neurotoxin 6-hydroxydopamine (6-OHDA) creating a unilateral 6-OHDA-induced lesion. Tongue force measurements were repeated 72 hours after lesions were made. This time period was chosen as limb and cranial behavioral deficits are apparent 72 hours following neurotoxin administration.[2,7,25,42] Following completion of all post-lesion data collection, the animals were transcardially perfused, euthanized and brain tissues were collected for immunohistochemistry. All behavioral assessments (cylinder test, apomorphine rotations) and tyrosine hydroxylase measures were done by an experienced rater. Inter and intra-rater reliability for these measures was performed on 20% of the data with Person’s product moment correlations. Inter-rater reliability was performed by a second experienced rater.
2.3.1 Parkinson disease model
After the introductory period and baseline testing, moderate to severe degeneration of presynaptic striatal neurons was induced in all rats by unilateral infusion of 6-OHDA into the medial forebrain bundle.[21,25,42,44] The rats were anesthetized with 2–4% inhaled isoflurane, and placed in a stereotaxic frame. All rats received unilateral infusions of 7 µg 6-OHDA hydrobromide (free base weight) dissolved in 3 µl artificial cerebrospinal fluid (composition: NaCl, KCl, CaCl2, MgCl2*6H20) containing 0.05% (w/v) ascorbic acid. Infusion coordinates were measured from bregma (−3.3 AP; ±1.7 ML; −8.0 DV from dural surface), and infusions were delivered at a rate of .3 µl/min for 10 minutes. Infusions were directed into the nigrostriatal projections in the left hemisphere, creating a unilateral 6-OHDA-induced lesion. Post-operative local analgesia (5 mg/kg 0.25% bupivicaine, subcutaneous around incision and 0.05 mg/kg bupenorphrine intraperitoneal) was administered after suturing. Following surgery, animals were placed on a warm surface to prevent hypothermia, and upon recovery were returned to their home cages.
2.3.2 Behavioral testing
To estimate the degree of 6-OHDA induced degeneration, two behavioral tests were administered: forelimb-use asymmetry and apomorphine-induced rotation. Rats were tested for forelimb-use asymmetry at baseline and 72 hours after 6-OHDA-induced lesions were made by placing them in an upright acrylic cylinder (diameter 20 cm) to encourage rearing and exploratory movements with the forepaws.[39,46] The number of wall contacts made by either forelimb or by both forelimbs simultaneously was recorded. The percentage of contacts made by the non-impaired forelimb (contralateral to the brain lesion) relative to the total number of contacts was calculated using the formula: (ipsilateral limb contacts + both (simultaneous or rapidly alternating) limb contacts)/total number of contacts (limited to 20 per test day to prevent habituation). Scores above 80 indicate a greater reliance on the ipsilateral limb for voluntary movement and have been well correlated with the degree of nigrostriatal dopamine depletion induced by 6-OHDA-induced lesions. [39] Apomorphine-induced rotational behavior was tested 7 days post-lesion. Rats were given 0.1 mg/kg apomorphine (s.c.), and the net number of contralateral turns made during a 2 min trial was recorded in revolutions per minute (25 min post injection) (modified from Herrera-Marschitz, Casas, & Ungerstedt, 1988). A net number of rotations contralateral to the deficit indicated a severe unilateral striatal dopamine deficit.
2.5.1 Tongue Force and Temporal Data Collection and Analysis
A custom instrument was designed based on previous research involving rodent models of licking behavior [12,18,28,29,41] that allowed us to modify and acquire tongue force and temporal measures during complex protrusive tongue movements. This set-up involved a traditional learning paradigm in which rats were trained to press a disk with their tongue by gradually restricting their access to water (see Connor et al, 2009 and Ciucci & Connor, 2009 for details). Throughout the experiment, animals were placed individually into a polycarbonate cage resembling the homecage, but equipped with a 1 × 1 centimeter (cm) aperture and force operandum that delivered aliquots of water based on tongue press behaviors.
Tongue force increment testing immediately followed a 6-day introductory period. Progressively increasing force targets were rapidly presented and resultant tongue force behaviors recorded prior to when 6-OHDA-induced lesions were made (baseline) and at 72 hours post lesion. These increment testing sessions were repeated on each of three days prior to and following surgery. Animals were monitored by direct visual observation for all training and data collection sessions to ensure that disk presses occurred only with the tongue and not with the teeth, which can artificially elevate tongue forces.
Tongue presses were recorded at 200 Hz using custom-designed computer data acquisition software (Matrix Product Development, Cottage Grove, WI) and analyzed with Matlab software using custom designed algorithms. All data were analyzed from the increment testing session (day) with the greatest number of tongue presses, and only for that session. The following variables were measured during the session: maximal tongue force (g), average tongue force (g), average press rate (presses/sec), average interpress interval (ms), force variability (g), time to peak force (ms), and average press time (ms). Maximum tongue force was the highest force achieved with a tongue press. Average tongue force was the average of the highest 10 presses during a session. Press rate was determined by the number of tongue presses that occurred per second and was determined over a two-minute period. For each rat, the two-minute period began when the rat approached the disk and initiated a press.
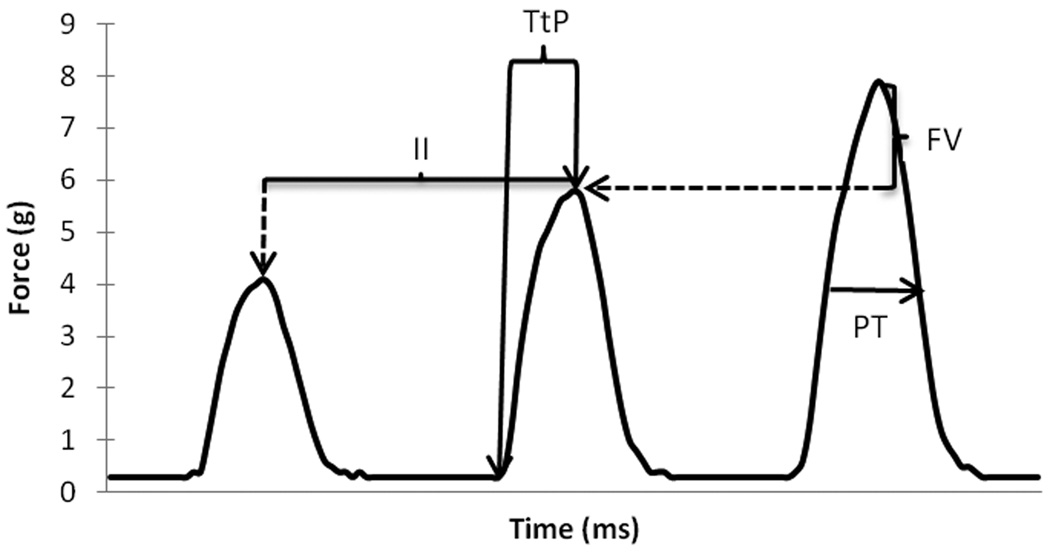
Individual tongue press profiles are illustrated in Figure 1. After peak forces were made for each tongue press observation, temporal measurements were made for observations of at least 2g (± .2 g) for one 2-second period for each animal. Temporal measures included: (1) Time to peak (TtP; ms), defined as the time between onset of the tongue press and peak force, (2) Press time (PT, ms) defined as the time between force onset and offset. Onset of a tongue press was defined as the baseline zero-cross point prior to peak force, while offset was defined as the point at which 50% of peak force was measured during the force declination phase. Variation in peak force (g) and average interpress interval (ms) were also measured. Interpress interval was defined as the average duration (ms) between individual press peaks over 2 seconds of continuous tongue presses. For the same 2-second period, force variability was also calculated by subtracting the peak force (g) from the next consecutive peak force (g). This measure was used to determine the ability of the animal to maintain the same force generation over a short period of time (two seconds). For analysis purposes, all observations were sorted into categories represented by their integer force level of either 2, 3, or 4 grams.
Figure 1.
A representation of three consecutive tongue pushes from one rat and measurement variables analyzed. Dashed lines indicate that the value is subtracted from the previous force peak. TtP=Time to peak, PT=Press time, II=Interpress interval FV=Force variability
2.4 Immunohistochemistry
The immunohistochemistry technique we used was modified from previous studies. [2, 42] After completion of post-lesion measures, rats were deeply anesthetized with 2.5 – 4.0% isoflurane and intra-aortically perfused with 250 mL physiological saline 1 minute after an intracardial injection of 100 units of heparin. Immediately following, 500 mL of ice cold 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) was perfused to fix brain tissue. Whole brains were removed and postfixed in ice cold fixative for 1–4 hours. Brains were then cryoprotected for 48–96 hours in a 20% sucrose/5% glycerol solution in 0.1M PBS at 4°C. Brains were mounted on a freezing microtome and one of every five 60 µm coronal slices throughout the basal ganglia were harvested and stored in PBS with 0.02% NaN3 at 4°C.
Floating slices were probed for tyrosine hydroxylase using a rabbit anti-tyrosine hydroxylase primary antibody (1:2000 dilution, Millipore, Billerica, MA, USA) and a biotinylated goat anti-rabbit secondary antibody (1:500 dilution, Millipore, Billerica, MA, USA). The signal was amplified using the VECTASTAIN Elite ABC avidin-biotin system (Vector Laboratories, Burlingame, CA, USA). Slices were incubated in primary antibody for 16 hours, in secondary for 3 hours, and avidin-biotin solution for 1 hour at room temperature. Labeling was visualized with 3,3'-diaminobenzidine (DAB) chromogen developed with a peroxidase reaction for 90 seconds. Slices were quenched, counterstained with hematoxylin, and mounted on gelatin-coated slides.
Brain slices were imaged on an Epson Perfection V500 Photo Scanner and uploaded to a computer (Dell Optiplex 960) and analyzed using ImageJ software (National Institutes of Health, Bethesda, MD). A custom-designed software program was developed in Image J to detect the optical density of thresholded values for neurons that were positive for tyrosine hydroxylase immunoreactivity. The region of interest (striatum in each hemisphere) was identified manually, run through the analysis script, and values were expressed as a percent of thresholded values as compared with the non-injured hemisphere. Thus, the injured hemisphere is expressed as percent loss of dopaminergic neurons in the striatum.
2.6 Statistical Analysis
All statistical analyses were performed with SAS (SAS Institute, Inc, Cary, NC). Comparisons between baseline and post-lesion measures were made with paired t-tests. If the test for equality of variances was significant, a Satterthwaite t-test was performed. The alpha level was set a priori at 0.05.
3. Results
Means and standard deviations of our measures as well as p-values from statistical analysis are presented in Table 1.
Table 1.
Results from tongue press measures at baseline and post-lesion. Values are reported as means and standard deviations (SD). Time to peak and press time are reported for each of the integer force levels studied (2,3, and 4 grams). Numbers in parenthesis are the n for that variable.
| Variables | Baseline | Post-Lesion | P-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Forelimb Asymmetry (%) | .55 (15) | .05 | .93 (15) | .09 | <.001* |
| Average Force (g) | 6.11 (15) | 1.57 | 3.37 (15) | 1.68 | <.001* |
| Maximum Force (g) | 8.36 (15) | 1.8 | 4.29 (15) | 2.05 | <.001* |
| Average Press Rate | 4.47 (15) | 1.19 | 4 (15) | 2.07 | NS |
| Interpress Interval (ms) | 0.26 (15) | 0.07 | 0.24 (15) | 0.05 | NS |
| Force Variability (g) | 0.87 (15) | n/a | 0.69 (15) | n/a | .03* |
| Time to Peak 2 (ms) | 21.97 (15) | 1.91 | 21.88 (14) | 2.54 | NS |
| Time to Peak 3 (ms) | 28.58 (15) | 3.52 | 27.70 (11) | 6.44 | NS |
| Time to Peak 4 (ms) | 33.09 (15) | 3.46 | 30.46 (8) | 6.94 | NS |
| Press Time 2 (ms) | 39.08 (15) | 3.56 | 41.54 (14) | 5.09 | NS |
| Press Time 3 (ms) | 42.72 (15) | 4.82 | 48.67 (11) | 5.93 | .005* |
| Press Time 4 (ms) | 48.27 (15) | 4.00 | 53.66 (8) | 6.01 | .007* |
denotes statistically significant outcome (p<.05)
3.1. Validation of lesion
3.1.1 Forelimb use asymmetry test
Data (box and whisker plots) for the forelimb asymmetry test are displayed in figure 2. The mean proportion of contralateral forelimb use (unimpaired limb) was 93 post-lesion, indicating a severe unilateral parkinsonian deficit.[15,46] There was a statistically significant difference in forelimb use asymmetry ratio after 6-OHDA-induced lesion (t = −15.08, df = 14, p<.001).
Figure 2.
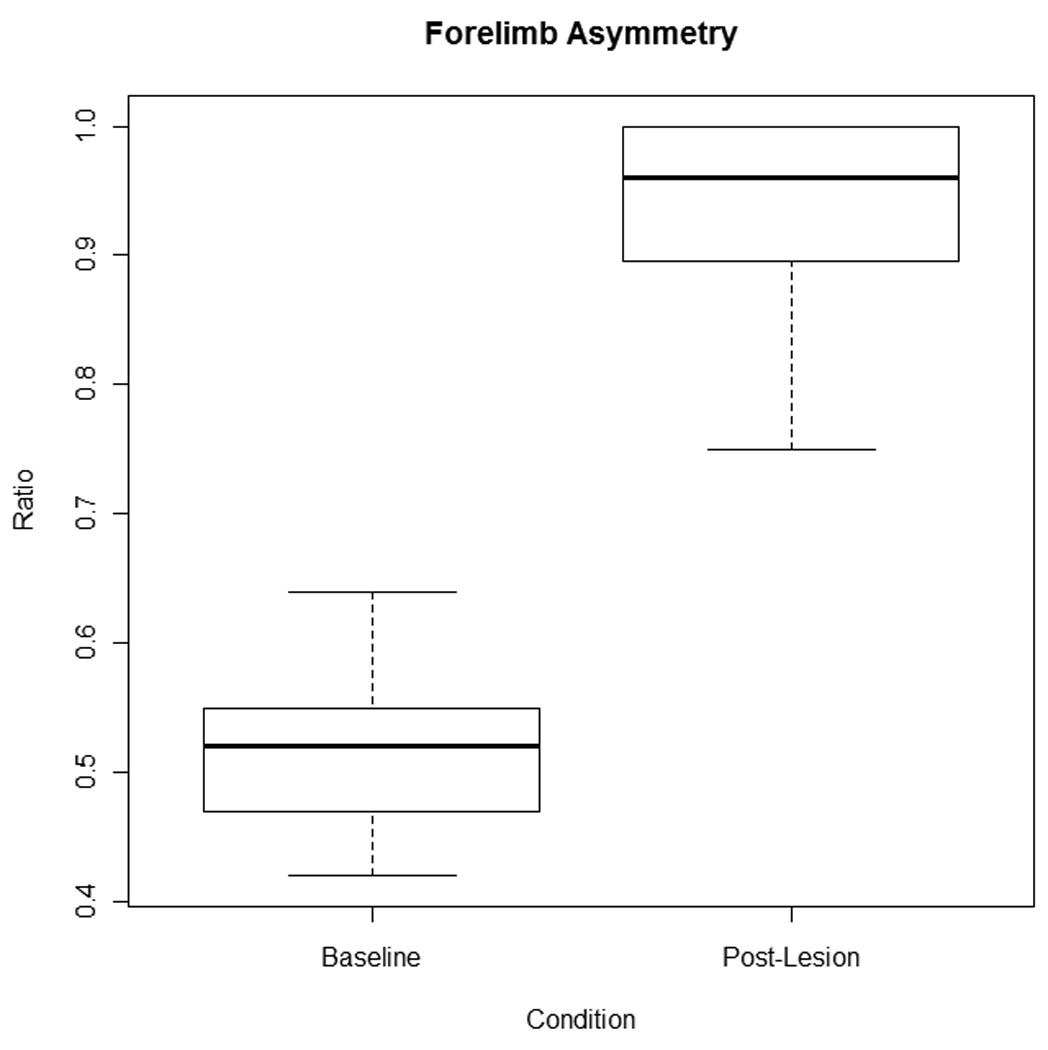
Forelimb Asymmetry box and whisker plots depicting the forelimb asymmetry ratio at baseline and in the post-lesion condition. The thick black line indicates the median of the data, the top and bottom of the box represent the upper (75%) and lower (25%) quartiles respectively, and the maximum and minimum values are represented by the whiskers. No outliers are present. Based on the means and standard deviations (presented in table 1) the forelimb asymmetry ratio was significantly higher in the post-lesion condition (p<.001) indicating a severe unilateral lesion.
3.1.2 Apomorphine rotation
The average number of revolutions per minute after 6-OHDA-induced lesion was 11, which is consistent with the number of rotations found in other studies with severe striatal dopamine loss.[22,25,43] Taken with the forelimb asymmetry test (Figure 2), these scores confirm that unilateral striatal dopamine depletion was severe.
3.1.3 Immunohistochemistry
Analysis of immunoreactivity to tyrosine hydroxylase revealed that the average percent striatal dopamine loss was 96%, confirming severe unilateral depletion of striatal dopaminergic neurons (Figure 3).
Figure 3.
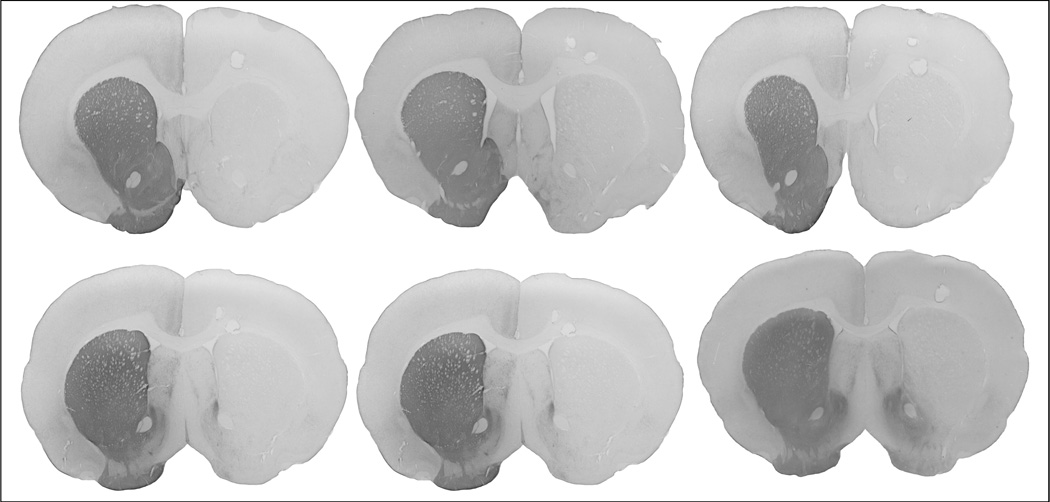
Montage of coronal slices of rat brains showing immunoreactivity to tyrosine hydroxylase in the striatum. Note the absence of staining in the left hemisphere (shown on the right side of the slice), indicating severe striatal dopamine depletions.
3.1.4 Reliability
The Pearson’s product moment correlations for both inter-rater and intra-rater reliability was r=0.99.
3.2 Tongue forces
3.2.1 Average tongue force
Data (box and whisker plots) for average tongue force are displayed in Figure 4. Average tongue force was significantly lower following unilateral 6-OHDA-induced lesion (t = 7.02 df = 14, p<.001).
Fig 4.
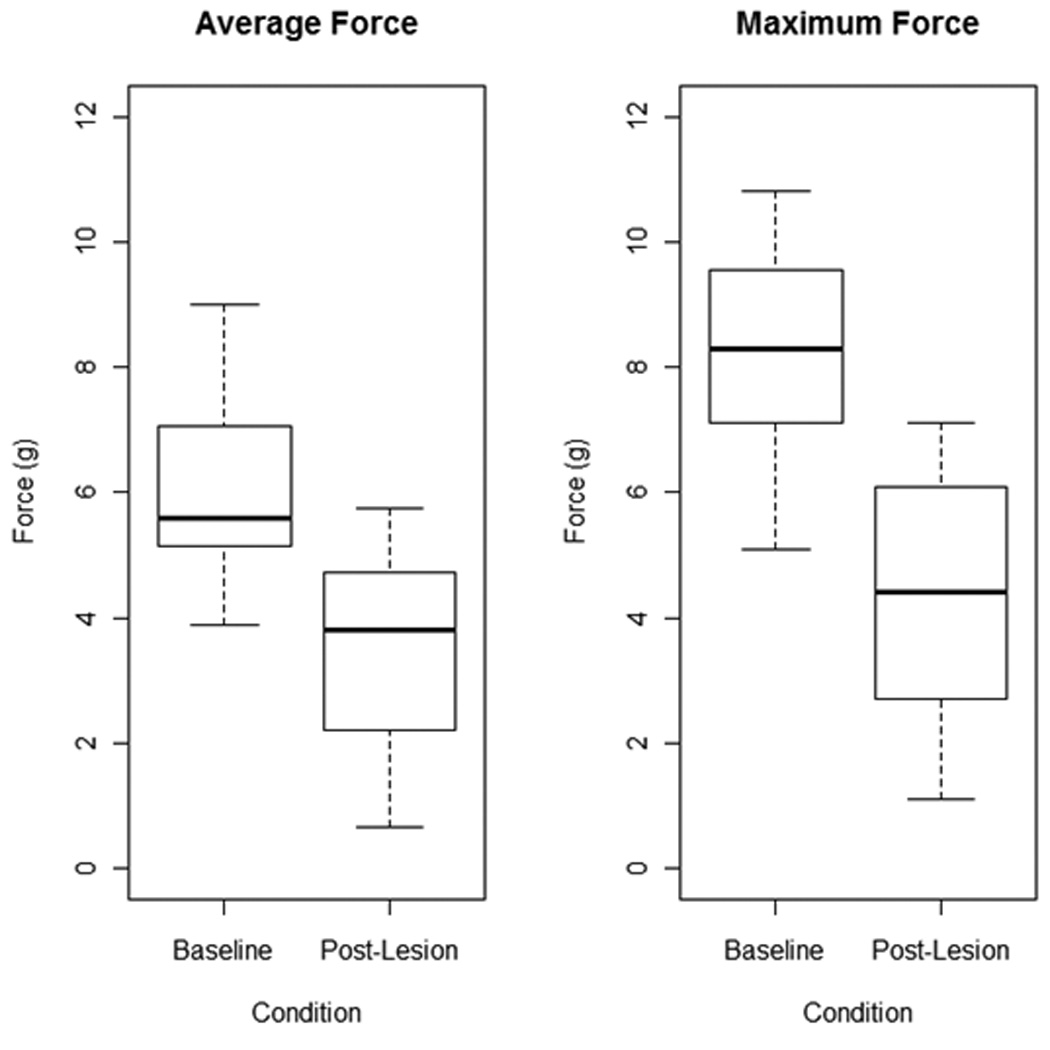
Box and whisker plots depicting average (left) and maximum (right) tongue forces (g) for animals at baseline and post-lesion. The thick black line indicates the median of the data, the top and bottom of the box represent the upper (75%) and lower (25%) quartiles respectively, and the maximum and minimum values are represented by the whiskers. No outliers are present. Mean values (shown in table 1) for both average and maximum force values were significantly lower in the post-lesion condition (p<.001).
3.2.2. Maximal tongue force
Data (box and whisker plots) for maximal tongue force are displayed in Figure 4. Maximal tongue force was significantly lower following unilateral 6-OHDA-induced lesion (t = 7.76, df = 14, p<.001).
3.2.3 Average press rate
Average press rate was not significantly different after 6-OHDA-induced lesion (t= 1.24, df=15, p=.19).
3.2.4 Force variability
Differences in peak to peak force generation were significantly lower following 6-OHDA lesion-induced lesion (t = 2.37, df = 14, p=.03).
3.3 Temporal Parameters
3.3.1 Average interpress interval
Average interpress interval was not significantly different after 6-OHDA-induced lesion (t = 0.61, df = 14, p=.55).
3.3.2 Time to peak force
Time to peak force was not significantly different after the 6-OHDA-induced lesion, at force levels of 2 g (t=0.02, df=13, p=.98), 3 g (t=0.72, df=10, p=.49), and 4 g (t=2.14, df=7, p=.07).
3.3.3 Press Time
Press time was not significantly different after 6-OHDA-induced lesion at the 2 g force level (t= 1.9, df=13, p=.08). However, press time was significantly longer after 6-OHDA-induced lesion at the 3 g (t= 3.62, df=10, p=.005) and 4 g (t= 3.73, df=7, p=.007) force categories.
4. Discussion
Parkinson disease, at all stages, is associated with oromotor deficiencies including lingual deficits and mild to profound dysphagia.[4,14,20,34] However, the degree to which nigrostriatal dopamine depletion is associated with these deficits has been unclear. The purpose of this study was to determine if unilateral disruption of dopaminergic signaling in the nigrostriatal pathway results in deficits of complex tongue protrusive behaviors during water consumption in rats (i.e. licking water from a disk). Our results demonstrated that severe unilateral lesions to the medial forebrain bundle with the neurotoxin 6-OHDA caused changes to force characteristics during a voluntary tongue press task. Specifically, maximal and average forces were significantly diminished after neurotoxin administration. Average peak force has also been reported in a similar model with lesions producing over 75% striatal dopamine depletion.[40] In contrast to what we predicted, force variability was also reduced after 6-OHDA-induced lesions. It is possible that the parkinsonian animals’ dynamic range for force production was limited to lower forces, thus reducing variability.
In this study, we also measured temporal aspects of tongue movement that may capture aspects of lingual bradykinesia and movement to movement variability during tongue press behavior: average interpress interval, time to peak force generation, and press time. After 6-OHDA-induced lesion, the interpress interval and time to peak force generation were not significantly different from baseline. However, press time was significantly longer. As force increased, tongue press time also increased and was significantly different from baseline levels at higher forces. Interestingly and contrary to our hypothesis, average press rate was not significantly reduced after 6-OHDA lesion. Previous studies have shown that interfering with dopaminergic synaptic transmission with the D2 antagonist haloperidol diminishes licking rate [8,13,17,19] especially at higher doses of the drug. Another study using a similar unilateral 6-OHDA lesion to the nigrostrial bundle, also showed severity-dependent decreases in the number of licks and lick rhythm, but this was only significant for animals with greater than 75% striatal dopamine depletion and was analyzed over a 40 second period.[40] However, the severe unilateral lesions to the medial forebrain bundle in our study do not appear to affect timing characteristics that we measured, with the exception of press time, which was longer after 6-OHDA lesion. Longer press times are likely reflective of bradykinesia that is characteristic in the unilateral 6-OHDA model. Unilateral lesions do not appear to affect movement to movement variation, at least in short bursts of licking.
As cranial sensorimotor functions are typically bilaterally innervated by descending cortical input that is modulated by the basal ganglia, it has been traditionally unclear if a unilateral striatal dopamine loss would yield deficits to oral and laryngeal functions. But, we have recently found that unilateral lesions to the medial forebrain bundle are associated with other cranial deficits, such as reductions in complexity, bandwidth and intensity of ultrasonic vocalizations.[7,9] However, this type of lesion does not cause devastating effects to the cranial sensorimotor systems, such as completely eliminating vocalization or severe dysphagia causing death of the animal. It is likely that the degeneration in the brainstem and other forebrain areas [5,6] as well as other PD-related phyisiopathies in cortico-striatal excitability [32,45] contribute to the severe oromotor deficits that manifest with late-stage idiopathic Parkinson disease.
Because oropharyngeal dysphagia is a prominent and debilitating aspect of Parkinson disease, it is important to develop appropriate behavioral and medical therapies to compensate for or ameliorate oromotor deficits. However, as nigrostriatal dopamine depletion is only one factor in a complex pathology in PD, [5,6,32,35,45] it is important to consider other brain areas and neurotransmitters when developing treatment strategies. We did not consider the role of norepinephrine in this study, as the animals were not pre-treated with desimpramine, which protects noradrenergic terminals. Because 6-OHDA is neurotoxic to catecholamines, it is possible that some of these oromotor deficits may be linked to alterations in noradrenergic synaptic activity as well.[37,38]
Our findings suggest that even unilateral deficits to the nigrostriatal dopamine system may be contributing to some of the lingual sensorimotor deficits seen in Parkinson disease. Because previous research in rat models of Parkinson disease have shown that targeted training of the limb can rescue the behavioral deficit and spare striatal dopamine neurons, early intervention for cranial sensorimotor deficits may be indicated. As such, identifying oromotor deficits at the early and unilateral stage of Parkinson disease may prompt earlier intervention and perhaps better outcomes for oromotor deficits, such as dysphagia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Michelle R Ciucci, Email: ciucci@surgery.wisc.edu.
John A Russell, Email: Russell@surgery.wisc.edu.
Allison J Schaser, Email: Schaser@surgery.wisc.edu.
Emerald J Doll, Email: wahoske@surgery.wisc.edu.
Lisa M Vinney, Email: vinney@surgery.wisc.edu.
Nadine P Connor, Email: connor@surgery.wisc.edu.
References
- 1.Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson's disease. Gastroenterology. 1996;110:383–392. doi: 10.1053/gast.1996.v110.pm8566584. [DOI] [PubMed] [Google Scholar]
- 2.Anstrom KK, Schallert T, Woodlee MT, Shattuck A, Roberts DC. Repetitive vibrissae-elicited forelimb placing before and immediately after unilateral 6-hydroxydopamine improves outcome in a model of Parkinson's disease. Behavioural Brain Research. 2007;179:183–191. doi: 10.1016/j.bbr.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 3.Bergman H, Deuschl G. Pathophysiology of Parkinson's disease: from clinical neurology to basic neuroscience and back. Movement Disorders. 2002;17 Suppl 3:S28–S40. doi: 10.1002/mds.10140. [DOI] [PubMed] [Google Scholar]
- 4.Bird MR, Woodward MC, Gibson EM, Phyland DJ, Fonda D. Asymptomatic swallowing disorders in elderly patients with Parkinson's disease: a description of findings on clinical examination and videofluoroscopy in sixteen patients. Age and Ageing. 1994;23:251–254. doi: 10.1093/ageing/23.3.251. [DOI] [PubMed] [Google Scholar]
- 5.Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Rüb U, Gai WP, Del Tredici K. Idiopathic Parkinson's disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. Journal of Neural Transmission. 2003;110:517–536. doi: 10.1007/s00702-002-0808-2. [DOI] [PubMed] [Google Scholar]
- 7.Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: Degradation of 50-khz ultrasonic vocalization in rats. Behavioral Neuroscience. 2009;123:328–336. doi: 10.1037/a0014593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ciucci MR, Connor NP. Dopaminergic influence on rat tongue function and limb movement initiation. Experimental Brain Research. 2009;194:587–596. doi: 10.1007/s00221-009-1736-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behavioural Brain Research. 2007;182:284–289. doi: 10.1016/j.bbr.2007.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciucci MR, Ma ST, Kane JR, Ahrens AM, Schallert T. Limb use and complex ultrasonic vocalization in a rat model of Parkinson's disease: deficit-targeted training. Parkinsonism & Related Disorders. 2008;14 Suppl 2:S172–S175. doi: 10.1016/j.parkreldis.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85:299–305. doi: 10.1046/j.1471-4159.2003.01657.x. [DOI] [PubMed] [Google Scholar]
- 12.Connor NP, Russell JA, Wang H, Jackson MA, Mann L, Kluender K. Effect of tongue exercise on protrusive force and muscle fiber area in aging rats. Journal of Speech, Language, and Hearing Research. 2009;52:732–744. doi: 10.1044/1092-4388(2008/08-0105). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Das S, Fowler SC. An update of Fowler and Das: anticholinergic reversal of haloperidol-induced, within-session decrements in rats' lapping behavior. Pharmacol Biochem Behav. 1996;53:853–855. doi: 10.1016/0091-3057(95)02094-2. [DOI] [PubMed] [Google Scholar]
- 14.Ertekin C, Tarlaci S, Aydogdu I, Kiylioglu N, Yuceyar N, Turman AB, Secil Y, Esmeli F. Electrophysiological evaluation of pharyngeal phase of swallowing in patients with Parkinson's disease. Movement Disorders. 2002;17:942–949. doi: 10.1002/mds.10240. [DOI] [PubMed] [Google Scholar]
- 15.Fleming SM, Delville Y, Schallert T. An intermittent, controlled-rate, slow progressive degeneration model of Parkinson's disease: antiparkinson effects of Sinemet and protective effects of methylphenidate. Behavioural Brain Research. 2005;156:201–213. doi: 10.1016/j.bbr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Foley P, Riederer P. Pathogenesis and preclinical course of Parkinson's disease. Journal of Neural Transmission Supplementum. 1999;56:31–74. doi: 10.1007/978-3-7091-6360-3_2. [DOI] [PubMed] [Google Scholar]
- 17.Fowler SC, Das S. Haloperidol-induced decrements in force and duration of rats' tongue movements during licking are attenuated by concomitant anticholinergic treatment. Pharmacol Biochem Behav. 1994;49:813–817. doi: 10.1016/0091-3057(94)90228-3. [DOI] [PubMed] [Google Scholar]
- 18.Fowler SC, Mortell C. Low doses of haloperidol interfere with rat tongue extensions during licking: a quantitative analysis. Behavioral Neuroscience. 1992;106:386–395. doi: 10.1037//0735-7044.106.2.386. [DOI] [PubMed] [Google Scholar]
- 19.Fowler SC, Wang G. Chronic haloperidol produces a time- and dose-related slowing of lick rhythm in rats: implications for rodent models of tardive dyskinesia and neuroleptic-induced parkinsonism. Psychopharmacology. 1998;137:50–60. doi: 10.1007/s002130050592. [DOI] [PubMed] [Google Scholar]
- 20.Fuh JL, Lee RC, Wang SJ, Lin CH, Wang PN, Chiang JH, Liu HC. Swallowing difficulty in Parkinson's disease. Clinical Neurology and Neurosurgery. 1997;99:106–112. doi: 10.1016/s0303-8467(97)00606-9. [DOI] [PubMed] [Google Scholar]
- 21.Fulceri F, Biagioni F, Lenzi P, Falleni A, Gesi M, Ruggieri S, Fornai F. Nigrostriatal damage with 6-OHDA: validation of routinely applied procedures. Ann N Y Acad Sci. 2006;1074:344–348. doi: 10.1196/annals.1369.032. [DOI] [PubMed] [Google Scholar]
- 22.Herrera-Marschitz M, Casas M, Ungerstedt U. Caffeine produces contralateral rotation in rats with unilateral dopamine denervation: comparisons with apomorphine-induced responses. Psychopharmacology. 1988;94:38–45. doi: 10.1007/BF00735878. [DOI] [PubMed] [Google Scholar]
- 23.Johnson AM, Ciucci MR, Russell JA, Hammer MJ, Connor NP. Ultrasonic output from the excised rat larynx. Journal of the Acoustical Society of America. 2010;128:EL75–EL79. doi: 10.1121/1.3462234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson's disease. Dysphagia. 1997;12:11–18. doi: 10.1007/pl00009512. discussion 19-. [DOI] [PubMed] [Google Scholar]
- 25.Marshall JF. Somatosensory inattention after dopamine-depleting intracerebral 6-OHDA injections: spontaneous recovery and pharmacological control. Brain Research. 1979;177:311–324. doi: 10.1016/0006-8993(79)90782-0. [DOI] [PubMed] [Google Scholar]
- 26.Miller N, Noble E, Jones D, Burn D. Hard to swallow: dysphagia in Parkinson's disease. Age and Ageing. 2006;35:614–618. doi: 10.1093/ageing/afl105. [DOI] [PubMed] [Google Scholar]
- 27.Monte FS, da Silva-Júnior FP, Braga-Neto P, Nobre e Souza MA, de Bruin VM. Swallowing abnormalities and dyskinesia in Parkinson's disease. Movement Disorders. 2005;20:457–462. doi: 10.1002/mds.20342. [DOI] [PubMed] [Google Scholar]
- 28.Moss SJ, Birkestrand B, Fowler SC. The neuroimmunophilin GPI-1046 partially protects against 3-acetylpyridine toxicity in the rat. Neurosci Lett. 2002;321:53–56. doi: 10.1016/s0304-3940(01)02571-x. [DOI] [PubMed] [Google Scholar]
- 29.Moss SJ, Wang G, Chen R, Pal R, Fowler SC. 3-acetylpyridine reduces tongue protrusion force but does not abolish lick rhythm in the rat. Brain Research. 2001;920:1–9. doi: 10.1016/s0006-8993(01)02790-1. [DOI] [PubMed] [Google Scholar]
- 30.Nagaya M, Kachi T, Yamada T. Effect of swallowing training on swallowing disorders in Parkinson's disease. Scandinavian Journal of Rehabilitation Medicine. 2000;32:11–15. doi: 10.1080/003655000750045677. [DOI] [PubMed] [Google Scholar]
- 31.Nagaya M, Kachi T, Yamada T, Igata A. Videofluorographic study of swallowing in Parkinson's disease. Dysphagia. 1998;13:95–100. doi: 10.1007/PL00009562. [DOI] [PubMed] [Google Scholar]
- 32.Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, Holschneider DP, Nacca A, Walsh JP, Jakowec MW. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Movement Disorders. 2010;25 Suppl 1:S141–S145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plowman-Prine EK, Sapienza CM, Okun MS, Pollock SL, Jacobson C, Wu SS, Rosenbek JC. The relationship between quality of life and swallowing in Parkinson's disease. Movement Disorders. 2009;24:1352–1358. doi: 10.1002/mds.22617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potulska A, Friedman A, Królicki L, Spychala A. Swallowing disorders in Parkinson's disease. Parkinsonism & Related Disorders. 2003;9:349–353. doi: 10.1016/s1353-8020(03)00045-2. [DOI] [PubMed] [Google Scholar]
- 35.Przuntek H, Müller T, Riederer P. Diagnostic staging of Parkinson's disease: conceptual aspects. Journal of Neural Transmission. 2004;111:201–216. doi: 10.1007/s00702-003-0102-y. [DOI] [PubMed] [Google Scholar]
- 36.Robbins JA, Logemann JA, Kirshner HS. Swallowing and speech production in Parkinson's disease. Annals of Neurology. 1986;19:283–287. doi: 10.1002/ana.410190310. [DOI] [PubMed] [Google Scholar]
- 37.Rommelfanger KS, Edwards GL, Freeman KG, Liles LC, Miller GW, Weinshenker D. Norepinephrine loss produces more profound motor deficits than MPTP treatment in mice. Proc Natl Acad Sci U S A. 2007;104:13804–13809. doi: 10.1073/pnas.0702753104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 39.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 40.Skitek EB, Fowler SC, Tessel RE. Effects of unilateral striatal dopamine depletion on tongue force and rhythm during licking in rats. Behavioral Neuroscience. 1999;113:567–573. doi: 10.1037//0735-7044.113.3.567. [DOI] [PubMed] [Google Scholar]
- 41.Smittkamp SE, Brown JW, Stanford JA. Time-course and characterization of orolingual motor deficits in B6SJL-Tg(SOD1-G93A)1Gur/J mice. Neuroscience. 2008;151:613–621. doi: 10.1016/j.neuroscience.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ungerstedt U. Striatal dopamine release after amphetamine or nerve degeneration revealed by rotational behaviour. Acta Physiologica Scandinavica Supplementum. 1971;367:49–68. doi: 10.1111/j.1365-201x.1971.tb10999.x. [DOI] [PubMed] [Google Scholar]
- 44.Ungerstedt U, Arbuthnott GW. Quantitative recording of rotational behavior in rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. Brain Research. 1970;24:485–493. doi: 10.1016/0006-8993(70)90187-3. [DOI] [PubMed] [Google Scholar]
- 45.VanLeeuwen JE, Petzinger GM, Walsh JP, Akopian GK, Vuckovic M, Jakowec MW. Altered AMPA receptor expression with treadmill exercise in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Journal of Neuroscience Research. 2010;88:650–668. doi: 10.1002/jnr.22216. [DOI] [PubMed] [Google Scholar]
- 46.Woodlee MT, Schallert T. The interplay between behavior and neurodegeneration in rat models of Parkinson's disease and stroke. Restorative Neurology and Neuroscience. 2004;22:153–161. [PubMed] [Google Scholar]